Abstract
Vicinal aminoalcohols are widespread structural motifs in bioactive molecules. We report the development of a new dioxazolone reagent containing a p‐nitrophenyldifluoromethyl group, which 1. displays a good safety profile; 2. shows a remarkably high reactivity in the oxime‐directed iridium(III)‐catalyzed amidation of unactivated C(sp3)−H bonds; 3. leads to amide products which can be hydrolyzed under mild conditions. The amidation reaction is mild, general and compatible with both primary C−H bonds of tertiary and secondary alcohols, as well as secondary C−H bonds of cyclic secondary alcohols. This method provides an easy access to free 1,2‐aminoalcohols after efficient and mild cleavage of the oxime directing group and activated amide.
Keywords: 1,2-amino alcohol; amidation; C−H activation; dioxazolone; iridium
A safe and bench‐stable dioxazolone reagent was developed for the oxime‐directed iridium(III)‐catalyzed amidation of unactivated C(sp3)−H bonds in tertiary and secondary alcohol‐based substrates. The amidation reaction is mild, general and compatible with both primary and secondary C−H bonds.
Introduction
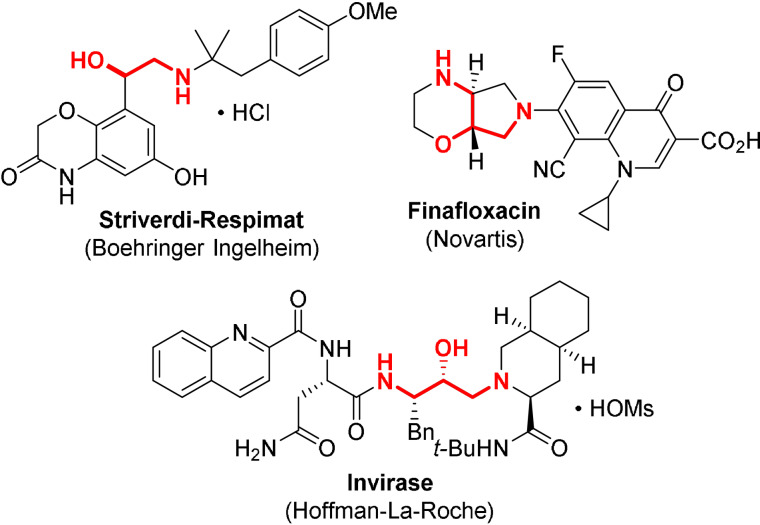
The 1,2‐aminoalcohol motif is a recurrent feature found across a large panel of natural products, agrochemicals and active pharmaceutical ingredients (Figure 1). The traditional elaboration of this motif calls upon aminohydroxylation of olefins, epoxide/aziridine ring‐opening with a suitable nucleophile, or multistep synthetic sequences often involving carbonyl/imine reduction steps. [1] Over the past decade, the construction of the C−N bond through metal‐catalyzed C−H functionalization has emerged as a powerful new synthetic tool, allowing chemists to devise shorter retrosynthetic routes. [2] In this respect, the directed β‐C(sp3)‐H amidation of alcohols can be regarded as a highly attractive and straightforward approach towards vicinal aminoalcohol derivatives. This can be achieved with the assistance of a suitable directing group appended to the alcohol, with oximes having proven to be optimal via the so‐called “exo” directing mode (Scheme 1 a).[ 3 , 4 , 5 ] Mechanistically, the coordination of the oxime nitrogen atom to the metal center is followed by a concerted metallation–deprotonation (CMD) process, affording a five‐membered metallacycle.[ 2f , 2g , 6 ] The subsequent amido‐transfer step is believed to proceed via an inner‐sphere mechanism involving a high‐valent metal–nitrenoid intermediate. Several classes of reagents are known to affect such a transformation, including azides, iminoiodanes (RN=IAr), and 1,4,2‐dioxazol‐5‐ones.[ 4 , 5 ]
Figure 1.
Examples of active pharmaceutical ingredients displaying the 1,2‐aminoalcohol motif.
Scheme 1.
Literature precedents on oxime‐directed C(sp3)−H (sulfon)amidation and current work.
In particular, iridium(III)‐based catalytic systems have been combined with sulfonylazides, [4a] acylazides, [5b] and azidoformates,[ 5d , 5e ] to affect primary C−H bonds and furnish the corresponding (sulfon)amide or carbamate products. These reagents allow for a facile generation of the iridium‐nitrenoid intermediate complex, with nitrogen gas being the sole byproduct of the reaction. However, they are prone to explosive decomposition, and their hydrolysis leads to the release of toxic/volatile/explosive hydrazoic acid, making them highly hazardous for large‐scale operations. Additionally, rhodium(III) complexes have successfully been employed in combination with sulfonyl‐iminoiodanes[ 4b , 4e ] and aryl‐ or alkyldioxazolones,[ 4d , 5c ] also affecting primary C−H bonds, and giving rise to the corresponding sulfonamides and aryl‐ or alkylamides. However, in these cases, the cleavage of the (sulfon)amide to generate the free amine is problematic, and requires reaction conditions that are either harsh or inapplicable on larger scale. Ideally, this issue might be circumvented by relying on reagents capable of transferring highly labile perfluoroalkylamide groups. Unfortunately, known reagents of this type are extremely sensitive to hydrolysis and undergo explosive decomposition. [7] In recent years, dioxazolones have emerged as versatile, highly robust and easily accessible nitrogen sources in combination with transition‐metal catalysts. [2g] Methodologies for the functionalization of unactivated C(sp3)−H bonds using these reagents have so far quasi‐solely relied on cobalt(III) [8] and rhodium(III)[ 4d , 5c , 9 ] catalytic systems. Very recently, Baik, Chang and co‐workers reported two low‐yielding examples under nickel(II)‐catalysis. [10] To the best of our knowledge, only a single example (from 8‐methylquinoline) employing an iridium(III)‐catalyst has been reported thus far. [11]
Herein, we report an efficient and scalable iridium(III)‐catalyzed directed amidation method affecting both unactivated primary and secondary C(sp3)−H bonds for the synthesis of 1,2‐aminoalcohol derivatives (Scheme 1 b). Our approach relies on the use of a novel and bench‐stable 3‐fluoroalkyl‐1,4,2‐dioxazol‐5‐one, which provides a high reactivity and furnishes amide products that are cleavable under very mild hydrolytic conditions.
Results and Discussion
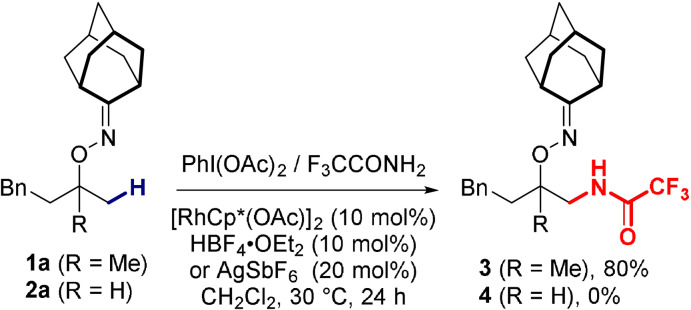
During our preliminary investigations, we first considered the possibility to employ known trifluoroacetyliminoiodane (F3CCON=IAr) reagents to obtain easily‐cleavable amide products. Given the reported explosiveness of such reagents, [7b] by its very nature incompatible with our safety requirements, in situ generation appeared mandatory. This was done by simply combining stoichiometric amounts of (diacetoxyiodo)benzene and trifluoroacetamide in the reaction mixture (Scheme 2). After optimization, it was found that the desired transformation could easily be achieved using a Cp*‐rhodium(III) catalyst (see the supporting information, Table S1). Tertiary alcohol‐derived substrate 1 a, bearing an adamantanone‐based oxime directing group, could be transformed into the corresponding amide 3 with up to 80 % yield. However, the unstable iminoiodane species undergoes rapid degradation at higher temperatures (>50 °C), rendering this approach inapplicable to less reactive secondary alcohol‐derived substrates such as 2 a.
Scheme 2.
Preliminary results.
Our attention was then naturally drawn to the highly robust and stable dioxazolone reagent class. [2g] Their facile preparation from carboxylic acids and their uniquely high reactivity towards the amido‐transfer process in metal‐catalyzed transformations made them immensely appealing. However, the stability and applicability of these reagents is intrinsically linked to the nature of the only possible substituent in the 1,4,2‐dioxazol‐5‐one scaffold. 3‐Alkyl and 3‐aryl groups give rise to highly stable dioxazolones, widely employed in the literature, but for which cleavage of the resulting amide tends to be severely problematic. On the other hand, highly electron‐deficient substituents (e.g., CF3) result in notoriously unstable and explosive compounds, [7a] although milder cleavage of the N‐acyl bond in the product can then be foreseen. On that account, we decided to explore potential alternatives to trifluoromethyldioxazolone 5, with the aim of retaining the benefits of its electron‐withdrawing character, while improving the stability of the reagent (Scheme 3 a). Our investigations led us to design the new dioxazolone reagent 6, incorporating a p‐nitrophenyldifluoromethyl group, which we named K‐diox.
Scheme 3.
Development and synthesis of a new dioxazolone reagent: K‐diox.
The synthesized compound is an off‐white crystalline solid, remarkably stable to moisture and air, which can be stored for more than 1 year in the freezer (−18 °C) without any degradation. Differential Scanning Calorimetry (DSC) displays a strong exothermic event with an initiation temperature of 164 °C and an enthalpy of decomposition of −726 J g−1 (−187 kJ mol−1) (see the supporting information for details). Comparatively, the simpler and widely used 3‐phenyl‐1,4,2‐dioxazol‐5‐one exhibits a lower onset at 142 °C while a similar decomposition energy of −171 kJ mol−1 is recorded. By contrast, 2,2,2‐trichloroethoxycarbonyl azide (TrocN3) displays a much stronger exothermal process (−248 kJ mol−1) initiating at a far lower temperature of 95 °C. The straightforward preparation of K‐diox starts with the copper‐mediated difluoroacetylation of 4‐iodonitrobenzene 7, followed by conversion of the resulting ethyl ester into the corresponding hydroxamic acid, which is finally reacted with CDI (CDI=carbonyldiimidazole) to construct the dioxazolone ring (Scheme 3 b). It was found that adding one equivalent of PPTS (PPTS=pyridinium p‐toluenesulfonate) to the reaction mixture was of crucial importance to the reproducibility and scalability of the process. Indeed, in the absence of PPTS, the highly electron‐deficient hydroxamic acid tends to precipitate as the corresponding imidazolium salt, preventing any further reaction. Overall, K‐diox 6 could easily be obtained on decagram scale with a highly‐reproducible 63 % yield over a 3‐step sequence requiring no purification by column chromatography.
With this new reagent in hand, we went back to exploring the catalytic C(sp3)‐H amidation of compound 1 a. We hypothesized that an IrIII‐based catalytic system could potentially outperform its RhIII equivalent under the appropriate reaction conditions. Indeed, recently published DFT calculations highlighted the fact that IrIII might accelerate the often rate‐determining CMD step through a metal‐assisted proton transfer. [6b] Additionally, a drastically lower kinetic barrier for the metal‐nitrenoid formation step was shown by DFT calculations to originate from the stronger relativistic contraction of iridium. [6a] Delightfully, when K‐diox was used in combination with [IrCp*Cl2]2, AgSbF6 and AgOAc at 35 °C in dichloromethane, not only could the desired product 8 a be isolated in 66 % yield, but the reaction also furnished diamide 9 a in 15 % yield (Table 1, entry 1). Replacing the silver carboxylate additive with sodium acetate led to the full conversion of the starting material within 24 h (entry 2). The reactivity could be adjusted by using potassium or cesium acetate, hence effectively reducing the amount of diamidated product 9 a while retaining a full conversion of 1 a (entries 3, 4). Satisfyingly, employing cesium pivalate almost completely suppressed the formation of 9 a, while the desired product was isolated in an excellent 95 % yield (entry 5). From that point on, halving the catalyst loading proved detrimental to the conversion, as 70 % of the starting material was then recovered (entry 6). To our delight, a high reactivity was regained when switching the additive to acetic acid, with full conversion of the starting material at 2.5 mol % of iridium dimer (entry 7). 1,2‐Aminoalcohol derivative 8 a could then be isolated in 83 % yield using only 1.1 equiv of K‐diox, while the reaction also furnished 13 % of the over‐amidated product. Our optimization study revealed that pivalic acid, when used in place of acetic acid, was enhancing even further the reactivity of the system, resulting in a large propensity towards diamidation (Table S2).
Table 1.
Optimization of the IrIII‐catalyzed C(sp3)−H amidation on tertiary alcohols.

|
Entry |
n (mol %) |
Additive |
K‐diox equiv |
%Yield[a] 1 a |
8 a |
9 a |
|---|---|---|---|---|---|---|
|
1[b] |
5 |
AgOAc |
1.2 |
13 (10) |
71 (66) |
16 (15) |
|
2 |
5 |
NaOAc |
1.2 |
n.d. |
84 |
14 |
|
3 |
5 |
KOAc |
1.2 |
n.d. |
82 |
8 |
|
4 |
5 |
CsOAc |
1.2 |
n.d. |
79 |
10 |
|
5 |
5 |
CsOPiv |
1.2 |
n.d. (1.6) |
92 (95) |
4 (3) |
|
6 |
2.5 |
CsOPiv |
1.1 |
70 |
30 |
n.d. |
|
7 |
2.5 |
AcOH |
1.1 |
n.d. |
85 (83) |
15 (13) |
[a] 1H NMR yields using CH2Br2 as the internal standard, yield of the isolated product in parentheses. [b] Reaction time: 72 h. n.d.=not determined. Reactions were run on 0.2 mmol scale with a catalyst loading of 5 mol %, or on 0.4 mmol scale with a catalyst loading of 2.5 mol % (a constant concentration of 0.25 M was employed).
At this stage, we wished to compare the reactivity of dioxazolone 6 with other amidation reagents under our reaction conditions (Table 2). 3‐Phenyldioxazolone 10 a, undoubtedly the most commonly employed dioxazolone in the literature, only furnished a 59 % mono‐C(sp3)−H amidation yield (entry 2). Moreover, amide 8 aa was isolated as a mixture of products originating from ortho‐C(sp2)−H amidation on the phenyl ring. This issue was however predictable, as dioxazolone 10 a exhibited a similar behavior under rhodium(III)‐catalyzed conditions in the recent work of Xu and co‐workers. [4d] The authors showed that its 2‐furyl analogue 10 b displayed superior reactivity while suppressing the formation of the undesired byproducts. However, under our reaction conditions 10 b only furnished a low 14 % yield of product 8 ab (entry 3). The pivaloyl dioxazolone 10 c performed noticeably better, as the monoamidation product 8 ac could be isolated with an excellent 95 % yield while no diamidation was observed (entry 4). Azidoformate TrocN3, which has proven highly competent in several metal‐catalyzed C−H amidation processes,[ 5e , 12 ] only furnished a moderate 58 % yield of the corresponding product 8 ad (entry 5). Overall, K‐diox displayed a remarkably superior reactivity in comparison to the other tested nitrene sources (entry 1). Nonetheless, we deemed necessary to tame this reactivity and minimize the formation of the diamidated product 9 a. We surmised that changing the nature of the oxime directing group, and in particular providing the substrate with a higher conformational flexibility, could potentially allow us to achieve this goal. Satisfyingly, the cyclooctanone‐derived oxime 1 b performed as intended, and diamide 9 b was isolated in only 7 % yield (entry 6). The yield of monoamidation product (8 c) could be further increased to 92 % when exchanging for a cyclohexanone‐derived oxime (entry 7). On the other hand, smaller cyclopentanone and cyclobutanone‐derived oximes displayed product ratios shifting towards the formation of 9, while overall yields dropped below 90 % (entries 8, 9). In light of these results, the cyclohexanone‐based oxime appeared as the optimum directing group for this transformation.
Table 2.
Optimization of the IrIII‐catalyzed C(sp3)−H amidation on tertiary alcohols: alternative nitrogen sources and directing groups.

|
Entry |
1 (R1) |
10 (R2) |
%Yield[a] 1 |
8 |
9 |
|---|---|---|---|---|---|
|
1 |
1 a (Ad) |
K‐diox |
0 (1 a) |
83 (8 a) |
13 (9 a) |
|
2 |
1 a (Ad) |
10 a |
26 (1 a) |
59[b] (8 aa) |
0 (9 aa) |
|
3 |
1 a (Ad) |
10 b |
76 (1 a) |
14 (8 ab) |
0 (9 ab) |
|
4 |
1 a (Ad) |
10 c |
4 (1 a) |
95 (8 ac) |
0 (9 ac) |
|
5 |
1 a (Ad) |
TrocN3 |
10 (1 a) |
58 (8 ad) |
0 (9 ad) |
|
6 |
1 b (Cyo) |
K‐diox |
– (1 b) |
87 (8 b) |
7 (9 b) |
|
7 |
1 c (Cy) |
K‐diox |
– (1 c) |
92 (8 c) |
6 (9 c) |
|
8 |
1 d (Cyp) |
K‐diox |
– (1 d) |
79 (8 d) |
10 (9 d) |
|
9 |
1 e (Cyb) |
K‐diox |
– (1 e) |
74 (8 e) |
12 (9 e) |
[a] Yields of the isolated products. [b] Combined yield of C(sp3)‐H monoamidation and C(sp2)−H polyamidation products. Reactions were run on 0.4 mmol scale at a concentration of 0.25 M. Troc= C(O)OCH2CCl3.
We then set out to investigate the scope of the reaction on tertiary alcohol‐derived substrates under these mild optimized conditions (Scheme 4). Unsurprisingly, aliphatic alcohol‐based substrates performed very well, with a slightly larger propensity towards diamidation (11 a,b, 12 a,b). Nevertheless, the corresponding diamidated products could be separated off. When a single methyl position was available for functionalization and steric bulk was minimal such as in 1‐methylcyclohexan‐1‐ol, the reaction proceeded quantitively in a matter of minutes to deliver compound 13. Pleasingly, in the presence of a competing benzylic site such as in the case of 14 a,b, the reaction occurred exclusively at the primary position(s). The reaction tolerated the presence of an α‐trifluoromethyl group, although harsher conditions (pivalic acid, DCE, 60 °C) were required to reach a good yield (72 %) of amide 15. Presumably, the coordination strength of the oxime directing group is weakened by the presence of this strongly electron‐withdrawing group, although the acidity of the β protons is simultaneously enhanced. Similarly, the presence of an ethyl ester at the α‐position to the oxime‐decorated alcohol proved to be slightly detrimental to the reaction kinetics. Longer reactions times (72 h) were required to reach full conversion of the starting material (16 a,b). Steric congestion was sometimes observed to be challenging, as per the example of (+)‐sclareolide derivative 17, for which enhanced conditions furnished a moderate 53 % yield of the amide product. Comparatively, the less hindered (+)‐cedrol aminoalcohol derivative 18 could be obtained in a very satisfying 82 % yield under standard reaction conditions. The system exhibited a tendency towards competing C(sp2)−H activation on benzylic alcohol scaffolds. Indeed, in the case of compounds 19, the undesired γ‐C(sp2)−H product 19γ was formed in 8 % yield while the reaction only furnished 28 % of the expected product 19β. Additionally, 49 % the starting material could be recovered. To circumvent this problem, we surmised that, in analogy to what was observed for rhodium, [4d] reverting to an adamantanone‐based oxime directing group might suppress the formation of the 6‐membered iridacycle leading to the undesired product. Gratifyingly, the intended transformation could be achieved with excellent regioselectivity when opting for this bulkier oxime, and 20 a was isolated in 76 % yield. Similarly, employing an adamantanone‐derived oxime proved mandatory for the α‐terpineol scaffold to avoid the competing allylic amidation. [13] In this way, the required transformation took place nearly quantitatively (21).
Scheme 4.
Reaction scope for tertiary alcohols. [a] Reaction time: 5 min. [b] PivOH (15 mol %), DCE, 60 °C, 14 h. [c] Reaction time: 72 h. [d] d.r. 1:1. Reactions were run on a 0.4 mmol scale at a concentration of 0.25 M.
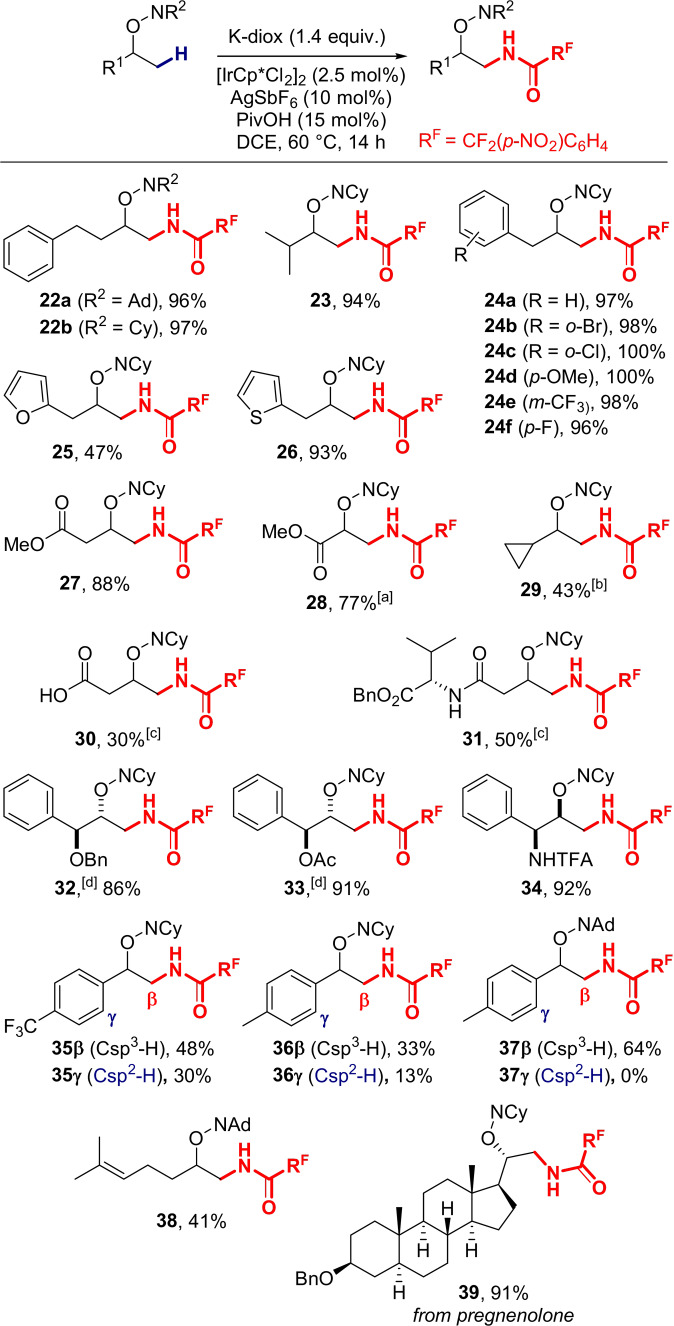
We then turned our attention to the functionalization of secondary alcohols, for which our original methodology had shown to be completely ineffective (see Scheme 2). After a quick reoptimization (Table S4), the adamantanone‐ and cyclohexanone‐derived oximes 22 a,b were obtained in excellent yields (96–97 %) using K‐diox (1.4 equiv) and a catalytic system composed of [IrCp*Cl2]2 (2.5 mol %), AgSbF6 (10 mol %) and pivalic acid (15 mol %) in DCE at 60 °C (Scheme 5). With these optimal conditions in hand, we started to explore the scope of the reaction. As previously observed, the reaction went smoothly for purely aliphatic compounds such as in the case of 23, which was isolated in 94 % yield. At this temperature of 60 °C, we expected to observe appreciable competition between the desired terminal C(sp3)−H bonds and more activated benzylic positions. Pleasingly, no such effect was observed, and homobenzylic alcohol‐derived substrates all exhibited perfect regiocontrol for primary C−H bonds, with the electronics of the aryl ring having no influence in the process (o‐Br, o‐Cl, p‐OMe, m‐CF3, p‐F). Compounds 24 a–f were all obtained in nearly quantitative yields. When the aryl group was replaced with a furan ring, the reaction furnished product 25 in a more moderate yield (47 %). By contrast, when a thiophene ring was instead introduced, no side‐reaction was observed, and amide 26 was produced in excellent yield (93 %). Interestingly, the presence of an ester group at the β‐position to the alcohol was inconsequential to the reaction, which proceeded with 88 % yield and full regioselectivity at the primary C−H bond (27). However, when the ester moiety was installed directly α‐ to the directing group, the transformation occurred at a slower pace and full conversion was only observed after 72 h (28). The same observation had previously been made for the tertiary alcohol‐based analogue 16 (Scheme 4). The presence of a sensitive α‐cyclopropane moiety was tolerated, although milder conditions and longer reaction times (40 °C, 96 h) were required to afford aminoalcohol derivative 29 in a moderate 43 % yield. Surprisingly, the catalytic system was also compatible with the presence of a carboxylic acid and a potentially coordinative valine‐derived amide, although a stronger activation via microwave‐assisted heating was required to generate the corresponding products in 30 % (30) and 50 % (31) yields. The iridium‐catalyzed transformation proved compatible with benzyl‐ and acetyl‐ protected alcohols, affording the corresponding terminal amides 32 and 33 with very good yields. Additionally, trifluoroacetyl‐protected amines also proved suitable, as diamide 34 was formed in very good yield (92 %). The stronger reaction conditions were also accompanied by a higher degree of competition with the undesired γ‐C(sp2)−H amidation in benzylic alcohol‐derived substrates. Indeed, in the case of compounds 35, the amide moiety was largely introduced at the ortho position of the p‐trifluoromethyl‐phenyl ring (35γ, 30 %). Functionalization at the expected aliphatic position accounted for a moderate 48 % yield (35β), while 15 % of the starting material could be recovered. With a more electron rich p‐tolyl ring, the transformation proceeded with a lower global yield (36, 46 %), a significant third of the material being amidated at the γ‐position, while the substrate could be recovered in 24 % yield. Gratifyingly, trading the directing group for an adamantanone‐derived oxime in 37 resulted in a significantly higher yield of the desired product 37β (64 %), while the side‐reaction was completely shut down. The same directing‐group‐swapping strategy also proved valuable in the presence of an olefin moiety, thus limiting the competing allylic amidation process, as per the example of 38 which was isolated in a moderate 41 % yield. At last, our methodology was successfully applied to the functionalization of the pregnenolone scaffold, providing an excellent yield (91 %) of aminoalcohol derivative 39.
Scheme 5.
Reaction scope for secondary alcohols. [a] Reaction time: 72 h. [b] Temperature: 40 °C, reaction time: 96 h. [c] [IrCp*(MeCN)3](SbF6)2 (10 mol %), PivOH (30 mol %), microwave, 100 °C, 1 h. [d] The starting material was engaged as a mixture of anti/syn diastereoisomers (d.r. 75:25). Reactions were run on a 0.4 mmol scale at a concentration of 0.25 M.
Bearing in mind that our K‐diox reagent had been able to withstand prolonged reaction times at temperatures as high as 80 °C, we contemplated the possibility to amidate less reactive secondary C(sp3)−H bonds (Table 3). Encouragingly, under the optimized conditions for secondary alcohols, cyclopentanol‐based substrate 40 was successfully converted to cis‐aminoalcohol derivative 41 with 12 % yield (93 % based on the recovery of 40, Table S5). However, the [IrCp*Cl2]2 precatalyst appeared too reactive and was leading to substantial degradation of the substrate. Switching to the dicationic [IrCp*(MeCN)3](SbF6)2 complex proved largely beneficial in tempering the uncontrolled side‐reactions. After 24 h in the presence of 10 mol % of the dicationic complex at a temperature of 70 °C, the desired compound 41 could be isolated in 32 % yield (entry 1), and inspection of the crude NMR spectra revealed that no degradation had occurred.
Table 3.
Optimization of the amidation of secondary C(sp3)−H bonds.

|
Entry |
n (mol %) |
T [°C] |
Heating mode |
t [h] |
%Yields[a] 40 |
41 |
|---|---|---|---|---|---|---|
|
1 |
10 |
70 |
convection |
24 |
65 (56) |
34 (32) |
|
2 |
10 |
70 |
convection |
40 |
51 |
44 |
|
3 |
5 |
70 |
convection |
24 |
87 |
11 |
|
4 |
5 |
100 |
convection |
24 |
34 |
18 |
|
5 |
10 |
100 |
microwave |
0.5 |
62 |
38 |
|
6 |
10 |
100 |
microwave |
1 |
34 (33) |
61 (61) |
|
7 |
10 |
100 |
microwave |
1.5 |
20 |
64 |
|
8 |
10 |
120 |
microwave |
1 |
10 |
64 |
[a] 1H NMR yields using CH2Br2 as the internal standard, yield of the isolated product in parentheses. [b] Isolated yields after column chromatography. [c] Reaction time: 72 h. n.d.=not determined. Reactions were run on a 0.2 mmol scale at a concentration of 0.25 M.
Prolonging the reaction time to 40 h allowed the conversion to reach 44 %, although up to 5 % degradation was then observed (entry 2). A lower catalyst loading of 5 mol % was subsequently employed, but only 11 % of amide 41 was formed under these conditions (entry 3). As the precatalyst was leading to a fully homogenous mixture at room temperature, we surmised that this system would be suitable for microwave‐assisted heating. Gratifyingly, using 10 mol % of the precatalyst, the desired product was then formed in 38 % yield after only 30 min in the microwave reactor at 100 °C (entry 5), which proved superior to conventional heating (entry 4). Doubling the reaction time to 1 h led to the isolation of 41 in 61 % yield while the starting oxime was recovered in 33 % yield (entry 6). However, prolonging the reaction time or increasing the temperature to 120 °C only led to degradation of the starting material with no significant further conversion into the product (entries 7, 8).
The scope of the methylene functionalization on cyclic systems under our best amidation conditions was then explored (Scheme 6). As expected, the more strained cyclobutanol scaffold behaved better than the 5‐membered model substrate, affording compound 42 in 76 % yield as a single cis diastereomer. On the other hand, larger rings, which are less prone to angular strain and for which the s‐character and hence the acidity of the C−H bonds are decreased, displayed lower reactivity towards the desired transformation. The six‐membered 1,2‐aminoalcohol derivative 43 was indeed isolated in moderate yield (50 %). This trend was confirmed with its 7‐membered analogue, for which the expected product was obtained in a lower yield of 25 % as a single diastereomer.
Scheme 6.
Reaction scope for methylene bonds. Reactions were run on a 0.4 mmol scale at a concentration of 0.25 M.
Interestingly, 5‐membered oxygen‐ and nitrogen‐heterocycles could also be amidated, albeit in lower yields of 31 % (45) and 10 % (46), with very good cis‐diastereoselectivity. These reactions were accompanied by significant degradation, possibly originating from functionalization of the more activated C−H bonds in α‐position to the endocyclic heteroatom, leading to unstable (hemi)aminal products. Furthermore, their 6‐membered counterparts 47 and 48 were isolated in 44 % and 29 % yield, respectively. Unexpectedly, those systems exhibited a noticeable reversal in diasteroselectivity, as the trans products were predominantly formed.
We then wished to gain some insight into the mechanism of this transformation. A significant primary kinetic isotope effect (k H/k D=5.0) was observed by measuring the initial reaction rate in the transformation of protiated and deuterated oximes 49/49‐d 3 (Figure 1). Given the high reactivity observed with these substrates, the catalyst loading and temperature had to be reduced to 1 mol % and 25 °C, respectively. This result clearly implicates that the breaking of the C−H bond occurs in the rate‐determining step of the reaction. A mechanistic proposal based on literature precedents is shown in the Supporting Information (Scheme S1). [2g]
Figure 2.
Initial reaction rate comparison for the protiated (49) and deuterated (49‐d 3 ) substrates. Reactions were performed in parallel.
To assess the scalability of the transformation, a 10‐fold scale‐up of the reaction was performed starting from (+)‐cedrol‐ and pregnenolone‐derived substrates 51 and 53 (Scheme 7). In both cases, the catalyst loadings could be decreased while retaining excellent yields of the corresponding amides 18 (85 %) and 39 (93 %). The hydrolysis of the amide groups proceeded effortlessly in the presence of aqueous sodium hydroxide at room temperature in ethanol, to furnish the corresponding free amines in excellent yields (96–100 %). [14] The oximes could then be swiftly deprotected by simple treatment with sodium borohydride and molecular iodine in refluxing THF, yielding after recrystallization the pure 1,2‐aminoalcohols 52 and 54 in overall yields exceeding 80 % over 3 steps.
Scheme 7.
Tenfold scale‐up of the reaction and subsequent deprotection.
Finally, the applicability of our new amidation reagent to other systems was investigated. To this end, a number of commercially available or quickly accessed substrates bearing a variety of different directing groups, and possessing C(sp3)−H or C(sp2)−H bonds, were tested under our established set of conditions for secondary alcohols without any further optimization (Scheme 8). Activation of the C−H bond endo to the oxime coordinating group proceeded smoothly as demonstrated with 55, which was β‐amidated in 85 % yield. By contrast, the benzylic C−H amidation of 8‐methylquinoline occurred less efficiently (56). Surprisingly, not only was 1‐acetylindoline readily functionalized on the benzene ring (57 a), but a significant third of the isolated material accounted for overamidation at the meta position (57 b). This seemed to indicate that even though the resulting amide is highly electron‐deficient, it is still able to direct the functionalization of the adjacent C(sp2)−H bonds. [15]
Scheme 8.
Applicability of K‐diox to the directed amidation of other systems.
A similar observation was made from N‐(tert‐butyl)benzamide, which was transformed into the desired amide 58 a with 79 % yield, while an inseparable mixture of ortho‐ortho and ortho‐meta diamidated products 58 b was isolated in 12 % yield. Interestingly, the same trend was not observed for 2,2‐dimethylpropiophenone, as only the monofunctionalized product 59 was formed in 70 % yield. In line with what was previously observed for compounds 19, 35 and 36, γ‐oxime‐directed amidation occurred smoothly on the aromatic ring, delivering product 60 in 67 % yield. Moreover, using a more classical pyridine directing group was also effective, as the targeted C(sp2)−H bond was affected in 65 % yield (61).
Conclusion
We have established a robust, mild, efficient and scalable methodology for the synthesis of 1,2‐aminoalcohol derivatives through an iridium(III)‐catalyzed C(sp3)−H amidation process. Our approach relies on the use of a new bench‐stable and yet electron‐deficient fluoroalkyldioxazolone, which proved significantly superior to traditional alkyl/aryl‐dioxazolones in both reactivity and amide cleavability aspects. The synthetic utility and applicability of this method was demonstrated over a broad range of substrates, including terpene and steroid derivatives. We anticipate that this reaction will be broadly applicable to introduce the amino group in a medicinal chemistry context.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
This work was financially supported by Novartis AG and the University of Basel. We thank Prof. Dr. D. Häussinger (University of Basel) for NMR experiments, S. Mittelheisser and Dr. M. Pfeffer (University of Basel) for MS analyses, and Dr. P. Hoehn (Novartis Pharma AG) for DSC experiments. Open access funding provided by CSAL.
K. Antien, A. Geraci, M. Parmentier, O. Baudoin, Angew. Chem. Int. Ed. 2021, 60, 22948.
References
- 1.
- 1a. Ager D. J., Prakash I., Schaad D. R., Chem. Rev. 1996, 96, 835–876; [DOI] [PubMed] [Google Scholar]
- 1b. O'Brien P., Angew. Chem. Int. Ed. 1999, 38, 326–329; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 1999, 111, 339–342; [Google Scholar]
- 1c. Bergmeier S. C., Tetrahedron 2000, 56, 2561–2576; [Google Scholar]
- 1d. Sweeney J. B., Chem. Soc. Rev. 2002, 31, 247–258; [DOI] [PubMed] [Google Scholar]
- 1e. Kobayashi S., Mori Y., Fossey J. S., Salter M. M., Chem. Rev. 2011, 111, 2626–2704; [DOI] [PubMed] [Google Scholar]
- 1f. Karjalainen O. K., Koskinen A. M. P., Org. Biomol. Chem. 2012, 10, 4311–4326; [DOI] [PubMed] [Google Scholar]
- 1g. Sehl T., Maugeri Z., Rother D., J. Mol. Catal. B-Enzym. 2015, 114, 65–71. [Google Scholar]
- 2.
- 2a. Dick A. R., Sanford M. S., Tetrahedron 2006, 62, 2439–2463; [Google Scholar]
- 2b. Collet F., Dodd R. H., Dauban P., Chem. Commun. 2009, 5061–5074; [DOI] [PubMed] [Google Scholar]
- 2c. Collet F., Lescot C., Dauban P., Chem. Soc. Rev. 2011, 40, 1926–1936; [DOI] [PubMed] [Google Scholar]
- 2d. Roizen J. L., Harvey M. E., Du Bois J., Acc. Chem. Res. 2012, 45, 911–922; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2e. Jeffrey J. L., Sarpong R., Chem. Sci. 2013, 4, 4092; [Google Scholar]
- 2f. Park Y., Kim Y., Chang S., Chem. Rev. 2017, 117, 9247–9301; [DOI] [PubMed] [Google Scholar]
- 2g. Hong S. Y., Hwang Y., Lee M., Chang S., Acc. Chem. Res. 2021, 54, 2683–2700. [DOI] [PubMed] [Google Scholar]
- 3.Seminal work: Ren Z., Mo F., Dong G., J. Am. Chem. Soc. 2012, 134, 16991–16994. [DOI] [PubMed] [Google Scholar]
- 4.Metal-catalyzed exo-directed (sulfon)amidation with oximes:
- 4a. Kang T., Kim H., Kim J. G., Chang S., Chem. Commun. 2014, 50, 12073–12075; [DOI] [PubMed] [Google Scholar]
- 4b. Huang X., Wang Y., Lan J., You J., Angew. Chem. Int. Ed. 2015, 54, 9404–9408; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 9536–9540; [Google Scholar]
- 4c. Dong Y., Liu G., J. Org. Chem. 2017, 82, 3864–3872; [DOI] [PubMed] [Google Scholar]
- 4d. Dong Y., Chen J., Xu H., Chem. Commun. 2018, 54, 11096–11099; [DOI] [PubMed] [Google Scholar]
- 4e. Liu B., Xie P., Zhao J., Wang J., Wang M., Jiang Y., Chang J., Li X., Angew. Chem. Int. Ed. 2021, 60, 8396–8400; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 8477–8481. [Google Scholar]
- 5.Metal-catalyzed endo-directed (sulfon)amidation with oximes:
- 5a. Thu H.-Y., Yu W.-Y., Che C.-M., J. Am. Chem. Soc. 2006, 128, 9048–9049; [DOI] [PubMed] [Google Scholar]
- 5b. Kang T., Kim Y., Lee D., Wang Z., Chang S., J. Am. Chem. Soc. 2014, 136, 4141–4144; [DOI] [PubMed] [Google Scholar]
- 5c. Wang H., Tang G., Li X., Angew. Chem. Int. Ed. 2015, 54, 13049–13052; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 13241–13244; [Google Scholar]
- 5d. Kim H., Park G., Park J., Chang S., ACS Catal. 2016, 6, 5922–5929; [Google Scholar]
- 5e. Zhang T., Hu X., Dong X., Li G., Lu H., Org. Lett. 2018, 20, 6260–6264. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Park Y., Heo J., Baik M.-H., Chang S., J. Am. Chem. Soc. 2016, 138, 14020–14029; [DOI] [PubMed] [Google Scholar]
- 6b. Ma N., Liu Z., Huang J., Dang Y., Org. Biomol. Chem. 2021, 19, 3850–3858. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a.3-Perfluoroalkyl-1,4,2-dioxazol-5-ones: Middleton W. J., J. Org. Chem. 1983, 48, 3845–3847; [Google Scholar]
- 7b.Trifluoroacetyliminoiodane: Mansuy D., Mahy J.-P., Dureault A., Bedi G., Battioni P., J. Chem. Soc. Chem. Commun. 1984, 1161–1163. [Google Scholar]
- 8.
- 8a. Barsu N., Rahman A., Sen M., Sundararaju B., Chem. Eur. J. 2016, 22, 9135–9138; [DOI] [PubMed] [Google Scholar]
- 8b. Tan P. W., Mak A. M., Sullivan M. B., Dixon D. J., Seayad J., Angew. Chem. Int. Ed. 2017, 56, 16550–16554; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16777–16781; [Google Scholar]
- 8c. Fukagawa S., Kato Y., Tanaka R., Kojima M., Yoshino T., Matsunaga S., Angew. Chem. Int. Ed. 2019, 58, 1153–1157; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 1165–1169; [Google Scholar]
- 8d. Sekine D., Ikeda K., Fukagawa S., Kojima M., Yoshino T., Matsunaga S., Organometallics 2019, 38, 3921–3926. [Google Scholar]
- 9.
- 9a. Ma Q., Yu X., Lai R., Lv S., Dai W., Zhang C., Wang X., Wang Q., Wu Y., ChemSusChem 2018, 11, 3672–3678; [DOI] [PubMed] [Google Scholar]
- 9b. Shi H., Dixon D. J., Chem. Sci. 2019, 10, 3733–3737; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c. Fukagawa S., Kojima M., Yoshino T., Matsunaga S., Angew. Chem. Int. Ed. 2019, 58, 18154–18158; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 18322–18326. [Google Scholar]
- 10. Kim Y. B., Won J., Lee J., Kim J., Zhou B., Park J.-W., Baik M.-H., Chang S., ACS Catal. 2021, 11, 3067–3072. [Google Scholar]
- 11. Hwang Y., Park Y., Chang S., Chem. Eur. J. 2017, 23, 11147–11152. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Lee M., Jung H., Kim D., Park J.-W., Chang S., J. Am. Chem. Soc. 2020, 142, 11999–12004; [DOI] [PubMed] [Google Scholar]
- 12b. Dong X., Ma P., Zhang T., Jalani H. B., Li G., Lu H., J. Org. Chem. 2020, 85, 13096–13107; [DOI] [PubMed] [Google Scholar]
- 12c. Lee J., Jin S., Kim D., Hong S. H., Chang S., J. Am. Chem. Soc. 2021, 143, 5191–5200. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Knecht T., Mondal S., Ye J.-H., Das M., Glorius F., Angew. Chem. Int. Ed. 2019, 58, 7117–7121; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 7191–7195; [Google Scholar]
- 13b. Burman J. S., Harris R. J., Farr C. M. B., Basca J., Blakey S. B., ACS Catal. 2019, 9, 5474–5479; [Google Scholar]
- 13c. Sihag P., Jeganmohan M., J. Org. Chem. 2019, 84, 13053–13064; [DOI] [PubMed] [Google Scholar]
- 13d. Lei H., Rovis T., Nat. Chem. 2020, 12, 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The deprotection of the p-nitrophenyldifluoroacetamide group was benchmarked against those of the more common trifluoroacetamide and pivalamide groups. See the supporting information for details (Table S6).
- 15. Park J., Lee J., Chang S., Angew. Chem. Int. Ed. 2017, 56, 4256–4260; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 4320–4324. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information