Abstract
Integrins have been shown to play important roles in embryonic development, wound healing, metastasis, and other biological processes. αvβ5 is a receptor for RGD-containing extracellular matrix proteins that has been suggested to be important in cutaneous wound healing and adenovirus infection. To examine the in vivo function of this receptor, we have generated mice lacking β5 expression, using homologous recombination in embryonic stem cells. Mice homozygous for a null mutation of the β5 subunit gene develop, grow, and reproduce normally. Keratinocytes harvested from β5−/− mice demonstrate impaired migration on and adhesion to the αvβ5 ligand, vitronectin. However, the rate of healing of cutaneous wounds is not different in β5−/− and β5+/+ mice. Furthermore, keratinocytes and airway epithelial cells obtained from null mice show adenovirus infection efficiency equal to that from wild-type mice. These data suggest that αvβ5 is not essential for normal development, reproduction, adenovirus infection, or the healing of cutaneous wounds.
Integrins are heterodimeric receptors for extracellular matrix and cell surface counterreceptors, which play important roles in diverse biological processes including embryonic development, inflammation, and wound healing (16, 24). αv integrins (αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8) mediate cell adhesion to various matrix proteins including fibronectin, vitronectin, tenascin, osteopontin, and fibrinogen at sites containing the tripeptide sequence arginine-glycine-aspartic acid (RGD). Although the first-described member of this subfamily, αvβ3, appears to bind to virtually all of these proteins, the other αv integrins have been reported to be more restrictive in their interactions with ligands. For example, αvβ5 (25) and αvβ8 (21) have been reported to be principally vitronectin receptors, and αvβ6 has been reported to principally bind fibronectin and, to a lesser extent, vitronectin and tenascin (4, 15, 22, 26, 29).
In addition to recognizing distinct subsets of RGD-containing ligands, αv integrins have been reported to exert distinct effects on cell behavior. αvβ5 is widely expressed on epithelial cells including keratinocytes, airway epithelial cells, fibroblasts, osteoclasts, and monocytes. αvβ5 has been suggested to play important roles in activation-dependent cell migration (18, 19), in promoting adenovirus-mediated gene delivery (11, 27), and in cutaneous wound healing. To investigate the role of αvβ5 in vivo, we have generated mice lacking β5 expression, using homologous recombination in embryonic stem (ES) cells. Our results demonstrate an important role for β5 in keratinocyte adhesion and migration on vitronectin but suggest that functions of αvβ5 in cutaneous wound healing or adenovirus infection can be either developmentally or functionally replaced by other receptors.
MATERIALS AND METHODS
Generation of αvβ5-deficient mice.
A genomic clone containing one exon of the mouse β5 gene (corresponding to amino acids 114 to 230 in the reported human sequence [23]) was isolated from a genomic library derived from mouse strain 129/sv. We used an 8.2-kb XbaI/SalI fragment of this clone to construct a replacement vector that contained a neomycin resistance gene replacing part of the exon and an adjacent intron in our clone, and a thymidine kinase gene at one end for negative selection. This vector was introduced into mouse RF8 ES cells (14), and targeted clones were identified by Southern blotting with both external and internal probes. Chimeric mice were established and mated with C57BL/6J females to obtain mice carrying the mutated β5 allele.
Northern blotting.
Total RNA was isolated from primary cultured mouse keratinocytes with TRIzol solution (Gibco/BRL) as instructed by the manufacturer. Thirty micrograms of total RNA was separated on an agarose gel containing formaldehyde and transferred to nylon membranes. The membranes were blotted with a cDNA probe specific for mouse β5 (corresponding to nucleotides 744 to 1095 in the reported human sequence [23]).
Cells and cell culture.
Murine keratinocytes were obtained and grown in keratinocyte growth medium (KGM; Clonectics) as previously described (14). Briefly, mouse skin was kept in 0.1% bacterial protease (P8811; Sigma) overnight at 4°C. The following day, the epithelial layer was scraped off and incubated in 0.05% trypsin for 40 min at 37°C. Then the cells were disaggregated by pipetting and washed twice with phosphate-buffered saline (PBS). Finally, the cells were resuspended in KGM and plated onto dishes coated with type I collagen (10 μg/ml; Sigma, St. Louis, Mo.).
Excised murine tracheas were incubated in 0.05% protease (Sigma) for 1 h at 37°C. The tracheal epithelium was exposed by a dorsal longitudinal incision, and the luminal epithelial surface was scraped. The cells were disaggregated by pipetting and washed twice with PBS. Finally, the cells were resuspended in small airways growth medium (Clonetics) and plated onto dishes coated with type I collagen.
Immunoprecipitation.
Murine keratinocytes were labeled with 0.5 mCi of [35S]methionine overnight in methionine-free Dulbecco modified Eagle medium (DMEM) supplemented with 1% fetal bovine serum, 2% KGM, and penicillin-streptomycin and were lysed in immunoprecipitation buffer (20 mM Tris-HCl [pH 7.5], 1% Triton X-100, 100 mM NaCl, 1 mM CaCl2, 1 mM MgCl2). The lysates were immunoprecipitated with antiserum raised against the human β5 cytoplasmic domain (kindly provided by Martin Hemler, Dana-Farber Cancer Institute, Boston, Mass.) or with antiserum 4377 against the human β5 cytoplasmic domain (kindly provided by Louis Reichardt, University of California, San Francisco). Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% acrylamide gels. Gels were impregnated with 2,5-diphenyloxazole (PPO; Fisher Scientific) and exposed to film at −80°C.
Migration assay.
Cell migration assays were performed with matrix-coated transwell plates (8-μm pores; Costar, Cambridge, Mass.). The undersurface of the membrane was coated with collagen (10 μg/ml) or vitronectin (10 μg/ml) in PBS for 1 h at 37°C and blocked with 1% bovine serum albumin (BSA). Primary cultured keratinocytes were harvested with trypsin-EDTA, and trypsin was inactivated with soybean trypsin inhibitor. Cells were suspended in serum-free KGM and plated in the upper chamber at a density of 3.6 × 104 per well in 100 μl of medium in the presence or absence of phorbol myristate acetate (PMA; 10 ng/ml). After a 6-h incubation, cells were fixed with 2% paraformaldehyde and stained with 0.5% crystal violet in 1% formaldehyde. Cells in the upper chamber were removed, and cells on the lower surface were counted with a 10× grid at high-power magnification (×40). Multiple fields were counted and averaged for each condition studied.
Cell adhesion assay.
Ninety-six-well non-tissue culture-treated polystyrene multiwell microtiter plates (Linbro/Titertek; Flow Laboratories, McLean, Va.) were coated with vitronectin or collagen. A 100-μl solution containing various concentrations of each protein was added to the wells and incubated at 37°C for 1 h. After incubation, wells were washed with PBS and then blocked with 1% BSA in serum-free DMEM at 37°C for 30 min. Control wells were filled with 1% BSA in DMEM. Cells were harvested in the same way as for the migration assay, resuspended in serum-free KGM, and then added to each protein-coated well. The plates were centrifuged (top side up) at 10 × g for 5 min before incubation for 1 h at 34°C in humidified 7% CO2. Nonadherent cells were removed by centrifugation top side down at 48 × g for 5 min. The attached cells were fixed with 1% formaldehyde and stained with 0.5% crystal violet, and then the wells were washed with PBS. The relative number of cells in each well was evaluated by measuring the absorbance at 595 nm in a Microplate Reader (Bio-Rad).
In vivo wounding.
Animals were anesthetized with Metofane solution (Pitman-Moore Inc., Mundelein, Ill.). A small area of the animal's back was shaved, and 2- and 4-mm full-thickness punch biopsy specimens were taken. At day 2, 4, 6, 12, and 24 after wounding, the maximum diameter of the wounds was measured to evaluate the rate of wound closure.
Adenovirus-mediated gene delivery.
Adenovirus (H5.010CMVlacZ; a gift from Alan Davies, University of Pennsylvania Medical Center) expressing the lacZ gene under the control of the cytomegalovirus promoter was used at log dilutions from 2 × 1011 particles per ml to infect keratinocytes and airway epithelial cells from β5−/− and wild-type controls. We used this adenovirus construct because it has been reported to utilize integrin αvβ5 to infect human airway cells (11). Cells were incubated with infection medium (DMEM supplemented with 2% fetal bovine serum) for 90 min. The cells were then washed and returned to their normal growth medium. Forty hours after infection, the cells were fixed with 2% formaldehyde and 0.2% glutaraldehyde in PBS and stained for 2 h at 37°C with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 1 mg/ml; Promega, Madison, Wis.) dissolved in potassium ferrocyanide (5 mM) and magnesium chloride (2 mM) in PBS. The blue-stained cells expressing viral lacZ were counted under an inverted microscope, and the total number was expressed as a percentage of the number of positive-staining wild-type cells per well incubated with the highest concentration of virus particles in the infection medium.
RESULTS
β5−/− mice develop and grow normally.
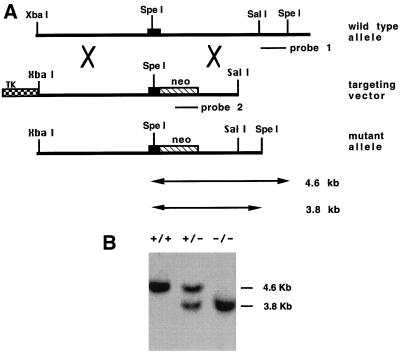
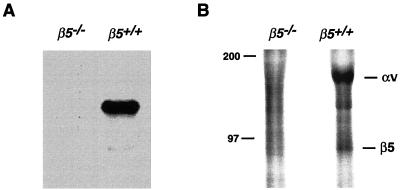
The murine αvβ5 gene was inactivated by homologous recombination in ES cells (5), using the strategy depicted in Fig. 1A. Mice bearing the desired mutant genotype were identified by Southern blot analysis (Fig. 1B). To test whether the mutation leads to a loss of β5 mRNA, we performed Northern blotting with a murine β5 cDNA probe. β5 mRNA was detected from RNA of β5+/+ keratinocytes, but no band was detectable from RNA of β5−/− cells (Fig. 2A). To confirm that β5−/− mice were not able to make β5 protein, we performed β5 immunoprecipitation from metabolically labeled lysates of cultured keratinocytes with antiserum raised against the cytoplasmic domain of human β5. The anti-β5 antiserum immunoprecipitated two bands of the appropriate molecular masses to be αv and β5 from β5+/+ keratinocytes; however, no bands were immunoprecipitated from β5−/− keratinocytes (Fig. 2B). Similar results were obtained with another antiserum, 4377, against the human β5 cytoplasmic domain (data not shown).
FIG. 1.
(A) Homologous recombination of the β5 gene in mouse ES cells. Top panel, 8.2 kb of normal genomic structure of the mouse β5 gene. One exon is shown as a solid box. Middle panel, the targeting plasmid in pBluescript is linearized at a unique SacII site. Neomycin resistance (neo) and herpes simplex virus thymidine kinase (TK) genes are shown as shaded boxes. Bottom panel, structure of the β5 gene after a correct targeting event. (B) Southern blot analysis of ES clones. SpeI-digested genomic DNA was blotted with the external probe (probe 1). The external probe gives a 4.6-kb band for the wild-type allele and a 3.8-kb band for the mutant allele since the neomycin resistance gene is shorter than the fragment replaced.
FIG. 2.
(A) Northern analysis of mRNA from β5+/+ and β5−/− mice. Total RNA was extracted from cultured keratinocytes and transcribed to cDNA. The specific cDNA probe of mouse β5 was used to detect message from wild-type but not deficient cells. (B) Immunoprecipitation of αvβ5 from wild-type but not β5−/− murine keratinocytes. [35S]methionine-labeled keratinocyte lysate was immunoprecipitated with anti-β5 antibody raised against the human β5 cytoplasmic domain. Immunoprecipitated proteins were analyzed by SDS-PAGE under nonreducing conditions. The positions of molecular mass marker (in kilodaltons) are shown to the left.
Viable β5−/− mice were born at the expected Mendelian frequency from heterozygous intercrosses (30% +/+, 45% +/−, and 25% −/− of 98 offspring analyzed), demonstrating that the β5−/− subunit is not required for mouse embryonic development. There were no gross abnormalities of the lungs, heart, skin, liver, kidney, spleen, or intestine of any of the β5−/− mice analyzed at up to 3 months of age.
Impaired adhesion and migration of β5−/− keratinocytes on vitronectin.
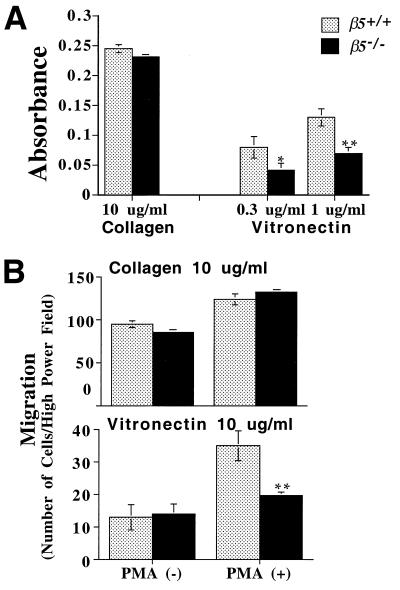
To determine whether the adhesive properties of cells lacking β5 were altered, cell adhesion assays were performed with keratinocytes harvested from β5−/− and wild-type mice. Cells were plated in wells coated with vitronectin or collagen. In comparison to wild-type cells, β5−/− cells showed decreased adhesion to vitronectin but no obvious alteration of adhesion to collagen (Fig. 3A), an extracellular matrix protein that is not a ligand for αvβ5. β5−/− keratinocytes did not completely lose the ability to adhere to vitronectin, presumably because this function is also mediated by the related integrin, αvβ6, as we have previously described (15).
FIG. 3.
(A) Cell adhesion of murine keratinocytes. Confluent keratinocytes from β5−/− and wild-type mice were harvested with trypsin and plated on 96-well plates coated with collagen or vitronectin. Cells were allowed to attach to the wells for an hour and were then fixed and stained. Adhesion was expressed as absorbance at 595 nm. Data calculated from triplicate wells were expressed as the mean (± standard error of the mean). Significantly less than wild type: *, P < 0.05; ∗∗, P < 0.01. (B) Cell migration of murine keratinocytes. Cells were harvested as described above and plated on transwell membranes coated with collagen (10 μg/ml) or vitronectin (10 μg/ml) in the presence or absence of PMA. Cells that had migrated onto the bottom side of the membrane were stained and counted by inverted microscopy at 40×. The data were expressed as the mean (± standard error of the mean). ∗∗, Significantly less than wild type, P < 0.01.
Studies utilizing anti-αvβ5 antibodies have previously implicated αvβ5 in cell migration on vitronectin of human keratinocytes and epithelial tumor cells. To determine whether genetic deletion of αvβ5 impaired cell migration, we used modified Boyden chambers as described previously (15). As for cell adhesion, the loss of αvβ5 had no effect on baseline or phorbol ester-stimulated migration of collagen but significantly inhibited stimulated migration on vitronectin in the presence of PMA (Fig. 3B).
Cutaneous wound healing was not altered in β5−/− mice.
Expression of αvβ5 has been previously reported to be upregulated on keratinocytes at the migrating edge of healing wounds. To investigate whether cutaneous wound healing is impaired in the absence of this integrin, 2- and 4-mm full-thickness cutaneous wounds were made in wild-type and β5−/− mice, and the rate of healing was determined by measuring the wound size at days 2, 4, 6, 12, and 24 after wounding. A similar healing rate was observed in null and wild-type mice (Table 1). In both groups, the wounds were completely healed by day 6 for 2-mm wounds and by day 12 for 4-mm wounds.
TABLE 1.
Mean maximal diameter of 4-mm wounds from wild-type and β5−/− mice
| Time point (day) | Mean wound diam (mm) ± SEM
|
|
|---|---|---|
| Wild type | β5−/− | |
| 2 | 4.3 ± 0.27 | 3.5 ± 0.2 |
| 4 | 2.8 ± 0.41 | 2.2 ± 0.2 |
| 6 | 1.5 ± 0.46 | 1.7 ± 0.52 |
| 12 | Closed | Closed |
Adenovirus infection of murine keratinocytes and airway epithelial cells is independent of αvβ5 integrin expression.
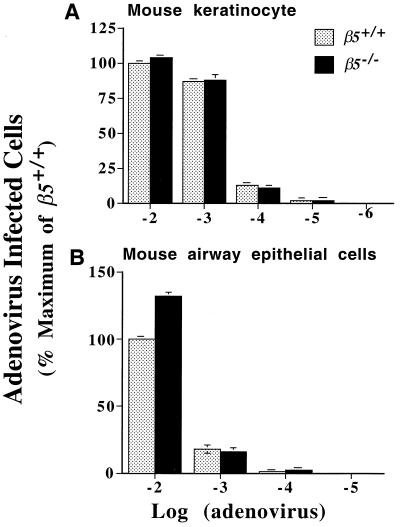
To test the role of the integrin αvβ5 in adenovirus infection, primary cultures of murine keratinocytes and airway epithelial cells were incubated with adenovirus expressing the β-galactosidase gene. Approximately 75% of murine keratinocytes and 30% of murine airway epithelial cells were stained by X-Gal following incubation with the highest concentration of viral particles in the infection medium. Cells from wild-type and β5−/− mice were equally efficiently infected by all concentrations of viral particles (Fig. 4). These data demonstrate that αvβ5 is not essential for infection of primary cultures of murine keratinocytes or airway epithelial cells with adenovirus.
FIG. 4.
Keratinocytes (A) and airway epithelial cells (B) from wild-type and β5−/− mice were infected with a range of concentrations of adenovirus H5.010CMVlacZ; 40 h after infection, cells were treated with X-Gal and blue-stained cells were counted. The results are expressed as a percentage of the number of positive wild-type cells after incubation with the highest concentration of adenovirus used. Results are the mean (± standard error of the mean) of three experiments.
DISCUSSION
We have generated mice with a null mutation of the integrin β5 subunit by homologous recombination in ES cells. Mice homozygous for a null mutation of the β5 subunit gene develop, grow, and reproduce normally. The mutant allele generated a true null as assayed by Northern blotting and immunoprecipitation. Keratinocytes from mutant animals have impaired attachment and migration on the principal αvβ5 ligand, vitronectin. However, the rate of healing of cutaneous wounds does not differ between wild-type and β5−/− mice. Also, keratinocytes and airway epithelial cells obtained from β5−/− mice are as susceptible to adenovirus infection as those from wild-type mice. Thus, we conclude that αvβ5 is not essential for normal development, reproduction, the healing of cutaneous wounds, or productive infection with adenovirus.
Recently, mice lacking the αv subunit have been described (2). These mice all die during embryonic development or immediately after birth, principally due to defects in the development of the blood vessels of the brain and gastrointestinal tract. β5−/− mice were born viable without obvious anatomic or histologic abnormalities. These results suggest that loss of αvβ5 is not, by itself, responsible for the developmental defect in αv−/− mice. Reports of the phenotype of mice expressing null mutations of the β6 (14) and β3 (13) subunits suggest that the developmental defect in αv null mice is not solely explained by the loss of αvβ3 or αvβ6 either. Thus, this phenotype must be due either to the loss of other specific αv integrins (e.g., αvβ1 or αvβ8) or to the combined effects of loss of multiple members of the αv integrin subfamily. Since the principal ligand for αvβ5 appears to be vitronectin, our results are consistent with the report that vitronectin knockout mice also develop normally (30).
One of the principal reasons for examining the in vitro migration of keratinocytes is to generate hypotheses about the mechanisms of processes that require keratinocyte migration in vivo. One of the most important of these is cutaneous wound healing. Several previous reports have suggested that αvβ5 would be likely to play important roles in the wound healing process (6, 10, 18), based largely on the findings that this integrin is rapidly induced on the keratinocytes along the wound edge, the cells that must migrate across the wound bed in order to facilitate wound closure. However, the results of the present study suggest that despite the important role that this integrin plays in in vitro migration of keratinocytes, it is not required for normal wound healing to occur in vivo. The β5−/− mice were fully capable of healing cutaneous wounds in the punch biopsy model. This result is not particularly surprising given the large number of matrix proteins present in wounds (8, 17), the large integrin repertoire expressed on keratinocytes (1, 20), and the biological importance of wound healing. We have reported similar findings for mice lacking the integrin αvβ6 (14), which is also highly induced at the edge of cutaneous wounds (3, 7, 12), and we have shown αvβ6 plays an important role in keratinocyte migration on defined ligands in vitro (15). Indeed, mice expressing null mutations in both the β6 and β5 subunits also heal cutaneous wounds normally (unpublished observations).
There is no impairment of adenovirus-mediated gene transfer and expression in cells from β5−/− mice, suggesting that this integrin is not critical for adenovirus infection, at least of keratinocytes and airway epithelial cells. Previous studies that have suggested a predominant role for αvβ5 in this process have used transfected cells infected in suspension (27, 28). The use of RGD peptide to antagonize adenovirus infection has been cited as evidence for a role for this integrin in this process. However, high concentrations of RGD (up to 4 mg/ml) have been required to produce significant decrements of infection (11). Our study, together with recent data implicating activated α5β1 in adenovirus infection (9), suggests that adenoviruses can readily infect epithelial cells by mechanisms independent of αvβ5.
The results of this study demonstrate that despite the contribution of αvβ5 to cellular response to vitronectin and other ligands, any role the integrin plays in murine development, wound healing, or adenovirus infection can be compensated for by other αvβ5-independent pathways. Identification of critical in vivo roles for the integrin may depend on use of these mice in additional disease models and/or the generation of mice lacking multiple β-subunit partners of αv.
ACKNOWLEDGMENTS
We thank Eric Sande for technical assistance in generating the β5−/− mice and Martin Hemler and Louis Reichardt for generously providing anti-β5 antiserum.
This work was supported by NIH grants HL/AI33259, HL47412, HL53949, and HL56385 (to Dean Sheppard) and the J. David Gladstone Institutes (to Robert V. Farese, Jr.).
REFERENCES
- 1.Adams J C, Watt F M. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991;115:829–841. doi: 10.1083/jcb.115.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bader B L, Rayburn H, Crowley D, Hynes R O. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 3.Breuss J M, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–1527. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 4.Busk M, Pytela R, Sheppard D. Characterization of the integrin αvβ6 as a fibronectin-binding protein. J Biol Chem. 1992;267:5790–5796. [PubMed] [Google Scholar]
- 5.Capecchi M R. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 6.Cavani A, Zambruno G, Marconi A, Manca V, Marchetti M, Giannetti A. Distinctive integrin expression in the newly forming epidermis during wound healing in humans. J Investig Dermatol. 1993;101:600–604. doi: 10.1111/1523-1747.ep12366057. [DOI] [PubMed] [Google Scholar]
- 7.Clark R, Ashcroft G, Spencer M, Larjava H, Ferguson M. Re-epithelialization of normal human excisional wounds is associated with a switch from alpha v beta 5 to alpha v beta 6 integrins. Br J Dermatol. 1996;135:46–51. [PubMed] [Google Scholar]
- 8.Clark R A, Folkvord J M, Wertz R L. Fibronectin, as well as other extracellular matrix proteins, mediate human keratinocyte adherence. J Investig Dermatol. 1985;84:378–383. doi: 10.1111/1523-1747.ep12265466. [DOI] [PubMed] [Google Scholar]
- 9.Davison E, Diaz R M, Hart I R, Santis G, Marshall J F. Integrin α5β1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J Virol. 1997;71:6204–6207. doi: 10.1128/jvi.71.8.6204-6207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gailit J, Welch M P, Clark R A. TGF-beta 1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Investig Dermatol. 1994;103:221–227. doi: 10.1111/1523-1747.ep12393176. [DOI] [PubMed] [Google Scholar]
- 11.Goldman M, Wilson J M. Expression of αvβ5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995;69:5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, Kramer R, Clark R, Uitto V, Larjava H. Keratinocytes in human wounds express alpha v beta 6 integrin. J Investig Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- 13.Hodivala-Dilke K M, McHugh K P, Tsakiris D A, Rayburn H, Crowley D, Ullman-Culleré M, Ross F P, Coller B S, Teitelbaum S, Hynes R O. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Investig. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Wu J, Cass D, Erle D, Corry D, Young S, Farese R J, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Wu J, Spong S, Sheppard D. The integrin alpha v beta 6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J Cell Sci. 1998;111:2189–2195. doi: 10.1242/jcs.111.15.2189. [DOI] [PubMed] [Google Scholar]
- 16.Hynes R O. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 17.Juhasz I, Murphy G F, Yan H C, Herlyn M, Albelda S M. Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol. 1993;143:1458–1469. [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J P, Zhang K, Chen J D, Kramer R H, Woodley D T. Vitronectin-driven human keratinocyte locomotion is mediated by the alpha v beta 5 integrin receptor. J Biol Chem. 1994;269:26926–26932. [PubMed] [Google Scholar]
- 19.Klemke R L, Yebra M, Bayna E M, Cheresh D A. Receptor tyrosine kinase signaling required for integrin alpha v beta 5-directed cell motility but not adhesion on vitronectin. J Cell Biol. 1994;127:859–866. doi: 10.1083/jcb.127.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larjava H, Salo T, Haapasalmi K, Kramer R H, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Investig. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura S L, Sheppard D, Pytela R. Integrin alpha v beta 8. Interaction with vitronectin and functional divergence of the beta 8 cytoplasmic domain. J Biol Chem. 1994;269:28708–28715. [PubMed] [Google Scholar]
- 22.Prieto A L, Edelman G M, Crossin K L. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc Natl Acad Sci USA. 1993;90:10154–10158. doi: 10.1073/pnas.90.21.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramaswamy H, Hemler M E. Cloning, primary structure and properties of a novel human integrin beta subunit. EMBO J. 1990;9:1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruoslahti E, Pierschbacher M D. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 25.Smith J W, Vestal D J, Irwin S V, Burke T A, Cheresh D A. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990;265:11008–11013. [PubMed] [Google Scholar]
- 26.Weinacker A, Chen A, Agrez M, Cone R I, Nishimura S L, Wayner E, Pytela R, Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- 27.Wickham T J, Filardo E J, Cheresh D A, Nemerow G R. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 29.Yokosaki Y, Palmer E L, Prieto A L, Crossin K L, Bourdon M A, Pytela R, Sheppard D. The integrin alpha 9 beta 1 mediates cell attachment to a non-RGD site in the third fibronectin type III repeat of tenascin. J Biol Chem. 1994;269:26691–26696. [PubMed] [Google Scholar]
- 30.Zheng X, Saunders T, Camper S, Samuelson L, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proc Natl Acad Sci USA. 1995;92:12426–12430. doi: 10.1073/pnas.92.26.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]