Abstract
Aim
To evaluate the efficacy and safety of dulaglutide 3.0 and 4.5 mg versus 1.5 mg when used as an add‐on to metformin in subgroups defined by age (<65 and ≥65 years).
Materials and Methods
Of 1842 patients included in this post hoc analysis, 438 were aged 65 years or older and 1404 were younger than 65 years. The intent‐to‐treat (ITT) population, while on treatment without rescue medication, was used for all efficacy analyses; the ITT population without rescue medication was used for hypoglycaemia analyses; all other safety analyses used the ITT population.
Results
Patients aged 65 years or older and those younger than 65 years had a mean age of 69.5 and 53.2 years, respectively. In each age subgroup, the reduction from baseline in HbA1c and body weight (BW), and the proportion of patients achieving a composite endpoint of HbA1c of less than 7% (<53 mmol/mol) with no weight gain and no documented symptomatic or severe hypoglycaemia, were larger for dulaglutide 3.0 and 4.5 mg compared with dulaglutide 1.5 mg, but the treatment‐by‐age interactions were not significant. The safety profile for the additional dulaglutide doses was consistent with that of dulaglutide 1.5 mg and was similar between the age subgroups.
Conclusion
Dulaglutide doses of 3.0 or 4.5 mg provided clinically relevant, dose‐related improvements in HbA1c and BW with no significant treatment‐by‐age interactions, and with a similar safety profile across age subgroups.
Keywords: body weight, dulaglutide, elderly, GLP‐1 RAs, glycaemic control, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D), which accounts for more than 90% of patients with diabetes, 1 is a health condition increasing in prevalence among the ageing population. 2 Approximately 40% of the adult population with diabetes in the United States is older than 65 years, 3 and the number of older adults living with this condition is expected to increase rapidly in the coming decades. 4 Older patients with T2D have an increased risk of many co‐morbidities, including cognitive dysfunction, cardiovascular disease (CVD), frailty, chronic kidney disease, retinopathy and peripheral neuropathy, affecting manual dexterity and physical ability. 5 , 6 , 7 These may predispose this patient population to an increased risk of recurring hypoglycaemia and hypoglycaemia‐associated morbidity, particularly in patients treated with insulin. Co‐morbidities may affect accurate insulin dosing and insulin clearance, and hypoglycaemic events increase the risk of falls and/or additional cardiovascular‐related morbidity. Therefore, the use of insulin therapy, particularly in elderly patients with T2D diabetes, is limited by the risk of hypoglycaemia. 8 , 9
Guideline changes by the American Diabetes Association (ADA) now recommend personalizing treatment for older patients with T2D based on their functional status and co‐morbidities. Less stringent glycaemic treatment goals are recommended for frail elderly patients to keep the risk of hypoglycaemia low while also taking co‐morbid illness and/or limited life expectancy into consideration. 4
Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) and sodium‐glucose co‐transporter‐2 inhibitors (SGLT‐2is) are recommended as first‐line therapy in combination with metformin, irrespective of the HbA1c level, in patients who either are at high risk or have pre‐existing atherosclerotic cardiovascular disease (ASCVD), heart failure or chronic kidney disease. Additionally, GLP‐1 RAs and SGLT‐2is are the preferred second treatment option after metformin for patients who would benefit from weight loss, while GLP‐1 RAs, SGLT‐2is, dipeptidyl peptidase‐4 inhibitors and thiazolidinedione are recommended after metformin for patients with increased hypoglycaemic risk. 10 , 11 These guidelines also pertain to older adults, particularly those with pre‐existing CVD and increased risk of hypoglycaemia 4 ; however, as with any other pharmacotherapy, they need to be used cautiously according to a patient's history, profile and individualized needs.
Prior Assessment of Weekly AdministRation of LY2189265 in Diabetes (AWARD) studies have shown that dulaglutide at 0.75 and 1.5 mg once‐weekly is effective for glycaemic control and well tolerated in elderly patients with T2D. 9 , 12 In the AWARD‐11 study, dulaglutide 3.0 and 4.5 mg once‐weekly provided clinically relevant, dose‐related improvements in glycaemic control and body weight (BW) in patients with T2D inadequately controlled with metformin monotherapy. 13 The safety profile was comparable with the 1.5 mg dose through 52 weeks and consistent with prior dulaglutide studies in the AWARD trial programme. The AWARD‐11 study enrolled nearly one quarter of patients aged 65 years or older. We conducted a post hoc analysis to examine the efficacy and safety of these additional dulaglutide doses in this elderly population.
2. MATERIALS AND METHODS
2.1. Study design
The study design of the AWARD‐11 trial was previously described in detail. 13 Briefly, this randomized, phase 3, double‐blind, multicentre, parallel‐arm study (ClinicalTrials.gov identifier: NCT03495102) included a 2‐week lead‐in period, followed by a 52‐week treatment period (with primary efficacy endpoint at 36 weeks), and a 4‐week safety follow‐up period. Patients initiated treatment with dulaglutide 0.75 mg for 4 weeks, followed by stepwise dose escalation every 4 weeks to the randomized dose of 1.5, 3.0 or 4.5 mg (Figure S1).
2.2. Key eligibility criteria
The key eligibility criteria of the AWARD‐11 trial were age 18 years or older, HbA1c of 7.5% or higher (≥58 mmol/mol) and 11.0% or less (≤97 mmol/mol), body mass index (BMI) of 25 kg/m2 or higher, and patients were taking commercially available metformin.
2.3. Efficacy measures and safety assessments
For this post hoc exploratory analysis, the primary efficacy measure was the change in HbA1c from baseline to 36 weeks in subgroups defined by age (<65 and ≥65 years). Secondary efficacy measures (all assessed at 36 weeks) were the change from baseline in BW and the proportion of patients achieving an HbA1c of less than 7.0% (<53 mmol/mol). Patients performed fasting plasma glucose measurements once‐daily, four‐point self‐monitored blood glucose (SMBG) measurements once‐weekly, and six‐point SMBG during the week preceding clinic visits. Safety assessments at 52 weeks included incidence of treatment‐emergent adverse events (TEAEs), discontinuation of study drug because of adverse events (AEs), adjudicated and confirmed cardiovascular (CV) and pancreatic AEs, and occurrence of hypoglycaemic episodes. Hypoglycaemic episodes were collected on a dedicated case report form, including cases where SMBG was 70 mg/dL or less (≤3.9 mmol/L), regardless of whether symptoms were experienced. As defined by the ADA, events were categorized as documented symptomatic hypoglycaemia any time patients felt they were experiencing symptoms and/or signs associated with hypoglycaemia and had a plasma glucose level of 70 mg/dL or less (≤3.9 mmol/L). Severe hypoglycaemia was defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions. Total hypoglycaemic events were defined as an episode with a plasma glucose level below the defined threshold, regardless of symptoms, an episode of symptomatic hypoglycaemia where the plasma glucose level was not measured, and all severe hypoglycaemia episodes. 14 , 15
Although 65 years is the most common age cut point used for subgroup analyses reported in clinical trials, 16 , 17 , 18 , 19 , 20 , 21 some studies define 70 years as the cut point for elderly patients. 22 Thus, we also compared the effects of dulaglutide dose escalation on change in HbA1c and BW from baseline and the proportion of patients achieving an HbA1c of less than 7.0% (<53 mmol/mol) at 36 weeks using a cut point of 70 years. Safety assessments were also carried out in these subgroups of patients aged less than 70 and 70 years or older.
2.4. Statistical analysis
The safety and efficacy analyses were performed using the intent‐to‐treat (ITT) population, defined as all patients randomized who received at least one dose of study drug. Efficacy analyses excluded measurements collected after discontinuation of study drug or initiation of another antihyperglycaemic medication (‘on‐treatment without rescue analysis’). The analysis for hypoglycaemia was performed using the ITT population excluding observations after rescue medication. All tests of treatment effects were conducted at a two‐sided alpha level of .05, unless otherwise specified. All tests of interactions between treatments and factors of interest were conducted at a two‐sided alpha level of .10.
3. RESULTS
3.1. Patient baseline characteristics
Of the 1842 patients included in this post hoc analysis, a total of 438 (dulaglutide 1.5 mg, 156; dulaglutide 3.0 mg, 150; dulaglutide 4.5 mg, 132) were aged 65 years or older, and 1404 (dulaglutide 1.5 mg, 456; dulaglutide 3.0 mg, 466; dulaglutide 4.5 mg, 482) were younger than 65 years of age.
At baseline, in the subgroup aged 65 years or older, the average age was 69.5 years, with a mean HbA1c of 8.4% (68.3 mmol/mol), mean BW of 90.0 kg and mean BMI of 32.9 kg/m2. The mean duration of disease was 9.9 years, and females comprised 48.4% of patients. In the subgroup younger than 65 years of age, the average age was 53.2 years, with a mean HbA1c of 8.7% (71.6 mmol/mol), mean BW of 97.5 kg and mean BMI of 34.7 kg/m2. The mean duration of disease was 6.9 years, and females comprised 48.9% of patients. Baseline data for fasting serum glucose, systolic blood pressure, diastolic blood pressure, mean baseline estimated glomerular filtration rate (eGFR), eGFR categories and patient CV risk factors are also presented in Table 1. Within each age subgroup, these baseline characteristics were comparable among treatment groups (Table 1). As expected, patients aged 65 years or older had a longer duration of T2D and a higher prevalence of renal impairment, hypertension, dyslipidaemia, history of CVD and atrial fibrillation.
TABLE 1.
Baseline characteristics and demographics of the <65 and ≥65 year subgroups
| Age group | <65 years | ≥65 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
DU 1.5 mg (N = 456) |
DU 3.0 mg (N = 466) |
DU 4.5 mg (N = 482) |
Total | P value |
DU 1.5 mg (N = 156) |
DU 3.0 mg (N = 150) |
DU 4.5 mg (N = 132) |
Total | P value | |
| Age (years) | 53.7 ± 7.6 | 52.9 ± 8.2 | 53.0 ± 8.2 | 53.2 ± 8.0 | .222 | 69.6 ± 3.5 | 69.3 ± 3.8 | 69.7 ± 4.0 | 69.5 ± 3.8 | .616 |
| Duration of diabetes (years) | 6.8 ± 5.3 | 6.9 ± 5.1 | 7.0 ± 5.2 | 6.9 ± 5.2 | .758 | 9.9 ± 6.6 | 9.8 ± 6.2 | 10.0 ± 7.3 | 9.9 ± 6.7 | .967 |
| Male | 227 (49.8) | 245 (52.6) | 246 (51.0) | 718 (51.1) | .697 | 71 (45.5) | 83 (55.3) | 72 (54.5) | 226 (51.6) | .164 |
| Female | 229 (50.2) | 221 (47.4) | 236 (49.0) | 686 (48.9) | 85 (54.5) | 67 (44.7) | 60 (45.5) | 212 (48.4) | ||
| Race | ||||||||||
| American Indian or Alaska native | 25 (5.5) | 20 (4.3) | 30 (6.2) | 75 (5.3) | .852 | 5 (3.2) | 6 (4.0) | 2 (1.5) | 13 (3.0) | .245 |
| Asian | 13 (2.9) | 16 (3.4) | 14 (2.9) | 43 (3.1) | 0 | 2 (1.3) | 0 | 2 (0.5) | ||
| Black or African American | 25 (5.5) | 27 (5.8) | 20 (4.1) | 72 (5.1) | 3 (1.9) | 4 (2.7) | 3 (2.3) | 10 (2.3) | ||
| Native Hawaiian or Pacific Islander | 1 (0.2) | 1 (0.2) | 3 (0.6) | 5 (0.4) | 0 | 0 | 0 | 0 | ||
| White | 383 (84.0) | 389 (83.5) | 404 (83.8) | 1176 (83.8) | 146 (93.6) | 132 (88.0) | 126 (95.5) | 404 (92.2) | ||
| Multiple | 9 (2.0) | 13 (2.8) | 11 (2.3) | 33 (2.4) | 2 (1.3) | 6 (4.0) | 1 (0.8) | 9 (2.1) | ||
| Ethnicity | ||||||||||
| Hispanic or Latino | 168 (36.8) | 175 (37.6) | 182 (37.8) | 525 (37.4) | .665 | 46 (29.5) | 39 (26.0) | 31 (23.5) | 116 (26.5) | .843 |
| Not Hispanic or Latino | 267 (58.6) | 261 (56.0) | 278 (57.7) | 806 (57.4) | 102 (65.4) | 102 (68.0) | 93 (70.5) | 297 (67.8) | ||
| Not reported | 21 (4.6) | 30 (6.4) | 22 (4.6) | 73 (5.2) | 8 (5.1) | 9 (6.0) | 8 (6.1) | 25 (5.7) | ||
| HbA1c (%) | 8.74 ± 1.0 | 8.71 ± 1.0 | 8.70 ± 0.9 | 8.72 ± 1.0 | .831 | 8.36 ± 0.7 | 8.38 ± 0.8 | 8.40 ± 0.9 | 8.38 ± 0.8 | .904 |
| BMI (kg/m2) | 34.8 ± 6.5 | 34.7 ± 6.5 | 34.5 ± 6.4 | 34.7 ± 6.4 | .700 | 33.3 ± 6.0 | 32.9 ± 4.9 | 32.3 ± 5.0 | 32.9 ± 5.4 | .258 |
| Weight (kg) | 97.3 ± 20.7 | 97.9 ± 21.0 | 97.3 ± 21.3 | 97.5 ± 21.0 | .874 | 90.0 ± 17.6 | 91.4 ± 16.5 | 88.3 ± 16.3 | 90.0 ± 16.9 | .305 |
| FSG (mg/dL) | 186.5 (52.5) | 186.2 (57.2) | 184.6 (49.1) | 185.8 (53.0) | .847 | 180.6 (50.6) | 176.8 (44.0) | 178.9 (43.7) | 178.8 (46.3) | .775 |
| Systolic BP (mmHg) | 131.1 (14.4) | 130.1 (14.1) | 131.1 (14.3) | 130.8 (14.3) | .471 | 135.0 (13.2) | 134.1 (13.7) | 135.6 (12.3) | 134.9 (13.1) | .601 |
| Diastolic BP (mmHg) | 79.4 (9.4) | 78.8 (8.9) | 79.9 (9.1) | 79.4 (9.2) | .194 | 76.8 (8.8) | 77.4 (7.9) | 75.9 (7.9) | 76.7 (8.2) | .312 |
| eGFR (mL/min/1.73m2) | 97.9 (16.7) | 97.9 (16.6) | 97.9 (16.5) | 97.9 (16.6) | >.999 | 80.3 (16.1) | 79.0 (13.3) | 78.2 (16.5) | 79.2 (15.3) | .488 |
| eGFR categories | ||||||||||
| ≥30‐60 | 11 (2.4) | 12 (2.6) | 13 (2.7) | 36 (2.6) | .994 | 22 (14.1) | 13 (8.7) | 20 (15.2) | 55 (12.6) | .341 |
| ≥60‐<90 | 95 (20.8) | 100 (21.5) | 109 (22.6) | 304 (21.7) | .930 | 76 (48.7) | 98 (65.3) | 75 (56.8) | 249 (56.8) | .035 |
| ≥90 | 350 (76.8) | 354 (76.0) | 360 (74.7) | 1064 (75.8) | .907 | 58 (37.2) | 39 (26.0) | 37 (28.0) | 134 (30.6) | .172 |
| Hypertension | 317 (69.5) | 302 (64.8) | 304 (63.1) | 923 (65.7) | .099 | 134 (85.9) | 119 (79.3) | 108 (81.8) | 361 (82.4) | .306 |
| Dyslipidaemia | 303 (66.5) | 294 (63.1) | 317 (65.8) | 914 (65.1) | .527 | 113 (72.4) | 111 (74.0) | 89 (67.4) | 313 (71.5) | .451 |
| History of CVD | 62 (13.6) | 54 (11.6) | 51 (10.6) | 167 (11.9) | .358 | 37 (23.7) | 39 (26.0) | 33 (25.0) | 109 (24.9) | .904 |
| Atrial fibrillation | 7 (1.5) | 7 (1.5) | 7 (1.5) | 21 (1.5) | >.999 | 10 (6.4) | 6 (4.0) | 7 (5.3) | 23 (5.3) | .666 |
Note: Data presented as mean ± SD or n (%).
Abbreviations: BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; DU, dulaglutide; eGFR, estimated glomerular filtration rate; FSG, fasting serum glucose; N, population size; n, sample size; SD, standard deviation.
3.2. Efficacy: glycaemic control and BW
In patients younger than 65 years, the least‐squares mean (LSM) change in HbA1c from baseline to week 36 was −1.74% and −1.94% with dulaglutide 3.0 and 4.5 mg, respectively, compared with −1.59% for dulaglutide 1.5 mg (Figure 1A). In the subgroup aged 65 years or older, similar results were observed, as the LSM change in HbA1c from baseline to week 36 was −1.58% and −1.65% with dulaglutide 3.0 and 4.5 mg, respectively, compared with −1.33% for dulaglutide 1.5 mg (Figure 1A). The results for both age subgroups were in line with those seen in the overall study population (Figure 1A), with no statistically significant treatment‐by‐age subgroup interaction for HbA1c reduction (interaction P = .591).
FIGURE 1.
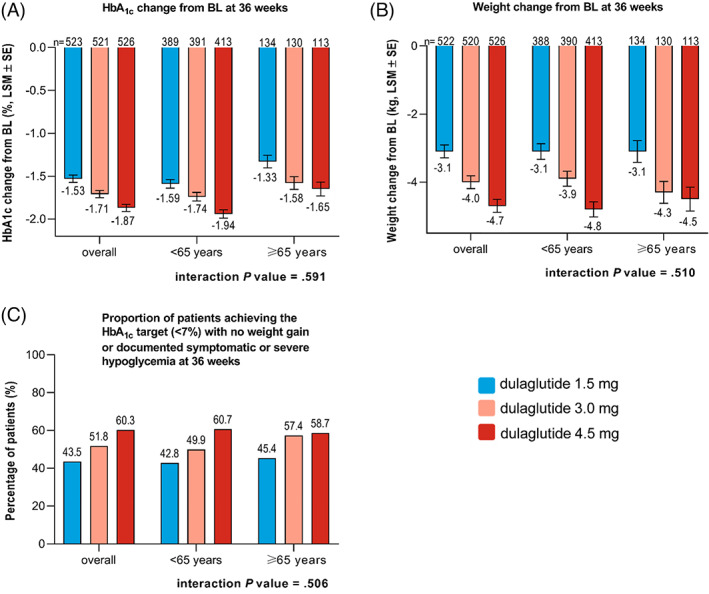
Primary and secondary efficacy outcomes in the <65 and ≥65 year subgroups. Change in A, HbA1c, B, weight, and, C, proportion of patients achieving the HbA1c target (<7%) with no weight gain or documented symptomatic or severe hypoglycaemia at 36 weeks. Data are presented as LSM ± SE unless otherwise indicated. Abbreviations: BL, baseline; LSM, least‐squares mean; n, sample size; SE, standard error
In patients younger than 65 years, the LSM change in BW from baseline to week 36 was −3.9 and −4.8 kg with dulaglutide 3.0 and 4.5 mg, respectively, compared with −3.1 kg for dulaglutide 1.5 mg (Figure 1B). Comparing these results with patients in the 65 years or older subgroup, a similar trend was observed. The LSM change in BW from baseline was −4.3 and −4.5 kg with dulaglutide 3.0 and 4.5 mg, respectively, compared with −3.1 kg for dulaglutide 1.5 mg at 36 weeks (Figure 1B). The results for both age subgroups were in line with those seen in the overall study population (Figure 1B), with no statistically significant treatment‐by‐age subgroup interaction for change in BW (interaction P = .510).
In both age subgroups, the proportion of patients achieving the HbA1c target of less than 7% with no weight gain or documented symptomatic or severe hypoglycaemia at the primary endpoint of 36 weeks was significantly higher (P ≤ .034) for the additional dulaglutide doses (3.0 and 4.5 mg) in comparison with dulaglutide 1.5 mg (Figure 1C). The proportions of patients achieving this composite target in both age subgroups were in line with those seen in the overall study population (Figure 1C), with no statistically significant treatment‐by‐age subgroup interaction (interaction P = .506).
Similar results were obtained for patients younger than 70 and those aged 70 years or older, with no statistically significant treatment‐by‐age subgroup interaction for HbA1c reduction from baseline (interaction P = .937), weight change from baseline (interaction P = .376), or the proportion of patients reaching the HbA1c target of less than 7% with no weight gain or documented symptomatic or severe hypoglycaemia (interaction P = .717) (Figure 2A‐C).
FIGURE 2.
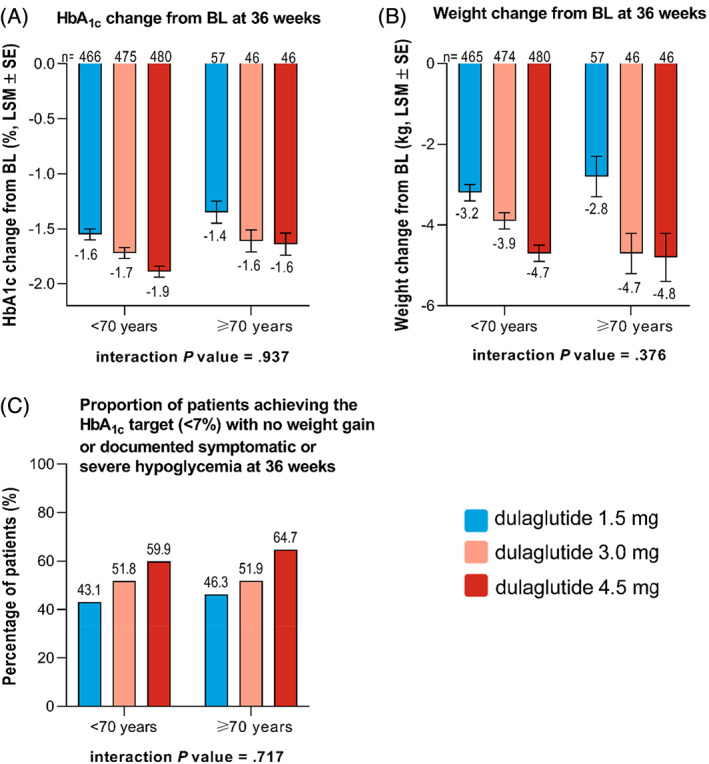
Primary and secondary efficacy outcomes in the <70 and ≥70 year subgroups. Change in A, HbA1c, B, weight, and, C, proportion of patients achieving the HbA1c target (<7%) with no weight gain or documented symptomatic or severe hypoglycaemia at 36 weeks. Data are presented as LSM ± SE unless otherwise indicated. Abbreviations: BL, baseline; LSM, least‐squares mean; n, sample size; SE, standard error
3.3. Safety
The most frequent TEAE experienced in both age subgroups was nausea, which ranged from 12.1% to 18.7%, followed by diarrhoea (range, 7.5% to 12.7%), vomiting (range, 6.4% to 10.7%), dyspepsia (range, 2.3% to 8.7%) and nasopharyngitis (range, 4.2% to 8.3%) (Table 2). There was no statistically significant treatment‐by‐age subgroup interaction for any of these TEAEs (interaction P = .383). The incidence of TEAEs related to a composite of supraventricular arrythmias, conduction disorders and adjudicated CV events was low and was similar across dose groups, with no significant treatment‐by‐age subgroup interaction (interaction P = .963).
TABLE 2.
Summary of adverse events through 52 weeks in the <65 and ≥65 year subgroups
| Age group | <65 years | ≥65 years | |||||
|---|---|---|---|---|---|---|---|
| Variable, n (%) |
DU 1.5 mg (N = 456) |
DU 3.0 mg (N = 466) |
DU 4.5 mg (N = 482) |
DU 1.5 mg (N = 156) |
DU 3.0 mg (N = 150) |
DU 4.5 mg (N = 132) |
Interaction P value |
| Composite incidence of nausea, vomiting and diarrhoea | 93 (20.4) | 115 (24.7) | 140 (29.0) | 35 (22.4) | 39 (26.0) | 31 (23.5) | .383 |
| Nausea | 62 (13.6) | 76 (16.3) | 90 (18.7) | 25 (16.0) | 23 (15.3) | 16 (12.1) | .201 |
| Vomiting | 29 (6.4) | 40 (8.6) | 48 (10.0) | 10 (6.4) | 16 (10.7) | 14 (10.6) | .887 |
| Diarrhoea | 34 (7.5) | 57 (12.2) | 61 (12.7) | 13 (8.3) | 17 (11.3) | 10 (7.6) | .366 |
| Dyspepsia | 13 (2.9) | 18 (3.9) | 14 (2.9) | 4 (2.6) | 13 (8.7) | 3 (2.3) | .229 |
| Nasopharyngitis | 19 (4.2) | 22 (4.7) | 27 (5.6) | 9 (5.8) | 10 (6.7) | 11 (8.3) | .988 |
| TEAEs related to supraventricular arrythmias, conduction disorders and adjudicated CV events | 22 (4.8) | 23 (4.9) | 24 (5.0) | 12 (7.7) | 11 (7.3) | 9 (6.8) | .963 |
| Overall discontinuation of study drug | 56 (12.3) | 75 (16.1) | 74 (15.4) | 23 (14.7) | 18 (12.0) | 20 (15.2) | .366 |
| Discontinuation of study drug because of an AE | 23 (5.0) | 33 (7.1) | 39 (8.1) | 14 (9.0) | 10 (6.7) | 13 (9.8) | .417 |
| Nausea | 2 (0.4) | 6 (1.3) | 7 (1.5) | 6 (3.8) | 2 (1.3) | 2 (1.5) | .124 |
| Diarrhoea | 1 (0.2) | 4 (0.9) | 6 (1.2) | 0 (0) | 2 (1.3) | 0 (0) | .548 |
| Vomiting | 0 (0) | 2 (0.4) | 8 (1.7) | 0 (0) | 3 (2.0) | 0 (0) | .194 |
| Overall serious adverse events | 34 (7.5) | 35 (7.5) | 24 (5.0) | 17 (10.9) | 7 (4.7) | 14 (10.6) | .057 |
| Hypoglycaemia incidences | |||||||
| Total a | 30 (6.6) | 22 (4.7) | 22 (4.6) | 13 (8.3) | 11 (7.3) | 15 (11.4) | .422 |
| Documented symptomatic (≤70 mg/dL) | 15 (3.3) | 10 (2.2) | 11 (2.3) | 4 (2.6) | 5 (3.3) | 8 (6.1) | .293 |
Note: Data presented as n (%).
Abbreviations: AE, adverse event; CV, cardiovascular; DU, dulaglutide; n, sample size; N, population size; TEAEs, treatment‐emergent adverse events.
Total hypoglycaemic incidences = any episode with plasma glucose level below the defined threshold (≤70 mg/dL), regardless of symptoms, an episode of symptomatic hypoglycaemia where plasma glucose level was not measured and all severe hypoglycaemia episodes.
The incidence of all AEs reported as serious (serious adverse events [SAEs]) was low across dose groups in each age subgroup (Table 2). The treatment‐by‐age subgroup interaction for SAEs was significant (interaction P = .057), but this was probably driven by the small number of patients and low incidence in the 3.0‐mg group of the 65 years or older subgroup (4.7%) relative to the 1.5‐mg (10.9%) and 4.5‐mg (10.6%) dose groups.
Incidence of documented symptomatic hypoglycaemia (<70 mg/dL) was not different between treatment groups, and the treatment‐by‐age subgroup interaction was not significant (interaction P = .293). Similarly, no difference was observed between treatment groups with a non‐significant treatment‐by‐age subgroup interaction (interaction P = .422) for total hypoglycaemia incidence (Table 2). Severe hypoglycaemic events occurred in one patient in the younger than 65 years subgroup receiving the 1.5 mg dose (45 years of age) and in one patient in the 65 years or older subgroup receiving the 4.5 mg dose (79 years of age).
The proportion of patients discontinuing study drug is presented in Table 2. Overall, discontinuation of study drug was similar across treatment groups in each age subgroup, less than 65 years (dulaglutide 1.5 mg, 12.3%; dulaglutide 3.0 mg, 16.1%; dulaglutide 4.5 mg, 15.4%; P = .213) and 65 years or older (dulaglutide 1.5 mg, 14.7%; dulaglutide 3.0 mg, 12.0%; dulaglutide 4.5 mg, 15.2%; P = .698). The treatment‐by‐age subgroup interaction was not significant (interaction P = .366). Similarly, study drug discontinuation specifically because of events of nausea, vomiting or diarrhoea overall and by treatment group was low, and was generally similar between age subgroups. Discontinuation of study drug because of overall AEs was also similar across treatment groups in each age subgroup (<65 years: dulaglutide 1.5 mg: 5.0%, 3.0 mg: 7.1%, 4.5 mg: 8.1%, P = .162; ≥65 years: dulaglutide 1.5 mg: 9.0%, 3.0 mg: 6.7%, 4.5 mg: 9.8%, P = .602), with no significant treatment‐by‐age subgroup interaction (interaction P = .417).
Similar safety results were obtained for patients younger than 70 and those aged 70 years or older with non‐statistically significant treatment‐by‐age subgroup interactions for safety assessments, except for nausea, which was higher in the 70 years or older subgroup (interaction P = .054) (Table 3). This was probably driven by the small number of patients in the 70 years or older subgroup in combination with the higher incidence in the lowest dulaglutide dose group (1.5 mg) relative to the higher dose groups (3.0 and 4.5 mg). No statistically significant treatment‐by‐age subgroup interactions were observed for discontinuation of study drug because of AEs, SAEs or incidences of hypoglycaemia (Table 3).
TABLE 3.
Summary of adverse events through 52 weeks in the <70 and ≥70 year subgroups
| Age group | <70 years | ≥70 years | |||||
|---|---|---|---|---|---|---|---|
| Variable, n (%) |
DU 1.5 mg (N = 543) |
DU 3.0 mg (N = 562) |
DU 4.5 mg (N = 561) |
DU 1.5 mg (N = 69) |
DU 3.0 mg (N = 54) |
DU 4.5 mg (N = 53) |
Interaction P value |
| Composite incidence of nausea, vomiting and diarrhoea | 107 (19.7) | 142 (25.3) | 157 (28.0) | 21 (30.4) | 12 (22.2) | 14 (26.4) | .171 |
| Nausea | 69 (12.7) | 91 (16.2) | 98 (17.5) | 18 (26.1) | 8 (14.8) | 8 (15.1) | .054 |
| Vomiting | 34 (6.3) | 49 (8.7) | 52 (9.3) | 5 (7.2) | 7 (13.0) | 10 (18.9) | .580 |
| Diarrhoea | 41 (7.6) | 69 (12.3) | 69 (12.3) | 6 (8.7) | 5 (9.3) | 2 (3.8) | .277 |
| Dyspepsia | 15 (2.8) | 26 (4.6) | 17 (3.0) | 2 (2.9) | 5 (9.3) | 0 (0) | .375 |
| Nasopharyngitis | 24 (4.4) | 27(4.8) | 36 (6.4) | 4 (5.8) | 5 (9.3) | 2 (3.8) | .402 |
| TEAEs related to supraventricular arrythmias, conduction disorders and adjudicated CV events | 29 (5.3) | 28 (5.0) | 28 (5.0) | 5 (7.2) | 6 (11.1) | 5 (9.4) | .730 |
| Overall discontinuation of study drug | 64 (11.8) | 84 (14.9) | 85 (15.2) | 15 (21.7) | 9 (16.7) | 9 (17.0) | .382 |
| Discontinuation of study drug because of an AE | 27 (5.0) | 36 (6.4) | 46 (8.2) | 10 (14.5) | 7 (13.0) | 6 (11.3) | .421 |
| Nausea | 5 (0.9) | 6 (1.1) | 9 (1.6) | 3 (4.3) | 2 (3.7) | 0 (0) | .379 |
| Diarrhoea | 1 (0.2) | 5 (0.9) | 6 (1.1) | 0 (0) | 1 (1.9) | 0 (0) | .762 |
| Vomiting | 0 (0) | 2 (0.4) | 8 (1.4) | 0 (0) | 3 (5.6) | 0 (0) | .166 |
| Overall serious adverse events | 44 (8.1) | 40 (7.1) | 32 (5.7) | 7 (10.1) | 2 (3.7) | 6 (11.3) | .282 |
| Hypoglycaemia incidences | |||||||
| Total a | 34 (6.3) | 28 (5.0) | 32 (5.7) | 9 (13.0) | 5 (9.3) | 5 (9.4) | .917 |
| Documented symptomatic (≤70 mg/dL) | 18 (3.3) | 14 (2.5) | 18 (3.2) | 1 (1.5) | 1 (1.9) | 1 (1.9) | .878 |
Note: Data presented as n (%).
Abbreviations: AE, adverse event; CV, cardiovascular; DU, dulaglutide; n, sample size; N, population size; TEAEs, treatment‐emergent adverse events.
Total hypoglycaemic incidences = any episode with plasma glucose level below the defined threshold (≤70 mg/dL), regardless of symptoms, an episode of symptomatic hypoglycaemia where plasma glucose level was not measured and all severe hypoglycaemia episodes.
4. DISCUSSION
The AWARD‐11 trial showed that dulaglutide 3.0 or 4.5 mg versus 1.5 mg once‐weekly provides dose‐related improvements in glycaemic control and BW reduction at 36 weeks that are sustained through 52 weeks. Similarly, this exploratory post hoc analysis showed that treatment with once‐weekly dulaglutide 3.0 and 4.5 mg resulted in clinically relevant reductions in HbA1c and BW compared with dulaglutide 1.5 mg in the younger than 65 and 65 years or older age subgroups, and also the younger than 70 and 70 years or older age subgroups. The safety profile for the additional dulaglutide doses was consistent with that of dulaglutide 1.5 mg and similar between age subgroups. These results were in line with the findings of previous published studies that showed similar glycaemic and BW effects and safety profiles irrespective of baseline age when treated with dulaglutide doses of 0.75 and 1.5 mg. 9 , 12 These additional dulaglutide doses may benefit elderly patients who are not achieving glycaemic targets on a lower dulaglutide dose, as they can remain on their current therapy with familiar administration and tolerability experience.
The growing demographic of elderly adults with T2D, spanning those who are healthier and robust and others who are more frail, will require individualized glycaemic targets based on each patient's health status. Medication classes with a low risk of hypoglycaemia such as GLP‐1 RAs have been recommended by the ADA and the European Association for the Study of Diabetes as the preferred treatment over insulin in adults with T2D with established and high‐risk ASCVD, obesity, and in those who are at an increased risk of hypoglycaemia. The current guidelines for treating T2D recommend an HbA1c of less than 7% (<53 mmol/mol) for adults who are considered comparatively healthy. For comparatively healthy older people with an extended life expectancy, a glycaemic target similar to that for younger patients may be most appropriate. Less stringent glycaemic treatment goals are recommended for more frail elderly patients to keep the risk of hypoglycaemia low while also taking co‐morbid illness and/or limited life expectancy into consideration. 4 , 10 , 14 , 23 The availability of four dulaglutide doses (0.75, 1.5, 3.0 and 4.5 mg) provides additional options to individualize a patient's treatment to achieve the respective glycaemic target tailored to the patient's health status and life expectancy.
The proportion of patients achieving the composite endpoint target of an HbA1c of less than 7% (<53 mmol/mol) with no weight gain and no documented or severe hypoglycaemia at 36 weeks was also similar, irrespective of baseline age. Sustaining glycaemic control targets over time is associated with a reduced risk of microvascular complications (retinopathy, nephropathy, neuropathy) and ASCVD in patients with diabetes 24 , 25 , 26 ; achieving such control with a low risk of hypoglycaemia is particularly important in older adults because they generally have a higher risk of hypoglycaemia, a longer duration of T2D and a higher risk of microvascular complications and ASCVD.
The incidence of total (including severe) hypoglycaemia and documented symptomatic hypoglycaemia was low, with no significant evidence of a differential treatment effect between age groups, consistent with prior studies with lower doses of dulaglutide. 9 , 12 These results are particularly reassuring in the treatment of elderly patients with T2D because of their increased risk of hypoglycaemia and complications associated with hypoglycaemia, including falls, fractures, depression, cardiac arrhythmias and other cardiac events, cognitive impairment, dementia, and an overall reduced quality of life. 27 , 28
The tolerability profile of dulaglutide across all three dose groups was comparable between age groups. As expected, the most common TEAEs reported across all doses in AWARD‐11 were nausea, vomiting and diarrhoea. In the overall study population, the incidence of nausea was similar across dose groups, whereas diarrhoea and vomiting were more frequently reported in the 3.0 and 4.5 mg groups. 13 In the current subgroup analysis, there was no dose relationship between reports of common gastrointestinal (GI) events in the older age subgroup compared with the younger age subgroup, and the overall incidences of these events among older patients was generally similar to those reported in the younger age group. Cardiac complications (arrythmias, conduction disorders and CV events) were also similar across doses and age subgroups. Discontinuations of study drug as a result of any AE or specifically because of nausea, vomiting or diarrhoea were not significantly different among older versus younger patients, further supporting the conclusion that overall and GI tolerability of dulaglutide across the dose range studied in AWARD‐11 are similar between age groups, consistent with prior studies with lower doses of dulaglutide. 9 , 12 The dulaglutide safety profile related to SAEs was also comparable between age groups across all doses. The incidence of SAEs was not dose‐related in either age subgroup.
This analysis has certain limitations that may influence the interpretation and generalizability of the results. With no evidence of treatment‐by‐age interaction, the most appropriate estimate of the effect size for any of the endpoints is that observed in the overall population of the trial; however, it is reassuring that further exploratory subgroup analysis by age remains consistent with the overall results for the relevant safety and efficacy measures. Because of an increased prevalence of asymptomatic hypogylcaemia in older patients, hypoglycaemia may be under‐reported in the elderly. 29 Study criteria excluded patients on sulphonylureas and insulin, as concomitant use with an insulin secretagogue or insulin may increase the risk of hypoglycaemia. Reducing the dose of insulin secretagogue or insulin may be necessary if combining with dulaglutide. 30 Study criteria also required patients to be on metformin only with an eGFR of less than 30 mL/min/1.73 m2, which may have excluded patients on other medications and those with severe renal disease. Exclusion criteria also included a minimum BMI of 25 kg/m2 at baseline, which may have excluded frail elderly participants from enrolment. Therefore, study results may vary from the real‐world elderly population with T2D in clinical practice and may not extrapolate to patients with criteria outside of this range.
In conclusion, these results show that dulaglutide 3.0 or 4.5 mg provided clinically relevant, dose‐related improvements in glycaemic control and BW for patients younger than 65 years and those aged 65 years or older, as well as patients younger than 70 years and those aged 70 years or older with a low risk of hypoglycaemia and a similar safety and tolerability profile, and can, therefore, be considered a treatment option independent of age for patients with T2D. Moreover, the availability of four dulaglutide doses (0.75, 1.5, 3.0 and 4.5 mg), now approved in the United States and European Union for clinical use, will allow physicians caring for older adults with T2D to better individualize treatment goals based on each patient's health status and life expectancy, as is recommended by current guidelines. 4
CONFLICT OF INTEREST
JPF: research support: Allergan, AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, Janssen, Madrigal, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi and Theracos; advisory boards and consulting: Altimmune, Axcella Health, Boehringer Ingelheim, Coherus Therapeutics, Eli Lilly, Gilead, Intercept, Merck, Novo Nordisk and Sanofi; and speaker bureau: Merck and Sanofi. EB: advisory boards: Abbott, AstraZeneca, Becton‐Dickinson, Boehringer Ingelheim, Bristol‐Myers Squibb, Bruno Farmaceutici, Daiichi‐Sanyo, Janssen, Johnson & Johnson, Eli Lilly, MSD, Mundipharma, Novartis, Novo Nordisk, Roche, Sanofi, Servier and Takeda; and research grants: AstraZeneca, Genzyme, Menarini Diagnostics, Novo Nordisk, Roche Diagnostics and Takeda. LNR: research support: Eli Lilly. SHH: research grants: American Diabetes Association and National Institutes of Health. HJ, SR, DAC, MAB and MK are current employees and shareholders of Eli Lilly and Company.
AUTHOR CONTRIBUTIONS
MK, MAB and HJ contributed to the design and conception of the study. DAC, JPF, LNR, SHH participated in the acquisition of the data. SR, DAC, HJ, EB, JPF, LNR, MAB and MK contributed to the analysis and interpretation of the data. All authors participated in writing and revising the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14469.
Supporting information
Figure S1 Study Design. Abbreviation: DU = dulaglutide.
ACKNOWLEDGEMENTS
The authors thank the trial investigators, trial staff and trial participants for their contributions, and Ciara O'Neill PhD for writing and editorial support. The AWARD‐11 trial was sponsored by Eli Lilly and Company.
Frias JP, Bonora E, Nevárez Ruiz L, et al. Efficacy and safety of dulaglutide 3.0 and 4.5 mg in patients aged younger than 65 and 65 years or older: Post hoc analysis of the AWARD‐11 trial. Diabetes Obes Metab. 2021;23(10):2279‐2288. 10.1111/dom.14469
Funding information Eli Lilly and Company
DATA AVAILABILITY STATEMENT
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at vivli.org.
REFERENCES
- 1. American Diabetes Association . Fast facts ‐ data and statistics about diabetes. 2020. https://professional.diabetes.org/sites/professional.diabetes.org/files/media/sci_2020_diabetes_fast_facts_sheet_final.pdf. Accessed May 19, 2021.
- 2. Caspard H, Jabbour S, Hammar N, Fenici P, Sheehan JJ, Kosiborod M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: an analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab. 2018;20(3):667‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laiteerapong N, Huang ES. Chapter 16. Diabetes in Older Adults. Diabetes in America. 3rd ed. National Institute of Diabetes and Digestive and Kidney Diseases; 2018. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/diabetes-in-america-3rd-edition. [PubMed] [Google Scholar]
- 4. American Diabetes Association . 12. Older adults: standards in medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S168‐S179. [DOI] [PubMed] [Google Scholar]
- 5. Onoviran OF , Li D, Toombs Smith S, Raji MA. Effects of glucagon‐like peptide 1 receptor agonists on comorbidities in older patients with diabetes mellitus. Ther Adv Chronic Dis. 2019;10:2040622319862691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Global report on diabetes, 2016. http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=3C9A02BAAB6F463CFEEA1DAD53C23D6B?sequence=1. Accessed December 1, 2020.
- 7. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed November 9, 2020. [Google Scholar]
- 8. Morley JE, Abbatecola AM, Woo J. Management of comorbidities in older persons with type 2 diabetes. J Am Med Dir Assoc. 2017;18(8):639‐645. [DOI] [PubMed] [Google Scholar]
- 9. Boustani MA, Pittman I, Yu M, Thieu VT, Varnado OJ, Juneja R. Similar efficacy and safety of once‐weekly dulaglutide in patients with type 2 diabetes aged ≥65 and <65 years. Diabetes Obes Metab. 2016;18(8):820‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetologia. 2020;63(2):221‐228. [DOI] [PubMed] [Google Scholar]
- 11. American Diabetes Association . Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes. Diabetes Care. 2021;44(Suppl. 1):S111‐S124. [DOI] [PubMed] [Google Scholar]
- 12. Kuang J, Zhu J, Liu S, Li Q. Efficacy and safety of once‐weekly dulaglutide in elderly Chinese patients with type 2 diabetes: a post hoc analysis of AWARD‐CHN studies. Diabetes Ther. 2020;11(10):2329‐2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frias JP, Bonora E, Nevarez Ruiz L, et al. Efficacy and safety of Dulaglutide 3.0 mg and 4.5 mg versus Dulaglutide 1.5 mg in metformin‐treated patients with type 2 diabetes in a randomized controlled trial (AWARD‐11). Diabetes Care. 2021;44(3):765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association . 6. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl. 1):S73‐S84. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association . 6. Glycemic targets. Diabetes Care. 2017;40(Suppl. 1):S48‐S56. [DOI] [PubMed] [Google Scholar]
- 16. Ochs‐Ross R, Daly EJ, Zhang Y, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment‐resistant depression—TRANSFORM‐3. Am J Geriatr Psychiatry. 2020;28(2):121‐141. [DOI] [PubMed] [Google Scholar]
- 17. Riddle MC, Gerstein HC, Xavier D, et al. Efficacy and safety of dulaglutide in older patients: a post hoc analysis of the REWIND trial. J Clin Endocrinol Metab. 2021;106(5):1345‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warren M, Chaykin L, Trachtenbarg D, Nayak G, Wijayasinghe N, Cariou B. Semaglutide as a therapeutic option for elderly patients with type 2 diabetes: pooled analysis of the SUSTAIN 1‐5 trials. Diabetes Obes Metab. 2018;20(9):2291‐2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monteiro P, Bergenstal RM, Toural E, et al. Efficacy and safety of empagliflozin in older patients in the EMPA‐REG OUTCOME® trial. Age Ageing. 2019;48(6):859‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pratley RE, Emerson SS, Franek E, et al. Cardiovascular safety and lower severe hypoglycaemia of insulin degludec versus insulin glargine U100 in patients with type 2 diabetes aged 65 years or older: results from DEVOTE (DEVOTE 7). Diabetes Obes Metab. 2019;21(7):1625‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou JH, Wei Y, Lyu YB, et al. Prediction of 6‐year incidence risk of chronic kidney disease in the elderly aged 65 years and older in 8 longevity areas in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(1):42‐47. [DOI] [PubMed] [Google Scholar]
- 22. Chung SM, Lee YY, Ha E, et al. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J. 2020;44(3):405‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee SJ, Eng C. Goals of glycemic control in frail older patients with diabetes. JAMA. 2011;305(13):1350‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korytkowski MT, Forman DE. Management of atherosclerotic cardiovascular disease risk factors in the older adult patient with diabetes. Diabetes Care. 2017;40(4):476‐484. [DOI] [PubMed] [Google Scholar]
- 25. American Diabetes Association . 9. Cardiovascular disease and risk management. Diabetes Care. 2017;40(Suppl. 1):S75‐S87. [DOI] [PubMed] [Google Scholar]
- 26. Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: an emerging public health burden. Curr Diab Rep. 2013;13(6):805‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang ES. Management of diabetes mellitus in older people with comorbidities. BMJ. 2016;353:i2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bansal N, Dhaliwal R, Weinstock RS. Management of diabetes in the elderly. Med Clin N Am. 2015;99:351‐377. [DOI] [PubMed] [Google Scholar]
- 29. Abdelhafiz AH, Rodríguez‐Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in older people ‐ a less well recognized risk factor for frailty. Aging Dis. 2015;6(2):156‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trulicity . Package Insert. Eli Lilly and Company; 2020. https://pi.lilly.com/us/trulicity-uspi.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study Design. Abbreviation: DU = dulaglutide.
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at vivli.org.


