Abstract
Purpose
To compare treatment outcomes of treatment‐naïve eyes with neovascular age‐related macular degeneration (nAMD) with bevacizumab as the first‐line treatment, according to the guidelines of the Dutch Ophthalmological Society, with those treated first with either ranibizumab or aflibercept, as used in many other countries, all treated using a treat‐and‐extend strategy.
Methods
Data were obtained from the prospectively designed Fight Retinal Blindness! outcomes registry. The primary outcome was the mean change from baseline in visual acuity of all treated eyes, after 12, 24 and 36 months of treatment. Secondary outcomes were the number of injections, the number of visits and the rate of switching to a second anti‐VEGF drug.
Results
The study included 703 treatment‐naïve eyes with nAMD with 12 months follow‐up, 373 eyes with 24 months follow‐up, and 171 eyes with 36 months follow‐up in the Netherlands, and 1131, 652, and 303 treatment‐naïve eyes with respectively 12, 24, and 36 months of follow‐up in all other countries. The change in visual acuity from baseline did not differ between the Netherlands and the other countries at any follow‐up time. The median number of injections, visits and the proportion of eyes switching treatment was significantly higher in the Netherlands than in the other countries.
Conclusion
Starting anti‐VEGF treatment for nAMD with bevacizumab, as is mandatory in the Netherlands, delivers outcomes similar to those starting treatment with either ranibizumab or aflibercept, but at a cost of more frequent injections, and visits, and more frequent switching treatment to a second drug.
Keywords: neovascular age‐related macular degeneration (nAMD), anti‐VEGF treatment, quality registration, real‐world data
Introduction
Dutch ophthalmologists tended to use bevacizumab as first‐line anti‐vascular endothelial growth factor (anti‐VEGF) treatment to reduce costs for the national healthcare system even before several important comparative trials had reported similar treatment outcomes with bevacizumab and ranibizumab for neovascular age‐related macular degeneration (nAMD) (Martin et al. 2012; Chakravarthy et al. 2013). After they were reported, the Dutch Ophthalmological Society issued an official guideline in 2014 that advised all ophthalmologists to use bevacizumab as the preferred drug to start anti‐VEGF treatment in nAMD, with a revision in 2017 after a randomized clinical trial in the Netherlands found that bevacizumab and ranibizumab had similar outcomes (Schauwvlieghe et al. 2016; Society 2017). Ophthalmologists in other parts of the world, however, continued to use the registered anti‐VEGF drugs, either ranibizumab or aflibercept, as first‐line treatment, instead of bevacizumab, which was an off‐label (not registered for ocular use) drug. The question arises whether the Dutch decision to start with bevacizumab in nAMD remains justified in ‘real‐world’ clinical practice, which often has outcomes that are different from those of randomized clinical trials.
One way to evaluate this is to compare data from a registry of anti‐VEGF treatment in nAMD patients from the Netherlands with other countries with different treatment regimens. A number of ophthalmological centres in the Netherlands began to use the nAMD module of the Fight Retinal Blindness! Registry in 2015. This database records real‐world treatment outcomes in patients with nAMD during routine clinical practice through a web‐based interface (Gillies et al., 2014b,). The registry, which has been endorsed by ICHOM (International Consortium for Health Outcomes Measurements) to collect their minimum, patient‐centred treatment outcomes of nAMD, is used in Australia, New Zealand, Switzerland and a growing number of other countries (Rodrigues et al. 2016).
In the present study, 12, 24 and 36 months results of the treatment of people with nAMD who were treated in the participating FRB! centres in the Netherlands, starting with bevacizumab, were compared to those of centres in other countries, also using the FRB! registry, where treatment was started with either ranibizumab or aflibercept. In addition, to prove the value of benchmarking with the FRB! registry, a comparison was made between one of the Dutch participating centres and all other participating centres in the Netherlands.
Methods
Data source
Thirteen centres in the Netherlands started using the FRB! registry for the treatment of wet AMD in 2015. New patients were immediately entered consecutively at all participating centres, and some centres chose to also ‘back enter’ data on patients who started treatment before 2015. For this study, only patients starting their treatment in 2016 and onward were included in order to prevent selectively entered data.
The mandatory, patient‐centred, minimum FRB! dataset was entered through a web‐based interface (Nguyen et al. 2020).
The FRB! System collected the following data for each patient visit: the number of letters read on a logarithm of the minimum angle of resolution VA chart (best of corrected, uncorrected or pinhole visual acuity); the drug given; activity of the choroidal neovascular lesion as judged by the treating ophthalmologist based on fundoscopy, OCT or FA alone or in combination (an active lesion was defined as presence of intra‐ or subretinal fluid on OCT, detection of a fresh haemorrhage, or leakage on FA), presence of macular atrophy or fibrosis or pigment epithelial detachment and ocular adverse events. At baseline, in addition, any previous treatment (all eyes in the present study were treatment‐naive), lesion subtype based on imaging and lesion size (greatest linear dimension, on FA or OCT) was recorded (Bhandari et al. 2020; Gillies et al., 2020a,2020b; Nguyen et al. 2020).
Participants
Due to its noninterventional character, approval of the use of the registry was not needed according to the Medical Ethics Committee of the Academic Medical University Centre, the Netherlands. All patients had to sign an informed consent before data were entered in the registry. The FRB! project adhered to the tenets of the Declaration of Helsinki.
Treatment‐naïve eyes with nAMD that were registered into the FRB! database in the Netherlands from January 2016 onwards that received bevacizumab at the start of the treatment as required by the Dutch guideline, with at least 12, 24 or 36 months of follow‐up, were included in this study. Patients entering the registry up until 1 January 2019 were considered for the 12‐month end‐point, 1 January 2018 for the 24‐month end‐point, and 1 January 2017 for the 36‐month end‐point to accurately determine the completion and dropout rate.
These eyes were compared to all the treatment‐naïve eyes with nAMD, registered in the FRB! registry in the same time period that were treated in Australia, New Zealand or Switzerland (hereafter referred to as ‘rest of FRB!’), and started treatment with either ranibizumab or aflibercept with identical follow‐up periods. We also made the same comparison between one of the participating centres, which entered the highest number of patients, in the Netherlands and all the other Dutch centres.
Outcome measures
The primary outcome measure was the change in VA from baseline at 12, 24 and 36 months. Secondary outcomes included the number of injections, the number of visits and the number of patients switching to another anti‐VEGF drug during the 36 months follow‐up period.
Statistical analysis
All analyses were performed using R version 4.0.0 (R Project – The R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics included mean, standard deviation (SD), median, range, quartiles and percentages where appropriate.
A LOESS (locally estimated scatterplot smoothing) curve was used to plot the visual acuity over time (Cleveland 2017). Unadjusted outcomes were compared using t‐tests and chi‐squared tests where appropriate. Adjusted change in VA was compared using mixed‐effects regression models. The number of injections and visits was compared using generalized Poisson regression models with an offset for log days of follow‐up. Analyses of time to switching to another anti‐VEGF drug were performed using Cox proportional hazards models. All models included adjustments for age and VA at baseline (fixed effects) and nesting of outcomes within doctors (for the Netherlands versus rest of FRB! analysis only) and patients with both eyes included (random effects).
Results
The Netherlands versus the rest of FRB!
This study included 703/851 eyes (17% dropout rate) from 623/748 patients treated at the participating centres in the Netherlands with at least 12 months follow‐up, 373/526 eyes (29% dropout rate) from 331/462 patients with at least 24 months follow‐up, and 171/249 eyes (31% dropout rate) from 153/225 patients with at least 36 months follow‐up. From the rest of FRB! cohort, 1131/1448 treatment‐naïve eyes (22% dropout rate) from 1009/1279 patients were included with at least 12 months follow‐up, 652/1014 eyes (36% dropout rate) from 594/918 patients with at least 24 months follow‐up, and 303/446 eyes (37% dropout rate) from 278/483 patients with at least 36 months follow‐up. Baseline demographics are summarized in Table 1.
Table 1.
Baseline demographics for the Netherlands versus rest of FRB!.
| 12‐Month Completers | 24‐Month Completers | 36‐Month Completers | ||||
|---|---|---|---|---|---|---|
| Netherlands | Rest of FRB! | Netherlands | Rest of FRB! | Netherlands | Rest of FRB! | |
| Eyes | 703 | 1131 | 373 | 652 | 171 | 303 |
| Patients | 623 | 1009 | 331 | 594 | 153 | 278 |
| Gender, % female patients | 62.1% | 62.2% | 61.6% | 64.3% | 64.1% | 66.9% |
| Age, mean (SD) | 79.1 (8.3) | 80.6 (8.2) | 78.6 (8.1) | 80.6 (7.8) | 77.9 (8.8) | 80.6 (7.8) |
| VA, mean letters (SD) | 59.2 (18.4) | 60.7 (18.6) | 59.3 (17.4) | 61.7 (17.3) | 58.7 (18.6) | 61.4 (17.2) |
| VA ≤ 35 letters, n (%) | 82 (11.7%) | 123 (10.9%) | 40 (10.7%) | 59 (9%) | 20 (11.7%) | 27 (8.9%) |
| VA ≥ 70 letters, n (%) | 249 (35.4%) | 463 (40.9%) | 127 (34%) | 274 (42%) | 62 (36.3%) | 127 (41.9%) |
| Lesion size, median µm (Q1, Q3) | 1759 (600, 3000) | 1700 (1000, 2580) | 1750 (500, 3000) | 1600 (1033, 2579) | 1576 (500, 2400) | 1500 (1000, 2384) |
| Angiographic lesion type, n (%) | ||||||
| Type 1 | 186 (26.5%) | 478 (42.3%) | 119 (31.9%) | 328 (50.3%) | 57 (33.3%) | 171 (56.4%) |
| Type 2 | 44 (6.3%) | 135 (11.9%) | 20 (5.4%) | 86 (13.2%) | 8 (4.7%) | 52 (17.2%) |
| Type 3 | 5 (0.7%) | 37 (3.3%) | 1 (0.3%) | 22 (3.4%) | 1 (0.6%) | 11 (3.6%) |
| Disciform scar | 3 (0.4%) | 3 (0.3%) | 0 (0%) | 3 (0.5%) | 0 (0%) | 1 (0.3%) |
| IPCV | 3 (0.4%) | 10 (0.9%) | 3 (0.8%) | 5 (0.8%) | 1 (0.6%) | 3 (1%) |
| Juxtapapillary | 12 (1.7%) | 15 (1.3%) | 6 (1.6%) | 7 (1.1%) | 2 (1.2%) | 3 (1%) |
| Not done | 450 (64%) | 453 (40.1%) | 224 (60.1%) | 201 (30.8%) | 102 (59.6%) | 62 (20.5%) |
| Initial injection type, n (%) | ||||||
| Avastin | 703 (100%) | 0 (0%) | 373 (100%) | 0 (0%) | 171 (100%) | 0 (0%) |
| Eylea | 0 (0%) | 571 (50.5%) | 0 (0%) | 329 (50.5%) | 0 (0%) | 128 (42.2%) |
| Lucentis | 0 (0%) | 560 (49.5%) | 0 (0%) | 323 (49.5%) | 0 (0%) | 175 (57.8%) |
Baseline characteristics were similar between both groups for the 12‐month, the 24‐month and the 36‐month completers.
The lesion size at baseline did not differ between the eyes treated in the Netherlands and the eyes treated in the other countries for the 12‐month, the 24‐month, and the 36‐month completers, but the longer the follow‐up the smaller the lesion size at baseline, going from around 1700 micron to 1500 micron.
The angiographic typing of the neovascular lesion was not done in most of the eyes so this information is only of limited use; however, lesion types between the Netherlands and the other countries were broadly similar.
Visual and treatment outcomes are summarized in Table 2. The mean (95% CI) unadjusted change in VA from baseline to 12, 24 and 36 months was 5.6 (4.3, 6.8), 6.1 (4.4, 7.7) and 5.7 (3.2, 8.2) letters in the Netherlands versus 5.1 (4.2, 5.9), 3.5 (2.3, 4.8) and 2.7 (0.8, 4.7) letters in the other countries, respectively. The change in VA (number of letters read) from baseline to 12, 24 and 36 months seemed better in the Netherlands, but the difference was only statistically significant (p = 0.017) for the 24 months completers. After adjusting the numbers for baseline visual acuity, age and nesting of outcomes from patients within treating ophthalmologists, the change in visual acuity from baseline did not differ between the Netherlands and the other countries at any point, including the proportions of eyes with a gain or loss of at least 10 or 15 letters.
Table 2.
Outcomes at 12, 24 and 36 months comparing Netherlands versus rest of FRB! for treatment‐naïve eyes initiating treatment from 2016 onwards completing 12, 24 and 36 months of treatment, respectively.
| 12 Months | p‐value | 24 Months | p‐value | 36 Months | p‐value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Netherlands | Rest of FRB! | Netherlands | Rest of FRB! | Netherlands | Rest of FRB! | ||||
| Eyes | 703 | 1131 | 373 | 652 | 171 | 303 | |||
| Baseline VA, mean letters (SD) | 59.2 (18.4) | 60.7 (18.6) | 0.105 | 59.3 (17.4) | 61.7 (17.3) | 0.035 | 58.7 (18.6) | 61.4 (17.2) | 0.119 |
| Final VA, mean letters (SD) | 64.8 (18.1) | 65.7 (18.8) | 0.286 | 65.4 (17.4) | 65.2 (19.9) | 0.891 | 64.4 (17.1) | 64.1 (20.7) | 0.868 |
| ≤35 letters, % baseline/% final | 11.7%/9.8% | 10.9%/9.5% | 0.867* | 10.7%/9.1% | 9.0%/10.3% | 0.623* | 11.7%/8.8% | 8.9%/12.2% | 0.319* |
| ≥70 letters, % baseline/% final | 35.4%/54.1% | 40.9%/59.2% | 0.036* | 34%// 53.6% | 42%/7.5% | 0.253* | 36.3%/52% | 41.9% / 53.1% | 0.895* |
| VA change, mean (95% CI) | 5.6 (4.3, 6.8) | 5.1 (4.2, 5.9) | 0.519 | 6.1 (4.4, 7.7) | 3.5 (2.3, 4.8) | 0.017 | 5.7 (3.2, 8.2) | 2.7 (0.8, 4.7) | 0.064 |
| ≥10 letter gain, n (%) | 259 (36.8%) | 390 (34.5%) | 0.329 | 144(38.6%) | 210 32.2%) | 0.045 | 60 (35.1%) | 90 (29.7%) | 0.268 |
| ≥10 letter loss, n (%) | 84 (11.9%) | 128 (11.3%) | 0.737 | 41 (11%) | 89 (13.7%) | 0.257 | 23 (13.5%) | 53 (17.5%) | 0.307 |
| ≥15 letter gain, n (%) | 164 (23.3%) | 250 (22.1%) | 0.581 | 102(27.3%) | 135(20.7%) | 0.019 | 39 (22.8%) | 65 (21.5%) | 0.821 |
| ≥15 letter loss, n (%) | 54 (7.7%) | 67 (5.9%) | 0.168 | 30 (8%) | 60 (9.2%) | 0.606 | 12 (7%) | 33 (10.9%) | 0.223 |
| Adjusted VA change, mean (95% CI) | 4.5 (3.0, 6.1) | 5.4 (4.4, 6.3) | 0.357 | 4.7 (2.6, 6.9) | 3.9 (2.6, 5.2) | 0.532 | 4.4 (1.5, 7.3) | 3.3 (1.4, 5.3) | 0.541 |
| Injections, median (Q1, Q3) | 10 (8, 12) | 8 (7, 10) | 0.001 † | 18 (13, 23) | 14 (11, 17) | 0.015 † | 26 (20, 33) | 20 (16, 25) | 0.001 † |
| Visits, median (Q1, Q3) | 13 (11, 15) | 9 (8, 10) | <0.001 † | 23 (18, 27) | 15 (12, 18) | <0.001 † | 34 (25.5, 40) | 21 (17, 26) | <0.001 † |
| Switched, n (%) | 185 (26.3%) | 180 (15.9%) | <0.001 † | 169 (45.3%) | 142 (21.8%) | 0.005 † | 100 (58.5%) | 87 (28.7%) | 0.010 † |
| To Avastin | 0 (0%) | 7 (0.6%) | 0 (0%) | 7 (1.1%) | 0 (0%) | 5 (1.7%) | |||
| To Eylea | 149 (21.2%) | 138 (12.2%) | 136 (36.5%) | 111 (17%) | 78 (45.6%) | 69 (22.8%) | |||
| To Lucentis | 36 (5.1%) | 35 (3.1%) | 33 (8.8%) | 24 (3.7%) | 22 (12.9%) | 13 (4.3%) | |||
Significant differences in bold.
CI = confidence interval; Q1 = first quartile (25th percentile); Q3 = third quartile (75th percentile); SD = standard deviation; VA = visual acuity
p‐values comparing percentages for final visual acuity
p‐value from mixed‐effects regression models, generalized Poisson regression models, or Cox proportional hazards models adjusted for baseline visual acuity, age and nesting of outcomes from patients within treating ophthalmologists
The change in visual acuity over 36 months is shown in Fig. 1. The curve from the Dutch patients improved less steeply than that of the other countries, with similar mean visual acuity after the first 12 months between the 2 groups.
Fig. 1.
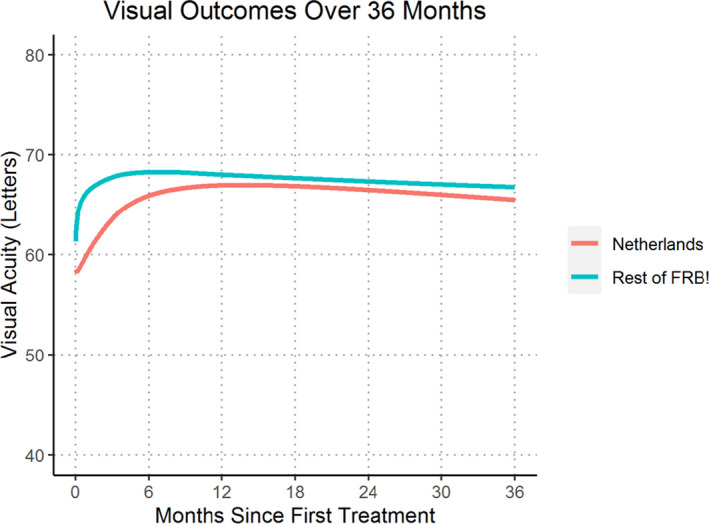
Locally weighted scatterplot smoothing (LOESS) regression curve of mean visual acuity for treatment‐naïve eyes initiating treatment from 2016 onwards completing 36 months of treatment comparing Netherlands versus Rest of FRB!
The number of injections after multivariate adjustment was significantly higher in the Netherlands than in the rest of FRB! for the 12‐month, the 24‐month, and the 36‐month completers. The median (Q1, Q3) number of injections for the Netherlands versus rest of FRB! was 10 (8, 12) versus 8 (7, 10) (p = 0.001) injections at 12 months, 18 (13, 23) versus 14 (11, 17) (p = 0.015) injections at 24 months, and 26 (20, 33) versus 20 (16, 25) (p = 0.001) at 36 months. This trend was also observed for the number of visits at all time‐points (Table 2).
The proportion of eyes switching treatment was significantly higher in the Netherlands than in the other countries. The percentage of switchers for the Netherlands versus rest of FRB! was 26% versus 16% (p < 0.001) at 12 months, 45% versus 22% (p = 0.005) at 24 months, and 59% and 29% (p = 0.010) at 36 months. The majority of switching in both the Netherlands and rest of FRB! was to aflibercept.
The number of adverse events, counting all events occurring within the 3 years of follow‐up, for all eligible eyes, including the dropouts, was low, and did not differ significantly between the Netherlands and the rest of FRB!. In total, 17 965 injections were recorded in the Netherlands and 21 397 in the rest of FRB! Haemorhage reducing BCVA > 15 letters was seen in 6 cases in the Netherlands (0.042%), versus 10 cases in the rest of FRB! (0.052%), infectious endophthalmitis in 2 (0.014%), versus 1 (0.005%) cases, noninfectious endophthalmitis in 7 (0.049%) versus 0 (0%) cases, and RPE tear in 16 (0.113%) versus 21 (0.11%) cases.
Benchmarking versus the rest of the Netherlands
A comparison could be made using the same data between one of the centres in the Netherlands (Centre A) that included most patients during the follow‐up period and the rest of the Netherlands. In Centre A, 257 eyes had at least 12 months follow‐up, 153 eyes at least 24 months follow‐up, and 74 eyes at least 36 months of follow‐up. In rest of the Netherlands, 446 treatment‐naïve eyes were included with at least 12, 220 eyes with at least 24, and 97 eyes with at least 36 months of follow‐up, respectively. Baseline demographics of both groups of patients are summarized in Table 3.
Table 3.
Baseline demographics for Centre A versus the rest of the Netherlands.
| 12‐Month Completers | 24‐Month Completers | 36‐Month Completers | ||||
|---|---|---|---|---|---|---|
| Centre A | Rest of Netherlands | Centre A | Rest of Netherlands | Centre A | Rest of Netherlands | |
| Eyes | 257 | 446 | 153 | 220 | 74 | 97 |
| Patients | 231 | 392 | 138 | 193 | 68 | 85 |
| Gender, % female patients | 60.2% | 63.3% | 58% | 64.2% | 63.2% | 64.7% |
| Age, mean (SD) | 78.9 (9.2) | 79.2 (7.8) | 78.9 (9.1) | 78.3 (7.4) | 78 (9.9) | 77.7 (7.9) |
| VA, mean letters (SD) | 59.1 (16.2) | 59.3 (19.6) | 58.3 (15.3) | 60 (18.8) | 58.4 (14.9) | 58.9 (21.1) |
| VA ≤ 35 letters, n (%) | 21 (8.2%) | 61 (13.7%) | 13 (8.5%) | 27 (12.3%) | 5 (6.8%) | 15 (15.5%) |
| VA ≥ 70 letters, n (%) | 78 (30.4%) | 171 (38.3%) | 43 (28.1%) | 84 (38.2%) | 20 (27%) | 42 (43.3%) |
| Lesion size, median µm (Q1, Q3) | 1500 (398, 2500) | 2000 (750, 3108) | 1500 (366, 2500) | 1955 (735, 3164) | 1350 (500, 2400) | 1750 (600, 2926) |
| Angiographic lesion type, n (%) | ||||||
| Type 1 | 29 (11.3%) | 157 (35.2%) | 20 (13.1%) | 99 (45%) | 9 (12.2%) | 48 (49.5%) |
| Type 2 | 4 (1.6%) | 40 (9%) | 4 (2.6%) | 16 (7.3%) | 2 (2.7%) | 6 (6.2%) |
| Type 3 | 0 (0%) | 5 (1.1%) | 0 (0%) | 1 (0.5%) | 0 (0%) | 1 (1%) |
| Disciform scar | 0 (0%) | 3 (0.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IPCV | 0 (0%) | 3 (0.7%) | 0 (0%) | 3 (1.4%) | 0 (0%) | 1 (1%) |
| Juxtapapillary | 8 (3.1%) | 4 (0.9%) | 2 (1.3%) | 4 (1.8%) | 1 (1.4%) | 1 (1%) |
| Not done | 216 (84%) | 234 (52.5%) | 127 (83%) | 97 (44.1%) | 62 (83.8%) | 40 (41.2%) |
Their baseline characteristics between Centre A and the rest of the Netherlands were similar.
The lesion size at baseline did not differ between the eyes treated in centre A and the eyes treated in the rest of the Netherlands for the 12‐month, the 24‐month, and the 36 month completers. The median lesion size was 1500 micron in centre A for the 12‐month completers, and 2000 micron for the rest of the Netherlands; for the 24 months completers, these numbers were 1500 micron and 1954 micron, and for the 36 months completers 1350 and 1750 micron.
The angiographic typing of the lesion provides only limited information because was not done in most eyes. There were no obvious differences between the two groups.
Visual and treatment outcomes are summarized in Table 2. The mean (95% CI) unadjusted change in VA from baseline to 12, 24 and 36 months was 7.8 (5.7, 9.9), 8.5 (6.2, 10.9) and 7.1 (4.0, 10.1) letters in centre A versus 4.3 (2.8, 5.8), 4.4 (95% CI 2.1, 6.7) and 4.7 (95% CI 0.9, 8.5) letters in the rest of the Netherlands, respectively. These differences were significant at 12 months (p = 0.008) and 24 months (p = 0.013). This difference in change in visual acuity from baseline remained significant after multivariate adjustment at 12 months (p = 0.003) and 24 months (p = 0.02). Visual acuity over the 36 months is shown Fig. 2.
Fig. 2.
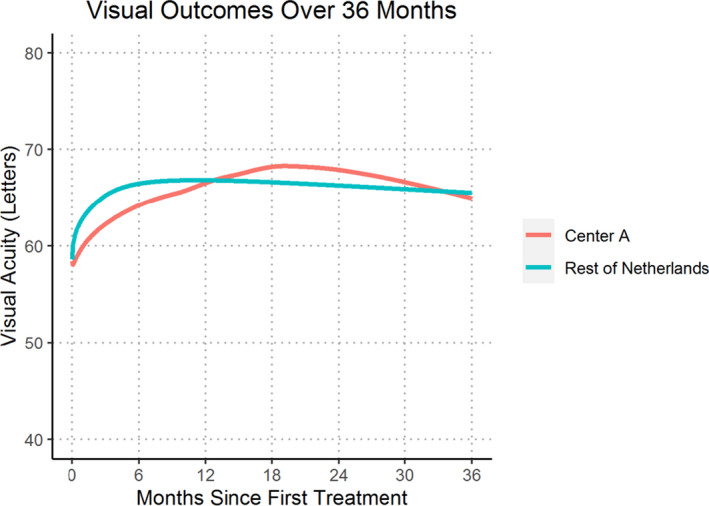
Locally weighted scatterplot smoothing (LOESS) regression curve of mean visual acuity for treatment‐naïve eyes initiating treatment from 2016 onwards completing 36 months of treatment comparing Centre A versus Rest of Netherlands
The number of injections after multivariate adjustment was significantly higher in Centre A compared to the rest of the Netherlands for the 12‐, 24‐ and 36‐month completers. The median (Q1, Q3) number of injections for Centre A versus the rest of Netherlands was 12 (10, 13) versus 9 (8, 11) (p < 0.001) injections at 12 months, 21 (15, 25) versus 17 (12, 20) (p < 0.001) injections at 24 months, and 31 (21, 35) versus 25 (19, 30) (p = 0.022) injections at 36 months. This trend was also observed for the number of visits at all time‐points (Table 4).
Table 4.
Outcomes at 12, 24 and 36 months comparing a single centre in the Netherlands versus the other centres in the Netherlands for treatment‐naïve eyes initiating treatment from 2016 onwards completing 12, 24 and 36 months of treatment respectively
| 12 Months | 24 Months | 36 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Centre A | Rest of Netherlands | p‐value | Centre A | Rest of Netherlands | p‐value | Centre A | Rest of Netherlands | p‐value | |
| Eyes | 257 | 446 | 153 | 220 | 74 | 97 | |||
| Baseline VA, mean letters (SD) | 59.1 (16.2) | 59.3 (19.6) | 0.858 | 58.3 (15.3) | 60 (18.8) | 0.333 | 58.4 (14.9) | 58.9 (21.1) | 0.861 |
| Final VA, mean letters (SD) | 66.9 (15.1) | 63.6 (19.5) | 0.012 | 66.8 (13.6) | 64.4 (19.5) | 0.155 | 65.5 (13.1) | 63.6 (19.6) | 0.462 |
| ≤35 letters, % baseline/% final | 8.2%/4.7% | 13.7%/12.8% | <0.001* | 8.5%/3.3% | 12.3%/13.2% | 0.002* | 6.8%/4.1% | 15.5%/12.4% | 0.103* |
| ≥70 letters, % baseline/% final | 30.4%/51.8% | 38.3%/55.4% | 0.395* | 28.1%/48.4% | 38.2%/57.3% | 0.112* | 27%/41.9% | 43.3%/59.8% | 0.030* |
| VA change, mean (95% CI) | 7.8 (5.7, 9.9) | 4.3 (2.8, 5.8) | 0.008 | 8.5 (6.2, 10.9) | 4.4 (2.1, 6.7) | 0.013 | 7.1 (4, 10.1) | 4.7 (0.9, 8.5) | 0.334 |
| ≥10 letter gain, n (%) | 115 (44.7%) | 144 (32.3%) | 0.001 | 69 (45.1%) | 75 (34.1%) | 0.041 | 25 (33.8%) | 35 (36.1%) | 0.881 |
| ≥10 letter loss, n (%) | 22 (8.6%) | 62 (13.9%) | 0.048 | 13 (8.5%) | 28 (12.7%) | 0.264 | 8 (10.8%) | 15 (15.5%) | 0.511 |
| ≥15 letter gain, n (%) | 69 (26.8%) | 95 (21.3%) | 0.114 | 47 (30.7%) | 55 (25%) | 0.271 | 15 (20.3%) | 24 (24.7%) | 0.612 |
| ≥15 letter loss, n (%) | 12 (4.7%) | 42 (9.4%) | 0.033 | 8 (5.2%) | 22 (10%) | 0.141 | 1 (1.4%) | 11 (11.3%) | 0.026 |
| Adjusted VA change, mean (95% CI) | 7.7 (5.9, 9.5) | 4.3 (2.9, 5.7) | 0.003 | 8.1 (5.9, 10.4) | 4.6 (2.7, 6.5) | 0.020 | 7.0 (3.8, 10.2) | 4.8 (2.0, 7.6) | 0.314 |
| Injections, median (Q1, Q3) | 12 (10, 13) | 9 (8, 11) | <0.001† | 21 (15, 25) | 17 (12, 20.2) | <0.001† | 31 (21, 35) | 25 (19, 30) | 0.022† |
| Visits, median (Q1, Q3) | 12 (10, 14) | 14 (12, 16) | <0.001† | 22 (17, 25) | 24 (19, 30) | <0.001† | 31 (22, 35.8) | 37 (31, 44) | <0.001† |
| Switched, n (%) | 66 (25.7%) | 119 (26.7%) | 0.094† | 66 (43.1%) | 103 (46.8%) | 0.130† | 36 (48.6%) | 64 (66%) | 0.095† |
| To Avastin | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| To Eylea | 66(25.7%) | 83 (18.6%) | 6643.1%) | 70 (31.8%) | 36 48.6%) | 42 (43.3%) | |||
| To Lucentis | 0 (0%) | 36 (8.1%) | 0 (0%) | 33 (15%) | 0 (0%) | 22 (22.7%) | |||
CI = confidence interval; Q1 = first quartile (25th percentile); Q3 = third quartile (75th percentile); SD = standard deviation; VA = visual acuity.
p‐values comparing percentages for final visual acuity.
p‐value from mixed‐effects regression models, generalized poisson regression models, or Cox proportional hazards models adjusted for baseline visual acuity, age and nesting of outcomes from patients within treating ophthalmologists.
The proportions switching treatment were not significantly different between Centre A and the rest of the Netherlands (around 25% of both groups in the 12‐month completers, 45% of the 24‐month completers, and 49% in the 36‐month completers from Centre A compared with 66% in the rest of the Netherlands.
Discussion
This analysis of a prospectively designed outcomes registry showed the results of treatment of nAMD with anti‐VEGF in the Netherlands starting obligatorily, according to the Dutch guidelines, with bevacizumab were comparable with results in other countries starting with either ranibizumab or aflibercept at 12, 24 and 36 months, but at the cost of more frequent injections and visits. Switching treatment to a second drug was also more prevalent in the Netherlands. Using the same data to compare the results of a single Dutch centre with the rest of the Dutch FRB! centres, we found better visual outcomes in this centre, again at a cost of more frequent injections and visits. The FRB! registry is a powerful tool to perform benchmarking clinical outcomes between different countries and centres who may be using different treatment guidelines and drugs.
The treatment strategy used in the Netherlands and in the other countries belonging to the comparison group was treat‐and‐extend. All treatment decisions were made by individual ophthalmologists without guidance from a central reading centre enforcing strict retreatment criteria. A comparison of treatments between groups of patients may therefore be biased by inconsistencies in treatment decisions. As the T&E treatment interval increment criteria were not standardized, comparison on treatment burden between the clinics/countries may better reflect variation how T&E is executed, rather than properties of the used anti‐VEGF drug itself.
However, the treatment results presented in this study are in line with other similar studies on treat‐and‐extend regimes (Berg et al. 2015; Berg et al. 2016; Gerding 2016; Kim et al. 2016; Ohji et al. 2020).
Recent meta‐analyses of 2‐year and 3‐year results following a treat‐and‐extend strategy with either ranibizumab or aflibercept found similar gains in visual acuity from baseline: after 12 months between 4.5 and 5.7 letters, after 24 months between 4.7 and 7.6 letters, and after 36 months between 2.2 and 3.5 letters. The mean number of injections given was 6.5 in the first year, 4.5 in the second year and 4.0 in the third year (Gerding 2016; Kim et al. 2016; Ohji et al. 2020).
In the LUCAS study comparing results of treatment with bevacizumab versus ranibizumab according to a treat‐and extend regime, the gain in VA from baseline after 1 year was comparable between the two groups, around 8.0 letters, around 7.0 letters after 2 years, in both groups, but with significantly more injections needed in the bevacizumab group: 8.9 in the first year and 18.2 in two years compared with 8.0 in the first year, and 16.0 in the second year, for ranibizumab. (Berg et al. 2015; Berg et al. 2016).
One may conclude that analysis of the data collected in routine clinical practice through the FRB! registry found largely comparable outcomes with regard to mean visual acuity gain at 12, 24 and 36 months of follow‐up in the Netherlands and the rest of FRB! The number of injections was higher in the present study not only in the Netherlands, but also in the rest of FRB, for all follow‐up periods. A previous study using the FRB! registry found that results from routine patient care were similar to those of a pivotal drug registration study if the observational cohort was filtered in the same way as the clinical trial (Gillies et al.,2014a).
The gain in mean visual acuity was faster in the first year in the other countries than in the Netherlands (see Fig. 1). This observation mirrors the results of the DRCR network study of the treatment of diabetic macular oedema with different anti‐VEGF drugs, where bevacizumab treated eyes responded with a slower rise to the maximum visual acuity gain than ranibizumab‐ or aflibercept‐treated eyes (Wells et al. 2016).
The small number of centres in the Netherlands participating in this study is a small sample of all the centres in the Netherlands treating nAMD, and therefore, it may not fully represent the entire country. It is possible that the participating centres were highly motivated to reach the best possible treatment results and therefore less tolerant of persistently active lesions, which could lead to a higher number of injections and visits compared to the general standard ophthalmological practice in the Netherlands. Several studies have demonstrated that the best results are achieved in patients treated with the highest number of injections irrespective of the drug used (Holz et al. 2015). The differences found in the present study could be simply due to the high number of injections and not caused by the difference in the drug, which was mainly bevacizumab in the Netherlands, and ranibizumab or aflibercept in the rest of FRB!.
General cardiovascular or other complications were not recorded in the registry. The number of ocular adverse events such as endophthalmitis was very low and seemed unrelated to the total number of injections. However, there were more incidences of noninfectious endophthalmitis in the Netherlands cohort compared with the rest of FRB! which is likely due to the use of bevacizumab as was reported in a previous FRB! analysis (Daien et al. 2018).
Many eyes in the Netherlands were switched to a second drug. Consistent with other studies, most switches were made to aflibercept (Barthelmes et al. 2016; Barthelmes et al. 2018). Switching was more frequent in the Netherlands than in the other countries. The indication to switch was based on the impression of the treating ophthalmologist that the first drug was not effective. This could be because visual acuity did not improve, the OCT failed to show an effect on retinal fluid, or the interval between injections could not be extended beyond 4 weeks. The precise reasons are not documented, but the higher proportion switching in the Netherlands, where they started with bevacizumab, suggests bevacizumab may be less effective than aflibercept or ranibizumab, the starting drug in the other countries. Practitioners’ perception of the relative efficacy of the different drugs may also have contributed to the higher switching rate in the Netherlands.
It would be interesting to include results of clinical practices in other countries having national guidelines for bevacizumab as a first‐line agent to substantiate the results of the present study.
We have analysed this in more detail for all patients who switched treatment in the Netherlands (see Table 5). The VA at the switch was considerably higher than at baseline. The final VA after switch was slightly higher than at the switch, so it appears there was still room for improvement even after a relatively long period of injections before the switch.
Table 5.
Treatment results for patients who switched therapy in the Netherlands
| 12 Months | 24 Months | 36 Months | ||||
|---|---|---|---|---|---|---|
| Switched to Ranibizumab | Switched to Aflibercept | Switched to Ranibizumab | Switched to Aflibercept | Switched to Ranibizumab | Switched to Aflibercept | |
| Eyes | 36 | 149 | 33 | 136 | 22 | 78 |
| Baseline VA, mean letters (SD) | 62.1 (14.4) | 59.7 (17.6) | 63.8 (14.4) | 57.3 (17.8) | 61.9 (17.1) | 57.5 (19.1) |
| VA at switch | 67.4 (15.4) | 63.9 (16.4) | 65.8 (17.1) | 63.6 (15.0) | 64.7 (18.5) | 64.8 (15.0) |
| Final VA, mean letters (SD) | 67.6 (18.7) | 64.6 (16.9) | 63.4 (21.2) | 65.3 (16.1) | 65.1 (20.4) | 66.2 (13.6) |
| Days til switch, median (Q1, Q3) | 189.5 (158.5, 276.5) | 202 (147, 287) | 283 (189, 382) | 290 (175, 444.5) | 381.5 (277.75, 728) | 308 (206, 588.5) |
| Injections til switch, median (Q1, Q3) | 7 (6.25, 10) | 7 (6, 10) | 10 (7, 12) | 10 (7, 13) | 11 (6, 16) | 13 (7.75, 19.75) |
| Injections per year Pre switch, median (Q1, Q3) | 11.6 (11.2, 12.0) | 12.4 (11.3, 13) | 11.5 (10.3, 11.9) | 12.0 (10.0, 12.7) | 11.0 (9.4, 11.6) | 11.7 (9.4, 12.7) |
| Injections per year Post‐switch, median (Q1, Q3) | 10.6 (9.0, 11.5) | 12.0 (10.4, 13.0) | 10.2 (9.2, 11.4) | 10.9 (8.0, 12.3) | 10.0 (8.0, 10.9) | 10.7 (8.7, 11.9) |
| Total injections, median (Q1, Q3) | 12 (12, 12) | 13 (11, 14) | 22 (20, 23) | 22 (18, 25) | 31 (25, 35) | 30 (29, 33) |
We have also analysed the median number of injections per year before and after the switch. The injection rates fell after the switch, but only by ˜1 injection per year which seems only a modest reduction and may have occurred over time in any case.
Interestingly patients who did not switch therapy received far fewer (median [Q1, Q3]) injections than the switchers (9 [8, 11] versus 13 [11, 13] at 12 months; 15 [11, 19] versus 22 [18, 24] at 24 months; and 20 [14.5, 24] versus 31 [26, 35] at 36 months) so it seems that the most important motivation for switching was to reduce the injection load for patients in need of a high frequency of injections.
General cardiovascular or other complications were not recorded in the register, but the number of ocular adverse events, like endophthalmitis, was very low, and seemed not related to the total number of injections.
Endorsed by ICHOM, FRB! provides many clinically helpful features such as a highly informative overview in a single graph of each individual patients treatment journey for as long as they are treated, which may be over a decade.(Invernizzi et al. 2019; Gillies et al., 2020a,2020b) Features such as the course of visual acuity over time, the time of injections provided, the drug(s) used and the grading of lesion activity at each visit were very helpful for the decision‐making process for retreatment and the choice of drug. Some centres changed their treatment regimens after seeing the outcomes from the other participating centres in order to improve their own.
Reducing the burden of data entry is essential to integrating a registry into the clinical workflow. The FRB! data set is the minimum, patient‐centred dataset that was identified by ICHOM for macular degeneration (Rodrigues et al. 2016). Still, many participating ophthalmologists preferred to enter the data after the outpatient clinic rather than when they saw the patient as the system is intended. Some centres stopped participating or only entered a limited the number of patients because of the extra time requirements. This is further evidence that the Dutch FRB! practitioners may not completely represent the Netherlands.
Single point data entry, in which all data already registered in the electronic patient file (EPF) is shared with the FRB! server, will make it easier to participate in registries although the minimum dataset will still have to be entered. The EPF needs to be adjusted to include all the FRB! required data in the correct format. Technically this can be realized; however, privacy legislation in Europe complicates this process especially because the central server is in Australia, outside the European Union. One prerequisite is an agreement between the participating centre and the FRB! organization in line with privacy and data protection legislation, and another is an informed consent to be signed by all patients describing the details and the go of the registry.
Conclusion
The outcomes of starting anti‐VEGF treatment for nAMD with bevacizumab, as is mandatory in the Netherlands, appear to be at least as good as those with either ranibizumab or aflibercept, but at a cost of more frequent injections, and visits, and more frequent switching treatment to a second drug. This study showed the power of using data from routine clinical practice acquired through a quality registry to make useful comparisons and benchmark countries and individual practices.
The authors acknowledge the project management support of Nga‐Chi Lau, PhD (employee of Bayer B.V.) in the start‐up phase of the FRB! Registry in the Netherlands and acknowledge the financial support of Bayer B.V. for the Dutch FRB! Platform.
References
- Barthelmes D, Campain A, Nguyen P et al. (2016): Effects of switching from ranibizumab to aflibercept in eyes with exudative age‐related macular degeneration. Br J Ophthalmol 100: 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelmes D, Nguyen V, Walton R, Gillies MC & Daien V (2018): A pharmacoepidemiologic study of ranibizumab and aflibercept use 2013–2016. The Fight Retinal Blindness! Project. Graefes Arch Clin Exp Ophthalmol 256: 1839–1846. [DOI] [PubMed] [Google Scholar]
- Berg K, Hadzalic E, Gjertsen I et al. (2016): Ranibizumab or bevacizumab for neovascular age‐related macular degeneration according to the lucentis compared to avastin study treat‐and‐extend protocol: two‐year results. Ophthalmology 123: 51–59. [DOI] [PubMed] [Google Scholar]
- Berg K, Pedersen TR, Sandvik L & Bragadóttir R (2015): Comparison of ranibizumab and bevacizumab for neovascular age‐related macular degeneration according to LUCAS treat‐and‐extend protocol. Ophthalmology 122: 146–152. [DOI] [PubMed] [Google Scholar]
- Bhandari S, Nguyen V, Arnold J, Young S, Banerjee G, Gillies M & Barthelmes D (2020): Treatment outcomes of ranibizumab versus aflibercept for neovascular age‐related macular degeneration: data from the fight retinal blindness! Registry. Ophthalmology 127: 369–376. [DOI] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA & Reeves BC (2013): Alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation: 2‐year findings of the IVAN randomised controlled trial. Lancet 382: 1258–1267. [DOI] [PubMed] [Google Scholar]
- Cleveland WSGE & Shyu WM (2017): Statistical models in S. Abingdon‐on‐Thames, England, UK: Routledge. [Google Scholar]
- Daien V, Nguyen V, Essex RW, Morlet N, Barthelmes D & Gillies MC (2018): Incidence and outcomes of infectious and noninfectious endophthalmitis after intravitreal injections for age‐related macular degeneration. Ophthalmology 125: 66–74. [DOI] [PubMed] [Google Scholar]
- Gerding H (2016): Long‐term results of intravitreal anti‐VEGF injections in wet AMD: A meta‐analysis. Klin Monbl Augenheilkd 233: 471–474. [DOI] [PubMed] [Google Scholar]
- Gillies M, Arnold J, Bhandari S, Essex RW, Young S, Squirrell D, Nguyen V & Barthelmes D (2020): Ten‐year treatment outcomes of neovascular age‐related macular degeneration from two regions. Am J Ophthalmol 210: 116–124. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Nguyen CL, Nguyen V, Daien V, Cohn A, Banerjee G & Arnold J (2020): Reply. Ophthalmology 127: e21–e22. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Walton RJ, Arnold JJ et al. (2014): Comparison of outcomes from a phase 3 study of age‐related macular degeneration with a matched, observational cohort. Ophthalmology 121: 676–681. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Walton R, Liong J et al. (2014): Efficient capture of high‐quality data on outcomes of treatment for macular diseases: the fight retinal blindness! Project. Retina 34: 188–195. [DOI] [PubMed] [Google Scholar]
- Holz FG, Tadayoni R, Beatty S et al. (2015): Multi‐country real‐life experience of anti‐vascular endothelial growth factor therapy for wet age‐related macular degeneration. Br J Ophthalmol 99: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi A, Nguyen V, Teo K, Barthelmes D, Fung A, Vincent A & Gillies M (2019): Five‐year real‐world outcomes of occult and classic choroidal neovascularization: data from the fight retinal blindness! Project. Am J Ophthalmol 204: 105–112. [DOI] [PubMed] [Google Scholar]
- Kim LN, Mehta H, Barthelmes D, Nguyen V & Gillies MC (2016): Metaanalysis of real‐world outcomes of intravitreal ranibizumab for the treatment of neovascular age‐related macular degeneration. Retina 36: 1418–1431. [DOI] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Fine SL et al. (2012): Ranibizumab and bevacizumab for treatment of neovascular age‐related macular degeneration: two‐year results. Ophthalmology 119: 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V, Leung KFC, Nguyen CL et al. (2020): Assessing the accuracy of a large observational registry of neovascular age‐related macular degeneration. Retina 40: 866–872. [DOI] [PubMed] [Google Scholar]
- Ohji M, Lanzetta P, Korobelnik JF, Wojciechowski P, Taieb V, Deschaseaux C, Janer D & Tuckmantel C (2020): Efficacy and treatment burden of intravitreal aflibercept versus intravitreal ranibizumab treat‐and‐extend regimens at 2 years: network meta‐analysis incorporating individual patient data meta‐regression and matching‐adjusted indirect comparison. Adv Ther 37: 2184–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues IA, Sprinkhuizen SM, Barthelmes D et al. (2016): Defining a minimum set of standardized patient‐centered outcome measures for macular degeneration. Am J Ophthalmol 168: 1–12. [DOI] [PubMed] [Google Scholar]
- Schauwvlieghe AM, Dijkman G, Hooymans JM et al. (2016): Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age‐related macular degeneration. The BRAMD study. PLoS One 11: e0153052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Society DO (2017): Guideline Age‐related Macular Degeneration.
- Wells JA, Glassman AR, Ayala AR et al. (2016): Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two‐year results from a comparative effectiveness randomized clinical trial. Ophthalmology 123: 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]


