Abstract
The main objective of diabetes control is to correct hyperglycaemia while avoiding hypoglycaemia, especially in insulin‐treated patients. Fear of hypoglycaemia is a hurdle to effective correction of hyperglycaemia because it promotes under‐dosing of insulin. Strategies to minimise hypoglycaemia include education and training for improved hypoglycaemia awareness and the development of technologies to allow their early detection and thus minimise their occurrence. Patients with impaired hypoglycaemia awareness would benefit the most from these technologies. The purpose of this systematic review is to review currently available or in‐development technologies that support detection of hypoglycaemia or hypoglycaemia risk, and identify gaps in the research. Nanomaterial use in sensors is a promising strategy to increase the accuracy of continuous glucose monitoring devices for low glucose values. Hypoglycaemia is associated with changes on vital signs, so electrocardiogram and encephalogram could also be used to detect hypoglycaemia. Accuracy improvements through multivariable measures can make already marketed galvanic skin response devices a good noninvasive alternative. Breath volatile organic compounds can be detected by dogs and devices and alert patients at hypoglycaemia onset, while near‐infrared spectroscopy can also be used as a hypoglycaemia alarms. Finally, one of the main directions of research are deep learning algorithms to analyse continuous glucose monitoring data and provide earlier and more accurate prediction of hypoglycaemia. Current developments for early identification of hypoglycaemia risk combine improvements of available ‘needle‐type’ enzymatic glucose sensors and noninvasive alternatives. Patient usability will be essential to demonstrate to allow their implementation for daily use in diabetes management.
Keywords: algorithms, devices, diabetes mellitus, hypoglycaemia, sensors
1. INTRODUCTION
Hypoglycaemia is one of the major threats for people with diabetes (PwD) in daily life, as it can result in symptoms that affect their mental agility and can lead to coma if not treated rapidly. 1 Psychological distress due to the fear of hypoglycaemia reduces the quality of life of PwD, 2 especially of persons with type 1 diabetes who have been reported as having twice higher occurrence of hypoglycaemia than those with type 2 diabetes. 3
The fear of hypoglycaemia and associated symptoms lead many PwD to accept hyperglycaemic glucose values, as the easiest solution to avoid dealing with the management of unpleasant hypoglycaemic events. 2
There are different definitions of hypoglycaemia based on the glucose levels, devices used and the impact of hypoglycaemia on the person with diabetes. 1 , 4 , 5 Alongside these differences in glucose thresholds to define hypoglycaemia, there are huge inter—as well as intraindividual variations in the glucose levels at which hypoglycaemic events are experienced. 6 For example, PwD with a long duration of diabetes and those with high glycaemic variability often have impaired awareness of hypoglycaemia and may not feel symptoms until glucose levels reach very low values, while those with sustained hyperglycaemia can feel symptoms of hypoglycaemia at much higher glucose values than the level of 3.9 mmol/L (70 mg/dl) defined in consensus documents. 4 , 7
In this paper, we provide an overview of new sensing technologies, other than electro‐enzymatic and fluorescence‐based ones present in currently marketed devices, which can be used to identify or prevent hypoglycaemia. Then, we address the algorithms being used or developed to enhance glucose monitoring and increase its accuracy, and to predict future glucose values, that can have a decisive role in predicting future hypoglycaemic events. Finally, we present hypoglycaemia detection techniques not based on glucose values but use one or different vital signs that are affected by low blood glucose values and hypoglycaemic symptoms. We finish with a discussion on the future of hypoglycaemia detection and prediction. The diversity of techniques presented in this review are represented in Figure 1 depending on the vital sign or fluid used for hypoglycaemia detection.
FIGURE 1.
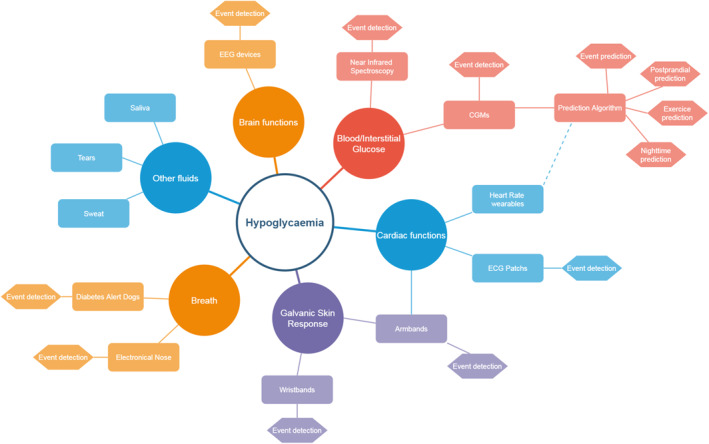
Mapping of hypoglycaemia detection and prediction techniques
2. METHODOLOGY
A literature review was performed using ‘PUBMED’, and ‘IEEE’ to find articles about technologies related to hypoglycaemia detection and prevention. After exploring and combining many search terms to ensure having the broadest results, we used the following terms: ‘hypoglycaemia’, ‘blood glucose’, ‘glycaemia’, ‘prediction’, ‘predictive’, ‘detection’, ‘forecast’, ‘continuous glucose monitoring’, ‘CGM’ and ‘diabetes’.
The search was performed in January 2019 and was restricted to articles from 2013 onward, and gave in total 1616 results. M.C. and O.D. made a first selection of 270 articles. In parallel, an alert was set to avoid missing articles published after January 2019 dealing with these topics. References of selected articles were analysed to extract other related articles, and a complementary search in ‘ScienceDirect’ and ‘Google Scholar’ was used to find further information when necessary and to complete the review with original works on each subtopic identified. Monthly alerts and complimentary alerts helped to add 25 articles.
Only articles published after 2013 were retained for this review. New glucose sensors having a linear range detection wide enough for blood or interstitial measurement were eligible. For prediction algorithms, selected articles had to deal with glucose prediction and present details on the datasets used, methodology and performance metrics. We included algorithms that predicted glucose values in a defined prediction horizon, as well as those that specifically predicted hypoglycaemic events up to a maximum of 24 h in the future. Solutions and technologies for hypoglycaemia correction like new glucagon formulations and closed‐loop algorithms were excluded. Figure 2 presents the PRISMA flow diagram of this review.
FIGURE 2.
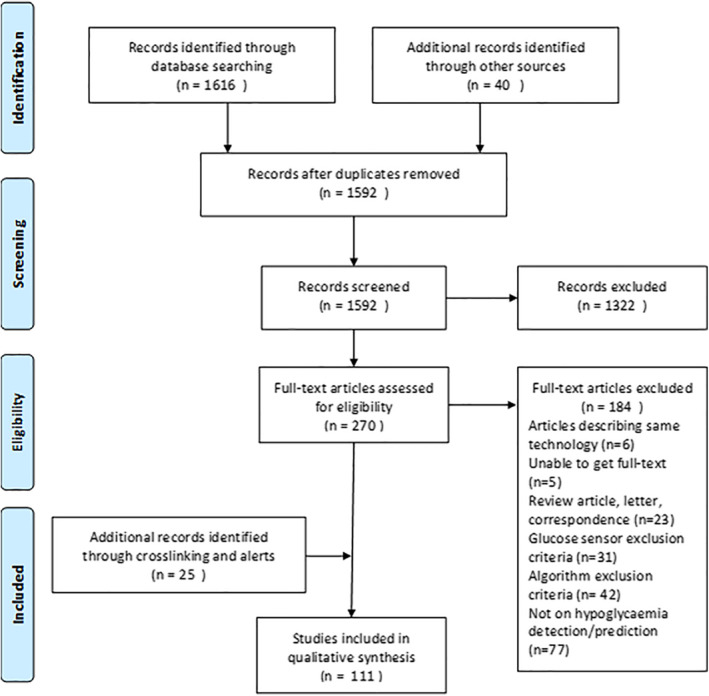
PRISMA flow diagram
3. SENSING TECHNOLOGIES FOR BLOOD OR INTERSTITIAL GLUCOSE MEASUREMENT
The advent of enzymatic glucose sensors was a major revolution in the lives of PwD by allowing accurate self‐monitoring of blood glucose (SMBG). However, SMBG allowed only intermittent measurement of blood glucose and PwD had to rely on their awareness of hypoglycaemia and appropriate timing of their daily tests to identify low glucose readings.
Continuous glucose monitoring (CGM) was an evolution that used the same principles of enzymatic glucose sensors, but through a new design of sensors allowed almost continuous (e.g., every 1–5 min) measurement of interstitial glucose (IG) for several consecutive days (e.g., 7–14 days). CGM empowered PwD to better manage their diabetes and minimise hypoglycaemia by allowing them to see their glucose at any time, along with information about the rate and direction of change, and with many systems, providing alerts of impending hypoglycaemia allowing the PwD to take preventative action.
Both SMBG and CGM rely on an enzymatic reaction, 8 mainly using glucose oxidase (GOx), but some systems can use glucose‐1‐dehydrogenase or hexokinase. After the first enzymatic reaction on glucose, subsequent reactions follow creating a current on an electrode. The measurement of the current allows calculation of glucose levels in the interstitial fluid by applying a calibration function. These electro‐enzymatic reactions were chosen for their high‐sensitivity (Se), the good linearity between the current and glucose level, and their range of detection going from 2 mmol/L (40 mg/dl) up to 40 mmol/L (400 mg/dl). 9 However, at the extremities of the range of detection, these sensors suffer from a reduction of accuracy, with an increase in mean absolute relative difference (MARD) from an average of 9%–15% in most of currently availably CGM systems 10 to percentages over 20% for low glucose values. 11 , 12 New advances in glucose sensor design may help to increase this accuracy at low glycaemia levels.
3.1. Evolutions of enzymatic and electrochemical sensors towards a better accuracy
Over the last decades, development of nanomaterials opened a new path to improve sensors thanks to their size, morphology, and higher surface‐to‐volume ratios providing improved Se that could benefit glucose measurement. Although many publications report on sensors that are at experimental levels, and are sometimes far from having the range of detection needed for blood glucose monitoring, some articles presented sensors that may fit these needs.
Balakrishnan et al. 13 propose a GOx surface modified with polysilicon nanogap, allowing a highly sensitive measurement with a limit of detection down to 0.6 µmol/L (0.011 mg/dl) and a good linear range up to 50 mmol/L (900 mg/dl) fitting the criteria for blood and IG. Another proposed system works by immobilising GOx into an oxide copper and gold nanostructure, 14 with a linear concentration range from 10 µmol/L (0.18 mg/dl) to 20 mmol/L (360 mg/dl). Many other systems that are close to the requirements for clinical sensors are described, like CeO2 nanorods, 15 multiwalled carbon nanotubes ZnO nanoparticles, 16 gold and ZnO nanorods 17 combinations of enzyme models and graphene, 18 , 19 all taking advantage of the nanostructures and materials to increase the Se of enzymatic sensors. 20 , 21
These nanomaterials are also proposed for nonenzymatic sensing, using platinum, gold, nickel, copper, palladium and other composites, 22 , 23 but only a few show a linear range inside the physiological levels of blood or IG in PwD. From this small number, we have a CuO NPs Ag electrode with a range from 0.05 to 18.45 mmol/L (0.9–332 mg/dl), 24 a Pd–Pt graphene electrode, 25 a Ni(OH)2 nanostructure with a glucose range detection of 0.01–30 mmol/L, 26 a Pd NPs silver electrode. 27
However, the path to market of these newly designed sensors is long and many steps are still needed. After initial laboratory accuracy assessments, reproducibility, biocompatibility and other fluid components' interferences must be evaluated. Toxicity evaluation is also necessary for these materials that may be present in invasive devices to ensure safety of use. They must also fit to industrialisation processes and storage requirements, and be amenable to scaling and quality control. Only after succeeding in these step will real world data collection from clinical studies be possible, that would allow us to know the true level of improvement of accuracy and MARD reduction of these next‐generation sensors.
3.2. Noninvasive blood glucose sensors
Noninvasive methods for CGM could change the life of PwD suffering from frequently occurring and severe hypoglycaemia.
Currently available noninvasive technologies do not reach the same accuracy as electrochemical minimally invasive sensors present nowadays in CGM. 28 , 29 It remains uncertain whether they will reach standards like ISO‐15197 or UCM 380327 for medical device approval that would make them suitable for closed‐loop systems and automated advisory systems. However, these devices could allow better acceptance by the patients due to their noninvasiveness, as well as more affordable access to CGM for many patients thanks to reduced need for expensive consumables.
Amongst the noninvasive continuous monitoring solutions we identified in development, many use optical technologies. For example, ‘Wear 2b©’ from Israel 30 and ‘Prediktor Medical©’ company from Norway 31 are developing devices with near‐infrared (NIR) spectroscopy, while ‘D‐pocket©’ and ‘D‐band©’ devices (DiaMonTech) 32 are using mid‐infrared spectroscopy and photothermal detection.
Other minimally invasive approaches need also to be mentioned, like “K'Watch©” device (PkVitality, France) 33 using micro‐needles to measure IG at skin surface and ‘SugarBeat©’ device (Nemaura Medical) 34 that uses reverse iontophoresis to extract interstitial fluid through two electrodes. These two devices are near‐to‐market and report MARDs almost similar to commercially available enzymatic CGM systems, despite using minimally invasive methods to access the interstitial fluid.
For a better insight in non‐invasive techniques, we recommend recent reviews by Avari et al. 28 and Gonzales et al. 29
4. ALGORITHMS FOR GLUCOSE AND HYPOGLYCAEMIA PREDICTION
In commercially available CGM devices that use an enzymatic sensor, algorithms are needed to convert measured electrical current into a glucose value, and in some cases to correlate the IG value to the blood glucose (BG) value that may have a different linear range, and also to reduce the delay of 10–15 min 35 , 36 , 37 between tissue and blood.
By consequence, the first steps to better detect hypoglycaemia are to improve the calibration algorithms that must be performed to calculate real blood glucose value allowing faster and more accurate detection of a low values.
4.1. Calibration algorithms
Early CGM systems used first‐order linear functions or Kalman filters as calibration algorithms, 38 , 39 with one or two SMBG entries per day. Recent developments in calibration algorithms pave the way to a reduction of capillary calibration needs and an improvement in MARD.
An algorithm presented by Mahmoudi et al. 40 was used to reduce requirement for calibration. 41 It uses three steps, starting by a rate‐limit filtering to ensure physiological limits of rate of change are not exceeded, then a selective smoothing to reduce the noise of the signal, followed by a regression that calculates the corresponding glucose value using the electrical signal and the SMBG entries. This algorithm demonstrated better accuracy in the range of low blood glucose levels. 41 , 42
Some other groups 43 , 44 , 45 used a regularised deconvolution to compensate for the difference between blood and IG, followed by linear regression using two SMBG calibration values. The algorithm was then further developed to use less calibrations using a Bayesian framework that revaluates calibration parameters at each iteration to a final version using no calibration, 46 making the simple first‐order linear functions more accurate by taking into account the whole 7‐day period and obtaining a reduction in MARD of 1.2%.
Other teams have tried to use past weeks' data of previous sensors, refining the calibration parameters for each new sensor insertion. 47 , 48 These algorithms cannot be easily used in real‐time in wearable devices, but the increasing usage of smartphones for glycaemic monitoring may open the way to such implementations.
These attempts show that using more complex techniques can help to reduce the MARD by more than 1%, and may improve performance in the hypoglycaemic range where CGM systems are currently less accurate. 12 , 42 , 46
4.2. Prediction algorithms
After improving the accuracy of CGM devices, through new sensors and more complex calibration algorithms as presented earlier, another step towards further improving the quality of life of PwD and their ability to counter hypoglycaemia are predictive algorithms and alarms as presented in Figure 3. They allow the patients to act early and effectively prevent episodes of hypoglycaemia.
FIGURE 3.
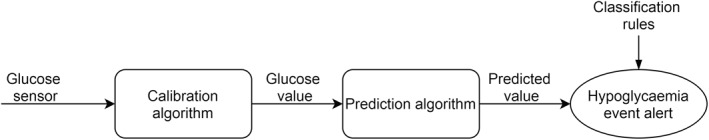
Process from glucose sensing to hypoglycaemic event alert found in many available devices
Similar to calibration algorithms, first‐generation of prediction algorithms used linear regressions or Kalman filters, 39 which are simple calculation techniques that can be embedded in monitoring devices and use past data to allow a short‐term prediction (20‐min prediction horizon for hypoglycaemia or hyperglycaemia alarm in current commercial devices).
While many articles present different prediction algorithms, only a few tried to assess their clinical efficacy and benefits in real life or at least in simulations.
A first in silico study led by Zecchin et al. 49 with 50 virtual patients from the UVA/PADOVA simulator compared the duration of hypoglycaemic events in three scenarios: one when hypoglycaemia was not detected by any device, one where the patient took rescue carbs when crossing hypoglycaemia threshold, and one when 30‐min predictions alerted about hypoglycaemia. The simulation results show that hypoglycaemia alert reduced time spent in hypoglycaemia from 17.7% to 4.7% in the scenario with threshold alert and to 1.2% in the scenario with predictive alert.
These predictions help patients to take clinical decisions in their treatment, as shown in an early study. 50 Twelve patients were asked to record their decision making around hypoglycaemia, comparing their usual care with a system that informed them of a 30‐min prediction. Twenty percent of the patient's decisions were changed and led to improved glycaemia. However, in some cases overtreatment led to hyperglycaemia.
An observational study 51 showed that the activation of such predictive hypoglycaemia alarms reduced time below 3 mmol/L (54 mg/dl) by almost 40%. Another retrospective study 52 found that predictive alarms prevented 59% of low glycaemic values.
Incoming years, we expect an increase of clinical evaluations of such algorithms, with the integration of prediction techniques in CGM systems and other devices, even though from a logical perspective the benefits for patients seem obvious through their empowerment to take proactive decisions before reaching critical glucose levels. However, education as for every new device will be necessary to prevent the counter effects of over reactions.
4.3. A diversity of modelling strategies
Numerous factors can affect blood glucose levels and induce hypoglycaemic events such as insulin dosage, meal consumption, physical activity, sleep quality, alcohol consumption, interfering medication, stress and emotions, leading to a diversity of features that can be used in addition to blood glucose history to develop predictive algorithms. The increasing availability of devices like CGM systems, insulin pumps or connected insulin pens, meal‐recognition apps, physical activity wearables and the increasing usage of CHO counting amongst PwD allow collection of a variety of nonglucose data that can be employed to predict blood glucose.
We found 48 relevant publications 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 that presented a prediction algorithm published between 2013 and 2020 showing the recent increasing interest for this topic. Information on these algorithms is presented in appendices.
4.4. Prediction inputs
In these publications, a diversity of strategies was used depending on the data collected and their complexity and the algorithm objective. More than half of the algorithms used one or two additional data inputs, usually insulin doses or carbohydrates (CHO) or both. These two inputs are easily accessible as they are almost always collected in bolus advisors used in sensor augmented pump trials with enough accuracy for modelling purposes. For a better interpretation by prediction algorithms, these two additional inputs are processed in almost one third of reviewed articles by physiological models to extract additional features describing the absorption dynamics of insulin action 101 , 102 , 103 , 104 or meals. 104 , 105 , 106 , 107
The addition of insulin and CHO data to CGM in prediction models can slightly improve the performance of algorithms. However, outside of clinical studies where patients are specifically selected for their ability to carb count, using the CHO input in real life setting seems difficult to achieve. To avoid this burdensome task for patients, three publications 70 , 92 , 95 proposed to use meal timestamps to inform the prediction algorithm of this event. Another research team 58 proposed that meal entries could be simplified by classifying them with a five‐level scale from very light to very heavy meal.
Physical activity is the other major input impacting blood glucose level. Nine studies investigated the use of armbands or wristbands to collect information on heart rate, 71 , 93 , 94 energy expenditure 75 , 76 , 85 , 99 , 100 or the number of steps, 87 to augment the predictability by this input. In all cases where comparisons have been made, the addition of a signal informing the algorithm about activity increased its prediction performance, even for commercial devices. This shows that in real‐life setting, many accessible devices are accurate enough for this purpose. The difficulty will be more on the technical side to integrate different models and take into account the diversity of data structure for each device. Finally, stress, 84 medication, 70 daily events and circadian rhythms 86 were also investigated as potential inputs, and could be helpful to differentiate prediction models for daytime and night‐time.
4.5. Prediction algorithms and outputs
After this step of input selection, and the use or non‐use of meal and insulin physiological models, an algorithm is selected depending on the purpose of the algorithm and its ability to process different type of inputs. For blood glucose prediction, where the output is a value a few steps ahead, the algorithms used are regressive models (ARM, ARMAX, ARIMAX ...), different types of neural networks (ANN, CNN, RNN, ELM, ESN …), genetic algorithms and grammatical evolution, support vector machines (SVMs) and support vector regressions, Gaussian processes, self‐organising maps (SOM) and kernel ridge regression. In some cases as illustrated in Figure 4, the authors added a final step with rules to create a hypoglycaemia or hyperglycaemia warning using computed blood glucose values from the prediction algorithms.
FIGURE 4.
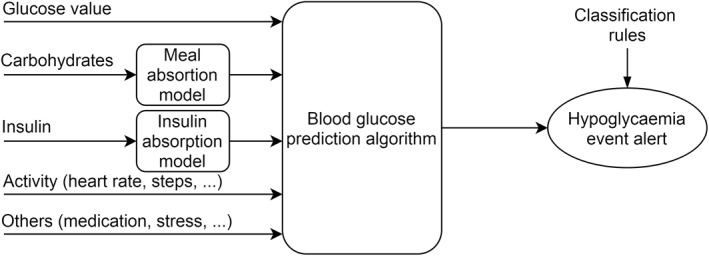
Multivariable blood glucose prediction. In addition to glucose value, others inputs are added to increase accuracy. A blood glucose prediction value is calculated, and classification rules allow evaluating the incoming hypoglycaemia event
Another strategy presented in Figure 5 is to predict directly the occurrence of a hypoglycaemic event in a defined prediction horizon, without computing blood glucose values. This strategy is mostly used for postprandial hypoglycaemia, exercise‐induced hypoglycaemia and night‐time hypoglycaemia, where the aim is to inform the PwD quite early, at meal time or bedtime or prior to the start of exercise to reduce the risk of having such adverse event in the following hours. The algorithms used are mainly classification algorithms like SVM, SOM, classification trees, linear discriminant functions, random forests and also ANN and grammatical evolution.
FIGURE 5.
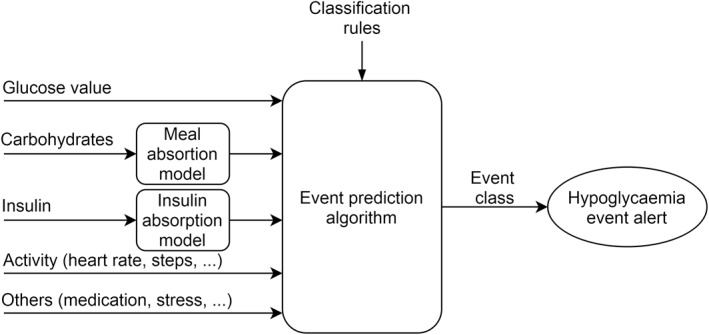
Multivariable event prediction. In this other model, the algorithm is trained directly for hypoglycaemia event prediction
Finally, some teams 53 , 59 , 62 , 71 , 73 , 78 , 81 , 83 merged different algorithms having the same output to increase the models' performance, taking advantage of each algorithms characteristics. Vehi et al. 87 took this strategy one step further, by proposing three algorithms for each situation, one predicting hypoglycaemia in the next hour using grammatical evolution, which can be enhanced by another algorithm for postprandial hypoglycaemia risk in the next 4 h following the meal using SVM, and another algorithm trained for night‐time hypoglycaemia using ANN.
The diversity of approaches, inputs used, algorithms and the number of publications in the last few years, shows that this field of research is expanding and researchers are still exploring all the capabilities that machine learning and artificial intelligence offer. New algorithms are created every year, 108 and are tested rapidly by different teams on different blood glucose datasets.
Most studies were performed in people with type 1 diabetes (T1D) who are most in need of this type of algorithm and are more challenging for algorithms due to their high glucose variability and Se to external factors than people with T2D. We can also highlight that in the last two years, many publications used large datasets of more than 80 patients with collection duration of several weeks or months. This shows an increasing availability of qualitative datasets collected during clinical studies that can be exploited for machine learning purpose.
4.6. Performance metrics
The performance metrics are key elements that allow determination of the accuracy of the developed model and its ability to predict correctly blood glucose or an adverse event. Some commonly used metrics are purely mathematical and give an insight on the level of errors made by the presented model. The most used mathematical metric is root main square error (RMSE), followed by mean absolute error (MAE), mean absolute difference (MAD), MARD, energy of the second‐order difference (ESOD), relative absolute difference (RAD) and sum of squares of the glucose prediction error (SSGPE). RMSE, MAE, MAD and SSGPE represent the errors between predicted and real values, while MARD and RAD are more sensitive to errors in low glucose values. ESOD is an indicator of the variability and the oscillations present in the predicted glucose profile, the lower the value the smoother is the profile.
For better clarity, some authors also use clinical evaluation metrics that can reflect the impact of prediction on clinical decisions and some were developed specifically for diabetes‐related technologies. Metrics like time lag or time gain 109 give an insight of the average time gained by patient before his real glucose level reaches the predicted value, and represent the true prediction horizon. ‘J Index’ 109 was developed specifically for blood glucose prediction algorithms, and combines ESOD and time gain to reflect the clinical usefulness of the predicted value.
Error grids were developed to assess the clinical accuracy of SMBG, classifying the error between the assessed device and a standard reference measured by laboratory test in different zones, each one corresponding to a level of clinical risk in case of difference between the real and the measured value. Many teams used the classical Clarke's error grid 110 and Parkes Error grids 111 that were intended for assessing the accuracy of SMBG. A few teams used the continuous glucose‐error grid analysis (CG‐EGA) 112 designed for assessing the accuracy of CGM, and one team used prediction (PRED)‐EGA. 113 This last error grid was developed specifically to assess the clinical accuracy of prediction algorithms, modifying the way rate of change is evaluated in CG‐EGA which results in a reduction of misclassified cases.
The accuracy of hypoglycaemia event prediction uses more standard metrics like receiver operating characteristics curves, Se, Sp, positive predictive value, false alarm rate, accuracy, Matthews correlation coefficient and F1‐score. However, we see in some publications differences in the way hypoglycaemic events are defined. Some authors considered the hypoglycaemic event as crossing the BG value of 3.9 mmol/L (70 mg/dl), others as having two or three BG values of 3.9 mmol/L, and others used also the second level of 3 (54 mg/dl) or 2.8 mmol/L (50 mg/dl).
This variety of performance metrics makes it difficult for an external reader to compare results from different publications. We also regret the lack of use of clinical performance metrics, especially the ones like J‐Index and PRED‐EGA or CG‐EGA and time gain that could be more pertinent to validate the accuracy of the presented algorithms.
5. NONGLYCAEMIA‐BASED DETECTION TECHNIQUES
Another axis of research in the last years was the development of technologies for hypoglycaemia detection using other sources than blood or IG, exploring other metabolic alterations that can occur during a hypoglycaemic event.
5.1. Electrocardiogram‐based hypoglycaemia detection
The impact of low blood glucose levels on the electrical activity of the heart has been studied in the last years. 114 , 115 , 116 , 117 , 118 Reports show a prolongation of the QT interval, an increase in heart rate variability (HRV), and changes in cardiac repolarisation during hypoglycaemic episodes. Thus, using electrocardiogram (ECG) changes could be a noninvasive way to detect the onset of hypoglycaemia.
The development in the last decade of new ECG wearables enabled easy collection of cardiac signals and opened the way to ECG‐based hypoglycaemia detection through deep learning algorithms.
First attempts to predict hypoglycaemia from ECG were made by the Nguyen HT team in many publications, 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 using the same dataset of 15 T1D children overnight, with an ECG Holter and CGM measurement. Many algorithms were used in these publications like neural networks or extreme learning machines, and inputs were QTc and HRV, with a learning approach that did not take into account interindividual variations. The best results obtained a Se of 80% and a Sp of 50% for only night‐time hypoglycaemia.
A publication in 2016 127 using data from an ECG Holter from outpatients over two days showed that significant prolongations of QT intervals can occur and be detected during daytime in real‐life setting.
Another proof of principle study published in 2019 128 used a wearable device in 23 T1D outpatients, the ‘VitalPatch©’ (VitalConnect) that can be placed on the chest for 5 days and can measure heart rate. Results showed that 55% of day and night hypoglycaemias were clearly detectable using only HRV, and 27% showed an atypical pattern.
These two studies showed that theoretically, QTc and HRV could be two ECG features that can be used for noninvasive hypoglycaemia detection, whether patients do or do not have diabetes, and that having cardiovascular autonomic neuropathy does not influence their ability to detect these measurable changes during hypoglycaemia. 129 This last characteristic will have to be further confirmed in patients with long‐standing diabetes or those with confirmed cardiac autonomic neuropathy. 130
While working on this review, an original work was published that leverages these approaches of hypoglycaemia detection through ECG to a next level, using a deep learning algorithm to detect the beginning of the event using the raw ECG signal directly without feature extraction. 131 After collecting and processing data from eight healthy patients using a wearable ECG patch that records ECG signal and actigraphy, two different algorithms were used to detect night‐time hypoglycaemia. They obtained good results with a Se, a Sp and an accuracy around 84% respectively for both models. The originality of this work, beside using real‐life raw data from commercialised ECG wearables, is using a person‐specific approach with a learning process for each individual patient. This allows the system to account for individual differences between subjects as visible in Figure 6, while all other studies were cohort‐based approaches.
FIGURE 6.
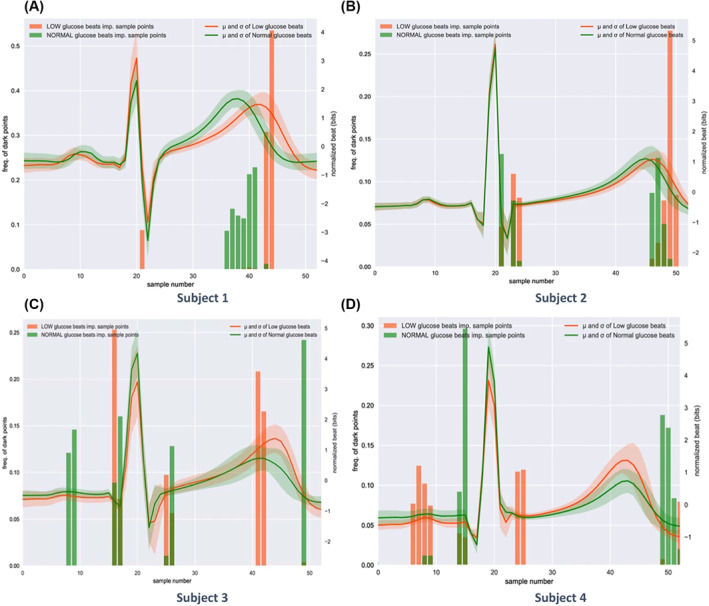
Illustration of interindividual differences in heartbeats during hypoglycaemia events. The solidlines represent the mean of all the heartbeats that correspond to each subject in the training dataset: green during normal glucose levels, red during hypoglycaemic events. The comparison among four different subjects highlighted the fact that each subject may have a different ECG waveform during hypoglycaemic events. For instance, Subjects 1 and 2 present a visibly longer QT interval during hypoglycaemic events, differently from Subjects 3 and 4. Reproduced from Porumb et al. 129
These different studies show that ECG could be used in real‐life conditions for helping patients to detect hypoglycaemic events. Improved devices and algorithms can make these algorithms more accurate and easier to implement in real‐life, which paves the way for new tools. If T1D patients may not be the first beneficiary of these technologies, ECG‐based algorithms could be useful for patients without diabetes suffering from night‐time hypoglycaemia, due to other conditions such as insulinoma, nesidioblastosis, endocrine, kidney, liver or heart disorders, anorexia, tumours.
5.2. Electroencephalogram detection
One of the other symptoms of hypoglycaemia is a decrease in cognitive function. Hence, time series electro‐encephalogram (EEG) can also be a good candidate for severe hypoglycaemia event detection (below 2.2 mmol/L), as it was confirmed by the first studies were EEG and blood glucose were recorded simultaneously. 132 , 133 , 134 Figure 7 presents an illustration of these difference of EEG signal during hypoglycaemia.
FIGURE 7.
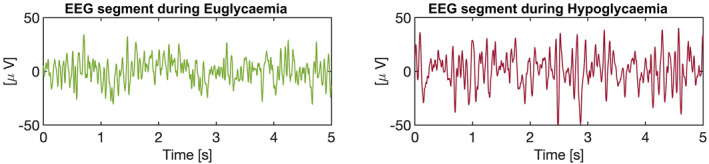
Encephalogram (EEG) segments during euglycaemia and hypoglycaemia. Each segment represents a 5‐s interval of EEG recordings during each phase, showing a higher amplitude in the low‐frequency bands and greater regularity during hypoglycaemia episodes
In recent years, in addition to their ECG studies, Nguyen and his team 135 , 136 have also conducted several studies with EEG. In their first attempts, they collected overnight EEG and BG data every 5 min through ‘YSI glucose analyser©’ (YSI Life Sciences) from five T1D adolescents, and performed insulin‐induced hypoglycaemia. Significant changes in EEG alpha band were observed during hypoglycaemia, and achieved detection performance results of 75% Se and 60% Sp. Further studies with three more volunteers and using other algorithms slightly improved their results 137 , 138 , 139
A Danish team in association with the company ‘UnEEG medical©’ (Denmark) have developed a wearable EEG for ambulatory and studied hypoglycaemia detection. In their first studies before 2013 where they investigated insulin‐induced hypoglycaemia, they concluded that EEG changes during low blood glucose events were detectable during both day‐ and night‐time. 140 , 141 This work was followed by another publication in 2016 where the same experiment was performed in eight prepubertal children with T1D in three phases to record EEG during daytime hypoglycaemia and during sleep. 142 The same algorithm developed for adults detected all hypoglycaemic events during daytime, but had too many false alarms during sleep, indicating that it needed a specific adjustment for children during night‐time. A later study 143 was performed with 24 T1D adults who underwent two hyperinsulinemic hypoglycaemic clamps on two subsequent days, to assess if brain changes were different after antecedent hypoglycaemia, and found no significant differences in EEG records. The conclusion was that EEG‐based detection can be used as hypoglycaemia alarm in daily use without suffering from loss of accuracy through successive episodes. On average, during these studies, EEG was able to detect a blood glucose level around 2.2 mmol/L (40 mg/dl), and between 15 and 20 min before reaching the nadir point, giving just enough time to prevent a severe hypoglycaemic episode.
After this series of clinically controlled trials, an ambulatory 3‐month study 144 was performed with eight T1D persons to evaluate the efficacy of an EEG alarm device in real‐life conditions. CGM was recorded for two periods of 5 days and SMBG was performed at each EEG alarm. One person withdrew from the study due to device discomfort, and 659 days were recorded. Sixteen alarms were triggered by the device at a median value of 2.4 mmol/L (43 mg/dl), and all subjects were able to take preventive actions before progression to severe hypoglycaemia. No false negative alarms were reported for hypoglycaemic events needing external help, but patients experienced 2.9 false alarms/week during daytime (and only 0.2/week during night‐time).
In all these studies, the ‘24/7 EEG SubQ©’ (unEEG medical) was used, a device in which a small part is implanted behind the ear, and an external device powers the implanted part, processes the signal in real‐time and launches the alarm if an event is detected.
New marketed devices opened the way for continuous real‐life measurement of ECG and EEG signals with enough accuracy to allow the development of algorithms for severe hypoglycaemia detection. These devices are not intended to enter in competition with CGM systems, but can be an alternative for some patients prone to hypoglycaemia. However, ECG seems more suitable for a daily use, being noninvasive and easier to wear and hide.
5.3. Other hypoglycaemia detection techniques or indirect blood glucose estimation
Beside EEG and ECG, other physiological signals that can be altered during a hypoglycaemic event have also been investigated.
Galvanic skin response (GSR) was studied extensively to develop alarms for night‐time hypoglycaemia since the 80s, taking into account alterations in skin temperature, perspiration and electrodermal activity at event onset. Two commercial devices were produced and tested in the 80s, ‘Diabalert©’ and ‘Sleep Sentry©’, but clinical studies showed a low accuracy and many false alarms. 145 , 146
Another available device on the market is ‘Diabetes Sentry©’ (Diabetes Sentry), 147 a wristband that measures the GSR and alerts patients in case of hypoglycaemia. The website states a rate of 90% alarm success, but no publication on this device was found to confirm these claims.
The ‘SenseWear Pro Armband©’ (Bodymedia) is a noninvasive multisensor that measures GSR, but also skin temperature, ECG and body motion. This device as mentioned previously has been used with CGM to increase prediction accuracy, 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 but it was also evaluated for glucose estimation. In a publication by Sobel et al. 148 the device was evaluated in 41 T1D and T2D patients. They underwent two tests to assess its accuracy in evaluating blood glucose level in dynamic conditions, during a glucose increase using an oral glucose tolerance test and during a glucose decrease during a treadmill test. During the study, reference blood glucose was measured every 5 min using an intravenous catheter, and a CGM was used for model training and for comparison. During the meal test, the device MARD was 26% versus 18% for the CGM, and for the exercise test the device MARD was 16% versus 12% for the CGM; and 95% of the readings were in Zone A + B in the CEG. However, both device and CGM were less accurate for low‐blood glucose readings, with only 20% hypoglycaemic values correctly estimated by the armband and 8.9% by the CGM.
This study, while confirming the limitations of CGM for the accurate measurement of high and low values and during rapid glucose variations, shows that this device measuring four separate physiological signals simultaneously can be almost as accurate as CGM. Nevertheless, these results need confirmation in larger trials and under real‐life conditions, to avoid the risks of overfitting of the learning algorithm than can occur in small datasets.
Other teams tried to detect blood glucose levels or hypoglycaemic events through volatile organic compounds (VOCs) present in breath, mainly acetone, methyl nitrate, ethanol, methanol 149 , 150 , 151 and isoprene, 152 that change with glucose variations. Shrestha et al. 153 collected breath samples from 52 PwD and analysed their composition, then trained a linear discriminant analysis to detect breath samples in hypoglycaemia and found seven main VOC that are significantly related to hypoglycaemic state. They succeeded in a classification of breath samples with a Se of 91% and Sp of 84%. In two other studies, an experimental device was presented and tested with artificial samples simulating VOC concentrations in real breath samples at different levels of blood glucose, and with different levels of acetone and ethanol. They obtained an accuracy of 97% for hypoglycaemia breath samples in their tests 154 and developed a prototype with the shape of a wristband. 155
Tronstad et al. 156 presented another prototype with ‘Prediktor Medical©’ from Norway, combining skin temperature, bioimpedance and NIR spectroscopy to predict glucose trends during hypoglycaemia. Their prototype was not reliable enough for low blood glucose threshold detection, and they concluded that a combination of other sensors with NIR could allow a noninvasive detection. Another publication by the same team 157 combined a sudomotor activity sensor, ECG (heart rate and QTc), NIR and bioimpedance spectroscopy. In this second publication, the aim was to detect hypoglycaemia using this combination of vital signs that are altered during low blood glucose events, and for classification purpose the threshold was defined at 4 mmol/L (72 mg/dl). The investigators succeeded in detecting almost all events with a Se of 95% and a Sp of 93%. In both trials, 20 T1D patients with impaired hypoglycaemia awareness were recruited, and data were collected during hypoglycaemic and euglycemic clamp studies.
Karunathilaka et al. 158 used also NIR spectroscopy to generate a noninvasive hypoglycaemia alarm, that was tested using an experimental setting that mimics glucose excursions in the human body, with an aqueous layer containing different levels of glucose and urea that can dynamically variate thanks to a pump and a tissue phantom composed of collagen and keratin to simulate human skin. This complex experimental setting allowed them to approach real life settings with blood glucose variations and different skin thicknesses and tissue orientations. They succeeded with this noninvasive technique with only 3.3% of false alarms in the first experimental procedure and a correct classification up to 93% in the second and more complex experimental procedure. Further testing with animal models is expected to complement these first results.
Even though we cannot categorise them as a technology or device, diabetes alert dogs were evaluated in some publications. 159 , 160 , 161 If investigational studies using perspiration samples show accuracy up to 90% for hypoglycaemia detection, real‐life studies show poorer efficacy. Two studies 159 , 160 with respectively 8 and 14 patients and their dogs using blinded CGM systems show a Se around 30% with a large variability between dogs (from 0% to 100%). Despite this lack in accuracy, these dogs help patients and their relatives in improving their quality of life and their confidence in managing their diabetes. 161
Some other fluids have been investigated for glucose monitoring, like tears, sweat, urine and saliva as their glucose concentrations seem to correlate with blood glucose concentrations, but due to higher lag‐times between these fluids and blood, they cannot be used for efficient hypoglycaemia detection or diabetes management.
The alternative techniques presented in this last chapter and resumed in appendix files used very different strategies, some being more realistic for ambulatory use than others. However, we must not forget that for an adoption by final users under real‐life conditions, such devices must achieve good accuracy, a continuous measure of vital signs to allow a real onset detection and not only point‐of‐care testing, and present a low burden for patients and affordable costs for payers.
6. DISCUSSION
In this review, we present many technologies for hypoglycaemia detection and prediction, with different levels of complexity and advancement that are summarised in Table 1.
TABLE 1.
Summary of main technologies for hypoglycaemia detection and prediction
| Next‐gen sensors | Prediction algorithms | EKG detection | EEG detection | NIR detection | Breath detection | Galvanic skin response detection | |
|---|---|---|---|---|---|---|---|
| Prevention level | ‐Detection at low threshold | ‐Prediction before reaching low threshold | ‐Detection at first symptoms | ‐Detection at first symptoms | ‐Detection at low threshold and at first symptoms | ‐Point of care test | ‐Detection at first symptoms |
| ‐Severe hypoprevention | ‐Severe hypoprevention | ‐Severe hypoprevention | ‐Severe hypoprevention | ||||
| Level of development | ‐In silico trials | ‐In silico trials for complex models | ‐Small cohort trials | ‐Small cohort trials | ‐In silico trials | ‐In silico trials | ‐Commercial devices existing |
| ‐Experimental sensors | ‐First algorithms available | ‐Existing wearable prototypes | ‐Existing wearable prototype | ‐Experimental prototypes | ‐Small cohort trials | ||
| ‐Experimental prototype | |||||||
| Remaining gaps | ‐Toxicity and biocompatibility trials | ‐Real‐life validation for multivariable algorithms | ‐Validation in patients with heart diseases and in large trials | ‐Validation in large trials for day and night | ‐Development of wearable NIR devices | ‐Development of a prototype for continuous use | ‐Accuracy improvements |
| ‐Industrialisation process | ‐Postmeal validation accuracy | ||||||
| PROs | ‐Better sensibility and accuracy | ‐Mid‐term and long‐term prediction | ‐Noninvasive measurement | ‐Good night‐time accuracy | ‐Noninvasive measurement | ‐Noninvasive measurement | ‐Noninvasive measurement |
| ‐MARD reduction | ‐Possibility to combine with closed‐loops | ‐Good accuracy | ‐Possibility to improve towards CGM | ||||
| ‐Possible reduction of invasiveness | |||||||
| CONs | ‐Invasive and expensive devices | ‐Risks of patients' overreactions | ‐Device lifetime of few days | ‐Invasive device | ‐Unknown CONs (early stage of development) | ‐Needs to breath towards the device in actual prototypes | ‐Low accuracy in patients with hypo unawareness |
| ‐Need to inform algorithm with data |
Abbreviations: CGM, continuous glucose monitoring; CON; MARD, mean absolute relative difference; NIR, near‐infrared; PRO.
Without any doubt, CGM will remain the first and main tool for hypoglycaemia detection. New enhancements in nanomaterials along with more complex and effective calibration algorithms will help the next generation of CGM systems to improve their accuracy, especially at low glucose levels.
Moreover, prediction algorithms provide a promising solution for PwD. Some algorithms are already implemented in commercial devices such as ‘G6©’ device (Dexcom) 51 and ‘Guardian Connect©’ (Medtronic) 52 and can reduce hypoglycaemic events by more than half. Connectivity of CGM systems, insulin pumps and activity wearables with smartphones gives the capacity to use multivariate algorithms and permits cloud‐based complex calculations. One of the major shared points between many reviewed prediction algorithms is that adding CHO intake, insulin doses or activity measurements helps increase the accuracy over a mid‐term or long‐term prediction horizon. Besides, combining different models may permit several levels of hypoglycaemia alarms, each one trained for a specific situation like meals, exercise or night‐time.
Unfortunately, we observed that most algorithms have been trained on small datasets or on clinically obtained datasets from highly motivated patients, which are far from real‐life settings where patients often do not routinely record events like CHO or exercise needed to inform these algorithms. Access of research teams to big datasets is needed for increasing the performance of these algorithms.
We can expect also the integration of these complex and multivariate algorithms in hybrid‐closed loop systems that are now entering the market.
Clinical assessment of the safety and efficacy of prediction algorithms is still in its infancy, with only a small number of papers in the last 2 years. Hence, it will be necessary to develop specific protocols to collect data on how patients use them in real‐life settings; so that clinical guidelines can be written and patients informed to avoid misuse and overreactions, as was observed in one publication. 50
People with T1D who require permanent monitoring of glucose levels are not the only ones exposed to hypoglycaemia. Other pathologies and some treatments can also induce hypoglycaemia. Alternative methods to CGM systems and CGM‐based prediction algorithms can be useful for patients who do not need an expensive system for real‐time and continuous glucose measurement.
GSR is already used in some marketed devices, and is still being investigated in other prototypes. Its combination with heart rate and other vital signs allows blood glucose estimation, albeit with a combined CGM‐use for the training phase. This technology that was explored since the eighties suffered from poor accuracy in early commercialised devices, but is still improving. It may become a good alternative to CGM systems for noninvasive hypoglycaemia detection.
One of the most promising methods, that seems near‐to‐market, is ECG‐based detection of hypoglycaemia. The most recent studies were performed with wearable noninvasive patches allowing real‐time ECG capture, and algorithms were tested in real‐life, during day‐ and night‐time, and showed good accuracy in hypoglycaemia detection. Some such devices are already on the market and are used for patients with heart diseases. Thus, we can expect companies that developed these devices may be able in the next years to add some extra features such as hypoglycaemia detection. But we need to point out that these devices only have a short duration of use, and consumable costs could make them expensive for long‐term continuous monitoring. Current smartwatches do not permit continuous ECG monitoring and need a manual input to start ECG measurement for a few seconds. The evolution of these smartwatches towards continuous monitoring would increase the acceptability of ECG‐based hypoglycaemia detection for patients.
The situation is the same for EEG detection technique with a similar level of advancement. However, current EEG‐based devices are more invasive than ECG‐based ones needing a small implant to measure brain activity.
In both cases, larger studies are needed for a final assessment of their performances and to evaluate the loss of accuracy in real‐life in patients affected by other metabolic disorders that can disturb the ECG‐ or EEG–based detection algorithms.
Finally, other techniques have been considered in this review. Breath‐based detection is one of them, previously known through diabetes alert dogs. A prototype device was developed for point‐of‐care testing with a good accuracy, but it seems difficult to imagine how such a technique and devices based on it could be used in real‐life for continuous evaluation. NIR spectroscopy, alone or combined with other techniques, has also been used to generate hypoglycaemia alarms. It also stands amongst the candidates for noninvasive glucose monitoring, which could become in the future a good alternative.
Some other fluids like tears, sweat, urine or saliva are other milieus usable for blood glucose estimation, using the fluids' glucose correlation with blood glucose variations. However, the large lag‐time between glucose variations in these fluids and in blood makes them less suitable candidates for hypoglycaemia detection. Thus, they are not detailed in this review. For further reading on these alternative modes of noninvasive and minimally invasive glucose monitoring, we recommend some recent reviews. 30 , 31
However, these technologies for the detection of hypoglycaemia cannot be proposed alone to patients, but have to be accompanied with a global strategy and plan of action to unveil their full potential in clinical practice. A recent report 162 highlighted the utmost importance of therapeutic education programs to help patients recognise hypoglycaemia events, know the effects of their medications and activities, and how to appropriately correct a detected hypoglycaemia. Medications may also have to be adapted in some cases depending on the type of diabetes, for physical activities and for specific situations like religious fasting. This education and medication management is essential to reduce hypoglycaemic risks, before implementing the appropriate tool or device that will assist the patient.
7. CONCLUSION
This extensive review presented a broad panel of devices and technologies related to hypoglycaemia detection and prediction techniques. PwD will almost certainly benefit from the next generation of CGM devices that will likely embed mid‐term or long‐term prediction features along with better accuracy and Se to glucose variations.
We believe other techniques and devices will struggle to compete with CGM devices in the near future as they are limited to event detection, and cannot inform PwD about their actual glucose levels. As such, they require reduced costs and acceptability related to noninvasive use to be successful.
CONFLICT OF INTERESTS
Omar Diouri, Monika Cigler and Martina Vettoretti reports no conflict of interests. Julia K. Mader is co‐founder and shareholder of decide Clinical Software GmbH. Julia K. Mader is a member in the advisory board of Boehringer Ingelheim, Eli Lilly, Medtronic, Prediktor A/S, Roche Diabetes Care, Sanofi‐Aventis and received speaker honoraria from Abbott Diabetes Care, Astra Zeneca, Dexcom, Eli Lilly, MSD, NovoNordisk A/S, Roche Diabetes Care, Sanofi, Servier and Takeda. Eric Renard reports consulting services to Abbott, Air Liquide SI, Cellnovo, Dexcom, Eli‐Lilly, Insulet, Johnson & Johnson (Animas, LifeScan), Medirio, Medtronic, Novo‐Nordisk, Roche and Sanofi and research support from Abbott, Dexcom, Insulet, Roche et Tandem. Pratik Choudhary has received speaker and consultancy fees from Novo Nordisk, Sanofi, Lilly, Insulet, Medtronic, Abbott, Diabits, Novartis and research support from Medtronic, Abbottm, Novo Nordisk and Dexcom.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design of this review, and to the critical appraisal of content, and have read and approved the final version.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Leandro Pecchia for providing electrocardiogram figure. This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under Grant Agreement No. 777460. The JU receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA and T1D Exchange, JDRF, International Diabetes Federation, The Leona M. and Harry B. Helmsley Charitable Trust. IMI website: htps://www.imi.europa.eu. This review was invited for publication in ‘Diabetes Metabolism Research and Review’ by chief editor Pr Paolo Pozzilli.
Diouri O, Cigler M, Vettoretti M, Mader JK, Choudhary P, Renard E. Hypoglycaemia detection and prediction techniques: a systematic review on the latest developments. Diabetes Metab Res Rev. 2021;37(7):e3449. doi: 10.1002/dmrr.3449
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a Workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98(5):1845‐1859. 10.1210/jc.2012-4127. [DOI] [PubMed] [Google Scholar]
- 2. Gonder‐Frederick LA, Vajda KA, Schmidt KM, et al. Examining the behaviour subscale of the hypoglycaemia fear survey: an international study. Diabet Med. 2013;30(5):603‐609. 10.1111/dme.12129. [DOI] [PubMed] [Google Scholar]
- 3. Bode BW, Schwartz S, Stubbs HA, Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care. 2005;28(10):2361‐2366. 10.2337/diacare.28.10.2361. [DOI] [PubMed] [Google Scholar]
- 4. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dl) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2017;40(1):155‐157. 10.2337/dc16-2215. [DOI] [PubMed] [Google Scholar]
- 5. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631‐1640. 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zammitt NN, Streftaris G, Gibson GJ, Deary IJ, Frier BM. Modeling the consistency of hypoglycemic symptoms: high variability in diabetes. Diabetes Technol Ther. 2011;13(5):571‐578. 10.1089/dia.2010.0207. [DOI] [PubMed] [Google Scholar]
- 7. Boyle PJ, Schwartz NS, Shah SD, Clutter WE, Cryer PE. Plasma glucose concentrations at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in nondiabetics. N Engl J Med. 1988;318(23):1487‐1492. 10.1056/NEJM198806093182302. [DOI] [PubMed] [Google Scholar]
- 8. Lane JE, Shivers JP, Zisser H. Continuous glucose monitors. Curr Opin Endocrinol Diabetes Obes. 2013;20(2):106‐111. 10.1097/MED.0b013e32835edb9d. [DOI] [PubMed] [Google Scholar]
- 9. Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev. 2008;108(7):2482‐2505. 10.1021/cr068069y. [DOI] [PubMed] [Google Scholar]
- 10. Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J. 2019;43(4):383‐397. 10.4093/dmj.2019.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodbard D. Characterizing accuracy and precision of glucose sensors and meters. J Diabetes Sci Technol. 2014;8:980. 10.1177/1932296814541810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zijlstra E, Heise T, Nosek L, Heinemann L, Heckermann S. Continuous glucose monitoring: quality of hypoglycaemia detection. Diabetes Obes Metabol. 2013;15(2):130‐135. 10.1111/dom.12001. [DOI] [PubMed] [Google Scholar]
- 13. Balakrishnan SR, Hashim U, Letchumanan GR, et al. Development of highly sensitive polysilicon nanogap with APTES/GOx based lab‐on‐chip biosensor to determine low levels of salivary glucose. Sens Actuator A Phys. 2014;220:101‐111. 10.1016/j.sna.2014.09.027. [DOI] [Google Scholar]
- 14. Ibupoto ZH, Khun K, Lu J, Willander M. The synthesis of CuO nanoleaves, structural characterization, and their glucose sensing application. Appl Phys Lett. 2013;102(10):103701. 10.1063/1.4795135. [DOI] [Google Scholar]
- 15. Patil D, Dung NQ, Jung H, Ahn SY, Jang DM, Kim D. Enzymatic glucose biosensor based on CeO2 nanorods synthesized by non‐isothermal precipitation. Biosens Bioelectron. 2012;31(1):176‐181. 10.1016/j.bios.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y‐T, Yu L, Zhu Z‐Q, Zhang J, Zhu J‐Z, Fan C‐h. Improved enzyme immobilization for enhanced bioelectrocatalytic activity of glucose sensor. Sens Actuator B Chem. 2009;136(2):332‐337. 10.1016/j.snb.2008.12.049. [DOI] [Google Scholar]
- 17. Hoo XF, Abdul Razak K, Ridhuan NS, Mohamad Nor N, Zakaria ND. Electrochemical glucose biosensor based on ZnO nanorods modified with gold nanoparticles. J Mater Sci Mater Electron. 2019;30(8):7460‐7470. 10.1007/s10854-019-01059-9. [DOI] [Google Scholar]
- 18. Shan C, Yang H, Song J, Han D, Ivaska A, Niu L. Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal Chem. 2009;81(6):2378‐2382. 10.1021/ac802193c. [DOI] [PubMed] [Google Scholar]
- 19. Unnikrishnan B, Palanisamy S, Chen S‐M. A simple electrochemical approach to fabricate a glucose biosensor based on graphene‐glucose oxidase biocomposite. Biosens Bioelectron. 2013;39(1):70‐75. 10.1016/j.bios.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 20. Taguchi M, Ptitsyn A, McLamore ES, Claussen JC. Nanomaterial‐mediated biosensors for monitoring glucose. J Diabetes Sci Technol. 2014;8:403. 10.1177/1932296814522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noah NM, Ndangili PM. Current trends of nanobiosensors for point‐of‐care diagnostics. J Anal Methods Chem. 2019:2179718. 10.1155/2019/2179718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaidi SA, Shin JH. Recent developments in nanostructure based electrochemical glucose sensors. Talanta. 2016;149:30‐42. 10.1016/j.talanta.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 23. Hwang D‐W, Lee S, Seo M, Chung TD. Recent advances in electrochemical non‐enzymatic glucose sensors—a review. Anal Chim Acta. 2018;1033:1‐34. 10.1016/j.aca.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 24. Ahmad R, Vaseem M, Tripathy N, Hahn Y‐B. Wide linear‐range detecting nonenzymatic glucose biosensor based on CuO nanoparticles inkjet‐printed on electrodes. Anal Chem. 2013;85(21):10448‐10454. 10.1021/ac402925r. [DOI] [PubMed] [Google Scholar]
- 25. Zhang H, Xu X, Yin Y, Wu P, Cai C. Nonenzymatic electrochemical detection of glucose based on Pd1Pt3‐graphene nanomaterials. J Electroanal Chem. 2013;690:19‐24. 10.1016/j.jelechem.2012.12.001. [DOI] [Google Scholar]
- 26. Subramanian P, Niedziolka‐Jonsson J, Lesniewski A, et al. Preparation of reduced graphene oxide‐Ni(OH)2 composites by electrophoretic deposition: application for non‐enzymatic glucose sensing. J Mater Chem. 2014;2(15):5525‐5533. 10.1039/C4TA00123K. [DOI] [Google Scholar]
- 27. Gutés A, Carraro C, Maboudian R. Nonenzymatic glucose sensing based on deposited palladium nanoparticles on epoxy‐silver electrodes. Electrochim Acta. 2011;56(17):5855‐5859. 10.1016/j.electacta.2011.04.128. [DOI] [Google Scholar]
- 28. Avari P, Reddy M, Oliver N. Is it possible to constantly and accurately monitor blood sugar levels, in people with type 1 diabetes, with a discrete device (non‐invasive or invasive)? Diabet Med, 2020;37(4):532‐544. 10.1111/dme.13942. [DOI] [PubMed] [Google Scholar]
- 29. Villena Gonzales W, Mobashsher A, Abbosh A. The progress of glucose monitoring‐a review of invasive to minimally and non‐invasive techniques, devices and sensors. Sensors. 2019;19(4):800. 10.3390/s19040800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hadar E, Chen R, Toledano Y, Tenenbaum‐Gavish K, Atzmon Y, Hod M. Noninvasive, continuous, real‐time glucose measurements compared to reference laboratory venous plasma glucose values. J Matern Fetal Neonatal Med. 2019;32(20):3393‐3400. 10.1080/14767058.2018.1463987. [DOI] [PubMed] [Google Scholar]
- 31. Prediktor Medical | BioMKR: Non Invasive CGM, a glucose smartwatch. prediktormedical . 2020. https://www.prediktormedical.com. [Google Scholar]
- 32. DiaMonTech . Non‐invasive glucose measurement. 2020. https://www.diamontech.de/home. [Google Scholar]
- 33. PKvitality ‐ Bio‐wearables Health & Sport Page d'accueil. PKVitality . 2020. https://www.pkvitality.com/fr/.
- 34. Nemaura Medical. Nemaura Medical . 2020. https://nemauramedical.com/.
- 35. Roe JN, Smoller BR. Bloodless glucose measurements. Crit Rev Ther Drug Carr Syst. 1998;15(3):199‐241. [PubMed] [Google Scholar]
- 36. Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol Endocrinol Metabol. 1999;277(3):E561‐E571. 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- 37. Aussedat B, Dupire‐Angel M, Gifford R, Klein JC, Wilson GS, Reach G. Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metabol. 2000;278(4):E716‐E728. 10.1152/ajpendo.2000.278.4.E716. [DOI] [PubMed] [Google Scholar]
- 38. Rossetti P, Bondia J, Vehí J, Fanelli CG. Estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors. 2010;10(12):10936‐10952. 10.3390/s101210936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bequette BW. Continuous glucose monitoring: real‐time algorithms for calibration, filtering, and alarms. J Diabetes Sci Technol. 2010;4(2):404‐418. 10.1177/193229681000400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahmoudi Z, Dencker Johansen M, Christiansen JS, Hejlesen OK. A multistep algorithm for processing and calibration of microdialysis continuous glucose monitoring data. Diabetes Technol Ther. 2013;15(10):825‐835. 10.1089/dia.2013.0041. [DOI] [PubMed] [Google Scholar]
- 41. Mahmoudi Z, Johansen MD, Christiansen JS, Hejlesen O. Comparison between one‐point calibration and two‐point calibration approaches in a continuous glucose monitoring algorithm. J Diabetes Sci Technol. 2014;8(4):709‐719. 10.1177/1932296814531356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahmoudi Z, Jensen MH, Dencker Johansen M, et al. Accuracy evaluation of a new real‐time continuous glucose monitoring algorithm in hypoglycemia. Diabetes Technol Ther. 2014;16(10):667‐678. 10.1089/dia.2014.0043. [DOI] [PubMed] [Google Scholar]
- 43. Guerra S, Facchinetti A, Sparacino G, De Nicolao G, Cobelli C. Enhancing the accuracy of subcutaneous glucose sensors: a real‐time deconvolution‐based approach. IEEE Trans Biomed Eng. 2012;59(6):1658‐1669. 10.1109/TBME.2012.2191782. [DOI] [PubMed] [Google Scholar]
- 44. Vettoretti M, Facchinetti A, Del Favero S, Sparacino G, Cobelli C. Online calibration of glucose sensors from the measured current by a time‐varying calibration function and Bayesian priors. IEEE Trans Biomed Eng. 2016;63(8):1631‐1641. 10.1109/TBME.2015.2426217. [DOI] [PubMed] [Google Scholar]
- 45. Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G, Cobelli C. From two to one per day calibration of Dexcom G4 platinum by a time‐varying day‐specific bayesian prior. Diabetes Technol Ther. 2016;18(8):472‐479. 10.1089/dia.2016.0088. [DOI] [PubMed] [Google Scholar]
- 46. Acciaroli G, Vettoretti M, Facchinetti A, Sparacino G, Cobelli C. Reduction of blood glucose measurements to calibrate subcutaneous glucose sensors: a Bayesian multiday framework. IEEE Trans Biomed Eng. 2018;65(3):587‐595. 10.1109/TBME.2017.2706974. [DOI] [PubMed] [Google Scholar]
- 47. Lee JB, Dassau E, Doyle FJ. A run‐to‐run approach to enhance continuous glucose monitor accuracy based on continuous wear. IFAC‐PapersOnLine. 2015;48(20):237‐242. 10.1016/j.ifacol.2015.10.145. [DOI] [Google Scholar]
- 48. Zavitsanou S, Lee JB, Pinsker JE, et al. A personalized week‐to‐week updating algorithm to improve continuous glucose monitoring performance. J Diabetes Sci Technol. 2017;11, 1070. 10.1177/1932296817734367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zecchin C, Facchinetti A, Sparacino G, Cobelli C. Reduction of number and duration of hypoglycemic events by glucose prediction methods: a proof‐of‐concept in silico study. Diabetes Technol Ther. 2013;15(1):66‐77. 10.1089/dia.2012.0208. [DOI] [PubMed] [Google Scholar]
- 50. Pérez‐Gandía C, García‐Sáez G, Subías D, et al. Decision support in diabetes care: the challenge of supporting patients in their daily living using a mobile glucose predictor. J Diabetes Sci Technol. 2018;12(2):243‐250. 10.1177/1932296818761457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Puhr S, Derdzinski M, Welsh JB, Parker AS, Walker T, Price DA. Real‐world hypoglycemia avoidance with a continuous glucose monitoring system's predictive low glucose alert. Diabetes Technol Ther. 2019;21(4):155‐158. 10.1089/dia.2018.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abraham SB, Arunachalam S, Zhong A, Agrawal P, Cohen O, McMahon CM. Improved real‐world glycemic control with continuous glucose monitoring system predictive alerts. J Diabetes Sci Technol. 2019;15:91. 10.1177/1932296819859334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mosquera‐Lopez C, Dodier R, Tyler N, Resalat N, Jacobs P. Leveraging a big dataset to develop a recurrent neural network to predict adverse glycemic events in type 1 diabetes. IEEE J Biomed Health Inform. 2019. 10.1109/JBHI.2019.2911701. [DOI] [PubMed] [Google Scholar]
- 54. Li N, Tuo J, Wang Y, Wang M. Prediction of blood glucose concentration for type 1 diabetes based on echo state networks embedded with incremental learning. Neurocomputing. 2020;378:248. 10.1016/j.neucom.2019.10.003. [DOI] [Google Scholar]
- 55. Li N, Tuo J, Wang Y. Chaotic time series analysis approach for prediction blood glucose concentration based on echo state networks: 2018 Chinese Control And Decision Conference (CCDC), Shenyang, China, 2018:2017‐2022. 10.1109/CCDC.2018.8407457. [DOI] [Google Scholar]
- 56. Yang J, Li L, Shi Y, Xie X. An ARIMA model with adaptive orders for predicting blood glucose concentrations and hypoglycemia. IEEE J Biomed Health Inform. 2019,23:1251. 10.1109/JBHI.2018.2840690. [DOI] [PubMed] [Google Scholar]
- 57. Gadaleta M, Facchinetti A, Grisan E, Rossi M. Prediction of adverse glycemic events from continuous glucose monitoring signal. IEEE J Biomed Health Inform. 2019;23(2):650‐659. 10.1109/JBHI.2018.2823763. [DOI] [PubMed] [Google Scholar]
- 58. Bidet S, Caleca N, Renard E, et al. First assessment of the performance of a personalized machine learning approach to predicting blood glucose levels in patients with type 1 diabetes: the CDDIAB study. Diabetes Technol Therap; 2019;61(S1):A75. [Google Scholar]
- 59. Hamdi T, Ben Ali J, Di Costanzo V, Fnaiech F, Moreau E, Ginoux J‐M. Accurate prediction of continuous blood glucose based on support vector regression and differential evolution algorithm. Biocybern Biomed Eng. 2018;38(2):362‐372. 10.1016/j.bbe.2018.02.005. [DOI] [Google Scholar]
- 60. Ben Ali J, Hamdi T, Fnaiech N, Di Costanzo V, Fnaiech F, Ginoux J‐M. Continuous blood glucose level prediction of type 1 diabetes based on artificial neural network. Biocybern Biomed Eng. 2018;38(4):828‐840. 10.1016/j.bbe.2018.06.005. [DOI] [Google Scholar]
- 61. Hamdi T, Ali JB, Fnaiech N, et al. Artificial neural network for blood glucose level prediction: 2017 International Conference on Smart, Monitored and Controlled Cities (SM2C), Sfax, Tunisia, 2017:91‐95. 10.1109/SM2C.2017.8071825. [DOI] [Google Scholar]
- 62. Sun Q, Jankovic MV, Bally L, Mougiakakou SG. Predicting blood glucose with an LSTM and Bi‐LSTM based deep neural network. In: 2018 14th Symposium on Neural Networks and Applications (NEUREL), Belgrade, Serbia, 2018:1‐5. 10.1109/NEUREL.2018.8586990. [DOI] [Google Scholar]
- 63. Mhaskar HN, Pereverzyev SV, van der Walt MD. A deep learning approach to diabetic blood glucose prediction. Front Appl Math Stat. 2017;3:14–25. 10.3389/fams.2017.00014. [DOI] [Google Scholar]
- 64. Hidalgo JI, Colmenar JM, Kronberger G, Winkler SM, Garnica O, Lanchares J. Data based prediction of blood glucose concentrations using evolutionary methods. J Med Syst. 2017;41(9):142. 10.1007/s10916-017-0788-2. [DOI] [PubMed] [Google Scholar]
- 65. Gyuk P, Vassányi I, Kósa I. Blood glucose level prediction with improved parameter identification methods: 2017 IEEE 30th Neumann Colloquium (NC), Budapest, 2017:000085‐000088. 10.1109/NC.2017.8263257. [DOI] [Google Scholar]
- 66. Fiorini S, Martini C, Malpassi D, et al. Data‐driven strategies for robust forecast of continuous glucose monitoring time‐series: 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Budapest, 2017:1680‐1683. 10.1109/EMBC.2017.8037164. [DOI] [PubMed] [Google Scholar]
- 67. Zhu T, Li K, Herrero P, Chen J, Georgiou P. A deep learning algorithm for personalized blood glucose prediction KHD@IJCAI; 2018:64‐78. [Google Scholar]
- 68. Chen J, Li K, Herrero P, Zhu T, Georgiou P. Dilated recurrent neural network for short‐time prediction of glucose concentration KHD@IJCAI; 2018:69‐73. [Google Scholar]
- 69. Zecchin C, Facchinetti A, Sparacino G, Cobelli C. How much is short‐term glucose prediction in type 1 diabetes improved by adding insulin delivery and meal content information to CGM data? A proof‐of‐concept study. J Diabetes Sci Technol. 2016;10(5):1149‐1160. 10.1177/1932296816654161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mirela F, Bogdan T, Diana L. A risk based neural network approach for predictive modeling of blood glucose dynamics. Stud Health Technol Inf. 2016;228:577‐581. 10.3233/978-1-61499-678-1-577. [DOI] [PubMed] [Google Scholar]
- 71. Jankovic MV, Mosimann S, Bally L, Stettler C, Mougiakakou S. Deep prediction model: the case of online adaptive prediction of subcutaneous glucose. In: 2016 13th Symposium on Neural Networks and Applications (NEUREL), Belgrade, Serbia, 2016:1‐5. 10.1109/NEUREL.2016.7800095. [DOI] [Google Scholar]
- 72. Contreras I, Vehi J. Mid‐term prediction of blood glucose from continuous glucose sensors, meal information and administered insulin. In: Kyriacou E, Christofides S, Pattichis CS, eds. XIV Mediterranean Conference on Medical and Biological Engineering and Computing 2016. IFMBE Proceedings. Cham: Springer International Publishing; 2016:1137‐1143. 10.1007/978-3-319-32703-7_222. [DOI] [Google Scholar]
- 73. Ahmed HB, Serener A. Effects of external factors in CGM sensor glucose concentration prediction. Procedia Comput Sci. 2016;102:623‐629. 10.1016/j.procs.2016.09.452. [DOI] [Google Scholar]
- 74. Zecchin C, Facchinetti A, Sparacino G, Cobelli C. Jump neural network for real‐time prediction of glucose concentration. In: Cartwright H, ed. Artificial Neural Networks. Methods in Molecular Biology. New York, NY: Springer New York; 2015:245‐259. 10.1007/978-1-4939-2239-0_15. [DOI] [PubMed] [Google Scholar]
- 75. Georga EI, Protopappas VC, Polyzos D, Fotiadis DI. Evaluation of short‐term predictors of glucose concentration in type 1 diabetes combining feature ranking with regression models. Med Biol Eng Comput. 2015;53(12):1305‐1318. 10.1007/s11517-015-1263-1. [DOI] [PubMed] [Google Scholar]
- 76. Georga EI, Protopappas VC, Polyzos D, Fotiadis DI. Online prediction of glucose concentration in type 1 diabetes using extreme learning machines: 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 2015:3262‐3265. 10.1109/EMBC.2015.7319088. [DOI] [PubMed] [Google Scholar]
- 77. Plis K, Bunescu R, Marling C, Shubrook J, Schwartz F. A machine learning approach to predicting blood glucose levels for diabetes management: Workshops at the Twenty‐Eighth AAAI Conference on Artificial Intelligence; 2014. https://www.aaai.org/ocs/index.php/WS/AAAIW14/paper/view/8737. [Google Scholar]
- 78. Botwey RH, Daskalaki E, Diem P, Mougiakakou SG. Multi‐model data fusion to improve an early warning system for hypo‐/hyperglycemic events: 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, 2014:4843‐4846. 10.1109/EMBC.2014.6944708. [DOI] [PubMed] [Google Scholar]
- 79. Hidalgo JI, Colmenar JM, Risco‐Martin JL, et al. Modeling glycemia in humans by means of Grammatical Evolution. Appl Soft Comput. 2014;20:40‐53. 10.1016/j.asoc.2013.11.006. [DOI] [Google Scholar]
- 80. Zecchin C, Facchinetti A, Sparacino G, Cobelli C. Jump neural network for online short‐time prediction of blood glucose from continuous monitoring sensors and meal information. Comput Methods Programs Biomed. 2014;113(1):144‐152. 10.1016/j.cmpb.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 81. Daskalaki E, Nørgaard K, Züger T, Prountzou A, Diem P, Mougiakakou S. An early warning system for hypoglycemic/hyperglycemic events based on fusion of adaptive prediction models. J Diabetes Sci Technol. 2013;7(3):689‐698. 10.1177/193229681300700314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zarkogianni K, Litsa E, Vazeou A, Nikita KS. Personalized glucose‐insulin metabolism model based on self‐organizing maps for patients with type 1 diabetes mellitus: 13th IEEE International Conference on BioInformatics and BioEngineering, Chania, Greece, 2013:1‐4. 10.1109/BIBE.2013.6701604. [DOI] [Google Scholar]
- 83. Wang Y, Wu X, Mo X. A novel adaptive‐weighted‐average framework for blood glucose prediction. Diabetes Technol Ther. 2013;15(10):792‐801. 10.1089/dia.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Rollins D, Cinar A. Hypoglycemia early alarm systems based on multivariable models. Ind Eng Chem Res. 2013;52(35):12329. 10.1021/ie3034015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Georga EI, Protopappas VC, Ardigò D, et al. Multivariate prediction of subcutaneous glucose concentration in type 1 diabetes patients based on support vector regression. IEEE J Biomed Health Inform. 2013;17(1):71‐81. 10.1109/TITB.2012.2219876. [DOI] [PubMed] [Google Scholar]
- 86. Bunescu R, Struble N, Marling C, Shubrook J, Schwartz F. Blood glucose level prediction using physiological models and support vector regression: 2013 12th International Conference on Machine Learning and Applications, Miami, FL, 2013:135‐140. 10.1109/ICMLA.2013.30. [DOI] [Google Scholar]
- 87. Vehí J, Contreras I, Oviedo S, Biagi L, Bertachi A. Prediction and prevention of hypoglycaemic events in type‐1 diabetic patients using machine learning. Health Inf J. 2019;26:703. 10.1177/1460458219850682. [DOI] [PubMed] [Google Scholar]
- 88. Cichosz SL, Frystyk J, Tarnow L, Fleischer J. Combining information of autonomic modulation and CGM measurements enables prediction and improves detection of spontaneous hypoglycemic events. J Diabetes Sci Technol. 2015;9(1):132‐137. 10.1177/1932296814549830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cichosz SL, Frystyk J, Hejlesen OK, Tarnow L, Fleischer J. A novel algorithm for prediction and detection of hypoglycemia based on continuous glucose monitoring and heart rate variability in patients with type 1 diabetes. J Diabetes Sci Technol. 2014;8(4):731‐737. 10.1177/1932296814528838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Eljil KS, Qadah G, Pasquier M. Predicting hypoglycemia in diabetic patients using data mining techniques: 2013 9th International Conference on Innovations in Information Technology (IIT), Al Ain, United Arab Emirates, 2013:130‐135. 10.1109/Innovations.2013.6544406. [DOI] [Google Scholar]
- 91. Oviedo S, Contreras I, Quirós C, Giménez M, Conget I, Vehi J. Risk‐based postprandial hypoglycemia forecasting using supervised learning. Int J Med Inf. 2019;126:1‐8. 10.1016/j.ijmedinf.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 92. Seo W, Lee Y‐B, Lee S, Jin S‐M, Park S‐M. A machine‐learning approach to predict postprandial hypoglycemia. BMC Med Inf Decis Mak. 2019;19(1):210. 10.1186/s12911-019-0943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Reddy R, Resalat N, Wilson LM, Castle JR, El Youssef J, Jacobs PG. Prediction of hypoglycemia during aerobic exercise in adults with type 1 diabetes. J Diabetes Sci Technol. 2019;13:919. 10.1177/1932296818823792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hobbs N, Hajizadeh I, Rashid M, Turksoy K, Breton M, Cinar A. Improving glucose prediction accuracy in physically active adolescents with type 1 diabetes. J Diabetes Sci Technol. 2019;13(4):718‐727. 10.1177/1932296818820550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jensen MH, Dethlefsen C, Vestergaard P, Hejlesen O. Prediction of nocturnal hypoglycemia from continuous glucose monitoring data in people with type 1 diabetes: a proof‐of‐concept study. J Diabetes Sci Technol. 2019;14:250. 10.1177/1932296819868727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kriukova G, Shvai N, Pereverzyev SV.Application of regularized ranking and collaborative filtering in predictive alarm algorithm for nocturnal hypoglycemia prevention. In: 2017 9th IEEE International Conference on Intelligent Data Acquisition and Advanced Computing Systems: Technology and Applications (IDAACS), Bucharest, Romania, 2017:634‐638. 10.1109/IDAACS.2017.8095169 [DOI] [Google Scholar]
- 97. Contreras I, Oviedo S, Vettoretti M, Visentin R, Vehí J. Personalized blood glucose prediction: a hybrid approach using grammatical evolution and physiological models. PLoS ONE. 2017;12(11):e0187754. 10.1371/journal.pone.0187754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tkachenko P, Kriukova G, Aleksandrova M, Chertov O, Renard E, Pereverzyev SV. Prediction of nocturnal hypoglycemia by an aggregation of previously known prediction approaches: proof of concept for clinical application. Comput Methods Programs Biomed. 2016;134:179‐186. 10.1016/j.cmpb.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 99. Zarkogianni K, Mitsis K, Litsa E, et al. Comparative assessment of glucose prediction models for patients with type 1 diabetes mellitus applying sensors for glucose and physical activity monitoring. Med Biol Eng Comput. 2015;53(12):1333‐1343. 10.1007/s11517-015-1320-9. [DOI] [PubMed] [Google Scholar]
- 100. Zarkogianni K, Mitsis K, Arredondo M‐T, Fico G, Fioravanti A, Nikita KS. Neuro‐fuzzy based glucose prediction model for patients with type 1 diabetes mellitus: IEEE‐EMBS International Conference on Biomedical and Health Informatics (BHI), Valencia, Spain, 2014:252‐255. 10.1109/BHI.2014.6864351. [DOI] [Google Scholar]
- 101. Wilinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type‐1 diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng. 2005;52(1):3‐12. 10.1109/TBME.2004.839639. [DOI] [PubMed] [Google Scholar]
- 102. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol Endocrinol Metabol. 1979;236(6):E667. 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 103. Tarin C, Teufel E, Pico J, Bondia J, Pfleiderer H‐J. Comprehensive pharmacokinetic model of insulin Glargine and other insulin formulations. IEEE Trans Biomed Eng. 2005;52(12):1994‐2005. 10.1109/TBME.2005.857681. [DOI] [PubMed] [Google Scholar]
- 104. Duke DL. Intelligent Diabetes Assistant: A Telemedicine System for Modeling and Managing Blood Glucose. The Robotics Institute Carnegie Mellon University. 2020. https://www.ri.cmu.edu/publications/intelligent‐diabetes‐assistant‐telemedicine‐system‐modeling‐managing‐blood‐glucose/. [Google Scholar]
- 105. Palumbo P, Pepe P, Panunzi S, De Gaetano A. Glucose control by subcutaneous insulin administration: a DDE modelling approach. IFAC Proc Vol. 2011;44(1):1471‐1476. 10.3182/20110828-6-IT-1002.01374. [DOI] [Google Scholar]
- 106. Hovorka R, Canonico V, Chassin LJ, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905‐920. 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 107. Man CD, Camilleri M, Cobelli C. A system model of oral glucose absorption: validation on gold standard data. IEEE Trans Biomed Eng. 2006;53(12 Pt 1):2472‐2478. 10.1109/TBME.2006.883792. [DOI] [PubMed] [Google Scholar]
- 108. Vecoven N, Ernst D, Wehenkel A, Drion G. Introducing neuromodulation in deep neural networks to learn adaptive behaviours. PLOS ONE. 2020;15(1):e0227922. 10.1371/journal.pone.0227922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Facchinetti A, Sparacino G, Trifoglio E, Cobelli C. A new index to optimally design and compare continuous glucose monitoring glucose prediction algorithms. Diabetes Technol Ther. 2011;13(2):111‐119. 10.1089/dia.2010.0151. [DOI] [PubMed] [Google Scholar]
- 110. Clarke WL, Cox D, Gonder‐Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self‐monitoring of blood glucose. Diabetes Care. 1987;10(5):622‐628. 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 111. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143‐1148. 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 112. Clarke W, Anderson S, Kovatchev B. Evaluating clinical accuracy of continuous glucose monitoring systems: continuous glucose‐error grid analysis (CG‐EGA). Curr Diabetes Rev. 2008;4(3):193‐199. 10.2174/157339908785294389. [DOI] [PubMed] [Google Scholar]
- 113. Sivananthan S, Naumova V, Man CD, et al. Assessment of blood glucose predictors: the prediction‐error grid analysis. Diabetes Technol Ther. 2011;13(8):787‐796. 10.1089/dia.2011.0033. [DOI] [PubMed] [Google Scholar]
- 114. Meinhold J, Heise T, Rave K, Heinemann L. Electrocardiographic changes during insulin‐induced hypoglycemia in healthy subjects. Horm Metab Res. 1998;30(11):694‐697. 10.1055/s-2007-978960. [DOI] [PubMed] [Google Scholar]
- 115. Christensen TF, Tarnow L, Randløv J, et al. QT interval prolongation during spontaneous episodes of hypoglycaemia in type 1 diabetes: the impact of heart rate correction. Diabetologia. 2010;53(9):2036‐2041. 10.1007/s00125-010-1802-0. [DOI] [PubMed] [Google Scholar]
- 116. Robinson RTCE, Harris ND, Ireland RH, Macdonald IA, Heller SR. Changes in cardiac repolarization during clinical episodes of nocturnal hypoglycaemia in adults with type 1 diabetes. Diabetologia. 2004;47(2):312‐315. 10.1007/s00125-003-1292-4. [DOI] [PubMed] [Google Scholar]
- 117. Laitinen T, Lyyra‐Laitinen T, Huopio H, et al. Electrocardiographic alterations during hyperinsulinemic hypoglycemia in healthy subjects. Ann Noninvasive Electrocardiol. 2008;13(2):97‐105. 10.1111/j.1542-474X.2008.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Marques JLB, George E, Peacey SR, et al. Altered ventricular repolarization during hypoglycaemia in patients with diabetes. Diabet Med. 1997;14(8):648‐654. . [DOI] [PubMed] [Google Scholar]
- 119. San PP, Ling SH, Nguyen HT. Combinational neural logic system and its industrial application on hypoglycemia monitoring system: 2013 IEEE 8th Conference on Industrial Electronics and Applications (ICIEA), Melbourne, VIC, Australia, 2013:947‐952. 10.1109/ICIEA.2013.6566503. [DOI] [Google Scholar]
- 120. San PP, Ling SH, Nguyen HT. Industrial application of evolvable block‐based neural network to hypoglycemia monitoring system. IEEE Trans Ind Electron. 2013;60(12):5892‐5901. 10.1109/TIE.2012.2228143. [DOI] [Google Scholar]
- 121. Ling SH, San PP, Lam HK, Nguyen HT. Non‐invasive detection of hypoglycemic episodes in Type 1 diabetes using intelligent hybrid rough neural system: 2014 IEEE Congress on Evolutionary Computation (CEC), Beijing, China, 2014:1238‐1242. 10.1109/CEC.2014.6900229. [DOI] [Google Scholar]
- 122. Nguyen LL, Su S, Nguyen HT. Neural network approach for non‐invasive detection of hyperglycemia using electrocardiographic signals: 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, 2014:4475‐4478. 10.1109/EMBC.2014.6944617. [DOI] [PubMed] [Google Scholar]
- 123. San PP, Ling SH, Nuryani, Nguyen H Evolvable rough‐block‐based neural network and its biomedical application to hypoglycemia detection system. IEEE Trans Cybern. 2014;44(8):1338‐1349. 10.1109/TCYB.2013.2283296. [DOI] [PubMed] [Google Scholar]
- 124. San PP, Ling SH, Soe NN, Nguyen HT. A novel extreme learning machine for hypoglycemia detection: 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, 2014:302‐305. 10.1109/EMBC.2014.6943589. [DOI] [PubMed] [Google Scholar]
- 125. Ling SH, San PP, Nguyen HT. Non‐invasive hypoglycemia monitoring system using extreme learning machine for type 1 diabetes. ISA (Instrum Soc Am) Trans. 2016;64:440‐446. 10.1016/j.isatra.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 126. San PP, Ling SH, Nguyen HT. Deep learning framework for detection of hypoglycemic episodes in children with type 1 diabetes: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, 2016:3503‐3506. 10.1109/EMBC.2016.7591483. [DOI] [PubMed] [Google Scholar]
- 127. Lee AS, Brooks BA, Simmons L, et al. Hypoglycaemia and QT interval prolongation: detection by simultaneous Holter and continuous glucose monitoring. Diabetes Res Clin Pract. 2016;113:211‐214. 10.1016/j.diabres.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 128. Bekkink MO, Koeneman M, Galan BE de, Bredie SJ. Early detection of hypoglycemia in type 1 diabetes using heart rate variability measured by a wearable device. Diabetes Care. 2019;42(4):689‐692. 10.2337/dc18-1843. [DOI] [PubMed] [Google Scholar]
- 129. Cichosz SL, Frystyk J, Tarnow L, Fleischer J. Are changes in heart rate variability during hypoglycemia confounded by the presence of cardiovascular autonomic neuropathy in patients with diabetes? Diabetes Technol Ther. 2017;19(2):91‐95. 10.1089/dia.2016.0342. [DOI] [PubMed] [Google Scholar]
- 130. Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Invest. 2013;4(1):4‐18. 10.1111/jdi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Porumb M, Stranges S, Pescapè A, Pecchia L. Precision medicine and artificial intelligence: a pilot study on deep learning for hypoglycemic events detection based on ECG. Sci Rep. 2020;10(1):1‐16. 10.1038/s41598-019-56927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pramming S, Thorsteinsson B, Stigsby B, Binder C. Glycaemic threshold for changes in electroencephalograms during hypoglycaemia in patients with insulin dependent diabetes. BMJ. 1988;296(6623):665‐667. 10.1136/bmj.296.6623.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Howorka K, Heger G, Schabmann A, Anderer P, Tribl G, Zeitlhofer J. Severe hypoglycaemia unawareness is associated with an early decrease in vigilance during hypoglycaemia. Psychoneuroendocrinology. 1996;21(3):295‐312. 10.1016/0306-4530(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 134. Rubega M, Sparacino G. Neurological changes in hypoglycemia. Diabetes Technol Ther. 2017;19(2):73‐75. 10.1089/dia.2017.0009. [DOI] [PubMed] [Google Scholar]
- 135. Nguyen LB, Nguyen AV, Ling SH, Nguyen HT. Analyzing EEG signals under insulin‐induced hypoglycemia in type 1 diabetes patients: 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 2013:1980‐1983. 10.1109/EMBC.2013.6609917. [DOI] [PubMed] [Google Scholar]
- 136. Nguyen LB, Nguyen AV, Ling SH, Nguyen HT. Combining genetic algorithm and Levenberg‐Marquardt algorithm in training neural network for hypoglycemia detection using EEG signals: 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 2013:5386‐5389. 10.1109/EMBC.2013.6610766. [DOI] [PubMed] [Google Scholar]
- 137. Ngo CQ, Truong BCQ, Jones TW, Nguyen HT. Occipital EEG activity for the detection of nocturnal hypoglycemia: 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, 2018:3862‐3865. 10.1109/EMBC.2018.8513069. [DOI] [PubMed] [Google Scholar]
- 138. Ngo CQ, Chai R, Nguyen TV, Jones TW, Nguyen HT. Electroencephalogram spectral moments for the detection of nocturnal hypoglycemia. IEEE J Biomed Health Inform. 2020;24:1237‐1245. 10.1109/JBHI.2019.2931782. [DOI] [PubMed] [Google Scholar]
- 139. Ngo CQ, Chai R, Nguyen TV, Jones TW, Nguyen HT. Nocturnal hypoglycemia detection using EEG spectral moments under natural occurrence conditions. Conf Proc IEEE Eng Med Biol Soc. 2019;2019:7177‐7180. 10.1109/EMBC.2019.8856695. [DOI] [PubMed] [Google Scholar]
- 140. Juhl CB, Højlund K, Elsborg R, et al. Automated detection of hypoglycemia‐induced EEG changes recorded by subcutaneous electrodes in subjects with type 1 diabetes‐The brain as a biosensor. Diabetes Res Clin Pract. 2010;88(1):22‐28. 10.1016/j.diabres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 141. Snogdal LS, Folkestad L, Elsborg R, et al. Detection of hypoglycemia associated EEG changes during sleep in type 1 diabetes mellitus. Diabetes Res Clin Pract. 2012;98(1):91‐97. 10.1016/j.diabres.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 142. Hansen GL, Foli‐Andersen P, Fredheim S, et al. Hypoglycemia‐associated EEG changes in prepubertal children with type 1 diabetes. J Diabetes Sci Technol. 2016;10(6):1222‐1229. 10.1177/1932296816634357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sejling A‐S, Kjaer TW, Pedersen‐Bjergaard U, et al. Hypoglycemia‐associated EEG changes following antecedent hypoglycemia in type 1 diabetes mellitus. Diabetes Technol Ther. 2017;19(2):85‐90. 10.1089/dia.2016.0331. [DOI] [PubMed] [Google Scholar]
- 144. Blaabjerg L, Remvig LS, Nielsen SS, et al. Prevention of severe hypoglycemia by use of the electroencephalography‐based alarm device, hyposafe™ SubQ. Diabetes Technol Ther. 2019;61(S1):A146. [Google Scholar]
- 145. Pickup JC. Preliminary evaluation of a skin conductance meter for detecting hypoglycemia in diabetic patients. Diabetes Care. 1982;5(3):326‐329. 10.2337/diacare.5.3.326. [DOI] [PubMed] [Google Scholar]
- 146. Johansen K, Ellegaard S, Wex S. Detection of nocturnal hypoglycemia in insulin‐treated diabetics by a skin temperature ‐ skin conductance meter. Acta Medica Scand. 1986;220(3):213‐217. 10.1111/j.0954-6820.1986.tb02753.x. [DOI] [PubMed] [Google Scholar]
- 147. Diabetes Sentry: Home. 2020. http://www.diabetessentry.com/index.htm [Google Scholar]
- 148. Sobel SI, Chomentowski PJ, Vyas N, Andre D, Toledo FGS. Accuracy of a novel noninvasive multisensor technology to estimate glucose in diabetic subjects during dynamic conditions. J Diabetes Sci Technol. 2014;8(1):54‐63. 10.1177/1932296813516182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Turner C, Walton C, Hoashi S, Evans M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J Breath Res. 2009;3(4):046004. 10.1088/1752-7155/3/4/046004. [DOI] [PubMed] [Google Scholar]
- 150. Wang C, Mbi A, Shepherd M. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sensor J. 2010;10(1):54‐63. 10.1109/JSEN.2009.2035730. [DOI] [Google Scholar]
- 151. Galassetti PR, Novak B, Nemet D, et al. Breath ethanol and acetone as indicators of serum glucose levels: an initial report. Diabetes Technol Ther. 2005;7(1):115‐123. 10.1089/dia.2005.7.115. [DOI] [PubMed] [Google Scholar]
- 152. Neupane S, Peverall R, Richmond G, et al. Exhaled breath isoprene rises during hypoglycemia in type 1 diabetes. Diabetes Care. 2016;39(7):e97‐e98. 10.2337/dc16-0461. [DOI] [PubMed] [Google Scholar]
- 153. Siegel AP, Daneshkhah A, Hardin DS, Shrestha S, Varahramyan K, Agarwal M. Analyzing breath samples of hypoglycemic events in type 1 diabetes patients: towards developing an alternative to diabetes alert dogs. J Breath Res. 2017;11(2):026007. 10.1088/1752-7163/aa6ac6. [DOI] [PubMed] [Google Scholar]
- 154. Boubin M, Shrestha S. Microcontroller implementation of support vector machine for detecting blood glucose levels using breath volatile organic compounds. Sensors. 2019;19(10):2283. 10.3390/s19102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shrestha S, Harold C, Boubin M, Lawrence L. Smart wristband with integrated chemical sensors for detecting glucose levels using breath volatile organic compounds: Proc. SPIE 11020, Smart Biomedical and Physiological Sensor Technology XVI, 110200R; 2 May 2019. 10.1117/12.2521365. [DOI] [Google Scholar]
- 156. Tronstad C, Elvebakk O, Staal OM, et al. Non‐invasive prediction of blood glucose trends during hypoglycemia. Anal Chim Acta. 2019;1052:37‐48. 10.1016/j.aca.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 157. Elvebakk O, Tronstad C, Birkeland KI, et al. A multiparameter model for non‐invasive detection of hypoglycemia. Physiol Meas. 2019;40(8):085004. 10.1088/1361-6579/ab3676. [DOI] [PubMed] [Google Scholar]
- 158. Karunathilaka SR, Small GW. Nocturnal hypoglycemic alarm based on near‐infrared spectroscopy: in vitro simulation studies. Anal Chim Acta. 2017;987:81‐90. 10.1016/j.aca.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 159. Gonder‐Frederick LA, Grabman JH, Shepard JA. Diabetes alert dogs (DADs): an assessment of accuracy and implications. Diabetes Res Clin Pract. 2017;134:121‐130. 10.1016/j.diabres.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Los EA, Ramsey KL, Guttmann‐Bauman I, Ahmann AJ. Reliability of trained dogs to alert to hypoglycemia in patients with type 1 diabetes. J Diabetes Sci Technol. 2017;11(3):506‐512. 10.1177/1932296816666537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lippi G, Plebani M. Diabetes alert dogs: a narrative critical overview. Clin Chem Lab Med. 2018;57(4):452. 10.1515/cclm-2018-0842. [DOI] [PubMed] [Google Scholar]
- 162. Ibrahim M, Baker J, Cahn A, et al. Hypoglycaemia and its management in primary care setting. Diabetes Metab Res Rev. 2020;36(8):e3332. 10.1002/dmrr. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


