Abstract
In the budding yeast Saccharomyces cerevisiae, Cdc37 is required for the productive formation of Cdc28-cyclin complexes. The cdc37-1 mutant arrests at Start with low levels of Cdc28 protein, which is predominantly unphosphorylated at Thr169, fails to bind cyclin, and has little protein kinase activity. We show here that Cdc28 and not cyclin is specifically defective in the cdc37-1 mutant and that Cdc37 likely does not act as an assembly factor for Cdc28-cyclin complex formation. We have also found that the levels and activity of the protein kinase Cak1 are significantly reduced in the cdc37-1 mutant. Pulse-chase analysis indicates that Cdc28 and Cak1 proteins are both destabilized when Cdc37 function is absent during but not after translation. In addition, Cdc37 promotes the production of Cak1, but not that of Cdc28, when coexpressed in insect cells. We conclude that budding yeast Cdc37, like its higher eukaryotic homologs, promotes the physical integrity of multiple protein kinases, perhaps by virtue of a cotranslational role in protein folding.
Activation of cyclin-dependent kinases (Cdks) is achieved through the binding of a cyclin subunit and phosphorylation of a conserved threonine residue in the T loop of the kinase. Cdk regulation is effected by the controlled synthesis and degradation of the cyclin partner, by inhibitory phosphorylation, and by the binding of modulatory proteins, including Cdk inhibitors (reviewed in reference 21). Phosphorylation of the activating threonine (T169 in Cdc28) is constitutive and in Saccharomyces cerevisiae is carried out by the essential Cdk-activating kinase, Cak1 (9, 10, 16, 29, 30).
Budding yeast has an additional requirement for activation of the Cdc28 kinase: Cdc37. CDC37 is essential for progression through Start (24, 25) and is required for the activation of Cdc28 (12). In cdc37-1 cells, Cdc28 fails to bind both G1 and mitotic cyclins, exhibits reduced phosphorylation at Thr169, and is found at three- to fivefold-lower levels (12). Yeast Cdc37 may also promote the function of other protein kinases: cdc37 mutations are synthetically lethal with mutations in the protein kinases Cdc28, Mps1, and Kin28 (24, 26, 31), and Mps1 activity is reduced in a cdc37 mutant. Likewise, Cdc37 is required for the activity of the protein kinase v-Src when it is expressed in yeast (8).
Higher eukaryotes contain Cdc37 homologs that also appear to play general roles in the maintenance of protein kinase integrity (4, 15, 28). In mammalian cells, the kinases v-Src, Cdk4, and Raf each exist in heterotrimeric complexes containing Hsp90 and Cdc37 (known originally as p50) (1–3, 5, 13, 19, 22, 23, 27, 28). Recent work suggests that Cdc37 acts by recruiting Hsp90 to Cdk4 and Raf, resulting in kinase stabilization and activation (13, 27, 28). Genetic interactions between cdc37 and hsp90 mutants in yeast and Drosophila also support the possibility that Cdc37 and Hsp90 act in concert (18). Furthermore, the experiments of Kimura et al. (18) demonstrate an in vitro activity for Cdc37 as an Hsp90-like molecule that can prevent the irreversible aggregation of a denatured reporter substrate.
Yeast Cdc37 is only distantly related to its mouse homolog (19% identity) (28), raising the possibility that the molecular mechanism of Cdc37 action in yeast is different from that in mammals. To better understand the function of yeast Cdc37 and to compare its function with that of the vertebrate protein, we analyzed in detail the molecular phenotypes that result in budding yeast with a cdc37 mutation. Our results support the hypothesis that Cdc37 is required for the stability of multiple protein kinases. In addition, our evidence suggests that Cdc37 is required during translation to prevent protein misfolding.
MATERIALS AND METHODS
Yeast strains and media.
The yeast strains referred to in this report are listed in Table 1. All media used are as described in reference 14.
TABLE 1.
Strains used in this study
| Strain | Genotype |
|---|---|
| RD488 | MATa leu2 ura3 trp1 BAR1 |
| RD249-2D | MATa cdc37-1 bar1::LEU2 ura3 pep4::TRP1 |
| RD249-2D p86-1 | MATa cdc37-1 CDC28-HA::URA3 pep4::TRP1 bar1::LEU2 |
| RD270-8B | MATa CDC28-HA::TRP1 pep4::TRP1 bar1::LEU2 |
| AF22 | MATa cdc37-1 ade2-1 can1-100 his3-11 leu2-3, 112 trp1-1 ura3-1 bar1::hinG CAK1-HA::LEU2 |
| AF23 | MATa ade2-1 can1-100 his3-11 leu2-3, 112 trp1-1 ura3-1 bar1::hinG CAK1-HA::LEU2 |
| AF40 | MATa cdc37-1 bar1::LEU2 pep4::TRP1 ura3::CAK1-HA3 |
| AF42 | MATa bar1::LEU2 pep4::TRP1 ura3::CAK1-HA3 |
| AL13 | MATa ade2-1 can1-100 his3-11 leu2-3, 112 trp1-1 ura3-1 bar1::hinG pGAL1-CAK1-HA–URA3 |
| AL14 | MATa cdc37-1 ade2-1 can1-100 his3-11 leu2-3, 112 trp1-1 ura3-1 bar1::hinG pGAL1-CAK1-HA–URA3 |
Yeast lysis.
Yeast strains were grown to an optical density at 600 nm (OD600) of 0.3 in YPD at 23°C and then shifted to 37°C for 3 h or maintained at 23°C. Cells were lysed in 170 μl of lysis buffer (20 mM Tris-HCl [pH 7.4], 50 mM NaF, 5 mM EDTA, 100 mM NaCl, 150 mM β-glycerophosphate, 0.2% Triton X-100, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 2 μg of aprotinin per ml, 1 μg of leupeptin per ml) with glass beads in a Biospec bead beater. Lysates were clarified by ultracentrifugation for 10 min at 4°C. For freeze-thaw lysis, cells were incubated in spheroplast buffer (1.1 M sorbitol, 50 mM Tris-HCl [pH 7.5], 1 mM MgCl2, 2 mM DTT) with 4 μl of Zymolase (5 mg/ml; Seikagaku Corporation) for 40 min at 23 or 37°C. Spheroplasts were washed in spheroplast buffer, lysed in hypotonic lysis buffer (20 mM HEPES-NaOH [pH 7.4], 25 mM NaCl, 1 mM EDTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, 2 μg of aprotinin per ml, 1 μg of leupeptin per ml) by freezing on dry ice and thawing in ice water three times, and then clarified by centrifugation.
Western blotting, immunoprecipitation, and kinase assays.
Western blotting was carried out essentially as described elsewhere (6), using rabbit anti-Cdc37 antibodies (1:1,000), rabbit anti-Cdc28 antibodies (1:1,000), or hemagglutinin (HA)-specific monoclonal antibody 12CA5 (1:1,000).
For immunoprecipitation of HA-tagged proteins, cell lysates (100 to 250 μg) were incubated with 1 μl of 12CA5, 20 μl of protein A-Sepharose beads, and yeast lysis buffer in a final volume of 300 μl for 1 to 2 h at 4°C on a rotator. Immunoprecipitates were washed three times with lysis buffer and once with 50 mM HEPES-NaOH (pH 7.4)–1 mM DTT. Histone H1 kinase assays were performed by adding to the immunoprecipitates 20 μl of histone H1 kinase mix (50 mM HEPES-NaOH [pH 7.4], 1 mM DTT, 10 mM MgCl2, 100 μM ATP, 0.25 mg of human histone H1 per ml, 0.125 mCi of [γ-32P]ATP per ml). After incubation at room temperature for 20 min, the reaction products were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 11% polyacrylamide gel.
Cak1 assays were performed as follows. Cell lysates (250 to 500 μg) were immunoprecipitated with anti-HA monoclonal antibody 12CA5 for 2 h at 4°C as described above. For direct determination of Cak1 activity, 1 μg of purified Cdc28 was added to each Cak1 immunoprecipitate in 20 μl of kinase reaction buffer (50 mM HEPES-NaOH [pH 7.4], 1 mM DTT, 10 mM MgCl2, 0.25 mCi of [γ-32P]ATP per ml, 50 μM ATP). Reaction mixtures were incubated for 20 min at 23°C and analyzed by SDS-PAGE and autoradiography. For indirect analysis of Cak1 activity, Cak1 immunoprecipitates were incubated with 0.24 μg of purified Cdc28-HA, 1 μg of purified glutathione S-transferase (GST)–Clb2, and 2 μg of HA peptide in 10 μl of activation buffer (50 mM HEPES-NaOH [pH 7.4], 1 mM DTT, 10 mM MgCl2, 1 mM ATP). Reaction mixtures were incubated for 20 min at 23°C. Activation of the Cdc28–GST-Clb2 complex was then measured by histone H1 kinase assay.
Pulse-chase labeling and analysis.
Wild-type and cdc37-1 cultures were grown at room temperature to an OD600 of 0.1 in YPD. Cells were harvested, washed twice in YNB, resuspended in YND minus methionine (YND-Met), and grown to an OD600 of 0.3 at 23°C (6 h). Cultures were split into two; one half was shifted to 37°C, and the other was left at room temperature. After 1 h, the cultures were harvested and resuspended in 3 ml of YND-Met supplemented with [35S]methionine (50 μCi/OD). Cells were incubated at room temperature or at 37°C for 15 min, harvested, and resuspended in 3 ml of YPD plus 40 μg of unlabeled methionine per ml. Samples were collected at various times thereafter and washed once in YND prior to freezing. In some experiments, cells were incubated with [35S]methionine (50 μCi/OD) for 30 min at 23°C, harvested, resuspended in 3 ml of prewarmed (37°C) YPD with 40 μg of unlabeled methionine per ml, and maintained at 37°C for the remainder of the experiment. Samples were lysed by bead beating, and lysates (100 to 250 μg) were immunoprecipitated with 12CA5. After separation of proteins by SDS-PAGE and gel fixation, gels were incubated for 30 min in Amplify (Amersham) prior to drying and autoradiography.
Protein purification. (i) Cdc28HA purification.
Sf9 insect cells infected with the Cdc28-HA virus were lysed in buffer A (20 mM HEPES-NaOH [pH 7.4], 25 mM NaCl, 1 mM EDTA, 10% glycerol, 1 mM DTT) containing 1 mM PMSF, 1 μg of leupeptin per ml, and 2 μg of aprotinin per ml. Following ultracentrifugation (60 min, 100,000 × g, 4°C), the lysate was applied to a HiTrap Q column (Pharmacia) preequilibrated in buffer A. The HiTrap Q column flowthrough containing Cdc28-HA was then applied to a HiTrap S column (Pharmacia) preequilibrated in buffer A, and proteins were eluted with a linear gradient of 25 to 500 mM NaCl in buffer B (20 mM HEPES-NaOH [pH 7.4], 1 mM EDTA, 10% glycerol, 1 mM DTT). Fractions containing Cdc28-HA were diluted with buffer B and applied to an ATP-agarose column preequilibrated with buffer A. Cdc28-HA was eluted from the ATP-agarose column with a linear gradient of 25 to 500 mM NaCl in buffer B, and final contaminants were removed by separation on a Superose 12 sizing column (Pharmacia) equilibrated in buffer C (150 mM NaCl, 20 mM HEPES-NaOH [pH 7.4], 1 mM EDTA, 10% glycerol, 1 mM DTT).
(ii) Cdc37 purification.
Insect cells were infected with a Cdc37 virus and lysed as described above for Cdc28-HA purification. Cdc37-containing lysate was applied to a HiTrap Q column preequilibrated in buffer A, and proteins were eluted with a linear gradient of 25 to 500 mM NaCl in buffer B. Fractions containing Cdc37 (250 to 350 mM NaCl) were diluted with buffer B to below 100 mM NaCl and applied to a HiTrap SP column preequilibrated with buffer A. The SP flowthrough containing Cdc37 was concentrated with a HiTrap Q column, and the eluted Cdc37 was injected on a Superose 12 column preequilibrated with buffer C. Cdc37 migrates as a 160-kDa species on gel filtration. Cdc37-containing fractions were pooled, (NH4)2SO4 was added to 1 M, and the sample was applied to a HiTrap octyl-Sepharose 4FF column (Pharmacia) preequilibrated with 20 mM HEPES-NaOH (pH 7.4)–1 M (NH4)2SO4 and eluted with a decreasing linear gradient of 1 M to 0 M (NH4)2SO4 in 20 mM HEPES-NaOH (pH 7.4)–1 mM EDTA–10% glycerol.
(iii) His6-Cdc37 purification.
A HiTrap chelating column (Pharmacia) was prebound with 200 mM CoCl2. Bacterial lysate containing six-histidine-tagged Cdc37 (His6-Cdc37) was applied to the column in sonication buffer (50 mM sodium phosphate [pH 7.8], 300 mM NaCl, 10% glycerol, 1 mM DTT, 0.5 mM PMSF) and eluted with a linear gradient of 7.5 to 300 mM imidazole (pH 7.8) in sonication buffer. His6-Cdc37-containing fractions were pooled and diluted to 100 mM NaCl with buffer B. Contaminating proteins were removed with a HiTrap Q column step as described for Cdc37 purification from insect cells.
Activation of Cdc28-HA or Cln2-HA3.
Insect cell lysate containing Cdc28-HA (5 μg) or Cln2-HA3 (30 μg) was mixed with increasing amounts of yeast lysate (0, 10, 50, 100, 250, and 500 μg of RD488 or RD249-2D), and reaction mixtures were incubated for 30 min at 23°C. Cdc28-HA or Cln2-HA3 was then immunoprecipitated with 12CA5, and histone H1 kinase activity was measured as described above.
Cdk complex assembly in vitro.
Purified Cdc28-HA (1 μg) was incubated with or without 1 μg of purified GST-Clb2, 1 μg of purified His6-Cdc37, or 50 ng of purified mammalian CAK (Cdk7-cyclin H-Mat1) in a final reaction volume of 10 μl (50 mM HEPES-NaOH [pH 7.4], 1 mM ATP, 1 mM DTT, 10 mM MgCl2). Reaction mixtures were incubated on ice for 15 min and then at 23°C for 15 min. To each reaction mixture was added 20 μl of histone H1 reaction mix (300 μM ATP, 0.125 mCi of [γ-32P]ATP per ml, 10 mM MgCl2, 50 mM HEPES-NaOH [pH 7.6], 0.25 mg of histone H1 per ml, 1 mM DTT). Reaction mixtures were incubated at room temperature for 20 min and analyzed by SDS-PAGE on an 11% polyacrylamide gel.
Gel filtration chromatography.
Insect cell lysate (500 μl) or yeast lysate (500 μg) was clarified by centrifugation for 30 min at 100,000 × g (4°C). Samples were applied in 500-μl aliquots to a Superose 12 sizing column (Pharmacia) preequilibrated with buffer C. Fractions (0.5 ml) were collected at 0.4 ml/min and analyzed by Western blotting. For analysis of the sizing profiles of Cdc28-HA in cdc37-1 cells and of Cak1-HA3 in wild-type and cdc37-1 cells, fractions were immunoprecipitated with 1 μl of 12CA5 and 20 μl of protein A-Sepharose beads for 1.5 h at 4°C as described above. Immunoprecipitated proteins were resolved by SDS-PAGE, and analyzed by immunoblotting with 12CA5.
RESULTS
Cdc28 and not cyclin is compromised in the cdc37-1 mutant.
In cdc37-1 cells at the restrictive temperature, Cdc28 binds neither G1 nor mitotic cyclins, resulting in low Cdc28-associated kinase activity (12). To determine whether this failure to bind cyclin is due to a defect in Cdc28 or in the cyclin itself, we examined the activation of an exogenous source of Cdc28 or cyclin by a cdc37-1 lysate. Insect cell lysates containing recombinant Cdc28 or Cln2, each fused to the HA epitope, were incubated with lysate from wild-type or cdc37-1 cells. Insect cell-derived Cdc28 and Cln2 were specifically immunoprecipitated with antibody directed against the HA epitope, and associated kinase activity was assessed by histone H1 phosphorylation. We found that incubation of exogenous Cln2 in wild-type lysate led to the appearance of abundant Cln2-associated kinase activity, whereas no kinase activity was observed after incubation of Cln2 with a lysate from cdc37-1 cells (Fig. 1A). The lack of Cln2-associated activity in the cdc37-1 lysate was accompanied by a failure of Cdc28 to bind exogenous Cln2 (Fig. 1B). In contrast, active kinase could be recovered by the addition of exogenous Cdc28 to both wild-type and cdc37-1 lysates, indicating that the cyclin subunits in these cells are competent to bind native Cdc28 (Fig. 1C). We conclude that Cdc28, rather than cyclin, is primarily affected by the cdc37-1 mutation.
FIG. 1.
Cyclins but not Cdc28 are competent to form active Cdk complexes in the cdc37-1 mutant. Crude insect cell lysate containing recombinant Cln2-HA3 was added to increasing amounts of wild-type (WT; RD488) or cdc37-1 (RD249-2D) lysate. Formation of an active Cdc28–Cln2-HA3 complex was measured by immunoprecipitation of Cln2-HA3 with 12CA5, followed by histone H1 phosphorylation (A) and by immunoblotting for Cdc28 protein associated with Cln2-HA3 (B). (C) Crude insect cell lysate containing Cdc28-HA was incubated with increasing amounts of wild-type (WT) or cdc37-1 lysate to assess the ability of endogenous cyclins to bind and activate Cdc28. Cdc28-HA was immunopurified, and the formation of active kinase complexes was determined by histone H1 kinase assay.
Cdc28 and Clb2 form an active complex in vitro in the absence of Cdc37.
Purified mammalian Cdk and cyclin homologues are able to assemble into functional kinase complexes in the absence of other factors (7), but the requirements for yeast Cdc28-cyclin complex formation are unknown. Moreover, it is not clear that all Cdk-cyclin complexes are able to assemble in the absence of other components. For example, in the absence of phosphorylation at T170, Cdk7 requires an additional protein (Mat1) for efficient binding to cyclin H (11). Cdk4 binds very low levels of its cyclin partner in insect cell lysate mixing experiments (17), and induction of cyclin D in quiescent fibroblasts does not promote active complex formation, suggesting the need for an additional factor (20). It is therefore possible that Cdc37 functions as an essential assembly factor in the binding of Cdc28 and cyclin. To test this prediction, we combined purified epitope-tagged Cdc28 (Cdc28-HA), a GST-Clb2 fusion protein, mammalian CAK (to phosphorylate Thr169), and Cdc37. We analyzed histone H1 kinase activity as a measure of successful complex formation (Fig. 2). We found that Cdc28, GST-Clb2, and CAK are sufficient to form an active Cdk complex. Cdc37 was not required for the binding of Cdc28 to cyclin or for the activation of the resultant complex by CAK. Addition of Cdc37 did not enhance the activity of the Cdk complex.
FIG. 2.
Assembly of active Cdc28-cyclin complexes in vitro is independent of Cdc37. The indicated combinations of purified Cdc28-HA, GST-Clb2, human CAK, and Cdc37 were incubated for 15 min on ice and then for 15 min at 23°C. The resulting histone H1 kinase activity was measured in solution. In the final lane, Cdc37 was not included in the incubation step but added immediately prior to the histone H1 kinase assay.
Cdc28 is aggregated in cdc37-1 cells.
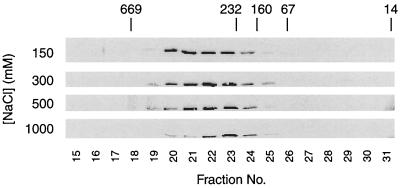
In wild-type cells, Cdc28 exists both as a monomer and associated with a variety of proteins to form a heterogeneous collection of both low- and high-molecular-weight complexes (data not shown). In contrast, we found that the Cdc28 in cdc37-1 extracts exists only in high-molecular-weight complexes and does not exist in a monomeric form (Fig. 3). Given that Cdc28 does not bind cyclins in a cdc37-1 cell at the nonpermissive temperature, this observation intimated that these Cdc28 complexes might contain an unidentified associated protein and that the absence of a monomeric form of Cdc28 results in its unavailability to bind cyclin. To test this hypothesis, we attempted to disrupt the Cdc28-containing complex by increasing the ionic strength of the lysate and monitored the size of the complex by gel filtration chromatography (Fig. 3). As the salt concentration was increased, however, Cdc28 was not chased to a monomeric form (fractions 28 and 29 in Fig. 3). Instead, at the highest salt concentrations, the complex focused more tightly (in fractions 22 to 24 at 1 M NaCl, compared to fractions 20 to 24 at 150 mM NaCl). This behavior suggests that the high-molecular-weight Cdc28 complex observed in cdc37-1 lysates is due to the limited aggregation of the Cdc28 protein rather than to its association with other protein(s). Although Cdc28 may form a salt-stable complex with proteins that are resistant to dissociation in 1 M NaCl, we have not been able to find any evidence of Cdc28-associated proteins in immunoprecipitates of Cdc28 from either radiolabeled or unlabeled cdc37-1 cells (data not shown). These results are consistent with the possibility that in the absence of Cdc37, Cdc28 is improperly folded, aggregates, and is ultimately targeted for degradation, thereby resulting in the low levels observed in the cdc37-1 mutant.
FIG. 3.
Cdc28 does not bind a salt-perturbable inhibitor in cdc37-1 cells. cdc37-1 (RD249-2D p86-1) lysate (1.5 mg) was injected onto a Superose 12 sizing column, and the elution of Cdc28-HA at increasing salt concentrations (150 mM, 300 mM, 500 mM, and 1 M NaCl) was monitored by immunoprecipitation of Cdc28-HA from each fraction, followed by immunoblotting against the HA epitope. Fractions in which proteins of 669, 232, 160, 67, and 14 kDa elute are marked with arrows. Cdc28 monomer in wild-type cells normally migrates in fractions 28 to 29 (data not shown).
Cdc37 is required for Cak1 activity.
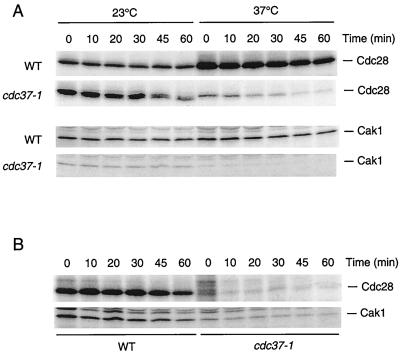
In the course of our analysis of the cdc37-1 mutant, we noted not only that the level of Cdc28 protein is dramatically reduced but also that Cdc28 is present predominantly in its slower-migrating, unphosphorylated form (12). This finding suggests either that Cak1 is unable to phosphorylate unfolded Cdc28 or that Cak1 itself is inactive in the cdc37-1 mutant. To determine if Cdc37 is also required for Cak1 function, we replaced the endogenous CAK1 gene with an HA epitope-tagged version and compared the levels of Cak1 protein in wild-type and cdc37-1 cells by immunoblotting. The level of Cak1 was significantly decreased in the cdc37-1 mutant at the permissive temperature, and this effect was further exacerbated at 37°C (Fig. 4A). We analyzed Cak1 activity in these cells by immunoprecipitation of Cak1 and measurement of its ability to phosphorylate Cdc28. Concomitant with the decrease in Cak1 protein, Cak1 activity was also greatly diminished in cdc37-1 cells (Fig. 4B) and was not rescued by the addition of exogenous Cdc37 (Fig. 4C). When Cak1 was expressed under the control of the GAL1 promoter in cdc37-1 strains, we observed a similar temperature-dependent loss of Cak1 activity (Fig. 4D), suggesting that the effects of the cdc37-1 mutation are not transcriptional. Thus, Cdc37 appears to be required to maintain the levels and activity of not only Cdc28 but also Cak1.
FIG. 4.
Cak1 protein and activity are reduced in the cdc37-1 mutant. (A) Wild-type (WT; AF42) and cdc37-1 (AF40) strains expressing HA-tagged Cak1 were incubated at 23 or 37°C for 3 h. Cells were lysed by bead beating, and Cak1 levels were compared by immunoblotting with 12CA5 against the HA epitope. (B) Wild-type (WT; AF23) and cdc37-1 (AF22) cells were incubated at 23 or 37°C for 3 h and lysed by freeze-thaw. HA epitope-tagged Cak1 was immunoprecipitated, and analysis of its kinase activity was performed by direct phosphorylation of Cdc28. (C) Wild-type and cdc37-1 lysates from the experiment in panel B were incubated with 1 μg of purified Cdc37 for 20 min at 23°C prior to immunoprecipitation of Cak1. Purified Cdc28 and GST-Clb2 were added to the Cak1 immunoprecipitate, and Cak1-dependent activation of the Cdk complex was determined by histone H1 phosphorylation. (D) Wild-type (WT; AL13) and cdc37-1 (AL14) strains expressing CAK1-HA under the control of the GAL1 promoter were incubated in galactose-containing medium at 23 or 37°C for 3 h. Cells were lysed, and Cak1 was immunoprecipitated with anti-HA monoclonal antibody. Cak1 activity was measured indirectly by its ability to phosphorylate and activate an exogenous Cdc28–GST-Clb2 complex. Activation of Cdc28–GST-Clb2 was measured by histone H1 kinase assay.
The loss of Cak1 activity in cdc37-1 cells does not appear to be directly responsible for the loss of Cdc28 protein or activity. Overexpression of CAK1 does not rescue the temperature sensitivity of the cdc37-1 mutant, nor does it raise the permissive temperature, unlike overexpression of CDC28 (data not shown and reference 12). Furthermore, a lack of Thr169 phosphorylation does not itself induce a decrease in Cdc28 protein. In cak1 mutants at the restrictive temperature, Thr169 phosphorylation of Cdc28 is absent, but the level of Cdc28 protein remains constant (10). Thus, although the loss of Cak1 activity leads to reduced activating phosphorylation of Cdc28, decreases in Cak1 and Cdc28 protein are two independent effects of the cdc37-1 mutation.
To determine if Cak1, like Cdc28, exists in high-molecular-weight aggregates in cdc37-1 cells, we analyzed the sizing profile of Cak1 in both wild-type and cdc37-1 cells. The sizing profile of Cak1 was not substantially altered in a cdc37-1 lysate, and Cak1 clearly existed in cdc37-1 cells only as a monomer (data not shown).
Cdc37 is required for Cdc28 and Cak1 stability.
If Cdc37 is necessary for the efficient folding of Cdc28 and Cak1, then the low levels of these two proteins in cdc37-1 cells may be explained by a lack of successful folding, resulting in the targeting of nonnative proteins for proteolysis. Furthermore, as a chaperone, Cdc37 may act either co- or posttranslationally. To explore these possibilities, we used pulse-chase analysis to estimate the half-lives of Cdc28 and Cak1 in wild-type and cdc37-1 cells at both the permissive and restrictive temperatures. Wild-type and cdc37-1 cells were incubated with 35S-labeled methionine at 23 or 37°C, and the label was chased for various times at both temperatures. These studies revealed that the loss of Cdc37 function decreased the stability of both Cdc28 and Cak1 (Fig. 5A). In wild-type cells, the half-lives of Cdc28 and Cak1 were both approximately 60 min at 23°C and did not change significantly at 37°C. In contrast, the half-life of Cdc28 in cdc37-1 cells was approximately 30 min at 23°C and was further decreased to less than 20 min at 37°C. Cak1 in cdc37-1 cells was quite stable at 23°C, with a half-life of approximately 45 min, but was rapidly turned over at 37°C (half-life of less than 20 min).
FIG. 5.
Pulse-chase analysis of the half-lives of Cdc28 and Cak1 in wild-type and cdc37-1 cells. (A) Wild-type (WT; RD270-8B) and cdc37-1 (RD249-2D p86-1) strains expressing HA epitope-tagged Cdc28 (Cdc28HA) were pulsed with [35S]methionine at 23 or 37°C for 15 min and chased at the same temperature with unlabeled medium for the indicated times. Similar studies were performed with wild-type (WT; AF42) and cdc37-1 (AF40) strains expressing HA epitope-tagged Cak1. Cell lysates were subjected to immunoprecipitation with an anti-HA monoclonal antibody. (B) Wild-type (WT; RD270-8B) and cdc37-1 (RD249-2D p86-1) strains expressing HA epitope-tagged Cdc28 were pulsed with [35S]methionine at 23°C for 30 min and chased with unlabeled medium at 37°C for the indicated times. Similar studies were performed with wild-type (WT; AF42) and cdc37-1 (AF40) strains expressing HA epitope-tagged Cak1. Cell lysates were subjected to immunoprecipitation with an anti-HA monoclonal antibody.
In an attempt to distinguish between roles for Cdc37 in cotranslational versus posttranslational protein folding, we allowed wild-type and cdc37-1 cells to synthesize radiolabeled Cdc28 and Cak1 at the permissive temperature (23°C) and then analyzed the effects of a shift to the restrictive temperature (37°C) upon simultaneous addition of unlabeled methionine (chase) (Fig. 5B). In wild-type cells, the stability of Cak1 and Cdc28 was comparable to that observed in our previous experiments (Fig. 5A). In cdc37-1 cells, Cak1 and Cdc28 were relatively stable following the shift to 37°C, contrasting with the instability evident when these proteins were synthesized at 37°C (Fig. 5A). Although the half-lives of both proteins appeared to be shorter than those observed in wild-type cells, there appeared to be a stable population of both Cdc28 and Cak1. Thus, the postulated chaperone activity of Cdc37 is necessary during but not after translation, arguing against a role for Cdc37 as a heat shock factor or scavenger of denatured proteins.
Cdc37 increases Cak1 protein level in insect cells.
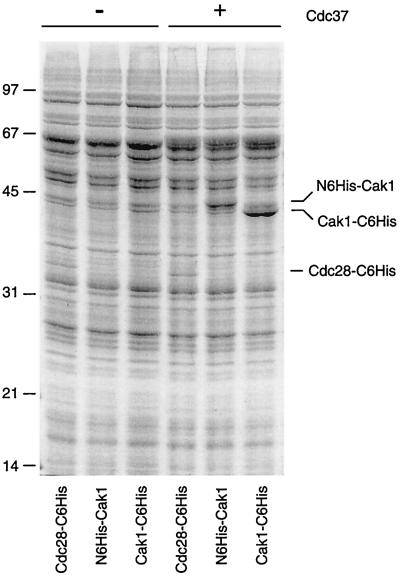
If Cdc37 is required directly or indirectly to stabilize the native conformation of Cak1 and Cdc28, then Cdc37 may be able to increase the level of protein expressed in a heterologous expression system. We tested this possibility by assessing the effects of Cdc37 on expression levels in Sf9 insect cells infected with baculoviruses encoding Cak1 and Cdc28. Coinfection with a Cdc37 baculovirus resulted in a three- to fivefold increase in the production of two different six-His-tagged Cak1 proteins (Fig. 6). Cak1 activity was also increased in insect cells coexpressing Cdc37 (F. H. Espinoza, personal communication). Interestingly, the expression of HA epitope-tagged Cak1 protein was unaffected by Cdc37 coexpression (data not shown), even though maximal expression of the same Cak1HA protein requires Cdc37 in the yeast cell. The six-His tag was not solely responsible for the effects of Cdc37 on Cak1 protein, however, as six-His-tagged Cdc28 was not similarly affected (Fig. 6). We have examined the expression patterns of five additional protein kinases in insect cells, including those of human Cdk7 and yeast Pho85 (data not shown), but in no other instance have we observed a consistent effect on protein level upon Cdc37 coexpression.
FIG. 6.
Cdc37 upregulates Cak1 expression in insect cells. Insect cells were infected with baculoviruses encoding the following kinases: Cdc28 with a C-terminal six-His tag (Cdc28-C6His), Cak1 with an N-terminal six-His tag (N6His-Cak1), and Cak1 with a C-terminal six-His tag (Cak1-C6His). Cells were infected either singly with each kinase virus (left lanes) or simultaneously with a baculovirus encoding Cdc37 (right lanes). The infected insect cells were lysed, and total cell protein was resolved by SDS-PAGE and stained with Coomassie brilliant blue. Sizes are indicated in kilodaltons.
Studies in mammalian cells have revealed that protein kinases such as Cdk4 form readily detectable complexes with Cdc37 and Hsp90 (5, 19, 28). In addition, overexpression of mouse Cdc37 in insect cells leads to the formation of tight complexes between Cdc37 and insect Hsp90 (28). We therefore pursued the possibility that yeast Cdc37 also forms complexes with protein kinases and/or Hsp90. However, we have not observed detectable binding of Cdc37 to any other protein in a variety of systems. When immunoprecipitated from yeast lysates, Cdc37 does not associate with other proteins, including Hsc82 and Cdc28 (reference 12 and data not shown), and Cdc37 is readily separated from Hsc82 upon gel filtration of yeast lysates (data not shown). When expressed in insect cells, yeast Cdc37 migrates on gel filtration at a position distinct from that of insect Hsp90, and purification of Cdc37 from these lysates does not reveal any associated proteins (data not shown). Coexpression of Cdc37 and Cdc28 or Cak1 in insect cells or in rabbit reticulocyte lysates does not lead to the formation of detectable Cdc37-kinase complexes (data not shown). Thus, yeast Cdc37 appears to promote protein kinase stability by low-affinity interactions or by indirect mechanisms that may be distinct from mechanisms employed by mammalian Cdc37.
DISCUSSION
In the cell cycle cdc37-1 mutant, the integrity of several protein kinases, among them Cdc28 and Mps1, appears to be impaired. In the case of Cdc28, the level of the protein is reduced, it fails to bind its cyclin partner, and phosphorylation at its activating threonine is diminished, contributing to its lack of activity. We show here that the lack of Thr169 phosphorylation is not a direct result of the absence of cyclin binding but rather is due to the effect of the cdc37-1 mutation on the activity of the Cdk-activating enzyme Cak1. We have demonstrated that Cdc37 is required for wild-type levels of both Cak1 protein and activity and that unlike Mps1 (26), Cak1 and Cdc28 proteins are destabilized in the cdc37-1 mutant. In this study, we have expanded the scope of activity of the CDC37 gene product and provided additional supportive evidence for the postulated role of Cdc37 as a molecular chaperone with protein kinase specificity.
Various experimental avenues have converged on the notion that Cdc37 is a molecular chaperone (15). Studies in vitro indicate that Cdc37, like Hsp90, can maintain an unfolded protein in a reactivation-competent state (18). Although Cdc37 appears promiscuous in these experiments (its effects are indistinguishable when the reporter protein luciferase, β-galactosidase, or casein kinase II is used [18]), Cdc37 has been linked primarily with protein kinases in vivo. Recent work in mammalian cells also indicates that Cdc37 does not act alone but instead may act as a kinase-targeting subunit of Hsp90 (13, 27, 28).
In contrast to mammalian Cdc37, yeast Cdc37 does not form tight complexes with protein kinases or Hsp90. Although previous studies have demonstrated genetic interactions in yeast between CDC37 and HSP90 (18), the absence of detectable physical interactions raises the possibility that yeast Cdc37 acts by mechanisms independent of Hsp90. Such mechanistic differences might be expected, given the rather distant relationship between yeast and mammalian Cdc37 proteins (19% identity). On the other hand, the absence of detectable Cdc37-Hsp90-kinase complexes in yeast lysates could simply be the result of low affinities that lead to rapid dissociation upon cell lysis, and further studies, perhaps involving chemical cross-linking agents, will be required to resolve this issue.
Cdc37 is probably not a refolding enzyme, as it lacks a consensus ATP binding site, has no homology to the Hsp70 family, and does not demonstrate any refolding activity in vitro. We do not, however, believe that Cdc37 is simply a heat shock factor, in part because it is not induced by extremes of temperature but also because of the temporal restriction of the effects of the cdc37-1 mutation that we have established. Cdc37 seems to be required at the time of protein synthesis, rather than to maintain the physical integrity of already synthesized proteins. These results are consistent with previous evidence that the Hsp90-Cdc37 complex associates primarily with newly synthesized v-Src (1, 3). It thus seems likely that Cdc37, possibly in conjunction with Hsp90 or Hsp70, is required for the productive folding of newly synthesized proteins. It remains to be determined if Cdc37 binds exposed hydrophobic residues and why it has thus far appeared to favor protein kinases.
We found that Cdc37 induces an increase in Cak1 expression in insect cells. This result is not true for Cdc28, another putative target of Cdc37, nor does it apply to all of our epitope-tagged versions of Cak1. Why and how Cdc37 should selectively increase the expression of six-His-tagged Cak1 is unclear, but this experiment has provided us with the first evidence of a positive effect of the yeast Cdc37 protein in vivo and a tractable assay for Cdc37 activity. Whether Cdc37 acts as an effector of cotranslational protein folding, at the level of protein stability, or in the prevention of premature protein degradation can now be addressed in studies using this system.
ACKNOWLEDGMENTS
We thank Doug Kellogg and Holly Chamberlin for reagents, Hernan Espinoza for assistance with Cdc37-Cak1 coexpression in insect cells, Julia Charles and Andrew Murray for comments on the manuscript, and Ira Herskowitz and members of the Morgan and Murray labs for helpful advice throughout the course of this work.
This work was supported by funding from the National Institute of General Medical Sciences (to D.O.M.) and a postgraduate scholarship from the Natural Sciences and Engineering Research Council of Canada (to A.F.).
REFERENCES
- 1.Brugge J, Yonemoto W, Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983;3:9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugge J S, Erikson E, Erikson R L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981;25:363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- 3.Courtneidge S A, Bishop J M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci USA. 1982;79:7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutforth T, Rubin G M. Mutations in Hsp83 and cdc37 impair signaling by the Sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 5.Dai K, Kobayashi R, Beach D. Physical interaction of mammalian CDC37 with CDK4. J Biol Chem. 1996;271:22030–22034. doi: 10.1074/jbc.271.36.22030. [DOI] [PubMed] [Google Scholar]
- 6.Desai D, Gu Y, Morgan D O. Activation of human cyclin-dependent kinases in vitro. Mol Biol Cell. 1992;3:571–582. doi: 10.1091/mbc.3.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai D, Wessling H C, Fisher R P, Morgan D O. The effect of phosphorylation by CAK on cyclin binding by CDC2 and CDK2. Mol Cell Biol. 1995;15:345–350. doi: 10.1128/mcb.15.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dey B, Lightbody J J, Boschelli F. CDC37 is required for p60v-src activity in yeast. Mol Biol Cell. 1996;7:1405–1417. doi: 10.1091/mbc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinoza F H, Farrell A, Erdjument-Bromage H, Tempst P, Morgan D O. A cyclin-dependent kinase-activating kinase (CAK) in budding yeast unrelated to vertebrate CAK. Science. 1996;273:1714–1717. doi: 10.1126/science.273.5282.1714. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza F H, Farrell A, Nourse J L, Chamberlin H M, Gileadi O, Morgan D O. Cak1 is required for Kin28 phosphorylation and activation in vivo. Mol Cell Biol. 1998;18:6365–6373. doi: 10.1128/mcb.18.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher R P, Jin P, Chamberlin H M, Morgan D O. Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell. 1995;83:47–57. doi: 10.1016/0092-8674(95)90233-3. [DOI] [PubMed] [Google Scholar]
- 12.Gerber M R, Farrell A, Deshaies R, Herskowitz I, Morgan D O. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grammatikakis N, Lin J-H, Grammatikakis A, Tsichlis P N, Cochran B H. p50cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol. 1999;19:1661–1672. doi: 10.1128/mcb.19.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guthrie C, Fink G R, editors. Methods in enzymology. 194. Guide to yeast genetics and molecular biology. San Diego, Calif: Academic Press; 1991. [PubMed] [Google Scholar]
- 15.Hunter T, Poon R Y C. Cdc37: a protein kinase chaperone? Trends Cell Biol. 1997;7:157–161. doi: 10.1016/S0962-8924(97)01027-1. [DOI] [PubMed] [Google Scholar]
- 16.Kaldis P, Sutton A, Solomon M J. The Cdk-activating kinase (CAK) from budding yeast. Cell. 1996;86:553–564. doi: 10.1016/s0092-8674(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 17.Kato J-Y, Matsuoka M, Strom D K, Sherr C J. Regulation of cyclin D-dependent kinases (cdk4) by cdk4-activating kinase. Mol Cell Biol. 1994;14:2713–2721. doi: 10.1128/mcb.14.4.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura Y, Rutherford S, Miyata Y, Yahara I, Freeman B C, Yue L, Morimoto R I, Lindquist S. Cdc37 is a molecular chaperone with signal transduction functions that overlap with but are distinct from those of Hsp90. Genes Dev. 1997;11:1775–1785. doi: 10.1101/gad.11.14.1775. [DOI] [PubMed] [Google Scholar]
- 19.Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Draetta G F, Gyuris J. Interaction between Cdc37 and Cdk4 in human cells. Oncogene. 1997;14:1999–2004. doi: 10.1038/sj.onc.1201036. [DOI] [PubMed] [Google Scholar]
- 20.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan D O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 22.Oppermann H, Levinson W, Bishop J M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci USA. 1981;78:1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perdew G H, Wiegand H, Vanden Heuvel J P, Mitchell C, Singh S S. A 50 kilodalton protein associated with raf and pp60v-src protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry. 1997;36:3600–3607. doi: 10.1021/bi9612529. [DOI] [PubMed] [Google Scholar]
- 24.Reed S I. The selection of amber mutations in genes required for completion of start, the controlling event of the cell division cycle of S. cerevisiae. Genetics. 1980;95:579–588. doi: 10.1093/genetics/95.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed S I. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics. 1980;95:561–577. doi: 10.1093/genetics/95.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schutz A R, Giddings T H, Steiner E, Winey M. The yeast CDC37 gene interacts with MPS1 and is required for proper execution of spindle pole body duplication. J Cell Biol. 1997;136:969–982. doi: 10.1083/jcb.136.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverstein A M, Grammatikakis N, Cochran B H, Chinkers M, Pratt W B. p50cdc37 binds directly to the catalytic domain of Raf as well as to a site on hsp90 that is topologically adjacent to the tetratricopeptide repeat binding site. J Biol Chem. 1998;273:20090–20095. doi: 10.1074/jbc.273.32.20090. [DOI] [PubMed] [Google Scholar]
- 28.Stepanova L, Leng X, Parker S B, Harper J W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 29.Sutton A, Freiman R. The Cak1p protein kinase is required at G1/S and G2/M in the budding yeast cell cycle. Genetics. 1997;147:57–71. doi: 10.1093/genetics/147.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thuret J-Y, Valay J-G, Faye G, Mann C. Civ1 (CAK in vivo), a novel Cdk-activating kinase. Cell. 1996;86:565–576. doi: 10.1016/s0092-8674(00)80130-0. [DOI] [PubMed] [Google Scholar]
- 31.Valay J-G, Simon M, Dubois M-F, Bensaude O, Facca C, Faye G. The KIN28 gene is required both for RNA polymerase II mediated transcription and phosphorylation of the Rpb1p CTD. J Mol Biol. 1995;249:535–544. doi: 10.1006/jmbi.1995.0316. [DOI] [PubMed] [Google Scholar]