Abstract
Salvia clandestina L. is a wild perennial species present in the Salento area of Italy. Here, we examined the in vitro effects of an aqueous extract of S. clandestina L. on the MG‐63 osteosarcoma cell line. The extract reduced osteosarcoma cell viability mainly by way of apoptosis, as we observed (1) upregulation of gene and protein expression of p53, cyclin‐dependent kinase inhibitors p21WAF1 and p27Kip1, and proapoptotic BAX; (2) activation of caspases; and (3) induction of a sub‐G1 peak in the cell cycle. The mitogen‐activated protein kinases (MAPKs) JNK1/2 and p38 are activated and involved in the intracellular effects of the S. clandestina extract, as preincubation with the JNK1/2 inhibitor SP600125 or the p38 inhibitor SB203580 significantly decreased S. clandestina extract–induced cytotoxicity and inhibited increase in p53, p21WAF1, p27Kip1, and BAX. SP600125 also inhibited mRNA levels for all the aforementioned proteins, while SB203580 only affected p53 mRNA. Furthermore, S. clandestina extract treatment counteracted epithelial‐to‐mesenchymal transition, inhibited cell migration, and decreased the expression and activity of matrix metalloproteinase MMP2. In addition, S. clandestina extract enhanced the cytotoxic activity of cisplatin on MG‐63 cells through downregulation of the Akt/PKB protein kinase. We conclude that S. clandestina extract may be a novel agent for osteosarcoma treatment.
Keywords: Salvia clandestina L., MG‐63 osteosarcoma, apoptosis, MAPKs, epithelial‐to‐mesenchymal transition, MMPs, phenolic compounds, danshensu
In this report, we examined the effects of an aqueous extract of Salvia clandestina L. on the MG‐63 osteosarcoma cell line. The extract reduced osteosarcoma cell viability mainly by inducing apoptosis; it also counteracted epithelial‐to‐mesenchymal transition, inhibited cell migration, and decreased the expression and activity of matrix metalloproteinase MMP2, as well as enhanced the cytotoxic activity of cisplatin. S. clandestina extract may therefore be a novel agent for osteosarcoma treatment.
Introduction
Osteosarcoma is the most frequent primary solid malignancy of the bone in pediatric and adolescent patients 1 since there exists a direct relationship between rapid bone growth and osteosarcoma. 2 Osteosarcoma is associated with resistance to chemotherapy and the tendency to metastasize to the lungs. 3 Lung metastases are associated with particularly poor prognoses among patients with osteosarcoma. It is estimated that approximately 20% of the sarcoma patients have detectable metastasis at diagnosis. 4 During metastasis, many regulatory events occur that include the involvement of several specific molecules. The main metastatic processes include migration, invasion, circulation, adhesion, and colonization. In the early stages, the alteration of the extracellular matrix (ECM), which is responsible for cell–cell contact, induces metastasis and migration. Thus, matrix metalloproteinases (MMPs) contribute to the ability of osteosarcoma cells to invade and metastasize. 5 , 6 For these reasons, MMPs have been considered as an attractive therapeutic target for the treatment of inflammation‐related diseases, such as cancer. Even when combined treatments are used, the prognosis for osteosarcoma metastatic disease remains poor, and more than half of the cases relapse; at present, the best treatment strategy to manage osteosarcoma patients with lung metastasis remains unclear. In addition, despite advances, chemotherapy often is difficult for patients due to the systemic toxicities of these agents. 7 Thus, there is a need to identify new and effective treatment strategies in order to overcome the limitations of current approaches. It has been demonstrated that diet has an impact on specific types of tumor; in addition, dietary natural compounds, such as phytochemicals, can influence cancer risk and tumor behavior, preventing invasion and metastatic phase. 8 Fruit, vegetables, and medicinal plants represent the main dietary sources of polyphenols, which exert many biological activities, including antioxidant, anti‐inflammatory, antimicrobial, antiproliferative, and proapoptotic activity. 9 Emerging evidence suggests that long‐term intake of polyphenols has positive effects on the incidence of several cancers, type II diabetes, cardiovascular disease, and neurodegenerative diseases. 10
We previously examined the composition and antioxidant activity of phenols from S. clandestina, a wild perennial species present in the Salento (Apulia, Italy) area. 11 Among the 29 compounds detected, a high content of danshensu (3‐(3,4‐dihydroxy‐phenyl) 2‐hydroxy‐propinic acid), a powerful antioxidant and a cardioprotective agent, was observed; the danshensu content in S. clandestina shoots (4.96 mg g–1 DW) 11 was more than fourfold the level reported for leaf extracts of S. miltiorrhiza. 12
Starting from recent studies demonstrating antitumor properties 13 of bioactive molecules contained in S. clandestina extracts, in the present study we tested for antitumor and antimigration effects of the extracts in an in vitro model of osteosarcoma using MG‐63 cancer cells.
Materials and methods
Materials
Eagle's minimum essential medium, antibiotics, glutamine, and fetal bovine serum (FBS) were purchased from Celbio (Milan, Italy). Antibodies to caspase‐9 and caspase‐3, Bax, Bid, Bcl‐2, poly (ADP‐ribose) polymerase (PARP), MMP2, MMP9, phospho‐Akt/PKB, phospho‐JNK1/2, and phospho‐p38 were obtained from Cell Signaling Technology (Celbio). Antibodies to p21WAF1/CIP1, p53, p27Kip1, CDKs, cyclin E, E‐cadherin, vimentin, JNK1/2, p38, and goat anti‐rabbit antibody conjugated with peroxidase and control antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Cell‐permeable broad‐spectrum caspase inhibitor z‐VAD‐fmk, JNK1/2 inhibitor SP600125, p38 inhibitor SB203580, and MMP inhibitor GM‐6001 were obtained from Calbiochem (Darmstadt, Germany). All other reagents were purchased from Sigma (Milan, Italy).
Plant material and phenolic extract preparation
S. clandestina L. wild plants were randomly collected from a fallow field in the town of Arnesano, near Lecce, Salento, Italy in April 2017. The plant material was harvested at the floral budding stage. Plants were identified by the Systematic Botanical Laboratory and voucher specimens deposited in the Herbarium of the Department of Biological and Environmental Sciences and Technologies, University of Salento, Italy (voucher no. Sc 24978). After collection from the field, shoots were oven dried at 35 °C until reaching a constant weight. A dried sample (1 g) was mixed with 100 °C H2O at a ratio of 1:20 (w/v) in a flask, and the suspensions were brought to boil with refluxing for 20 min, according to Zhou et al. 12 and as indicated in Nicolì et al. 11 After centrifugation, the resulting solutions were filtered using a 0.2‐μm PFTE membrane and analyzed as described below.
HPLC ESI/MS‐TOF analysis
Phenolic characterization (Fig. S1 and Table S1, online only) was carried out using an Agilent 1200 liquid chromatography system (Agilent Technologies, Palo Alto, CA) equipped with a standard autosampler. The HPLC column was an Agilent Extended C18 (1.8 μm, 2.1 × 50 mm). Separation was carried out at 40 °C with a gradient elution program at a flowrate of 0.5 mL/min. The mobile phases consisted of water plus 0.1% formic acid (A) and acetonitrile (B). The following multistep linear gradient was applied: 0 min, 5% B; 13 min, 25% B; and 19 min, 40% B. The injection volume in the HPLC system was 5 μL, and the extract was diluted 1:2 by phase A. The HPLC system was coupled to an Agilent 6320 TOF mass spectrometer equipped with a dual ESI interface (Agilent Technologies) operating in negative ion mode. Detection was carried out within a mass range of 50–1700 m/z. Accurate mass measurements of each peak from the total ion current chromatogram were obtained by means of an ISO pump (Agilent G1310B) using a dual nebulizer ESI source that introduces a low flow (20 μL/min) of a calibration solution that contains the internal reference masses at m/z 112.9856, 301.9981, 601.9790, and 1033.9881, in negative ion mode. The accurate mass data of the molecular ions were processed through the MassHunter (Agilent Technologies) software.
Quantitative analysis and total phenolics quantification
In order to establish the relationship between peak area and concentration, linear regression models were carried out using the following standards: danshensu, caffeic acid, rosmarinic acid, and salvianolic acid B. Phenolic compound contents were expressed in microgram per gram of dry matter (μg/g DW) and microgram per milliliter of extract (μg/mL) (Table S2, online only).
The total phenolic content (TPC) of the S. clandestina aqueous extract was determined according to the Folin‐Ciocalteu method 14 by reading the absorbance at 765 nm with a JASCO V‐550 UV/VIS spectrophotometer (JASCO Corporation, Tokyo, Japan); results were expressed in terms of caffeic acid equivalent (CAE) as mg/g DW and mg/mL of extract by using a caffeic acid standard curve (equation: y = 4.3039x) (Table S2, online only). All measurements were performed in triplicate.
Cell lines
The human osteosarcoma cell line MG‐63 was cultured in Eagle's minimum essential medium supplemented with 10% FBS and maintained at 37 °C in a humidified incubator with 5% CO2. Cells were grown to 70–80% confluence and then treated with extracellular nucleotides at various concentrations and for different incubation periods.
Cell viability assay and cell cycle analysis
The SRB (sulforhodamine B) assay and the conversion of MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenol tetrazolium bromide) by osteosarcoma cells were used as indicators of cell number. Viable cells were also counted by the trypan blue exclusion assay and light microscopy. The data presented are means ± standard deviation (SD) from eight replicate wells per microtiter plate.
MG‐63 cells were fixed in 70% cold ethanol and kept overnight at 4 °C. Cells were then resuspended in 20 μg/mL propidium iodide (PI) and 100 μg/mL RNase (final concentrations). Cell cycle was evaluated with the FL3 detector in the linear mode using FloMax software (Partec, Cornaredo, Milano, Italy).
Cell apoptosis assay
Cell apoptosis was determined by the annexin V‐FITC/PI assay. After treatment, cells were collected and resuspended with 500 mL binding buffer at a concentration of 106 cells/mL. After adding 5 mL annexin V‐FITC and 10 mL of propidium iodide solution, cells were mixed and incubated at room temperature in the dark for 10 minutes. Then, the samples were analyzed using a CyFlow® Space cytometer (Partec) and FloMax software (Partec), as described previously. 15
Preparation of subcellular fractions and western blotting
Subcellular fractions were obtained as previously reported. 16 Western blotting analysis, immunodetection, and densitometric analysis were performed as previously described. 16
Reverse transcriptase‐polymerase chain reaction
Total RNA from portions of tissue or cells was extracted using the SV Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's instructions. The procedures for the reverse transcriptase‐polymerase chain reaction and/or real‐time PCR were performed as described previously 16 in the presence of 2 μM of specific primers for different genes and for β‐actin (Celbio).
Cell wounding assay
The cells were seeded in 12‐well culture dishes and cultured until they reached confluence. The cells were then scraped with a 20‐μL micropipette tip, denuding a strip of the monolayer approximately 500 μm in width, as described previously. 16
MMP gelatin zymography
After treatment, the culture medium was collected and centrifuged at 14,000 rpm for 5 min at 4 °C to remove cells and debris as described previously. MMP gelatin zymography assay was performed as previously indicated. 16
Statistical analysis
The analysis was performed by a different person than the experimenters. Each experiment was repeated at least four times. Data points reported in the figures are given as means ± standard deviation (SD). The data were analyzed using GraphPad® Prism 5.0 Software (GraphPad Software, San Diego, CA). Either an unpaired Student's t‐test or one‐way ANOVA was performed. A P value of less than 0.05 was considered statistically significant. When the results were at least P < 0.05, we performed a post hoc analysis using the Bonferroni–Dunn test.
Results
Salvia clandestina L. extracts inhibit cell proliferation
In vitro proliferation data were obtained by the MTT assay and confirmed by the SRB assay to rule out potential effects of S. clandestina extracts on mitochondrial enzymes. Furthermore, comparable results were obtained when cell number was directly determined by cell counting (data not shown); consequently, we used the MTT assay in the combined experiments reported hereafter. Exposure of serum‐free medium starved MG‐63 cells to increasing doses of S. clandestina aqueous extracts (2.5–200 μg/mL) induced, starting from about 25 μg/mL TPC, a significant dose‐dependent reduction in cell viability (Fig. 1A). It should be noted that low doses of S. clandestina extract increased cell viability due to the polyphenol paradox effect. After 24‐h incubation with S. clandestina extract, the IC50 value was 60 μg/mL (Fig. 1A); therefore, this concentration was used for all subsequent experiments. Exposure of the MG‐63 cells to S. clandestina extract at concentrations ranging from 2.5 to 200 μg/mL for 24 h resulted in a dose‐dependent activation of apoptosis (Fig. S2, online only).
Figure 1.
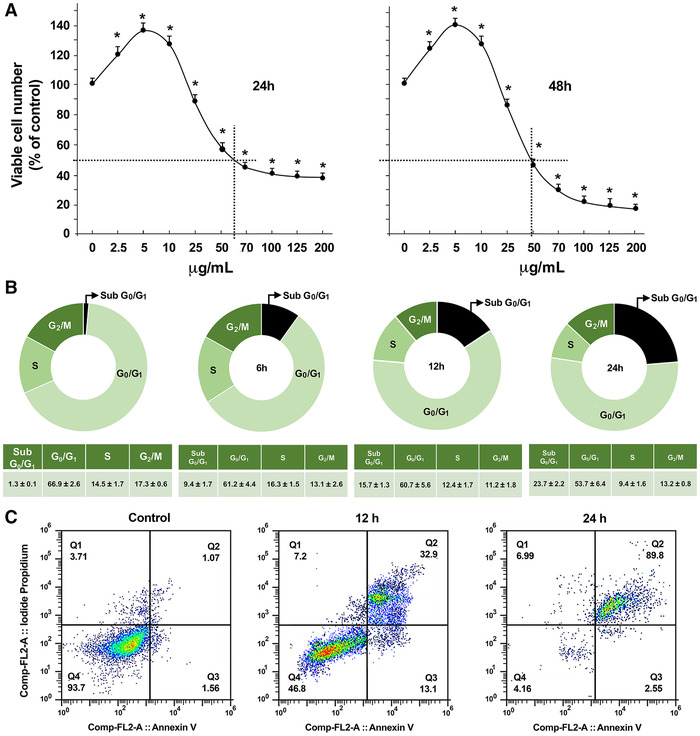
S. clandestina aqueous extract decreases MG‐63 cells viability. (A) Osteosarcoma cells treated or not with increasing concentrations of S. clandestina aqueous extract; cell viability was determined by the MTT assay and data are presented as percent of control (means ± SD of six independent experiments with eight replicates each); * P < 0.05 compared with untreated cells. (B) Top: The cell cycle was investigated by flow cytometry in propidium iodide–stained cells after treatment with 60 μg/mL of S. clandestina extract. Bottom: The percentage of cells in different cell cycle phases. (C) Cells treated or not for 12 and 24 h with 60 μg/mL of S. clandestina extract; apoptosis was determined by the annexin V‐FITC/PI assay.
Cell cycle arrest and induction of apoptosis
The effect of the extract on the cell cycle was investigated by flow cytometry in PI (propidium iodide)‐stained cells after treatment for 6, 12, and 24 hours. Notably, 60 μg/mL S. clandestina extract causes a significant and time‐dependent increase in the percentage of cells in the sub‐G1 phase (Fig. 1B). After 24 h of incubation, most of the cells treated have undergone apoptosis (Fig. 1C).
S. clandestina extract (60 μg/mL) caused the activation of caspase‐9, ‐7, and ‐3, with the generation of activated heterodimers of low molecular mass in a time‐dependent manner (Fig. 2A). Activation of caspases leads to the cleavage of several target proteins, such as PARP. Indeed, as shown in Figure 2A, the extract significantly increased the amounts of cleaved PARP after 12‐h treatment, thus confirming the induction of apoptosis. Caspase‐9 has been linked to the mitochondrial death pathway, and Bcl‐2 proteins regulate this pathway. Thus, we assessed the effects of 60 μg/mL S. clandestina extract on the expression of BAX and Bcl‐2 proteins by western blot analysis of mitochondrial fractions. S. clandestina extract increased the expression of BAX and Bid and decreased the expression of the antiapoptotic factor Bcl‐2. Furthermore, mitochondrial cytochrome c decreased significantly after 12 h of treatment (Fig. 2B). This finding suggests that apoptosis induced by S. clandestina extract is mediated through a cytochrome c–dependent activation of caspases.
Figure 2.
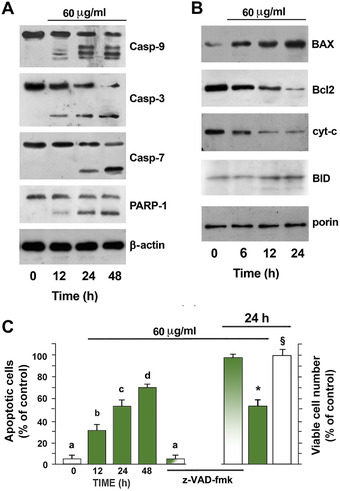
S. clandestina extract induces apoptosis in MG‐63 cells. Cytosolic nuclear and mitochondrial fractions were separated by SDS‐PAGE and analyzed by western blotting using (A) anti‐PARP‐1, anti‐caspase‐3, ‐7, and ‐9 and (B) anti‐BAX, anti‐Bcl2, anti‐cyt‐c, and anti‐BID antibodies. The purity of fractions was assessed by immunoblotting with anti‐β‐actin, A, or with anti‐porin, B, monoclonal antibodies. (C) Cells treated with and without S. clandestina extract and also pretreated with the cell‐permeable broad‐spectrum caspase inhibitor z‐VAD‐fmk (100 μM); left: quantification of the percentage of apoptotic nuclei was obtained by fluorescent staining with 4′,6‐diamidino‐2‐phenylindole (DAPI) (means ± SD; n = 6); P < 0.001 by one‐way ANOVA; values with shared letters are not significantly different according to Bonferroni/Dunn post hoc tests; right: viable cell number evaluation after 24‐h treatment. Statistically significant differences between cells treated with an aqueous extract plus z‐VAD‐fmk and with S. clandestina extract alone (* P < 0.01) or with untreated cells (§P > 0.05) by Student's t‐test.
In order to prove that the activation of caspases is an essential step in the apoptotic pathway induced by the S. clandestina extract, cells were pretreated with the cell‐permeable broad‐spectrum caspase inhibitor z‐VAD‐fmk (100 μM) for 1 h, and then treated with 60 μg/mL S. clandestina extract for 24 hours. Blockage of caspase activity markedly inhibited the cell death and apoptosis process induced by S. clandestina extract (Fig. 2C). These results suggest that the mechanism of cytotoxic effects induced by S. clandestina extract on MG‐63 cells is related to its apoptosis‐inducing activity.
Signal transduction pathways involved in S. clandestina extract–mediated effects
S. clandestina extract induced a time‐dependent phosphorylation of the mitogen‐activated protein kinases (MAPKs) JNK1/2 and p38, without affecting their basal levels (Fig. 3A). Preincubation with the JNK1/2 inhibitor SP600125 (1 and 10 μM) or with the p38 inhibitor SB203580 (1 and 10 μM) significantly decreased extract‐induced cytotoxicity (Fig. 3B). Thus, S. clandestina extract activated MAPKs, thereby forcing apoptosis.
Figure 3.
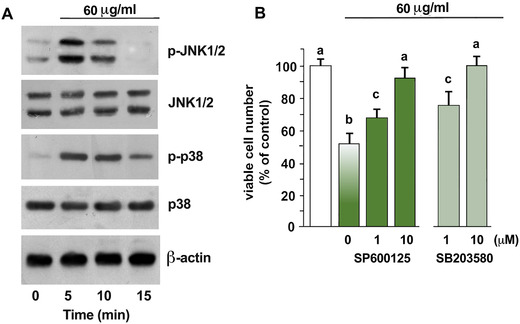
S. clandestina extract induces phosphorylation of JNK1/2 and p38. (A) Western blotting of total lysates was performed with anti‐JNK1/2 and anti‐phospho‐JNK1/2, or with anti‐p38 and anti‐phospho‐p38. Sequential incubation with anti‐β‐actin confirmed equal protein loading. The blots are representative of four independent experiments. (B) Cells were pretreated with the JNK1/2 inhibitor SP600125 or the p38MAPK inhibitor SB203580, and then incubated with 60 μg/mL of S. clandestina extract for 24 hours. The percentage of cell survival shown represents the mean ± SD of four independent experiments with eight replicates each. P < 0.001 by one‐way ANOVA (n = 4); values with shared letters are not significantly different according to Bonferroni/Dunn post hoc tests.
S. clandestina extract (60 μg/mL) caused a significant decrease in the expression of cyclin D1 and the cyclin‐dependent kinases CDK4 and CDK6 but no detectable changes in CDK2 protein expression (Fig. 4A). S. clandestina extract also modulated the expression of the CDK inhibitors p21WAF1 and p27Kip1 inasmuch as extract treatment produced a significant increase in both inhibitors (Fig. 4A). Since p21WAF1 expression may be regulated by p53, we checked the effect of the extract on p53 levels. Indeed, p53 levels were significantly increased (Fig. 4A). Therefore, we analyzed p53, p21WAF1, and p27Kip1 transcripts by real time‐PCR using specific primers, with β‐actin as an internal control. As shown in Figure 4B, S. clandestina extract induced the upregulation of these three transcripts in a time‐dependent manner.
Figure 4.
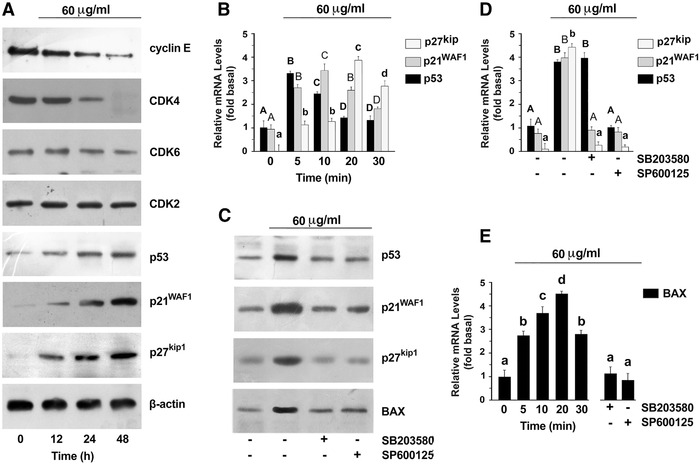
S. clandestina extract induces p53. (A) Cell lysates were separated on an SDS gel and immunoblotting was performed using antibodies to the indicated proteins. Sequential incubation with anti‐β‐actin antibody confirmed equal protein loading. These results are representative of six independent experiments. (B) Real‐time PCR; mRNA levels are presented as fold change values relative to control. Data are expressed as the mean ± SD of six different experiments. For p27Kip1, p21WAF1, and p53, P < 0.0001 by one‐way ANOVA (n = 6); values with shared lowercase (for p27Kip1), uppercase (for p21WAF1), or bold (for p53) letters are not significantly different according to Bonferroni/Dunn post hoc tests. (C) Cells were pretreated with the JNK1/2 inhibitor SP600125 or the p38 inhibitor SB203580, and then incubated with S. clandestina extract. Immunoblotting was done against the indicated proteins after 48‐h treatment. Sequential incubation with anti‐β‐actin antibody confirmed equal protein loading. (D) Cells were pretreated with the JNK1/2 inhibitor SP600125 or the p38 inhibitor SB203580 and then incubated with S. clandestina extract for different times (5 min for p53; 10 min for p21WAF1; and 20 min for p27Kip1). Real‐time PCR was performed with specific primers for p53, p21WAF1, p27Kip1, and the housekeeping gene β‐actin. mRNA levels are presented as fold change values relative to control. Data are expressed as the mean ± SD of six different experiments. For p27Kip1, p21WAF1, and p53, P < 0.0001 by one‐way ANOVA (n = 6); values with shared lowercase (for p27Kip1), uppercase (for p21WAF1), or bold (for p53) letters are not significantly different according to Bonferroni/Dunn post hoc tests. (E) MG‐63 cells were treated or not with S. clandestina extract for the indicated times or were pretreated with the JNK1/2 inhibitor SP600125 or the p38 inhibitor SB203580 and then incubated with S. clandestina extract for 20 minutes. Real‐time PCR was performed with specific primers for mRNAs for BAX and the housekeeping gene β‐actin. mRNA levels are presented as fold change values relative to control. Data are expressed as the mean ± SD of six different experiments. P < 0.0001 by one‐way ANOVA (n = 6); values with shared letters are not significantly different according to Bonferroni/Dunn post hoc tests.
The expression of p53, p21WAF1, and p27Kip1 was examined by both western blot and real‐time PCR analysis in MG‐63 cells pretreated with p38 or JNK1/2 inhibitors before S. clandestina extract incubation. Both SB203580 and SP600125 inhibited the effects of the extract on increasing p53, p21WAF1, and p27 protein expression (Fig. 4C). Furthermore, SP600125 also inhibited the levels of the three mRNAs considered, while SB203580 had effects on the p21WAF1 and p27Kip1 mRNAs but not on that of p53 (Fig. 4D). Thus, these results suggest that both p38 and JNK1/2 activations are necessary for induction of p53 by S. clandestina extract.
Transcriptional regulation of proapoptotic members of the Bcl‐2 family is involved in the initiation of apoptosis that is central to the activity of p53. The increased expression of the proapoptotic protein BAX, a Bcl‐2 family member, was observed following S. clandestina extract incubation (Fig. 2B), suggesting that p53‐mediated induction of Bcl‐2 proapoptotic family members may contribute to S. clandestina extract–induced apoptosis. Consistently, the upregulation of BAX mRNA (Fig. 4E) and protein (Fig. 4C) was inhibited by both SP600125 and SB203580, indicating that S. clandestina extract increased p53 transcriptional activity in an MAPK‐dependent manner.
S. clandestina extract inhibits the migration of osteosarcoma cells
Osteosarcoma cell migration is associated with its metastatic potential; 5 , 6 thus, we used in vitro cell culture wound closure assays to further study the effects of S. clandestina extract. Wounds were created in confluent cell cultures, and repopulation of the wound was evaluated by measuring the width of the wound over time. The width of the wound was plotted as the percentage of the control in order to quantify the effect of 20 μg/mL S. clandestina extract on cell migration (this concentration was used since it did not change cell viability, as shown in Fig. 1A). The 20 μg/mL extract significantly decreased cell migration (Fig. 5A and B), apparently not due to cytotoxicity (Fig. 1A). This effect was blocked by the JNK1/2 inhibitor SP600125 (Fig. 5B).
Figure 5.
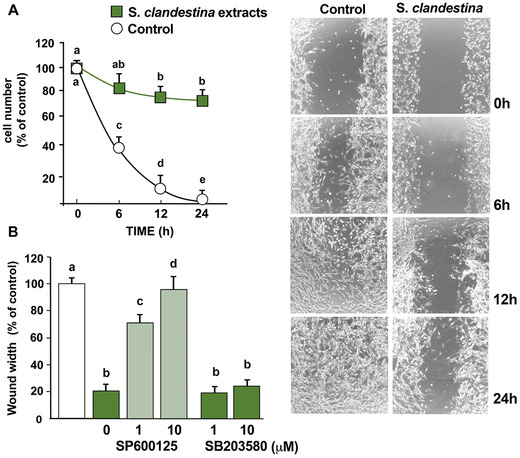
S. clandestina aqueous extract inhibits cell migration. Differential cell migration rate was examined using a wound closure assay. Cells were treated or not with 20 μg/mL of S. clandestina extract and monitored by microscopy at the indicated times. (A) Migration rate (average ± SD) and degree of wound closure were assessed by measuring the distance between wound edges at the indicated time intervals in at last eight randomly chosen regions of four different experiments normalized to 100% wound closure for control cells. P < 0.001 by one‐way ANOVA (n = 4); values with shared letters are not significantly different according to Bonferroni/Dunn post hoc test. (B) Cells were treated with the JNK1/2 inhibitor SP600125 or the p38 inhibitor SB203580 for 30 min before stimulation with or without S. clandestina extract. After 24 h, migration was evaluated. Results (means ± SD) from four independent experiments are presented. Migration was expressed as the percentage of unstimulated cells at 24 hours. P < 0.001 by one‐way ANOVA (n = 4); values with shared letters are not significantly different according to Bonferroni/Dunn post hoc tests.
S. clandestina extract induces downregulation of MMP2
MMPs play a central role in ECM degradation during osteosarcoma metastasis; 5 thus, to check whether S. clandestina extract affects the activity of MMP2 and MMP9, the main MMPs expressed by MG‐63 cells, we evaluated their activities in cell culture–conditioned medium using zymographic analysis. Treatment of cells with S. clandestina extract caused a time‐dependent decrease in MMP2 activity but no change in MMP9 activity (Fig. 6B). Consistently, S. clandestina extract also induced downregulation of MMP2 mRNA and protein levels (Fig. 6A and B).
Figure 6.
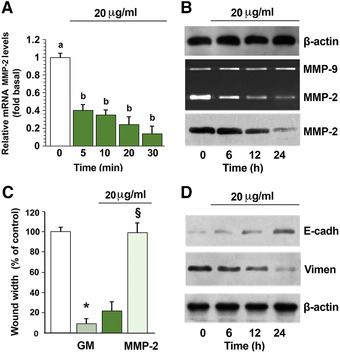
S. clandestina aqueous extract inhibits MMP2 mRNA and protein. (A) Real‐time PCR for MMP2 and for the housekeeping gene β‐actin. mRNA levels are presented as fold change values relative to control. Data were expressed as the mean ± SD of six different experiments. (B) Conditioned media and cell lysates were subjected to gelatin zymography and western blot analysis with anti‐MMP antibodies. (C) Cells were pretreated for 30 min with 10 nM of the MMP inhibitor GM‐6001 and then incubated with S. clandestina extract, in the presence or absence of 15 nM human recombinant active MMP2. After 24 h, migration was evaluated. Results (means ± SD) from four independent experiments are presented. Migration was expressed as the percentage of that of unstimulated cells. Statistically significant differences were observed between unstimulated cells and cells treated with GM‐6001 (* P < 0.0001), and between cells treated with S. clandestina extract alone and cells treated with S. clandestina extract plus human recombinant active MMP2 (§P < 0.0001) by Student's t‐test. (D) Western blotting of total lysates was performed with anti‐E‐cadherin (E‐cadh) and anti‐vimentin (Vimen) monoclonal antibodies. Sequential incubation with anti‐β‐actin antibody confirmed equal protein loading. The figures are representative of four independent experiments.
In order to understand whether decreased MMP2 was involved in the decrease in cell migration induced by the extract, we used the MMP inhibitor GM‐6001 to inhibit MG‐63 cell mobility. As shown in Figure 6C, preincubation of cells with 10 nM GM‐6001 markedly decreased cell migration, mimicking the inhibitory effect of S. clandestina extract. In addition, the use of human recombinant active MMP2 at the physiological concentration of 15 nM 17 reversed the effect of S. clandestina extract on cell migration (Fig. 6C).
S. clandestina extract reverses epithelial‐to‐mesenchymal transition in osteosarcoma cells
Epithelial‐to‐mesenchymal transition (EMT) is a crucial step for the invasion and metastasis of osteosarcoma cells. It was found that S. clandestina extract inhibited EMT, and this is accompanied by the upregulation of the epithelial marker E‐cadherin and downregulation of the protein levels of the mesenchymal marker vimentin, as shown by western blot (Fig. 6D).
Effects of S. clandestina extract and cisplatin on MG‐63 cells
Cisplatin is a well‐known chemotherapeutic agent, but tumor cell resistance to it often compromises its efficacy. We investigated the hypothesis that S. clandestina extract may be able to potentiate the cytotoxic activity of cisplatin. The viability of MG‐63 cells treated with increasing concentrations of cisplatin (from 0.1 to 200 μM) or with the combination of both cisplatin and S. clandestina extract (60 μg/mL). That concentration of the extract was chosen on the basis of the MTT assay after 24‐h incubation. Cisplatin caused dose‐dependent cytotoxicity (Fig. 7A), and the simultaneous addition of 60 μg/mL S. clandestina extract enhanced its antiproliferative potency (Fig. 7A; P < 0.001). We studied the effect on the cell cycle of S. clandestina extract in combination with cisplatin by using flow cytometry of PI‐stained cells after treatment for 24 hours. Addition of S. clandestina extract (60 μg/mL) caused a further significant increase in the percentage of apoptotic cells beyond that caused by cisplatin alone (Table 1).
Figure 7.
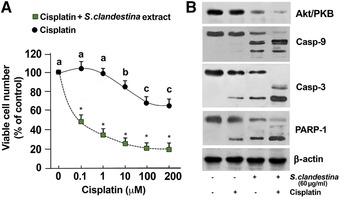
S. clandestina aqueous extract sensitizes osteosarcoma cells to cisplatin. Increasing concentrations of cisplatin in the presence or absence of 60 μg/mL of S. clandestina extract were added to MG‐63 cells. (A) Cell viability was assessed after 24 hours. Values are means ± standard deviation of five independent experiments with eight replicates in each and are presented as percent of control. * P < 0.0001 compared with cells treated with cisplatin alone, based on an unpaired Student's t‐test. (B) Cells were treated or not with cisplatin, with S. clandestina extract, or with cisplatin plus S. clandestina extract. Cell lysates were then subjected to western blot analysis. Sequential incubation with anti‐β‐actin antibody confirmed equal protein loading. The figures are representative of four independent experiments.
Table 1.
Cell cycle investigated by flow cytometry in propidium iodide
| S. clandestina (60 μg/mL) | Cisplatin (100 μM) | Sub G0/G1 | G0/G1 | S | G2/M |
|---|---|---|---|---|---|
| – | – | 1.39 ± 0.11 | 66.95 ± 2.57 | 14.49 ± 1.7 | 17.06 ± 0.61 |
| + | – | 24.97 ± 3.34* | 52.75 ± 0.48* | 9.56 ± 0.21* | 12.71 ± 0.47* |
| – | + | 6.65 ± 0.57* | 80.37 ± 2.23* | 7.67 ± 0.57* | 5.31 ± 0.37* |
| + | + | 37.48 ± 1.93 § | 45.36 ± 2.23 § | 14.05 ± 0.57 § | 3.11 ± 0.37 § |
P < 0.05 compared with untreated cells.
P < 0.05 compared with cells treated with S. clandestina extract alone.
Activation of caspase‐9 and caspase‐3 together with an increase in cleaved PARP was detected, thus revealing that S. clandestina extract could potentiate cisplatin‐induced apoptosis through the activation of the intrinsic apoptosis pathway (Fig. 7B). Cells exposed to 60 μg/mL extract for 24 h showed a significant decrease in the protein level of Akt/PKB, a kinase promoting survival and growth in response to many extracellular signals. Such downregulation of Akt/PKB was higher in cells treated with a combination of 60 μg/mL S. clandestina extract and 100 μM cisplatin compared with either agent alone (Fig. 7B).
Discussion
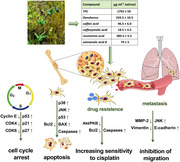
Osteosarcoma is the most common primary bone tumor in children and young adults. 1 The currently used treatment for osteosarcoma is a combination of doxorubicin, cisplatin, and methotrexate before and after surgery, with an overall 5‐year survival rate of less than 40%. 18 This therapy helps in the survival of patients with nonmetastatic conditions, but, since osteosarcoma metastasizes to the lungs in 80% of cases, 18 the survival rate is very low. 4 In addition, chemotherapeutic drugs often cause various side effects. 19 Therefore, the identification of efficient alternative drugs with less toxicity is crucial. The use of medicinal plants to improve health is an ancient practice and, more recently, many kinds of bioactive phytochemicals have been identified to exhibit antitumor and cytotoxic activities. 8 The screening and the evaluation of the antitumor features of phytochemicals could lead to the development of new pharmacologically active molecules for cancer therapy. In recent years, the potential use of Salvia as a new anticancer agent has been recognized. 13 The genus Salvia, consisting of nearly 1000 species, is among the important aromatic and medicinal plants worldwide. Among these species, we previously reported the phenolic characterization of S. clandestina L., a wild species present in the Salento (Apulia, Italy) area. 11 The main phytochemical components of S. clandestina extract are presented in the table in Figure 8. In this in vitro study, we have demonstrated that an extract obtained from this plant has antitumor effects in osteosarcoma cells.
Figure 8.
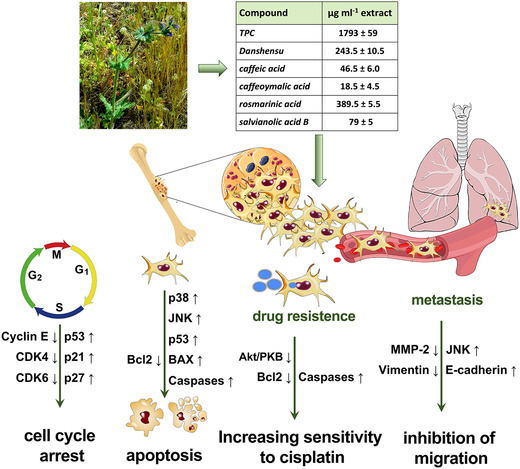
Anticancer effects and mechanisms of S. clandestina extract. The extract can directly inhibit cancer cell proliferation and kill cancer cells by induction of cell cycle arrest and apoptosis, respectively. It also overcomes drug resistance by increasing sensitivity to cisplatin, and, therefore, might be used in combination chemotherapy. In addition, S. clandestina extract decreases the metastatic potential of osteosarcoma cells.
Apoptotic death of cancer cells occurs in response to the administration of many natural compounds with antitumor activities, 13 and we show here that S. clandestina extract also exerts its antitumor effects through apoptosis. Specifically, it induces the characteristic features of apoptosis, including activation of caspase‐9, caspase‐3, and caspase‐7, and the cleavage of PARP. Caspase activation is regulated by various proteins, including the Bcl‐2 family, and after S. clandestina extract treatment, Bcl‐2 and BAX expression levels decreased and increased, respectively. It is noteworthy that many agents that inhibit cell cycle progression help to eliminate cancer cells via apoptotic or nonapoptotic mechanisms. 20
Cell cycle checkpoints are the most important cell cycle regulators, which act when DNA damage or other cell damage has occurred. If the damage cannot be properly repaired, cells will either continue to divide with aberrant DNA or undergo apoptosis. The cyclin‐dependent serine/threonine kinases and their regulatory cyclin subunits are responsible for governing the checkpoints in a cell division cycle. Normal progression through the G1 phase of the cell cycle is dependent upon the activities of the CDK4/cyclin D and CDK2/cyclin E complexes. 20 In our study, we showed that S. clandestina extract decreased the expression of CDK4 and cyclin E and, conversely, increased p21WAF1/CIP1 and p27Kip1 expression, thus indicating that the activity of the CDK4/cyclin D and CDK2/cyclin E complexes was inhibited.
The p53 tumor suppressor is involved in cell cycle regulation, DNA repair, and apoptosis. We show here that S. clandestina extract induces apoptosis by upregulating p53 mRNA and protein. Many pathways mediate the apoptosis caused by p53, including BAX and Bcl‐2 proteins. 21 The BAX gene promoter contains several consensus sequences for p53 binding. 21 BAX is able to foster cytochrome c release in the cytosol, thus activating caspase‐9 and leading to apoptosis. In addition, BAX may bind to Bcl‐2, thus inhibiting its apoptosis suppression function. In MG‐63 cells, p53 upregulates BAX and p21WAF1/CIP1 transcripts. It is not fully known why some cells undergo apoptosis in response to p53 activation, whereas others are simply arrested in the cell cycle. It may depend on p53 level, extracellular survival factors, or intrinsic activation of intracellular survival pathways. Multiple signaling pathways have been shown to influence p53 activation, including the MAPKs ERK1/2, JNK1/2, and p38. 22 Much evidence shows that activation of p38 and JNK1/2 by a variety of cell stimuli, including natural products from Salvia miltiorrhiza Bunge, is correlated with apoptosis. 23 We show that S. clandestina extract induces the activation of JNK1/2 and p38 and that their inhibition prevents apoptosis. Furthermore, p53 mRNA blockage after JNK1/2 inhibition suggests that the transcriptional induction of p53 signaling occurs downstream of JNK1/2. Consistent with this, various studies have demonstrated that JNK1/2 can, directly or indirectly, modulate p53 and its targets. 24 Phosphorylation of p53 can regulate its activity by altering protein stability, interacting with coactivators, and modulating the transcription of target genes 25 as part of the cellular response to stress. Here, we show that p38 activation contributes to p53‐dependent apoptosis, in accordance with reported data that p38 physically associates with and phosphorylates p53. Phosphorylation of p53 by p38 is crucial for subsequent p53 phosphorylation at other N‐terminal residues, allowing increased expression of p53‐responsive proapoptotic genes. 26 Thus, our findings suggest that S. clandestina extract exerts proapoptotic effects through p53 activity and modulation of its target genes.
In order to improve the overall effectiveness of cancer treatment and minimize toxicity and side effects, the use of natural compounds is promising because they have lower toxicities than conventional chemotherapy agents. 27 In addition, it has been shown that by combining phytochemicals with chemotherapy, the effectiveness of the cancer treatment can be improved. 27 Indeed, polyphenols are able to increase the sensitivity of different cancer cells to chemotherapy drugs. 27 Accordingly, a significant inhibition of cell viability and an increase in apoptosis were observed in MG‐63 cells treated with the combination of S. clandestina extract and cisplatin. The bioactive components modulate other cell signaling pathways in addition to MAPKs, among them the PI3K/Akt pathway, which is constitutively activated in various types of cancers, including osteosarcomas. 28 Akt/protein kinase B (PKB) is a key signaling kinase downstream of PI3K that regulates cell survival; it inhibits apoptosis through its ability to phosphorylate and inactivate several components of the intrinsic apoptosis machinery, including BAX and caspase‐9. 29 In osteosarcoma, Akt/PKB hyperactivation contributes to tumorigenesis, proliferation, invasion, cell cycle progression, inhibition of apoptosis, angiogenesis, metastasis, and chemotherapy resistance. Inhibition of this pathway through small‐molecule compounds represents an attractive potential therapeutic approach for osteosarcoma. 28 Decreased Akt/PKB phosphorylation was observed in MG‐63 cells treated with S. clandestina extract and cisplatin, suggesting that the effect of S. clandestina extract in enhancing the apoptosis induced by cisplatin may be Akt/PKB dependent.
Tumor metastasis is a complex process that depends upon the ability of tumor cells to undergo the EMT, to dissociate from the primary tumor in order to enter the blood or lymphatic vessels, and to reattach at distant sites. Since invasion and migration contribute to the recurrence and metastasis of cancer cells, the suppression of migration might be important for successful cancer therapy. 30 Some in vivo and in vitro studies have demonstrated that phytochemicals extracted from Salvia miltiorrhiza inhibit the invasion and migration of various cancer cells and also in osteosarcoma cells. 31 , 32 Here, we show that the migration of MG‐63 cells is decreased by low concentrations of S. clandestina extract through the downregulation of MMP2 and the inhibition of the EMT. MMP‐induced degradation of the ECM regulates the metastatic ability of cancer cells, 5 and, among them, MMP2 plays an important role in osteosarcoma invasion and metastasis. 6 , 33 In addition, the expression of MMP2 is associated with pulmonary metastasis and related to the prognosis of osteosarcoma. Consequently, MMP2 is considered a drug target in cancer treatment, inasmuch as its inhibition could limit cancer progression and metastasis. 6
EMT is a critical step in cancer metastasis. 34 It consists of epithelial cells temporarily losing their epithelial characteristics and acquiring the characteristics of mesenchymal cells. The regulation of the activities of the inducers of EMT is an obvious strategy to inhibit osteosarcoma progression. 35 Recent evidence indicates that natural plant compounds can modulate pathological EMT or its deleterious effects through acting on different cellular signal transduction pathways. 13 EMT is characterized by the loss of epithelial E‐cadherin and the acquisition of mesenchymal markers, such as vimentin. 33 In the present paper, we show that S. clandestina extract can effectively reverse the EMT, thus inhibiting osteosarcoma metastasis, by regulating the expression of E‐cadherin and vimentin.
The effects of the main components of S. clandestina extracts were also evaluated (Fig. S3, online only) on osteosarcoma cells, but the most important anticancer effects are due to a synergistic action of the aqueous extract components of S. clandestina, as demonstrated also for S. officinalis extracts. 36 These data support the administration of the extract as it is, as that would be less expensive and simpler compared with the isolation of single components.
The results presented here demonstrate that S. clandestina extract can counteract osteosarcoma (MG‐63 cells) by (1) apoptosis induction, (2) inhibition of metastatic potential, and (3) potentiation of cisplatin effects (Fig. 8). Although further investigation is required, including in vivo studies, our study supports our hypothesis that S. clandestina extract may be a novel and effective agent for the treatment of osteosarcoma. An appropriate method of administration of S. clandestina L. extract remains to be elucidated in order to overcome the limitation of the poor bioavailability of polyphenol compounds, as well as to develop appropriate formulations in order to use S. clandestina in human therapy.
Author contributions
A.M., S.M., and E.N. presented the concept. E.S. and C.N. performed methodology. E.S. carried out the investigation. A.M., L.D.B., and C.N. wrote the original draft. S.M. and L.D.B. helped in writing—reviewing and editing. S.M. and E.N. were involved in supervision. All authors read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Supporting information
Figure S1. The total ion current chromatogram of HPLC/MS‐TOF of the S. clandestina aqueous extract.
Figure S2. S. clandestina extract induces apoptosis in MG‐63 cells.
Figure S3. Effects of phenolic compounds of S. clandestina aqueous extract on MG‐63 cell viability.
Table S1. HPLC/ESI‐TOF‐MS accurate masses of [M–H]– ions of constituents of S. clandestina aqueous extract and tentative identification.
Table S2. Total phenolic content (TPC) and phenolic compounds quantification of the S. clandestina aqueous extract.
Acknowledgments
The authors thank Francesca Nicolì and Marzia Vergine of DiSTeBA for their technical support.
References
- 1. Lee, J.S. , DuBois S.G., Boscardin W.J., et al. 2014. Secondary malignant neoplasms among children, adolescents, and young adults with osteosarcoma. Cancer 120: 3987–3993. [DOI] [PubMed] [Google Scholar]
- 2. Longhi, A. , Errani C., De Paolis M., et al. 2006. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat. Rev. 32: 423–436. [DOI] [PubMed] [Google Scholar]
- 3. Wesolowski, R. & Budd G.T.. 2010. Use of chemotherapy for patients with bone and soft‐tissue sarcomas. Cleve. Clin. J. Med. 77: S23–S26. [DOI] [PubMed] [Google Scholar]
- 4. Kager, L. , Tamamyan G. & Bielack S.. 2017. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 13: 357. [DOI] [PubMed] [Google Scholar]
- 5. Bjørnland, K. , Flatmark K., Pettersen S., et al. 2005. Matrix metalloproteinases participate in osteosarcoma invasion. J. Surg. Res. 127: 151–156. [DOI] [PubMed] [Google Scholar]
- 6. Zhang, M. & Zhang X.. 2015. Association of MMP‐2 expression and prognosis in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 8: 14965–14970. [PMC free article] [PubMed] [Google Scholar]
- 7. Schirrmacher, V. 2019. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 54: 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soldati, L. , Di Renzo L., Jirillo E., et al. 2018. The influence of diet on anti‐cancer immune responsiveness. J. Transl. Med. 16: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spencer, J.P. , Abd El Mohsen M.M., Minihane A.M., et al. 2008. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br. J. Nutr. 99: 12–22. [DOI] [PubMed] [Google Scholar]
- 10. Del Bo, C. , Bernardi S., Marino M., et al. 2019. Systematic review on polyphenol intake and health outcomes: is there sufficient evidence to define a health‐promoting polyphenol‐rich dietary pattern? Nutrients 11: 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicolì, F. , Vergine M., Negro C., et al. 2019.. Salvia clandestina L.: unexploited source of danshensu. Nat. Prod. Res. 33: 439–442. [DOI] [PubMed] [Google Scholar]
- 12. Zhou, X. , Chan S.W., Tseng H.L., et al. 2012.. Danshensu is the major marker for the antioxidant and vasorelaxation effects of Danshen (Salvia miltiorrhiza) water‐extracts produced by different heat water‐extractions. Phytomedicine 19: 1263–1269. [DOI] [PubMed] [Google Scholar]
- 13. Fu, L. , Han B., Zhou Y., et al. 2020. The anticancer properties of tanshinones and the pharmacological effects of their active ingredients. Front. Pharmacol. 11: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Makkar, H.P.S. , Blummel M., Borowy N.K. & Becker K.. 1993. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 61: 161–165. [Google Scholar]
- 15. Muscella, A. , Vetrugno C., Antonaci G., et al. 2015. PKC‐δ/PKC‐α activity balance regulates the lethal effects of cisplatin. Biochem. Pharmacol. 98: 29–40. [DOI] [PubMed] [Google Scholar]
- 16. Muscella, A. , Vetrugno C., Cossa L.G. & Marsigliante S.. 2020. TGF‐β1 activates RSC96 Schwann cells migration and invasion through MMP‐2 and MMP‐9 activities. J. Neurochem. 153: 525–538. [DOI] [PubMed] [Google Scholar]
- 17. Sarker, H. , Hardy E., Haimour A., et al. 2019. Identification of fibrinogen as a natural inhibitor of MMP‐2. Sci. Rep. 9: 4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrison, D.J. & Schwartz C.L.. 2017. Osteogenic sarcoma: systemic chemotherapy options for localized disease. Curr. Treat. Options Oncol. 18: 24. [DOI] [PubMed] [Google Scholar]
- 19. Oun, R. , Moussa Y.E. & Wheate N.J.. 2018. The side effects of platinum‐based chemotherapy drugs: a review for chemists. Dalton Trans. 47: 6645–6653. [DOI] [PubMed] [Google Scholar]
- 20. Thangaraj, K. , Balasubramanian B., Park S., et al. 2019. Orientin induces G0/G1 cell cycle arrest and mitochondria mediated intrinsic apoptosis in human colorectal carcinoma HT29 cells. Biomolecules 9: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beckerman, R. & Prives C.. 2010. Transcriptional regulation by P53. Cold Spring Harb. Perspect. Biol. 2: a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haigis, K.M. & Sweet‐Cordero A.. 2001. New insights into oncogenic stress. Nat. Genet. 43: 177–178. [DOI] [PubMed] [Google Scholar]
- 23. Sui, X. , Kong N., Ye L., et al. 2014. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 344: 174–179. [DOI] [PubMed] [Google Scholar]
- 24. Saha, M.N. , Jiang H., Yang Y., et al. 2012. Targeting p53 via JNK pathway: a novel role of RITA for apoptotic signaling in multiple myeloma. PLoS One 7: e30215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashcroft, M. , Kubbutat M.H. & Vousden K.H.. 1999. Regulation of p53 function and stability by phosphorylation. Mol. Cell. Biol. 19: 1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bulavin, D.V. , Saito S., Hollander M.C., et al. 1999. Phosphorylation of human p53 by p38 kinase coordinates N‐terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18: 6845–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Youn, M.J. , So H.S., Cho H.J., et al. 2008. Berberine, a natural product, combined with cisplatin enhanced apoptosis through a mitochondria/caspase mediated pathway in HeLa cells. Biol. Pharm. Bull. 31: 789–795. [DOI] [PubMed] [Google Scholar]
- 28. Zhang, J. , Yu X.H., Yan Y.G., et al. 2015. PI3K/Akt signaling in osteosarcoma. Clin. Chim. Acta 444: 182–192. [DOI] [PubMed] [Google Scholar]
- 29. Liu, B. , Yan S., Qu L. & Zhu J.. 2017. Celecoxib enhances anticancer effect of cisplatin and induces anoikis in osteosarcoma via PI3K/Akt pathway. Cancer Cell Int. 17: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang, F. , Lv L.Z., Cai Q.C. & Jiang Y.. 2015. Potential roles of EZH2, Bmi‐1 and miR‐203 in cell proliferation and invasion in hepatocellular carcinoma cell line Hep3B. World J. Gastroenterol. 21: 13268–13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang, Y. , Wei R‐X., Zhu X‐B., et al. 2012. Tanshinone IIA induces apoptosis and inhibits the proliferation, migration, and invasion of the osteosarcoma MG‐63 cell line in vitro . Anticancer Drugs 23: 212–219. [DOI] [PubMed] [Google Scholar]
- 32. Xie, Z. , He B., Jiang Z. & Zhao L.. 2020. Tanshinone IIA inhibits osteosarcoma growth through modulation of AMPK‐Nrf2 signaling pathway. J. Recept. Signal. Transduct. Res. 40: 591–598. [DOI] [PubMed] [Google Scholar]
- 33. Jin, J. , Cai L., Liu Z.M. & Zhou X.S.. 2013. miRNA‐218 inhibits osteosarcoma cell migration and invasion by down‐regulating of TIAM1, MMP2 and MMP9. Asian Pac. J. Cancer Prev. 14: 3681–3684. [DOI] [PubMed] [Google Scholar]
- 34. Kalluri, R. & Weinberg R.A.. 2009. The basics of epithelial‐mesenchymal transition. J. Clin. Invest. 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang, G. , Yuan J. & Li K.. 2013. EMT transcription factors: implication in osteosarcoma. Med. Oncol. 30: 697. [DOI] [PubMed] [Google Scholar]
- 36. Ghorbani, A. & Esmaeilizadeh M.. 2017. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 7: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The total ion current chromatogram of HPLC/MS‐TOF of the S. clandestina aqueous extract.
Figure S2. S. clandestina extract induces apoptosis in MG‐63 cells.
Figure S3. Effects of phenolic compounds of S. clandestina aqueous extract on MG‐63 cell viability.
Table S1. HPLC/ESI‐TOF‐MS accurate masses of [M–H]– ions of constituents of S. clandestina aqueous extract and tentative identification.
Table S2. Total phenolic content (TPC) and phenolic compounds quantification of the S. clandestina aqueous extract.


