Abstract
Sialylated HEG1 has been reported as a highly specific and sensitive mesothelioma marker but a comprehensive evaluation of its expression in carcinomas in different organs, various sarcomas and reactive mesothelial proliferations has not been reported. The aim of this study was to evaluate the clinical applicability of HEG1 as a marker in the diagnosis of mesothelioma. HEG1 immunoreactivity was evaluated in whole sections of 122 mesotheliomas, 75 pulmonary carcinomas, 55 other carcinomas, 16 mesenchymal tumors, and 24 reactive mesothelial proliferations and in tissue microarrays containing 70 epithelioid (EM), 36 biphasic (BM), and 2 sarcomatoid mesotheliomas (SM). In whole sections and tissue microarrays, respectively, membranous HEG1 was expressed in 93.0% and 85.5% of EM, 81.3% and 69.4% of BM, 0% and 0% of SM. HEG1 was not expressed in pulmonary adenocarcinomas. HEG1 was expressed as cytoplasmic immunoreactivity in pulmonary squamous cell carcinomas (21.7%). Membranous HEG1 staining was seen in ovarian carcinomas (66.7%), thyroid carcinomas (100%), reactive conditions (16.7%), and mesenchymal tumors (18.8%). The sensitivity of membranous HEG1 expression to distinguish EM/BM from all carcinomas was 88.8%. The specificity for the differential diagnosis between EM/BM and all carcinomas and pulmonary carcinomas was 92.3% and 98.7%, respectively.
Keywords: HEG1, immunohistochemistry, mesothelial marker, mesothelioma
Abbreviations
- BM
biphasic mesothelioma
- CK5/6
cytokeratin 5/6
- EM
epithelioid mesothelioma
- ER
epitope retrieval solution
- FFPE
formalin‐fixed paraffin‐embedded
- HEG1
protein HEG homolog 1
- LCNEC
large cell neuroendocrine carcinoma
- PAX8
paired box 8
- SCC
squamous cell carcinoma
- SM
sarcomatoid mesothelioma
- TTF‐1
thyroid transcription factor‐1
- TMA
tissue microarray
- WDPM
well‐differentiated papillary mesothelioma
- WT1
Wilms' tumor‐1
INTRODUCTION
Malignant mesothelioma is an aggressive tumor arising from mesothelial cells. Approximately 80% of these neoplasms occur in patients with previous exposure to asbestos. In 2018 there were 30 000 newly diagnosed patients with mesothelioma and 26 000 deaths from mesothelioma world‐wide. 1
The diagnosis of malignant mesothelioma can be difficult and it is essential to consider the clinical history, physical examination, and imaging findings in combination with the pathology in order to accurately distinguish mesothelioma from other malignancies. 2 , 3 Current guidelines for a definitive pathologic diagnosis of malignant mesothelioma require two positive mesothelioma markers as well as two negative markers for other tumors in the morphological differential diagnosis. 2 Claudin 4 is the most sensitive and specific of the commonly used carcinoma markers (claudin 4, carcinoembryonic antigen, MOC31, and BER‐EP4). 2 Claudin 4 is also consistently negative in mesotheliomas. 4 , 5 Calretinin, WT1, podoplanin, and CK5/6 are the best commercially available mesothelioma markers for the histologic diagnosis of malignant mesothelioma. However, none of these markers is 100% sensitive or 100% specific for this diagnosis. Given that some epithelioid, biphasic or sarcomatoid mesotheliomas exhibit immunoreactivity for none or only one of these markers, there is a need for additional sensitive and specific mesothelial markers that could be used in selected cases.
Protein HEG homolog 1 (HEG1) was first reported as the “heart of glass” gene regulating the concentric growth of the zebrafish heart. 6 HEG1, a heart development protein with EGF‐like domains 1, is suggested to regulate an endothelial cell signaling pathway. 7 It has also been suggested that HEG1 expression may support the survival and proliferation of mesothelioma cells 8 and may promote metastasis of hepatocellular carcinoma. 9 , 10 Tsuji and colleagues recently reported that sialylated HEG1, a novel mucin‐like membrane protein, is a mesothelioma‐related antigen and that HEG1 protein expression is highly sensitive and specific for malignant mesothelioma. 8 More recently, Naso et al. also reported that HEG1 is a highly specific mesothelial marker in the differential diagnosis between sarcomatoid mesotheliomas and sarcomatoid carcinomas. 11 However, a comprehensive study of HEG1 immunohistochemical expression and staining patterns (membranous, cytoplasmic, or both) in mesotheliomas, carcinomas and sarcomas has not been previously reported.
MATERIALS AND METHODS
Tissue samples
Tissue microarrays (TMAs) provided by one of the authors (ANH) 12 included 87 malignant pleural mesotheliomas (51 epithelioid, 34 biphasic, 2 sarcomatoid) and 22 malignant peritoneal mesotheliomas (19 epithelioid, 3 biphasic).
Additional cases from the pathology department archives at Tokyo Women's Medical University, Yachiyo Medical Center, Chiba Rosai Hospital, and Cedars‐Sinai Medical Center, included biopsies of 120 malignant pleural mesotheliomas (53 epithelioid, 34 biphasic, 24 sarcomatoid, 3 desmoplastic, 3 transitional, 2 with heterologous elements, 1 lymphohistiocytoid), 5 malignant peritoneal mesotheliomas (4 epithelioid, 1 sarcomatoid), 1 malignant mesothelioma of tunica vaginalis (1 epithelioid), 1 well‐differentiated papillary mesothelioma, 75 pulmonary carcinomas (34 nonmucinous adenocarcinomas (15 papillary, 11 acinar, 4 lepidic, 3 solid adenocarcinomas, and 1 adenocarcinoma in situ), 2 invasive mucinous adenocarcinomas, 23 squamous cell carcinomas (12 keratinizing, 10 nonkeratinizing squamous carcinomas, and 1 squamous cell carcinoma in situ), 2 adenosquamous carcinomas, 9 pleomorphic carcinomas, and 1 mucoepidermoid carcinoma, 4 large cell neuroendocrine carcinomas), and 56 nonpulmonary carcinomas (9 esophageal squamous cell carcinomas, 6 gastric adenocarcinomas, 7 colon adenocarcinomas, 7 breast carcinomas, 9 ovarian serous carcinomas, 7 uterine cervical squamous cell carcinomas, 6 urothelial carcinomas, 3 thyroid carcinomas (2 papillary and 1 follicular), 1 pancreatic adenosquamous carcinoma, and 1 thymic pleomorphic carcinoma), 16 mesenchymal tumors (6 angiosarcomas, 6 leiomyosarcomas, 2 lymphomas, 1 solitary fibrous tumor, and 1 synovial sarcoma), 21 cases of fibrous pleuritis and 3 cases with reactive mesothelial cells.
Results of immunohistochemistry of the TMAs and whole sections of 88 malignant mesotheliomas, 57 pulmonary carcinomas, and 11 cases of fibrous pleuritis have been published in the congress proceedings. 13
This study, including anonymous use of redundant tissues from archived materials, was approved by the ethics committee of Tokyo Women's Medical University (approval number, 4553; approval date, November 2017). Informed consent was waived by the ethics committee due to minimal risk.
Immunohistochemistry
Specimens were processed at Tokyo Women's Medical University, Yachiyo Medical Center. Sections (4 μm) were cut from formalin‐fixed paraffin‐embedded (FFPE) blocks and placed on silanized slides (Muto Pure Chemicals, Tokyo, Japan), and unstained FFPE sections were submitted for the study from the other institutions. Immunohistochemistry was performed using the following primary antibodies: monoclonal anti‐sialylated HEG1 antibody (SKM9‐2; 20 μg/mL, produced by one of the coauthors [ST]) (Nichirei Biosciences Inc., Tokyo, Japan), monoclonal anti‐calretinin antibody (5A5, 1:200, #NCL‐CALRETININ; Leica Biosystems, Wetzlar, Germany), monoclonal anti‐WT1 antibody (WT49, 1:1, #PA0562; Leica Biosystems), and monoclonal anti‐podoplanin antibody (D2‐40, 1:10, #IR072; Dako, Glostrup, Denmark). To improve staining intensity, the tissue slides were heated in an autoclave at 121°C for 10 min in a citrate buffer (10 mM, pH 6.0) before staining with anti‐sialylated HEG1 antibody, heated for 20 min in BOND Epitope Retrieval Solution 2 (ER2) (Leica Biosystems) before staining with anti‐calretinin antibody or anti‐podoplanin antibody, or heated for 30 min in BOND ER2 before staining with anti‐WT1 antibody. Staining was performed on a Leica BOND Max automatic stainer (Leica Biosystems).
HEG1 Immunohistochemical staining scores
Immunohistochemical staining scores were calculated as previously described. 14 The staining intensity for HEG1 in biopsies was scored as follows: 0 (none), 1 (weak), 2 (moderate), and 3 (strong). The extent of staining was scored as follows: 0 (<1%), 1 (1%–24%), 2 (25%–49%), 3 (≥50%). A staining result was considered negative when the total staining score was ≤2 and considered positive when the total staining score was ≥3. HEG1 staining was also recorded as membranous or cytoplasmic for each case. Apical membranous staining was recorded as apical. In biphasic mesotheliomas, staining was based on the epithelioid component. Membranous immunoreactivity was interpreted as a positive stain in all tumors and reactive mesothelial proliferations. Positive HEG1 cytoplasmic staining of endothelial cells served as the internal positive control. Absence of HEG1 staining in lymphocytes served as the internal negative control.
Conventional mesothelial markers
Results of immunohistochemical staining for calretinin, WT1, and podoplanin in biopsies of epithelioid and biphasic mesotheliomas were obtained from the patients’ medical records.
Statistical analysis
Fisher's exact test, using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA), was used to compare the frequency of expression of mesothelial markers. All statistical tests were two‐sided and used a 5% level of significance.
RESULTS
Staining for HEG1 and conventional mesothelial markers in TMAs
As shown in Table 1, the frequency of expression of HEG1 is higher than that of WT1 and podoplanin in epithelioid and biphasic mesotheliomas. Both sarcomatoid mesotheliomas in the TMA were negative for HEG1, calretinin, WT1, and podoplanin.
Table 1.
Expression frequency of HEG1 and conventional mesothelial markers in mesotheliomas
| EM | BM | SM | ||||
|---|---|---|---|---|---|---|
| No. I/T | (%) | No. I/T | (%) | No. I/T | (%) | |
| TMA | ||||||
| m + cHEG1 | 66/69 | 95.7 | 34/36 | 94.4 | 2/2 | 100 |
| mHEG1 | 59/69 | 85.5 | 25/36 | 69.4 | 0/2 | 0 |
| Calretinin | 64/70 | 91.4 | 19/36 | 52.8 | 0/2 | 0 |
| WT1 | 45/70 | 64.3* | 10/36 | 27.8* | 0/2 | 0 |
| Podoplanin | 47/70 | 67.1* | 10/36 | 27.8* | 0/2 | 0 |
| Whole sections | ||||||
| m + cHEG1 | 56/57 | 98.2 | 30/32 | 93.8 | 20/25 | 80.0 |
| mHEG1 | 53/57 | 93.0 | 26/32 | 81.3 | 0/25 | 0 |
| Calretinin | 41/43 | 95.3 | 16/19 | 84.2 | NA | |
| WT1 | 30/38 | 78.9 | 10/17 | 58.8 | NA | |
| Podoplanin | 36/42 | 85.7 | 14/17 | 82.4 | NA | |
Abbreviations: BM, biphasic mesothelioma; EM, epithelioid mesothelioma; m + cHEG1, membranous and/or cytoplasmic HEG1 staining; mHEG1, membranous HEG1 staining; NA, not analyzed; No. I/T, number of immunoreactive/total cases; SM, sarcomatoid mesothelioma; TMA, tissue microarray.
Statistically significant difference between expression frequency of membranous HEG1 staining and that of conventional mesothelial marker (p < 0.05).
There was a statistically significant difference between the expression frequencies of HEG1 and WT1 (p = 0.0058), between that of HEG1 and podoplanin (p = 0.0161), between that of calretinin and WT1 (p = 0.0002), and between that of calretinin and podoplanin (p = 0.0007) in epithelioid mesotheliomas. There was also a statistically significant difference between the expression frequencies of HEG1 and WT1 (p = 0.0008), and between that of HEG1 and podoplanin (p = 0.0008) in biphasic mesotheliomas. However, no statistically significant difference in expression frequency was observed between any of the markers in sarcomatoid mesotheliomas.
Staining in whole sections
Five cases were excluded from the study (one epithelioid mesothelioma, two biphasic mesotheliomas, one mesothelioma with heterologous elements, and one uterine cervical carcinoma) because tissues were not well preserved and immunostains were uninterpretable. The 293 cases analyzed included 122 malignant mesotheliomas (57 epithelioid, 32 biphasic, 25 sarcomatoid, 3 desmoplastic, 3 transitional, 1 with heterologous elements, 1 lymphohistiocytoid), 1 well‐differentiated papillary mesothelioma, 75 pulmonary carcinomas, 55 nonpulmonary carcinomas, 16 mesenchymal tumors, and 24 reactive mesothelial proliferations.
HEG1 expression in mesotheliomas
Immunoreactivity for HEG1 in whole sections from mesotheliomas is shown in Tables 1 and 2; 79 of 89 (88.8%) epithelioid components of epithelioid/biphasic mesotheliomas were positive for HEG1. Fifty three of 57 (93.0%) epithelioid mesotheliomas showed membranous staining (Figure 1a,b) and three (5.3%) showed cytoplasmic staining (Figure 1c–f); one was negative for HEG1. Twenty‐six of 32 (81.3%) biphasic mesotheliomas showed membranous staining and four (12.5%) showed cytoplasmic staining (Figure 2a,b); two were negative for HEG1. Twenty of 25 (80.0%) sarcomatoid mesotheliomas (Figure 2c,d), two of three (66.7%) desmoplastic mesotheliomas, and one (100%) lymphohistiocytoid mesothelioma showed cytoplasmic staining. None of these showed membranous staining. Intratumoral heterogeneity of staining from field to field was observed in some mesotheliomas. However, the staining in most epithelioid mesotheliomas and in most of the epithelioid component in biphasic mesotheliomas was strong and diffuse, while that in sarcomatoid mesotheliomas was variable. One well‐differentiated papillary mesothelioma showed strong, diffuse apical HEG1 staining.
Table 2.
HEG1 immunostaining in tumors analyzed in this study (whole sections)
| Staining score | Staining pattern | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | (%) | 0–2 | 3 | 4 | 5 | 6 | m | (%) | c | (%) | |
| Mesotheliomas | 122 | 112 | (91.8) | 10 | 5 | 5 | 21 | 81 | 81 | (66.4) | 31 | (25.4) |
| Epithelioid and biphasic | 89 | 86 | (96.6) | 3 | 1 | 0 | 12 | 73 | 79 | (88.8) | 7 | (7.9) |
| Epithelioid | 57 | 56 | (98.2) | 1 | 1 | 0 | 7 | 48 | 53 | (93.0) | 3 | (5.3) |
| Biphasic (epithelioid component) | 32 | 30 | (93.8) | 2 | 0 | 0 | 5 | 25 | 26 | (81.3) | 4 | (12.5) |
| Sarcomatoid | 25 | 20 | (80.0) | 5 | 4 | 4 | 7 | 5 | 0 | (0.0) | 20 | (80.0) |
| Desmoplastic | 3 | 2 | (66.7) | 1 | 0 | 0 | 2 | 0 | 0 | (0.0) | 2 | (66.7) |
| Transitional | 3 | 3 | (100.0) | 0 | 0 | 1 | 0 | 2 | 2 | (66.7) | 1 | (33.3) |
| Heterologous elements | 1 | 0 | (0.0) | 1 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Lymphohistiocytoid | 1 | 1 | (100.0) | 0 | 0 | 0 | 0 | 1 | 0 | (0.0) | 1 | (100.0) |
| WDPM | 1 | 1 | (100.0) | 0 | 0 | 0 | 0 | 1 | 1 | (100.0) | 0 | (0.0) |
| Lung carcinomas | 75 | 11 | (14.7) | 64 | 4 | 3 | 3 | 1 | 1 | (1.3) | 10 | (13.3) |
| Nonmucinous adenocarcinoma | 34 | 0 | (0.0) | 34 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Invasive mucinous adenocarcinoma | 2 | 0 | (0.0) | 2 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| SCC | 23 | 5 | (21.7) | 18 | 2 | 1 | 2 | 0 | 0 | (0.0) | 5 | (21.7) |
| Keratinizing SCC | 12 | 2 | (16.7) | 10 | 1 | 1 | 0 | 0 | 0 | (0.0) | 2 | (16.7) |
| Nonkeratinizing SCC | 10 | 2 | (20.0) | 8 | 1 | 0 | 1 | 0 | 0 | (0.0) | 2 | (20.0) |
| SCC in situ | 1 | 1 | (100.0) | 0 | 0 | 0 | 1 | 0 | 0 | (0.0) | 1 | (100.0) |
| Adenosquamous carcinoma | 2 | 2 | (100.0) | 0 | 2 | 0 | 0 | 0 | 1 | (50.0) | 1 | (50.0) |
| Pleomorphic carcinoma | 9 | 4 | (44.4) | 5 | 0 | 2 | 1 | 1 | 0 | (0.0) | 4 | (44.4) |
| Mucoepidermoid carcinoma | 1 | 0 | (0.0) | 1 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| LCNEC | 4 | 0 | (0.0) | 4 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Other carcinomas | ||||||||||||
| Esophageal carcinoma | 9 | 1 | (11.1) | 8 | 0 | 0 | 0 | 1 | 0 | (0.0) | 1 | (11.1) |
| Gastric carcinoma | 6 | 0 | (0.0) | 6 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Colon carcinoma | 7 | 0 | (0.0) | 7 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Breast carcinoma | 7 | 0 | (0.0) | 7 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Ovarian serous carcinoma | 9 | 6 | (66.7) | 3 | 5 | 0 | 0 | 1 | 6 | (66.7) | 0 | (0.0) |
| Uterine cervical carcinoma | 6 | 2 | (33.3) | 4 | 1 | 1 | 0 | 0 | 0 | (0.0) | 2 | (33.3) |
| Urothelial carcinoma | 6 | 0 | (0.0) | 6 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Thyroid carcinoma | 3 | 3 | (100.0) | 0 | 1 | 0 | 1 | 1 | 3 | (100.0) | 0 | (0.0) |
| Pancreatic carcinoma | 1 | 0 | (0.0) | 1 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Thymic carcinoma | 1 | 0 | (0.0) | 1 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Mesenchymal tumors | ||||||||||||
| Angiosarcoma | 6 | 6 | (100.0) | 0 | 0 | 2 | 4 | 0 | 3 | (50.0) | 3 | (50.0) |
| Leiomyosarcoma | 6 | 6 | (100.0) | 0 | 1 | 2 | 1 | 2 | 0 | (0.0) | 6 | (100.0) |
| Lymphoma | 2 | 0 | (0.0) | 2 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Solitary fibrous tumor | 1 | 1 | (100.0) | 0 | 0 | 0 | 1 | 0 | 0 | (0.0) | 1 | (100.0) |
| Synovial sarcoma | 1 | 0 | (0.0) | 1 | 0 | 0 | 0 | 0 | 0 | (0.0) | 0 | (0.0) |
| Reactive mesothelial proliferations | ||||||||||||
| Fibrous pleuritis | 21 | 13 | (61.9) | 0 | 3 | 6 | 3 | 1 | 1 | (4.8) | 12 | (57.1) |
| Reactive mesothelial cells | 3 | 3 | (100.0) | 0 | 0 | 0 | 0 | 3 | 3 | (100.0) | 0 | (0.0) |
Note: Discrepancy in the sum of percentages in a tabulation is due to rounding of numbers.
Abbreviations: c, cytoplasmic; LCNEC, large cell neuroendocrine carcinoma; m, membranous; N, total; P, positive; SCC, squamous cell carcinoma; WDPM, well‐differentiated papillary mesothelioma.
Figure 1.
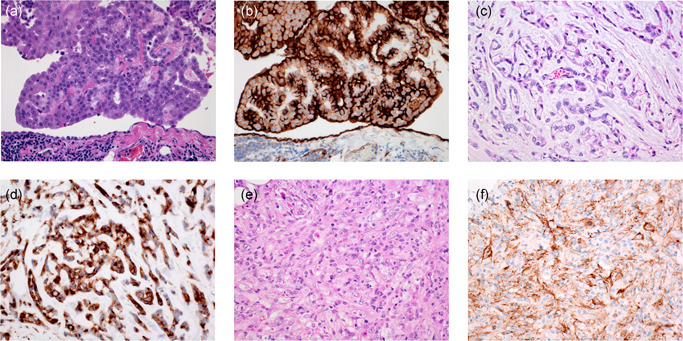
Epithelioid mesothelioma with tumor cells growing in papillary pattern (a) and showing strong membranous HEG1 staining (b). Reactive mesothelial cells also show membranous or cytoplasmic HEG1 staining. Epithelioid mesothelioma with tumor cells growing in trabeculae (c). Mesothelioma cells show strong cytoplasmic HEG1 staining (d). Pleomorphic mesothelioma (e) with tumor cells showing granular cytoplasmic HEG1 staining (f)
Figure 2.
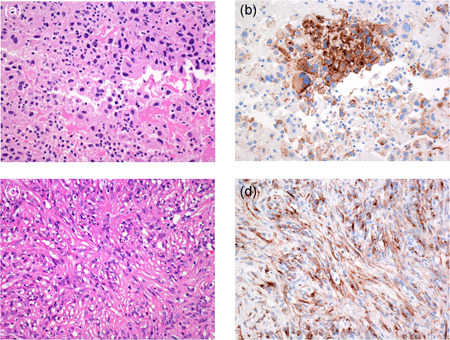
Biphasic mesothelioma (a) with tumor cells showing strong granular cytoplasmic HEG1 staining (b). Sarcomatoid mesothelioma (c) with spindle‐shaped tumor cells showing granular cytoplasmic HEG1 staining (d)
HEG1 expression in carcinomas
As shown in Table 2, although various subtypes of pulmonary adenocarcinomas were studied, HEG1 was not expressed in any of 36 adenocarcinomas (Figure 3a,b), the pulmonary mucoepidermoid carcinoma, or four large cell neuroendocrine carcinomas evaluated. HEG1 was focally expressed in 5 of 23 (21.7%) pulmonary squamous cell carcinomas, two of two (100%) pulmonary adenosquamous carcinomas (in the squamous component only), and four of nine (44.4%) pulmonary pleomorphic carcinomas (Figure 3c,d). Staining was cytoplasmic, weak to moderate and focal in squamous cell carcinomas and pleomorphic carcinomas. None of the squamous cell carcinomas or pleomorphic carcinomas showed membranous staining. Tumor cells at the periphery of the nests of squamous cell carcinoma with differentiation to basal cells tended to be positive for HEG1; keratinizing cells were negative. Staining was weak to moderate, focal, and membranous in one adenosquamous carcinoma and cytoplasmic in another adenosquamous cell carcinoma. Six of nine (66.7%) ovarian serous carcinomas exhibited membranous staining for HEG1 (Figure 3e,f) with total staining scores of 3 (five cases) and 6 (one case). In two ovarian serous carcinomas that were considered negative for HEG1, staining intensity was 1 and staining extent was 0 or 1. Total staining score was 0 in one ovarian serous carcinoma. All (100%) of three thyroid carcinomas were positive for HEG1 (Figure 3g,h); staining was membranous, moderate to strong, and of variable extent. One of nine (11.1%) esophageal carcinomas and two of six (33.3%) uterine cervical carcinomas were positive for HEG1, while all six gastric carcinomas, seven colonic carcinomas, one pancreatic carcinoma, six urothelial carcinomas, seven breast carcinomas, and one thymic carcinoma in the study were negative. Staining in the esophageal and uterine cervical squamous carcinomas was cytoplasmic. Most fibroblasts in the tumor stroma stained with the HEG1 antibody in our study, thereby making the visualization of HEG1 staining in the tumor cells difficult at low power magnification.
Figure 3.
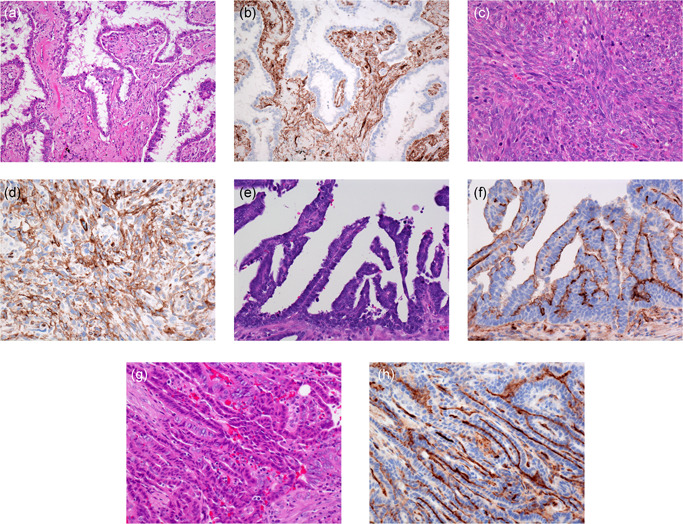
Pulmonary adenocarcinoma (a). Adenocarcinoma cells do not express HEG1 (b). Capillary endothelium and stromal cells show cytoplasmic HEG1 staining. Pulmonary pleomorphic carcinoma (c) with spindle‐shaped tumor cells showing granular cytoplasmic HEG1 staining (d). Ovarian serous carcinoma (e) showing membranous HEG1 staining (f). Thyroid papillary carcinoma (g) showing strong membranous HEG1 staining (h)
Overall, membranous HEG1 staining had a sensitivity of 88.8% and a specificity of 92.3% in distinguishing epithelioid/biphasic mesotheliomas from carcinomas (Table 3). When the differential was restricted to epithelioid/biphasic mesotheliomas versus pulmonary carcinomas, membranous HEG1 staining had a sensitivity of 88.8% and a specificity of 98.7% for mesotheliomas.
Table 3.
Sensitivity and specificity of membranous HEG1 staining for diagnosis of mesothelioma (whole sections)
| N | Positive | Negative | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| EM or BM | 89 | 79 | 10 | ||
| Carcinoma | 130 | 10 | 120 | 88.8 | 92.3 |
| Reactive mesothelial cells | 3 | 3 | 0 | 88.8 | 0 |
| SM | 28 | 0 | 28 | ||
| Mesenchymal tumor | 16 | 3 | 13 | 0 | 81.3 |
| Fibrous pleuritis | 21 | 1 | 20 | 0 | 95.2 |
Abbreviations: BM, biphasic mesothelioma; EM, epithelioid mesothelioma; N, total; SM, sarcomatoid mesothelioma.
HEG1 expression in mesenchymal tumors
Three angiosarcomas exhibited moderate diffuse membranous immunoreactivity. Three angiosarcomas, six leiomyosarcomas, and one solitary fibrous tumor in the study exhibited strong, mostly diffuse cytoplasmic immunoreactivity for HEG1 (Table 2). Both lymphomas and the synovial sarcoma were negative. Overall, 3 of 16 (18.8%) mesenchymal tumors studied showed membranous HEG1 staining. Membranous HEG1 had a low sensitivity in distinguishing sarcomatoid mesothelioma including desmoplastic mesothelioma from mesenchymal tumors (Table 3).
HEG1 expression in reactive mesothelial proliferations
As shown in Tables 2 and 3, 13 of 21 (61.9%) biopsies of fibrous pleuritis showed weak, diffuse staining for HEG1 (Figure 4a,b). Twelve showed cytoplasmic and one showed membranous staining. The eight other cases of fibrous pleuritis were negative for HEG1. HEG1 had a low sensitivity in distinguishing sarcomatoid mesothelioma from fibrous pleuritis (Table 3). All three cases with reactive mesothelial cells showed strong and diffuse apical staining (Figure 4c,d). HEG1 had a low specificity in distinguishing epithelioid/biphasic mesothelioma from reactive mesothelial cells (Table 3). Overall, 4 of 24 (16.7%) reactive mesothelial proliferations showed membranous HEG1 staining.
Figure 4.
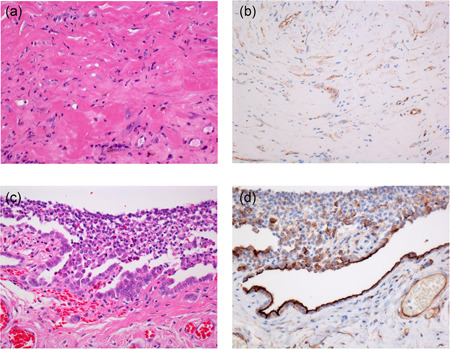
Fibrous pleuritis (a) with spindle cells showing weak cytoplasmic HEG1 staining (b). Pleural reactive mesothelial proliferation associated with pneumothorax (c) showing strong apical HEG1 staining in reactive mesothelial cells (d)
Expression of conventional mesothelial markers in epithelioid and biphasic mesotheliomas
As shown in Table 1, there was no statistically significant difference between the expression frequency of two markers except between that of calretinin and WT1 (p = 0.0399) in epithelioid mesotheliomas in whole sections.
Immunohistochemical staining results for all three conventional mesothelial markers (calretinin, WT1, podoplanin) were available from the medical records of 51 patients with malignant mesothelioma (35 epithelioid, 16 biphasic). One, two, and all three conventional mesothelial markers were positive in 13.7%, 25.5%, and 60.8%, respectively, of the 51 mesotheliomas whereas HEG1 was positive in 88.2% of the mesotheliomas. Although 2 of 35 epithelioid mesotheliomas and 5 of 16 biphasic mesotheliomas stained with only 1 conventional mesothelial marker, all epithelioid mesotheliomas and 3 of 5 biphasic mesotheliomas that stained with only 1 conventional mesothelial marker (71.4%) showed membranous HEG1 staining. The remaining 2 biphasic mesotheliomas that stained with only 1 conventional mesothelial marker showed cytoplasmic HEG1 staining.
DISCUSSION
Our results show that membranous HEG1 is a useful mesothelial marker and could be a valuable addition to the current panel of markers in the differential diagnosis of epithelioid/biphasic mesothelioma versus carcinomas. The HEG1 staining in most epithelioid mesotheliomas and in the epithelioid component of biphasic mesotheliomas was membranous, strong, and diffuse. HEG1 was not expressed in any of the pulmonary adenocarcinomas. Focal cytoplasmic staining for HEG1 was present in 21.7% of the pulmonary squamous cell carcinomas; none showed diffuse staining. In pulmonary squamous cell carcinomas, HEG1 staining was observed in basal cells but not in keratinizing cells and was independent of the degree of tumor differentiation. In both adenosquamous carcinomas, only the squamous carcinoma component was positive for HEG1; the adenocarcinoma component was negative for HEG1.
Membranous HEG1 staining had a specificity of 92.3% in distinguishing epithelioid/biphasic mesotheliomas from all carcinomas and 98.7% in distinguishing epithelioid/biphasic mesotheliomas from pulmonary carcinomas. Importantly, HEG1 was positive in 71.4% of the cases that stained positive with only one conventional mesothelioma marker.
HEG1 is predicted to be a type I membrane protein and is predominantly localized on the membrane of epithelioid mesothelioma, 8 but cytoplasmic localization was observed in areas of solid growth in 5% of the epithelioid mesotheliomas and in the epithelioid component in 12% of the biphasic mesotheliomas in our study. Such a shift in cellular localization has been reported for some membrane‐associated mucins in other tumors. MUC13 is usually localized in the apical region of ovarian carcinoma cells, but its localization is also observed in the cytoplasm. A shift in cellular localization might facilitate detachment of carcinoma cells from the primary site as well as stromal invasion. 15 Breast carcinomas with cytoplasmic and membranous MUC1 expression have poorer prognosis compared to those with pure luminal expression. 16 It is suggested that differences in HEG1 expression between epithelioid mesothelioma and epithelioid component of biphasic mesothelioma are related to differences in biological behavior of these tumors.
In our study four (44.4%) of nine pulmonary pleomorphic carcinomas stained with HEG1. However, Naso et al. reported that none of 21 pulmonary sarcomatoid carcinomas showed cytoplasmic or membranous staining for HEG1 and that HEG1 has high specificity (100%) for sarcomatoid mesotheliomas when compared with sarcomatoid carcinoma. 11 Possible explanations for the differences in HEG1 staining results reported by the two groups include differences in staining protocol and/or differences in size of the tissue samples. We used the HEG1 antibody at a concentration of 20 μg/mL and heated the tissues in an autoclave at 121°C for 10 min before staining with HEG1 antibody. Despite this strong antigen retrieval protocol, most carcinomas did not express HEG1 and all subtypes of mesotheliomas expressed HEG1. In addition, Naso et al. used TMAs whereas we used whole sections; hence we analyzed a larger area of tumor in all cases. 11
In our study HEG1 staining in ovarian serous carcinomas was weak to moderate in all but one case in which staining was strong and diffuse. We previously reported HEG1 staining in 28% of cell blocks from effusions containing metastatic ovarian carcinoma; all of the ovarian carcinomas that expressed HEG1 were serous carcinomas; none was a clear cell carcinoma. 14 HEG1 staining was membranous, moderate to strong in all thyroid carcinomas, and diffuse in some thyroid carcinomas. However, ovarian serous carcinomas could be correctly identified because they characteristically express estrogen receptor, 17 PAX8, and claudin 4, 18 while thyroid carcinomas characteristically stain positive for TTF‐1 19 and claudin 4. 20 , 21
We previously reported strong membranous staining for HEG1 in most solitary and clustered reactive mesothelial cells and weak cytoplasmic staining in some solitary reactive mesothelial cells in cell blocks of effusions. 14 Here we have confirmed strong, diffuse membranous staining for HEG1 in reactive mesothelial cells. Fibrous pleuritis also stained with HEG1, but the staining was weak and diffuse. Twelve showed cytoplasmic and one showed membranous staining. Therefore, we consider that HEG1 is not useful to distinguish between mesothelioma and reactive mesothelial proliferation.
To improve the intensity of HEG1 staining, heat‐induced epitope retrieval in a citrate buffer was performed with an autoclave in this study. However, most laboratories use an immunohistochemical automatic stainer and do not use an autoclave. Therefore, we compared HEG1 staining intensity using the methods in this study with that obtained heating slides in BOND ER2 (Leica Biosystems) with Bond Max automatic stainer. The staining patterns seen in slides from mesotheliomas did not differ between the two methods and background staining was slightly weaker with heating in BOND ER2 with an automatic stainer.
Limitations to our study include its retrospective nature. The cases analyzed were selected from pathology archives and were not consecutive. Although the cases originated from a variety of organs, tumors originating from additional sites such as brain, salivary gland, kidney, adrenal gland, liver, testis, and others were not represented. Also, because the sensitivity and specificity of HEG1 for the pathological diagnosis of mesothelioma was calculated from the results of this study, these percentages cannot be interpreted as universal and/or applicable in daily pathology practices. Sensitivity and specificity should be assessed from consecutive surgical specimens received in the laboratory.
In conclusion, membranous HEG1 staining shows excellent sensitivity and specificity in the differential diagnosis between epithelioid/biphasic mesothelioma and carcinomas, especially pulmonary carcinomas including squamous cell carcinoma. However, it cannot distinguish epithelioid mesothelioma from ovarian serous carcinoma and thyroid carcinoma. Although HEG1 was not expressed in selected urothelial, breast or colon carcinomas, future studies with larger numbers of cases are needed to confirm this finding and study HEG1 in additional tumors.
CONFLICT OF INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
Conception and design: Kenzo Hiroshima, Shoutaro Tsuji, Yohei Miyagi, Kohzoh Imai; Immunohistochemistry and analysis of data: Kenzo Hiroshima, Di Wu; Acquisition of subjects: Eitetsu Koh, Yasuo Sekine, Toshikazu Yusa; Pathological diagnosis: Kenzo Hiroshima, Daisuke Ozaki, Tadao Nakazawa; Draft of the manuscript: Kenzo Hiroshima, Di Wu; Review and editing: Ann E. Walts, Alberto M. Marchevsky, Aliya N. Husain; Funding acquisition: Kenzo Hiroshima, Kohzoh Imai. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We acknowledge the hisototechnologists at Tokyo Women's Medical University, Yachiyo Medical Center, University of Chicago, and Cedars‐Sinai Medical Center, for their technical contribution to this study. This work was supported by the Ministry of the Environment of Japan, the Japan Agency for Medical Research and Development under Grant Number JP20cm0106406, and the Nichias Corporation, Tokyo, Japan.
Hiroshima K, Wu D, Koh E, Sekine Y, Ozaki D, Yusa T, et al. Membranous HEG1 expression is a useful marker in the differential diagnosis of epithelioid and biphasic malignant mesothelioma versus carcinomas. Pathology International. 2021;71:604–613. 10.1111/pin.13140
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018;142:89–108. [DOI] [PubMed] [Google Scholar]
- 3. Woolhouse I, Bishop L, Darlison L, De Fonseka D, Edey A, Edwards J, et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax. 2018;73:i1–i30. [DOI] [PubMed] [Google Scholar]
- 4. Facchetti F, Lonardi S, Gentili F, Bercich L, Falchetti M, Tardanico R, et al. Claudin 4 identifies a wide spectrum of epithelial neoplasms and represents a very useful marker for carcinoma versus mesothelioma diagnosis in pleural and peritoneal biopsies and effusions. Virchows Arch. 2007;451:669–80. [DOI] [PubMed] [Google Scholar]
- 5. Ordonez NG. Value of claudin‐4 immunostaining in the diagnosis of mesothelioma. Am J Clin Pathol. 2013;139:611–9. [DOI] [PubMed] [Google Scholar]
- 6. Mably JD, Mohideen MA, Burns CG, Chen JN, Fishman MC. Heart of glass regulates the concentric growth of the heart in zebrafish. Curr Biol. 2003;13:2138–47. [DOI] [PubMed] [Google Scholar]
- 7. Kleaveland B, Zheng X, Liu JJ, Blum Y, Tung JJ, Zou Z, et al. Regulation of cardiovascular development and integrity by the heart of glass‐cerebral cavernous malformation protein pathway. Nat Med. 2009;15:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsuji S, Washimi K, Kageyama T, Yamashita M, Yoshihara M, Matsuura R, et al. HEG1 is a novel mucin‐like membrane protein that serves as a diagnostic and therapeutic target for malignant mesothelioma. Sci Rep. 2017;7:45768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dewdney B, Hebbard L. A novel function for HEG1 in promoting metastasis in hepatocellular carcinoma. Clin Sci (Lond). 2019;133:2019–22. [DOI] [PubMed] [Google Scholar]
- 10. Zhao YR, Wang JL, Xu C, Li YM, Sun B, Yang LY. HEG1 indicates poor prognosis and promotes hepatocellular carcinoma invasion, metastasis, and EMT by activating Wnt/beta‐catenin signaling. Clin Sci (Lond). 2019;133:1645–62. [DOI] [PubMed] [Google Scholar]
- 11. Naso JR, Tsuji S, Churg A. HEG1 is a highly specific and sensitive marker of epithelioid malignant mesothelioma. Am J Surg Pathol. 2020;44:1143–8. [DOI] [PubMed] [Google Scholar]
- 12. McGregor SM, Dunning R, Hyjek E, Vigneswaran W, Husain AN, Krausz T. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol. 2015;46:1670–8. [DOI] [PubMed] [Google Scholar]
- 13. Chapel DB, Churg A, Santoni‐Rugiu E, Tsujimura T, Hiroshima K, Husain AN. Molecular pathways and diagnosis in malignant mesothelioma: A review of the 14th International Conference of the International Mesothelioma Interest Group. Lung Cancer. 2019;127:69–75. [DOI] [PubMed] [Google Scholar]
- 14. Hiroshima K, Wu D, Hamakawa S, Tsuruoka S, Ozaki D, Orikasa H, et al. HEG1, BAP1, and MTAP are useful in cytologic diagnosis of malignant mesothelioma with effusion. Diagn Cytopathol. 2021;49:622–32. [DOI] [PubMed] [Google Scholar]
- 15. Chauhan SC, Vannatta K, Ebeling MC, Vinayek N, Watanabe A, Pandey KK, et al. Expression and functions of transmembrane mucin MUC13 in ovarian cancer. Cancer Res. 2009;69:765–74. [DOI] [PubMed] [Google Scholar]
- 16. Rakha EA, Boyce RW, Abd El‐Rehim D, Kurien T, Green AR, Paish EC, et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–304. [DOI] [PubMed] [Google Scholar]
- 17. Ordonez NG. Value of estrogen and progesterone receptor immunostaining in distinguishing between peritoneal mesotheliomas and serous carcinomas. Hum Pathol. 2005;36:1163–7. [DOI] [PubMed] [Google Scholar]
- 18. Ordonez NG. Value of PAX8, PAX2, claudin‐4, and h‐caldesmon immunostaining in distinguishing peritoneal epithelioid mesotheliomas from serous carcinomas. Mod Pathol. 2013;26:553–62. [DOI] [PubMed] [Google Scholar]
- 19. Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R. Diagnostic utility of thyroid transcription factors Pax8 and TTF‐2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol. 2008;21:192–200. [DOI] [PubMed] [Google Scholar]
- 20. Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tzelepi VN, Tsamandas AC, Vlotinou HD, Vagianos CE, Scopa CD. Tight junctions in thyroid carcinogenesis: diverse expression of claudin‐1, claudin‐4, claudin‐7 and occludin in thyroid neoplasms. Mod Pathol. 2008;21:22–30. [DOI] [PubMed] [Google Scholar]


