Abstract
Introduction
Studies on the association of cancer and risk of dementia are inconclusive due to result heterogeneity and concerns of survivor bias and unmeasured confounding.
Methods
This study uses data from the Memento cohort, a French multicenter cohort following persons with either mild or isolated cognitive complaints for a median of 5 years. Illness‐death models (IDMs) were used to estimate transition‐specific hazard ratios (HRs) and 95% confidence intervals (CIs) for incident cancer in relation to dementia from time since study entry.
Results
The analytical sample (N = 2258) excluded 65 individuals without follow‐up information. At the end of follow‐up, 286 individuals were diagnosed with dementia, 166 with incident cancer, and 95 died. Incident cancer was associated with a reduced risk of dementia (HR = 0.58, 95% CI = 0.35‐0.97), with a corresponding E‐value of 2.84 (lower CI = 1.21).
Discussion
This study supports a protective relationship between incident cancer and dementia, encouraging further investigations to understand potential underlying mechanisms.
Keywords: Alzheimer's disease, cancer, dementia, epidemiology, illness‐death model, selection bias
1. BACKGROUND
Cancer has been identified by several epidemiological studies to be protective against dementia, particularly of Alzheimer's disease (AD) type. 1 , 2 , 3 In a large prospective community cohort study, Driver et al. estimated that incident cancer was associated with an ≈30% reduction in risk of AD dementia. 1 Similarly, a U.K.‐based prospective cohort estimated that cancer not only reduced the risk of any AD‐related dementia, but also reduced future cancer risk by up to 70%. 4 However, results are not always consistent across literature. 5 , 6 , 7 , 8 For example, although a recent nationwide Danish cohort study also showed cancer to be initially protective against AD, the strength of this effect diminished over time. 2 Such results could suggest an effect of surveillance bias due to interval censoring of dementia, the impact of competing risks of death, or potentially differential misclassification.
Arguments against an apparent protective effect have been discussed in previous literature. 9 Individuals with cancer may be less likely to survive long enough to develop dementia—or those that do survive may have more favorable characteristics that are protective against dementia, all of which could result in a protective effect due to selection (survivor bias). In addition, individuals with cancer may be less likely to be screened for dementia potentially due to suspicions of cognitive deficits resulting from “chemo brain” rather than dementia (surveillance bias). Alternatively, individuals with dementia may be less likely to be diagnosed with cancer due to a reduced likelihood for medical practitioners to screen for cancer, thereby potentially leading to erroneous conclusions. Finally, potential unmeasured confounders associated with treatment effects– whereas treatment for one disease may influence risk for the other disease—have also been proposed as a driver for the observed protective effects. 9
Statistical methodology exists to tackle many of the potential biases, although most previous research has been limited, for example, to the use of standard survival models, which ignores the potential role of competing events, or to simulation studies. 10 Illness‐death models (IDMs), similar to competing risk analyses, can help to account for survivor bias by accurately considering differential mortality while concurrently modeling risk of disease progression (ie, competing risks). This study investigates the effect of incident cancer on dementia risk in a large clinical cohort of persons with either mild cognitive impairment (MCI) or isolated cognitive complaints, with the hypothesis that processes related to cancer and dementia are inversely related.
RESEARCH IN CONTEXT
Systematic review: Relevant background literature was identified through PubMed using multiple search terminologies; published research was also screened for relevant citations. Overall, previous research suggests a protective effect of incident cancer on risk of dementia, albeit results are inconclusive regarding whether the observed effect is a result of bias.
Interpretation: Incident cancer was associated with an ≈50% reduction in the risk of dementia in a large cohort of participants with either mild cognitive impairment (MCI) or isolated cognitive impairment at study entry. Implementation of alternative statistical models aimed at targeting biases had little or no impact on effect estimates.
Future directions: The results from this large clinical study support previous evidence that has found cancer to be associated with a reduction in the risk of dementia. Research investigating changes in brain endophenotypes could aid in clarifying underlying mechanisms of this association and offer new perspectives into dementia prevention.
2. METHODS
2.1. Memento cohort
The Memento cohort is a French, multicenter cohort that aims to contribute to improving current knowledge on the natural history of Alzheimer's disease and related disorders (ADRD), and to identify new patient phenotypes associated with risk of developing dementia. Included in the Memento cohort are patients from the 26 participating memory clinics across France between 2011 and 2014. Upon inclusion in the Memento cohort, participants were followed at least annually for a median of 5 years. 11 Individuals were eligible for inclusion if they (1) were 18 years or older; presented with at least one cognitive deficit defined as performing worse than 1 SD to the mean in one or more cognitive domains (considered as MCI), or (2) presented with an isolated cognitive complaint and were 60 years of age or older. They also had to score at clinical dementia rating (CDR) scale ≤0.5 (ie, not demented); have sufficient visual and auditory abilities to partake in neuropsychological testing; and have health insurance, as required by the French government (France has universal access to health care for all legal residents, independent of age, professional standing, or revenue). 12 All participants signed an informed consent form.
2.2. Cancer identification
Malignant cases of prevalent and incident cancer were identified from a questionnaire on antecedent medical or surgical events and reported adverse events during the follow‐up period administered by medical doctors, nurses, or neuropsychologists. All medical events were coded using the Medical Dictionary for Regulatory Activities (MedRA) terms (https://www.meddra.org/). Cancer cases were identified using the MedRA term Neoplasms benign, malignant, and unspecified (incl. cysts and polyps) at the “system organ class” level. Non‐malignant cancer cases were then identified using “Preferred Terms” and subsequently excluded from all analyses. Participants without recorded information related to antecedent cancer diagnosis or related therapeutic intervention were assumed to be cancer‐free. To avoid potential survivor bias associated with prevalent cancer cases, this study restricted to incident cancer cases only.
2.3. Outcome evaluation
Participants’ dementia status during follow‐up was assessed by neurologists according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Revision (DSM‐IV) criteria. Following initial diagnosis, case files were provided to members of an independent committee composed of expert neurologists/geriatricians that reviewed available data individually; if consensus was reached, the individual's dementia status in question was confirmed. Dementia possible etiology was also ascertained using criteria put forth by National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) for AD dementia. 13 Individuals wrongly included with prevalent dementia were excluded from all analyses (N = 14).
2.4. Statistical analyses
Descriptive statistics were stratified based on dementia status at the end of the follow‐up period.
An IDM (multi‐state model, MSM) was estimated using the mstate package in R. 14 Individuals’ transition intensities between states (non‐demented, AD dementia, death) were modeled using a clock‐forward (ie, continuous time) approach, for which time‐at‐risk started with date of study entry and ended with either date of dementia diagnosis, date of death, or study exit. Individuals were recorded as dead (yes/no) if death occurred or were censored at last known date of contact. Incident cancer was included as a time‐invariant covariate, such that cancer status did not change during follow‐up. Estimated transition‐specific hazard ratios (HRs) and 95% confidence intervals (CIs) represent the ratio of the instantaneous risk of moving from one state to another, for example, individuals diagnosed with incident cancer with reference to those without.
Potential confounders were identified using directed acyclic graphs (DAGs) displayed in Figure S1. An initial model (Model 1) included sociodemographic factors (1) age at study entry (centered), sex (reference: males), and education level (reference: Highschool degree or lower), as well as the dementia‐specific risk factor (2) apolipoprotein E (APOE) genotype (none [reference] vs. at least one ε4 allele). In a second step (Model 2), body mass index (BMI) and smoking status (never smoker [reference], previous smoker, and current smoker) were included in the model given the established impact on cancer and particularly cancer‐related mortality. 15 , 16 Interactions between cancer and other covariates were assessed using a likelihood ratio test (P ≤ 0.05).
To determine the potential differential effect of cancer on dementia type, two definitions of the outcome were compared: (1) all dementia regardless of etiology (dementia); and (2) AD dementia including dementias of mixed etiology (AD dementia). To note, an individual diagnosed with a dementia of mixed etiology is diagnosed with both AD dementia in addition to, for example, vascular dementia. To assess the robustness of results in the potential presence of bias, two alternate modeling approaches were used: (1) a traditional Cox proportional hazards model including incident cancer as a time‐invariant covariate, as well as (2) a Cox proportional hazards model including incident cancer as a time‐varying covariate. 17 , 18 Creation of a time‐varying covariate was done by splitting follow‐up time into separate risk sets (ie, pre‐ and post‐incident cancer diagnosis); partitioning follow‐up time into stratified risk sets can aid in controlling for surveillance bias. For these models, time‐at‐risk started with time at study entry and ended with dementia diagnosis, death, or study completion; whichever came first. The proportional hazards assumption was assessed for all models. 19
To determine the strength of results in the presence of unmeasured confounders, E‐values were calculated using the EValue package in R. 20 , 21 Briefly, an E‐value provides an estimate of the required effect size needed for an unmeasured confounder, or set of confounders, to explain away an observed association. For example, an E‐value of 3.0 with an associated lower limit of the confidence interval (LCI) of 2.1 would mean that an unmeasured confounder with an effect size of 3.0 (LCI = 2.1)—in association with both the exposure and the outcome—could render the observed effect estimate of a study null. This implies that for higher E‐values, a strong unmeasured confounder would be required to explain away study effect estimates, whereas low E‐values suggest that study results are likely not robust and thus susceptible to unmeasured confounding.
All statistical analyses were performed using R statistical software, version 3.5.3 (https://www.R‐project.org/).
3. RESULTS
The original Memento cohort included 2323 participants. Following the exclusion of participants with no follow‐up data (N = 51) and with prevalent dementia (N = 14), the analytical sample included 2258 participants with 9738.8 person‐years of follow‐up time. During follow‐up (median = 5.0 years), the incidence rate for dementia diagnosis was 29.4 per 1000 person‐years (N = 286) and 16.9 per 1000 person‐years (N = 166) for development of malignant cancer. Malignant cancer occurred prior to dementia in 61% (N = 11) of incident cancer cases. The mortality rate during follow‐up was 9.8 per 1000 person‐years (N = 95). Table 1 presents baseline characteristics of the analytical sample stratified by incident cancer status. Overall, the average age was 72.8 years at study entry, more than 60% of the sample (N = 1399) was female, roughly two‐thirds (N = 1709) had a degree beyond high school (e.g., Bachelor's degree or higher), and nearly 30% had at least one ε4 allele of the APOE genotype (N = 636). Most cancers were classified as either “Other” (52.7%) or skin cancer (10.9%).
TABLE 1.
Study characteristics of analytical sample stratified by incident cancer status. The Memento cohort 2011‐2019
| Incident cancer status | ||
|---|---|---|
| Characteristic [Missing] | No cancer (N = 2093) | Cancer (N = 165) |
| Age at entry, mean (SD) | 70.9 (8.8) | 72.6 (7.9) |
| Body mass index (BMI), mean (SD) | 25.6 (4.4) | 25.6 (4.4) |
| Age at entry [0]; n (%) | ||
| 60 and younger | 226 (10.8) | 9 (5.5) |
| 61‐70 | 662 (31.6) | 50 (30.3) |
| 71‐80 | 910 (43.5) | 78 (47.3) |
| 80+ | 295 (14.1) | 28 (17.0) |
| Sex, female [0]; n (%) | ||
| Male | 779 (37.2) | 80 (48.5) |
| Female | 1314 (62.8) | 85 (51.5) |
| Education level [2]; n (%) | ||
| High school diploma or lower | 511 (24.4) | 36 (21.8) |
| Professional or technical degree | 763 (36.5) | 60 (36.4) |
| Bachelor's degree or higher | 817 (39.1) | 69 (41.8) |
| Smoking status [13]; n (%) | ||
| Never | 1228 (59.0) | 84 (50.9) |
| Previous smoker | 705 (33.9) | 66 (40.0) |
| Current smoker | 147 (7.1) | 15 (9.1) |
| Incident cancer, Yes [0]; n (%) | ||
| Breast cancer | – | 16 (9.7) |
| Colon cancer | – | 3 (1.8) |
| Leukemia | – | 6 (3.6) |
| Lung cancer | – | 4 (2.4) |
| Lymphomas | – | 3 (1.8) |
| Prostate cancer | – | 15 (9.1) |
| Skin cancer | – | 18 (10.9) |
| Thyroid cancer | – | 13 (7.9) |
| Other cancer | – | 87 (52.7) |
| At least one APOE ε4 allele [118]; n (%) | 496 (26.5) | 140 (51.9) |
| No ε4 alleles | 1396 (70.4) | 108 (68.8) |
| At least one ε4 allele | 587 (29.6) | 49 (31.2) |
| Dementia status (at end of follow‐up) | ||
| No dementia | 1825 (87.2) | 147 (89.1) |
| Dementia | 268 (12.8) | 18 (10.9) |
Incident cancer was associated with a roughly 42% reduction in risk of dementia (HR) = 0.58, 95% CI) = 0.35‐0.97), whereas for AD dementia it was associated with a 55% reduction in risk (HR = 0.45, 95% CI = 0.24‐0.85) (Figure 1). Results from multistate models using alternative definitions of the outcome are presented in Figure 1. In comparison to the less‐restrictive outcome definition including all cases of dementia regardless of etiology, the effect of incident cancer on the transition intensity to AD dementia was increasingly protective (Figure 1). In comparison to Model 1, additionally adjusting for smoking status and BMI had no meaningful impact on estimated transition intensities (Figure 1). There was no evidence for an interaction between cancer and other covariates, notably with APOE ε4 allele carriers (P = 0.09). The estimated E‐values were 2.84 (LCI = 1.21) and 3.87 (LCI = 1.63) for dementia and AD dementia, respectively. Incident cancer was associated with an elevated transition intensity from healthy to death (HR = 3.23, 95% CI = 1.85‐5.62), with a similarly elevated risk from dementia to death, albeit not significant (HR = 2.75; 95% CI = 0.79‐9.50) (Table S1).
FIGURE 1.
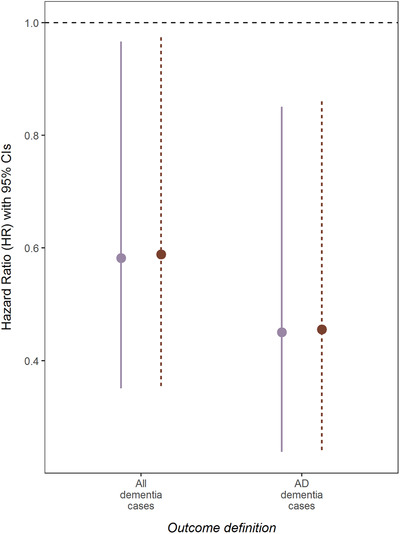
Multi‐state results: Hazard ratios (HRs) and 95% confidence intervals (CIs) of incident cancer on the transition to dementia using alternative outcome definitions. The solid line depicts estimated coefficients adjusted for: Age at study entry, sex, education level, and apolipoprotein E (APOE) genotype (Model 1). The dashed line depicts estimated coefficients adjusted for: Age at study entry, sex, education level, APOE genotype, smoking status, and body mass index (BMI) (Model 2)
In the sensitivity analyses using the traditional survival analysis, the intensity of the protective effect of incident cancer on AD dementia remained unchanged (HR = 0.45, 95% CI = 0.24‐0.85). When including cancer as a time‐varying covariate, the effect size remained constant, but was no longer significant (HR = 0.45, 95% CI = 0.18‐1.11) (Table S2).
4. DISCUSSION
4.1. Summary of results
Incident cancer was associated with an ≈50% reduction in the risk of dementia in a large cohort of participants with either MCI or isolated cognitive impairment at study entry. This reduction in risk was observed regardless of the outcome definition (ie, all dementias or only AD dementia). These results remained generally unchanged even when using alternative statistical models.
4.2. Comparison with previous literature
This observed protective effect of cancer against dementia is largely in line with previous research. 1 , 4 , 10 , 22 , 23 For example, a recent prospective cohort study found that individuals diagnosed with cancer during follow‐up had a 3‐fold reduction in risk of AD. 4 Recent meta‐analyses similarly reflect a protective effect in extant literature, estimating an overall pooled effect size associated with a 15% to 37% decrease in risk of AD. 24 , 25 In contrast, a study on community‐dwelling persons between 1992 and 2008 found that although individuals diagnosed with incident cancer were initially estimated to have up to a 35% reduction in dementia risk, when including cancer as a time‐updated variable effect estimates were attenuated and no longer indicative of a protective effect in younger ages. 5 However, these discrepancies could be partly attributable to induced immortal time bias, given that the analyses including cancer as a time‐dependent covariate included prevalent cases of cancer pre‐1992, but incident AD dementia cases were restricted to only those that occurred after 1994. 5 , 26 Variation in follow‐up time may also affect risk estimates, as the protective effect of cancer on dementia risk may be influential for only a limited period post‐cancer diagnosis. 2 , 5 Supporting this, in the study by Ording et al., cancer was found to be protective against AD only up to 10 years after diagnosis. 2 A limited time‐window for reduction in risk may also explain why incident cancer has more often been found to be protective against dementia, but not prevalent cancer. 6 , 7 Finally, results in the present study are consistent with previous research that found a stronger effect of cancer on reducing the risk of AD dementia etiology. For example, a recent prospective cohort study found evidence for a protective effect of cancer on AD, but not on vascular dementia. 4 Unfortunately, given the limited number of non‐AD cases, further investigation into the role of dementia etiologies was not plausible in the current study.
Implementation of alternative statistical models aimed at targeting biases had little or no impact on effect estimates in this study. In addition, the estimated E‐values of 2.8 and 3.9 (models considering dementia and AD dementia, respectively) demonstrate that results are reasonably robust and that a relatively strong confounder would be required to nullify the present results. For the sake of comparison, the estimated E‐value for APOE—the covariate with the strongest association with risk of dementia (HR = 2.87, 95% CI = 2.26‐3.65) in the present analysis—was 3.53 (LCI = 2.9) (results not shown). A potential unmeasured confounder not considered may be cancer treatment insomuch that cancer treatment is associated with a cancer diagnosis, but also may engender temporary effects on cognition, such as “chemobrain.” 27 In addition, cancer‐related changes in cognition may be influenced by cognitive resilience, insomuch as an individual's psychological and biological capacity to handle stress may contribute to cognitive deficits or even cognitive improvements post‐diagnosis. 28 , 29 However, a recent population‐based study by Ospina‐Romero et al. found that overall cancer treatment did not impact cognitive trajectories among patients with cancer. 30 Adding to this, results from a recent study that investigated the evidence for a causal association between cancer and AD using Mendelian randomization techniques found that genetically predicted cancer was associated with a reduced likelihood of AD dementia. 31 Taken together, these results contribute to evidence for a true protective effect of cancer on reducing the risk of dementia. However, although it appears likely that cancer reduces the risk of dementia, understanding how or what pathways contribute to this putative effect remain in question and likely contribute to continued skepticism. 9 , 28 , 31 , 32 , 33 Research investigating potential mechanisms contributing to the observed effect of cancer on dementia, but also the potential role of resilience and cancer treatment is thus needed. To this effect, research on the impact of cancer on changes to brain endophenotypes could aid in clarifying disease mechanisms by determining whether cancer acts on dementia risk through limiting neurodegeneration.
4.3. Strengths and limitations
This study uses data collected in a large clinic‐based cohort study. Although this study design may limit generalizability to the general population and contribute to differing results in comparison with other population‐based cohort studies, it ensures a well‐phenotyped, accurate characterization of individuals’ dementia status and an adequately powered sample to investigate the topic in question. However, data available on cancer status were based on self‐reported information. Although efforts were undertaken by study personnel to verify the accuracy of all reported adverse events during follow‐up, inaccurate recall could be possible. Furthermore, the present study employed multiple alternative statistical methodologies to account for several potential biases that reinforced the robustness of the finding. Likewise, the relative uniformity of results regardless of the statistical model implemented suggest the limited role of survivor and surveillance bias in the present sample. Moreover, participants included in the Memento cohort enter the study with a cognitive complaint. Because older individuals presenting with cognitive complaints are less likely to be screened for cancer in the primary care setting, 34 this restriction may aid in limiting the effects of surveillance bias given that all study participants are equally susceptible to a reduced likelihood of cancer screening or referral. However, our data are not in favor of such a bias as we did not find an association between self‐reported cognitive complaints level at baseline (estimated using a 1–10 Likert scale) and likelihood of incident cancer during follow‐up (results not shown). Because a general population (GP)‐based cohort necessarily includes a mix of individuals with cognitive complaints (but without dementia diagnosis) and individuals without cognitive complaints, there is potentially a greater risk for systematic differences in cancer screening (ie, differential misclassification). Although this could engender nondifferential misclassification, the observed results would rather be attenuated toward the null. 35 Finally, the Memento cohort collects extensive information on behavioral, genetic, and sociodemographic risk factors, which allowed for the adjustment for potential confounders identified in the DAG.
Some limitations exist, most notable being the limited number of cancer cases, particularly of those diagnosed with both incident cancer and dementia (6.3%). Although this is indicative of the protective role that cancer appears to play in dementia risk, it impeded further investigations into the potential differential impact of specific cancer types on risk as well as a more sophisticate multi‐state model (ie, including incident cancer diagnosis as a separate state). 8 , 22 , 35 , 36 Information on cancer stage was also not available; due to the influence of cancer stage on reductions in survival, stratification by cancer stage could have permitted a further in‐depth investigation into survival bias. However, in a national cohort study on US veterans, adjustment for cancer severity was not found to impact results in previous studies. 6 Adding to this, due to the limited cases, it was not possible to stratify analyses by cancer type. Therefore, it was not possible to investigate potential differential effects on dementia risk according to cancer type. To this effect, previous research has found that the protective effect of cancer on dementia risk may be limited to certain types of cancer, notably head and neck cancers. 2 , 23 , 35 Finally, due to the limited amount of follow‐up time, it was not possible to investigate the duration of protective effect of cancer on dementia risk.
5. CONCLUSION
The results from this large clinical study support previous evidence that has found cancer to be associated with a reduction in the risk of dementia. 23 Future research that investigates the mechanistic underpinnings driving this relationship are needed to improve the understanding of the mechanistic behind this putative relationship. Epidemiological research aimed at investigating changes in brain endophenotypes could aid in this endeavor. In addition, investigations into the potential mediating role of cancer treatment could help delineate further the relationship between incident cancer and dementia risk. Given the pressing need for therapeutic options to reduce or even prevent neurocognitive decline, 37 such research could provide insights into the potential of using cancer‐related therapies in dementia treatment. 6 , 9
CONFLICTS OF INTEREST
None declared.
Supporting information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
ACKNOWLEDGEMENTS
Please refer to the appendix for the complete list of the Memento Study Group.
Chamberlain JD, Rouanet A, Dubois B, et al. Investigating the association between cancer and the risk of dementia: Results from the Memento cohort. Alzheimer's Dement. 2021;17:1415–1421. 10.1002/alz.12308
Geneviève Chêne and Carole Dufouil have contributed equally
Funding information
The MEMENTO cohort is funded by the Fondation Plan Alzheimer (Alzheimer Plan 2008‐2012), and the French Ministry of Research (MESRI, DGRI) through the Plan Maladies Neurodégénératives (2014‐2019). This work was also supported by CIC 1401‐EC, Bordeaux University Hospital (CHU Bordeaux, sponsor of the cohort), Inserm, and the University of Bordeaux. This work was undertaken using resources on the Dementias Platform UK (DPUK) Data Portal; the Medical Research Council supports DPUK through grant MR/L023784/2.
REFERENCES
- 1. Driver JA, Beiser A, Au R, et al. Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study. BMJ. 2012; 344: e1442. 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ording AG, Horváth‐Puhó E, Veres K, et al., Cancer and risk of Alzheimer's disease: Small association in a nationwide cohort study. Alzheimer's & Dementia, 2020;16(7):953–964. 10.1002/alz.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shafi O. Inverse relationship between Alzheimer's disease and cancer, and other factors contributing to Alzheimer's disease: a systematic review. BMC Neurol. 2016;16(1):236. 10.1186/s12883-016-0765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology. 2010;74(2):106‐112. 10.1212/WNL.0b013e3181c91873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanson HA, Horn KP, Rasmussen KM, Hoffman JM, Smith KR. Is Cancer Protective for Subsequent Alzheimer's Disease Risk? Evidence From the Utah Population Database. J Gerontol B Psychol Sci Soc Sci. 2017;72(6):1032‐1043. 10.1093/geronb/gbw040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frain L, Swanson D, Cho K, et al. Association of cancer and Alzheimer's disease risk in a national cohort of veterans. Alzheimers Dement. 2017;13(12):1364‐1370. 10.1016/j.jalz.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowles EJA, Walker RL, Anderson ML, Dublin S, Crane PK, Larson EB. Risk of Alzheimer's disease or dementia following a cancer diagnosis. PLoS ONE. 2017;12(6):e0179857. 10.1371/journal.pone.0179857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Willik KD, Ruiter R, Wolters FJ, et al. Mild Cognitive Impairment and Dementia Show Contrasting Associations with Risk of Cancer. Neuroepidemiology. 2018;50(3‐4):207‐215. 10.1159/000488892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganguli M. Cancer and Dementia: it's Complicated. Alzheimer Dis Assoc Disord. 2015;29(2):177‐182. 10.1097/WAD.0000000000000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayes‐Larson E, Ackley SF, Zimmerman SC, et al., The competing risk of death and selective survival cannot fully explain the inverse cancer‐dementia association. Alzheimer's & Dementia, 2020;16(12):1696–1703. 10.1002/alz.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dufouil C, Dubois B, Vellas B, et al. Cognitive and imaging markers in non‐demented subjects attending a memory clinic: study design and baseline findings of the MEMENTO cohort. Alzheimers Res Ther. 2017;9(1):67. 10.1186/s13195-017-0288-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nay O, Béjean S, Benamouzig D, Bergeron H, Castel P, Ventelou B. Achieving universal health coverage in France: policy reforms and the challenge of inequalities. Lancet. 2016;387(10034):2236‐2249. 10.1016/S0140-6736(16)00580-8 [DOI] [PubMed] [Google Scholar]
- 13. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939‐944. 10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- 14. de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non‐ and semi‐parametric multi‐state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261‐274. 10.1016/j.cmpb.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 15. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet. 2008;371(9612):569‐578. 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 16. Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non‐linear dose‐response meta‐analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ, 2016; i2156. 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. tmerge function | R Documentation. Accessed June 17, 2020. https://www.rdocumentation.org/packages/survival/versions/3.1‐11/topics/tmerge
- 18. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi‐state models. Stat Med. 2007;26(11):2389‐2430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 19. Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81(3):515‐526. 10.2307/2337123 [DOI] [Google Scholar]
- 20. VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: introducing the E‐Value. Ann Intern Med. 2017;167(4):268‐274. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 21. Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Website and R Package for Computing E‐Values. Epidemiology. 2018;29(5):e45‐e47. 10.1097/EDE.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Musicco M, Adorni F, Di Santo S, et al. Inverse occurrence of cancer and Alzheimer disease: a population‐based incidence study. Neurology. 2013;81(4):322‐328. 10.1212/WNL.0b013e31829c5ec1 [DOI] [PubMed] [Google Scholar]
- 23. Ospina‐Romero M, Glymour MM, Hayes‐Larson E, et al. Association Between Alzheimer Disease and Cancer With Evaluation of Study Biases. JAMA Network Open, 2020;3(11):e2025515. 10.1001/jamanetworkopen.2020.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma L‐L, Yu J‐T, Wang H‐F, et al. Association between cancer and Alzheimer's disease: systematic review and meta‐analysis. J Alzheimers Dis. 2014;42(2):565‐573. 10.3233/JAD-140168 [DOI] [PubMed] [Google Scholar]
- 25. Papageorgakopoulos TN, Moraitou D, Papanikolaou M, Tsolaki M. The association between Alzheimer's disease and cancer: systematic review ‐ Meta‐analysis. Hell J Nucl Med. 2017;20 Suppl:45‐57. [PubMed] [Google Scholar]
- 26. Yang X, Weng J. Increased cancer risk with drug use among patients with diabetes: are the biased methods the culprit? J Diabetes Investig. 2012;3(6):479‐480. 10.1111/jdi.12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen LD, Ehrlich BE. Cellular mechanisms and treatments for chemobrain: insight from aging and neurodegenerative diseases. EMBO Mol Med. 2020;12(6):e12075. 10.15252/emmm.202012075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parsons MW, Traeger L, Perez GK, Hirschberg A, Park ER. Resilience and cognitive symptoms in cancer: an exploratory study. JCO. 2020;38(15_suppl):e24079‐e24079. 10.1200/JCO.2020.38.15_suppl.e24079 [DOI] [Google Scholar]
- 29. Seiler A, Jenewein J. Resilience in Cancer Patients. Front Psychiatry. 2019;10:208. 10.3389/fpsyt.2019.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ospina‐Romero M, Abdiwahab E, Kobayashi L, et al. Rate of Memory Change Before and After Cancer Diagnosis. JAMA Netw Open. 2019;2(6):e196160. 10.1001/jamanetworkopen.2019.6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seddighi S, Houck AL, Rowe JB, Pharoah PDP. Evidence of a Causal Association Between Cancer and Alzheimer's Disease: a Mendelian Randomization Analysis. Sci Rep. 2019;9(1):13548. 10.1038/s41598-019-49795-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu T, Ren D, Zhu X, et al. Transcriptional signaling pathways inversely regulated in Alzheimer's disease and glioblastoma multiform. Sci Rep. 2013;3(1):3467. 10.1038/srep03467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Behrens MI, Lendon C, Roe CM. A common biological mechanism in cancer and Alzheimer's disease? Curr Alzheimer Res. 2009;6(3):196‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapelet G, Berrut G, Bourbouloux E, Campone M, Derkinderen P, de Decker L. Cancer screening practices in elderly with dementia. Gériatrie et Psychologie Neuropsychiatrie du Viellissement. 2015;13(2):133‐140. 10.1684/pnv.2015.0540 [DOI] [PubMed] [Google Scholar]
- 35. van der Willik KD, Schagen SB, Ikram MA. Cancer and dementia: Two sides of the same coin?. European Journal of Clinical Investigation, 2018;48(11):e13019. 10.1111/eci.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun M, Wang Y, Sundquist J, Sundquist K, Ji J (2020) The Association Between Cancer and Dementia: A National Cohort Study in Sweden. Frontiers in Oncology, 10 10.3389/fonc.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casey DA, Antimisiaris D, O'Brien J. Drugs for Alzheimer's disease: are they effective? P T. 2010;35(4):208‐211. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information
Supplementary information


