Abstract
Objective
To compare differences in efficacy during maintenance treatment for bipolar disorder (BD) according to lithium serum levels. A multicenter retrospective cohort study and a dose‐response meta‐analysis were conducted.
Methods
The cohort study was conducted in Taiwan from 2001 to 2019 to identify patients with euthymic BD according to different serum levels (<0.4, 0.4–0.8, and 0.8–1.2 mmol/L). We adopted adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for time to the recurrence of mood episodes having the <0.4 mmol/L group as the reference group. Moreover, we systematically searched for related articles in major databases before January 31, 2021 (PROSPERO: CRD42021235812). We used random‐effects modeling to estimate the dose‐response relationships between lithium serum levels and recurrence of mood episodes, which were depicted as odds ratios (ORs) with 95% CIs.
Results
A total of 1406 participants (cohort: 466; meta‐analysis: 940) were included. In the cohort study, the 0.4–0.8 mmol/L group was associated with a significantly lower risk of recurrences (aHR: 0.75), while the 0.8–1.2 mmol/L group had a lower risk without statistical significance (aHR: 0.77). The dose‐response meta‐analysis showed that with the increase in lithium serum levels, the risk decreased (linear model OR: 0.85, for every 0.1 mmol/L increase; non‐linear model OR: 1.00 at 0.0 mmol/L, 0.42 at 0.4 mmol/L, and 0.27 at 0.8 mmol/L).
Conclusion
Although confounding by indication cannot be excluded, the combined results suggest a significant preventative effect on the recurrence of major affective episodes among those with serum levels of 0.4–0.8 mmol/L.
Keywords: bipolar disorder, dose‐response meta‐analysis, lithium, maintenance treatment, serum level
Summations
A multicenter retrospective cohort with a dose‐response meta‐analysis investigated the association between lithium serum levels and treatment response in bipolar disorder during maintenance treatment.
Patients with lithium serum levels of 0.4–0.8 mmol/L had a significantly lower risk of recurrence of mood episodes.
For patients with lithium serum levels of 0.4–0.8 mmol/L, the older the age, the lower the risk.
Limitations
The study lacks information on the relationship between lithium serum levels and the risk of recurrence of mood episodes of different polarities.
1. INTRODUCTION
Lithium is well recognized as the first‐line maintenance treatment for bipolar disorder (BD) in most treatment guidelines. 1 , 2 , 3 Lithium can effectively reduce the risk of recurrence of mood episodes, 4 prevent suicide, 5 and might have a protective effect on certain types of cancers. 6 Although lithium has been proven to have a meaningful beneficial therapeutic effect, its narrow therapeutic index may limit its prescription despite being an archetypal mood stabilizer. 7 However, there is uncertainty in the literature regarding which lithium serum levels would be more effective for the prevention of major mood episodes during the maintenance treatment of BD. Accordingly, some reviews suggest that different ranges of lithium serum levels would be efficacious for the maintenance treatment of BD, the lowest level being from 0.4 mmol/l to 0.8 mmol/L, 8 , 9 and the highest level from 0.8 mmol/L to 1.2 mmol/L. 1 , 10 A putative reason for those distinct recommendations could be that relatively few studies have been conducted to compare the effectiveness of different lithium serum levels for the prevention of recurring mood episodes. 11 In keeping with this view, real‐world studies may provide useful insights in this regard and may add evidence to existing randomized controlled trials (RCTs) by providing results relevant to clinical practice by the enrollment of a broader spectrum of patient populations. 12 Hence, real‐world studies are essential to support clinical decision‐making, particularly in a scenario where relatively few RCTs have investigated which serum levels of lithium would provide optimal efficacy for the prevention of recurring mood episodes during the long‐term treatment of BD.
Moreover, the course of BD is clinically characterized by chronic and recurring mood episodes of different polarities ([hypo] manic, depressive, and mixed episodes), and manifests throughout the lifespan, 13 with the peak age of onset of BD appearing to be around mid‐adolescence, notwithstanding that even elderly patients may present with BD. 14 , 15 The aforementioned recommendations regarding serum levels of lithium are based on evidence predominantly derived from adults with BD. People of different ages may have various co‐occurring physical conditions, 16 , 17 , 18 which may affect the safety of lithium at higher serum levels in some circumstances. For example, the common use of diuretics, anti‐inflammatory drugs, and concurrent psychotropic drugs in the elderly may interact with lithium (increase its serum levels), thereby increasing the risk of toxicity. 19 , 20 To the best of our knowledge, there is still a lack of evidence on which serum levels of lithium would be more efficacious for the prophylaxis of major affective episodes in younger versus older patients with BD during maintenance treatment. 11
1.1. Aims of the study
First, we used data from a multicenter retrospective cohort study to investigate the associations between specific serum levels of lithium and treatment response during maintenance therapy. These associations were also explored across different age groups. Second, we summarized the current findings and results of previous studies by conducting a systematic review and dose‐response meta‐analysis.
2. MATERIAL AND METHODS
2.1. Data source
The study protocol was approved by the institutional review board of Chang Gung Memorial Hospital (No: 202100131B0). This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Appendix 1). 21 We extracted data from the medical claims of the Chang Gung Research Database (CGRD) from January 1, 2001, to December 31, 2019. The CGRD is a multicenter electronic medical record (EMR), which includes de‐identified personal data on demographics (gender, age); disease diagnoses; medical visits (outpatient, emergency room, and inpatient); pharmacy records (medication type, dosage, and duration of supply); laboratory data (lithium serum levels); and examination reports from seven medical institutes throughout Taiwan. 22 From 1997 to 2010, the CGRD covered 21.2% of outpatients in Taiwan's total medical population. 23
2.2. Participants
Figure S1 (Appendix 2) depicts the flowchart of the selection process for this retrospective cohort study and outlines its analytic procedures. We enrolled participants diagnosed with BD (disease code of International Classification of Disease, Ninth Revision [ICD‐9]: 296.0–296.8, except 296.2, 296.3, or ICD‐10: F30 and F31) and treated with lithium. They also had laboratory records of lithium serum levels. The first date when patients’ lithium serum levels were available comprised the index date of this cohort, and those participants were followed up for 2 years from the index date. Moreover, we a priori established the following exclusion criteria to define (by proxy) patients with euthymic BD who initiated lithium maintenance treatment and also had their lithium serum levels within a specific range: (1) Patients who had either inpatient or emergency department treatment within 180 days before the index date; (2) Patients who had not taken the same lithium dosage within 180 days before the index date; (3) Compared to the lithium dose at the index date, the patients’ lithium dose was reduced during the follow‐up period; (4) Whenever patients had their serum lithium levels assessed during the follow‐up, the difference in the serum level between the index date and any follow‐up was >0.1 mmol/L; and (5) Patients’ data of lithium serum levels was not a trough level (if lithium is taken in divided doses, the trough level is measured at least 12 h after the last dose). 24 , 25 A total of 466 participants participated in this cohort.
2.3. Participant characteristics and outcome measures
Participants were divided into three groups according to lithium serum levels (<0.4 mmol/L, 0.4–0.8 mmol/L, and 0.8–1.2 mmol/L). Those groups were chosen a priori based on previous treatment guidelines. 2 , 3 , 26 Characteristics of participants with BD were assessed and included age; gender; medical comorbidities (heart diseases, cerebral vascular diseases, peripheral vascular diseases, any bleeding, pulmonary diseases, peptic ulcer diseases, liver diseases, renal diseases, diabetes mellitus, hypertension, hyperlipidemia, rheumatic diseases, and any cancer); and concomitant medications (mood stabilizers, antidepressants, antipsychotics, and benzodiazepines). Detailed information on medical comorbidities and concomitant medications is provided in Table S2 (Appendix 3). The outcome measure of treatment efficacy was the recurrence of any major mood episode, which was defined as the occurrence of psychiatric hospitalization or any changes (increase the dosage of the original drugs or add other new drugs) in psychotropic medications (mood stabilizers [lithium, carbamazepine, lamotrigine, and valproic acid], antidepressants, and antipsychotics). These indicators were documented elsewhere in previous similar studies. 24 , 27 , 28 , 29 All included participants were observed from the index date (lithium serum level test date) to the date of outcome occurrence over a 2‐year follow‐up period.
2.4. Statistical analysis
The analysis was based on an intention‐to‐treat (ITT) scenario, where participants who were lost during the 2‐year follow‐up period in the CGRD were also censored. We used Cox regression models to analyze the time to recurrence of any mood episode. Analyses were adjusted for age, gender, all medical comorbidities, and all concomitant medications. Table 1 shows the detailed variables. Adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for time to the event were determined having the <0.4 mmol/L group as the reference group. We also adopted Kaplan‐Meier curves for survival analysis. Moreover, we conducted two sensitivity analyses to assess the robustness of our findings. First, some previous studies defined the index date for maintenance treatment of BD as the one in which participants achieved stabilization for at least 20 weeks or 6 months. 24 , 27 Thus, we expanded the inclusion criteria to enroll participants without a history of hospitalization or emergency department treatment within 150 days before the index date in model 1. Second, the outcome of the participants treated using lithium monotherapy was assessed in model 2.
TABLE 1.
Characteristics of all included participants and their lithium groups from 2001 to 2019
| Characteristics |
All participants (N = 466) |
<0.4 mmol/L (n = 153) |
0.4–0.8 mmol/L (n = 287) |
0.8–1.2 mmol/L (n = 26) |
|---|---|---|---|---|
| Age, year | 42.25 ± 14.90 | 40.52 ± 14.70 | 43.21 ± 14.88 | 41.86 ± 15.98 |
| Gender | ||||
| Female | 245 (52.6) | 87 (56.9) | 145 (50.5) | 13 (50.0) |
| Male | 221 (47.4) | 66 (43.1) | 142 (49.5) | 13 (50.0) |
| Comorbidities | ||||
| Heart diseases | 1 (0.2) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Cerebral vascular diseases | 9 (1.9) | 1 (0.7) | 8 (2.8) | 0 (0.0) |
| Peripheral vascular diseases | 2 (0.4) | 0 (0.0) | 2 (0.7) | 0 (0.0) |
| Any bleeding | 32 (6.9) | 7 (4.6) | 25 (8.7) | 0 (0.0) |
| Pulmonary diseases | 17 (3.7) | 4 (2.6) | 13 (4.5) | 0 (0.0) |
| Peptic ulcer diseases | 22 (4.7) | 5 (3.3) | 17 (5.9) | 0 (0.0) |
| Liver diseases | 28 (6.0) | 6 (3.9) | 21 (7.3) | 1 (3.9) |
| Renal diseases | 5 (1.1) | 0 (0.0) | 5 (1.7) | 0 (0.0) |
| Diabetes mellitus | 35 (7.5) | 9 (5.9) | 23 (8.0) | 3 (11.5) |
| Hypertension | 45 (9.7) | 9 (5.9) | 33 (11.5) | 3 (11.5) |
| Hyperlipidemia | 31 (6.7) | 9 (5.9) | 21 (7.3) | 1 (3.9) |
| Rheumatic diseases | 3 (0.6) | 0 (0.0) | 3 (1.1) | 0 (0.0) |
| Any cancer | 6 (1.3) | 1 (0.7) | 4 (1.4) | 1 (3.9) |
| Medications | ||||
| Mood stabilizers | ||||
| Carbamazepine | 27 (5.8) | 13 (8.5) | 12 (4.2) | 2 (7.7) |
| Lamotrigine | 38 (8.2) | 9 (5.9) | 26 (9.1) | 3 (11.5) |
| Valproic acid | 144 (30.9) | 43 (28.1) | 93 (32.4) | 8 (30.8) |
| Antidepressants | 164 (35.2) | 51 (33.3) | 103 (35.9) | 10 (38.5) |
| Antipsychotics | 340 (73.0) | 114 (74.5) | 206 (71.8) | 20 (76.9) |
| Benzodiazepines | 350 (75.1) | 113 (73.9) | 218 (76.0) | 19 (73.1) |
Data was expressed as N (percentage) or mean ± standard deviation.
Furthermore, we conducted subgroup analysis stratified by age to investigate the association between lithium serum levels and prevention of mood episodes. 11 Participants were divided into four age subgroups (<20, 20–40, 40–60, and ≥60 years). All analyses were performed using SAS Software version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as a two‐tailed p‐value < 0.05. MedCalc® Statistical Software version 19.8 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021) was used to draw Kaplan‐Meier curves.
2.5. Systematic review and a dose‐response meta‐analysis
We further performed a systematic review and dose‐response meta‐analysis on the treatment efficacy of different lithium serum levels by summarizing the results of our research and previous studies. We used data from an a priori registered protocol (PROSPERO: CRD42021235812), which followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (Appendix 4). 30 Two investigators (CW Hsu and PT Tseng) independently searched the PubMed/MEDLINE, Embase, and Cochrane CENTRAL electronic databases from their inception until January 31, 2021, for studies that compared treatment outcomes in participants with BD and included a minimum of three groups according to lithium serum levels. Detailed search strings are provided in the Supplement (Appendix 5). We included both cohort studies (retrospective or prospective) and clinical trials without language restrictions, including participants with BD receiving maintenance therapy with lithium. Publications initially removed by title/abstract screening were letters, comments, case series or reports, conference papers, protocols, and non‐peer‐reviewed articles. Two investigators (CW Hsu and PT Tseng) independently appraised the methodological quality/risk of bias of the included studies and extracted data. The methodological quality of cohort studies was assessed using the Newcastle‐Ottawa Scale, and the Cochrane risk‐of‐bias tool was used for randomized trials. 31 , 32 The primary outcome was the efficacy of different lithium serum levels to prevent the recurrence of mood episodes of any polarity during maintenance treatment of participants with BD. We primarily used data from ITT participants; if this was unavailable, data from per‐protocol participants were used.
Odds ratios (ORs) and 95% CIs were used to measure the association between different lithium serum levels and the risk of recurrence of any mood episode. Due to the different ranges of lithium serum levels in the included studies, we used the following criteria to define the value of serum levels in the comparison group: (1) the mean or median of each serum level category; (2) when the ranges of each serum level category were reported, the average value of the lower and upper bounds of each category was used; (3) when the lowest category was open‐ended, the average value of its upper bound and 0 was used; and (4) when the highest category was open‐ended, the average value was assumed to be 1.2 times its lower boundary. 33
Linear trends were estimated using random‐effects modeling by pooling study‐specific linear slopes. A two‐stage, random‐effects multivariate meta‐analysis described by Greenland 34 and Orsini 35 was used to evaluate linear or non‐linear trends in the association between exposure and outcomes. First, we used restricted cubic splines with three knots at fixed percentiles of 10%, 50%, and 90% of the distribution of lithium serum levels to model the non‐linear dose‐response relationship within each study. 35 , 36 The models were then estimated using the generalized least squares method, which considers the correlation within each set of reported ORs. 37 Second, we pooled study‐specific dose‐response curves to obtain an overall average curve using random‐effects modeling. 37 Finally, a likelihood ratio test was used to compare differences between linear and non‐linear models. 36 Furthermore, we also estimated heterogeneity with the I 2 statistic. 38 All analyses were conducted with the R software (dosresmeta package version 2.0.1). 39 A two‐tailed p‐value < 0.05 was regarded as statistically significant.
3. RESULTS
3.1. Baseline characteristics of cohort patients
A total of 466 participants in this cohort (type I BD: 429 cases; type II or other BD: 37 cases) were followed up and divided into groups according to lithium serum levels: <0.4 mmol/L (153 cases, mean with a standard deviation = 0.30 ± 0.06 mmol/L), 0.4–0.8 mmol/L (287 cases, 0.55 ± 0.11 mmol/L), and 0.8–1.2 mmol/L (26 cases, 0.86 ± 0.06 mmol/L). Table 1 summarizes the characteristics of the participants enrolled in this cohort. In general, the average age of participants was 42 years, and they most often had co‐occurring hypertension (9.7%), and concurrently took antipsychotics (73.0%).
3.2. Primary outcome and sensitivity analyses
In the 267 person‐years of follow‐up, either a psychiatric hospitalization or any increase in the doses of concomitant psychopharmacological agents occurred in 329 participants over the 2‐year follow‐up period. Table 2 and Figure 1 show the results of the primary and sensitivity analyses (model 1: stable lithium serum levels for 150 days; model 2: lithium monotherapy). Compared to participants with lithium serum levels below 0.4 mmol/L, those with lithium serum levels between 0.4 and 0.8 mmol/L exhibited a significant lower rate of recurring mood episodes (primary analysis: aHR [95% CI], 0.75 [0.59–0.95]; model 1: aHR [95% CI], 0.76 [0.60–0.96]; and model 2: aHR [95% CI], 0.19 [0.07–0.51]). For participants with lithium serum levels between 0.8 and 1.2 mmol/L, the risk of recurring mood episodes was reduced, although it did not reach a statistical significance (primary analysis: aHR [95% CI], 0.77 [0.47–1.27]; model 1: aHR [95% CI], 0.82 [0.51–1.31]; and model 2: aHR [95% CI], 0.40 [0.06–2.66]).
TABLE 2.
Comparing the adjusted effectiveness of all patients in different lithium groups through the Cox proportional hazard model, with two sensitivity analyses
| Group | Incident/total cases | Person–years | Adjusted HR a | p |
|---|---|---|---|---|
| All patients | ||||
| <0.4 mmol/L | 114/153 | 73.86 | 1.00 [Reference] | |
| 0.4–0.8 mmol/L | 196/287 | 178.37 | 0.75 (0.59–0.95) | 0.019* |
| 0.8–1.2 mmol/L | 19/26 | 14.42 | 0.77 (0.47–1.27) | 0.302 |
| Model 1 (stabilization for 150 days) | ||||
| <0.4 mmol/L | 118/158 | 74.52 | 1.00 [Reference] | |
| 0.4–0.8 mmol/L | 205/298 | 182.04 | 0.76 (0.60–0.96) | 0.022* |
| 0.8–1.2 mmol/L | 22/29 | 14.90 | 0.82 (0.51–1.31) | 0.401 |
| Model 2 (lithium monotherapy) | ||||
| <0.4 mmol/L | 13/17 | 7.44 | 1.00 [Reference] | |
| 0.4–0.8 mmol/L | 11/30 | 33.64 | 0.19 (0.07–0.51) | < 0.001* |
| 0.8–1.2 mmol/L | 2/4 | 1.73 | 0.40 (0.06–2.66) | 0.342 |
Abbreviation: HR, hazard ratio.
Hazard ratio was adjusted for age, gender, all comorbidities, and all medications.
Indicated p < 0.05.
FIGURE 1.
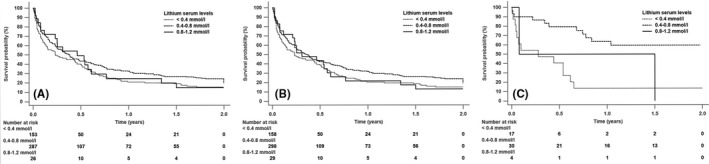
Time to recurrence of any mood episode (Kaplan‐Meier curves). (A) primary analysis of all participants, (B) sensitivity analysis of participants on stable serum levels of lithium for 150 days, and (C) sensitivity analysis of participants on lithium monotherapy
3.3. Subgroup analysis of the cohort study
Table 3 presents analysis stratified by age groups, with lithium serum levels <0.4 mmol/L as the reference group. For participants with lithium serum levels of 0.4–0.8 mmol/L, the aHR decreased in older age groups (aHR [95% CI] <20 years: 1.97 [0.60–6.43]; 20–40 years: 0.74 [0.49–1.12]; 40–60 years: 0.67 [0.46–0.99]; ≥60 years: 0.59 [0.21–1.68]), and only participants between 40 and 60 years exhibited a significant decrease in the occurrence of recurring mood episodes (aHR [95% CI], 0.67 [0.46–0.99]). For participants with serum levels of 0.8–1.2 mmol/L, there was no influence of age on the recurrence of mood episodes.
TABLE 3.
Comparing the adjusted effectiveness of all participants in different lithium groups through Cox proportional hazard models, stratified analysis by age
| Age group | <0.4 mmol/L | 0.4–0.8 mmol/L | 0.8–1.2 mmol/L | |||
|---|---|---|---|---|---|---|
| Incident/total cases | Adjusted HR a | Incident/total cases | Adjusted HR a | Incident/total cases | Adjusted HR a | |
| <20 years | 9/14 | 1.00 [Reference] | 11/13 | 1.97 (0.60–6.43) | 1/2 | 0.76 (0.06–10.04) |
| 20–40 years | 46/61 | 1.00 [Reference] | 81/111 | 0.74 (0.49–1.12) | 5/9 | 0.39 (0.14–1.07) |
| 40–60 years | 46/62 | 1.00 [Reference] | 81/125 | 0.67 (0.46–0.99)* | 12/13 | 1.35 (0.66–2.75) |
| ≥60 years | 13/16 | 1.00 [Reference] | 23/38 | 0.59 (0.21–1.68) | 1/2 | 0.76 (0.07–8.07) |
Abbreviation: HR, hazard ratio.
Hazard ratio was adjusted for gender, all comorbidities, and all medications.
Indicated p < 0.05.
3.4. Dose‐response meta‐analysis
Five studies (including our retrospective cohort study) with a total of 1406 participants were included in the dose‐response meta‐analysis. 24 , 27 , 28 , 40 The flowchart of study selection along with the reasons for exclusion of studies following full‐text review as well as the characteristics of the included studies, and the methodological quality/risk of bias assessment of eligible studies are detailed in the Supplement (Appendix 6–9). Figure 2 depicts the linear and non‐linear dose‐response relationships between lithium serum levels and recurring mood episodes. In the linear model, the pooled OR of recurrence of any mood episode for each 0.1 mmol increase in lithium serum levels was 0.85 (95% CI, 0.81–0.89), with no heterogeneity across the included studies (I 2 = 0.0%, p = 0.742). In the non‐linear model, as lithium serum levels increased, the risk of recurring mood episodes exhibited a concave upward trend with a negative slope. Odds ratios with 95% CIs are presented in Table S6 (Appendix 10). However, the likelihood ratio test suggested that there were no statistically significant differences between the two models (p = 0.192). Furthermore, in this systematic review, there was a lack of data on the treatment efficacy of different lithium serum levels in younger vs. older participants; thus, a dose‐response meta‐analysis stratified by age subgroups could not be conducted.
FIGURE 2.
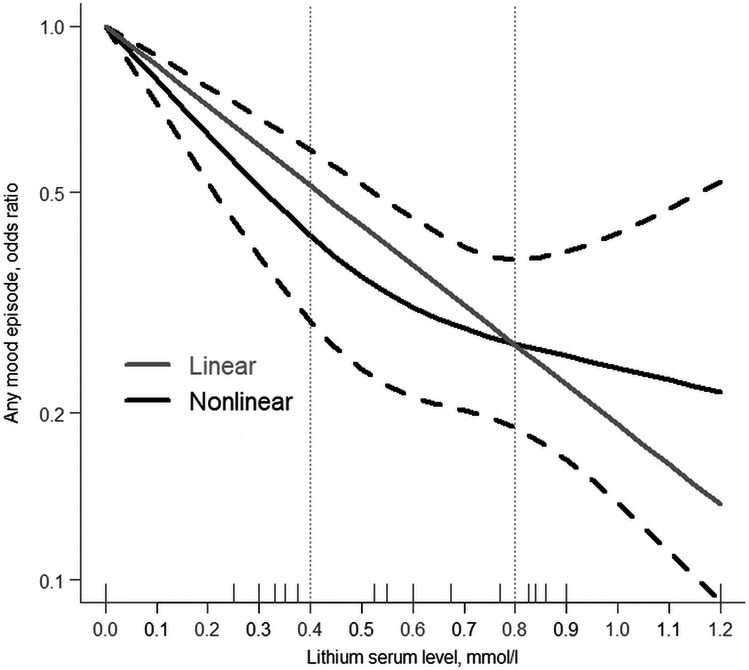
The linear and non‐linear relationships of lithium serum levels and any recurring major mood episode
4. DISCUSSION
This real‐world retrospective cohort study of participants with BD during maintenance treatment across multiple medical institutions evaluated the clinical efficacy of different lithium serum levels for the prophylaxis of mood episodes of any polarity. Compared to lithium serum levels below 0.4 mmol/L, those participants with serum lithium levels of 0.4–0.8 mmol/L exhibited a significant decrease in the risk of recurring major mood episodes. Additionally, participants with lithium serum levels of 0.8–1.2 mmol/L also presented a lower risk of recurring mood episodes, although these findings did not reach statistical significance. Furthermore, these associations were confirmed in the sensitivity analysis, thus adding robustness to our primary findings. These results were further confirmed in our dose‐response meta‐analysis, which showed that higher serum lithium levels reduced the risk of recurrence of mood episodes. Moreover, subgroup analysis stratified by age found that among participants with lithium serum levels of 0.4–0.8 mmol/L, the risk of recurring mood episodes decreased as a function of older age; only participants aged 40–60 years exhibited a significantly reduced risk of recurring mood episodes.
Our data provide real‐world prescription patterns for lithium during the maintenance treatment of BD. In terms of serum levels, most participants belonged to the 0.4–0.8 mmol/L group (n = 287) and few to 0.8–1.2 mmol/L group (n = 26). Compared to a survey conducted among psychiatrists from Spain 41 and recommendations from some national guidelines, 42 , 43 much fewer participants in our cohort belonged to the 0.8–1.2 mmol/L group. This counterintuitive finding could be explained by the difference between psychiatrists’ thoughts and patients’ expectations. Higher serum levels of lithium during the maintenance phase of treatment of BD generally require the prescription of more daily doses of lithium, 44 which may lead to a higher incidence of side effects and poor compliance. 45 , 46 This issue may prevent some clinical psychiatrists from increasing lithium dosages to reach the target serum levels recommended by the treatment guidelines. 47
A recent systematic review 11 and previous treatment guidelines from the British Association for Psychopharmacology (BAP) 1 and the Royal Australian and New Zealand College of Psychiatrists (RANZCP) 2 recommend target lithium serum levels of 0.6–0.8 mmol/L as an efficacious range. However, there is limited evidence to support these recommendations. For example, the aforementioned systematic review included seven studies and 1274 participants. 11 Our retrospective cohort study provided evidence from 466 participants; thus, enriched the evidence base indicating that lithium serum levels of 0.4–0.8 mmol/L (serum level: 0.55 ± 0.11 mmol/L) could be associated with a notably lower risk of recurring mood episodes when compared to lithium serum levels <0.4 mmol/L (serum level: 0.30 ± 0.06 mmol/L). Moreover, the subsequent dose‐response meta‐analysis that included data from 1406 participants further supports this view, which may indicate that these serum levels could be the best choice for patients with BD during lithium maintenance treatment, considering both evidence from real‐world evidence and previous clinical trials.
Regarding the efficacy of the 0.8–1.2 mmol/L group, the results of our cohort and dose‐response meta‐analysis showed a reduced risk of recurring mood episodes, albeit with a wide interval. This partially reflects the inconsistent recommendations across different guidelines. For instance, the Canadian Network for Mood and Anxiety Treatments/International Society for Bipolar Disorders (CANMAT/ISBD) guideline 3 and the International College of Neuro‐Psychopharmacology (CINP) guideline 10 propose different upper limits of lithium serum levels during maintenance treatment for BD (CANMAT/ISBD: 0.6–1.0 mmol/L and CINP: 0.6–1.2 mmol/L). At least two explanations may explain these discrepancies. First, the low sample size of the participants with lithium serum levels of 0.8–1.2 mmol/L may result in a wider CI range. 48 In our cohort study and in the dose‐response meta‐analysis, the number of cases in the 0.8–1.2 mmol/L group was much lower than those in other groups (our cohort: Table 2; dose‐response meta‐analysis: 0.8–1.2 mmol/L, 82 cases; 0.4–0.8 mmol/L, 555 cases; and <0.4 mmol/L, 769 cases). Second, participants with higher serum levels (0.8–1.2 mmol/L) may have a more severe illness, leading clinicians to prescribe higher lithium doses to achieve higher serum levels. This potential indication bias might have affected treatment efficacy, 49 both in our cohort and in three studies included in this meta‐analysis (Table S5). 24 , 27 , 40 In addition, BD in special populations can only benefit from lower lithium serum levels rather than higher lithium serum levels, which may be another reason. For example, one review indicated that perinatal BD with serum levels <0.64 mmol/L had more reactive newborns without an increased risk of cardiac malformations 50 ; the other study suggested that the serum level should be reduced to 0.40–0.60 mmol/L in BD with poor tolerance to lithium. 11 Collectively, for patients treated using lithium serum levels of 0.8–1.2 mmol/L, further studies are needed to establish the true prophylactic efficacy.
Due to the lack of research on the comparative prophylactic efficacy of lithium serum levels in younger versus older patients with BD, 15 , 51 we conducted a subgroup analysis stratified by age. For lithium serum levels of 0.4–0.8 mmol/L across different age subgroups, the current results suggest that older the age, the lower was the risk of recurring mood episodes; however, when analyzing patients with lithium serum levels of 0.8–1.2 mmol/L, the aforementioned pattern was not observed (Table 3). This risk distribution pattern may partly support previous studies, which suggest that a more conservative (lower) lithium serum level (0.4–0.8 mmol/L) could be more efficacious particularly among the elderly. 11 , 15 Additionally, the relationship between serum lithium levels and the risk of recurrent mood episodes in perinatal BD is another issue. Physiological adaptation of body volumes may affect lithium concentration, thereby affecting its potential efficacy and safety. 50 The evidence for the above issues is not yet sufficient. Therefore, further research is needed on issues involving BD special populations for the forthcoming meta‐analysis. 15 , 50 , 51
This study had several strengths. First, our cohort came from large EMRs from multiple institutions across Taiwan, providing real‐world data for patients with BD on maintenance treatment using lithium, thereby providing further evidence for this relatively scarce literature. 52 Second, our study used sensitivity analysis and dose‐response meta‐analysis to enhance the robustness of our findings and their universality to real‐world patients with BD treated using different serum levels of lithium. However, some limitations deserve discussion. First, using a database‐based retrospective cohort study, it is difficult to avoid non‐random allocation, possible indication bias, and unmeasured (residual) confounding. 53 To minimize those potential limitations, we extracted observable differences in baseline characteristics, applied the aHRs model, and performed further sensitivity analysis and a dose‐response meta‐analysis. However, some unobserved confounders, such as the history of BD, the polarity of recent episodes, and previous response to lithium, are lacking. Second, lithium serum levels at the index date were used to classify participants into different groups; however, follow‐up measurements of lithium serum levels were not routinely implemented. We considered two criteria to reduce this uncertainty and ensure that participants were accurately allocated into each group according to lithium serum levels (no reduction in lithium dose and an acceptable difference in lithium serum levels of <0.1 mmol/L during the 2‐year follow‐up). Third, the current medical records of CGRD do not provide scores from validated rating scales for depressive or manic symptoms, 53 such as the Young Mania Rating Scale 54 or the Montgomery‐Asberg Depression Rating Scale. 55 Therefore, we adopted the most common definition in previous studies (psychiatric hospitalization and the use of any additional psychotropic drug) as proxies for recurring mood episodes. 24 , 27 , 28 Nevertheless, we had no information about the polarity of mood episodes. Fourth, notwithstanding this multi‐institutional study accounting for more than 20% of the outpatient population in Taiwan, patients might still receive care at other medical institutions outside of the CGRD system. This lack of continuity of medical visits can affect the completeness of our patient records, such as the recurrence of mood episodes. Finally, since relatively few studies were included (k < 10) in the dose‐response meta‐analysis, 56 publication bias could not be reliably assessed.
In our real‐world retrospective cohort of participants with BD during lithium maintenance treatment, those with serum levels of 0.4–0.8 mmol/L had a significantly lower risk of recurring mood episodes; those with serum levels of 0.8–1.2 mmol/L may also exhibit a reduced risk of recurring mood episodes; however, the risk interval was wider possibly due to the relatively few participants in this group rendering the results non‐significant. A dose‐response meta‐analysis also provided similar findings. Further analysis of age subgroups showed that older the age, the lower was the risk of recurring mood episodes at lithium serum levels of 0.4–0.8 mmol/L. Our findings provide a clinical reference for the most efficacious lithium serum levels for the maintenance treatment of BD.
CONFLICT OF INTEREST
Prof. Vieta has received grants and served as a consultant, advisor, or CME speaker for the following entities: AB‐Biotics, Abbott, Allergan, Angelini, AstraZeneca, Bristol‐Myers Squibb, Dainippon Sumitomo Pharma, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo‐Smith‐Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, SAGE, Sanofi‐Aventis, Servier, Shire, Sunovion, Takeda, the Brain and Behaviour Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the EU Horizon 2020 and the Stanley Medical Research Institute. Other authors declare no financial interests or potential conflicts of interest regarding the authorship and publication of this article.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/acps.13346.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Pei‐Ying Yang, Mr. Chien‐An Hu, and the Institute of Epidemiology & Preventive Medicine, College of Public Health, National Taiwan University for the technical support. This study is supported by grants from the Ministry of Science and Technology, Taiwan (MOST 109‐2314‐B‐182A‐009‐MY2), and the funding sources had no role in the design of the study. The detailed author contributions are as follows CW Hsu and AF Carvalho involved in research idea and study design. data acquisition: CW Hsu for cohort, CW Hsu and PT Tseng for meta‐analysis; CW Hsu, AF Carvalho, LJ Wang, and M Solmi involved in data interpretation. CW Hsu involved in statistical analysis and manuscript drafting. CW Hsu, AF Carvalho, SY Tsai, LJ Wang, PT Tseng, PY Lin, YK Tu, E Vieta, M Solmi, CF Hung, and HY Kao involved in manuscript revision. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Hsu C‐W, Carvalho AF, Tsai S‐Y, et al. Lithium concentration and recurrence risk during maintenance treatment of bipolar disorder: Multicenter cohort and meta‐analysis. Acta Psychiatr Scand. 2021;144:368–378. 10.1111/acps.13346
Hsu and Carvalho equally contributed as first author.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available but can be accessed with permission from the Chang Gung Memorial Hospital in Taiwan.
REFERENCES
- 1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence‐based guidelines for treating bipolar disorder: Revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malhi GS, Bell E, Bassett D, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2021;55:7‐117. [DOI] [PubMed] [Google Scholar]
- 3. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long‐term lithium therapy for bipolar disorder: systematic review and meta‐analysis of randomized controlled trials. Am J Psychiatry. 2004;161:217‐222. [DOI] [PubMed] [Google Scholar]
- 5. Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta‐analysis. BMJ. 2013;346:f3646. [DOI] [PubMed] [Google Scholar]
- 6. Anmella G, Fico G, Lotfaliany M, et al. Risk of cancer in bipolar disorder and the potential role of lithium: international collaborative systematic review and meta‐analyses. Neurosci Biobehav Rev. 2021;126:529‐541. [DOI] [PubMed] [Google Scholar]
- 7. Medić B, Stojanović M, Stimec BV, et al. Lithium ‐ pharmacological and toxicological aspects: the current state of the art. Curr Med Chem. 2020;27:337‐351. [DOI] [PubMed] [Google Scholar]
- 8. Severus WE, Kleindienst N, Seemüller F, Frangou S, Möller HJ, Greil W. What is the optimal serum lithium level in the long‐term treatment of bipolar disorder–a review? Bipolar Disord. 2008;10:231‐237. [DOI] [PubMed] [Google Scholar]
- 9. Mcintyre RS, Mancini DA, Parikh S, Kennedy SH. Lithium revisited. Can J Psychiatry. 2001;46:322‐327. [DOI] [PubMed] [Google Scholar]
- 10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro‐Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP‐BD‐2017), Part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20:180‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nolen WA, Licht RW, Young AH, et al. What is the optimal serum level for lithium in the maintenance treatment of bipolar disorder? A systematic review and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Bipolar Disord. 2019;21:394‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parker G, Mccraw S. The ‘disconnect’ between initial judgments of lamotrigine vs. its real‐world effectiveness in managing bipolar disorder. A tale with wider ramifications. Acta Psychiatr Scand. 2015;132:345‐354. [DOI] [PubMed] [Google Scholar]
- 13. Carvalho AF, Firth J, Vieta E. Bipolar disorder. N Engl J Med. 2020;383:58‐66. [DOI] [PubMed] [Google Scholar]
- 14. Duffy A, Heffer N, Goodday SM, et al. Efficacy and tolerability of lithium for the treatment of acute mania in children with bipolar disorder: A systematic review: A report from the ISBD‐IGSLi joint task force on lithium treatment. Bipolar Disord. 2018;20:583‐593. [DOI] [PubMed] [Google Scholar]
- 15. Shulman KI, Almeida OP, Herrmann N, et al. Delphi survey of maintenance lithium treatment in older adults with bipolar disorder: An ISBD task force report. Bipolar Disord. 2019;21:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Firth J, Siddiqi N, Koyanagi A, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6:675‐712. [DOI] [PubMed] [Google Scholar]
- 17. Colomer L, Anmella G, Vieta E, Grande I. Physical health in affective disorders: a narrative review of the literature. Braz J Psychiatr. 2020. Epub ahead of print. 10.1590/1516-4446-2020-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kessing LV, Ziersen SC, Andersen PK, Vinberg M. A nation‐wide population‐based longitudinal study mapping physical diseases in patients with bipolar disorder and their siblings. J Affect Disord. 2021;282:18‐25. [DOI] [PubMed] [Google Scholar]
- 19. Juurlink DN, Mamdani MM, Kopp A, Rochon PA, Shulman KI, Redelmeier DA. Drug‐induced lithium toxicity in the elderly: a population‐based study. J Am Geriatr Soc. 2004;52:794‐798. [DOI] [PubMed] [Google Scholar]
- 20. Hsu CW, Lee Y, Lee CY, Lin PY. Neurotoxicity and nephrotoxicity caused by combined use of lithium and risperidone: a case report and literature review. BMC Pharmacol Toxicol. 2016;17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344‐349. [DOI] [PubMed] [Google Scholar]
- 22. Shao SC, Chan YY, Kao Yang YH, et al. The Chang Gung Research Database‐A multi‐institutional electronic medical records database for real‐world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28:593‐600. [DOI] [PubMed] [Google Scholar]
- 23. Tsai MS, Lin MH, Lee CP, et al. Chang Gung Research Database: A multi‐institutional database consisting of original medical records. Biomed J. 2017;40:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double‐blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15:100‐109. [DOI] [PubMed] [Google Scholar]
- 25. Reddy DS, Reddy MS. Serum lithium levels: ideal time for sample collection! Are we doing it right? Indian J Psychol Med. 2014;36:346‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fountoulakis KN, Yatham L, Grunze H, et al. The International College of Neuro‐Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP‐BD‐2017), part 2: Review, grading of the evidence, and a precise algorithm. Int J Neuropsychopharmacol. 2017;20:121‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prien RF, Caffey EM Jr. Relationship between dosage and response to lithium prophylaxis in recurrent depression. Am J Psychiatry. 1976;133:567‐570. [DOI] [PubMed] [Google Scholar]
- 28. Johnson FN, Johnson S. Lithium in Medical Practice: Proceedings of the First British Lithium Congress, University of Lancaster, England, 15‐19 July 1977. University Park Press; 1978.
- 29. Waters B, Lapierre Y, Gagnon A, et al. Determination of the optimal concentration of lithium for the prophylaxis of manic‐depressive disorder. Biol Psychiatry. 1982;17:1323‐1329. [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603‐605. [DOI] [PubMed] [Google Scholar]
- 32. JaC S, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 33. Il'yasova D, Hertz‐Picciotto I, Peters U, Berlin JA, Poole C. Choice of exposure scores for categorical regression in meta‐analysis: a case study of a common problem. Cancer Causes Control. 2005;16:383‐388. [DOI] [PubMed] [Google Scholar]
- 34. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301‐1309. [DOI] [PubMed] [Google Scholar]
- 35. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037‐1057. [DOI] [PubMed] [Google Scholar]
- 37. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6:40‐57. [Google Scholar]
- 38. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 39. Crippa A, Orsini N. Multivariate dose‐response meta‐analysis: the dosresmeta R package. J Stat Softw. 2016;72:1‐15. [Google Scholar]
- 40. Maj M, Starace F, Nolfe G, Kemali D. Minimum plasma lithium levels required for effective prophylaxis in DSM III bipolar disorder: a prospective study. Pharmacopsychiatry. 1986;19:420‐423. [DOI] [PubMed] [Google Scholar]
- 41. Pérez De Mendiola X, Hidalgo‐Mazzei D, Vieta E, González‐Pinto A. Overview of lithium's use: a nationwide survey. Int J Bipolar Disord. 2021;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bschor T, Baethge C, Grunze H, et al. German S3 guidelines on bipolar disorders‐first update 2019: What is new in pharmacotherapy? Nervenarzt. 2020;91:216‐221. [DOI] [PubMed] [Google Scholar]
- 43. Bai YM, Chang CJ, Tsai SY, et al. Taiwan consensus of pharmacological treatment for bipolar disorder. J Chin Med Assoc. 2013;76:547‐556. [DOI] [PubMed] [Google Scholar]
- 44. Abou‐Auda HS, Al‐Yamani MJ, Abou‐Shaaban RR, Khoshhal SI. A new accurate method for predicting lithium clearance and daily dosage requirements in adult psychiatric patients. Bipolar Disord. 2008;10:369‐376. [DOI] [PubMed] [Google Scholar]
- 45. Jamison KR, Gerner RH, Goodwin FK. Patient and physician attitudes toward lithium: relationship to compliance. Arch Gen Psychiatry. 1979;36:866‐869. [DOI] [PubMed] [Google Scholar]
- 46. Gitlin MJ, Cochran SD, Jamison KR. Maintenance lithium treatment: side effects and compliance. J Clin Psychiatry. 1989;50:127‐131. [PubMed] [Google Scholar]
- 47. Schoot TS, Molmans THJ, Grootens KP, Kerckhoffs APM. Systematic review and practical guideline for the prevention and management of the renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16‐32. [DOI] [PubMed] [Google Scholar]
- 48. Biau DJ, Kernéis S, Porcher R. Statistics in brief: the importance of sample size in the planning and interpretation of medical research. Clin Orthop Relat Res. 2008;466:2282‐2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol. 1999;149:981‐983. [DOI] [PubMed] [Google Scholar]
- 50. Fornaro M, Maritan E, Ferranti R, et al. Lithium exposure during pregnancy and the postpartum period: a systematic review and meta‐analysis of safety and efficacy outcomes. Am J Psychiatry. 2020;177:76‐92. [DOI] [PubMed] [Google Scholar]
- 51. Amerio A, Ossola P, Scagnelli F, et al. Safety and efficacy of lithium in children and adolescents: A systematic review in bipolar illness. Eur Psychiatry. 2018;54:85‐97. [DOI] [PubMed] [Google Scholar]
- 52. Franklin JM, Pawar A, Martin D, et al. Nonrandomized Real‐world evidence to support regulatory decision making: process for a randomized trial replication project. Clin Pharmacol Ther. 2020;107:817‐826. [DOI] [PubMed] [Google Scholar]
- 53. Taipale H, Tiihonen J. Registry‐based studies: What they can tell us, and what they cannot. Eur Neuropsychopharmacol. 2021;45:35‐37. [DOI] [PubMed] [Google Scholar]
- 54. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429‐435. [DOI] [PubMed] [Google Scholar]
- 55. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382‐389. [DOI] [PubMed] [Google Scholar]
- 56. Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are not publicly available but can be accessed with permission from the Chang Gung Memorial Hospital in Taiwan.


