Summary
Strains belonging to the Pseudomonas protegens and Pseudomonas chlororaphis species are able to control soilborne plant pathogens and to kill pest insects by producing virulence factors such as toxins, chitinases, antimicrobials or two‐partner secretion systems. Most insecticidal Pseudomonas described so far were isolated from roots or soil. It is unknown whether these bacteria naturally occur in arthropods and how they interact with them. Therefore, we isolated P. protegens and P. chlororaphis from various healthy insects and myriapods, roots and soil collected in an agricultural field and a neighbouring grassland. The isolates were compared for insect killing, pathogen suppression and host colonization abilities. Our results indicate that neither the origin of isolation nor the phylogenetic position mirror the degree of insecticidal activity. Pseudomonas protegens strains appeared homogeneous regarding phylogeny, biocontrol and insecticidal capabilities, whereas P. chlororaphis strains were phylogenetically and phenotypically more heterogenous. A phenotypic and genomic analysis of five closely related P. chlororaphis isolates displaying varying levels of insecticidal activity revealed variations in genes encoding insecticidal factors that may account for the reduced insecticidal activity of certain isolates. Our findings point towards an adaption to insects within closely related pseudomonads and contribute to understand the ecology of insecticidal Pseudomonas.
Introduction
The Pseudomonas genome is complex and plastic, which allows these bacteria to colonize a wide variety of habitats such as plant roots, animals or polluted water (Silby et al., 2011; Rumbaugh, 2014). Different Pseudomonas species are either generalists or form specific interactions with certain hosts. For instance, human pathogenic multidrug‐resistant Pseudomonas aeruginosa strains are more adapted to cystic fibrosis patients than to healthy humans (AbdulWahab et al., 2014; Freschi et al., 2018), whereas different Pseudomonas syringae pathovars are able to infect and cause disease in specific plant hosts (reviewed by O'Brien et al., 2011; Thynne et al., 2015). Some Pseudomonas are also adapted to live as endophytes which may allow the bacteria to exploit the resources of the plant host more efficiently (reviewed by Afzal et al., 2019). Also, Pseudomonas stutzeri and Pseudomonas fluorescens show preferences for colonizing wheat roots over cucumber roots (Tovi et al., 2019).
Insect microbiomes include bacteria, archaea, fungi, viruses and protists which can protect the host against invading pathogens, increase the availability of nutrients (reviewed by Dillon and Dillon, 2004; Engel and Moran, 2013; Paniagua Voirol et al., 2018; Gurung et al., 2019) or even confer resistance to pesticides (Gomes et al., 2020; Wang et al., 2020). The physiology, the diet, the behaviour and the niche of the different insect orders highly influence the composition of the microbiota and its transmission (Engel and Moran, 2013; Esposti and Romero, 2017; Paniagua Voirol et al., 2018; Hannula et al., 2019). Here, we will focus on holometabolous insects including Coleoptera, Diptera and Lepidoptera. They mostly obtain their microbial communities from their diet or the environment and the communities change throughout the different life stages of the insect (Vasanthakumar et al., 2008; Lauzon et al., 2009; Aharon et al., 2013; Hammer et al., 2014; Montagna et al., 2015a; Montagna et al., 2015b; Chen et al., 2016; Malacrino et al., 2018; González‐Serrano et al., 2019). Yet, Diptera and Coleoptera retain some core microbiota that is transmitted vertically through generations (Lauzon et al., 2009; Ziganshina et al., 2018; Chouaia et al., 2019; Kolasa et al., 2019). On the other hand, it has been proposed that Lepidoptera larvae do not have a core microbiota probably due to the demanding conditions of their guts such as high pH, antimicrobial peptides and the lack of pouches or cavities that can accommodate microbial communities (Engel and Moran, 2013; Hammer et al., 2017). Indeed, it has been shown that Lepidoptera larvae can obtain their microbiota from their diet and interactions with the soil (Hammer et al., 2017; Hannula et al., 2019). Other arthropods such as myriapods have not been studied as detailed as insects but one biorxiv study described Proteobacteria associated with centipedes belonging to the Geophilidae family (Geli‐Cruz et al., 2019). Pseudomonas have been detected within the arthropod microflora in several studies (reviewed by Esposti and Romero, 2017; Paniagua Voirol et al., 2018). Although pseudomonads were detected in field and laboratory‐reared Lepidoptera (Çakici et al., 2014; Chen et al., 2016; Mashtoly et al., 2019; Skowronek et al., 2020) and Coleoptera (Bahar and Demirbağ, 2007; Montagna et al., 2015a; Montagna et al., 2015b), and in laboratory‐reared Diptera (Wong et al., 2015), it is still uncertain whether they have established a relationship with these insects or if they are just transient bacteria in their gut.
Some plant‐beneficial pseudomonads belonging to the Pseudomonas protegens, Pseudomonas chlororaphis and P. fluorescens species can also be pathogens of diverse pest insect species (Péchy‐Tarr et al., 2008; Jang et al., 2013; Kupferschmied et al., 2013, 2014; Péchy‐Tarr et al., 2013; Ruffner et al., 2013, 2015; Flury et al., 2016, 2019; Loper et al., 2016; Vacheron et al., 2019; Vesga et al., 2020). Pseudomonas protegens and P. chlororaphis are known root colonizers and they can control soil‐borne fungal pathogens (Haas and Défago, 2005). However, they can, after oral uptake, also invade the insect gut, transmigrate into the hemocoel, cause a systemic infection and kill the insect (Kupferschmied et al., 2013; Flury et al., 2019; Vacheron et al., 2019). Insecticidal activity in these two species was first related to the Fit toxin encoded by the fitD gene (Péchy‐Tarr et al., 2008). This toxin is very similar to the Mcf (make caterpillars floppy) toxins of Photorhabdus luminescens and Xenorhabdus nematophila (Daborn et al., 2002; Waterfield et al., 2003; Ruffner et al., 2015). Subsequent work established that insecticidal activity in P. protegens and P. chlororaphis is a multifactorial trait that involves, besides the Fit toxin, various elements such as lipopolysaccharide O‐antigens (Kupferschmied et al., 2014), the type VI secretion system (Vacheron et al., 2019), the cyclic lipopeptide orfamide A (Jang et al., 2013; Flury et al., 2016), a chitinase and a phospholipase C (Flury et al., 2016), hydrogen cyanide (Flury et al., 2017), the toxins rhizoxin (Loper et al., 2016) or IPD072Aa (Schellenberger et al., 2016), and two‐partner secretion (TPS) systems (Vesga et al., 2020).
Pseudomonas chlororaphis and P. protegens cause high levels of mortality in larvae of different Lepidoptera species such as Spodoptera littoralis, Pieris brassicae, Manduca sexta, Plutella xylostella and Heliothis virescens (Péchy‐Tarr et al., 2008; Péchy‐Tarr et al., 2013; Ruffner et al., 2013, 2015; Flury et al., 2019) and they also negatively affect pupation rates in Delia radicum (Flury et al., 2019). Specifically, P. protegens strain CHA0 is able to persist throughout different D. radicum, P. xylostella and P. brassicae life stages and can even be transmitted to new host plants by D. radicum flies (Flury et al., 2019). However, no oral effect was found for larvae of the Coleoptera Otiorhynchus sulcatus even though P. protegens CHA0 was detected in pupae and adults (Flury et al., 2019).
Most of the published P. protegens and P. chlororaphis isolates with described insecticidal activity were isolated from soil or plant environments. Thus, the first aim of this study was to isolate P. protegens and P. chlororaphis from arthropods collected in agricultural and neighbouring undisturbed grassland in order to characterize their natural association with these animals. The second aim was to investigate whether root and arthropod isolates differ in their abilities or efficacies to colonize and kill an insect host and to colonize plant roots and protect them against a soilborne pathogen. Finally, a variant calling approach was performed on known virulence traits to determine whether genetic modifications may explain the different killing ability of these Pseudomonas. This study indicates that P. protegens species is more homogeneous, whereas P. chlororaphis is more phylogenetically and phenotypically diverse for the studied traits, independently of their isolation origin. We identified several variations in key insecticidal genes that may be linked to an attenuation of insecticidal activity in certain Pseudomonas isolates, potentially reflecting an ongoing adaptation to their host.
Results and discussion
Pseudomonas protegens and P. chlororaphis strains are naturally associated with arthropods, plants and soil of agricultural fields
Pseudomonas protegens and P. chlororaphis strains have demonstrated insecticidal activity (Péchy‐Tarr et al., 2008; Loper et al., 2012, 2016; Jang et al., 2013; Flury et al., 2016, 2017, 2019; Vacheron et al., 2019; Vesga et al., 2020). However, it was not known, whether these two species are naturally associated with insects. The first aim of our study was thus to search for the presence of P. protegens and P. chlororaphis in insects and other arthropods collected from soil. We collected a total of 120 arthropods belonging to 51 different genera over 2 years at the Reckenholz facilities of Agroscope (Switzerland), i.e. in an agricultural field (cropped with wheat in 2016 and with potato in 2017) and in a patch of neighbouring grassland. A total of 660 bacteria were isolated from those arthropods after a strict surface disinfection previously validated by Flury et al. (2019), from roots or from soil. Ninety isolates were characterized as Pseudomonas spp. based on their 16S rRNA gene sequence. From those, 14 arthropod isolates were identified as P. protegens (~15%) and 14 as P. chlororaphis (~15%). Additionally, eight (~9%) P. chlororaphis were isolated from grassland roots, potato roots or soil. We sequenced 10 genomes of each species for this study. Surprisingly, no P. protegens strains were detected in the root or soil samples collected (Table S1). The Pseudomonas isolates that were not assigned as P. protegens or P. chlororaphis as well as the non‐Pseudomonas isolates were not further characterized.
The P. protegens isolates are closely related to each other but phylogenetically divergent from other previously described P. protegens root isolates (Figs 1 and S1). A proteome‐wide analysis showed that the new isolates harbour similar orthologue groups compared to closely related strains (Fig. S1). In particular, these isolates harbour the same plant beneficial and insecticidal traits as other P. protegens strains already described, which were mainly isolated from soil or plants (Fig. 1; Table S2). This confirms that the distribution pattern of specific insecticidal and plant‐beneficial traits is very conserved within this Pseudomonas subgroup. An exception is rhizoxin, a toxin with insecticidal activity so far only detected in a specific phylogenetic clade within the P. protegens subgroup harbouring strains Pf‐5 and PF. This toxin is absent in the new isolates, which is in line with their phylogenetic position (Fig. 1).
Fig. 1.
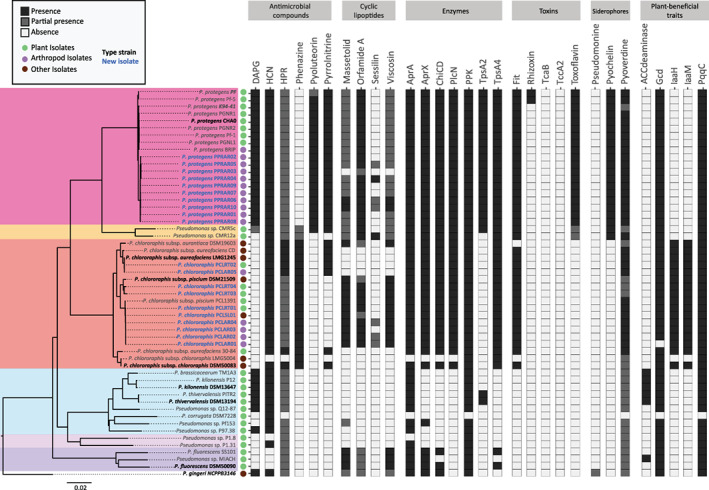
Distribution of functional traits related to insecticidal and/or plant‐beneficial activity among fluorescent Pseudomonas. The phylogenetic tree of 54 Pseudomonas genomes is based on the whole protein sequence extracted from the NCBI or predicted with Prokka. The squares correspond to the presence of amino acid sequences related to a functional trait. Black squares: present; grey squares: partially present; white squares: absent. Type strains are marked in bold and new isolates in blue. The origin of isolation is indicated with green dots for strains isolated from plants and with purple dots for arthropod isolates. Brown dots indicate other origins of isolation. References from all the strains are provided in Supplementary Tables S1 and S2.
In contrast to P. protegens, P. chlororaphis were isolated from roots, soil and invertebrates. These isolates are closely related to the type strain P. chlororaphis subsp. piscium DSM 21509T, P. chlororaphis subsp. aureofaciens LMG1245T, or to P. chlororaphis subsp. piscium PCL1391 (Fig. 1, Fig. S1). Interestingly, P. chlororaphis isolates did not cluster according to the niche of isolation (soil, root or arthropod; Figs 1 and S1). Even though the P. chlororaphis subgroup was much more heterogeneous regarding the distribution of plant‐beneficial/insecticidal traits, new P. chlororaphis isolates possess the same traits as the other strains they are clustering with (Fig. 1). Additionally, a group of strains closely related to P. chlororaphis subsp. piscium PCL1391 has more orthologue groups than the rest of the chlororaphis cluster (Fig. S1).
Although pseudomonads have been isolated from different laboratory‐reared or field insects, none of them was fully characterized to the species level or were found to be insecticidal (Bahar and Demirbağ, 2007; Çakici et al., 2014; Bensidhoum et al., 2016; Mashtoly et al., 2019; Skowronek et al., 2020). Our results indicate that plant‐beneficial P. protegens and P. chlororaphis are indeed commonly associated with apparently healthy arthropods and harbour the same traits as closely related strains isolated from soil or roots.
Pseudomonas protegens and P. chlororaphis isolates show variable insecticidal activity independent of their root or arthropod origin
Since P. chlororaphis and P. protegens isolated from arthropods and roots seem to display largely similar patterns of insecticidal traits, we wondered whether they would differ in insecticidal activity. To evaluate the insecticidal capabilities of the new isolates, second instar P. xylostella larvae were fed with artificial diet pellets spiked with 4 × 106 bacterial cells. Mortality was regularly assessed which allowed to calculate the LT50 and the survival rates of the larvae. LT50 and mortality curves were compared to those of the well‐studied reference strains P. protegens CHA0 and P. chlororaphis PCL1391, which are both plant‐root isolates. These comparisons are summarized in Table 1 and further described in detail in Tables S3 and S4.
Table 1.
Summary of comparisons of insecticidal activity of different Pseudomonas chlororaphis and Pseudomonas protegens isolates with the reference strains P. chlororaphis PCL1391 and P. protegens CHA0.
| LT50 | Log‐rank test | Total experiments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Strain | Origin of isolation | + | = | − | + | = | − | =NaCl | |
| P. chlororaphis | PCL1391 | Tomato root | ||||||||
| P. chlororaphis | 30.84 | Wheat seed | 1 | 1 | 2 | 2 | ||||
| P. chlororaphis | PCLAR01 | Aphodiinae (Coleoptera) | 3 | 3 | 3 | |||||
| P. chlororaphis | PCLAR02 | Aphodiinae (Coleoptera) | 2 | 2 | 2 | |||||
| P. chlororaphis | PCLAR03 | Undefined Diptera sp. | 2 | 3 | 1 | 4 | 5 | |||
| P. chlororaphis | PCLAR04 | Scarabaeidae pupa (Coleoptera) | 1 | 5 | 3 | 3 | 6 | |||
| P. chlororaphis | PCLAR05 | Scarabaeidae larva (Coleoptera) | 1 | 2 | 1 | 1 | 2 | 1 | 4 | |
| P. chlororaphis | PCLRT01 | Grass land, roots | 2 | 1 | 1 | 2 | ||||
| P. chlororaphis | PCLRT02 | Potato root | 3 | 1 | 2 | 3 | ||||
| P. chlororaphis | PCLRT03 | Potato root | 1 | 2 | 1 | 2 | 3 | |||
| P. chlororaphis | PCLRT04 | Potato root | 1 | 2 | 1 | 2 | 3 | |||
| P. chlororaphis | PCLSL01 | Potato field, soil | 1 | 1 | 1 | 1 | 2 | |||
| P. protegens | CHA0 | Tobacco root | ||||||||
| P. protegens | K94.41 | Cucumber root | 1 | 2 | 1 | 2 | 3 | |||
| P. protegens | Pf‐1 | Tobacco root | 2 | 1 | 2 | 1 | 3 | |||
| P. protegens | Pf‐5 | Soil | 2 | 2 | 2 | |||||
| P. protegens | PGNL1 | Tobacco root | 2 | 2 | 2 | |||||
| P. protegens | PGNR2 | Tobacco root | 2 | 2 | 2 | |||||
| P. protegens | PPRAR01 | Lithobius (Myriapod) | 2 | 1 | 2 | 1 | 3 | |||
| P. protegens | PPRAR02 | Lithobius (Myriapod) | 2 | 1 | 2 | 1 | 3 | |||
| P. protegens | PPRAR03 | Larva (undefined Lepidoptera sp.) | 3 | 3 | 3 | |||||
| P. protegens | PPRAR04 | Agriotes (Coleoptera) | 3 | 3 | 3 | |||||
| P. protegens | PPRAR05 | Staphylinidae (Coleoptera) | 2 | 1 | 1 | 1 | 1 | 3 | ||
| P. protegens | PPRAR06 | Agriotes (Coleoptera) | 2 | 1 | 1 | 2 | ||||
| P. protegens | PPRAR07 | Lithobius (Myriapod) | 2 | 1 | 1 | 2 | ||||
| P. protegens | PPRAR08 | Geophilidae (Myriapod) | 1 | 1 | 2 | 2 | ||||
| P. protegens | PPRAR09 | Curculionidae (Coleoptera) | 1 | 1 | 2 | 2 | ||||
| P. protegens | PPRAR10 | Agrypnus murinus (Coleoptera) | 2 | 2 | 2 | |||||
Numbers indicate the number of experiments in which a strain was significantly more insecticidal (+), less insecticidal (−) or not significantly different (=) compared to PCL1391 (reference for P. chlororaphis) or CHA0 (reference for P. protegens). Comparisons are based on LT50 and log‐rank tests performed on Kaplan–Meyer survival curves. Plutella xylostella larvae were fed with pellets spiked with 4 × 106 bacterial cells and survival was recorded over time. LT50 values were calculated using the ‘ecotox’ package of R and considered significantly different if the 95% confidence intervals of the studied strains and references did not overlap. Insect survival curves were evaluated using the log‐rank test in the ‘survival’ package of R and considered significantly different at p < 0.05. Detailed results of individual experiments are shown in Figs S2 and S3 (Kaplan–Meyer survival curves), Table S3 (LT50 values) and Table S4 (p‐values of log‐rank tests).
The performance of P. protegens strains was quite consistent as the killing speed and the global mortality of the larvae only differed occasionally compared to the reference P. protegens CHA0 (Table 1; Fig. S2A). Pseudomonas chlororaphis strains displayed more variability as several of them killed significantly slower or faster compared to the reference strain PCL1391 (Table 1) with particular isolates only causing partial mortality (Fig. S2B). Indeed, the LT50 values presented in Table S3 show a similar scenario with P. protegens strains differing much less among each other in the individual experiments than the P. chlororaphis strains. Kaplan–Meyer plots of individual experiments are shown in Fig. S3 and according to log‐rank values in Table S4. In general, P. protegens killed P. xylostella larvae faster than P. chlororaphis, which was already observed by Flury et al. (2016). There were differences in larval susceptibility between the first sets of experiments (1–5) and the experiments performed 1 year later (6–16). In the first set, larvae died generally at a slower pace compared to the second, which was probably due to different fitness of larvae at different rearing times. LT50 values for larvae in the first set of experiments ranged from 37 to 70 h in the P. protegens and from 66 to 191 h in the P. chlororaphis treatments and in the second set from 20 and 37 h for P. protegens and from 20 to 57 h for P. chlororaphis (Table S3).
In summary, for both species root and arthropod isolates show similar levels of insecticidal activities. The variation observed for P. chlororaphis strains does not seem to be related to the phylogenetic position or the niche of isolation (plant, soil vs. arthropod). Root isolates of the P. chlororaphis species from crops or grassland showed similar variability in activity against P. xylostella larvae as did arthropod isolates (Table 1). A comparison of the clusters of orthologue genes (COG) revealed that there are no major differences in COG content according to phylogeny, origin of isolation or insecticidal capabilities (Fig. S4). This supports that the insecticidal capabilities of the strains are independent of their origin of isolation and phylogeny. Therefore, regarding the differences of killing speed and insect mortality, it could be hypothesized that these two bacterial species have the potential to modulate their virulence towards their hosts, leading to a continuum of possible interactions ranging from pathogenic to commensal under certain circumstances. Indeed, the new strains were isolated from healthy myriapods or healthy insects belonging to different orders, mostly to Coleoptera. However, all of them were insecticidal in our feeding system with P. xylostella, even if they showed different progression of the infection. This might indicate species‐specific adaptation. This is supported by several studies describing variable effects of P. protegens and P. chlororaphis on insects of different orders such as differences in mortality, in persistence or in causing anomalous morphologies in adults (Saravanakumar et al., 2007; Olcott et al., 2010; Kupferschmied et al., 2013; Flury et al., 2019). We speculate that the association of P. chlororaphis and P. protegens strains with Coleoptera might be predominantly commensal and only becomes pathogenic when the bacteria have access to the hemolymph. Moreover, under laboratory rearing conditions, the insects are not exposed to the nutrients and microorganisms of their natural habitat, which are relevant for the insect fitness and protection against invader microbes respectively (reviewed by Chambers and Schneider, 2012; Gurung et al., 2019). The intake of different diets has been shown to affect the immune response of several insect species such as Grammia incorrupta and Bombus terrestris (Chambers and Schneider, 2012; Singer et al., 2014; Roger et al., 2017). Thus, further quantitative studies with field‐collected Lepidoptera and Coleoptera would be necessary to confirm pathogenic or commensal species‐ or order‐specific interactions of P. protegens and P. chlororaphis with insects.
Closely related P. chlororaphis strains display high variability in insect colonization and killing activity but similar plant‐protection abilities
Considering the previous results, we aimed at further characterizing a subset of closely related strains that displayed divergent (i) orthologue groups, (ii) insecticidal activities towards the insect and (iii) origin of isolation (root vs. insect). We selected four P. protegens strains, i.e. two root isolates (CHA0 and K94.41) and two insect isolates (PPRAR03 and PPRAR04) and five P. chlororaphis strains including two root isolates (PCL1391 and PCLRT03) and three closely related insect isolates (PCLAR01, PCLAR03 and PCLAR04) (Fig. 1). These selected bacterial strains shared at least 96.61% of genome identity (based on ANIm values, Table S1). Strains of the same species were compared to each other for insecticidal activity, insect colonization ability, root colonization ability and efficacy in controlling root disease caused by the oomycete pathogen Pythium ultimum. Besides the mortality monitoring, a subset of insects was dedicated to assess the insect colonization at 5 and 30 h after infection.
Pseudomonas protegens isolates from roots and insects killed and colonized the P. xylostella larvae equally well in all experiments (Figs 2A–C and 3A), except the strain K94.41 that displayed a faster killing speed compared to the reference strain CHA0 in one out of three experiments (Fig. 2A). All P. protegens strains were able to kill 100% of the larvae within 50 h and the insect colonization by P. protegens reached log 5 CFU per larvae after 30 h (Fig. 3A). In contrast, the killing activity and the insect colonization by P. chlororaphis strains were more disparate at 30 h post‐infection (Figs 2D–F and 3B). Indeed, the Coleoptera isolates (PCLAR01 and PCLAR04), closely related to PCL1391 in the phylogeny, displayed delayed killing speed in all three experiments compared to PCL1391. In contrast, the Diptera isolate (PCLAR03) killed similarly to PCL1391, or even faster in one of the experiments (Fig. 2D–F). Overall, we observed a higher mortality when there was a higher colonization of the insect by the P. chlororaphis strains (Fig. 3B).
Fig. 2.
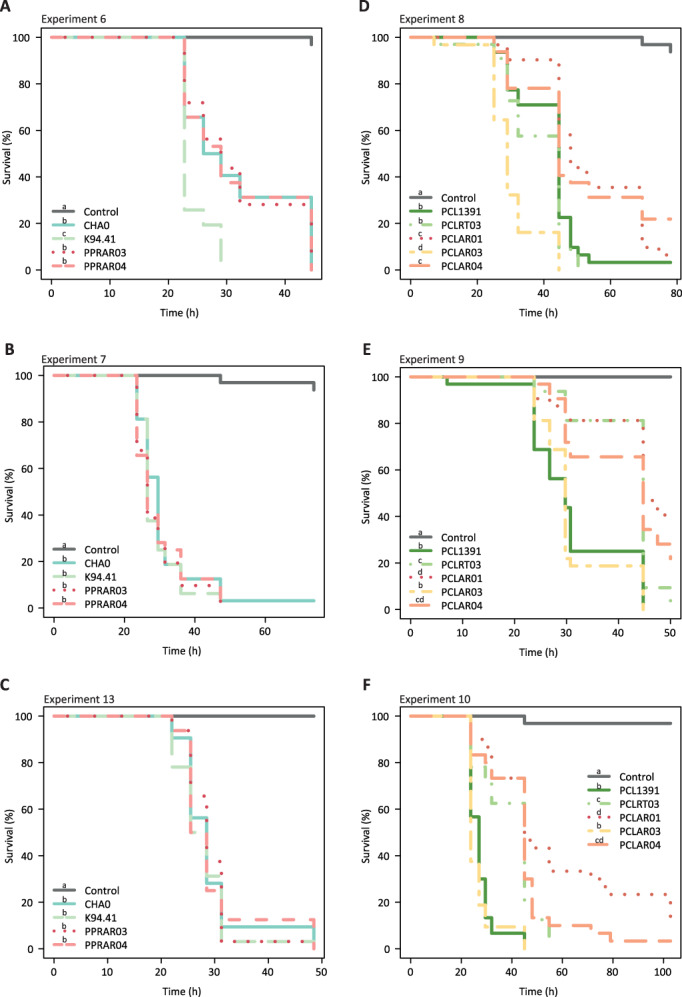
Survival of Plutella xylostella larvae after oral uptake of different Pseudomonas protegens (A, B, C) and Pseudomonas chlororaphis (D, E, F) strains. Second instar larvae were fed with artificial diet pellets spiked with 4 x 106 bacterial cells and mortality was assessed periodically by poking the insects. Strains were isolated from roots (P. protegens CHA0, P. protegens K94.41, P. chlororaphis PCL1391 and P. chlororaphis PCLRT03) or from arthropods (P. protegens PPRAR03, P. protegens PPRAR04, P. chlororaphis PCLAR01, P. chlororaphis PCLAR03 and P. chlororaphis PCLAR04). Statistical differences between the survival of the insects exposed to the different bacteria are depicted as different letters in the legend (Log‐rank test p < 0.05). Thirty‐two larvae were used in each experiment.
Fig. 3.
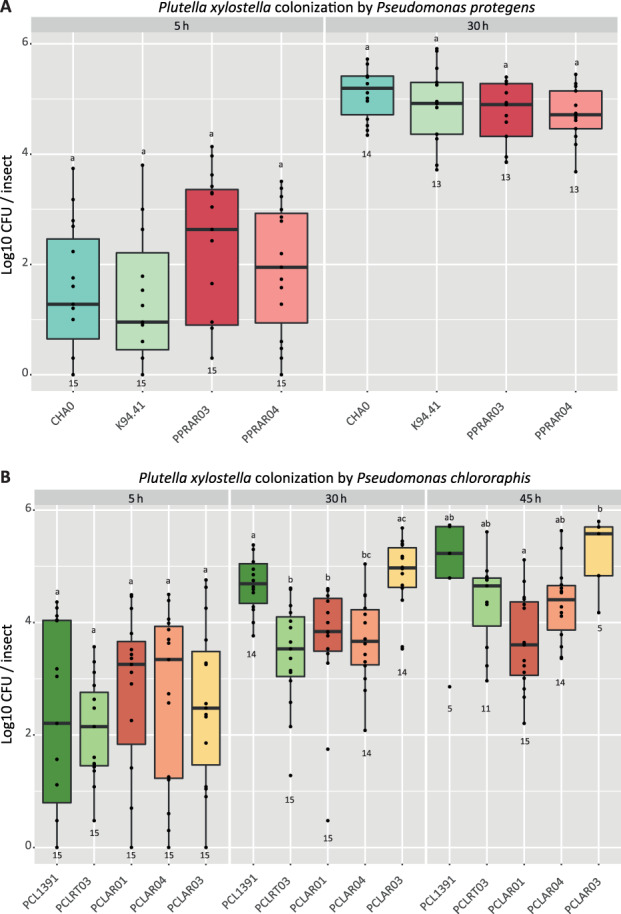
Colonization of Plutella xylostella by different strains of Pseudomonas protegens (A) and Pseudomonas chlororaphis (B) after oral uptake. Second instar larvae were fed with pellets of artificial diet spiked with 4 × 106 bacterial cells. Larvae were surface sterilized and homogenated after 5, 30 or 45 h and serial dilutions of homogenates plated on selective medium. Strains were isolated from roots (P. protegens CHA0, P. protegens K94.41, P. chlororaphis PCL1391 and P. chlororaphis PCLRT03) or from arthropods (P. protegens PPRAR03, P. protegens PPRAR04, P. chlororaphis PCLAR01, P. chlororaphis PCLAR03 and P. chlororaphis PCLAR04). For each time point, significant differences between strains are indicated with different letters on top of the boxplots (Dunn's test p < 0.05). Numbers at the bottom of boxplots indicate numbers of extracted larvae.
To evaluate whether closely related strains exhibit similar root colonization and plant protection patterns, we inoculated cucumber plants with the different Pseudomonas strains and infected the plants with the oomycete plant‐pathogen Pythium ultimum or left them untreated. After 9 days, the plant protection ability, assessed by measuring the disease severity (characteristic symptoms are presented in Fig. S5), the shoot fresh weight as well as the bacterial root colonization were determined. All the tested P. protegens and P. chlororaphis strains colonized roots similarly in absence of the plant pathogen (Figs S6 and S7). Moreover, the root colonization by these bacterial strains did not affect the shoot weight in the absence of the plant pathogen (Figs S8C‐D and S9C‐D). Regarding the plant protection ability, all P. protegens strains reduced disease in both experiments. Compared to infected control plants without added bacteria the number of dead plants was reduced by 50%–75% (Fig. S8A‐B) and shoot weights were significantly higher (Fig. S8‐D). Moreover, the root colonization levels of P. protegens strains slightly increased in presence of the pathogen but overall, all P. protegens strains colonized the roots similarly (Fig. S7). Regarding the P. chlororaphis strains, all of them reduced plant mortality (Fig. S8A‐B), especially in the second replicate; however, their positive effect on shoot weights under P. ultimum attack was mostly not significant (Fig. S8A‐C). Root colonization levels were similar among strains with the exception of PCLAR01 which established significantly higher population sizes on pathogen‐infected roots compared to PCLRT03 and PCLAR03 in the second experiment (Fig. S9). In contrast to treatments with P. protegens, the root colonization by P. chlororaphis strains was only stimulated by the presence of the pathogen in individual cases (PCL1391, exp.1 and PCLAR03, exp. 2, Fig. S7).
In summary, the studied P. protegens isolates performed equally well on plants and in insects. In contrast, P. chlororaphis strains were quite homogenous in colonizing roots and suppressing the root pathogen but showed markedly higher variability regarding insect colonization and oral activity. Our findings show that, among three phylogenetically closely related insect isolates, the two beetle isolates (PCLAR01 and PCLAR04) were less efficient P. xylostella colonizers/killers than the Diptera isolate. This might point to a possible adaptation to different insect species/orders that should be explored in future research, especially, since the isolated strains were not tested in the same arthropod species where they were found. These phenotypic differences could be related to small genetic and regulatory variations as shown for antimicrobial resistance in P. aeruginosa (Freschi et al., 2018), or to other genomic plasticity mechanisms of importance for bacterial adaptation (reviewed by Silby et al., 2011; Darmon and Leach, 2014; Li et al., 2021).
Genetic differences between closely related Pseudomonas may reflect recent host adaptation
Insecticidal activity of Pseudomonas is a multifactorial trait, relying mainly on the production of the entomotoxin Fit, chitinases, antimicrobials and phospholipases (Péchy‐Tarr et al., 2008; Loper et al., 2012; Kupferschmied et al., 2013, 2016; Péchy‐Tarr et al., 2013; Flury et al., 2016, 2017, 2019). As we highlighted a differential behaviour of P. chlororaphis isolates during insect assays, we analysed the COG terms associated to the strains PCLAR01, PCLAR03 and PCLAR04 and we performed a single nucleotide polymorphism (SNP) analysis comparing these insect isolates with the closely related root isolate P. chlororaphis subsp. piscium PCL1391.
The COG analysis revealed that the Coleoptera isolates PCLAR01 and PCLAR04 have more specific genes related to the ‘replication, recombination and repair’, ‘mobilome, prophages and transposons’, ‘lipid transport and metabolism’ and ‘signal transductions mechanisms’ terms than PCLAR03. On the other hand, the Diptera isolate PCLAR03 harbours more genes related to ‘amino acid transport and metabolism’, ‘cell wall/membrane/envelope biogenesis’, ‘coenzyme transport and metabolism’, ‘energy production and conversion’, ‘nucleotide transport and metabolism’ and ‘translation, ribosomal structure and biogenesis’ (Fig. S10). The extra metabolism and transduction‐related genes in the Coleoptera isolates could be important to establish a commensal relationship with the insect. The higher number of genes related to the ‘mobilome and recombination’ COG category attests a higher genome plasticity that might be important to orient the bacteria to non‐pathogenic lifestyles on insects.
Although these four strains shared at least 99% of genomic identity (Table S1), we identified more than 25 000 SNPs between the three insect isolates and PCL1391 (Table S5). PCLAR01 and PCLAR04 have ~25 000 variations and PCLAR03 ~29 000 compared to the PCL1391 genome (NZ_CP027736.1) but only between 15% and 19% of the total changes were intragenic non‐synonymous variations, i.e. cause an amino acid or structural change in the resulting protein (Table S5). The presence of additional genes in PCLAR01 and PCLAR04 (Figs S1 and S10) together with small genetic changes in specific regions (Table S5) might be another reason why the Coleoptera strains are delayed in insect killing compared to the Diptera isolate PCLAR03. It has been shown in Yersinia pestis and Salmonella enterica serovar Typhi that, in order to adapt to a specific host, it is necessary to inactivate certain coding regions of the genome (Chain et al., 2004; Klemm and Dougan, 2016). On the other hand, intergenic modifications could result in important changes in regulatory sequences such as small RNAs, riboswitches, promoters, terminators and regulator binding sites (Waters and Storz, 2009). These non‐coding regions were shown to be under selective pressure in several pathogenic bacterial species, e.g. Escherichia coli, Klebsiella pneumoniae, S. enterica, Staphylococcus aureus and Streptococcus pneumoniae (Thorpe et al., 2017).
Then, we focused our attention on the SNPs affecting genes known as keystones for insecticidal activity like those of the fit cluster (encoding entomotoxin Fit along with its type I secretion system, Péchy‐Tarr et al., 2008), plcN (encoding a phospholipase, Flury et al., 2016; Vesga et al., 2020), chiD (encoding a chitinase, Flury et al., 2016) and also genes encoding effector proteins of TPS systems (Vesga et al., 2020). The fit cluster has numerous SNPs in the three strains but the regions of the Coleoptera isolates PCLAR01 and PCLAR04 related to the transport and regulation of the production of the Fit toxin had fewer SNPs than the corresponding region of the Diptera isolate PCLAR03 (Fig. 4A). The fitD gene showed numerous SNPs in all three strains, several of them affected the TcdA‐TcdB pore‐forming domain, which is the active domain of the protein (Ruffner et al., 2015). PCLAR01 and PCLAR04 have nine changes in amino acids in this active domain while PCLAR03 has 13. Additionally, PCLAR03 has a SNP that could lead to a stop codon inside the TcdA‐TcdB domain but the insecticidal activity of this strain was even enhanced in one experiment (Fig. 4A). This change does not affect the virulence of the strain which supports the already known involvement of other mechanisms in insect pathogenesis in pseudomonads (Ruffner et al., 2013; Flury et al., 2016; Keel, 2016; Vesga et al., 2020).
Fig. 4.
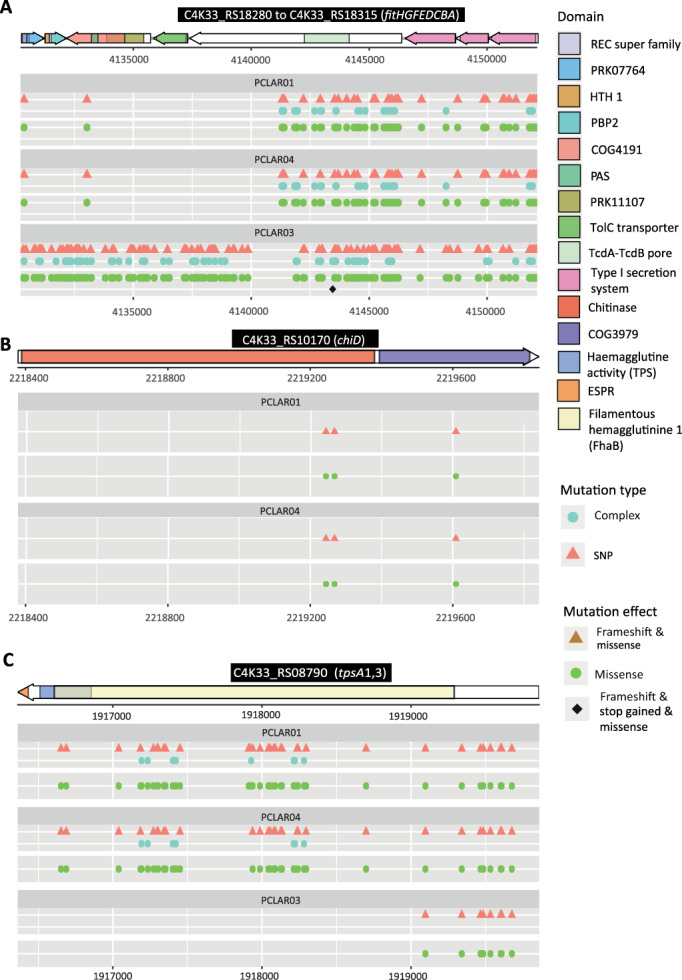
Non‐synonymous variations in the hemagglutinin‐like coding sequences of the gene cluster encoding the insecticidal toxin FitD of P. chlororaphis strains (A), the chitinase ChiD (B) and the two‐partner secretion system TpsA1/3 (A). Genes are depicted by arrows on top of the panels A, B and C, and the protein domain coding sequences are indicated within each gene. Lower panels show the variations harboured by strains PCLAR01 (Coleoptera isolate), PCLAR04 (Coleoptera isolate) and PCLAR03 (Diptera isolate) compared to the genome of the reference strain P. chlororaphis PCL1391. fitHGF are the regulatory genes of the fit cluster. REC: response regulator domain; HTH 1 and PBP2: typical domains of signal transducers; PRK11107 and COG4191: part of a sensor histidine‐kinase; PAS: is a structural domain. The TcdA‐TcdB domain of the FitD toxin is predicted to form a pore in the target cell (Daborn et al., 2002; Waterfield et al., 2003; Ruffner et al., 2015). The fitABCE genes harbour the domains of a type I secretion system predicted to secrete the FitD toxin to the extracellular space. The chitinase domain has the active function of degrading chitin; COG3979: chitodextrinase domain that catalyses the hydrolysis of chitin oligosaccharides. The hemagglutinin activity (TPS) domain is used to interact with the transporter protein for membrane translocation; the filamentous hemagglutinins are repeats used to attach to the host cells and translocate into the host; Filamentous hemagglutinin 1 (FhaB): filamentous‐hemagglutinin domain of Bordetella pertussis; ESPR: signal domain related to type V secretion systems. Mutation type: SNP = single nucleotide polymorphisms; complex = insertions, deletions or larger sequence changes. Mutation effect: modifications that the mutations will cause in the protein, i.e. missense when they cause a change of amino acid and frame‐shift when the translation frame is affected.
Only the Coleoptera isolates had SNPs in the chiD gene while the Diptera isolate showed neither synonymous nor non‐synonymous changes compared to PCL1391 (Fig. 4B; Table S6). Two out of three detected SNPs in the Coleoptera isolates affected the chitinase domain, one of them leading to a change of an amino acid (Fig.4B). Finally, the plcN gene in all three isolates had only one change from serine to proline that might not have a functional effect since these two amino acids are polar non‐charged amino acids (Fig. S11A).
In addition to the insecticidal factors described above, we took a closer look at SNPs in three genes homologous to the tpsA genes encoding effector proteins of TPS systems involved in macrophage pyroptosis (Basso et al., 2017). C4K33_RS19905 and C4K33_RS21280 of PCL1391 have 77.97% and 80.08% nucleotide identity with the tpsA2 and tpsA4 of P. protegens CHA0 respectively. CK33_ RSA08790 has 80.13% nucleotide identity with tpsA1 and 80.6% with tpsA3 of CHA0. We, therefore, adopt the CHA0 annotation for these genes and will further refer to C4K33_RS19905 as tpsA2, to C4K33_RS21280 as tpsA4 and to CK33_ RSA08790 as tpsA1/A3. We observed that compared to PCL1391, tpsA4 did not have SNPs in any of the analysed strains and PCLAR03 did not show any changes in the tpsA2 either (Table S6). On the other hand, the two Coleoptera isolates PCLAR01 and PCLAR04 displayed many missense variations in tpsA2 encoding the TpsA2 effector, i.e. SNPs that lead to a predicted amino acid change in the protein (Fig. S11). In P. protegens CHA0, tpsA2 is suggested to be involved in insect gut colonization/transmigration from gut to hemolymph (Reboud et al., 2017a; Reboud et al., 2017b; Vesga et al., 2020). It is tempting to speculate that the SNPs detected in the tpsA2 gene of the two Coleoptera isolates could lead to reduced respectively, slowed down oral activity observed for these isolates in the Plutella feeding assays (Table 1; Fig. S3; Tables S3 and S4).
Compared to PCL1391, the three P. chlororaphis strains show more synonymous than non‐synonymous variations for all these insecticidal factors, i.e. FitD, ChiD, PlcN and TpsA2. (Table S6). This is not the case for the third analysed TpsA encoding gene tpsA1/A3, which exhibits more non‐synonymous than synonymous variations (Table S6). This gene shows a high concentration of missense variations throughout the coding sequence in the two Coleoptera isolates but only at the 5′ end of the gene for the Diptera isolates (Fig. 4C). TpsA encoding genes have been related to insecticidal activity in P. protegens CHA0 (Vesga et al., 2020) and macrophage killing and pathogenesis in P. aeruginosa PA7 and Serratia marcescens (Reboud et al., 2017a; Reboud et al., 2017b). The diversification of TpsA proteins might be important in the establishment of the diverse functional host–pathogen interactions. Therefore, the TpsA produced by PCLAR01 and PCLAR04 might be defective due to the different SNPs in the nucleotide sequence which leads to a delayed insecticidal activity.
Both COG and SNP analyses of the present study highlight the genomic diversity in the insecticidal Pseudomonas isolates. High genetic variation allows a bacterial population to rapidly expand and fill a new niche or colonize a new host. The easy switch of hosts, as it seems to be the case for P. protegens and P. chlororaphis, indicates that these bacteria have developed as generalists rather than specialists. Pseudomonas are well known to undergo numerous genetic re‐arrangements during host colonization, which increases the variability within the population and allows it to adapt to and survive in a new environment (Broek et al., 2005).
Conclusion
Pseudomonas are present in very different environments as free bacteria and also associated to other organisms in pathogenic, commensal or beneficial relationships. Our study shows that the two species P. protegens and P. chlororaphis naturally occur in several insect and myriapod classes. Interestingly all P. protegens strains isolated in this study were always associated with insects, whereas we found insecticidal P. chlororaphis associated with insects, myriapods, roots and soil which is an indication for the variability and adaptability of this latter species. All P. protegens and P. chlororaphis strains we have characterized so far have the ability to colonize plant and insect hosts, exercise a certain insect‐killing activity and control root pathogens. The findings presented here provide evidence that P. protegens isolates are much more consistent in insecticidal abilities while P. chlororaphis are more variable which might reflect a certain degree of adaptation to different hosts. However, this remains purely speculative since also all P. protegens isolates obtained in this study, although highly lethal to P. xylostella, were isolated from healthy arthropods, plant roots or soil. Altogether, our observations raise the question of whether these species have evolved towards commensal, pathogenic and symbiotic interactions with arthropods as a new niche or if they are just very versatile colonizers able to adapt to any new environment. This highlights how far we actually are from fully understanding their ecology and the particular mechanisms allowing them to colonize such contrasting environments. We observed that the isolation site or the phylogeny does not always reflect the insecticidal capabilities of fluorescent Pseudomonas as closely related strains exhibit differential insecticidal activities. In two P. chlororaphis insect isolates displaying lower insecticidal activity we have identified non‐synonymous variations in some insecticidal factors which potentially could explain their reduced or slowed down insect‐killing capacities. Our findings corroborate that these bacteria are multi‐talented and able to conquer very different niches and exploit cross‐kingdom hosts. The ability of P. protegens and P. chlororaphis to successfully colonize plant roots, to enhance plant growth and control pests and pathogens, makes them promising candidates for future biocontrol applications.
Experimental procedures
Isolation of Pseudomonas from different habitats
To assess the natural occurrence of P. protegens and P. chlororaphis, arthropods, soil and plant roots were collected in spring 2016 and spring 2017 in an agricultural field (wheat in 2016, potato in 2017) and a neighbouring undisturbed grassland area. The field is located at Agroscope Reckenholz, Zurich, Switzerland (47° 25′ 32″ N; 8° 30′ 57″ E). Arthropod, root and soil samples were collected up to 50 cm of soil depth (soil horizon characteristics are included in Table S7). The collected arthropods were visually classified as detailed as possible.
For the arthropod samples, all animals were surface disinfected first with 96% ethanol and then with 70% ethanol (20 s each). After each disinfection step, the animals were rinsed with distilled H2O amended with 0.05% SDS. The animals were homogenized in 1 ml or 15 ml 0.9% NaCl depending on their size with a Polytron PT‐DA 2112 blender (Kinematica, Littau, Switzerland). For root samples, the soil attached to the roots was removed with tap water. From 0.5–2 g of roots were incubated in 50 ml of sterile 0.9% NaCl solution overnight at 3 °C without shaking. Roots were shaken for 30 min at 3 °C and 350 rpm to detach the bacteria from the root surface. For the soil samples, 20 g of sieved soil was suspended in 100 ml of 0.9% NaCl and solution shaken for 3 h at 150 rpm and 3 °C.
Undiluted and serially diluted suspensions derived from arthropods, roots and soil were plated on the Pseudomonas isolation medium King's B supplemented with ampicillin, 40 μl ml−1; chloramphenicol, 13 μl ml−1; and cycloheximide, 100 μl ml−1 (KB+++) and kept at 24°C (King et al., 1954; Landa et al., 2002). Growing colonies were purified twice successively in KB+++. All strains were stored in 43% glycerol at −80°C.
Identification of P. chlororaphis and P. protegens
A single colony from each isolate was picked and cultured overnight at 24°C in lysogeny broth (LB, Bertani, 1951), diluted and used for PCR with the DreamTaq polymerase (ThermoFisher Scientific, MA, USA). To confirm that the bacteria isolated belong to the Pseudomonas genus, a PCR was performed on all isolated strains using a Pseudomonas‐specific 16S rRNA primer (forward Pse435F 5′‐ACT TTA AGT TGG GAG GAA GGG‐3′; reverse Pse686R 5′‐ACA CAG GAA ATT CCA CCA CCC‐3′; annealing temperature 60°C, expected fragment of 252 bp; Bergmark et al., 2012). Then to detect specifically the isolates assigned to P. protegens and P. chlororaphis subgroups, positive strains were then screened using a fitD‐specific primer (forward FitD66F 5′‐CTA TCG GGT SCA GTT CAT CA‐3′; reverse FitD308R 5′‐TTC TTG TCG GSA AAC CAC T‐3′; annealing temperature 60°C; expected fragment of 242 bp; Flury et al., 2017). So far, the presence of the Fit toxin was exclusively found in strains belonging to P. protegens and P. chlororaphis species (Flury et al., 2016). Finally, to confirm the assignation of the isolates to either P. protegens or P. chlororaphis species, DNA from fitD‐positive Pseudomonas strains was extracted with Wizard Genomic DNA purification Kit (Promega, WI, USA) and a larger fragment of the 16S rRNA (1465 bp) was amplified using the following primers: forward 16F27 5′‐AGA GTT TGA TCM TGG CTC AG‐3′; reverse 16R1492 5′‐TAC GGY TAC CTT GTT ACG ACT T‐3′ and annealing temperature of 55°C (Lane, 1991). The PCR product was purified with the FastAP Thermosensitive Alkaline phosphatase and the Exonuclease I (ThermoFisher Scientific). The sequencing reaction was set up with the purified PCR product using the Big Dye v3.1 Cycle Sequencing Kit (Applied Biosystems, CA, USA) and further cleaned with Sephadex GM‐50 (GE‐Healthcare, MA USA). PCR fragment sequencing was performed by Sanger sequencing following manufacturer's instructions (3130xl DNA analyser, Applied Biosystems) to identify P. chlororaphis and P. protegens. The data generated for bacterial phylogenetic identification were generated in collaboration with the Genetic Diversity Center (GDC), ETH Zurich.
Pseudomonas protegens and P. chlororaphis genome sequencing
The DNA from 10 P. chlororaphis strains and 13 P. protegens strains was extracted with Wizard Genomic DNA purification Kit (Promega) and the genome sequenced by MiSeq (2 × 300 paired‐end v3 with Nextera XT library kit, Illumina, CA, USA). Alternatively, DNA was extracted with the MagAttract high‐molecular‐weight DNA kit (Qiagen, Germany) and sequenced by PacBio (SMRT v3, SMRTbell template preparation kit v1.0, Pacific Biosciences, CA, USA). The de novo genome assembly was performed using MIRA v4.0.2 with standard settings in accurate mode (MiSeq‐sequenced strains) or RS_HGAP_Assembly.4 protocol in SMRT Link v6.0 for (PacBio sequenced strains). Genomes were deposited in NCBI's genome repository under the Bioproject ID PRJNA637204.
The GC content, the average nucleotide identity and the tetranucleotide analysis indexes were assessed with JSpecies v1.2.1 and are listed in Table S2, which also indicates the DNA extraction method and the sequencing technology for each strain.
Insect assays
To assess the insecticidal activities of the new isolates, a P. xylostella feeding assay was performed as described in detail in Flury et al. (2019). Briefly, P. xylostella eggs obtained from Syngenta Crop Protection AG (Stein, Switzerland) were reared in a growth chamber at 25°C, 60% of relative humidity and a cycle of 16 h day (162 μmol m−2 s−1) and 8 h of darkness. Second instar larvae (about 5 days old) were fed with artificial diet pellets spiked with 10 μl of sterile 0.9% NaCl solution or of cell suspensions of the different bacterial strains (4 × 108 colony‐forming units (CFU)/pellet). Cell suspensions were prepared with washed cells from overnight LB cultures. To avoid cannibalism, larvae were kept individually in 128‐well bioassay trays (Frontier Agricultural Sciences, Delaware, USA) at the same conditions used for rearing. Survival was regularly assessed by poking the larvae with a tip. Survival curves of different treatments were compared using the log‐rank test (p‐value <0.05) from the ‘survival 3.1.8’ package (https://github.com/therneau/survival, Therneau and Grambsch, 2000) of R 3.6.0 (www.r-project.org). Statistical differences were calculated for the individual experiments but pooled experiments were plotted in a Kaplan–Meier graph as well to show an overview of all the experiments together. LT50 values were estimated using the ‘ecotox 1.4.1’ R package (https://www.rdocumentation.org/packages/ecotox/versions/1.4.1) using LT_probit option and considered significantly different when the 95% intervals were not overlapping between the strains. All LT50 values are included in Table S3 and p‐values of the log‐rank test in Table S4. All strains were tested at least twice in independent experiments. In total, 16 experiments were performed. In every experiment, reference strains P. chlororaphis PCL1391 and P. protegens CHA0 were included as an internal standard.
A subset of P. xylostella larvae in the feeding assays was used to assess the ability of the strains to colonize insects. Five larvae per treatment were collected at different time points (5 and 30 h for P. protegens strains and 5, 30 and 45 h for P. chlororaphis strains) and washed twice in 70% EtOH for 20 s with subsequent rinsing with sterile H2O amended with 0.05% SDS. Insects were homogenized in 1 ml of sterile 0.9% NaCl solution with a Polytron PT‐DA 2112 blender (Kinematica). Homogenates were serially diluted and plated onto KB+++ medium. CFUs were assessed after incubating plates for 2 days at 27°C. There were no statistical differences between the experiments (Kruskal–Wallis p‐value <0.05), therefore, the data were pooled. Statistical differences in colonization numbers among strains were assessed using a Dunns's test (p‐value <0.05) from the ‘FSA’ R package (https://github.com/droglenc/FSA). In the boxplot graphs, boxes correspond to the 25th and 75th percentiles, lines inside the boxes indicate the median and whiskers correspond to 1.5 times the interquartile range.
Plant assays
The ability of isolated strains to colonize roots and to protect plants against a root pathogen was tested in a cucumber pot assay with natural soil and the oomycete pathogen Pythium ultimum. The soil collected at the sampling site at Agroscope Reckenholz, Switzerland (see above) was used because we wanted to test the root colonization ability and disease suppressive capacity of the new isolates under competitive conditions, i.e. in the presence of the native soil microorganisms from where the isolates were obtained.
Cultivation of pathogen, bacteria and plants
Pythium ultimum strain Pu‐11 was grown on a malt agar plate at 18°C for 7 days. Three plugs with fungal mycelium were transferred to twice‐autoclaved millet (organic millet, Migros, Switzerland) and the pathogen was cultivated at 18°C for another 7 days. Pseudomonas isolates were grown overnight in LB at 180 rpm and 24°C. From these cultures, aliquots of 200 μl were spread onto KB+++ plates and incubated at 27°C for 24 h. Bacteria were scraped from plates and washed with sterile 0.9% NaCl solution. Suspensions used for pot inoculation were adjusted to an OD600 of 4.0 (approx. 3.2 × 109 cells ml−1). Cucumber seeds of the variety ‘Chinese Snake’ (Bigler Samen AG, Thun, Switzerland) were sterilized in 1.4% NaClO for 30 min, washed thoroughly with sterile water and left to germinate on moistened filter paper for 1.5 days at 24°C in the dark.
Set up of pot experiments
250 ml pots were filled with 320 g of a 4:1 soil/quartz sand (1.5–2.2 mm diameter) mixture. Each pot received 1 g of the P. ultimum‐millet inoculum. Control treatments without pathogen received 1 g of autoclaved P. ultimum–millet mixture. The pathogen inoculum was thoroughly mixed into the soil. Then, 5 ml of a Pseudomonas cell suspension was added per pot resulting in a final concentration of 107 cells per gram of soil. Control treatments were amended with the same volume of sterile 0.9% NaCl solution. Finally, pots received 45 ml of sterile water and three cucumber seedlings were planted per pot. Plants were grown for 10 days in a growth chamber set at 70% of relative humidity with a cycle of 16 h of light (210 mmol m2 s−1) at 22°C, followed by an 8 h dark period at 20°C. Six replicate pots with pathogen and six without pathogen were prepared per bacterial strain. Two independent experiments were performed over time.
Evaluation of pot experiments
Nine days after inoculation, plant mortality and disease severity were assessed (see Fig. S5 for disease classification) and shoots fresh weight was assessed. Roots were washed, weighted and incubated overnight at 3 °C in 50 ml of autoclaved sterile 1% NaCl solution. The next day, roots were shaken at 400 rpm and 3 °C for 30 min to detach the bacteria from the root surface. Serial dilutions of the obtained root suspensions were plated onto KB+++ and CFU numbers were assessed after 2 days of incubation at 27°C.
Since the factor experiment had a significant impact on root colonization and shoot weight data (Kruskal–Wallis p‐value <0.05), the data of individual experiments could not be pooled. Data were first subjected to Shapiro–Wilk normality test (p‐value <0.05) to check normal distribution. Differences in root colonization were assessed with one‐way ANOVA (p‐value <0.05) for experiments with normally distributed data (both P. protegens experiments and the second P. chlororaphis experiment) or Kruskal–Wallis (p‐value <0.05) if data were not normally distributed (first P. chlororaphis experiment). Shoot weight data of all experiments were not normally distributed. Therefore, a Dunn's test (p‐value <0.05) was used to assess the differences between the shoot weights of plants treated with the different Pseudomonas strains. Kruskal–Wallis and ANOVA test are part of the ‘stats’ package built‐in within R. In the boxplot graphs, boxes correspond to the 25th and 75th percentiles, lines inside the boxes indicate the median and whiskers correspond to 1.5 times the interquartile range.
Genome analyses
Orthologue analysis
Genomes from 54 Pseudomonas strains were annotated with Prokka 1.14.6 (Seemann, 2014) using the standard settings. Further information about the strains is displayed in Table S1. The resulting 54 amino acid sequences were analysed with Orthofinder 2.3.12 (Emms and Kelly, 2015) to obtain a presence–absence matrix of orthologue groups across the different strains. The phylogenetic information was processed with FigTree 1.4.4 to generate the tree (https://github.com/rambaut/figtree/releases/tag/v1.4.4).
Secondary metabolites genome mining
The distribution of insecticidal and plant‐beneficial traits within the Pseudomonas fluorescens species complex was done by screening a total of 126 amino acid sequences by BLASTp (https://blast.ncbi.nlm.nih.gov/Blast.cgi). A function was considered present inside a genome when showing at least 65% of identity over 70% of the length of the amino acid sequences, a lower than 50 bit‐score and an e‐value of less than 0.01. Gene clusters were considered present only when all the coding sequences fulfilled the previously mentioned requirements and partial when there were matches for some of the coding sequences of the cluster. The amino acid sequences of the studied traits are included in Supplementary File S2.
Clusters of orthologue genes counting
The 54 Pseudomonas genomes were compared using Roary 3.13.0 (Page et al., 2015) to assess the gene presence. Prokka (Seemann, 2014) annotations were used to assess the frequency of different COG in different strain categories, namely, phylogeny, insecticidal activity and origin of isolation. The same analysis was performed on the Coleoptera and Diptera interesting strains using only the specific genes of each of these strains.
SNPs in Coleoptera and Diptera isolates
To detect the genetic differences between the characterized isolates and their reference (P. chlororaphis subsp. piscium PCL1391, accession number NZ_CP027736.1), Snippy was used indicating that the inputs were contigs and not raw reads. Non‐synonymous variations were selected to identify which genes had the highest content of changes among the strains. Specific regions corresponding to the genes C4K33_RS18280 to C4K33_18315 (fitHGFEDCBA cluster), C4K33_RS15780 (plcN), C4K33_RS10170 (chiD), C4K33_RSA19905 (tpsA2), C4K33_RS08790 (tpsA1/3) and C4K33_RS21280 (tpsA4) in PCL1391 (NZ_CP027736.1) were analysed more in detail. To check if any of the variations were affecting the predicted protein domains, the nucleotide sequence of the genes was used to find conserved domains in the Conserved Domain database of the NCBI (www.ncbi.nlm.nih.gov/cdd/).
Supporting information
File S1. Supplementary figures.
File S2. Coding sequences used in the BLAST analysis.
Table S1. Genome information of the sequenced strains of this study.
Table S2. List of used genomes.
Table S3. LT50 raw data.
Table S4. Pairwise p‐values of the survival log‐rank test.
Table S5. Summary of the SNPs found in the PCLAR01, PCLAR03 and PCLAR04 strains.
Table S6. Summary of the SNPs found in the genes of interest of PCLAR01, PCLAR03, PCLAR04.
Table S7. Soil characteristics.
Appendix S1. Supporting information.
Acknowledgements
We are very grateful to Giselher Grabenweger (Reckenholz, Agroscope, Switzerland) for his help in identifying the arthropods collected in the field. We thank Oliver Kindler (Syngenta Switzerland) for kindly providing P. xylostella eggs. We also thank Theo Smits and Joel Pothier (ZHAW, Zürich, Switzerland) for sequencing and assembling several of our genomes and Danilo dos Santos Pereira for his valuable comments regarding the SNP analysis. Finally, we gratefully acknowledge the work of Jana Schneider, Francesca Dennert, Patrick Brunner and our laboratory assistants Camille Tinguely, Caroline Meier, Florence Guilleron and Peter Egli who contributed to the isolation, screening and identification of the bacterial strains as well as to the P. xylostella feeding assays of this project.
Contributor Information
Christoph Keel, Email: christoph.keel@unil.ch.
Monika Maurhofer, Email: monika.maurhofer@usys.ethz.ch.
Jordan Vacheron, Email: jordan.vacheron@unil.ch.
Data Availability Statement
The genome sequences were deposited on the NCBI under the BioProject ID PRJNA637204.
References
- AbdulWahab, A. , Taj‐Aldeen, S.J. , Ibrahim, E. , Abdulla, S.H. , Muhammed, R. , Ahmed, I. , et al. (2014) Genetic relatedness and host specificity of Pseudomonas aeruginosa isolates from cystic fibrosis and non‐cystic fibrosis patients. Infect Drug Resist 7: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal, I. , Shinwari, Z.K. , Sikandar, S. , and Shahzad, S. (2019) Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol Res 221: 36–49. [DOI] [PubMed] [Google Scholar]
- Aharon, Y. , Pasternak, Z. , Ben Yosef, M. , Behar, A. , Lauzon, C. , Yuval, B. , and Jurkevitch, E. (2013) Phylogenetic, metabolic, and taxonomic diversities shape mediterranean fruit fly microbiotas during ontogeny. Appl Environ Microbiol 79: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar, A. , and Demirbağ, Z. (2007) Isolation of pathogenic bacteria from Oberea linearis (Coleptera: Cerambycidae). Biologia 62: 13–18. [Google Scholar]
- Basso, P. , Wallet, P. , Elsen, S. , Soleilhac, E. , Henry, T. , Faudry, E. , and Attrée, I. (2017) Multiple Pseudomonas species secrete exolysin‐like toxins and provoke Caspase‐1‐dependent macrophage death. Environ Microbiol 19: 4045–4064. [DOI] [PubMed] [Google Scholar]
- Bensidhoum, L. , Nabti, E. , Tabli, N. , Kupferschmied, P. , Weiss, A. , Rothballer, M. , et al. (2016) Heavy metal tolerant Pseudomonas protegens isolates from agricultural well water in northeastern Algeria with plant growth promoting, insecticidal and antifungal activities. Eur J Soil Biol 75: 38–46. [Google Scholar]
- Bergmark, L. , Poulsen, P.H.B. , Al‐Soud, W.A. , Norman, A. , Hansen, L.H. , and Sørensen, S.J. (2012) Assessment of the specificity of Burkholderia and Pseudomonas qPCR assays for detection of these genera in soil using 454 pyrosequencing. FEMS Microbiol Lett 333: 77–84. [DOI] [PubMed] [Google Scholar]
- Bertani, G. (1951) Studies on lysogenesis I: the mode of phage liberation by lysogenic Escherichia coli . J Bacteriol 62: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek, D.V.D. , Bloemberg, G.V. , and Lugtenberg, B. (2005) The role of phenotypic variation in rhizosphere Pseudomonas bacteria. Environ Microbiol 7: 1686–1697. [DOI] [PubMed] [Google Scholar]
- Çakici, F.Ö. , Sevií, M.,.A. , Demiírbağ, Z. , and Demií, R.,.İ. (2014) Investigating internal bacteria of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) larvae and some Bacillus strains as biocontrol agents. Turk J Agric For 38: 12. [Google Scholar]
- Chain, P.S.G. , Carniel, E. , Larimer, F.W. , Lamerdin, J. , Stoutland, P.O. , Regala, W.M. , et al. (2004) Insights into the evolution of Yersinia pestis through whole‐genome comparison with Yersinia pseudotuberculosis . Proc Natl Acad Sci U S A 101: 13826–13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, M.C. , and Schneider, D.S. (2012) Pioneering immunology: insect style. Curr Opin Immunol 24: 10–14. [DOI] [PubMed] [Google Scholar]
- Chen, B. , Teh, B.‐S. , Sun, C. , Hu, S. , Lu, X. , Boland, W. , and Shao, Y. (2016) Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis . Sci Rep 6: 29505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaia, B. , Goda, N. , Mazza, G. , Alali, S. , Florian, F. , Gionechetti, F. , et al. (2019) Developmental stages and gut microenvironments influence gut microbiota dynamics in the invasive beetle Popillia japonica Newman (Coleoptera: Scarabaeidae). Environ Microbiol 21: 4343–4359. [DOI] [PubMed] [Google Scholar]
- Daborn, P.J. , Waterfield, N. , Silva, C.P. , Au, C.P.Y. , Sharma, S. , and ffrench‐Constant, R.H. (2002) A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc Natl Acad Sci U S A 99: 10742–10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon, E. , and Leach, D.R.F. (2014) Bacterial genome instability. Microbiol Mol Biol Rev 78: 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon, R.J. , and Dillon, V.M. (2004) The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49: 71–92. [DOI] [PubMed] [Google Scholar]
- Emms, D.M. , and Kelly, S. (2015) OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol 16: 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, P. , and Moran, N.A. (2013) The gut microbiota of insects – diversity in structure and function. FEMS Microbiol Rev 37: 699–735. [DOI] [PubMed] [Google Scholar]
- Esposti, M.D. , and Romero, E.M. (2017) The functional microbiome of arthropods. PLoS One 12: e0176573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flury, P. , Aellen, N. , Ruffner, B. , Péchy‐Tarr, M. , Fataar, S. , Metla, Z. , et al. (2016) Insect pathogenicity in plant‐beneficial pseudomonads: phylogenetic distribution and comparative genomics. ISME J 10: 2527–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flury, P. , Vesga, P. , Dominguez‐Ferreras, A. , Tinguely, C. , Ullrich, C.I. , Kleespies, R.G. , et al. (2019) Persistence of root‐colonizing Pseudomonas protegens in herbivorous insects throughout different developmental stages and dispersal to new host plants. ISME J 13: 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flury, P. , Vesga, P. , Péchy‐Tarr, M. , Aellen, N. , Dennert, F. , Hofer, N. , et al. (2017) Antimicrobial and insecticidal: cyclic lipopeptides and hydrogen cyanide produced by plant‐beneficial Pseudomonas strains CHA0, CMR12a, and PCL1391 contribute to insect killing. Front Microbiol 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi, L. , Bertelli, C. , Jeukens, J. , Moore, M.P. , Kukavica‐Ibrulj, I. , Emond‐Rheault, J.‐G. , et al. (2018) Genomic characterisation of an international Pseudomonas aeruginosa reference panel indicates that the two major groups draw upon distinct mobile gene pools. FEMS Microbiol Lett 365: 1–11. [DOI] [PubMed] [Google Scholar]
- Geli‐Cruz, O.J. , Cafaro, M.J. , Santos‐Flores, C.J. , Ropelewski, A.J. , and Van Dam, A.R. (2019) Taxonomic survey of Anadenobolus monilicornis gut microbiota via shotgun nanopore sequencing. BioRxiv. [Google Scholar]
- Gomes, A.F.F. , Omoto, C. , and Cônsoli, F.L. (2020) Gut bacteria of field‐collected larvae of Spodoptera frugiperda undergo selection and are more diverse and active in metabolizing multiple insecticides than laboratory‐selected resistant strains. J Pest Sci 93: 833–851. [Google Scholar]
- González‐Serrano, F. , Pérez‐Cobas, A.E. , Rosas, T. , Baixeras, J. , Latorre, A. , and Moya, A. (2019) The gut microbiota composition of the moth Brithys crini reflects insect metamorphosis. Microb Ecol 79: 960–970. [DOI] [PubMed] [Google Scholar]
- Gurung, K. , Wertheim, B. , and Salles, J.F. (2019) The microbiome of pest insects: it is not just bacteria. Entomol Exp Appl 167: 156–170. [Google Scholar]
- Haas, D. , and Défago, G. (2005) Biological control of soil‐borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3: 307–319. [DOI] [PubMed] [Google Scholar]
- Hammer, T.J. , Janzen, D.H. , Hallwachs, W. , Jaffe, S.L. , and Fierer, N. (2017) Caterpillars lack a resident gut microbiome. 114: 9641–9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, T.J. , McMillan, W.O. , and Fierer, N. (2014) Metamorphosis of a butterfly‐associated bacterial community. PLoS One 9: e86995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula, S.E. , Zhu, F. , Heinen, R. , and Bezemer, T.M. (2019) Foliar‐feeding insects acquire microbiomes from the soil rather than the host plant. Nat Commun 10: 1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, J.Y. , Yang, S.Y. , Kim, Y.C. , Lee, C.W. , Park, M.S. , Kim, J.C. , and Kim, I.S. (2013) Identification of orfamide A as an insecticidal metabolite produced by Pseudomonas protegens F6. J Agric Food Chem 61: 6786–6791. [DOI] [PubMed] [Google Scholar]
- Keel, C. (2016) A look into the toolbox of multi‐talents: insect pathogenicity determinants of plant‐beneficial pseudomonads. Environ Microbiol 18: 3207–3209. [DOI] [PubMed] [Google Scholar]
- King, E.O. , Ward, M.K. , and Raney, D.E. (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301–307. [PubMed] [Google Scholar]
- Klemm, E. , and Dougan, G. (2016) Advances in understanding bacterial pathogenesis gained from whole‐genome sequencing and phylogenetics. Cell Host Microbe 19: 599–610. [DOI] [PubMed] [Google Scholar]
- Kolasa, M. , Ścibior, R. , Mazur, M.A. , Kubisz, D. , Dudek, K. , and Kajtoch, Ł. (2019) How hosts taxonomy, trophy, and endosymbionts shape microbiome diversity in beetles. Microb Ecol 78: 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmied, P. , Chai, T. , Flury, P. , Blom, J. , Smits, T.H.M. , Maurhofer, M. , and Keel, C. (2016) Specific surface glycan decorations enable antimicrobial peptide resistance in plant‐beneficial pseudomonads with insect‐pathogenic properties. Environ Microbiol 18: 4265–4281. [DOI] [PubMed] [Google Scholar]
- Kupferschmied, P. , Maurhofer, M. , and Keel, C. (2013) Promise for plant pest control: root‐associated pseudomonads with insecticidal activities. Front Plant Sci 4: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmied, P. , Péchy‐Tarr, M. , Imperiali, N. , Maurhofer, M. , and Keel, C. (2014) Domain shuffling in a sensor protein contributed to the evolution of insect pathogenicity in plant‐beneficial Pseudomonas protegens . PLoS Pathog 10: e1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa, B.B. , de Werd, H.A.E. , McSpadden Gardener, B.B. , and Weller, D.M. (2002) Comparison of three methods for monitoring populations of different genotypes of 2,4‐diacetylphloroglucinol‐producing Pseudomonas fluorescens in the rhizosphere. Phytopathology 92: 129–137. [DOI] [PubMed] [Google Scholar]
- Lane, D. (1991) 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics. Chichester, England: John Wiley and Sons, E. Stackebrandt and M. Goodfellow, pp. 115–147. [Google Scholar]
- Lauzon, C.R. , McCombs, S.D. , Potter, S.E. , and Peabody, N.C. (2009) Establishment and vertical passage of Enterobacter (Pantoea) agglomerans and Klebsiella pneumoniae through all life stages of the mediterranean fruit fly (Diptera: Tephritidae). Ann Entomol Soc Am 102: 85–95. [Google Scholar]
- Li, E. , Zhang, H. , Jiang, H. , Pieterse, C.M.J. , Jousset, A. , Bakker, P.A.H.M. , and de Jonge, R. (2021) Experimental evolution‐driven identification of Arabidopsis rhizosphere competence genes in Pseudomonas protegens . mBio 12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loper, J.E. , Hassan, K.A. , Mavrodi, D.V. , Davis, E.W. , Lim, C.K. , Shaffer, B.T. , et al. (2012) Comparative genomics of plant‐associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet 8: e1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loper, J.E. , Henkels, M.D. , Rangel, L.I. , Olcott, M.H. , Walker, F.L. , Bond, K.L. , et al. (2016) Rhizoxin analogs, orfamide A and chitinase production contribute to the toxicity of Pseudomonas protegens strain Pf‐5 to Drosophila melanogaster . Environ Microbiol 18: 3509–3521. [DOI] [PubMed] [Google Scholar]
- Malacrino, A. , Campolo, O. , Medina, R.F. , and Palmeri, V. (2018) Instar‐ and host‐associated differentiation of bacterial communities in the Mediterranean fruit fly Ceratitis capitata . PLoS One 13: e0194131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashtoly, T.A. , El‐Zemaity, M.S. , Abolmaaty, A. , Abdelatef, G.M. , Marzouk, A.A. , and Reda, M. (2019) Phylogenetic characteristics of novel Bacillus weihenstephanensis and Pseudomonas sp. to desert locust, Schistocerca gregaria Forskål (Orthoptera: Acrididae). Egypt J Biol Pest Control 29: 85. [Google Scholar]
- Montagna, M. , Chouaia, B. , Mazza, G. , Prosdocimi, E.M. , Crotti, E. , Mereghetti, V. , et al. (2015a) Effects of the diet on the microbiota of the red palm weevil (Coleoptera: Dryophthoridae). PLoS One 10: e0117439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna, M. , Gómez‐Zurita, J. , Giorgi, A. , Epis, S. , Lozzia, G. , and Bandi, C. (2015b) Metamicrobiomics in herbivore beetles of the genus Cryptocephalus (Chrysomelidae): toward the understanding of ecological determinants in insect symbiosis. Insect Sci 22: 340–352. [DOI] [PubMed] [Google Scholar]
- O'Brien, H.E. , Thakur, S. , and Guttman, D.S. (2011) Evolution of plant pathogenesis in Pseudomonas syringae: a genomics perspective. Annu Rev Phytopathol 49: 269–289. [DOI] [PubMed] [Google Scholar]
- Olcott, M.H. , Henkels, M.D. , Rosen, K.L. , Walker, F.L. , Sneh, B. , Loper, J.E. , and Taylor, B.J. (2010) Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains. PLoS One 5: e12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, A.J. , Cummins, C.A. , Hunt, M. , Wong, V.K. , Reuter, S. , Holden, M.T.G. , et al. (2015) Roary: rapid large‐scale prokaryote pan genome analysis. Bioinformatics 31: 3691–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniagua Voirol, L.R. , Frago, E. , Kaltenpoth, M. , Hilker, M. , and Fatouros, N.E. (2018) Bacterial symbionts in Lepidoptera: their diversity, transmission, and impact on the host. Front Microbiol 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péchy‐Tarr, M. , Borel, N. , Kupferschmied, P. , Turner, V. , Binggeli, O. , Radovanovic, D. , et al. (2013) Control and host‐dependent activation of insect toxin expression in a root‐associated biocontrol pseudomonad. Environ Microbiol 15: 736–750. [DOI] [PubMed] [Google Scholar]
- Péchy‐Tarr, M. , Bruck, D.J. , Maurhofer, M. , Fischer, E. , Vogne, C. , Henkels, M.D. , et al. (2008) Molecular analysis of a novel gene cluster encoding an insect toxin in plant‐associated strains of Pseudomonas fluorescens . Environ Microbiol 10: 2368–2386. [DOI] [PubMed] [Google Scholar]
- Reboud, E. , Basso, P. , Maillard, A. , Huber, P. , and Attrée, I. (2017a) Exolysin shapes the virulence of Pseudomonas aeruginosa clonal outliers. Toxins 9: 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboud, E. , Bouillot, S. , Patot, S. , Béganton, B. , Attrée, I. , and Huber, P. (2017b) Pseudomonas aeruginosa ExlA and Serratia marcescens ShlA trigger cadherin cleavage by promoting calcium influx and ADAM10 activation. PLoS Pathog 13: e1006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger, N. , Michez, D. , Wattiez, R. , Sheridan, C. , and Vanderplanck, M. (2017) Diet effects on bumblebee health. J Insect Physiol 96: 128–133. [DOI] [PubMed] [Google Scholar]
- Ruffner, B. , Péchy‐Tarr, M. , Höfte, M. , Bloemberg, G. , Grunder, J. , Keel, C. , and Maurhofer, M. (2015) Evolutionary patchwork of an insecticidal toxin shared between plant‐associated pseudomonads and the insect pathogens Photorhabdus and Xenorhabdus . BMC Genomics 16: 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffner, B. , Péchy‐Tarr, M. , Ryffel, F. , Hoegger, P. , Obrist, C. , Rindlisbacher, A. , et al. (2013) Oral insecticidal activity of plant‐associated pseudomonads. Environ Microbiol 15: 751–763. [DOI] [PubMed] [Google Scholar]
- Rumbaugh, K.P. (2014) Genomic complexity and plasticity ensure Pseudomonas success. FEMS Microbiol Lett 356: 141–143. [DOI] [PubMed] [Google Scholar]
- Saravanakumar, D. , Muthumeena, K. , Lavanya, N. , Suresh, S. , Rajendran, L. , Raguchander, T. , and Samiyappan, R. (2007) Pseudomonas‐induced defence molecules in rice plants against leaffolder (Cnaphalocrocis medinalis) pest. Pest Manag Sci 63: 714–721. [DOI] [PubMed] [Google Scholar]
- Schellenberger, U. , Oral, J. , Rosen, B.A. , Wei, J.‐Z. , Zhu, G. , Xie, W. , et al. (2016) A selective insecticidal protein from Pseudomonas for controlling corn rootworms. 354: 634–637. [DOI] [PubMed] [Google Scholar]
- Seemann, T. (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30: 2068–2069. [DOI] [PubMed] [Google Scholar]
- Silby, M.W. , Winstanley, C. , Godfrey, S.A.C. , Levy, S.B. , and Jackson, R.W. (2011) Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev 35: 652–680. [DOI] [PubMed] [Google Scholar]
- Singer, M.S. , Mason, P.A. , and Smilanich, A.M. (2014) Ecological immunology mediated by diet in herbivorous insects. Integr Comp Biol 54: 913–921. [DOI] [PubMed] [Google Scholar]
- Skowronek, M. , Sajnaga, E. , Pleszczyńska, M. , Kazimierczak, W. , Lis, M. , and Wiater, A. (2020) Bacteria from the midgut of common cockchafer (Melolontha melolontha L.) larvae exhibiting antagonistic activity against bacterial symbionts of entomopathogenic nematodes: isolation and molecular identification. Int J Mol Sci 21: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau, T.M. , and Grambsch, P.M. (2000) Modeling Survival Data: Extending the Cox Model. New York: Springer‐Verlag. [Google Scholar]
- Thorpe, H.A. , Bayliss, S.C. , Hurst, L.D. , and Feil, E.J. (2017) Comparative analyses of selection operating on nontranslated intergenic regions of diverse bacterial species. Genetics 206: 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thynne, E. , McDonald, M.C. , and Solomon, P.S. (2015) Phytopathogen emergence in the genomics era. Trends Plant Sci 20: 246–255. [DOI] [PubMed] [Google Scholar]
- Tovi, N. , Frenk, S. , Hadar, Y. , and Minz, D. (2019) Host specificity and spatial distribution preference of three Pseudomonas isolates. Front Microbiol 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron, J. , Péchy‐Tarr, M. , Brochet, S. , Heiman, C.M. , Stojiljkovic, M. , Maurhofer, M. , and Keel, C. (2019) T6SS contributes to gut microbiome invasion and killing of an herbivorous pest insect by plant‐beneficial Pseudomonas protegens . ISME J 13: 1318–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar, A. , Handelsman, J. , Schloss, P.D. , Bauer, L.S. , and Raffa, K.F. (2008) Gut microbiota of an invasive subcortical beetle, Agrilus planipennis fairmaire, across various life stages. Mol Ecol Evol 37: 1344–1353. [DOI] [PubMed] [Google Scholar]
- Vesga, P. , Flury, P. , Vacheron, J. , Keel, C. , Croll, D. , and Maurhofer, M. (2020) Transcriptome plasticity underlying plant root colonization and insect invasion by Pseudomonas protegens. ISME J 14: 2766–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G.‐H. , Berdy, B.M. , Velasquez, O. , Jovanovic, N. , Alkhalifa, S. , Minbiole, K.P.C. , and Brucker, R.M. (2020) Changes in microbiome confer multigenerational host resistance after sub‐toxic pesticide exposure. Cell Host Microbe 27: 213–224. [DOI] [PubMed] [Google Scholar]
- Waterfield, N.R. , Daborn, P.J. , Dowling, A.J. , Yang, G. , Hares, M. , and Ffrench‐Constant, R.H. (2003) The insecticidal toxin makes caterpillars floppy 2 (Mcf2) shows similarity to HrmA, an avirulence protein from a plant pathogen. FEMS Microbiol Lett 229: 265–270. [DOI] [PubMed] [Google Scholar]
- Waters, L.S. , and Storz, G. (2009) Regulatory RNAs in bacteria. Cell 136: 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, A.C.‐N. , Luo, Y. , Jing, X. , Franzenburg, S. , Bost, A. , and Douglas, A.E. (2015) The host as the driver of the microbiota in the gut and external environment of Drosophila melanogaster . Appl Environ Microbiol 81: 6232–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziganshina, E.E. , Mohammed, W.S. , Shagimardanova, E.I. , Vankov, P.Y. , Gogoleva, N.E. , and Ziganshin, A.M. (2018) Fungal, bacterial, and archaeal diversity in the digestive tract of several beetle larvae (Coleoptera). Biomed Res Int 2018: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Supplementary figures.
File S2. Coding sequences used in the BLAST analysis.
Table S1. Genome information of the sequenced strains of this study.
Table S2. List of used genomes.
Table S3. LT50 raw data.
Table S4. Pairwise p‐values of the survival log‐rank test.
Table S5. Summary of the SNPs found in the PCLAR01, PCLAR03 and PCLAR04 strains.
Table S6. Summary of the SNPs found in the genes of interest of PCLAR01, PCLAR03, PCLAR04.
Table S7. Soil characteristics.
Appendix S1. Supporting information.
Data Availability Statement
The genome sequences were deposited on the NCBI under the BioProject ID PRJNA637204.


