Abstract
Background
Erenumab is a human anti‐calcitonin gene‐related peptide receptor monoclonal antibody approved for migraine prevention. We sought to further assess the temporal patterns of response to erenumab in patients with chronic migraine (CM), specifically the onset and sustainability of monthly migraine day (MMD) response.
Methods
This is a post hoc analysis of a 12‐week, randomized, double‐blind, placebo‐controlled study of erenumab for migraine prevention in patients with CM (≥15 headache days/month, including ≥8 migraine days/month). Onset and sustainability were assessed according to MMD reduction from baseline, with the following response categories: responders (≥50% reduction), partial responders (≥30% and <50%), or nonresponders (<30%).
Results
Among the erenumab 140 mg group (n = 187), 54.0% (101/187) achieved a response at any month during the study with a median time to onset of monthly response of 1 month. This improvement was maintained in most patients with continued treatment. An initial response was achieved at Month 1 by 28.3% (53/187) of patients; 69.8% (37/53) of whom maintained a response at Months 2 and 3. Although many patients responded early, some patients required longer treatment to achieve a response; 79.4% (27/34) of initial partial responders and 21.0% (21/100) of initial nonresponders subsequently achieved a response. Similar findings were observed for the erenumab 70mg group (n = 188).
Conclusion
A majority of erenumab‐treated patients with CM who achieved an initial response at Month 1 sustained this benefit. Many patients responded later with continued treatment. Our data support recommendations to assess outcomes after ≥3 months of preventive treatment with erenumab in CM.
Keywords: chronic migraine, erenumab, response patterns
Abbreviations
- AMSM
acute migraine‐specific medication
- CGRP
calcitonin gene‐related peptide
- CM
chronic migraine
- MMD
monthly migraine day
INTRODUCTION
Erenumab (erenumab‐aooe in the United States) 1 is a fully human monoclonal antibody against the canonical calcitonin gene‐related peptide (CGRP) receptor 2 and is approved for the prevention of migraine in adults. 3 In a pivotal, double‐blind, placebo‐controlled trial in chronic migraine (CM; NCT02066415), erenumab 70 and 140 mg administered subcutaneously every 4 weeks significantly decreased monthly migraine days (MMDs) compared with placebo and decreased the number of acute migraine‐specific medication (AMSM) days. 4
MMD analyses are a useful way to evaluate month‐by‐month efficacy and are recommended for the assessment of migraine‐preventive treatments. 5 A ≥50% reduction in MMD (often assessed after ≥2–3 months of treatment) is a common goal for migraine prevention 6 and is the primary threshold for response used in this post hoc analysis. Because of the high burden of the disease, a ≥30% reduction in MMD may also be considered clinically meaningful for CM. 5
For patients who do not achieve a robust initial response to preventive treatment, clinicians must determine the optimal time frame to reassess the treatment response. 6 In particular, clinicians should be able to advise patients when a benefit might occur after continued use of preventive medication and/or when to make a modification to treatment, if necessary. 6 The objectives of this post hoc analysis were to assess the onset and sustainability of response to erenumab in patients with CM to better inform clinical decision‐making.
METHODS
Overview of study design
Erenumab was compared with placebo in a pivotal, double‐blind, randomized, multicenter, 12‐week trial in patients with CM across sites in North America and Europe (NCT02066415). 4 Briefly, patients aged 18–65 years with CM (≥15 headache days per month, with at least 8 migraine days per month) were included if they had not received migraine‐preventive treatment in the 2 months (4 months for botulinum toxin) before baseline screening. Patients with overuse of acute headache medication, including triptans, ergot derivatives, analgesics, and combination drugs could be enrolled and were not required to withdraw from acute headache medications. Patients with opioid overuse (>12 days during the 3 months before screening or >4 days during baseline) were excluded. 7 Those with no therapeutic response to more than three categories of preventive treatment (no benefit in the frequency, severity, or duration of headache after ≥6 weeks of treatment at recommended doses, as reported by the study investigator based on the patient's medical records and/or history) were excluded. Independent ethics committee or institutional review board approval was required for each study center, and all patients provided written informed consent before enrollment. 4
Patients meeting enrollment criteria were randomly assigned 3:2:2 to placebo, erenumab 70 mg, or erenumab 140 mg administered subcutaneously once every 4 weeks (Figure 1), stratified by region and medication overuse status. 4
FIGURE 1.
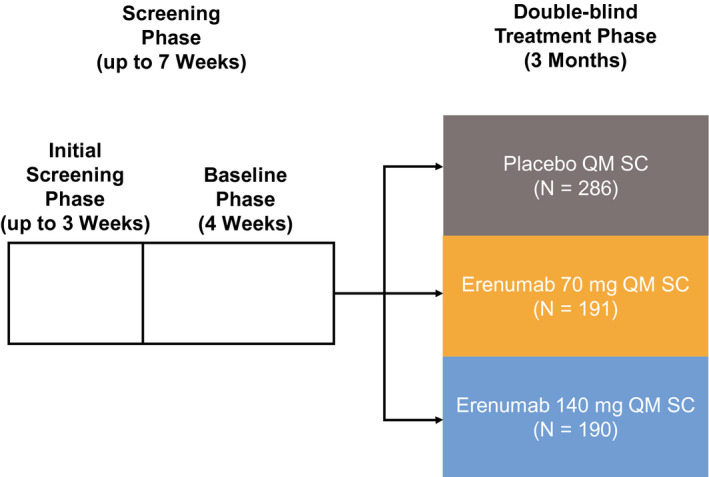
Overview of study design. QM, once monthly; SC, subcutaneously
Outcomes
Patients entered headache data, including the use of acute headache medications, into an electronic daily diary throughout the trial. 4 Change in MMD from the baseline period for Weeks 1–4, Weeks 5–8, and Weeks 9–12 was assessed. 4 The primary efficacy endpoint of the study was change in MMD from baseline at Week 12; achievement of a ≥50% reduction in MMD from baseline at Week 12 was a prespecified secondary endpoint. Results for the primary and secondary endpoints have been previously published. 4
In the current post hoc analysis, response was defined as ≥50% reduction from baseline in MMD (Table 1). Response was further classified as a good response (≥50% to <75% reduction in MMD) or an excellent response (≥75% reduction in MMD). In addition, we defined a partial responder as a ≥30% to <50% reduction in MMD from baseline in the 4‐week period being evaluated. Nonresponse for migraine frequency was defined as a <30% reduction in MMD from baseline in the 4‐week period being evaluated. Nonresponse was further classified as limited reduction in MMD (>0% to <30% reduction in MMD) and no change or worsening of outcome (no change or an increase in MMD). An initial responder was defined as achieving ≥50% reduction from baseline in MMD in the first 4‐week period (at Month 1). An initial partial responder and an initial nonresponder had a ≥30% to <50% and <30% reduction in MMD from baseline at Month 1, respectively.
TABLE 1.
Summary of definitions of MMD response
| Definition | Assessment criteria |
|---|---|
| Response | ≥50% reduction from baseline in MMD |
| Excellent | ≥75% reduction from baseline in MMD |
| Good | ≥50% and <75% reduction from baseline in MMD |
| Partial response | ≥30% and <50% reduction from baseline in MMD |
| Insufficient/nonresponse | <30% reduction from baseline in MMD |
| Limited MMD reduction | >0% and <30% reduction from baseline in MMD |
| No change or worsening | No change or increase in MMD from baseline |
| Assessment timeframe | |
|---|---|
| Initial response | Assessed at Month 1 |
| Subsequent response | Assessed at Months 2 and 3 |
| Delayed | Assessed at Months 2 and 3 among initial nonresponders (<50% reduction in MMD) |
| Sustained | Assessed at Months 2 and 3 among initial responders (≥50% reduction in MMD) |
Abbreviation: MMD, monthly migraine day.
Among the initial responders at Month 1, response to continued erenumab treatment was assessed at Months 2 and 3, and at Month 2 or 3 to evaluate the durability of the response to erenumab. A sustained good response was a response at both Months 2 and 3 in patients who had an initial response; a sustained excellent response was defined as an excellent response at both Months 2 and 3 in patients who had an initial response. Among patients who achieved a response in any study month, time to onset of monthly response was summarized.
Among initial partial responders or initial nonresponders, the patterns of response to erenumab in the two subsequent months (at Months 2 and 3 [i.e., a delayed sustained response], or at Month 2 or 3 [i.e., a delayed response]) were determined.
Statistical analyses
These post hoc analyses were primarily descriptive and included all patients who received ≥1 dose of erenumab and had baseline and ≥1 postbaseline MMD measurement. Patients with missing MMD at a particular month due to drop out or low compliance on electronic diary reporting were analyzed in the “no change or worsening response” category (Table 1). Data were reported as counts and percentages for dichotomous variables and mean (SD) for continuous variables. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). All authors had access to study data.
RESULTS
Patients
A total of 667 patients were included in the randomization analysis data set in the original study. 4 The 286 patients randomized to placebo were not included in this post hoc descriptive analysis of the timing of response to erenumab. Of the 381 patients randomized to erenumab, 375 patients (erenumab 70 mg, n = 188; erenumab 140 mg, n = 187) received ≥1 dose of erenumab and had ≥1 postbaseline MMD value period and were therefore included in this post hoc analysis.
Patient demographics at baseline, previously reported, 4 were similar between treatment arms, with a mean (SD) age of 41.4 (11.3) years for erenumab 70 mg and 42.9 (11.1) years for 140 mg. The majority of patients were female (70 mg: 86.9%; 140 mg: 84.2%), with a similar mean (SD) age of onset for both groups (70 mg: 21.1 [10.5] years; 140 mg: 21.5 [10.6] years) and use of prior preventive medication (72.3% vs. 71.6%). Two‐thirds of patients in each group experienced failure on ≥1 migraine‐preventive therapy, and approximately 50% had experienced failure on ≥2 migraine‐preventive therapies. Mean (SD) MMD was similar across both groups (erenumab 70 mg: 17.9 [4.4] days; erenumab 140 mg: 17.8 [4.7] days). The key differences between the two treatment groups were a lower percentage of patients in the erenumab 70 mg group compared with patients in the erenumab 140 mg group who ever used topiramate (46.6% vs. 51.1%, respectively), and a slightly higher percentage who ever used onabotulinumtoxinA (26.2% vs. 22.6%, respectively). In addition, mean (SD) monthly acute headache medication days were slightly lower for erenumab 70 mg (8.8 [7.2] days) than for erenumab 140 mg (9.7 [7.0] days). Baseline demographics by initial response status (in Month 1) are presented in Table 2. Generally, these characteristics were consistent with those of the original study population with one notable difference among the groupings of patients: a lower percentage of initial responders were noted to have ever used topiramate or onabotulinumtoxinA than initial nonresponders.
TABLE 2.
Patient demographics and clinical characteristics at baseline by initial MMD response status at Month 1
| Erenumab 70 mg (N = 188) | Erenumab 140 mg (N = 187) | |||||
|---|---|---|---|---|---|---|
| Responders (N = 45) | Partial responders (N = 45) | Nonresponders (N = 98) | Responders (N = 53) | Partial responders (N = 34) | Nonresponders (N = 100) | |
| Age, years | 40.8 (9.9) | 41.8 (11.8) | 41.0 (11.7) | 43.1 (10.8) | 46.3 (7.8) | 42.0 (11.9) |
| Female, n (%) | 42 (93.3) | 39 (86.7) | 84 (85.7) | 45 (84.9) | 31 (91.2) | 83 (83.0) |
| Disease duration, years | 20.1 (12.3) | 20.7 (11.3) | 20.3 (13.4) | 22.0 (11.8) | 23.1 (10.8) | 21.8 (12.2) |
| History of previous prevention treatment failure, n (%) | ||||||
| Failure of ≥1 category | 28 (62.2) | 27 (60.0) | 69 (70.4) | 32 (60.4) | 25 (73.5) | 68 (68.0) |
| Failure of ≥2 categories | 18 (40.0) | 21 (46.7) | 52 (53.1) | 24 (45.3) | 17 (50.0) | 52 (52.0) |
| Previous use of preventives, n (%) | ||||||
| Topiramate | 16 (35.6) | 21 (46.7) | 50 (51.0) | 26 (49.1) | 20 (58.8) | 51 (51.0) |
| OnabotulinumtoxinA | 9 (20.0) | 10 (22.2) | 29 (29.6) | 9 (17.0) | 6 (17.6) | 28 (28.0) |
| Headache characteristics during the baseline period | ||||||
| Monthly headache days | 19.4 (3.1) | 20.3 (3.5) | 21.3 (4.0) | 20.1 (3.5) | 20.5 (3.1) | 21.2 (4.0) |
| MMD | 16.2 (3.6) | 17.6 (3.5) | 18.9 (4.8) | 17.0 (4.5) | 17.7 (4.0) | 18.2 (5.0) |
| Monthly AMSM days | 7.5 (7.0) | 9.8 (7.0) | 8.9 (7.4) | 9.6 (7.2) | 11.2 (6.5) | 9.2 (7.1) |
| AMSM use, n (%) | 28 (62.2) | 35 (77.8) | 77 (78.6) | 39 (73.6) | 31 (91.2) | 77 (77.0) |
| Monthly AMSM days among AMSM users | 12.0 (4.7) | 12.6 (5.2) | 11.3 (6.5) | 13.1 (5.0) | 12.3 (5.7) | 11.9 (5.7) |
Data are mean (SD), unless otherwise stated.
Abbreviations: AMSM, acute migraine‐specific medication; MMD, monthly migraine day.
No patients had missing MMD data at Month 1; at Months 2 and 3, missing data were reported for 8 and 15 patients, respectively.
Average time to monthly response
The cumulative percentage of patients with a ≥50% reduction in MMD over the course of the 3‐month treatment period is shown in Figure 2A. 57.4% (108/188) of patients who received erenumab 70 mg and 54.0% (101/187) of patients who received 140 mg had a response in any month during the 3‐month treatment period, with a median (Q1, Q3) time to onset of 2 (1, 2) and 1 (1, 2) month, respectively. Among responders in the 70 mg group, 41.7% (45/108) had their first ≥50% reduction in MMD at Month 1, and 77.8% (84/108) of patients responded by Month 2. Among responders in the 140 mg group, 52.5% (53/101) had a response at Month 1, and 84.2% (85/101) responded by Month 2.
FIGURE 2.
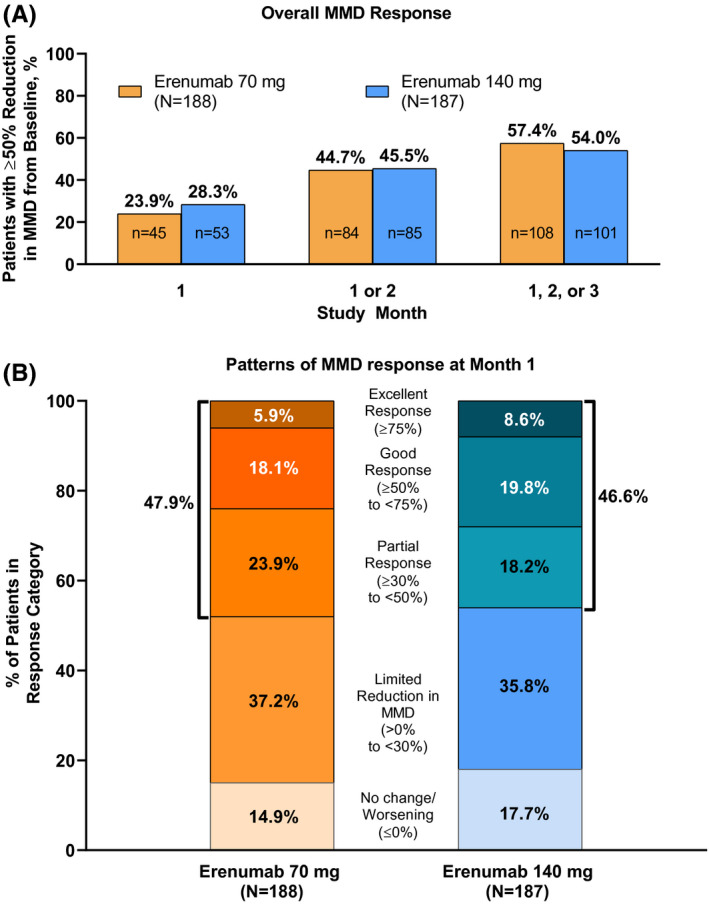
Overall MMD response and patterns of initial response at Month 1. (A) Cumulative number of patients who achieved a ≥50% reduction in MMD from baseline by each month during the 3‐month treatment period and (B) percentages of patients in each MMD response category*, based on reduction in MMD at Month 1. *Response categories are defined as follows: excellent, ≥75% reduction in MMD; good, ≥50% to <75% reduction in MMD; partial, ≥30% to <50% reduction in MMD; limited, >0% to <30% reduction in MMD; no change/worsening, no change or an increase in MMD. MMD, monthly migraine day
Sustained response in initial responders
In some patients, onset of efficacy occurred rapidly following the initiation of erenumab treatment, and early responses were generally maintained with continued erenumab treatment. The percentages of patients belonging to all response categories at Month 1 are shown in Figure 2B. A total of 23.9% (45/188) of patients receiving erenumab 70 mg were initial responders; 18.1% (34/188) had a good response, and 5.9% (11/188) had an excellent response. Of initial responders, 84.4% (38/45) had a good or better response at Month 2 or 3, including 55.6% (25/45) who had an excellent response. Similarly, 48.9% (22/45) of initial responders had a sustained response at Months 2 and 3, including 17.8% (8/45) who had a sustained excellent response (Table 3; Figure 3).
TABLE 3.
Overview of patients achieving a reduction in MMD at Month 2 or 3 and at Months 2 and 3, based on initial response at Month 1
| Initial response status | N1 | Treatment | |||||
|---|---|---|---|---|---|---|---|
| Subsequent response at Month 2 or 3, n (%) | Subsequent response at Months 2 and 3, n (%) | ||||||
| Partial | Good or better | Excellent | Partial | Good or better | Excellent | ||
| Erenumab 70 mg (N = 188) | |||||||
| Good or better | 45 | 3 (6.7) | 38 (84.4) | 25 (55.6) | 11 (24.4) | 22 (48.9) | 8 (17.8) |
| Partial | 45 | 5 (11.1) | 29 (64.4) | 8 (17.8) | 12 (26.7) | 15 (33.3) | 4 (8.9) |
| Limited | 70 | 15 (21.4) | 30 (42.9) | 9 (12.9) | 16 (22.9) | 8 (11.4) | 3 (4.3) |
| No change or worsening | 28 | 2 (7.1) | 4 (14.3) | 2 (7.1) | 1 (3.6) | 2 (7.1) | 0 |
| Erenumab 140 mg (N = 187) | |||||||
| Good or better | 53 | 3 (5.7) | 48 (90.6) | 31 (58.5) | 6 (11.3) | 37 (69.8) | 17 (32.1) |
| Partial | 34 | 3 (8.8) | 27 (79.4) | 10 (29.4) | 11 (32.4) | 12 (35.3) | 4 (11.8) |
| Limited | 67 | 19 (28.4) | 14 (20.9) | 5 (7.5) | 6 (9.0) | 7 (10.4) | 1 (1.5) |
| No change or worsening | 33 | 12 (36.4) | 7 (21.2) | 0 | 6 (18.2) | 0 | 0 |
Abbreviations: MMD, monthly migraine day; %, n/N1.
FIGURE 3.
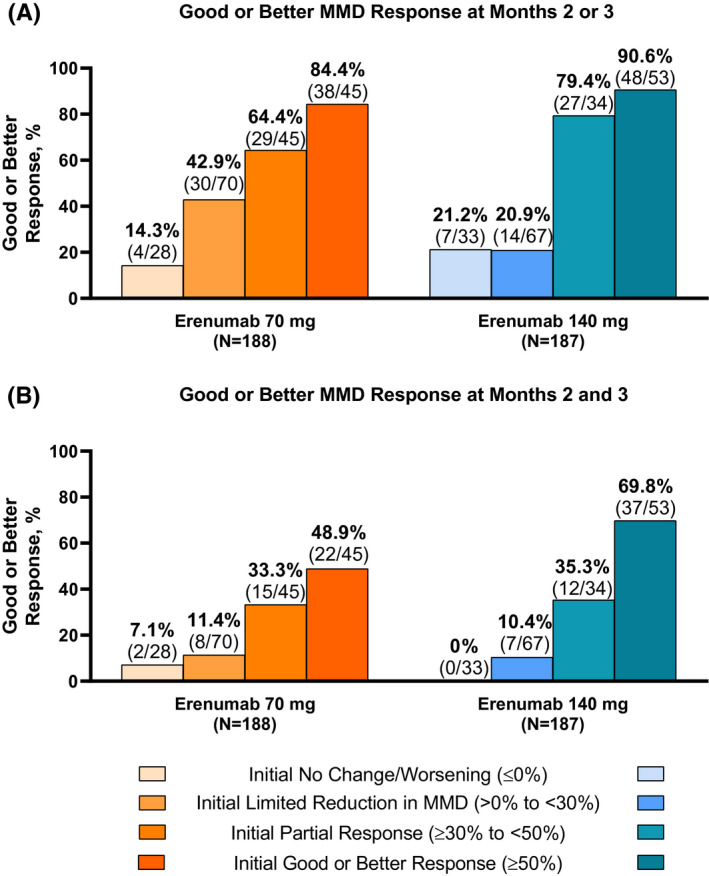
Patterns of continued and delayed response by the level of initial response at Month 1 after treatment with erenumab 70 and 140 mg. (A) Response at Month 2 or 3. (B) Response at Months 2 and 3. MMD, monthly migraine day
A total of 28.3% (53/187) of patients receiving erenumab 140 mg were initial responders (Figure 2B); 19.8% (37/187) had a good response and 8.6% (16/187) had an excellent response. Of initial responders, 90.6% (48/53) also had a response at Month 2 or 3, including 58.5% (31/53) who had an excellent response. Similarly, 69.8% (37/53) of initial responders had a sustained response at Months 2 and 3, including 32.1% (17/53) who had a sustained excellent response (Table 3; Figure 3).
Improved response in initial partial responders
Patients who achieved an initial partial response had a high likelihood of achieving a subsequent response (≥50% reduction from baseline in MMD) with continued erenumab treatment. At Month 1, 23.9% (45/188) of patients in the erenumab 70 mg group and 18.2% (34/187) of patients in the 140 mg group were partial responders (Figure 2B). A subsequent response was achieved at Month 2 or 3 by 64.4% (29/45) of initial partial responders in the 70 mg group (Figure 3A), including 17.8% (8/45) who further achieved an excellent response (Table 3). Similarly, 33.3% (15/45) of initial partial responders subsequently achieved and sustained a response at Months 2 and 3 (Figure 3B), including 8.9% (4/45) who further achieved an excellent response consistently at Months 2 and 3.
Among the initial partial responders in the 140 mg group, a subsequent response was achieved at Month 2 or 3 by 79.4% (27/34) (Figure 3A), including 29.4% (10/34) who had an excellent response (Table 3). Similarly, 35.3% (12/34) of initial partial responders subsequently achieved and sustained a response at Months 2 and 3 (Figure 3B), including 11.8% (4/34) who further achieved an excellent response consistently at Months 2 and 3.
Subsequent response in initial nonresponders
A total of 52.1% (98/188) of the patients in the 70 mg group did not achieve a response at Month 1 (Figure 2B). Most (71.4% [70/98]) of these initial nonresponders had a limited reduction in MMD, and fewer (28.6% [28/98]) showed no change or worsening MMD. In the 70 mg group, 37.2% (70/188) of patients overall had limited reduction, and 14.9% (28/188) of patients overall showed no change or worsening MMD (Figure 2B). A total of 53.5% (100/187) of patients in the erenumab 140 mg group were initial nonresponders, with most of these patients (67.0% [67/100]) having a limited reduction in MMD. In the 140 mg group, 35.8% (67/187) of patients overall had limited reduction and 17.7% (33/187) of patients showed no change or worsening MMD (Figure 2B).
Although many patients responded early to erenumab treatment, some patients required longer treatment to achieve a response (Figure 3; Table 3). Of initial nonresponders, 34.7% (24/98) of patients in the 70 mg erenumab group and 21.0% (21/100) of patients in the 140 mg erenumab group subsequently achieved a response at Month 2 or 3. Patients with an initial limited reduction in MMD generally improved with continued erenumab treatment with 64.3% (45/70) of the 70 mg group and 49.3% (33/67) of the 140 mg group achieving a partial or better response at Month 2 or 3. Generally, patients with an initial limited reduction in MMD eventually achieved and/or sustained a good or better response with continued erenumab treatment more so than those with no initial reduction in MMD (Figure 3; Table 3). Furthermore, those with an initial partial response appeared to have a better chance of achieving and/or sustaining a good or better response than those with an initial limited or no reduction in MMD in subsequent study months. This was true in all cases with one exception: in the 140 mg group, 20.9% (14/67) of the initial nonresponders with a limited reduction in MMD achieved a subsequent response at Month 2 or 3, and 21.2% (7/33) of patients with no change or worsening MMD initially subsequently achieved a response at Month 2 or 3.
DISCUSSION
Temporal response patterns to preventive treatments for migraine are important to understand. It is now well accepted that headache and migraine day frequencies are subject to natural fluctuation. 8 Longer‐term assessment of outcome is, therefore, important to clearly establish that effects observed are related to treatment and not simply natural variation. 8 Our results demonstrate that erenumab is associated with a meaningful treatment‐related reduction in MMD that is sustained over 3 months. Furthermore, over the 3‐month assessment period, the benefits of erenumab continued to accrue, with at least one‐half of initial partial responders experiencing response over the subsequent 2 months, as has been observed with other preventive therapies targeting the CGRP pathway. 9
In addition, our results suggest that erenumab 140 mg may be associated with better outcomes than erenumab 70 mg in patients with CM. Onset of first monthly response was earlier in patients receiving erenumab 140 mg versus 70 mg, with a median time of 1 versus 2 months. The majority of patients with an initial partial response to erenumab experienced a good or better response at Month 2 or 3, with higher rates of subsequent response reported for erenumab 140 mg versus erenumab 70 mg (79.4% vs. 64.4%). This was also true when looking at those who responded at Months 2 and 3. For Month 2 or 3, the higher subsequent response to erenumab 140 mg compared with erenumab 70 mg was not consistently observed among initial nonresponders; it is possible that a longer period of follow‐up will be required for a differential dose response to be observed in this subgroup. When analyses were undertaken using a lower threshold for response (≥30% reduction in MMD from baseline), overall patterns of response were similar between the two erenumab doses although, as would be expected, numerically greater numbers of patients achieved a response compared with using the ≥50% threshold.
Patients’ expectations for rapid onset of efficacy may be a factor leading to low adherence to preventive migraine treatments in the real world. However, there is evidence that responders show cumulative benefits leading to better response rates over time. 10 For this reason, it is advised that the overall efficacy of a treatment should be judged after several treatment cycles. 10 It has been established previously that erenumab may have early onset of efficacy for some patients, with separation from placebo within the first week of treatment. 11 Our analysis assessed time to observe a reduction in MMD, with many patients achieving a response at the earliest monthly time point evaluated, Month 1, with the 140 mg dose (i.e., weekly migraine days or probability of a migraine on each day was not assessed here). Importantly, for patients with robust initial responses, our results suggest that the majority will have sustained improvement in headache measures over 3 months. Hence, being equipped with this type of information early on may reduce some of the attrition introduced by patient expectations.
For patients who do not achieve a robust initial response to erenumab, clinicians need to determine the optimal time frame over which to reassess treatment response. 6 The American Headache Society (AHS) suggests that for anti‐CGRP monoclonal antibody‐preventive treatment with a monthly dosing schedule, outcomes should be assessed after 3 months of treatment. 6 The European Headache Federation (EHF) guideline suggests treatment should be continued for ≥3 months, even in those experiencing no benefit, and for 6–12 months in those experiencing benefit. 12 The results of the current analyses support these recommendations regarding a 3‐month observation period before assessing therapeutic outcome. We found that the majority of patients with a partial reduction in MMD after 1 month of treatment are likely to achieve a good or better response over time if continued on erenumab treatment for up to 3 months. A proportion of those with an initial limited or no reduction in MMD also went on to achieve a good or better response in MMD in subsequent study months. Furthermore, after the 3‐month double‐blind period of this study, patients were enrolled into a 52‐week open‐label study of active study drug only (erenumab only); MMD continued to decrease from baseline throughout this extension study, with 65% of patients on erenumab monthly achieving ≥50% reduction in MMD. 13 Likewise, longer‐term evaluations have demonstrated that benefits continue to accrue for more than 4 years with ongoing treatment of episodic migraine with erenumab, with 77% of patients achieving ≥50% reduction in MMD, 14 suggesting further reassessment of treatment response beyond 3 months would be required to fully assess patient response to erenumab. Although only analyzed over 3 months, our results show that even in initial nonresponders, continued erenumab treatment may be associated with increased benefit over time.
The underlying mechanism of action leading to delayed onset of effect observed in a subgroup of patients with migraine is not well understood but may have a neurobiological basis in some patients. Central sensitization appears to be involved in the development of CM, with CGRP playing a key role in increased nociceptive sensitivity. 15 It is possible that delayed onset of action is, in part, related to the time between the reversal of peripheral sensitization and the subsequent reduction in central sensitization. Removal of the peripheral stimulus, per se, appears to be only the first step in treating CM; resolution of central sensitization may also be required and reversal of central sensitization is likely time dependent. 16 , 17 This could explain the delayed onset of action observed with other preventive treatments, 9 , 16 especially those with a peripheral mechanism of action (e.g., botulinum toxin). 16 It is also possible that delayed onset is related to interindividual pharmacokinetic differences. In an analysis of 108 patients weighing 49–104 kg, for every 10 kg increase in patient body weight, the clearance of erenumab increased by 11%, and the central volume of distribution increased by 14%. 18 The magnitude of these changes may be sufficient to increase the time to achieve steady‐state erenumab concentrations. Lastly, these results may partially reflect a tendency for the number of MMD to regress toward a mean. 8
Strengths and limitations
A key strength of this study is that the data are from a well‐designed, double‐blind, placebo‐controlled study, the results of which have previously been reported in full. 4 The study excluded patients refractory to more than three categories of preventive therapies, limiting the generalizability of study results to this very treatment‐refractory subgroup of patients with migraine. For this analysis, in particular, the 3‐month study duration is an important limitation; while the patterns of response to erenumab over 12 weeks can be described, the temporal response to erenumab over longer periods is also important to understand. In our analysis the percentage of patients achieving a good or better response to erenumab continued to increase over the 3‐month period; analysis of data over a longer period is required to determine the time from initiation of erenumab treatment to optimal response. In addition, the definition of response in this study is strictly in terms of MMD reduction. As mentioned earlier in the discussion, other analyses such as weekly migraine days and probability of a migraine day comparison have indicated some patients responding at earlier time points, within the first week. Furthermore, we know that patients can still be considered “responders” in other clinically meaningful parameters (e.g., reduction in severity, associated nonpain symptoms, functional disability) not assessed here. Finally, this was a post hoc analysis, which prevented the use of inferential testing because there is an associated risk of statistical significance inflation. 19
CONCLUSIONS
Using ≥50% reduction in MMD from baseline as the response threshold, a majority of patients with CM who achieved an initial response at Month 1 with erenumab sustained that benefit with continued treatment. Although some patients may respond early within the first week or month, there are some who respond later with continued treatment. Our data therefore support recommendations made by the American Headache Society and European Headache Federation about assessing treatment outcomes after ≥3 months of migraine‐preventive treatment. Additionally, ≥30% reduction in MMD from baseline may be an appropriate threshold in patients with CM for assessing response, as many patients who initially achieve a partial response (≥30% reduction in MMD) achieved a response (≥50% reduction in MMD) with continued treatment. Whether the timing of the first response is associated with the level of response, as well as other non‐MMD parameters, merits further investigation.
CONFLICT OF INTEREST
SJT has received grants for research (no personal compensation) from Allergan/Abbvie, Amgen, Eli Lilly, Lundbeck, Neurolief, Novartis, Satsuma, and Zosano, has served as a consultant for Aeon, Allergan/Abbvie, AlphaSights, Amgen, Atheneum, Axsome Therapeutics, Becker Pharmaceutical Consulting, ClearView Healthcare Partners, CoolTech, CRG, Currax, DRG, Eli Lilly, ExpertConnect, FCB Health, GLG, Guidepoint Global, Health Science Communications, HMP Communications, Impel, InteractiveForums, Krog and Partners, Lundbeck, M3 Global Research, MJH Holdings, Neurolief, Novartis, Palion Medical, Pulmatrix, SAI MedPartners, Satsuma, Spherix Global Insights, Strategy Inc, System Analytic, Taylor and Francis, Teva, Theranica, UnityHA, Xoc, and Zosano, has received salary from Dartmouth‐Hitchcock Medical Center, American Headache Society, and the Thomas Jefferson University, and has received continuing medical education honoraria from the Annenberg Center for Health Sciences, American Academy of Neurology, American Headache Society, Catamount Medical Education, Diamond Headache Clinic, Forefront Collaborative, Haymarket Medical Education, Peerview, Medical Education Speakers Network, Migraine Association of Ireland, North American Center for CME, The Ohio State University, Physicians’ Education Resource, PlatformQ Education, Primed, Texas Neurological Society, WebMD/Medscape. SL has received advisory or consulting fees from Amgen, Alder, Biohaven, Impel, Eli Lilly, Lundbeck, and Teva, and receives compensation from Premera as a member of the Pharmacy & Therapeutics committee. MA has received personal fees from Alder, Allergan, Amgen, Alder, Eli Lilly, Novartis, and Teva; has participated in clinical trials as the principal investigator for Alder, Allergan, Amgen, ElectroCore, Eli Lilly, Novartis and Teva; has received a research grant form Novartis; has no ownership interest and does not own stocks of any pharmaceutical company; serves as associate editor of Cephalalgia, associate editor of The Journal of Headache and Pain, and associate editor of Headache, and is President of the International Headache Society. TS has received consulting fees from Alder, Allergan, Amgen, Aural Analytics, Avanir, Cipla, Dr. Reddy's, Eli Lilly, Ipsen Bioscience, Nocira, Novartis, Second Opinion, and Teva. JA has received consulting fees from Alder, Allergan, electroCore, Eli Lilly, Impel, Promius, and Teva; honoraria from Alder, Allergan, Amgen, Avanir, electroCore, Eli Lilly, Promius, and Teva; and serves as section editor for Current Pain and Headache Reports. JS, DEC, AW, and GPdSL are employees and stockholders of Amgen. JK is an employee and stockholder of Novartis.
AUTHOR CONTRIBUTIONS
Study concept and design: Gabriel Paiva da Silva Lima, Denise E. Chou, Jan Klatt, James Scanlon. Acquisition of data: Gabriel Paiva da Silva Lima, Denise E. Chou, Jan Klatt, James Scanlon. Analysis and interpretation of data: Stewart J. Tepper, Sylvia Lucas, Messoud Ashina, Todd J. Schwedt, Jessica Ailani, James Scanlon, Jan Klatt, Denise E. Chou, Andrea Wang, Gabriel Paiva da Silva Lima. Drafting of the manuscript: Gabriel Paiva da Silva Lima, Denise E. Chou, Jan Klatt, James Scanlon. Revising it for intellectual content: Stewart J. Tepper, Sylvia Lucas, Messoud Ashina, Todd J. Schwedt, Jessica Ailani, James Scanlon, Jan Klatt, Denise E. Chou, Andrea Wang, Gabriel Paiva da Silva Lima. Final approval of the completed manuscript: Stewart J. Tepper, Sylvia Lucas, Messoud Ashina, Todd J. Schwedt, Jessica Ailani, James Scanlon, Jan Klatt, Denise E. Chou, Andrea Wang, Gabriel Paiva da Silva Lima.
CLINICAL TRIALS REGISTRATION NUMBER
NCT02066415 (clinicaltrials.gov).
ACKNOWLEDGMENTS
The authors thank Lee Hohaia, PharmD (ICON, North Wales, PA), whose work was funded by Amgen Inc., and Jon Nilsen, PhD (Amgen Inc.) for medical writing assistance in the preparation of this manuscript.
Tepper SJ, Lucas S, Ashina M, et al. Timing and durability of response to erenumab in patients with chronic migraine. Headache. 2021;61:1255–1263. 10.1111/head.14193
Funding information
This analysis was funded by Amgen Inc. and Novartis
DATA AVAILABILITY STATEMENT
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
REFERENCES
- 1. Aimovig (erenumab‐aooe). Full prescribing information. Thousand Oaks, CA: Amgen Inc.; 2020. [Google Scholar]
- 2. Shi L, Lehto SG, Zhu DXD, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene‐related peptide receptor. J Pharmacol Exp Ther. 2016;356:223‐231. [DOI] [PubMed] [Google Scholar]
- 3. Edvinsson L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol. 2015;80:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Neurol. 2017;16:425‐434. [DOI] [PubMed] [Google Scholar]
- 5. Tassorelli C, Diener H‐C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815‐832. [DOI] [PubMed] [Google Scholar]
- 6. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 7. Tepper SJ, Diener H‐C, Ashina M, et al. Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology. 2019;92:e2309‐e2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nichols R, Doty E, Sacco S, Ruff D, Pearlman E, Aurora SK. Analysis of initial nonresponders to galcanezumab in patients with episodic or chronic migraine: results from the EVOLVE‐1, EVOLVE‐2, and REGAIN randomized, double‐blind, placebo‐controlled studies. Headache. 2019;59:192‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dodick DW. CGRP ligand and receptor monoclonal antibodies for migraine prevention: evidence review and clinical implications. Cephalalgia. 2019;39:445‐458. [DOI] [PubMed] [Google Scholar]
- 11. Schwedt T, Reuter U, Tepper S, et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J Headache Pain. 2018;19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sacco S, Bendtsen L, Ashina M, et al. European Headache Federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ashina M, Dodick D, Goadsby PJ, et al. Erenumab (AMG 334) in episodic migraine: interim analysis of an ongoing open‐label study. Neurology. 2017;89:1237‐1243. [DOI] [PubMed] [Google Scholar]
- 14. Ashina M, Goadsby PJ, Reuter U, et al. Long‐term efficacy and safety of erenumab in migraine prevention: results from a 5‐year, open‐label treatment phase of a randomized clinical trial. Eur J Neurol. 2021;28:1716‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene‐related peptide enhances release of native brain‐derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006;99:1338‐1350. 10.1111/j.1471-4159.2006.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silberstein SD, Dodick DW, Aurora SK, et al. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia. 2014;34:853‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vu T, Ma P, Chen JS, et al. Pharmacokinetic–pharmacodynamic relationship of erenumab (AMG 334) and capsaicin‐induced dermal blood flow in healthy and migraine subjects. Pharm Res. 2017;34:1784‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Srinivas TR, Ho B, Kang J, Kaplan B. Post hoc analyses: after the facts. Transplantation. 2015;99:17‐20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.


