Abstract
Background
The usefulness of D‐dimer measurement to rule out venous thromboembolism (VTE) during pregnancy is debated.
Objectives
We performed a systematic review and meta‐analysis to investigate the safety of D‐dimer to rule out acute VTE in pregnant women with suspected pulmonary embolism and/or deep vein thrombosis.
Methods
Two reviewers independently identified studies through PubMed and Embase until June 2021, week 1. We supplemented our search by manually reviewing reference lists of all retrieved articles, clinicalTrials.gov, and reference literature. Prospective or retrospective studies in which a formal diagnostic algorithm was used to evaluate the ability of D‐dimer to rule out VTE during pregnancy were eligible.
Results
We identified 665 references through systematic database and additional search strategies; 45 studies were retrieved in full, of which four were included, after applying exclusion criteria. Three studies were prospective, and one had a retrospective design. The 3‐month thromboembolic rate in pregnant women left untreated after a negative D‐dimer was 1/312 (0.32%; 95% CI, 0.06–1.83). The pooled estimate values were 99.5% for sensitivity (95% CI, 95.0–100.0; I², 0%) and 100% for negative predictive value (95% CI, 99.19–100.0; I², 0%). The prevalence of VTE and the yield of D‐dimer were 7.4% (95% CI, 3.8–12; I², 83%) and 34.2% (95% CI, 15.9–55.23; I², 89%) respectively.
Conclusion
Our results suggest that D‐dimer allows to safely rule out VTE in pregnant women with suspected VTE and a disease prevalence consistent with a low/intermediate or unlikely pretest probability.
Keywords: clinical probability, D‐dimer., diagnostic strategy, pregnancy, pulmonary embolism
Essentials.
The usefulness of D‐dimer measurement to rule out venous thromboembolism (VTE) during pregnancy is debated.
A systematic meta‐analysis to investigate the safety of D‐dimer to rule out acute VTE in pregnant women was performed
The pooled estimate values were 99.5% for sensitivity (95% CI, 95.0–100.0; I², 0%) and 100% for negative predictive value (95% CI, 99.19–100.0; I², 0%).
Our results suggest that D‐dimer allows to safely rule out VTE in pregnant women with suspected VTE and a disease prevalence consistent with a low/intermediate or unlikely pre‐test probability.
1. BACKGROUND
The risk of venous thromboembolism (VTE) increases during pregnancy. Acute pulmonary embolism (PE) is still one of the leading causes of maternal death in Western countries. 1 , 2 , 3 , 4 Diagnosis of VTE in pregnant women remains a challenge because of physiological changes of pregnancy that can overlap with signs and symptoms of PE or deep vein thrombosis (DVT). 5 Moreover, because of the heightened awareness that missing a diagnosis could result in severe maternal consequences, the threshold to test for VTE during pregnancy is low. This leads to a low prevalence of confirmed VTE among women investigated for the disease of 2% to 7%, compared with 15% to 20% in a nonpregnant population. 6 , 7 , 8
Plasma D‐dimer measurement, a noninvasive, simple, and inexpensive blood test, has been extensively investigated for excluding the diagnosis of VTE in nonpregnant patients. In the nonpregnant population, the combination of a non‐high pretest probability (PTP) with a negative D‐dimer result safely rules out the diagnosis in one‐third of outpatients with suspected PE. 9 , 10 , 11 This is also the case in outpatients with suspected DVT and an “unlikely” PTP. 12 , 13 , 14 However, these strategies might have limitations in pregnant women. Indeed, most VTE diagnostic studies that derived and validated models assessing PTP in the past have excluded pregnant patients. 15 , 16 , 17 , 18 Also, D‐dimer levels physiologically increase throughout pregnancy, reducing their specificity for VTE in this setting and limiting the chance of a negative result. 19 , 20 Given these limitations, most pregnant women with suspected DVT require lower limb venous compression ultrasound (CUS) and most patients with suspected PE need chest imaging, such as ventilation–perfusion (V/Q) lung scan or computed tomography pulmonary angiography (CTPA). This has raised concerns about potential deleterious consequences of exposure to ionizing radiation and intravenous contrast for the mother and the fetus. 6 , 21 , 22
Over the past few years, new data regarding VTE diagnosis during pregnancy have emerged. Two recent prospective management outcome studies assessed different PE diagnostic strategies, both based on a sequential algorithm applying a clinical decision rule (CDR) followed by D‐dimer measurement in patients with a non‐high PTP or a disease prevalence consistent with unlikely PTP, and imaging when needed (i.e., in patients with a positive D‐dimer result or a high/likely PTP). 23 , 24 These studies suggested the potential role of D‐dimer to safely rule out PE in pregnant women with a non‐high PTP. Nevertheless, despite these recent prospective data, international guidelines remain conflicting regarding the use of D‐dimer in pregnant women. 25 , 26 Hence, to further assess the safety and usefulness of D‐dimer to exclude VTE in pregnant women, we performed a systematic review and meta‐analysis of available data in this setting.
2. OBJECTIVES
2.1. Primary outcome
Our primary objective was to investigate the safety of D‐dimer to rule out acute VTE, by assessing the sensitivity and negative predictive value (NPV) of D‐dimer in pregnant women with suspected PE and/or DVT with a disease prevalence consistent with a low/intermediate or unlikely PTP (expected to be 10% and 30% in low and moderate probability and less than 10% in the unlikely category). 25 , 27 , 28
2.2. Secondary outcomes
Our secondary objectives were to assess the diagnostic yield of D‐dimer during pregnancy (i.e., the proportion of patients with negative D‐dimer among pregnant women with suspected PE and/or DVT and a disease prevalence consistent with a low/intermediate or unlikely PTP) and to evaluate for each pregnancy trimester (first, second, or third trimester) and puerperium, the sensitivity and NPV of D‐dimer to rule out VTE, as well as the diagnostic yield of the test.
3. METHODS
This systematic review was performed according to the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) 29 and the Preferred Reporting Items for a Systematic Review and Meta‐analysis of Diagnostic Test Accuracy Studies (PRISMA‐DTA) 30 guidelines.
3.1. Criteria for study selection
Prospective and retrospective studies that used plasma D‐dimer measurement to rule out VTE in pregnant women with suspected PE and/or DVT were considered. We included studies using D‐dimer (index test), combined or not with PTP assessment, in which an imaging test (V/Q lung scan, CTPA, pulmonary angiography, lower limb venous CUS) or clinical follow‐up at 3 months were used as the reference standard. Studies were eligible if a 2 × 2 contingency table was supplied or could be back‐calculated (true positives, true negatives, false positives, and false negatives), or if the sensitivity and NPV of D‐dimer could be calculated.
3.2. Population
The study's population consisted of adult (≥18 years) pregnant women during their first, second, or third trimester of pregnancy presenting to an outpatient clinic or emergency department with symptoms and/or signs suggestive of acute PE and/or DVT, namely acute or new‐onset dyspnea and/or chest pain or lower limb pain and/or edema without an obvious explanation. The present analysis targets a population of pregnant women estimated with a disease prevalence consistent with a low/intermediate or unlikely PTP according to a CDR (prespecified PTP) or irrespective of PTP when a CDR had not been applied (PTP not used) in the original study.
3.3. Index test
The index test was the measurement of plasma D‐dimer levels on a blood test performed at the time of patient presentation with suspected VTE using a quantitative, semiquantitative, or a qualitative assay. D‐dimer threshold assessed in the original study was used (i.e., 500 ng/ml) as a standard widely validated cutoff and 1000 ng/ml in one study, where a specific algorithm was applied, using a higher D‐dimer cutoff in patients with no items of the specific PTP assessment rule used.
3.4. Target conditions
The target conditions were acute symptomatic PE and/or DVT. Suspected PE is usually defined as acute onset of new or worsening shortness of breath or chest pain, with or without hemoptyses, tachycardia, increased respiratory rate, low blood pressure or fainting, without an obvious explanation, and suspected DVT as unilateral lower limb pain and/or edema without an obvious explanation.
3.5. Reference standards
The reference standard was a final confirmation/exclusion of the diagnosis by validated imaging tests (positive reference standards) used within VTE diagnostic algorithms—lower limb venous CUS, V/Q lung scan, CTPA, pulmonary angiography for PE; lower limb venous CUS for DVT—and/or the rate of VTE events during the 3‐month clinical follow‐up (negative reference standard).
3.6. Data sources and searches
Studies using VTE diagnostic algorithms including D‐dimer to rule out the diagnosis of PE and/or DVT in pregnant women were systematically searched using the MEDLINE (1966 to June 2021, week 1) and EMBASE (1980 to June 2021, week 1) electronic databases. The search strategy was developed without any language restriction using www.embase.com: “venous thromboembolism” OR “lung embolism” OR “deep vein thrombosis” AND “pregnancy” OR “puerperium” AND “D‐dimer.” We supplemented our search by manually reviewing the reference lists of all retrieved articles, clinicalTrials.gov, and reference literature (guidelines and systematic reviews) and questioned experts in VTE diagnostic strategies for possible missing studies.
3.7. Data collection and analysis
3.7.1. Selection of studies
Two investigators (M.B. and C.D.) independently reviewed titles and abstracts from the initial search to determine whether the inclusion criteria were satisfied. According to prespecified selection criteria, any study evaluating D‐dimer to rule out VTE during pregnancy was eligible, if the results provided or allowed the calculation of sensitivity and NPV for VTE. Decisions regarding inclusion were made independently, the results were compared, and any disagreement was resolved through discussion or by involving a third reviewer (M.R.), when necessary. We included prospective and retrospective studies in which a formal diagnostic algorithm was used.
The authors of the eligible studies were contacted for additional information, in particular to check possible duplicate publications. No language restrictions were applied.
3.7.2. Data extraction and management
Two reviewers (M.B. and M.R.) independently extracted data on study and patient characteristics and on sensitivity and diagnostic performance of D‐dimer in this setting, using a standardized form. Any disagreement concerning the extracted data was resolved by consensus and, if necessary, by involving a third reviewer (H.R.E.).
We extracted 2 × 2 contingency tables (true positive, true negative, false positive, and false negative results) to estimate the accuracy of D‐dimer test compared with the reference standard for each study, and its sensitivity as an exclusion test.
We considered as acceptable a PE or DVT diagnosis definition which was in line with well‐established diagnostic criteria reported in the literature. 25 , 31 , 32 , 33
3.7.3. Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) checklist was used to assess the risk of bias of the primary studies. 34
3.7.4. Statistical analysis
Proportions were combined across studies using the method of the inverse of the variance. The Freeman‐Tukey Double arcsine transformation was applied to proportion before pooling and the pooled estimates were back transformed. 35 The level of heterogeneity between studies was assessed by the I 2 statistic. 36 If a high level of heterogeneity was detected (I 2 > 50%), a model with random effects was used (Der Simonian and Laird's approach). 37 All statistical analyses were conducted with the package meta for R software. 38 , 39
3.7.5. Sensitivity analysis
We planned to perform separate meta‐analyses on groups of studies according to the assessment of PTP, type and cutoff of D‐dimer assay, prospective or retrospective design, and type of reference standard (imaging, 3‐month follow‐up, or both).
We also planned to repeat the analyses after stratifying studies according to the median QUADAS‐2 score (by calculating median score after arbitrary transformation of quality judgment into a quantitative score) to highlight potential distortions in our sensitivity and NPV estimates driven by low‐quality studies.
4. RESULTS
4.1. Study identification and selection
We identified 662 potentially relevant studies from the electronic search strategy. Three additional studies were identified with additional search strategies through other sources. We excluded 620 studies after title and abstract screening, using the predefined inclusion and exclusion criteria. The remaining 45 studies were retrieved in full for detailed evaluation. Of the retrieved studies, 41 were excluded for the following reasons: duplicate data (n = 10); not addressing our question (n = 21); selection criteria of the study population not clear (n = 2), with suspected selection bias for a population with very high prevalence of PE in the first one 40 , 41 ; study design not reaching our inclusion/exclusion criteria (n = 6), 42 , 43 , 44 , 45 , 46 , 47 of which three were publication of the same cohort of patients and its secondary analysis 42 , 43 , 46 ; validation study of a diagnostic algorithm using the same cohort of patient already analyzed in another included paper (n = 1). 48 We also excluded one study in which there was a concern about overlapping groups of subjects in two different publications (n = 1) 49 and included only the first publication (prospective cohort). 50 Therefore, four studies were finally included in this systematic review. 23 , 24 , 50 , 51 The study identification and selection progression is detailed in Figure 1.
FIGURE 1.
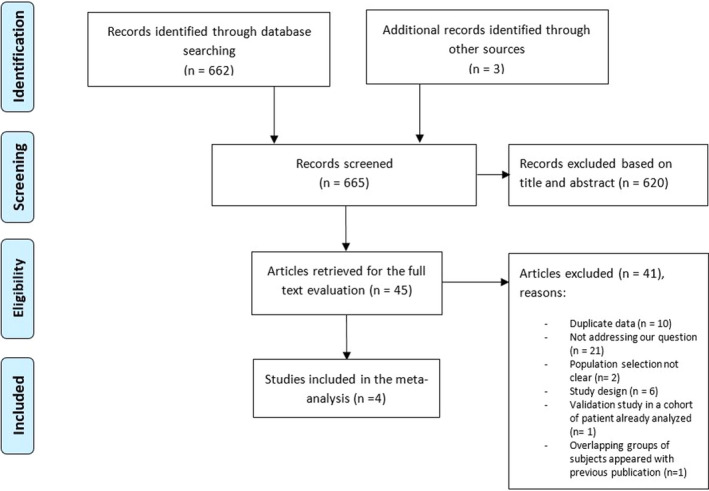
PRISMA flow diagram of study selection, included and excluded studies
4.2. Study characteristics
Characteristics of included studies and baseline characteristics of patients enrolled in the studies are summarized in Tables 1 and 2, respectively. Study size ranged from 149 47 to 498 24 patients. The total overall population of the four studies was of 1194 patients, of whom 836 were eligible for the present analysis. Of the four included studies, three were prospective 23 , 24 , 50 and only one study had a retrospective design. 51 Of all studies, only one was specifically designed for suspected DVT, 50 whereas the other three focused on suspected PE. 23 , 24 , 51
TABLE 1.
Characteristics of included studies
| Study | Country (n Centers) | Design | Period | Population | Target Condition | Setting | Inclusion Criteria | Exclusion Criteria | Type of D‐dimer Assay | D‐dimer Cutoff | Reference Standard | Type of PTP | Diagnostic Algorithm | Follow‐up (days) | Overall VTE Prevalence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chan 2007 50 | Canada (5) | Prospective | March 2000‐November 2005 | Pregnant women | DVT | Women from emergency departments/primary practitioners referred to thrombosis or pregnancy's center | Consecutive pregnant women suspected of having DVT | History of VTE, treatment with “full‐dose” anticoagulation for >24 hours, concomitant symptoms consistent with PE, inability or unwillingness to return for follow‐up, geographic inaccessibility, failure of patient or attending physician to provide consent | SimpliRED assay (Agen Biomedical, Brisbane, Australia). Red Blood Cell Agglutination assay. Qualitative assay | <500 µg/L | CUS (at day 0, 3, 7 based on PTP) and 3 months follow‐up | PTP low vs non‐low (intermediate and high) based on clinician's earlier impression | Yes | 90 | 8.7% (13/149) |
| Choi 2018 51 | London (1) | Retrospective | January 2007‐January 2011 | Pregnant and puerperium women | PE | Emergency department or antenatal clinic | Consecutive pregnant and postpartum patients with clinically suspected acute PE, who underwent V/Q scan, CTPA, or pulmonary angiography | NR | MDA Auto‐Dimer. Latex agglutination (immunoturbidimetric assay). Quantitative assay | <500 ng/ml | V/Q scan or CTPA result at baseline | NA | No | NR | 15.1% (14/93) |
| Righini 2018 23 | France, Switzerland (11) | Prospective | August 2008‐July 2016 | Pregnant women | PE | Women from emergency department, outpatients | Outpatient pregnant women with clinically suspected PE, defined as acute onset of new or worsening shortness of breath or chest pain without another obvious cause | Age <18 years, allergy to iodinated contrast agent, impaired renal function (defined as creatinine clearance <30 ml/min), diagnosis before presentation, indication for or current receipt of full‐dose anticoagulation, inaccessibility for follow‐up | Vidas assay (bioMérieux), ELISA assay. Quantitative assay | <500 ng/ml for low or intermediate PTP | CUS or CTPA or V/Q scan at baseline or 3 months follow‐up | Revised Geneva score | Yes | 90 | 7.1% (28/387) |
| Van der Pol 2019 24 | Netherlands, France, Ireland (18) | Prospective | October 2013‐May 2018 | Pregnant women | PE | Women referred to emergency department or obstetrical ward | Consecutive pregnant in‐ and outpatients with suspected PE | Age <18 years, life expectancy <3 months, treatment with full‐dose therapeutic LMWH or UH that was initiated 24 hours or more before eligibility assessment, treatment with VKA, ultrasonography proven symptomatic proximal DVT, unable to give consent, and contraindication to helical CT | VIDAS d‐Dimer Exclusion (bioMérieux), Tina‐quant (Roche Diagnostica), STA‐Liatest (Diagnostica Stago), Innovance (Siemens), HemosIL (Instrumentation Laboratory). Quantitative assays | <1000 ng/ml if 0 adapted YEARS criteria; <500 ng/ml if ≥1 adapted YEARS criteria | CTPA or V/Q scan at baseline or 3 months follow‐up | YEARS algorithm | Yes | 90 | 4.2% (21/498) |
Abbreviations: CTPA, computed tomography pulmonary angiography; CUS, compression ultrasound scans; DVT, deep vein thrombosis; LMWH, low‐molecular‐weight heparin; NA, not applicable; NR, not reported; PE, pulmonary embolism; PTP, pretest probability; UH, unfractionated heparin; V/Q, ventilation–perfusion lung scan; VKA, vitamin K antagonist; VTE, venous thromboembolism.
TABLE 2.
Baseline characteristics of patients included in the studies and outcomes
| Study | n Enrolled | n Included in Our Analysis | Mean Age or Age Group | n First Trimester | n Second Trimester | n Third Trimester | n Puerperium | VTE at Baseline | VTE at Follow‐up | n Lost to Follow‐up | PTP Allocation: n (%) | VTE Prevalence (in Low‐intermediate PTP) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chan 2007 50 | 149 | 105 |
n < 35 y: 75 n > 35 y: 74 |
8 | 54 | 87 | 0 | 12 |
1 (1 PE within 2 months follow‐up; patient with positive D‐dimer at baseline) |
0 |
Low PTP: 105 (68.9%) Not low PTP: 43 (29.1%) |
2.9% (3/105) Low PTP |
| Choi 2018 51 | 152 | 93 |
31.4 (± 5.1 SD) |
23 | 42 | 59 | 28 | 14 | NA | NA | NA |
15.1% (14/93) Irrespective of PTP |
| Righini 2018 23 | 395 | 387 |
31 (27–36 IQR) |
83 | 170 | 142 | 0 | 28 | 0 | 0 |
Low PTP: 192 (48.6%) Intermediate PTP: 200 (50.6%) High PTP: 3 (0.8%) |
6.5% (25/387) Low‐intermediate PTP |
| Van der Pol 2019 24 | 498 | 251 |
30 (±5.8 SD) |
74 | 193 | 231 | 0 |
20 (4 proximal DVT diagnosed with CUS before apply diagnostic algorithm) |
1 (1 proximal DVT at day 90 of follow‐up; patient in 0 YEARS group, with D‐baseline dimer level of 480 ng/ml) |
1 (patient in 0 YEARS group and dimer level <1000 ng/ml) |
0 YEARS criteria: 252 (51%) ≥1 YEARS criteria: 246 (49%) |
0.8% (2/251) Low PTP |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; PTP, pretest probability; VTE, venous thromboembolism.
Regarding the D‐dimer test, highly sensitive quantitative assays ELISA and immuno‐turbidimetric assays, expressing results as fibrinogen equivalent units, were used in three studies. Of these, two used the same commercial assay for all their patients, 23 , 51 whereas one allowed different types of commercially available assays, 24 as detailed in Table 1. The prespecified D‐dimer cutoff used was the standard cutoff of 500 ng/ml in two studies, for all patients in one and for low‐intermediate PTP patients in the other. 23 , 51 One study used a higher prespecified cutoff (<1000 ng/ml) in patients with low PTP and a standard 500 μg/L cutoff in other patients, based on a defined diagnostic algorithm. 24 Only one study used a red blood cell agglutination assay (qualitative assay), 50 known to have a lower sensitivity (the SimpliRED test).
The three prospective studies included in this analysis applied a standard diagnostic algorithm including PTP assessment. Details about diagnostic algorithm used by single studies are summarized in the Supplementary Appendix. Only the retrospective study by Choi et al. did not stratify patients according to PTP and did not apply a diagnostic strategy. 51 Indeed, this study retrospectively enrolled pregnant women with suspected PE who underwent thoracic imaging (V/Q lung scan or CTPA at baseline). The two prospective management outcome studies in suspected PE by Righini et al. and Van der Pol et al. 23 , 24 used PTP assessment integrated in a diagnostic management algorithm. In both studies, patients with negative results on the diagnostic workup were considered as not having PE, did not receive anticoagulant treatment, and were followed for 3 months and assessed for any suspected and confirmed VTE. 23 , 24 The prospective study by Chan et al. 50 focused on pregnant women with suspected DVT. PTP was established based on the clinician's empirical judgment. CUS of the symptomatic leg(s) was performed in all patients on initial presentation and, if negative, was repeated at day 3 and/or day 7 based on the clinician's standard of practice. Patients who did not have DVT on CUS were left without anticoagulant treatment and underwent clinical follow‐up for at least 3 months. 50
According to our prespecified inclusion criteria, our final population (Table 2) included all patients from Choi et al. in whom both D‐dimer and an imaging test were performed (n = 93) 51 ; patients from Chan et al. 50 with low PTP based on clinical judgment (n = 105); patients with low‐intermediate PTP from Righini et al. 23 (n = 387); patients at “low” PTP according to YEARS (patients showing clinical signs of DVT, hemoptysis, and PE as the most likely diagnosis) criteria (zero YEARS item) from Van der Pol et al. 24 (n = 251). Almost all patients included in our review were pregnant women (Table 2), with only a limited proportion of women in the puerperium period from Choi’ s study (28/152, 18.4%) 51 ; the exact number of postpartum women of the 93 patients included in our analysis from Choi's study was not specified in the paper. 51
4.2.1. Risk of bias
Quality assessment is reported in Table 3 and Figure 2. Overall, included studies were high‐quality studies. Only one of four studies was considered at high risk of bias regarding patient selection.
TABLE 3.
Quality assessment, QUADAS‐2 results

FIGURE 2.

Quality assessment, QUADAS‐2 results
4.2.2. PE and DVT prevalence among pregnant women with suspected VTE
The prevalence of VTE varied importantly across studies (Table 1 and Table 2): the level of heterogeneity was extremely high (I 2 = 90%). Therefore, a model with random effects was used. Weighted mean prevalence of VTE for the random‐effect model was 5.0% (95% CI, 1.1–11.4; I², 90%).
4.2.3. Sensitivity and NPV of D‐dimer to exclude VTE during pregnancy
Sensitivity and NPV estimates reported in the individual studies were all close to 100% and the level of heterogeneity was low. Models with fixed effects were used. The pooled estimates were close to 100% both for sensitivity and NPV: 99.5% (95% CI, 95.0–100.0; I², 0%) and 100% (95% CI, 99.1–100.0; I², 0%), respectively. In Figure 3, forest plots of the meta‐analysis of sensitivities and NPV (primary objective of our analysis) are presented.
FIGURE 3.

Forest plot of the meta‐analysis of sensitivities (A), of negative predictive values (NPV) (B), and of the diagnostic yield of D‐dimer
4.2.4. Diagnostic yield of D‐dimer during pregnancy
The diagnostic yield of D‐dimer to exclude VTE (i.e., the proportion of patients with a negative D‐dimer result, sometimes also called the “efficiency” of the test) varied importantly across studies (Figure 3), with an extremely high level of heterogeneity (I 2 = 98%). The yield of D‐dimer for the random‐effects model was 34.2% (95% CI, 15.9–55.2; I², 98%). A summary of these main results is presented in Table 4.
TABLE 4.
Summary of main results
| Pooled Estimate (95% CI) | I 2 (%) | Model Used | |
|---|---|---|---|
| VTE prevalence | 7.4% (3.8–12.0) | 83 | Random effects |
| Sensitivity of D‐dimer to rule out VTE | 99.5% (95–100.0) | 0 | Fixed effect |
| Diagnostic yield of D‐dimer (proportion of patients with a negative test) | 34.2% (15.9–55.2) | 98 | Random effects |
| Negative predictive value | 100% (99.1–100.0) | 0 | Fixed effect |
The diagnostic yield of D‐dimer varied importantly across studies: the level of heterogeneity was extremely high (I 2 = 98%; therefore, a model with random effects was used. The same was true for the prevalence. Contrarily, for both sensitivity and negative predictive value, the estimates reported in studies were all close to 100% and the level of heterogeneity was low. Models with fixed effects were used. The pooled estimates were close to 100%.
Abbreviations: CI, confidence interval; VTE, venous thromboembolism.
4.2.5. VTE rate during follow‐up
In patients in whom VTE was ruled out based on the combination of a non‐high PTP and a negative D‐dimer, the 3‐month VTE event rates were: 0/46 (0.0%, 95% CI, 0.0‐7.7) in women with a low‐intermediate PTP and D‐dimer <500 μg/L in the study by Righini et al. 23 and 1/164 (0.61%, 95% CI, 0.1‐3.5) in patients with zero YEARS item and D‐dimer <1000 μg/L in the study by Van der Pol et al. 24 This latter event occurred in a patient with a baseline D‐dimer level of 480 μg/L and was a proximal DVT diagnosed at day 90 of follow‐up. In the study by Chan et al., 50 among patients with low PTP of DVT and D‐dimer <500 μg/L, no VTE event was reported during follow‐up (0/69; 0.0%, 95% CI, 0–5.6). In the study by Choi et al., 51 no PE was diagnosed by imaging among the 33 women with negative D‐dimer results: 0/33 (0.0%, 95% CI, 0.0–10.4). Overall, the pooled "failure rate” of D‐dimer was 1/312 (0.32%; 95% CI, 0.06‐1.8).
4.2.6. VTE excluded on the basis of a negative diagnostic workup
In the CT‐PE‐Pregnancy study, 23 the 3‐month thromboembolic rate in patients in whom PE was ruled out on the basis of a negative diagnostic strategy was of 0.0% (0/367; 95% CI, 0.0‐1.0). 23 In the ARTEMIS study, the 3‐month VTE rate among patients in whom PE was ruled out on the basis of a negative diagnostic strategy was of 0.21% (1/477; 95% CI, 0.04‐1.2). 24 Only one patient left untreated on the basis of the diagnostic algorithm for DVT, comprehensive of both D‐dimer test and CUS at presentation, developed PE within 2 months’ follow‐up in the study of Chan et al. 50 ; the patient who developed PE did so 3 days after a second‐trimester pregnancy loss, 55 days after her initial presentation with suspected DVT. Therefore, the 3‐month thromboembolic rate after a negative diagnostic work‐up for DVT in the study of Chan et al. was 0.7% (1/137; 95% CI, 0.1–4.0). 50
Overall, the 3‐month thromboembolic rate in pregnant women left untreated after a negative diagnostic algorithm was low: 0.2% (2/981; 95% CI, 0.06‐0.74).
In the study by Choi et al., 51 all 93 women with D‐dimer performed underwent CTPA at baseline and PE was ruled out in 79 patients, without applying a further diagnostic algorithm and no 3‐month follow‐up was available; thus, we excluded Choi's study 51 from the calculation of the overall 3 months VTE risk on the basis of a negative diagnostic workup.
4.2.7. Sensitivity, NPV, and the yield of D‐dimer according to different trimesters
There were insufficient data available to evaluate sensitivity, NPV, and the yield of D‐dimer test to rule out VTE during different trimesters of pregnancy and puerperium, as initially planned. Only Righini et al. 23 reported the proportion of negative D‐dimer results during each trimester, showing a decreased proportion of negative D‐dimer results with increasing gestational age (25%, 11% and 4% during the first, second, and third trimester, respectively). The same trend was shown in the study of Chan et al., 50 who provided data about the proportion of negative D‐dimer result during each trimester, in the absence of DVT (100%, 76%, and 51% during the first, second, and third trimester, respectively). In Van der Pol et al., 24 the median D‐dimer level was 505 μg/L (interquartile range, 292 to 963) during the first trimester, 730 μg/L (interquartile range, 505 to 1260) during the second trimester, and 1120 μg/L (interquartile range, 818 to 1718) during the third trimester.
4.2.8. Sensitivity analyses
Given the small number of studies included, we could not perform the planned sensitivity analyses. However, we performed a leave‐one‐out sensitivity analysis. Results are shown in detail in Table 5. The pooled yield was most influenced by this analysis. Indeed, when the study by Righini et al. 23 was excluded, the yield raised to 55.8%; when the study by Chan et al. 50 or Van der Pol et al. 24 were excluded, yield decreased to 35.9%. Conversely, the pooled NPV and sensitivity remained stable. The pooled prevalence varied slightly but remained low in all analyses.
TABLE 5.
Leave‐one‐out sensitivity analysis
| Removed Study | Pooled Estimates | |||
|---|---|---|---|---|
| Yield (95%CI) | NPV (95% CI) | Sensitivity (95% CI) | Prevalence (95% CI) | |
| Chan 2007 50 | 27.8 (9.8‐50.6) | 99.9 (98.8‐100.0) | 99.3 (93.8‐100.0) | 7.1 (2.9‐12.9) |
| Choi 2018 51 | 33.8 (12.0‐60.0) | 99.9 (98.9‐100.0) | 99.3 (93.7‐100.0) | 5.7 (3.1‐9.2) |
| Righini 2018 23 | 43.2 (32.9‐53.9) | 99.9 (98.9‐100.0) | 98.8 (91.5‐100.0) | 8.1 (2.5‐16.3) |
| Van der Pol 2019 24 | 32.5 (8.0‐63.7) | 100.0 (98.8‐100.0) | 100.0 (96.3‐100.0) | 9.2 (5.1‐14.3) |
5. DISCUSSION
The results of this systematic review and meta‐analysis suggest that D‐dimer measurement is a useful and safe diagnostic test to rule out VTE during pregnancy.
Until recently, points usually advocated as reasons against the use of D‐dimer in pregnant women were: (1) availability of limited and inconsistent data on sensitivity and NPV of D‐dimer for suspected VTE in pregnancy; (2) lack of CDR specific for pregnancy to stratify patient according to PTP; (3) unclear appropriate cutoff of D‐dimer test because of D‐dimer physiological increase during pregnancy. These three points need to be specifically addressed and evaluated, also in light of our review's results.
In the past 2 years, two prospective management outcome studies bridging these gaps were published 23 , 24 and suggested that D‐dimer measurement can be included in diagnostic algorithms. Pooling together these available data adds further evidence, claiming the safety of D‐dimer use to exclude VTE in pregnant patients with a disease prevalence consistent with a low/intermediate or unlikely PTP. Indeed, the 3‐month thromboembolic risk in pregnant women left untreated after a negative diagnostic algorithm was low: 0.2% (2/981; 95% CI, 0.06‐0.74). This is perfectly in line with the recent recommendations from the International STH suggesting that the upper bound of the 3‐month VTE risk should be below 2% in diagnostic strategies for VTE. 52 Also, the 3‐month thromboembolic risk in pregnant women left untreated in case of a non‐high clinical probability and negative D‐dimer was 1/312 (0.32%; 95% CI, 0.06–1.83).
Our results showed a high sensitivity and NPV of 99.5% (95% CI, 95.0–100.0; I², 0%) and 100% (95% CI, 99.1–100.0; I², 0%), respectively, for D‐dimer testing. Of note, for sensitivities and NPV, the estimates reported by single studies were close to 100%, with a low level of heterogeneity. These findings are in line with sensitivity observed in general population, 53 supporting the use of D‐dimer test for safely ruling out VTE in non‐high‐risk pregnant women, without the need for imaging.
A main limitation for the use of D‐dimer test is represented by its general poor specificity. 53 , 54 In pregnant women, D‐dimer concentrations are even less specific because of physiological increase of D‐dimer levels during normal pregnancies, 19 reducing the diagnostic yield of the test. In our review, the estimated proportion of patients with negative D‐dimer was 34.2% (95% CI, 15.9–55.1; I², 98%). Despite the wide CI and high level of heterogeneity across studies, this proportion could be considered clinically significant, avoiding further testing in a considerable number of patients.
Because a negative D‐dimer test has to be combined with an assessment of the pretest clinical probability to exclude VTE, an accurate pretest stratification of pregnant women is mandatory. However, most diagnostic studies that derived and validated models assessing PTP of VTE have excluded pregnant patients in the past. 15 , 16 , 17 , 18 Only recently, as shown in our included studies, Righini et al. 23 and Van der Pol et al. 24 took a step forward to address this point for PE diagnosis. In the CT‐PE‐Pregnancy study, 23 the Revised Geneva score was incorporated in the diagnostic algorithm for PTP evaluation in pregnant patient with suspected PE; this score effectively stratified pregnant patients with an increasing prevalence of the disease: 4% (7/192) in the low‐probability group, 9% (18/200) in the intermediate‐probability group, and 100% (3/3) in the high‐probability group. In the ARTEMIS study, 24 the YEARS algorithm was used and it stratified pregnant women in two groups with an increasing prevalence of PE. Indeed, PE prevalence was 0.4% (1 /252) in the group with no YEARS criteria, and of 6.2% (15/242) in the group who met one or more of the three YEARS criteria. Both of these diagnostic algorithms incorporating the Revised Geneva score and the three most predictive criteria of Well's CDR, appeared useful in the initial stratification of pregnant women with suspected PE, as suggested by the 0% (0/367; 95% CI, 0.0‐1.0) and the 0.21% (1/477; 95% CI, 0.04‐1.2) 3‐month thromboembolic rate in pregnant patients who were left untreated after a negative diagnostic algorithm based on these PTP assessment tools. 23 , 24 Of note, in the ARTEMIS study, 24 the pregnancy‐adapted YEARS algorithm was driven largely by the criterion "PE as the most likely diagnosis," which was present in 89% of patients. Clinicians could have difficulty assessing this criterion, especially in the context of pregnancy where symptoms and signs of normal pregnancy and PE can overlap. 5 Despite these steps forward, further studies are needed to develop specific scores derived and validated in pregnant women for PTP evaluation. In this direction, the LEFt rule, a CDR for pregnant women with suspected DVT, was recently derived and externally validated. 33 , 55 , 56 Moreover, a prospective validation is ongoing (NCT01708239). 57
As in the general population, the use of a D‐dimer cutoff adjusted for age or adapted to clinical probability was retrospectively explored by different studies. 58 , 59 , 60 However, some prospective validation is also available. In the study by Van der Pol al. 24 a higher D‐dimer cutoff (1000 ng/ml) was combined with zero YEARS criteria. The adoption of a diagnostic algorithm using a higher D‐dimer cutoff in patient with low PTP improved the yield of D‐dimer test, without compromising the safety. 48 Indeed, in the study by Righini et al., 23 the proportion of women in whom PE could be ruled out on the basis of a negative algorithm including usual D‐dimer threshold of 500 ng/ml was of 11.6%. When the YEARS algorithm was applied to the same sample of women, 21% had PE excluded without apparent loss in safety. 48
Strengths of our study need to be underlined. To our knowledge, after the emergence of new evidence driven by recent prospective management diagnostic studies, 23 , 24 this is the first systematic review and meta‐analysis assessing the safety of using D‐dimer to rule out suspected VTE in pregnant women when the disease prevalence is consistent with a low/intermediate or unlikely PTP. Previous studies, 40 , 42 with unclear criteria to select population and high PE prevalence 40 or using different design compared with our target, 42 showed lower sensitivity of D‐dimer test in pregnant patients. In the DiPEP study, 42 existing CDR and D‐dimer showed little diagnostic value, suggesting against their use to select pregnant or postpartum women with suspected PE for further investigation. However, the DiPEP study 42 was a retrospective analysis of two cohorts (one of women with suspected PE recruited in 11 centers and one of women with confirmed PE across the United Kingdom) and included pregnant and postpartum women. The DiPEP study 42 used different D‐dimer assays with different thresholds. It did not specify the timing of D‐dimer measurement, but most of women were already receiving anticoagulant treatment at the time of testing, which can have a major impact on D‐dimer results. Also, the final comparator to assess the diagnostic performance of this testing, the presence or absence of PE, was based on clinical grounds in about 15% of patients. 42 Therefore, the conclusions reported in the DiPEP study 42 may have important limitations. Contrarily, our meta‐analysis, based on strict prespecified inclusion and exclusion criteria, defines with more accuracy the role of D‐dimer in non‐high‐risk pregnant patient. In particular, despite the low number of included studies, our data come from a relatively large number of patients pooled together and our main results are affected by low heterogeneity, showing high pooled sensitivity and NPV with narrow confidence intervals. Another strength of our review is the overall high quality of included studies, with the majority of them having a prospective design.
Currently, the use of D‐dimer to rule out PE in pregnancy is recommended in only two of the available guidelines (European Society of Cardiology [ESC] and Working Group in Women’s Health of the Society of Thrombosis and Haemostasis), 25 , 61 whereas the remaining five guidelines (Australasian Society of Thrombosis and Haemostasis‐Society of Obstetric Medicine of Australia and New Zealand, American Thoracic Society‐Society of Thoracic Radiology, EANM, Royal College of Obstetricians and Gynaecologists, and Society of Obstetricians and Gynaecologists of Canada) 62 , 63 , 64 , 65 , 66 recommend against the use of D‐dimer in this setting. 26 In the latest ESC guidelines, 25 D‐dimer measurement and CDR should be considered to rule out PE during pregnancy or the postpartum period; moreover, the strength of this recommendation was upgraded in the current version of ESC guideline, 25 in line with new evidence emerged from the prospective management outcome trials included in our review (from class IIb to IIa). 67 , 68 Our findings may support an evidence‐based update of future guidelines and reduce controversy.
Our systematic review has potential limitations. First, a limited number of studies were available in literature addressing our question, and our inclusion and exclusion criteria allowed us to include only four studies. As mentioned previously, although included studies were overall high‐quality studies and the total number of population analyses count was of 836 patients in total, we included only 312 pregnant women in whom VTE was excluded on the basis of D‐dimer measurement. Second, the D‐dimer assays used on two of the four studies are no longer available. 50 , 51 Third, given the small number of studies included, we did not attempt to perform any planned sensitivity analyses; in particular, it was not possible to perform separate meta‐analyses on groups of studies according to the use of PTP, type and cutoff of D‐dimer assay, prospective or retrospective design, and type of reference standard. Nevertheless, we performed a leave‐one‐out sensitivity analysis, showing no relevant effect on sensitivity and NPV. Fourth, to be noted as potential bias, three authors of this review are also the main authors of one of the large studies included in this meta‐analysis. 23
In conclusion, the results of our meta‐analysis suggest that D‐dimer may be a safe and useful diagnostic tool in the management of pregnant women with suspected VTE. However, limited data exist and further trials are needed to derive/validate specific CDR, and to identify the optimal D‐dimer cutoff during pregnancy.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
AUTHOR CONTRIBUTIONS
Marta Bellesini, Helia Robert‐Ebadi, Christophe Combescure, Cristina Dedionigi, Grégoire Le Gal, Marc Righini: performed the conception and design, acquisition of data, analysis and interpretation of data; and drafted the article. All authors provided final approval of the version to be published.
Supporting information
Supplementary Material
Bellesini M, Robert‐Ebadi H, Combescure C, Dedionigi C, Le Gal G, Righini M. D‐dimer to rule out venous thromboembolism during pregnancy: A systematic review and meta‐analysis. J Thromb Haemost. 2021;19:2454–2467. 10.1111/jth.15432
Marta Bellesini and Helia Robert‐Ebadi contributed equally to this work.
Manuscript handled by: Claire McLintock
Final decision: Claire McLintock, 22‐Jun‐2021
REFERENCES
- 1. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30‐year population‐based study. Ann Intern Med. 2005;143:697‐706. [DOI] [PubMed] [Google Scholar]
- 2. Liu S, Rouleau J, Joseph KS, et al. Epidemiology of pregnancy‐associated venous thromboembolism: a population‐based study in Canada. J Obstet Gynaecol Can. 2009;31:611‐620. [DOI] [PubMed] [Google Scholar]
- 3. Sultan AA, West J, Tata LJ, Fleming KM, Nelson‐Piercy C, Grainge MJ. Risk of first venous thromboembolism in and around pregnancy: a population‐based cohort study. Br J Haematol. 2012;156:366‐373. [DOI] [PubMed] [Google Scholar]
- 4. MBRRACE‐UK Update . Key messages from the UK and Ireland Confidential Enquiries into Maternal Death and Morbidity 2017. Obstet Gynaecol. 2018;20:75‐79. [Google Scholar]
- 5. Soma‐Pillay P, Nelson‐Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27:89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Mens TE, Scheres LJ, de Jong PG, Leeflang MM, Nijkeuter M, Middeldorp S. Imaging for the exclusion of pulmonary embolism in pregnancy. Cochrane Database Syst Rev. 2017;(1):CD011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamilton EJ, Green AQ, Cook JA, Nash H. Investigating for pulmonary embolism in pregnancy: five year retrospective review of referrals to the acute medical unit of a large teaching hospital. Acute Med. 2016;15:58‐62. [PubMed] [Google Scholar]
- 8. Kline JA, Richardson DM, Than MP, Penaloza A, Roy P‐M. Systematic review and meta‐analysis of pregnant patients investigated for suspected pulmonary embolism in the emergency department. Acad Emerg Med. 2014;21:949‐959. [DOI] [PubMed] [Google Scholar]
- 9. Righini M, Le Gal G, Aujesky D, et al. Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non‐inferiority trial. Lancet. 2008;371:1343‐1352. [DOI] [PubMed] [Google Scholar]
- 10. Carrier M, Righini M, Djurabi RK, et al. VIDAS D‐dimer in combination with clinical pre‐test probability to rule out pulmonary embolism. A systematic review of management outcome studies. Thromb Haemost. 2009;101:886‐892. [PubMed] [Google Scholar]
- 11. Le Gal G, Righini M, Wells PS. D‐dimer for pulmonary embolism. JAMA. 2015;313:1668‐1669. [DOI] [PubMed] [Google Scholar]
- 12. Perrier A, Desmarais S, Miron MJ, et al. Non‐invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999;353:190‐195. [DOI] [PubMed] [Google Scholar]
- 13. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D‐dimer in the diagnosis of suspected deep‐vein thrombosis. N Engl J Med. 2003;349:1227‐1235. [DOI] [PubMed] [Google Scholar]
- 14. Righini M, Perrier A, De Moerloose P, Bounameaux H. D‐dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost. 2008;6:1059‐1071. [DOI] [PubMed] [Google Scholar]
- 15. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep‐vein thrombosis in clinical management. Lancet. 1997;350:1795‐1798. [DOI] [PubMed] [Google Scholar]
- 16. Bates SM, Kearon C, Crowther M, et al. A diagnostic strategy involving a quantitative latex D‐dimer assay reliably excludes deep venous thrombosis. Ann Intern Med. 2003;138:787‐794. [DOI] [PubMed] [Google Scholar]
- 17. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D‐dimer. Thromb Haemost. 2000;83:416‐420. [PubMed] [Google Scholar]
- 18. Le Gal G, Righini M, Roy P‐M, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva Score. Ann Intern Med. 2006;144:165. [DOI] [PubMed] [Google Scholar]
- 19. Kline JA, Williams GW, Hernandez‐Nino J. D‐dimer concentrations in normal pregnancy: new diagnostic thresholds are needed. Clin Chem. 2005;51:825‐829. [DOI] [PubMed] [Google Scholar]
- 20. Murphy N, Broadhurst DI, Khashan AS, Gilligan O, Kenny LC, O’Donoghue K. Gestation‐specific D‐dimer reference ranges: a cross‐sectional study. BJOG. 2015;122:395‐400. [DOI] [PubMed] [Google Scholar]
- 21. Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64‐slice computed tomography coronary angiography. JAMA. 2007;298:317‐323. [DOI] [PubMed] [Google Scholar]
- 22. Tromeur C, van der Pol LM, Le Roux P‐Y, et al. Computed tomography pulmonary angiography versus ventilation‐perfusion lung scanning for diagnosing pulmonary embolism during pregnancy: a systematic review and meta‐analysis. Haematologica. 2019;104:176‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Righini M, Robert‐Ebadi H, Elias A, et al.; CT‐PE‐Pregnancy Group . Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med. 2018;169:766‐773. [DOI] [PubMed] [Google Scholar]
- 24. van der Pol LM, Tromeur C, Bistervels IM, et al. Pregnancy‐adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019;380:1139‐1149. [DOI] [PubMed] [Google Scholar]
- 25. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS)The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:543‐603. [DOI] [PubMed] [Google Scholar]
- 26. Cohen SL, Feizullayeva C, McCandlish JA, et al. Comparison of international societal guidelines for the diagnosis of suspected pulmonary embolism during pregnancy. The Lancet Haematology. 2020;7:e247‐e258. [DOI] [PubMed] [Google Scholar]
- 27. Wells P, Hirsh J, Anderson D, et al. Accuracy of clinical assessment of deep‐vein thrombosis. The Lancet Elsevier. 1995;345:1326‐1330. [DOI] [PubMed] [Google Scholar]
- 28. Ceriani E, Combescure C, Gal GL, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta‐analysis. J Thromb Haemost. 2010;8:957‐970. [DOI] [PubMed] [Google Scholar]
- 29. Shamseer L, Moher D, Clarke M, et al.; PRISMA‐P Group . Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ. 2015;2015(350): g7647. [DOI] [PubMed] [Google Scholar]
- 30. McInnes MDF, Moher D, Thombs BD, et al.; Preferred reporting items for a systematic review and meta‐analysis of diagnostic test accuracy studies. JAMA. 2018;319(4):388‐396. [DOI] [PubMed] [Google Scholar]
- 31. Righini M, Robert‐Ebadi H, Le Gal G. Diagnosis of acute pulmonary embolism. J Thromb Haemost. 2017;15:1251‐1261. [DOI] [PubMed] [Google Scholar]
- 32. Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous thromboembolism: advances in diagnosis and treatment. JAMA. 2018;320:1583‐1594. [DOI] [PubMed] [Google Scholar]
- 33. Mazzolai L, Aboyans V, Ageno W, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2018;39:4208‐4218. [DOI] [PubMed] [Google Scholar]
- 34. Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529‐536. [DOI] [PubMed] [Google Scholar]
- 35. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health. 2013;67:974‐978. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 38. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 39. Schwarzer G. meta: an R package for meta‐analysis. R News. 2007;7:40‐45. [Google Scholar]
- 40. Damodaram M, Kaladindi M, Luckit J, Yoong W. D‐dimers as a screening test for venous thromboembolism in pregnancy: Is it of any use? J Obstet Gynaecol. 2009;29:101‐103. [DOI] [PubMed] [Google Scholar]
- 41. Hassanin IMA, Shahin AY, Badawy MS, Karam K. D‐dimer testing versus multislice computed tomography in the diagnosis of postpartum pulmonary embolism in symptomatic high‐risk women. Int J Gynecol Obstet. 2011;115:200‐201. [DOI] [PubMed] [Google Scholar]
- 42. Goodacre S, Horspool K, Nelson‐Piercy C, et al. The DiPEP study: an observational study of the diagnostic accuracy of clinical assessment, D‐dimer and chest x‐ray for suspected pulmonary embolism in pregnancy and postpartum. BJOG. 2019;126:383‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hunt BJ, Parmar K, Horspool K, Shephard N, Nelson‐Piercy C, Goodacre S. The DiPEP (Diagnosis of PE in Pregnancy) biomarker study: An observational cohort study augmented with additional cases to determine the diagnostic utility of biomarkers for suspected venous thromboembolism during pregnancy and puerperium. Br J Haematol. 2018;180:694‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parilla BV, Fournogerakis R, Archer A, et al. Diagnosing pulmonary embolism in pregnancy: are biomarkers and clinical predictive models useful? AJP Rep. 2016;6:e160‐e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le Gal G, Kercret G, Ben Yahmed K, et al.; EDVIGE Study Group . Diagnostic value of single complete compression ultrasonography in pregnant and postpartum women with suspected deep vein thrombosis: prospective study. BMJ. 2012;344:e2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goodacre S, Nelson‐Piercy C, Hunt BJ, Fuller G. Accuracy of PE rule‐out strategies in pregnancy: secondary analysis of the DiPEP study prospective cohort. Emerg Med J. 2020;37:423‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Al Oweidat K, Al Ryalat SA, Al Husban N, et al. Additive evidence of the competence of pregnancy‐adapted YEARS algorithm in reducing the need for CTPA, Q and/or V/Q scintiscan. Hell J Nucl Med. 2020;23:165‐172. [DOI] [PubMed] [Google Scholar]
- 48. Langlois E, Cusson‐Dufour C, Moumneh T, et al. Could the YEARS algorithm be used to exclude pulmonary embolism during pregnancy? Data from the CT‐PE‐pregnancy study. J Thromb Haemost. 2019;17:1329‐1334. [DOI] [PubMed] [Google Scholar]
- 49. Chan W‐S, Lee A, Spencer FA, et al. D‐dimer testing in pregnant patients: towards determining the next ‘level’ in the diagnosis of deep vein thrombosis. J Thromb Haemost. 2010;8:1004‐1011. [DOI] [PubMed] [Google Scholar]
- 50. Chan W‐S, Chunilal S, Lee A, Crowther M, Rodger M, Ginsberg JS. A red blood cell agglutination D‐dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med. 2007;147:165‐170. [DOI] [PubMed] [Google Scholar]
- 51. Choi H, Krishnamoorthy D. The diagnostic utility of D‐dimer and other clinical variables in pregnant and post‐partum patients with suspected acute pulmonary embolism. Int J Emerg Med. 2018;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dronkers CEA, van der Hulle T, Le Gal G, et al.; Subcommittee on Predictive and Diagnostic Variables in Thrombotic Disease . Towards a tailored diagnostic standard for future diagnostic studies in pulmonary embolism: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15:1040‐1043. [DOI] [PubMed] [Google Scholar]
- 53. Di Nisio M, Squizzato A, Rutjes AWS, Büller HR, Zwinderman AH, Bossuyt PMM. Diagnostic accuracy of D‐dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5:296‐304. [DOI] [PubMed] [Google Scholar]
- 54. Kearon C. Diagnosis of suspected venous thromboembolism. Hematology. 2016;2016:397‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chan W‐S, Lee A, Spencer FA, et al. Predicting deep venous thrombosis in pregnancy: out in “LEFt” field? Ann Intern Med. 2009;151:85. [DOI] [PubMed] [Google Scholar]
- 56. Righini M, Jobic C, Boehlen F, et al.; EDVIGE Study Group . Predicting deep venous thrombosis in pregnancy: external validation of the LEFT clinical prediction rule. Haematologica. 2013;98:545‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Left Rule, D‐Dimer Measurement and Complete Ultrasonography to Rule Out Deep Vein Thrombosis During Pregnancy. ClinicalTrials.gov. (ongoing study).
- 58. Righini M, Van Es J, Den Exter PL, et al. Age‐adjusted D‐dimer cutoff levels to rule out pulmonary embolism: the ADJUST‐PE study. JAMA. 2014;311:1117‐1124. [DOI] [PubMed] [Google Scholar]
- 59. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289‐297. [DOI] [PubMed] [Google Scholar]
- 60. Kearon C, de Wit K, Parpia S, et al.; PEGeD Study Investigators . Diagnosis of pulmonary embolism with d‐dimer adjusted to clinical probability. N Engl J Med. 2019;381:2125‐2134. [DOI] [PubMed] [Google Scholar]
- 61. Linnemann B, Bauersachs R, Rott H, et al. Diagnosis of pregnancy‐associated venous thromboembolism – position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87‐101. [DOI] [PubMed] [Google Scholar]
- 62. Mclintock C, Brighton T, Chunilal S, et al. Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. Aust N Z J Obstet Gynaecol. 2012;52:14‐22. [DOI] [PubMed] [Google Scholar]
- 63. Leung AN, Bull TM, Jaeschke R, et al. American Thoracic Society Documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline—evaluation of suspected pulmonary embolism in pregnancy. Radiology. 2012;262:635‐646. [DOI] [PubMed] [Google Scholar]
- 64. Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B. EANM guidelines for ventilation/perfusion scintigraphy. Eur J Nucl Med Mol Imaging. 2009;36:1528‐1538. [DOI] [PubMed] [Google Scholar]
- 65. Thrombosis and Embolism during Pregnancy and the Puerperium: Acute Management (Green‐top Guideline No. 37b). Royal College of Obstetricians & Gynaecologists. 2015.
- 66. Chan W‐S, Rey E, Kent NE, et al.; VTE in Pregnancy Guideline Working Group . Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36(6):527‐553. [DOI] [PubMed] [Google Scholar]
- 67. Vedovati MC, Giustozzi M, Franco L. Beyond the guidelines: Novelties, changes and unsolved issues from the 2019 ESC guidelines on pulmonary embolism. Eur J Intern Med. 2020;72:1‐4. [DOI] [PubMed] [Google Scholar]
- 68. Robert‐Ebadi H, Righini M. The 2019 ESC guidelines on pulmonary embolism: some further insights. Eur J Intern Med. 2020;77:6‐8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


