Abstract
Objective
To investigate the rate of and the risk factors for breakthrough‐IFI (b‐IFI) after orthotopic liver transplantation (OLT) according to the new definition proposed by Mycoses‐Study‐Group‐Education‐and‐Research‐Consortium (MSG‐ERC) and the European‐Confederation‐of‐Medical‐Mycology (ECMM).
Methods
Multicenter prospective study of adult patients who underwent OLT at three Italian hospitals, from January 2015 to December 2018. Targeted antifungal prophylaxis (TAP) protocol was developed and shared among participating centers. Follow‐up was 1‐year after OLT. B‐IFI was defined as infection occurring during exposure to antifungal prophylaxis. Risk factors for b‐IFI were analyzed among patients exposed to prophylaxis by univariable analysis.
Results
We enrolled 485 OLT patients. Overall compliance to TAP protocol was 64.3%, 220 patients received antifungal prophylaxis, 172 according to TAP protocol. Twenty‐nine patients were diagnosed of IFI within 1 year after OLT. Of them, 11 presented with b‐IFI within 17 (IQR 11‐33) and 16 (IQR 4‐30) days from OLT and from antifungal onset, respectively. Then out of 11 patients with b‐IFI were classified as having high risk of IFI and were receiving anti‐mould prophylaxis, nine with echinocandins and one with polyenes. Comparison of patients with and without b‐IFI showed significant differences for prior Candida colonization, need of renal replacement therapy after OLT, re‐operation, and CMV infection (whole blood CMV‐DNA >100 000 copies/mL). Although non‐significant, a higher rate of b‐IFI in patients on echinocandins was observed (8.2% vs 1.8%, P = .06).
Conclusions
We observed 5% of b‐IFI among OLT patients exposed to antifungal prophylaxis. The impact of echinocandins on b‐IFI risk in this setting should be further explored.
Keywords: antifungal prophylaxis, breakthrough IFI, infection risk, invasive fungal infection, liver transplantation
1. INTRODUCTION
The incidence of invasive fungal infection (IFI) after solid organ transplantation (SOT) has decreased over the last two decades owing to improvements in managing end‐stage disease, enhanced surgical techniques, safer immunosuppressive therapy, and improved anti‐infective preventive measures. 1 , 2 However, IFI‐associated morbidity and mortality in SOT recipients are still high. 3 In addition, detection of IFIs can be difficult because the classical signs and symptoms may be missing or confounded with other conditions, and diagnostic techniques have limited sensitivity and specificity. 4
In patients undergoing orthotopic liver transplantation (OLT), guidelines recommend targeted antifungal prophylaxis (TAP) rather than universal prophylaxis. 5 , 6 , 7 The feasibility and efficacy of this approach have been investigated in observational studies. 8 , 9 , 10 , 11 The adherence to TAP varied from 49.4% to 97%, as well as the rates of IFI from 2.8% to 28%. Although in prior studies the occurrence of IFI during prophylaxis was reported, 11 , 12 the definition of breakthrough IFI (b‐IFI) varied across clinical trials and observational studies. 7 , 8 , 9 , 11 , 12 These differences may limit comparison between studies and hinder epidemiological interpretation. For such reasons, the Mycoses Study Group Education and Research Consortium (MSG‐ERC) and the European Confederation of Medical Mycology (ECMM) have recently proposed a broadly applicable definition of b‐IFI for clinical research. 13
With these assumptions, we performed a multicenter prospective study of OLT recipients managed with a common TAP strategy, to assess the rate of and the risk factors for b‐IFI after liver transplantation.
2. MATERIALS AND METHODS
2.1. Setting
This prospective study was performed in three University‐affiliated hospitals with OLT programs. S. Orsola‐Malpighi Hospital is a 1420‐bed tertiary teaching care hospital in Bologna, with an average of 72 000 admissions per year. The hospital has an active OLT program, performing an average of 90 procedures per year. The hospital of Padua is a 1400‐bed tertiary teaching care hospital in Padua, with an average of 70 000 admissions per year. The local OLT program performs an average of 100 procedures per year. The hospital of Modena is a 1100‐bed tertiary teaching hospital in Modena, with an average of 46,000 admissions per year. The local OLT program performs an average of 50 procedures per year.
2.2. Study design and participants
We performed a prospective multicenter cohort study. We enrolled consecutive adult (≥18 years) patients undergoing OLT, from January 2015 to December 2018 at participating centers. This study was approved by the Institutional Review Board of coordinating centre (N°89/2015/O/Oss) and for each participating hospital, informed consent was obtained before enrolling patients.
The only exclusion criterion was patient refusal to provide consent. Patients were followed‐up to one year after OLT by hospital and outpatient clinical assessments.
2.3. Transplant management
In all centers, ampicillin‐sulbactam was used as standard perioperative prophylaxis. The standard immunosuppressive treatment consisted of induction with high‐dose prednisone, followed by tacrolimus and low‐dose prednisone.
CMV seronegative patients who received an organ from a CMV positive donor received oral valganciclovir 900 mg/daily as prophylaxis until 90 days after transplant. All CMV seropositive patients were screened weekly for CMV DNAemia by whole‐blood quantitative polymerase chain reaction until 90 days after OLT. If CMV DNAemia exceeded ≥100 000 copies/mL, patients were started on induction treatment with ganciclovir 5 mg/kg/bid or oral valgancyclovir 900 mg/bid. 14 All patients were administered 160/800 mg trimethoprim/sulfamethoxazole three times weekly for 12 months post‐transplant as prophylaxis for Pneumocystis jirovecii.
2.4. Management of antifungal prophylaxis
Before study onset, two meetings between participating centres were performed in order to establish a common approach to antifungal prophylaxis. A study protocol was written and summarized in supplementary Tables 1 and 2.
TABLE 1.
General characteristic of study population
|
Total N = 485 (%) |
|
|---|---|
| Demographic data | |
| Age (y) [median (IQR)] | 56 (49‐61) |
| Sex, male | 354 (73) |
| Comorbidities | |
| Charlson index [median (IQR)] | 6 (4‐7) |
| Underlying liver disease | |
| Viral hepatitis | 240 (49.5) |
| Alcohol | 120 (24.7) |
| Hepatocellular carcinoma | 230 (47.4) |
| Fulminant liver failure | 11 (2.3) |
| Combined transplant | 13 (2.7) |
| MELD at inclusion in waiting list (median, IQR) | 16 (10‐23) |
| MELD at OLT (median, IQR) | 17 (12‐25) |
| Pre‐operative variables (90 d prior OLT) | |
| Steroid treatment | 5 (1) |
| ICU stay | 54 (11.1) |
| Candida colonization | 55 (11.3) |
| Graft characteristics | |
| Donor age | 62 (49‐75) |
| Cold ischemia time (h) (median, IQR) | 7:00 (6:15‐8:00) |
| Intervention duration (h (median, IQR) | 7:30 (6:45‐8:30) |
| Intraoperative variables | |
| Prolonged operation (≥8 h) | 204 (42.1) |
| Choledochojejunostomy | 72 (14.9) |
| Transfusion of ≥40 units of blood products | 6 (1.2) |
| Post‐operative complications | |
| Mechanical ventilation ≥48 h | 59 (12.2) |
| PGNF | 25 (5.2) |
| Acute renal failure | 136 (28) |
| Renal replacement therapy | 72 (14.8) |
| Blood CMV‐DNA >100 000 copies/mL | 16 (3.3) |
| Rejection within 2 wk after OLT | 53 (10.9) |
| Rejection threated with ATG, OKT3, or ALB | 7 (1.4) |
| Re‐transplantation | 38 (7.8) |
| Reoperation | 109 (22.5) |
| Risk class | |
| None | 154 (31.8) |
| Low | 101 (20.8) |
| High | 230 (47.4) |
| Antifungal prophylaxis | |
| None | 265 (54.6) |
| Fluconazole | 22 (4.5) |
| Echinocandin | 110 (22.7) |
| L‐AmB | 88 (18.1) |
| Time from OLT to antifungal prophylaxis initiation (d) (median, IQR) | 1 (0‐5) |
| Antifungal prophylaxis duration (d) (median, IQR) | 13 (7‐20) |
| Overall compliance to TAP protocol | 311 (64.1) |
| Compliance to TAP protocol among patients receiving antifungal prophylaxis | 172/220 (78.2) |
| Outcome | |
| Length of hospital stay after OLT (d) (median, IQR) | 17 (11‐30) |
| All‐cause 1‐y mortality | 48 (9.9) |
Abbreviations: ALB, alemtuzumab; ATG, anti‐thymocyte‐globuline; CMV, cytomegalovirus; ICU, intensive care unit; IFI, invasive fungal infection; IQR, interquartile range; L‐AmB, liposomal‐amphotericin‐B; MELD, model for end stage liver disease; OKT3, anti‐CD3 monoclonal antibody; OLT, orthotopic liver transplantation; PGNF, primary graft non‐function; TAP, targeted antifungal prophylaxis.
TABLE 2.
Distribution of antifungal prophylaxis according to risk group
|
No risk N = 154 (%) |
Low risk N = 101 (%) |
High risk N = 230 (%) |
Total N = 485 (%) |
|
|---|---|---|---|---|
| No prophylaxis | 139 (90.3) a | 72 (71.3) | 54 (23.5) | 265 (54.7) |
| Fluconazole | 4 (2.6) | 7 (6.9) a | 11 (4.8) | 22 (4.5) |
| Anti‐mould drug | 11 (7.1) | 22 (21.8) | 165 (71.7) a | 198 (40.8) |
| LamB | 8 (5.2) | 10 (9.9) | 70 (30.4) | 88 (18.1) b |
| Echinocandins | 3 (1.9) | 12 (11.9) | 95 (41.3) | 110 (22.7) |
| Overall IFI | 2 (1.29) | 5 (5) | 22 (9.6) | 29 (6) |
| b‐IFI | 0 (0) | 1 (1) | 10 (4.3) | 11 (2.3) |
Bold figures refer to antifungal management according to TAP protocol.
Seven patients received LamB 3 mg/kg/d, 81 LamB 10 mg/kg/wk.
Briefly, risk factors for IFI were checked daily within the first week after OLT and then weekly until the end of the first month. After this period, systematic assessment of IFI risk factors was not performed per protocol, but only based on patient clinical course.
Risk factors for invasive candidiasis (IC) include pre‐operative variables such as intensive care unit (ICU) hospitalization within 90 days before OLT and peri‐operative Candida spp. colonization (defined as isolation of Candida spp. from ≥three of five surveillance samples obtained in the last 7 days before transplantation); intraoperative variables, defined as choledochojejunostomy, prolonged operation (≥8 hours),and/or massive bleeding requiring transfusion of more than 40 units of cellular blood products. Finally, post‐operative variables were acute renal failure defined according to KDIGO criteria, 15 biopsy‐proven rejection managed with any type of immunosuppressive regimen within two weeks after OLT, and CMV infection/reactivation (defined by finding of whole blood CMV‐DNA levels>100 000 copies/mL). 14
Regarding risk factors for invasive aspergillosis (IA), pre‐operative variables included OLT secondary to fulminant hepatic failure, steroid treatment within one‐month prior transplantation (at least an equivalent of 16 mg/d prednisone for ≥15 days), and multi‐visceral transplantation. Among post‐operative variables, the requirement of any renal replacement therapy, biopsy‐proven rejection requiring anti‐thymocyte globulin, anti‐CD3 monoclonal antibody or alemtuzumab, re‐transplantation, and re‐operation were included.
According to risk factors described above, patients were classified in three groups: no risk, low risk (1 RF for IC) and high risk for IFI (≥2 RF for IC or ≥1RF for IA).
Patients assigned to no‐risk group were not recommended for antifungal prophylaxis. Fluconazole 400 mg/d after a loading dose of 600 mg for 7‐14 days was proposed in low‐risk patients. Fluconazole dose was reduced of 50% if creatinine clearance was less than 50 mL/min, a reduction of 75% was scheduled if creatinine clearance was under 10 mL/min. Echinocandin or polyenes were recommended for patients in the high‐risk group according to physician's choice. No reduction dose of caspofungin, micafungin, anidulafungin, and LamB for renal impairment was scheduled. To minimize the risk of drug‐drug interactions, echinocandin and polyene classes were preferred to new azoles. The suggested duration of prophylaxis in high‐risk patients was 21 days.
2.5. Variables
All variables considered in the analysis were collected in a standardized electronic case report form (eCRF).
The primary endpoint was b‐IFI defined by the first sign, symptom, or finding of IFI that occurs during antifungal prophylaxis according to the latest definitions stated by Mycoses Study Group Education and Research Consortium and the European Confederation of Medical Mycology. 13 It was assessed from the administration of the first dose up to one dosing interval after drug discontinuation (one day for fluconazole, echinocandin and daily LamB, and one week for weekly LamB administrations, respectively).
IFI was defined according to the revised European Organization for Research and Treatment of Cancer criteria/Mycosis Study Group (EORTC‐MSG). 16 We consider only the first episode of IFI during the study period.
Exposure variables included demographic data (age and sex), comorbidities according to Charlson index, etiology of liver disease, and Model for End‐Stage Liver Disease (MELD) at inclusion in the waiting list, and at the time of OLT. Primary graft non‐function (PGNF) was defined as an aggravated form of reperfusion injury resulting in reversible graft failure without detectable technical or immunological problems. 17 The risk factors for IC and IA in pre‐operative, intra‐operative and post‐operative periods, as defined by the study protocol, were collected. Antifungal prophylaxis, including antifungal drugs administered with respective dates of onset and discontinuation.
Exposure variables were assessed from OLT to the first IFI episode, death or one year after transplantation whichever occurred first.
2.6. Statistical analysis
Categorical variables were expressed as absolute numbers and their relative frequencies. Continuous variables were expressed as mean ± standard deviation (SD) if normally distributed, or as median and interquartile range (IQR) if non‐normally distributed.
At univariable analysis, categorical variables were compared for patients with and without b‐IFI using Pearson chi‐square or Fisher's exact test where appropriate. Continuous variables were compared using Student's t or Mann‐Whitney U test according to their distribution.
Cumulative incidence of b‐IFI according to antifungal prophylaxis, and stratified by risk class, was analyzed with Kaplan‐Meyer curves, patients were considered from the day of transplant up to b‐IFI diagnosis, death or 40 days after transplant, whichever occurred first.
The level of significance was set at P < .05 for all the tests. All analyses were performed using SPSS 21.00.
3. RESULTS
Overall, 485 patients who underwent OLT during the study period were enrolled. The distribution of number of enrolled patients per site was 324 (66.8%) Bologna, 104 (21.4%) Padova, and 57 (11.8%) Modena. General characteristics of the study cohort are shown in Table 1.
Stratifying patients by risk factors for IFI, 154 (31.8%) showed no risk, 101 (20.8%) low risk, and 230 (47.4%) high risk (see Figure 1). Antifungal prophylaxis was administered to 220 (45.3%) patients, for a median of 13 (IQR 7‐20) days. Twenty‐one (4.3%) patients had an adverse event related to administration of antifungal prophylaxis. However, all of them were considered as mild by the attending physicians and drug discontinuation or other medical interventions were not required.
FIGURE 1.
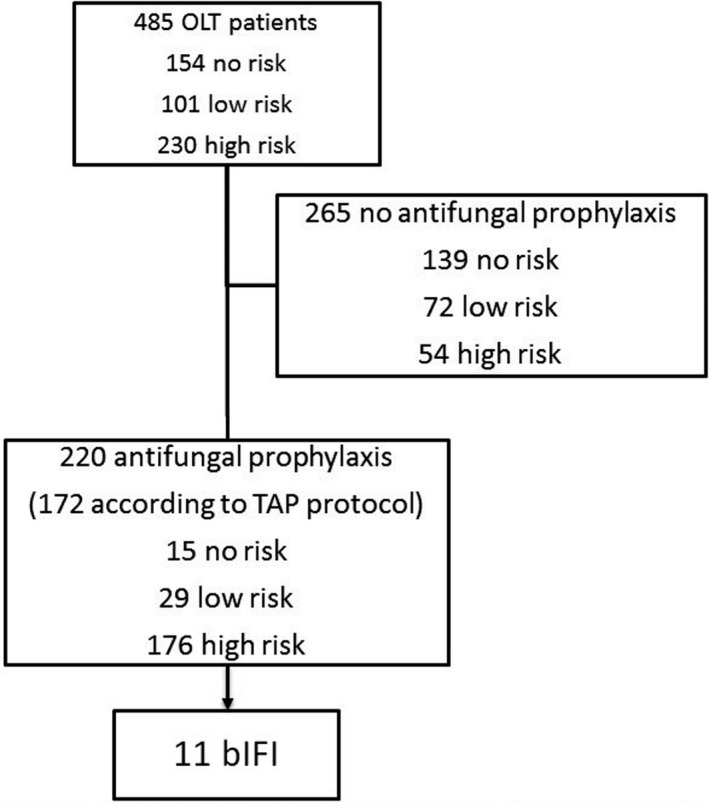
Study flow‐chart
The overall adherence to TAP protocol was 64.3%, varying from 90.3%, 6.9%, and 71.7% in no‐risk, low‐risk and high‐risk patients, respectively (data shown in Table 2).
Twenty‐nine (6%) patients were diagnosed of IFI within 1 year after OLT, consisting of 17 IC and 12 IA (details of IFI episodes are shown in supplementary Table 3). Of them, 11 were b‐IFI, 6 IC and 5 IA (details are shown in Table 3). The median time to b‐IFI was 16 (IQR 4‐30) days after the onset of antifungal prophylaxis, and 17 (IQR 11‐33) days after OLT. The rate of b‐IFI among patients receiving antifungal prophylaxis was 5%. Patients who developed b‐IFI had been considered at low and high risk for IFI in 1 and 10 cases, respectively. The patient at low risk was receiving fluconazole upon the diagnosis of b‐IFI; among the patients at high risk for IFI, nine were receiving echinocandins and one liposomal amphotericin B (data shown in Table 4). Only one fluconazole resistant C albicans was isolated from blood cultures in the patient on fluconazole prophylaxis. No echinocandin or L‐AmB resistance was observed in patients exposed to such drugs who developed b‐IFI.
TABLE 3.
Breakthrough invasive fungal infection characteristics
| N = 11 (%) | |
|---|---|
| Distribution of bIFI according to risk group | |
| Low risk | 1 (9.1) |
| High risk | 10 (90.9) |
| Invasive candidiasis | 6 (54.5) |
| Intra‐abdominal a | 4 (36.4) |
| BSI | 2 (18.2) |
| Candida species | |
| C albicans | 3 (27.3) |
| C parapsilosis | 1 (9.1) |
| C glabrata | 1 (9.1) |
| C tropicalis | 1 (9.1) |
| Time to IC development from onset of antifungal prophylaxis (median, IQR) | 4 (1.5‐26) |
| Invasive aspergillosis | 5 (45.5) |
| Proven | 1 (9.1) |
| Probable b | 4 (36.4) |
| Site of infection | |
| Lung | 5 (100) |
| Aspergillus species | |
| A fumigatus | 1 (9.1) |
| Time to IA development from onset of antifungal prophylaxis (median, IQR) | 16 (12‐31) |
| Antifungal treatment | |
| L‐AmB | 5/11(45.5) |
| Caspofungin | 3/11 (27.3) |
| Anidulafungin | 1/11 (9.1) |
| Fluconazole | 1/11 (9.1) |
| Voriconazole | 1/11(9.1) |
Abbreviations: bIFI, breakthrough invasive fungal infection; BSI, bloodstream infections; IA, invasive aspergillosis; IC, invasive candidiasis; IQR, interquartile range; L‐AmB, liposomal‐amphotericin‐B; TAP, targeted antifungal prophylaxis; TAP, targeted antifungal prophylaxis.
Monomicrobial candida isolation from intraoperativeabdominal samples.
Diagnostic criteria: in all cases the diagnosis was established according to risk factors, suggestive imaging and positive galactomannan (index >1) on BAL. In addition, in one patient A fumigatus grew from BAL.
TABLE 4.
Distribution of b‐IFI, invasive candidiasis and invasive aspergillosis, according to risk group and antifungal drug received
| Breakthrough IFI | Fluconazole | Echinocandins | LamB |
|---|---|---|---|
| Invasive candidiasis (n = 6) | |||
| No risk n = 15 | 0/4 (0) | 0/3 (0) | 0/8 (0) |
| Low risk n = 29 | 1/7 (14.3) | 0/12 (0) | 0/10 (0) |
| High risk n = 176 | 0/11 (0) | 5/95 (5.3) | 0/70 (0) |
| Invasive aspergillosis (n = 5) | |||
| No risk n = 15 | 0/4 (0) | 0/3 (0) | 0/8 (0) |
| Low risk n = 29 | 0/7 (0) | 0/12 (0) | 0/10 (0) |
| High risk n = 176 | 0/11 (0) | 4/95 (4.2) | 1/70 (1.4) a |
Abbreviations: IFI, invasive fungal infections; LamB, liposomal amphotericin B.
LamB 10 mg/kg weekly.
Comparison of patients with and without b‐IFI showed significant differences for the following variables: peri‐operative Candida colonization (45.5% vs 16.3%), whole blood CMV‐DNA >100 000 cps/mL (27.3% vs 2.9%), renal replacement therapy (54.5% vs 28.7%) and reoperation (72.7% vs 37.8%) (data shown in Table 5).
TABLE 5.
Comparison of patients with and without breakthrough IFI
| Patients with bIFI, n = 11 (%) | Patients without bIFI, n = 209 (%) | P | |
|---|---|---|---|
| Demographic data | |||
| Age (y) [median (IQR)] | 51 (45‐59) | 54 (48‐60) | 0.52 |
| Sex, male | 9 (81.8) | 147 (70.3) | 0.5 |
| Comorbidities | |||
| Charlson index [median (IQR)] | 5 (4‐6) | 5 (4‐7) | 0.7 |
| Underlying liver disease | |||
| Viral hepatitis | 3 (27.3) | 90 (43.1) | 0.36 |
| Alcohol | 4 (36.4) | 55 (26.3) | 0.49 |
| Hepatocellular carcinoma | 2 (18.2) | 72 (34.4) | 0.34 |
| Fulminant liver failure | 0 | 9 (4.3) | 0.44 |
| Combined transplant | 1 (9.1) | 4 (1.9) | 0.23 |
| MELD at inclusion in waiting list (median, IQR) | 22 (11‐24) | 19 (12‐26.5) | 0.89 |
| MELD at OLT (median, IQR) | 24 (11‐32) | 21 (13‐30) | 0.69 |
| Preoperative variables (90 d prior OLT) | |||
| Steroid treatment | 0 (0) | 4 (1.9) | 1 |
| ICU stay | 3 (27.3) | 39 (18.7) | 0.44 |
| Candida colonization | 5 (45.5) | 34 (16.3) | 0.03 |
| Graft characteristics | |||
| Donor age | 61.5 (53‐73) | 59 (44.5‐73.5) | 0.53 |
| Cold ischemia time (h) (median, IQR) | 7:19 (6:30‐9) | 7 (6:23‐8) | 0.68 |
| Intervention duration (h) (median, IQR) | 7:30 (6‐10.25) | 7:45 (6:55‐9) | 0.78 |
| Intraoperative variables | |||
| Prolonged operation (≥8 h) | 8 (72.7) | 130 (62.2) | 0.75 |
| Choledochojejunostomy | 1 (9.1) | 53 (25.4) | 0.3 |
| Transfusion of ≥40 units of blood products | 1 (9.1) | 4 (1.9) | 0.23 |
| Post‐operative complications | |||
| Mechanical ventilation ≥48 h | 2 (18.2) | 46 (22) | 1 |
| PGNF | 1 (9.1) | 22 (10.5) | 1 |
| Acute renal failure | 7 (63.6) | 87 (41.6) | 0.21 |
| Renal replacement therapy | 6 (54.5) | 60 (28.7) | 0.09 |
| Blood CMV‐DNA >100 000 copies/mL | 3 (27.3) | 6 (2.9) | 0.01 |
| Rejection within 2 wk after OLT | 0 (0) | 36 (17.2) | 0.22 |
| Rejection treated with ATG, OKT3, or ALB | 0 (0) | 4 (2.6) | 1 |
| Re‐transplantation | 1 (9.1) | 36 (17.2) | 0.69 |
| Reoperation | 8 (72.7) | 79 (37.8) | 0.03 |
| Risk class | |||
| None | 0 (0) | 15 (7.2) | 1 |
| Low | 1 (9.1) | 28 (13.4) | 0.8 |
| High | 10 (90.9) | 166 (79.4) | 0.7 |
| Antifungal prophylaxis | |||
| Fluconazole | 1 (9.1) | 21 (10) | 1 |
| Echinocandin | 9 (81.8) | 101 (48.3) | 0.06 |
| L‐AmB | 1 (9.1) | 87 (41.6) | 0.05 |
| Compliance to TAP | 11 (100) | 161 (77) | 0.13 |
| Outcome | |||
| Length of hospital stay after OLT (d) (median, IQR) | 45 (24‐96) | 26 (16‐42) | 0.01 |
| All‐cause 1‐y mortality | 3 (27.3) | 33 (15.8) | 0.39 |
Abbreviations: ALB, alemtuzumab; ATG, anti‐tymocite‐globuline; CMV, cytomegalovirus; ICU, intensive care unit; IFI, invasive fungal infection; IQR, interquartile range; L‐AmB, liposomal‐amphotericin‐B; MELD, model for end stage liver disease; OKT3, anti‐CD3 monoclonal antibody; OLT, orthotopic liver transplantation; PGNF, primary graft non‐function; TAP, targeted antifungal prophylaxis.
Kaplan‐Meier curves showed higher cumulative risk of b‐IFI for echinocandin prophylaxis in patients classified as having high risk of IFI (Log Rank 4.47, P = .03) (data shown in Figure 2).
FIGURE 2.
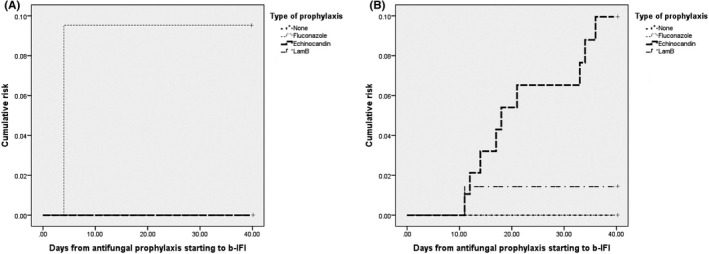
Kaplan‐Meier curves of cumulative risk of b‐IFI among low (panel a) and high‐risk (panel b) patients according to the antifungal prophylaxis
4. DISCUSSION
Our study showed a 5% of b‐IFI after liver transplantation in a multicenter cohort of OLT recipients managed with TAP strategy. CMV infection was the main predictor of b‐IFI. However, also the use of echinocandin and the need of reoperation were associated with an increased risk of b‐IFI.
The overall compliance to TAP protocol (64.3%) in our study was lower than that reported in other observational studies. 8 , 9 , 10 , 11 The adherence was lowest among patients classified as having a low risk. The majority of IFI episodes were observed in high‐risk class (76%). These findings may suggest that a two‐tiered risk class differentiation using a cut‐off of 1 or more risk factors for IFI could allow to a higher overall adherence without negatively affecting IFI rates. Indeed, a recent retrospective single‐center study reached high overall levels of compliance (94.1%) through this method, reporting an IFI rate of 2.8%. 11 To note, that most IFI in this study occurred during antifungal prophylaxis.
Breakthrough invasive fungal infection is an emerging problem in patients with hematologic malignancies, hematopoietic stem cell transplant (HSCT) recipients, solid organ transplant (SOT) recipients, and critically ill patients managed with antifungal prophylaxis or pre‐emptive treatment. 18 Recently the Mycoses Study Group Education and Research Consortium (MSG‐ERC) and the European Confederation of Medical Micology (ECMM) proposed broadly applicable definitions for b‐IFI. 13 Risk factors for b‐IFI have been investigated mostly among hematological patients. They include host factors, fungal pathogen‐related factors, and antifungal drug‐related factors.
Among host factors: acute leukemia, neutropenia, steroid use, mucositis, central venous catheter, and broad spectrum antibitoics have been reported. 18 Historically, CMV infection in OLT recipients has been associated with an increased incidence of IFI. 19 , 20 , 21 In our study, we confirmed the key role of CMV infection/replication also in patients under antifungal prophylaxis.
Fungal factors include virulence, resistance and biofilm formation. 18 Resistance is the most common reported mechanism. In OLT recipients, it was well described in a review on the use of antifungal prophylaxis showing a shift toward non‐albicans Candida spp. associated with the universal use of fluconazole prophylaxis. 22 On this regard, one of our b‐IFI was due to a fluconazole‐resistant C albicans isolate from the blood cultures of a patient receiving fluconazole. Recently, b‐IFI to echinocandins have been reported in the TRANSNET cohort. 23 The authors found that prophylaxis with echinocandins was associated with C parapsilosis breakthrough, consistent with reduced susceptibility of this species. Furthermore, a clinical trial that compared micafungin vs. amphotericin B lipid complex in high‐risk OLT recipients reported b‐IFI rate of 5.9% vs 0%, respectively. 24 It is possible that intra‐abdominal penetration of echinocandins could be suboptimal and may lead to treatment failure and resistance development. 25 , 26 In our study, all patients who developed b‐IFI during prophylaxis with echinocandins were in the high‐risk group, but none of the isolates exhibited MICs above current EUCAST echinocandin susceptibility breakpoints.
Iatrogenic and treatment‐related factors include three major groups: inappropriate antifungal therapy, insufficient plasma and tissue drug levels, fungal biofilm infections of vascular devices or foreign bodies. 18 All patients who developed b‐IFI in our study were receiving an appropriate antifungal drug according to their risk class and at recommended dosage. However, drug exposure levels were not available for any of the antifungal administered. Despite we did not observed intravascular device‐related b‐IFI, when present central venous catheters were promptly removed at diagnosis in all patients.
Our study has some limitations. First, although we performed a multicenter cohort enrolling a large number of patients who underwent OLT, the number of b‐IFI events was low hampering to explore the association with several covariables in a stepwise logistic regression analysis to confirm our hypothesis that echinocandin use may be associated with an independent increased risk of b‐IFI. Second, most of the enrolled patients were from Bologna; thus local epidemiology could have influenced the incidence and types of observed IFIs. Third, we did not record the reasons for no compliance to the TAP protocol.
In conclusion, we observed a rate of b‐IFI among OLT recipients managed with TAP strategy of 5%. We confirmed a strong impact of CMV infection/reactivation on IFI development also in patients on antifungal prophylaxis. Further studies are needed to explore the relationship between echinocandin use and b‐IFI in OLT recipients.
CONFLICT OF INTEREST
Authors have no conflict of interest related to the present study.
AUTHOR CONTRIBUTION
MR contributed to manuscript drafting and data analysis. MB contributed to supervision and data analysis. AF, EF, CC, SC, RP and ST contributed to data collection. MG contributed to revision process. MC contributed to data collection. SA, AS, and MCM contributed to project design. MC, UC, and FDB contributed to supervision. PB, CM, and FC contributed to project design. RL contributed to supervision and project design. PV contributed to supervision and project design. MG contributed to project design, data analysis, manuscript drafting, and supervision.
Supporting information
Supplementary Material
Rinaldi M, Bartoletti M, Ferrarese A, et al. Breakthrough invasive fungal infection after liver transplantation in patients on targeted antifungal prophylaxis: A prospective multicentre study. Transpl Infect Dis. 2021;23:e13608. 10.1111/tid.13608
REFERENCES
- 1. Pappas P, Alexander B, Andes D, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50(8):1101‐1111. [DOI] [PubMed] [Google Scholar]
- 2. Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12(3):220‐229. [DOI] [PubMed] [Google Scholar]
- 3. Hosseini‐Moghaddam SM, Ouédraogo A, Naylor KL, et al. Incidence and outcomes of invasive fungal infection among solid organ transplant recipients: a population‐based cohort study. Transpl Infect Dis. 2020;22(2):e13250. [DOI] [PubMed] [Google Scholar]
- 4. Anesi JA, Baddley JW. Approach to the solid organ transplant patient with suspected fungal infection. Infect Dis Clin North Am. 2016;30(1):277‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Husain S, Camargo JF. Invasive Aspergillosis in solid‐organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13544. [DOI] [PubMed] [Google Scholar]
- 6. Aslam S, Rotstein C, Practice AIDCo . Candida infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13623. [DOI] [PubMed] [Google Scholar]
- 7. Gavaldà J, Meije Y, Fortún J, et al. Invasive fungal infections in solid organ transplant recipients. Clin Microbiol Infect. 2014;20(Suppl 7):27‐48. [DOI] [PubMed] [Google Scholar]
- 8. Eschenauer GA, Kwak EJ, Humar A, et al. Targeted versus universal antifungal prophylaxis among liver transplant recipients. Am J Transplant. 2015;15(1):180‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saliba F, Delvart V, Ichaï P, et al. Fungal infections after liver transplantation: outcomes and risk factors revisited in the MELD era. Clin Transplant. 2013;27(4):E454‐E461. [DOI] [PubMed] [Google Scholar]
- 10. Giannella M, Bartoletti M, Morelli M, et al. Antifungal prophylaxis in liver transplant recipients: one size does not fit all. Transpl Infect Dis. 2016;18(4):538‐544. [DOI] [PubMed] [Google Scholar]
- 11. Lavezzo B, Patrono D, Tandoi F, et al. A simplified regimen of targeted antifungal prophylaxis in liver transplant recipients: A single‐center experience. Transpl Infect Dis. 2018;20(2):e12859. [DOI] [PubMed] [Google Scholar]
- 12. Winston DJ, Limaye AP, Pelletier S, et al. Randomized, double‐blind trial of anidulafungin versus fluconazole for prophylaxis of invasive fungal infections in high‐risk liver transplant recipients. Am J Transplant. 2014;14(12):2758‐2764. [DOI] [PubMed] [Google Scholar]
- 13. Cornely OA, Hoenigl M, Lass‐Flörl C, et al. Defining breakthrough invasive fungal infection‐Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses. 2019;62(9):716‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girmenia C, Lazzarotto T, Bonifazi F, et al. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: a multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies. Clin Transplant. 2019;33(10):e13666. [DOI] [PubMed] [Google Scholar]
- 15. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179‐c184. [DOI] [PubMed] [Google Scholar]
- 16. De Pauw B, Walsh T, Donnelly J, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lock JF, Schwabauer E, Martus P, et al. Early diagnosis of primary nonfunction and indication for reoperation after liver transplantation. Liver Transpl. 2010;16(2):172‐180. [DOI] [PubMed] [Google Scholar]
- 18. Jenks JD, Cornely OA, Chen SC, Thompson GR, Hoenigl M. Breakthrough invasive fungal infections: who is at risk? Mycoses. 2020;63(10):1021‐1032. [DOI] [PubMed] [Google Scholar]
- 19. Fortún J, Martín‐Dávila P, Moreno S, et al. Risk factors for invasive aspergillosis in liver transplant recipients. Liver Transpl. 2002;8(11):1065‐1070. [DOI] [PubMed] [Google Scholar]
- 20. Collins LA, Samore MH, Roberts MS, et al. Risk factors for invasive fungal infections complicating orthotopic liver transplantation. J Infect Dis. 1994;170(3):644‐652. [PubMed] [Google Scholar]
- 21. Hadley S, Karchmer AW. Fungal infections in solid organ transplant recipients. Infect Dis Clin North Am. 1995;9(4):1045‐1074. [PubMed] [Google Scholar]
- 22. Cruciani M, Mengoli C, Malena M, Bosco O, Serpelloni G, Grossi P. Antifungal prophylaxis in liver transplant patients: a systematic review and meta‐analysis. Liver Transpl. 2006;12(5):850‐858. [DOI] [PubMed] [Google Scholar]
- 23. Andes DR, Safdar N, Baddley JW, et al. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis. 2016;18(6):921‐931. [DOI] [PubMed] [Google Scholar]
- 24. Sun HY, Cacciarelli TV, Singh N. Micafungin versus amphotericin B lipid complex for the prevention of invasive fungal infections in high‐risk liver transplant recipients. Transplantation. 2013;96(6):573‐578. [DOI] [PubMed] [Google Scholar]
- 25. Zhao Y, Prideaux B, Nagasaki Y, et al. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra‐abdominal abscess model. Antimicrob Agents Chemother. 2017;61(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shields RK, Nguyen MH, Press EG, Clancy CJ. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother. 2014;58(12):7601‐7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


