Abstract
The process of wound healing includes four phases: Hemostasis, inflammation, proliferation, and remodeling. Many wound dressings and technologies have been developed to enhance the body's ability to close wounds and restore the function of damaged tissues. Several advancements in wound healing technology have resulted from innovative experiments by individual scientists or physicians working independently. The interplay between the medical and scientific research fields is vital to translating new discoveries in the lab to treatments at the bedside. Tracing the history of wound dressing development reveals that there is an opportunity for deeper collaboration between multiple disciplines to accelerate the advancement of novel wound healing technologies. In this review, we explore the different types of wound dressings and biomaterials used to treat wounds, and we investigate the role of multidisciplinary collaboration in the development of various wound management technologies to illustrate the benefit of direct collaboration between physicians and scientists.
Keywords: biomedical engineering, interdisciplinary teams, negative pressure wound therapy, wound dressings, wound healing
1. INTRODUCTION
A glossary of terms found in the introduction is provided in Table 1.
TABLE 1.
Glossary of terms found in introduction
| Term | Description |
|---|---|
| Basal lamina | Part of the basement membrane that is in contact with the bottom‐most surface of the epithelial and endothelial cells |
| Epidermal growth factor | Protein that stimulates growth and proliferation of epithelial cells and fibroblasts |
| Factor VII | Initiating protein of coagulation cascade |
| Factor XIII | Enzyme that stabilizes fibrin |
| Fibrinogen | The inactive precursor of fibrin |
| Fibroblasts | Cells that produce proteins that make up the extracellular matrix such as collagen |
| Fibronectin | Glycoprotein involved in cell adhesion |
| Glycoprotein Ib/IX/V | Platelet adhesion receptor involved in hemostasis |
| IL‐1β |
Interleukin‐1beta Proinflammatory cytokine that can mediate fever |
| IL‐6 |
Interleukin‐6 Proinflammatory cytokine that stimulates acute phase protein production by the liver and induces inflammation |
| IL‐8 |
Interleukin‐8 Chemoattractant for granulocytes, especially neutrophils |
| Interferon‐γ | Cytokine that activates macrophages, induces helper T cell differentiation (TH1), and induces IgG antibody production |
| Keratinocyte growth factor 2 | Protein that stimulates growth of epidermal keratinocytes |
| Keratinocytes | Cells that make up a majority of the epidermis and produce keratin and the critical stratum lucidum layer |
| Laminin 5 β‐3 | Protein involved in adhesion of keratinocytes of the epidermis to the dermal skin layer |
| Leukocytes | White blood cells |
| Macrophages | A type of larger monocyte of the innate immune response that is involved in phagocytosis |
| Monocyte chemoattractant protein‐1 | Proinflammatory protein that attract monocytes |
| Myofibroblasts | Fibroblasts capable of contraction |
| Natural killer cell | A type of white blood cell of the innate immune response. This type of lymphocyte plays a major role in the direct early host rejection of both tumors and virally infected cells. |
| Neutrophil | Polymorphonuclear granulocytes abundant in acute phase of inflammation. |
| Phagocytize | Engulfment or “eating” of cells and foreign material |
| Phosphatidylserine | Phospholipid located in cell membrane |
| Platelet derived growth factor | Glycoprotein produced by platelets and activated macrophages that acts as a chemoattractant for neutrophils and can induce cell division in mesenchymal cell types |
| T cells | A type of white blood cell seen in the adaptive immunity responsible for cell mediated immunity. T cells, along with B cells, are the two primary types of lymphocytes that determine the specificity of immune response to antigens. |
| TGF‐β |
Transforming growth factor‐beta Cytokine that stimulates proliferation of epithelial cells and inhibits inflammation |
| Thrombin | Protease that cleaves inactive fibrinogen to fibrin in coagulation cascade |
| TNF‐α |
Tumor necrosis factor‐alpha Proinflammatory cytokine |
| Transforming growth factor beta | Inhibits function of immune cells such as T cells, B cells, and monocytes/macrophages173 |
| Type III collagen | Collagen predominantly found in skin, blood vessels, and granulation tissue |
| VEGF |
Vascular endothelial growth factor A signaling protein that stimulates vasculogenesis and angiogenesis |
| VonWillebrand Factor | Glycoprotein produced by endothelial cells that stimulates platelet adhesion and aggregation |
Abbreviations: TGF, transforming growth factor; TNF‐α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
1.1. Overview of wound healing
Wound healing involves four phases: Hemostasis, inflammation, proliferation, and remodeling. 1 Wound healing begins with transient vasoconstriction of injured vessels. 2 In the initial phase, damage to the skin exposes the subendothelial collagen and tissue factor, leading to platelet aggregation. 3 In this process, Von Willebrand factor (vWF) binds to both the subendothelial collagen and the platelet receptor glycoprotein Ib/IX/V. 4 This adhesion, as well as thrombin generated by tissue factor, results in platelet activation, degranulation, and conformational change. 5 Conformational change in glycoprotein IIB/IIIA allows the binding of fibrinogen, a crucial step in platelet aggregation and formation of the platelet plug. Tissue factor and phosphatidylserine, located on the surface of platelets and endothelial cells, form a complex with circulating activated Factor VII, leading to activation of a coagulation cascade which results in the cleavage of fibrinogen to fibrin. 5 Fibrin activates Factor XIII, which acts to crosslink the fibrin monomers. The final result is a strong blood clot. 6
The hemostasis process of wound healing leads to the inflammatory process in multiple ways. Platelets can activate the complement cascade, leading to inflammation and the formation of the membrane attack complex (MAC). 7 Activated complement proteins C3a and C5a cause the degranulation of mast cells, which releases factors, such as histamine, heparin, and proteases. 8 Mast cells express the enzymes cyclooxygenase (COX) 1 and 2, which generate prostaglandins from the arachidonic acid precursor. 8 The histamine and prostaglandins that are released as a result of mast cell activation lead to increased vascular permeability 9 causing the sign localized swelling, 10 which can be visualized. In addition to increasing vascular permeability, prostaglandins sensitize pain receptors, 9 act as a neutrophilic chemoattractant, and cause vasodilation. 11 Prostaglandin induced receptor sensitization leads to pain, 11 while vasodilation leads to redness and heat 10 ; all of which can be appreciated by the patient.
Platelet aggregation leads to the release of neutrophil chemotactic factors such as platelet derived growth factor (PDGF) and transforming growth factor (TGF) beta. 12 Neutrophils migrate to the site of injury and become trapped in the platelet plug. 12 There, the neutrophils phagocytize dead tissue and release reactive oxygen species that create an unfavorable environment for bacteria. 12 An excellent review written by Ellis et al 13 details the inflammatory phase of wound healing. During the inflammatory phase, the neutrophils express cytokines that recruit additional leukocytes (neutrophils, T cells, and macrophages) to the site of injury, such as tumor necrosis factor alpha (TNF‐α), interleukin (IL) 1β, IL‐6, IL‐8, monocyte chemoattractant protein −1 (MCP‐1). 13 A study by Theilgaard‐Mönch et al 14 found that neutrophils upregulate factors that promote wound healing by stimulating angiogenesis (e.g., vascular endothelial growth factor [VEGF] and MCP‐1), stimulating the proliferation of fibroblasts and keratinocytes (e.g., IL‐8, IL‐1β, and MCP‐1), and promoting the adhesion of keratinocytes to the dermal layer (laminin 5β3). Recruited macrophages are activated into the proinflammatory M1 phenotype by damage associated molecular patterns (DAMPs), pathogen associated molecular patterns (PAMPs), and interferon‐gamma (IFN‐γ). 13 The proinflammatory macrophages phagocytize microbes and cellular debris as well as secrete proinflammatory cytokines and chemokines that increase the number of natural killer cells, helper T cells, and macrophages at the site of injury. 13 Some macrophages will differentiate into anti‐inflammatory and pro‐regenerative M2 macrophages. 15 The M2 subset of macrophages assist in wound healing by stimulating fibroblasts, stimulating angiogenesis (e.g., PDGF and VEGF), producing collagen precursors, producing factors that attenuate inflammation (e.g., IL‐10 and TGF‐β), and producing of metalloproteinases (MMPs). 15
The proliferative phase consists of the epithelization, revascularization, and formation of granulation tissue. 16 , 17 Epithelial cells located at the wound edges, upon stimulation by the epidermal growth factor (EGF) and TGF‐α produced by the platelets, begin to proliferate. Stimulated fibroblasts secrete factors, such as keratinocyte growth factor (KGF)‐2, which induce keratinocyte migration. 16 Keratinocytes migrate along the fibrin blood clot by lamellipodial crawling, until they begin to contact each other. 17 Growth factors bind to endothelial cells and set off a signaling cascade that results in secretion of proteolytic enzymes that break down the basal lamina, allowing the endothelial cells to proliferate and migrate into the wound to form blood vessels. 17 Fibroblasts initially synthesize a provisional matrix consisting of collagen, fibronectin, and other substances. In the final step of the proliferation phase, granulation tissue replaces the provisional matrix. 17 Granulation tissue mainly consists of fibroblasts, new blood vessels, and type III collagen. 18 Some fibroblasts will differentiate into myofibroblasts, which contract to bring the wound edges closer together. 18
The remodeling phase (also referred to as the maturation phase) is the longest phase of wound healing and is dependent on a delicate balance between synthesis and degradation. 18 The type III collagen is lysed by collagenases and subsequently denatured and digested by various proteases. 19 The type III collagen is replaced by type I collagen, which orients itself in an organized manner that gives it maximal strength against stress forces. Fibroblasts begin to decrease in number, resulting in a relatively acellular scar. 20 Reduced biological activity leads to apoptosis of vascular tissue, 19 which can be visualized clinically as a reduction in redness. 19 , 20
1.2. Intro to wound dressings
Throughout history, many innovations have been made in order to enhance wound healing. As far back as about 2000 BC, there are accounts of Sumerians utilizing poultice‐like materials to cover wounds. In 1550 BC, an Egyptian medical papyrus, currently known as Ebers Papyrus, mentions the use of honey, lint, and grease in wound care, which respectively have antimicrobial, absorbent, and barrier properties that are currently considered essential in wound therapy. 21 Much later, a Greek physician known as Galen of Pergamum, emphasized the importance of keeping the wound moist. 21 Prior to the late 1800s, dressings were prepared from unsterilized materials that could be reused, such as old linens, rags, and rope. 22 In the late 1800s, Gamgee of Birmingham had the idea to combine absorbent cotton with compressing gauze in an aseptic manner for use in wound care. 22 The concept of keeping the wound moist was not scientifically proven until 1962 when George D. Winter published research that showed epithelization of wounds occurs more rapidly in a moist environment, as opposed to wounds that are exposed to air. 21 , 23
According to Weir and C. Tod Brindle, two nurse clinicians, in their chapter on wound dressings, 24 the ideal dressing should provide a moist environment, remove excess exudate, be able to provide antimicrobial properties if needed, facilitate autolytic debridement, prevent contamination by bacteria, be non‐allergenic, minimize pain, be compatible with support needs, be easily applied and removed, thermally insulate the wound, and be cost effective. If, according to our hypothesis, we also look at the recommendations for an ideal dressing by a research scientist, in this case G.D. Winter, father of moist wound healing, 25 we would add to this list the qualities of “prevents dehydration and scab formation, is permeable to oxygen, is sterilizable–supplies mechanical protection to the wound–is non‐toxic–conforms to anatomical contours, resists tearing, resists soiling, is not inflammable, has constant properties in a range of temperatures and humidities encountered in use, has long shelf life, has small bulk, is compatible with medicaments…” This approach exemplifies the benefits of combining the work of clinician and scientist, even if after the fact rather than contemporaneously. The basic categories for the wet‐to‐dry wound dressings currently being used include contact layer dressings, film dressings, hydrogels, hydrocolloids, foam dressings, alginates, and antimicrobial dressings. 24 , 26 These dressings will be further elaborated in the subsequent section. This review article examines wound dressings and the biomaterials used for them, how the wound dressings are used, and the makeup of the research teams involved in creating the dressings and how that may play a role in the translation into clinical practice.
2. TYPES OF WOUND DRESSING
There are a variety of different types of wound dressings available. Each wound dressing has unique properties that can be exploited to fit the needs of a patient. Furthermore, wound dressings can be combined to enhance wound healing. The major types of occlusive wound dressings are described below. Table 2 summarizes the advantages and disadvantages of the following wound dressings.
TABLE 2.
Summary of advantages and disadvantages of several wound dressings
| Advantages | Disadvantages | |
|---|---|---|
| Contact layer |
|
|
| Semipermeable film |
|
|
| Hydrocolloid |
|
|
| Hydrogel |
|
|
| Foam |
|
|
| NPWT |
|
|
| Antimicrobial dressings |
|
Abbreviation: NPWT, negative pressure wound therapy.
2.1. Contact layer dressings
Contact layer wound dressings are single layer dressings that are in direct contact with the wound surface. Contact layer wound dressings typically have low adherence with the wound bed thus preventing trauma to the wound bed upon removal. 24 They function to allow exudate to pass through to a secondary wound dressing 24 , 26 and can be used in conjunction with other wound therapies. 24 Some contact layer dressings, such as gauze impregnated with paraffin, may trap water vapor and lead to maceration, a condition in which exposure to moisture causes the surrounding skin to breakdown. 27 , 28 If contact dressings such as gauze impregnated with paraffin and tulle dry out, they may adhere to the wound bed, leading to trauma and pain upon removal of the dressing. 27 , 29 Because of these problems, gauze traditionally has attempted to control these factors by the closeness of its weave and the addition of other moisturizing agents. Gauze can be described as having a fine or coarse cotton mesh, depending upon the thread count per inch. 30 Gauze with a thread count of 41–47, referred to as “fine mesh gauze,” 31 has been empirically determined to prevent granulation ingrowth through the contact layer, thus enhancing epithelial growth beneath it. When coated with Vaseline™ (Vaseline™ Petrolatum Gauze), or petrolatum, it allows moisture vapor to escape preventing maceration but retains enough moisture to prevent drying. When coated with Bismuth (Xeroform Gauze™) it has the same moisture properties but adds some antimicrobial effect. These two dressings because of their petrolatum base, adhere less to the open wound than other contact layers. Even though they never received the label, these dressings appear to represent the first example of “composite dressings” or, in G.D. Winter's terms, dressings composed of more than one component each with different individual properties. In addition, “Scarlet Red” (a blend of 5% o‐tollazo‐o‐tolyl‐azo‐B‐naphthol, lanolin, white petrolatum and olive oil 32 ) added to fine mesh gauze forms a composite contact layer dressing that deliberately seeks to escarify or scab the wound surface. Finally, other materials besides gauze are used as contact layers. Historically the most important perhaps is rayon (Owen's rayon 33 ). This extremely tight weave allows no cellular penetration, but wound exudate quickly incorporates the layer and it becomes part of the more quickly forming, thinner scab when this dressing is used. Contact layer dressings are permeable to bacteria. 27 Figure 1 illustrates the usage of a contact layer dressing on a wound. Interprofessional cooperation on the front end and review afterward would help problems like the fact that the above information on contact layer dressings does not appear in any one published description.
FIGURE 1.
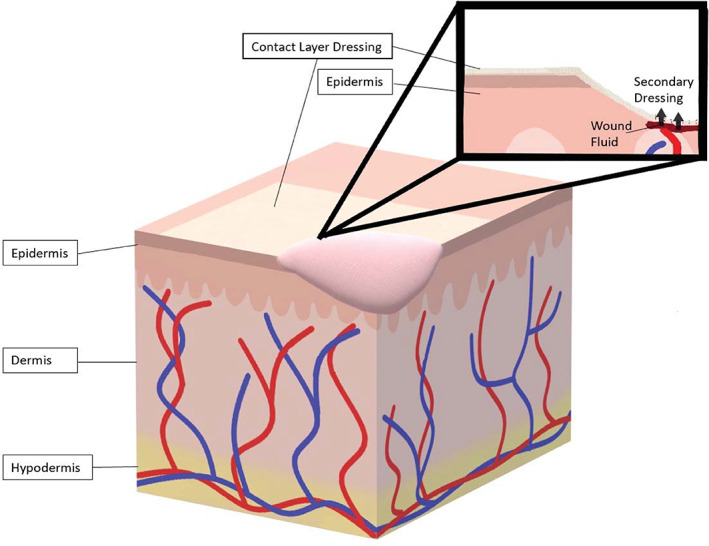
Contact layers. Contact layer dressings provide a cover over of the wound that allows wound fluid such as blood and exudate to flow through to a secondary dressing
2.2. Semipermeable films
Semipermeable films (Figure 2) are transparent films that are permeable to gases and impermeable to liquids and bacteria. 27 The transparency of the films permits visualization of the wound, without requiring removal of the dressing. 27 Semipermeable films allow enough oxygen to penetrate and affect growth of anaerobic bacteria, but not enough to support the underlying tissue growth. 24 The waterproof nature of the films trap the bodily fluids in the wound, supporting autolytic debridement. 24 Though semipermeable films support autolytic debridement, the impermeability to liquids can lead to a buildup of exudate which makes the surrounding wound tissue susceptible to maceration. The amount of water vapor escape through the film is influenced by the material of the film and the thickness of the material. It is measured as “mean vapor transmission rate” or MVTR. For example, just adding adhesive to the plastic film decreases the MVTR. A low MVTR can lead to the accumulation of exudate. So, this is an important parameter to know and understand in the use of films.
FIGURE 2.
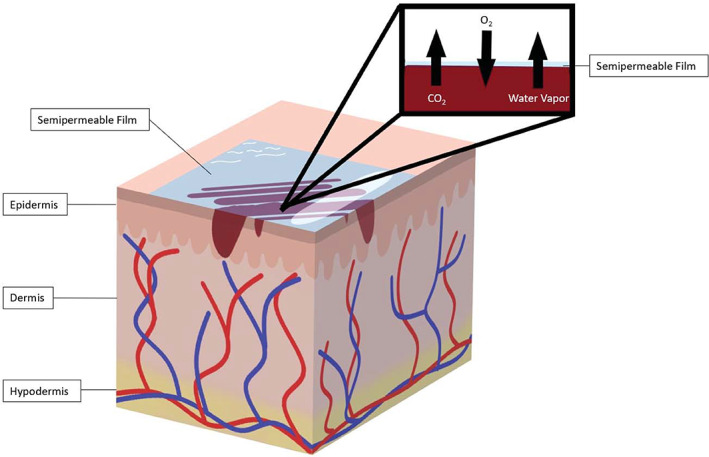
Semipermeable films. Semipermeable films are transparent, fluid impermeable wound dressings that allow the exchange of O2, CO2, and water vapor between the wound and the environment
2.3. Hydrocolloids
Hydrocolloid wound dressings are usually composed of two layers: An outer semi‐occlusive layer and a hydrocolloid layer. 27 , 34 The outer occlusive layer is usually a foam or film. The hydrocolloid layer consists of a crosslinked polymer matrix that, in the presence of exudate, absorbs fluid to form a gel. 27 This reaction results in a moist environment for the wound bed and stimulates autolytic debridement. 27 Evidence suggests that hydrocolloid wound dressings are more effective for complete wound healing of chronic wounds than saline or paraffin gauze. 35 Hydrocolloid dressings are usually opaque, which complicates wound inspection. 36 Upon contact with wound exudate, hydrocolloids form a thick, yellow malodorous gel that can be mistaken for infection upon removal of the dressing. 36 Though the gel both looks and smells unpleasant, it provides a cushioning effect and prevents the dressing from sticking to the wound, facilitating painless removal. 37 Patients should be educated that this is a normal effect believed to be due to the breakdown products of gelatin. 38 Clinicians should use other parameters such as warmth and erythema to assess for wound infection when using hydrocolloid wound dressings. 38 See Figure 3 for an illustration of a hydrocolloid dressing.
FIGURE 3.
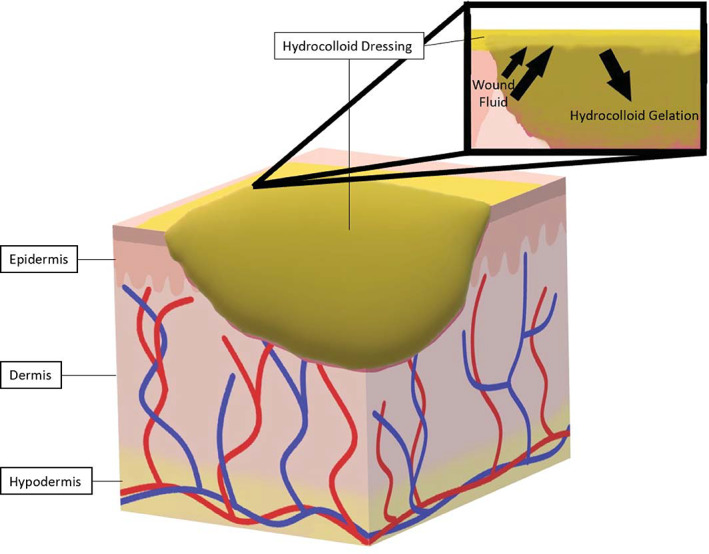
Hydrocolloids. Hydrocolloid dressings are usually opaque wound dressings that provide moisture to the wound by gelling on contact with wound fluid such as blood and exudate
2.4. Hydrogels
Hydrogel wound dressings consist of insoluble polymers in matrix with up to a 96% water content. 36 Hydrogels are available in a multitude of physical forms: amorphous (Figure 4), sheets, and impregnated within gauze, 36 nanoparticles, films, and coatings. 39 Hydrogel properties can be manipulated by a developer's choice of polymeric materials and the method of crosslinking. 40 They are also being explored as drug delivery systems. 39 For example, Yan et al 41 compared the effects treating deep partial thickness burns with a hydrogel containing recombinant granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) to a hydrogel without additives and found that the hydrogel containing GM‐CSF had a faster healing time than hydrogel alone. Researchers at Xi'an Jiaotong University have actively been engineering hydrogels capable of self‐healing, hemostasis, antibacterial, and antioxidant capabilities. 42 , 43 , 44 , 45
FIGURE 4.
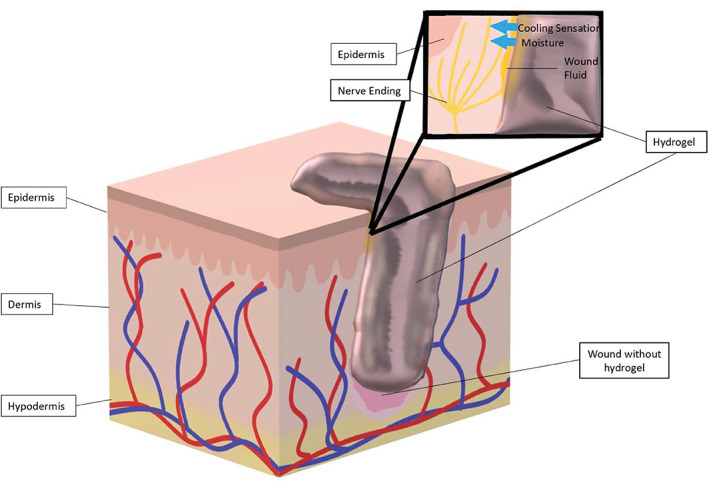
Hydrogels. A transparent amorphous hydrogel filling a cavitary wound is illustrated above. Hydrogels provide moisture to a wound and deliver soothing relief with via a cooling sensation
Hydrogels maintain a moist environment in the wound bed and even provide a cooling effect when applied, which can act to soothe and reduce inflammation. 28 , 36 Hydrogels tend to have a low absorptive capacity due to its inherent high water content. 28 Low absorptive capacity can facilitate exudate accumulation, which can lead to wound maceration and bacterial proliferation. 34 They have low adherence to the wound bed, which necessitates use of a secondary dressing such as film or foam. 36 Hydrogels have small pore sizes which can impede cell migration and the diffusion of proteins, waste products, nutrients, and oxygen. 38 Cryogels are able to overcome the limitations imposed by the nanoporous hydrogel.
Cryogels are a subtype of hydrogel that are formed at sub‐zero temperatures, resulting in a macroporous structure. 38 Cryogels have been used in various applications, such as tissue engineering, cosmetics, cell transplantation, and immunotherapy. 38 Cryogels are able to be compressed at over 90% strain and regain their original configuration. 46 The macroporous nature and injectability of the cryogels make them excellent candidates for hemostatic application. 47 , 48 , 49
2.5. Foam
Foam wound dressings (Figure 5) are absorbent dressings that come in sheets and cavity filling chips. 50 Foam's ability to absorb large amounts of exudate is dependent upon the composition and vapor transmission rate of the foam. 51 Foam dressings provide protection from trauma and thermal insulation to the wound. 26 Reticulated foam dressings can be combined with negative pressure wound therapy (NPWT) to remove drainage and exudate and further debride the wound. 50 These open‐cell foam dressings are generally of a material that is hydrophobic so without the vacuum associated with NPWT they will not absorb or remove any fluid. Also, because they are reticulated and open‐celled, tissue ingrowth is rapid, and this prevents the migration of epithelium beneath the foam so this foam cannot be used on intact skin and cannot be used for epithelialization. Many available foam dressings are of hydrophilic material or configuration. They absorb fluid without the need for vacuum drainage. These foam materials can have the contact surface compressed so that the pore size, just like fine mesh gauze, does not admit any tissue ingrowth and these particular foams promote epithelial migration. In fact some of the fastest rates of epithelial migration reported by G. D. Winter were with these materials as dressings (e.g., Lyofoam). 52 , 53 It is recommended that highly absorbent foam dressings be avoided in patients with little to no exudate due to the risk of drying. 28
FIGURE 5.
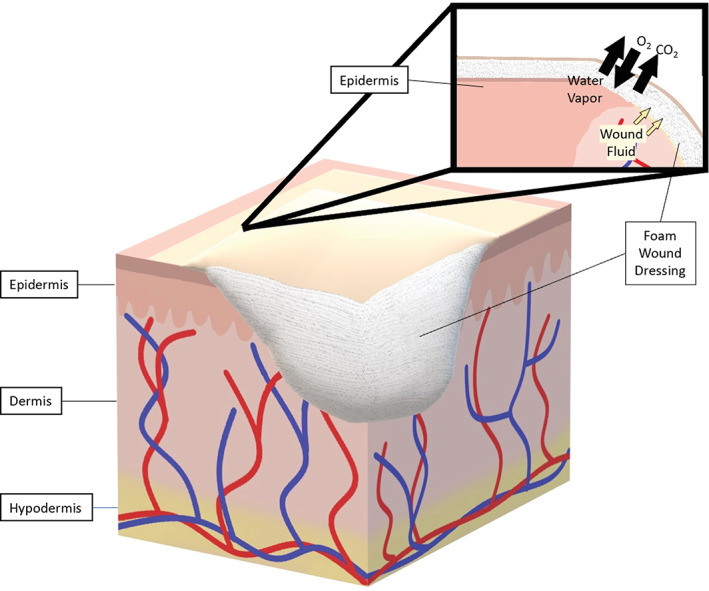
Foams. Foam wound dressings absorb blood and exudate, while allowing for the exchange of water vapor and gas between the environment and the wound bed
2.6. Antimicrobial dressings
Antimicrobial agents can be added to wound dressings to prevent wound contamination. To prevent microbial wound invasion, wound dressings have incorporated substances, such as antibiotics, nanoparticles, and natural products. 54 Simões et al 54 excellently summarized recent investigations of antimicrobial wound dressings in a review. Table 3 provides a list of these antimicrobial agents and respective mechanisms of action that have been used in wound dressings.
TABLE 3.
Antimicrobials found in wound dressings
| Antimicrobial | Mechanism | Examples of pathogen coverage |
|---|---|---|
| Antibiotics | ||
| β‐Lactams | Prevents synthesis of bacterial cell wall174 |
Streptococcus pneumoniae 175 Listeria monocytogenes 175 Escherichia choli 175 Haemophilus influenzae 175 |
| Tetracycline | Binds to 30S ribosomal subunit to prevent protein synthesis174 |
Mycoplasma pneumoniae 176 Chlamydiae trachomatis 176 Rickettsia rickettsii 176 Borrelia burgdorferi 176 |
| Aminoglycosides | Inhibition of protein synthesis174 |
Escherichia coli Klebsiella pneumoniae Yersinia pestis Staphylococcus aureus |
| Quinolones | Inhibition of DNA gyrase and topoisomerase IV174 |
Salmonella enterica 177 Shigella sonnei 177 Escherichia coli 177 Pseudomonas aeruginosa 177 |
| Sulphonamides | Competitively inhibit dihydropteroate synthetase in folic acid synthesis pathway174 |
Nocardia brasiliensis 177 Toxoplasma gondii 177 |
| Glycopeptides | Prevention of transglycosylation step in cell wall synthesis174 |
Staphylococcus aureus 178 Streptococcus pnumoniae 178 |
| Nanoparticles | ||
| Iron oxide | Disruption of DNA and enzymatic processes | |
| Titanium dioxide | Disruption of cell wall, plasma membrane, and DNA | |
| Zinc oxide | Disruption of cell wall and plasma membrane | |
| Silver | Disruption of cell wall and plasma membrane, DNA replication, transcription, and enzymatic pathways | Neisseria gonorrhoeae 179 |
| Natural products | ||
| Honey | Provides unfavorable environment for microbes, prevents microbial growth, damages cell walls, lipids, proteins, and nucleic acids | Staphylococcus aureus 180 |
| Henna | Contains quinones which form stable free radicals that irreversibly complex with amino acids to inactivate proteins181 | |
| Curcumin | Inhibition of cell division182 | |
| Aloe vera | Contains anthraquinones, which is a structural analogue of tetracycline (mechanism of action listed above)183 | |
| Thymol | Disrupts cell membranes184 | |
Essential oils:
|
Disrupts cell membranes |
2.7. Wound dressings by injury
There is currently insufficient evidence to draw conclusions about the best use of specific wound dressings in treating burns. 55 The dressings that are typically used for Stage 1 and 2 pressure ulcers are polyurethane films, hydrocolloid wafers, and foam dressings. 56 For full thickness ulcers (Stage 3 and 4 pressure ulcers) that are highly exudative, absorptive dressings, such as those consisting of calcium alginate, and gauze packing are recommended. 56 Table 4 provides a quick reference for suitable wound dressings based upon wound types.
TABLE 4.
Preferred wound types of various wound dressings26
| Wound type | Low exudate | Medium exudate | High exudate |
|---|---|---|---|
| Flat, shallow wounds |
Semipermeable Film Contact layer dressing Hydrocolloid Hydrogel |
Semipermeable film Hydrocolloid Foam26,28 Alginates |
Foam26,28 Alginates |
| Cavity |
Hydrocolloid Hydrogel |
Hydrocolloid Foam Alginates |
Foam Alginates |
| Infected wounds | Antimicrobial | Antimicrobial | Antimicrobial |
2.8. Other wound management techniques
NPWT is a system that utilizes subatmospheric pressure to enhance wound healing. NPWT devices consist of a semipermeable film covering a wound filler, most commonly polyurethane foam though gauze and polyvinyl foam can also be used, which either directly contacts the wound bed or rests on top of a low adherent contact layer. 57 , 58 The dressing is connected to a drainage tube which is also connected to a device responsible for generating the negative pressure. 58 Figure 6 demonstrates an example of a NPWT system. NPWT pulls wound edges together, removes edematous fluid, stimulates fibroblast proliferation, and, with the use of a liner or contact layer, stimulates epithelial cell proliferation. 57 Some contraindications of NPWT include malignancy, osteomyelitis, and the presence of necrotic tissue. 59
FIGURE 6.
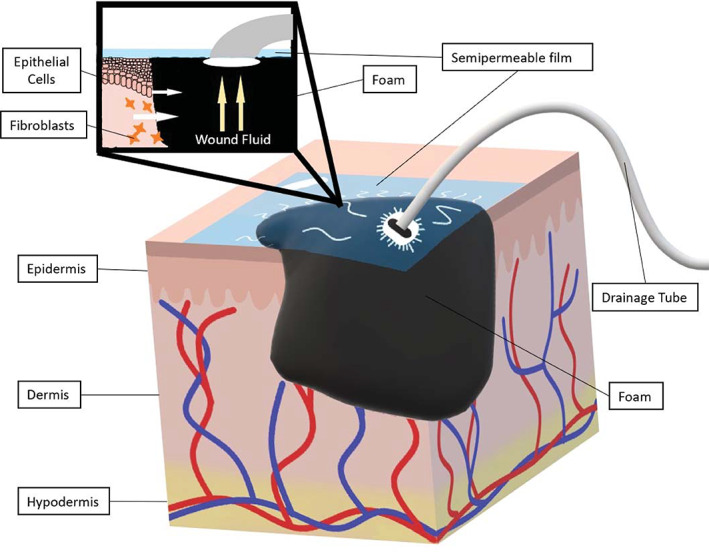
Negative pressure wound therapy. Negative pressure wound therapy involves the creation of a sub‐atmospheric environment in the wound, which enables wound edges to come together, facilitates fluid drainage, and stimulates fibroblasts and epithelial cell activity. The vacuum device is not pictured
Another wound management technique is shock wave therapy, which is hypothesized to utilize external deformations to the cytoskeleton via mechanotransduction. 60 Shock wave therapy involves the absorption of biphasic high energy acoustic waves by tissue through electrohydraulics. 60 Larking et al was able to demonstrate the effectiveness of extracorporeal shock wave therapy (ESWT) on chronic decubitus ulceration through a cross‐over study. 61 Both study groups demonstrated wound healing after the introduction of ESWT. 61 Current use of this wound management technique is limited due to a lack studies with high level evidence and a lack of optimization of shock wave parameters. 60
Hyperbaric oxygen is being investigated as a treatment modality to promote wound healing. Hyperbaric oxygen therapy (HBOT) involves patients breathing 100% oxygen while being exposed to increased atmospheric pressure. 62 One of the proposed mechanisms for this treatment modality's efficacy is through stimulation of neovascularization by increasing VEGF expression. 62 A cross‐sectional study conducted in Brazil found venous ulcers, traumatic injuries, and diabetic foot wounds as the most common indications for HBOT. 63 A systematic review including 29 studies on the effects of HBOT on skin wound healing found that 18 of those studies showed at least one positive outcome including wound closure and vascular perfusion. 64 However, the studies included varying levels of evidence; five of the studies were randomized control trials with small sample sizes, five were prospective cohort studies, eighteen were clinical studies, and three were case series. 64
Photobiomodulation (PBM) is a form of light therapy that uses nonionizing light sources to elicit photochemical and photophysical biological responses through absorption by chromophores. 65 PBM is known to decrease pain and inflammation as well as promote wound healing and tissue regeneration. 66 The exact mechanisms leading to these effects are still under investigation, though studies have shown that absorption of specific wavelength of light are absorbed by cytochrome C oxidase, initiating a cascade that results in generation of increased adenosine triphosphate and reactive oxygen species. 65 PBM also results in activation of TGF‐β1 as well as the modulation of various ions, such as calcium, proton, sodium, and potassium between the cytosol and extracellular matrix. 65
Ultrasonic debridement is a method of wound bed preparation used in the UK, Europe, and Australia. 67 With ultrasonic debridement, saline runs through a tube that is attached to a headpiece; this headpiece vibrates at ultrasound frequency, creating bubbles which subsequently implode, producing a shockwave that is transmitted from the saline to the wound bed. 67 The resulting shockwaves destroy dead and dying tissues while stimulating the more elastic membranes of healthy cells. 67 , 68 Ultrasonic debridement has been shown to promote angiogenesis, increased collagen deposition, mast cell degranulation, and increased intracellular calcium. 68
3. BIOMATERIALS
3.1. Introduction to synthetic biomaterials
The biomaterials that are used to make wound dressings can be classified as either synthetic or natural. Synthetic biomaterials usually have good mechanical properties, thermal stability, and a shape that can be manipulated. 69 Synthetic biomaterials can be further classified into metals, polymers, ceramics, and composites. 70 Polymeric and composite materials will be considered below.
3.2. Polyurethane
Polyurethane is a synthetic polymer consisting of repetitive urethane groups that is, used as a biomaterial in both semipermeable films and foam wound dressings. 71 It is synthesized through polymerization of diisocyanates and polyols. 72 Properties of the polyurethane may vary based upon the choice of diisocyanate, polyol, and chain extender used in its synthesis. 72 For example, Yoo et al 73 synthesized polyethylene glycol‐based waterborne polyurethane hydrogels with desirable water absorption percentage and water vapor transmission rates. Polyurethane is viewed as a desirable biomaterial for wound dressings due to its biocompatibility, strength, and lack of toxicity. 71
3.3. Silicone
Silicone is a biomaterial used in foam dressings and low adherent contact wound dressings, such as Adaptic™ and Mepitel®. 74 It is a polymer of repeating units of siloxane, which is composed of silicon, oxygen, and an alkane. 75 Most medical products that contain silicone utilize the organic polymer polydimethylsiloxane. 75 Silicone is nontoxic, biocompatible, and resistant to biodegradation in the short term. 70 A review by Truong et al found that silicone foam dressings were more effective than traditional methods, such as regular repositioning and skin care, at preventing pressure ulcers. 76
3.4. Carboxymethylcellulose
Carboxymethylcellulose is a derivative of cellulose that contains a carboxymethyl group bound to the hydroxyl groups found in the backbone of the cellulose molecule. 70 It is synthesized in a two‐step reaction that consists of the alkalization of cellulose, followed by carboxymethylation using chloroacetic acid. 77 Carboxymethylcellulose is a nontoxic, nonimmunogenic, and biocompatible polymer. 78 It is hydrophilic and rapidly undergoes gelation in an aqueous environment. 79 Examples of wound dressings that carboxymethylcellulose is used for include hydrocolloids, hydrogels, and hydrofibers. DuoDERM® is a hydrocolloid wound dressings offered by the company ConvaTec Inc that contains a proprietary mix of carboxymethylcellulose, gelatin, and pectin.
3.5. Poly (vinyl alcohol)
Poly (vinyl alcohol) is a synthetic polymer synthesized from vinyl acetate by controlled free radical polymerization. 80 Subsequently, the acetate group is removed from the resultant poly (vinyl acetate) via ester hydrolysis. 80 The degree of hydrolysis, tacticity, and average molecular weight of the poly (vinyl alcohol) determine its solubility, biodegradability, and mechanical strength. 81 Poly (vinyl alcohol) is biocompatible, easily prepared, odorless, and has a low permeability to oxygen and aroma. 81 Due to poly (vinyl alcohol) having poor stability in water, inadequate elasticity, and a rigid structure, 81 it is usually blended with synthetic polymers, such as polyvinylpyrrolidone, polyethylene glycol, poly (N‐isopropylacrylamide), and montmorillonite, or natural polymers, such as chitosan, alginate, starch, dextran, glucan, gelatin, and hyaluronan. An example of such a dressing would be Hydrofera Blue®, which is composed of polyurethane and poly (vinyl alcohol). 82
3.6. Poly (ethylene glycol)
Poly (ethylene glycol), also referred to as poly(ethylene oxide) when the molecule weight exceeds 20,000 is a neutral polyether used in for a wide range of biomedical and biotechnical applications. 83 Poly (ethylene glycol) is a nonimmunogenic and biocompatible hydrophilic polymer. 84 Poly (ethylene glycol) is soluble in water and many organic solvents, but becomes insoluble in water when exposed to temperatures above 100°C. 83 Though poly (ethylene glycol) is bioinert in nature, it can be modified to incorporated bioactive moieties. 85 , 86 Poly (ethylene glycol) can be functionalized by either replacing the terminal hydroxyl groups with a functional group or by reacting the poly (ethylene glycol) with a molecule that has two functional groups, one of which will be replaced with poly (ethylene glycol). 87 Poly (ethylene glycol) modifies both the solubility and size of a molecule to which it is attached. 87
3.7. Composites
Composite wound dressings consist of two or more distinct elements. A biocomposite material consists of two or more matrices. 88 Biocomposite materials have improved properties compared to the individual components. For example, Park et al 89 synthesized a composite wound dressing consisting of chitosan and silica that was shown to lead to an increase in collagen deposition compared to a chitosan sponge alone. One of the first “composite” dressings proposed as such (1981) was by a group of scientists at 3M that included G.D. Winter posthumously. 25 This was a combination of polytetrafluoroethylene fibril matrix, hydrophilic absorptive particles enmeshed in the matrix and a semi‐occlusive film on the outer surface. This has become a model for many subsequent dressing products.
3.8. Introduction to natural biomaterials
Biomaterials that are derived from animals, plants, and microbes are considered to be natural. 70 Natural biomaterials are typically biocompatible, biodegradable, and possess similar properties to the extracellular matrices found in connective tissues. 90 In addition, natural biomaterials are advantageous because of their ability to interact at the molecular level with tissues, thereby playing an active role in the wound healing process. 70
3.9. Collagen
Collagen is the most abundant protein found in mammals, comprising a third of one's total protein content. 91 The collagen polypeptide is rich in proline, hydroxyproline, and glycine that displays a characteristic triple helix conformation. 92 The enzyme lysyl oxidase crosslinks the lysine and hydroxylysine residues, providing strength and stability to mature collagen fibrils. 92 Collagen affects all phases of wound healing; collagen acts as a matrix for blood clot formation thereby promoting hemostasis, scavenges reactive oxygen and reactive nitrogen species, acts as a sacrificial substrate for collagenases, and promotes migration of fibroblasts, macrophages, and epithelial cells into the wound area. 93 Collagen wound dressings are flexible, nontoxic, biodegradable, and have low immunogenicity. 93 Collagen wound dressings are absorbent and can decrease the pH of wound fluid, decreasing the likelihood of bacterial colonization as well as the activity of proteases. 93 Collagen wound dressings are recommended for full and partial thickness wounds with little‐to‐moderate exudate. 70 Collagen wound dressings are available in various forms, such as powder (e.g., Stimulen™) that dissolves in wound fluid to form a gel, matrices (e.g., Biostep™ by Smith & Nephew), and freeze dried composites (e.g., Promogran Prisma).
3.10. Alginate
Alginate is derived from brown seaweed in the Phaeophyceae class. 94 It is composed of linear copolymers of (1,4)‐linked β‐D‐mannuronate and α‐L‐guluronate blocks. 94 Alginate forms gels in the presence of acid and divalent and trivalent cations through an ionotropic mechanism. 95 In the case of sodium alginate gelation, sodium alginate exchanges its sodium cation that was bonded to guluronate with the divalent or trivalent cations, which link the polymers together. 94 The proportion of mannuronic and guluronic acid determines alginate's physical and chemical properties; a greater mannuronate content promotes gelation while a greater guluronate content promotes fiber integrity. 96 Alginates can be used to form many different types of wound dressings including hydrogels, semipermeable films, foams, and nanofibers. 95 According to a chapter in Advanced Wound Repair Therapies written by Agarwal et al, 96 the benefits of using alginate based wound dressing include gelation providing a moist wound healing environment, ability to absorb 20–30 times its weight in exudate, hemostatic capabilities, modulation of wound macrophages, and, reportedly, enhanced epithelialization and granulation tissue formation. Antimicrobials, medications, and nanoparticles can be added to alginate based wound dressings to enhance its antimicrobial activity. It is recommended that alginates be avoided in wounds that contain little exudate to no exudate and changed often due to the possibility of desiccation of the dressing. This can cause adherence of the dressing to the wound, which would result in painful removal. 24 , 26 , 36 One example of an alginate wound dressing is the Kaltostat® calcium sodium alginate dressing sold by ConvaTec.
3.11. Chitin and chitosan
Chitin is the second most abundant natural polysaccharide, and consists of polymers of β (1, 4) linked N‐acetylglucosamine units. 97 The main source of chitin used for commercial purposes are the shells of crustaceans, though it can also be found in the exoskeletons of other arthropods as well as in the cell walls of fungi and yeast. 98 Chitosan can be synthesized by partial deacetylation of chitin. 98 Chitin and chitosan are both biocompatible, biodegradable, nontoxic, and nonimmunogenic. 97 When chitin and chitosan are degraded by lysozymal activity they form N‐acetyl‐β‐D‐glucosamine which has been found to stimulate (a) fibroblast proliferation, (b) ordered collagen deposition, and (c) hyaluronic acid synthesis. 97 Both chitosan and chitin can be used as a biomaterial for various types of wound dressings, including hydrogels, sponges, films, and porous membranes. 99 A couple of chitosan product examples include the HemCon® Bandage Pro, Smith & Nephew BST‐CarGel®.
3.12. Pectin
Pectin is a heteropolysaccharide consisting of several different structural elements, such as homogalacturonan and rhamnogalacturonan, that can be found in the cell walls of dicotyledons. 91 Pectins that have greater than 50% methylation in the homogalacturonan region will gel in an acidic environment (pH <4.0) in the presence of a high concentration of sugar while pectins with a lower degree of methylation gel in the presence of divalent cations regardless of the presence of soluble solids. 91 Pectin is useful in biomedical applications due to its biocompatibility, biodegradability, low cost of production, and availability. 100 Pectin has been used as a biomaterial for hydrocolloids, 91 hydrogels, and films. 100 Two examples of wound dressings that contain pectin are ConvaTect Ltd.'s DuoDERM® and GranuGEL®. 101
3.13. Hyaluronan
Hyaluronan (also known as hyaluronic acid) is a polysaccharide consisting of D‐glucuronic acid and N‐acetyl‐D‐glucosamine disaccharide. 102 Hyaluronan is highly absorbent; when hydrated, it forms stiff helices that can contain as much as 1,000‐fold more water than polymer. 103 Hyaluronan is naturally found in the extracellular matrix, vitreous humor, cartilage, synovial fluid, epidermis, and dermis. 91 It is able to stimulate wound healing through multiple mechanisms. Hyaluronan modulates the inflammatory process, which may contribute to granulation tissue stabilization. 104 It is able to soften the fibrin clot, facilitating cell colonization. 104 In addition, hyaluronan modulates angiogenesis and stimulates fibroblasts. 104
3.14. Gelatin
Gelatin is a protein derived from type I collagen present in bones and skin. 91 Gelatin contains an amino acid sequence similar to arginylglycylaspartic acid, which is known to promote cell adhesion and migration. 105 Gelatin forms a thermoreversible gel; it gels at lower temperatures and melts when the temperature increases. 106 At physiological temperatures, gelatin gel is unstable unless it is chemically or enzymatically crosslinked. Gelatin has been used in hydrocolloids, hydrogels, and foams. Two examples of gelatin based sponges are Surgifoam® Absorbable Gelatin Sponge (Johnson & Johnson) and Gelfoam® (Pfizer). Gelatin is also present in some hydrocolloid dressings, such as Granuflex®, 91 and the aforementioned DuoDERM.
4. DISCIPLINES OF WOUND DRESSING DEVELOPERS
According to a paper published in 2017 by Gwak et al, 107 the top five applicants for patents for wound healing technologies with the United States Patent and Trademark Office (USPTO) include Kinetic Concepts, Inc (KCI), Smith & Nephew, 3M, Johnson & Johnson, and Tyco Healthcare. With the recent (October 2019) purchase of KCI by 3M, this puts 3M in an even stronger position in this list. This section will describe wound dressings assigned to the aforementioned top companies submitting patents, wound dressings sold by a relatively new company, and the respective inventor backgrounds. Tyco Healthcare will not be considered below due to the fact it was spun off by Tyco International in 2007. See Figure 7 for a timeline of the events described below.
FIGURE 7.
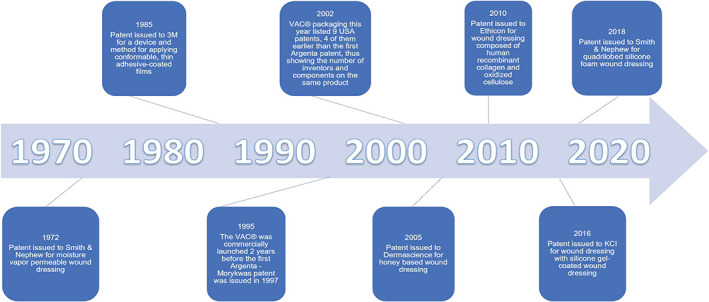
A timeline of the issuance of patents for various wound healing technology companies
4.1. Johnson & Johnson
According to its company website, Johnson & Johnson was founded in 1886 by three brothers: James Wood Johnson, Robert Johnson, and Edward Johnson. Johnson & Johnson offers many products addressing different aspects of a wound healing including Instat® and Surgicel®. Instat is a collagen sponge sold by Johnson & Johnson indicated for hemostasis. According to its trademark history, Instat was first used commercially in April of 1985. The first collagen sponge patent from Johnson & Johnson was published in 1962. 108 The patent was assigned to Ethicon, Inc., a Johnson & Johnson subsidiary, and outlined a process for preparing a collagen sponge that was water insoluble and able to retain its original rigid structure. The inventor listed on this project is scientist Dr. Charles Artandi, Ph.D. Dr. Charles Artandi was the former Associated Director of Research at Ethicon, Inc. He has multiple research publications, most relating to radiation and the use of radiation for sterilization. 109 , 110 , 111 , 112 , 113 , 114 The process of producing a collagen sponge was an improvement upon the method patented by scientist, Dr. Robert H Sifferd, and chemical engineer, Ralph J Schmitt. 115 Dr. Sifferd has written research papers with biochemists involving amino acids and sterols. 116 , 117 , 118 , 119 The patent describes the use of surgical sponges to stop the flow of blood and fluid, and the use of collagen to provide a stronger sponge with a “highly desirable physical structure.”
Surgicel is an absorbable hemostat consisting of oxidized regenerated cellulose. According to the USPTO, the Surgicel trademark was first used in commerce in November of 1957. Wound dressings containing cellulose; however, have been available for over a century. In 1901, chemical engineer Henry P Weidig filed an application with the USPTO for a surgical dressing consisting of cellulose and pyroxylin (a form of nitrocellulose). 120 A year later, engineer Robert W. Johnson, better known as the co‐founder of Johnson & Johnson, submitted a patent application for an absorbent surgical dressing. 121 This dressing consisted of absorbent layers of “rumpled cellulose tissue‐paper,” separated by a thin layer of absorbent cotton. In 1938, chemists Edward Yackel and Dr. William Kenyon file a patent, assigned to Eastman Kodak Co, describing a method of preparing oxidized cellulose. 122 Eastman Kodak Co. supplied oxidized cellulosic gauze through Parke, Davis & Company for physicians to use for research. 123 According to the USPTO, Parke, Davis & Company trademarked the absorbable cellulose dressing as Oxycel® in 1944. Many physicians published research using Oxycel soon after. 124 , 125 , 126 , 127 , 128 Chemist and former head of the Johnson & Johnson research department David F. Smith filed two patents involving hemostats consisting of cellulose in 1950 and 1957. 129 , 130 Johnson & Johnson filed for the Surgicel trademark in 1958.
A patent was issued for a collagen and oxidized regenerated cellulose sponge in October of 2001 with Johnson & Johnson as the assignee. In April of 2002, Johnson & Johnson announced the release of Promogran™ Matrix Wound Dressing, a wound dressing comprised of oxidized regenerated cellulose, collagen, and silver. During that year, Dr. Breda Cullen, Ph.D., along with other scientists at Ethicon published research papers regarding the effects of oxidized regenerated cellulose and collagen on wound healing. 131 , 132 , 133 , 134 Physicians also begin to publish research using the Promogran Matrix Wound Dressing, some of which received a grant from Johnson & Johnson. 135 In December of 2005, Dr. Breda Cullen and two other scientists from Ethicon filed an application for a wound dressing composed of human recombinant collagen and oxidized cellulose. 136 The patent was issued by USPTO in November of 2010. Research involving the collaboration between the inventors and physicians was published many years after the initial filing of the patent regarding the use of oxidized regenerated cellulose and collagen as wound dressings. 137 , 138 , 139 , 140 The wound dressings that have the trademark Promogran are now sold by KCI.
4.2. Kinetic Concepts, Inc
KCI was an Acelity Company that, according to a 3M news release, was be acquired by 3M in October of 2019. In 1995, KCI trademarked Vacuum Assisted Closure, a brand of NPWT that, according to the KCI website, has more published clinical evidence than any other NPWT system. In 1991, Dr. Louis Argenta, a plastic surgeon, and Michael Morykwas, a biomedical engineer, from Wake Forest School of Medicine filed a patent with the USPTO for the NPWT system that would become Vacuum Assisted Closure®. 141 However, at the time of commercial launch of the product in the early 1990's, Wake Forest had still not received an issued patent for their format for NPWT. KCI purchased patents from another physician and used patents from its own development engineers to launch the product under patent protection. The package label from 2002 for the product shows 15 patent labels; this demonstrates the work of multiple “inventors” necessary to develop the components for a complex device or product. Here again we see the combined work of practicing clinicians and company engineers represented in the patent literature. But for commercial success, there is no substitute for a clinical champion, and KCI had that in the Wake Forest pair of Drs. Argenta and Morykwas. 142 , 143
Another product offered by KCI, trademarked as Adaptic Touch™, is a low adherent contact layer dressing. Adaptic Touch consists of a cellulose acetate gauze coated with soft tack silicone. A USPTO patent, currently assigned to KCI, for a silicone gel‐coated wound dressing was filed by scientists in 2011, with a United Kingdom patent filed in 2010 taking priority. 144 The patent for this invention was issued in March of 2016. A divisional patent, also assigned to KCI, for a wound dressing with a tacky silicone coating was filed by scientists in 2016. 145 The patent was recently issued by USPTO in June of 2019. This patent cites many resources, including research publications primarily written by physicians.
4.3. 3M
One of the patents cited was filed in 1981 for a polytetrafluoroethylene wound dressing coated with a silicone film, designed to prevent wound desiccation. 25 It was issued by the USPTO in February of 1983. The patent was assigned to 3M and lists the inventors as Louis A. Errede, James D. Stoescz, and George D. Winter, all of whom are scientists. To date, 3M sells multiple styles of silicone foam wound dressings and the patent has been cited over 500 times. There are experimental results reported in this patent application that are not searchable in any other publication. The “trade” that a patent applicant makes is to agree to the publication of the application in exchange for proprietary patent protection for a specified period of time. That is the difference between the patent literature and “trade secrets” which are preserved inside a company and not accessible at all outside that company.
4.4. Smith & Nephew
Smith & Nephew is a medical technology company founded in England in 1856 by Thomas James Smith. Smith & Nephew has many signature brands including OpSite™, Allevyn™ Life, and Durafiber®. Smith & Nephew offers a uniquely shaped silicone foam wound dressing under the trademark Allevyn Life. Allevyn Life comes in a quadrilobed shape, as well as shapes that contour to the heel and sacrum. In 2016, inventors Ella Mumby and Dr. Helene Anne Lecomte, Ph.D., filed a patent application for a wound dressing with the characteristic four lobed shape. 146 The patent inventors have a chemistry background and listed Smith & Nephew as the assignee. The wound dressing described in the patent contains an adhesive layer, a perforated wound contact layer, a polyurethane foam layer, a layer of activated charcoal cloth, a superabsorbent layer with cellulose fibers and polyacrylate, a shielding layer (consisting of either foam, woven fabric, or nonwoven fabric), and a top film layer. This patent was issued by the USPTO in August of 2018.
4.5. Comparison of Smith & Nephew and 3M: Semipermeable film
Opsite is a polyurethane film wound dressing that was introduced in 1977. Five years earlier, two patents were published by scientists listing Smith & Nephew as the assignee for polyurethane film wound dressings. 147 , 148 In 1975, Smith & Nephew filed to trademark the name OpSite for the selectively permeable films. In between the time of publishing the patent and the introduction of OpSite, plastic surgeons published research on the product. 149 , 150 , 151 , 152 , 153 , 154
In 1978, 3M began working on its own semipermeable film. 3M filed an application with the Canadian Intellectual Patent Office in 1981 for a method and device for applying an adhesive coated polymeric film, listing Steven Heinecke as the inventor. 155 Steven Heinecke was an engineer at 3M that described going to a local hospital and interacting with critical care nurses to discover a need for a transparent IV site dressing. 156 That same year, 3M test launched a semipermeable polymeric film known as Tegaderm™ in the United States. Six years later, 3M became the US market share leader in transparent film wound dressings. In 2005, the Tegaderm brand was extended to other 3M wound dressings including hydrocolloids, alginates, silicone foam, and so forth. Tegaderm is also the brand of adhesive film packaged with the KCI products VAC® and Prevena™.
4.6. Integra LifeScience
Integra LifeScience is a company that, even though it was founded relatively recently in 1989, has become one of the world leaders in medical devices. Integra LifeScience moved to acquire the tissue regeneration company Dermasciences, Inc in 2017. In July of 2015, inventors Keith Johnson, Rehan Khanzada, Mario Neto, and Barry Wolfenson filed a patent application for a foam wound dressing impregnated with honey assigned to Dermasciences, Inc. 157 Two of the inventors have engineering backgrounds: One chemical, the other biomedical. Interestingly, all, except one inventor, have a background or education in business. To date, the patent status is listed as ready for examination. Integra LifeScience now offers Leptospermum honey‐based pastes, hydrogels, hydrocolloid wound dressings, and calcium alginate dressings under the trademark MEDIHONEY®. MEDIHONEY previously listed four issued patents on the Dermascience website. 158 , 159 , 160 , 161 One of the patents was filed by an inventor with a background in engineering. 160 Two of the patents were filed by an inventor with a background in biochemistry. 158 , 159 The other patent was filed by an inventor with a background in farming and engineering. 161 Multiple publications were cited in each of the patents, which were authored by primarily by biochemists, physicians, and chemists. 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169
5. DISCUSSION
One question that should be explored is if early collaboration with different fields leads to a better wound dressing. A great example of this dynamic is seen with the product Tegaderm. It cannot be ignored that this product discovery was influenced by an engineer physically visiting a hospital and interacting with health care professionals that harbored a need. That engineer worked with a team of scientists to build a product that met those needs. Even though OpSite had been introduced around 6 years prior to Tegaderm's introduction to the US market, Tegaderm was able to surpass OpSite as a US market share leader in transparent films.
Another example of early collaboration leading to a better wound dressing is the advent of the Vacuum Assisted Closure device. This device not only introduced a new product, but it also introduced a new way of achieving wound healing, NPWT. Ten years after the original VAC® product was launched, work in a hospital setting by a plastic surgeon and an orthopedic surgeon working on difficult, infected joint replacements, produced a new modification of the original NPWT product and the patent was purchased by KCI. 170 Working with the company engineers the Prevena™, an incisional dressing, was developed and launched. A meta‐analysis performed by Strugala et al 171 demonstrated on the Prevena that a single use of NPWT resulted in the reduction of surgical site infection, wound dehiscence, and length of stay when used prophylactically.
Early collaboration between those in different fields is also seen with the development of the Medihoney products. The backgrounds of the Medihoney product inventors were diverse, including fields, such as business, farming, biochemistry, and engineering. One of the inventors even collaborated with physicians on research involving using honey as a dressing for leg ulcers and included the resulting publication on the patent application. 166 Medihoney has since grown into the global leading medical honey‐based product line.
Early collaboration between professionals in different disciplines has been shown to be advantageous for innovations in wound management technologies. One possible reason could be that representatives of separate disciplines can bring different perspectives that can be brought together to solve a problem. Another possible reason is that early input from medical professionals that will be using the technologies or have firsthand experience of the problem that the product is designed to solve can help shape the development in a way that better addresses the issue experienced in clinical practice. In the future, deliberately utilizing interdisciplinary collaboration may prove beneficial when developing novel wound care products. There are untapped viewpoints and solutions that could enhance the creation of future medical technologies. Valuing diverse perspectives from differing fields of study and expertise can drive this innovation. Therefore, inventors should prioritize receiving early input from both scientists and clinicians when developing products intended for use in the delivery of health care.
There continues to be promising research that is likely to result in innovation in wound dressing technology. Current areas of active research in wound dressings include further exploring the use of chitosan as a biomaterial, experimenting with different biomaterials in the synthesis of hydrogels, utilizing electrospinning in the construction of the biomaterials, and incorporating a drug delivery system and nanoparticles in the matrix of wound dressings. For example, Long et al 172 was able to 3D print a chitosan‐pectin hydrogel impregnated with the lidocaine hydrochloride that could release the anesthetic in a controlled manner over the course of hours in vitro. In this study, the release of lidocaine hydrochloride resulted in degradation of the hydrogel. This illustrates an ever‐present challenge that wound dressing pioneers are faced with, which is manipulating the delicate balance between structure and function in a way enhances the dressings wound healing capabilities.
CONFLICT OF INTEREST
Ms. Hawthorne has no conflict of interest to disclose. Mr. Simmons has no conflict of interest to disclose. Mr. Stuart has no conflict of interest to disclose. Dr. Tung has no conflict of interest to disclose. Dr. Zamierowski reports relevant financial activity from KCI, Inc., outside the submitted work. In addition, Dr. Zamierowski has a patent 4,969,880 sold to KCI, and a patent 7,976,519 with royalties paid by KCI. Dr. Mellott reports relevant financial activity from Zam Research LLC and from Ronawk LLC, outside the submitted work. In addition, Dr. Mellott has a patent Expandable cell culture substrate (WO2018044990A1) licensed, and a patent Biopsy punch device and method (US201802349988A1) pending.
ACKNOWLEDGMENTS
Authors would like to sincerely thank the entire R3 lab for their support, feedback, and encouragement.
Hawthorne, B. , Simmons, J. K. , Stuart, B. , Tung, R. , Zamierowski, D. S. , & Mellott, A. J. (2021). Enhancing wound healing dressing development through interdisciplinary collaboration. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 109(12), 1967–1985. 10.1002/jbm.b.34861
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gross PL, Weil PA, Rand ML. Hemostasis & thrombosis. In: Rodwell VW, Bender DA, Botham KM, Kennelly PJ, Weil PA, eds. Harper's Illustrated Biochemistry, 31e. New York, NY: McGraw‐Hill Education; 2018. [Google Scholar]
- 3. Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81(2):94‐101. [DOI] [PubMed] [Google Scholar]
- 4. McPherson RA, Pincus MR. Henry's clinical diagnosis and management by laboratory methods. Online resource (xvii, 1565 pages).
- 5. Bunn HF, Furie B. Overview of hemostasis. In: Aster JC, Bunn HF, eds. Pathophysiology of Blood Disorders, 2e. New York, NY: McGraw‐Hill Education; 2016. [Google Scholar]
- 6. Pardo M, Miller RD, Miller RD. Basics of anesthesia. Online resource.
- 7. Peerschke EI, Yin W, Ghebrehiwet B. Platelet mediated complement activation. Adv Exp Med Biol. 2008;632:81‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krystel‐Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi‐functional master cell. Front Immunol. 2015;6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eming SA. Biology of wound healing. Dermatology. 3rd ed. Philadephia: Elsevier Saunders; 2012. [Google Scholar]
- 10. Burkitt HG, Quick CR, Reed JB. Essential Surgery: Problems, Diagnosis and Management. Oxford: Churchill Livingstone Elsevier; 2007. [Google Scholar]
- 11. Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Health Sciences; 2014. [Google Scholar]
- 12. Childs DR, Murthy AS. Overview of wound healing and management. Surg Clin North Am. 2017;97(1):189‐207. [DOI] [PubMed] [Google Scholar]
- 13. Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep. 2018;7(4):350‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theilgaard‐Monch K, Knudsen S, Follin P, Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol. 2004;172(12):7684‐7693. [DOI] [PubMed] [Google Scholar]
- 15. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro‐wound healing phenotypes. Front Physiol. 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 suppl):12S‐34S. [DOI] [PubMed] [Google Scholar]
- 17. Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 18. Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am. 2011;19(3):441‐453. [DOI] [PubMed] [Google Scholar]
- 19. Chandawarkar RY, Miller MJ, Kellogg BC, Schulz SA, Valerio IL, Kirschner RE. Plastic and reconstructive surgery. In: Brunicardi FC, Andersen DK, Billiar TR, et al., eds. Schwartz's Principles of Surgery, 11e. New York, NY: McGraw‐Hill Education; 2019. [Google Scholar]
- 20. Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31(6):674‐686. [DOI] [PubMed] [Google Scholar]
- 21. Nazzal M, Osman MF, Albeshri H, Abbas DB, Angel CA. Wound healing. In: Brunicardi FC, Andersen DK, Billiar TR, et al., eds. Schwartz's Principles of Surgery, 11e. New York, NY: McGraw‐Hill Education; 2019. [Google Scholar]
- 22. Scales JT. Wound healing and the dressing. Br J Ind Med. 1963;20(2):82‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293‐294. [DOI] [PubMed] [Google Scholar]
- 24. Weir D, Brindle CT. Wound Dressings. In: Hamm RL, ed. Text and Atlas of Wound Diagnosis and Treatment, 2e. New York, NY: McGraw‐Hill Education; 2019. [Google Scholar]
- 25. Errede LANO, Stoesz, James D , Winter Deceased., George D. (late of St. Paul, MN); Minnesota Mining and Manufacturing Company (St. Paul, MN), assignee. Composite wound dressing. United States 1983.
- 26. Jones V, Grey JE, Harding KG. Wound dressings. BMJ. 2006;332(7544):777‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weller C, Sussman G. Wound dressings update. J Pharm Pract Res. 2006;36(4):318‐324. [Google Scholar]
- 28. Landriscina A, Rosen J, Friedman AJ. Systematic approach to wound dressings. J Drugs Dermatol. 2015;14(7):740‐744. [PubMed] [Google Scholar]
- 29. Tan PW, Ho WC, Song C. The use of Urgotul in the treatment of partial thickness burns and split‐thickness skin graft donor sites: a prospective control study. Int Wound J. 2009;6(4):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madihally SV. Principles of Biomedical Engineering. Norwood: Artech House; 2010. [Google Scholar]
- 31. Rice R. Home Care Nursing Practice: Concepts and Application. St. Louis: Mosby Elsevier; 2006. [Google Scholar]
- 32. Dhanraj P. A clinical study comparing helicoll with scarlet red and OpSite in the treatment of split thickness skin graft donor sites‐a randomized controlled trial. Indian J Surg. 2015;77(suppl 2):385‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Owens N. Rayon, an ideal surgical dressing for surface wounds. Surgery. 1946;19(4):482‐485. [PubMed] [Google Scholar]
- 34. Dhivya S, Padma VV, Santhini E. Wound dressings‐a review. Biomedicine. 2015;5(4):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chaby G, Senet P, Vaneau M, et al. Dressings for acute and chronic wounds: a systematic review. Arch Dermatol. 2007;143(10):1297‐1304. [DOI] [PubMed] [Google Scholar]
- 36. Broussard KC, Powers JG. Wound dressings: selecting the most appropriate type. Am J Clin Dermatol. 2013;14(6):449‐459. [DOI] [PubMed] [Google Scholar]
- 37. Bolognia J, Schaffer JV, Cerroni L. Dermatology. Online resource (2 volumes).
- 38. Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. A clinical review. Dermatol Surg. 1995;21(7):583‐590. [DOI] [PubMed] [Google Scholar]
- 39. Narayanaswamy Radhika, Torchilin Vladimir P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019;24(3):603. 10.3390/molecules24030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Op't Veld RC, Walboomers XF, Jansen JA, Wagener F. Design considerations for hydrogel wound dressings: strategic and molecular advances. Tissue Eng Part B Rev. 2020;26(3):230‐248. [DOI] [PubMed] [Google Scholar]
- 41. Yan H, Chen J, Peng X. Recombinant human granulocyte‐macrophage colony‐stimulating factor hydrogel promotes healing of deep partial thickness burn wounds. Burns. 2012;38(6):877‐881. [DOI] [PubMed] [Google Scholar]
- 42. Liang Y, Zhao X, Hu T, et al. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full‐thickness skin regeneration during wound healing. Small. 2019;15(12):1900046. [DOI] [PubMed] [Google Scholar]
- 43. Zhang B, He J, Shi M, Liang Y, Guo B. Injectable self‐healing supramolecular hydrogels with conductivity and photo‐thermal antibacterial activity to enhance complete skin regeneration. Chem Eng J. 2020;400:125994. [Google Scholar]
- 44. Qu J, Zhao X, Liang Y, Zhang T, Ma PX, Guo B. Antibacterial adhesive injectable hydrogels with rapid self‐healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185‐199. [DOI] [PubMed] [Google Scholar]
- 45. Li M, Liang Y, He J, Zhang H, Guo B. Two‐pronged strategy of biomechanically active and biochemically multifunctional hydrogel wound dressing to accelerate wound closure and wound healing. Chem Mater. 2020;32(23):9937‐9953. [Google Scholar]
- 46. Bencherif SA, Sands RW, Bhatta D, et al. Injectable preformed scaffolds with shape‐memory properties. Proc Natl Acad Sci U S A. 2012;109(48):19590‐19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao X, Liang Y, Guo B, Yin Z, Zhu D, Han Y. Injectable dry cryogels with excellent blood‐sucking expansion and blood clotting to cease hemorrhage for lethal deep‐wounds, coagulopathy and tissue regeneration. Chem Eng J. 2021;403:126329. [Google Scholar]
- 48. Zhao X, Guo B, Wu H, Liang Y, Ma PX. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat Commun. 2018;9(1):2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang Y, Zhao X, Zhang Z, et al. Degradable gelatin‐based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem Mater. 2020;32(15):6595‐6610. [Google Scholar]
- 50. Pfenninger JL, Fowler GC. Pfenninger and Fowler's Procedures for Primary Care E‐Book. Philadelphia: Elsevier Health Sciences; 2010. [Google Scholar]
- 51. Seaman S. Dressing selection in chronic wound management. J Am Podiatr Med Assoc. 2002;92(1):24‐33. [DOI] [PubMed] [Google Scholar]
- 52. Johnson A. Lyofoam in the treatment of open wounds. Nurs Stand. 1989;3(40):8‐14. [DOI] [PubMed] [Google Scholar]
- 53. Winter GD. Epidermal wound healing under a new polyurethane foam dressing (Lyofoam). Plast Reconstr Surg. 1975;56(5):531‐537. [DOI] [PubMed] [Google Scholar]
- 54. Simoes D, Miguel SP, Ribeiro MP, Coutinho P, Mendonca AG, Correia IJ. Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm. 2018;127:130‐141. [DOI] [PubMed] [Google Scholar]
- 55. Wasiak J, Cleland H. Burns: dressings. BMJ Clin Evid. 2015;2015:1903. [PMC free article] [PubMed] [Google Scholar]
- 56. Harper GM, Johnston CB, Landefeld CS. Management of Common Geriatric Problems. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis and Treatment 2020. New York, NY: McGraw‐Hill Education; 2020. [Google Scholar]
- 57. Mellott AJ, Zamierowski DS, Andrews BT. Negative pressure wound therapy in maxillofacial applications. Dent J. 2016;4(3):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Glass GE, Nanchahal J. The methodology of negative pressure wound therapy: separating fact from fiction. J Plast Reconstr Aesthet Surg. 2012;65(8):989‐1001. [DOI] [PubMed] [Google Scholar]
- 59. Jaffe L, Wu SC. Dressings, topical therapy, and negative pressure wound therapy. Clin Podiatr Med Surg. 2019;36(3):397‐411. [DOI] [PubMed] [Google Scholar]
- 60. Qureshi AA, Ross KM, Ogawa R, Orgill DP. Shock wave therapy in wound healing. Plast Reconstr Surg. 2011;128(6):721e‐727e. [DOI] [PubMed] [Google Scholar]
- 61. Larking AM, Duport S, Clinton M, Hardy M, Andrews K. Randomized control of extracorporeal shock wave therapy versus placebo for chronic decubitus ulceration. Clin Rehabil. 2010;24(3):222‐229. [DOI] [PubMed] [Google Scholar]
- 62. Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(suppl 1):131S‐141S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Andrade SM, Santos IC. Hyperbaric oxygen therapy for wound care. Rev Gaucha Enferm. 2016;37(2):e59257. [DOI] [PubMed] [Google Scholar]
- 64. de Smet GHJ, Kroese LF, Menon AG, et al. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017;25(4):591‐608. [DOI] [PubMed] [Google Scholar]
- 65. Mosca RC, Ong AA, Albasha O, Bass K, Arany P. Photobiomodulation therapy for wound care: a potent, noninvasive photoceutical approach. Adv Skin Wound Care. 2019;32(4):157‐167. [DOI] [PubMed] [Google Scholar]
- 66. Khan I, Arany P. Biophysical approaches for oral wound healing: emphasis on photobiomodulation. Adv Wound Care. 2015;4(12):724‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Butcher G, Pinnuck L. Wound bed preparation: ultrasonic‐assisted debridement. Br J Nurs. 2013;22(6):S36‐S38. [DOI] [PubMed] [Google Scholar]
- 68. Chang YR, Perry J, Cross K. Low‐frequency ultrasound debridement in chronic wound healing: a systematic review of current evidence. Plast Surg. 2017;25(1):21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: review. Prog Polym Sci. 2011;36(9):1254‐1276. [Google Scholar]
- 70. Aramwit P. Introduction to biomaterials for wound healing. In: Ågren MS, ed. Wound Healing Biomaterials. Duxford: Woodhead Publishing; 2016:3‐38. [Google Scholar]
- 71. Ghadi R, Jain A, Khan W, Domb AJ. Microparticulate polymers and hydrogels for wound healing. In: Ågren MS, ed. Wound Healing Biomaterials. Duxford: Woodhead Publishing; 2016:203‐225. [Google Scholar]
- 72. Hepburn C. Polyurethane Elastomers. Netherlands: Springer; 2012. [Google Scholar]
- 73. Yoo HJ, Kim HD. Synthesis and properties of waterborne polyurethane hydrogels for wound healing dressings. J Biomed Mater Res B Appl Biomater. 2008;85(2):326‐333. [DOI] [PubMed] [Google Scholar]
- 74. Vowden K, Vowden P. Wound dressings: principles and practice. Surgery. 2017;35(9):489‐494. [Google Scholar]
- 75. Gurtner GC, Neligan PC. Plastic Surgery E‐Book: Volume 1: Principles. London: Elsevier Health Sciences; 2017. [Google Scholar]
- 76. Truong B, Grigson E, Patel M, Liu X. Pressure ulcer prevention in the hospital setting using silicone foam dressings. Cureus. 2016;8(8):e730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lakshmi DS, Trivedi N, Reddy CRK. Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr Polym. 2017;157:1604‐1610. [DOI] [PubMed] [Google Scholar]
- 78. Lee S, Park YH, Ki CS. Fabrication of PEG–carboxymethylcellulose hydrogel by thiol‐norbornene photo‐click chemistry. Int J Biol Macromol. 2016;83:1‐8. [DOI] [PubMed] [Google Scholar]
- 79. Bhattarai S, Bunt C, Rathbone M, Alany RG. Phase behavior, rheological and mechanical properties of hydrophilic polymer dispersions. Pharm Dev Technol. 2011;16(3):259‐268. [DOI] [PubMed] [Google Scholar]
- 80. Alves MH, Jensen BE, Smith AA, Zelikin AN. Poly (vinyl alcohol) physical hydrogels: new vista on a long serving biomaterial. Macromol Biosci. 2011;11(10):1293‐1313. [DOI] [PubMed] [Google Scholar]
- 81. Teodorescu M, Bercea M, Morariu S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: perspectives and challenges. Biotechnol Adv. 2019;37(1):109‐131. [DOI] [PubMed] [Google Scholar]
- 82. Kamoun EA, Kenawy ES, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA‐based hydrogel dressings. J Adv Res. 2017;8(3):217‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Harris JM. Poly (ethylene glycol) Chemistry: Biotechnical and Biomedical Applications. USA: Springer; 2013. [Google Scholar]
- 84. Mir M, Ali MN, Barakullah A, et al. Synthetic polymeric biomaterials for wound healing: a review. Prog Biomater. 2018;7(1):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhu J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639‐4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Choe G, Park J, Park H, Lee JY. Hydrogel biomaterials for stem cell microencapsulation. Polymers. 2018;10(9):997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li J, Kao WJ. Synthesis of polyethylene glycol (PEG) derivatives and PEGylated−peptide biopolymer conjugates. Biomacromolecules. 2003;4(4):1055‐1067. [DOI] [PubMed] [Google Scholar]
- 88. Zagho M, Hussein E, Elzatahry A. Recent Overviews in Functional Polymer Composites for Biomedical Applications. Polymers 2018;10(7):739. 10.3390/polym10070739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Park JU, Jung HD, Song EH, et al. The accelerating effect of chitosan‐silica hybrid dressing materials on the early phase of wound healing. J Biomed Mater Res B Appl Biomater. 2017;105(7):1828‐1839. [DOI] [PubMed] [Google Scholar]
- 90. Mogosanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463(2):127‐136. [DOI] [PubMed] [Google Scholar]
- 91. Smith AM, Moxon S, Morris GA. Biopolymers as wound healing materials. In: Ågren MS, ed. Wound Healing Biomaterials. Duxford: Woodhead Publishing; 2016:261‐287. [Google Scholar]
- 92. Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pallaske F, Pallaske A, Herklotz K, Boese‐Landgraf J. The significance of collagen dressings in wound management: a review. J Wound Care. 2018;27(10):692‐702. [DOI] [PubMed] [Google Scholar]
- 94. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Alginate in Wound Dressings. Pharmaceutics 2018;10(2):42. 10.3390/pharmaceutics10020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Agarwal A, McAnulty JF, Schurr MJ, Murphy CJ, Abbott NL. Polymeric materials for chronic wound and burn dressings. In: Farrar D, ed. Advanced Wound Repair Therapies. Cambridge: Woodhead Publishing; 2011:186‐208. [Google Scholar]
- 97. Singh R, Shitiz K, Singh A. Chitin and chitosan: biopolymers for wound management. Int Wound J. 2017;14(6):1276‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13(3):1133‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322‐337. [DOI] [PubMed] [Google Scholar]
- 100. Mishra R, Banthia A, Majeed ABA. Pectin based formulations for biomedical applications: a review. Asian J Pharm Clin Res. 2012;5(4):1‐7. [Google Scholar]
- 101. Munarin F, Tanzi MC, Petrini P. Advances in biomedical applications of pectin gels. Int J Biol Macromol. 2012;51(4):681‐689. [DOI] [PubMed] [Google Scholar]
- 102. Mero A, Campisi M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers. 2014;6(2):346‐369. [Google Scholar]
- 103. Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6(7):2397‐2404. [PubMed] [Google Scholar]
- 104. Longinotti C. The use of hyaluronic acid based dressings to treat burns: a review. Burns Trauma. 2014;2(4):162‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan‐gelatin scaffolds for tissue engineering. Biomaterials. 2005;26(36):7616‐7627. [DOI] [PubMed] [Google Scholar]
- 106. Yang Z, Hemar Y, Hilliou L, et al. Nonlinear behavior of gelatin networks reveals a hierarchical structure. Biomacromolecules. 2016;17(2):590‐600. [DOI] [PubMed] [Google Scholar]
- 107. Gwak JH, Sohn SY. Identifying the trends in wound‐healing patents for successful investment strategies. PLOS One. 2017;12(3):e0174203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Charles A; ETHICON INC, assignee. Preparation of collagen sponge. United States 1964.
- 109. Artandi C, Van Winkle Jr W. Electron‐beam sterilization of surgical sutures. Nucleonics. 1959;17:86‐90. [Google Scholar]
- 110. Artandi C, Stonehill A. Polyvinyl chloride. New high‐level dosimeter. Nucleonics. 1958;16:118‐120. [Google Scholar]
- 111. Artandi C. Porduction experiences with radiation sterilization. Bull Parenter Drug Assoc. 1964;18:2‐9. [PubMed] [Google Scholar]
- 112. Artandi C, Stonehill AA. Rigid vinyl film dosimeter. Nucl Instrum Methods. 1959;6:279‐282. [Google Scholar]
- 113. Artandi C. Sterilization by ionizing radiation. Int Anesthesiol Clin. 1972;10(2):123‐130. [DOI] [PubMed] [Google Scholar]
- 114. Artandi C. Successful and promising applications of radiation processing‐sterilization. Radiat Phys Chem. 1977;9(1–3):183‐191. [Google Scholar]
- 115. Sifferd RH, Schmitt, Ralph J .; ARMOUR & CO, assignee. Surgical sponge and the preparation thereof. United States 1952.
- 116. Vigneaud VD, Sifferd RH, Miller G. On the absence of Thiolhistidine in insulin. Proc Soc Exp Biol Med. 1935;33(3):371‐373. [Google Scholar]
- 117. Sifferd RH, Vigneaud VD. Oxidation of cystine sulfur to sulfate by ferric chloride. Proc Soc Exp Biol Med. 1934;32(2):332‐333. [Google Scholar]
- 118. Sifferd R, Anderson R. Über das Vorkommen von Sterinen in Bakterien. Hoppe‐Seyler´ s Zeitschrift für physiologische Chem 1936;239(4–6):270–272. [Google Scholar]
- 119. du Vigneaud V, Sifferd RH, Irving G Jr. The utilization of l‐carnosine by. Animals on a histidinedeficient diet. J Biol Chem. 1937;117:589‐597. [Google Scholar]
- 120. Weidig HP; Weidig Henry P, Surgical dressing. United States.
- 121. Johnson RW; ROBERT W. JOHNSON, assignee. Surgical absorbent dressing United States
- 122. Yackel EC, Kenyon, William O .; EASTMAN KODAK CO, assignee. Preparation of oxycellulose. United States 1941.
- 123. Frantz VK, Clarke HT, Lattes R. Hemostasis with absorbable gauze (oxidized cellulose). Ann Surg. 1944;120(2):181‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. VANDERHOOF ES, MERENDINO KA. Unfavorable reactions to oxidized cellulose (oxycel) in the bed of the gallbladder: the retained oxycel sponge syndrome. Arch Surg. 1949;58(2):182‐188. [DOI] [PubMed] [Google Scholar]
- 125. Goldstein AE, Hollander A. Experiences with oxycel gauze in genito‐urinary surgery. J Urol. 1948;59(2):195‐201. [DOI] [PubMed] [Google Scholar]
- 126. Hatch WE. The use of oxycel in prostatic resections. J Urol. 1948;59(5):887‐889. [DOI] [PubMed] [Google Scholar]
- 127. Hinman F, Babcock KO. Local reaction to oxidized cellulose and gelatin hemostatic agents in experimentally contaminated renal wounds. Surgery. 1949;26(4):633‐640. [PubMed] [Google Scholar]
- 128. Ault GW, Madigan EP. Clinical use of oxycel in minor anorectal surgery. Am J Surg. 1949;77(3):352‐355. [DOI] [PubMed] [Google Scholar]
- 129. Smith DF; Smith, David F , assignee. Cellulosic hemostatic composition. United States 1959.
- 130. Smith DF; Smith, David F , assignee. Hemostatic surgical dressings. United States 1964.
- 131. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10(1):16‐25. [DOI] [PubMed] [Google Scholar]
- 132. Cullen B, Watt PW, Lundqvist C, et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. 2002;34(12):1544‐1556. [DOI] [PubMed] [Google Scholar]
- 133. Hart J, Silcock D, Gunnigle S, Cullen B, Light ND, Watt PW. The role of oxidised regenerated cellulose/collagen in wound repair: effects in vitro on fibroblast biology and in vivo in a model of compromised healing. Int J Biochem Cell Biol. 2002;34(12):1557‐1570. [DOI] [PubMed] [Google Scholar]
- 134. Cullen B. The role of oxidized regenerated cellulose/collagen in chronic wound repair. Part 2. Ostomy Wound Manage. 2002;48(6 suppl):8‐13. [PubMed] [Google Scholar]
- 135. Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002;137(7):822‐827. [DOI] [PubMed] [Google Scholar]
- 136. Cullen BMS, Silcock, Derek Walter , Boyle, Claire ; Wound dressings comprising oxidized cellulose and human recombinant collagen. United States 2007.
- 137. Bourdillon KA, Delury CP, Cullen BM. Biofilms and delayed healing ‐ an in vitro evaluation of silver‐ and iodine‐containing dressings and their effect on bacterial and human cells. Int Wound J. 2017;14(6):1066‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wu S, Applewhite AJ, Niezgoda J, et al. Oxidized Regenerated Cellulose/Collagen Dressings: Review of Evidence and Recommendations. Adv Skin Wound Care. 2017;30(11S Suppl 1):S1‐S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cullen BM, Serena TE, Gibson MC, Snyder RJ, Hanft JR, Yaakov RA. Randomized controlled trial comparing collagen/oxidized regenerated cellulose/silver to standard of care in the management of venous leg ulcers. Adv Skin Wound Care. 2017;30(10):464‐468. [DOI] [PubMed] [Google Scholar]
- 140. Gottrup F, Cullen BM, Karlsmark T, Bischoff‐Mikkelsen M, Nisbet L, Gibson MC. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen. 2013;21(2):216‐225. [DOI] [PubMed] [Google Scholar]
- 141. Argenta LCW‐S, Morykwas, Michael J ; Wake Forest University Health Sciences Wound treatment employing reduced pressure. United States 2007.
- 142. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553‐562. [DOI] [PubMed] [Google Scholar]
- 143. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563‐576. [PubMed] [Google Scholar]
- 144. Addison DK, Stephens, Sally , Hadley, Rachel ; SYSTAGENIX WOUND MANAGEMENT IP. CO. B.V Silicone gel‐coated wound dressing. United States 2013.
- 145. Addison DVL, Stephens, Sally , Hadley, Rachel ; KCI USA, INC . Silicone gel‐coated wound dressing. United States 2019.
- 146. Mumby ELH, Lecomte, Helene Anne ; Smith & Nephew PLC Wound dressing and method of treatment. United States 2018.
- 147. Donald Edwin Seymour NMDC, Hodgson Martin Edward, Dow James; T J Smith and Nephew Ltd, Smith and Nephew PLC Adhesive materials. United Kingdom 1972.
- 148. E HM; T.J. SMITH & NEPHEW LTD , Moisture‐vapor‐permeable pressure‐sensitive adhesive materials. United States 1972.
- 149. Smith P, Robinson P. Burns of the scalp involving bone: an alternative technique. Burns. 1976;2(4):254‐260. [Google Scholar]
- 150. Snow R. An improved ear prosthesis in silicone rubber incorporating an adhesive surface. Br J Plast Surg. 1975;28(4):289‐291. [DOI] [PubMed] [Google Scholar]
- 151. Townsend P. The quest for a cheap and painless donor‐site dressing. Burns. 1976;2(2):82‐85. [Google Scholar]
- 152. Winter GD. Transcutaneous implants: reactions of the skin‐implant interface. J Biomed Mater Res. 1974;8(3):99‐113. [DOI] [PubMed] [Google Scholar]
- 153. Pontén B, Nordgaard JO. The use of collagen film (Cutycol®) as a dressing for donor areas in split skin grafting. Scand J Plast Reconstr Surg. 1976;10(3):237‐240. [DOI] [PubMed] [Google Scholar]
- 154. James J, Watson A. The use of opsite1, a vapour permeable dressing, on skin graft donor sites. Br J Plast Surg. 1975;28(2):107‐110. [PubMed] [Google Scholar]
- 155. Heinecke SB; 3M, assignee. Device and method for applying comfortable, thin adhesive‐coated films. Canada 1985.
- 156. Fischer J. Seeing through a nurse's need: A scientist's transparent solution. https://www.3m.com/3M/en_US/particles/all-articles/article-detail/~/tegaderm-dressings-surgery-solution-nurses-need/?storyid=eb703b4a-5f60-4d22-a2f8-912dc43fb8c8 .
- 157. Johnson KAD, Khanzada, Rehan N , Neto, Mario BF ., Wolfenson, Barry J ; Derma Sciences, Inc Honey‐based foam compositions. United States 2017.
- 158. Molan PH; Apimed Medical Honey Limited . Honey based wound dressing. United States 2011.
- 159. Molan PH; Waikatolink Limited Honey based wound dressing. United States 2005.
- 160. Peter Moloney AQ; Medihoney Pty Ltd . Therapeutic composition comprising honey or a honey derivative. United States 2014.
- 161. Caskey PRC; Apimed Medical Honey Limited Use of honey in dressings. United States 2010.
- 162. Habashy RR, Abdel‐Naim AB, Khalifa AE, Al‐Azizi MM. Anti‐inflammatory effects of jojoba liquid wax in experimental models. Pharmacol Res. 2005;51(2):95‐105. [DOI] [PubMed] [Google Scholar]
- 163. Patel S, Nelson DR, Gibbs AG. Chemical and physical analyses of wax ester properties. J Insect Sci. 2001;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Molan PC, Allen KL. The effect of gamma‐irradiation on the antibacterial activity of honey. J Pharm Pharmacol. 1996;48(11):1206‐1209. [DOI] [PubMed] [Google Scholar]
- 165. Subrahmanyam M. Honey impregnated gauze versus polyurethane film (OpSite) in the treatment of burns–a prospective randomised study. Br J Plast Surg. 1993;46(4):322‐323. [DOI] [PubMed] [Google Scholar]
- 166. Wood B, Rademaker M, Molan P. Manuka honey, a low cost leg ulcer dressing. N Z Med J. 1997;110(1040):107. [PubMed] [Google Scholar]
- 167. Molan PC. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol. 2001;2(1):13‐19. [DOI] [PubMed] [Google Scholar]
- 168. Postmes T, van den Bogaard AE, Hazen M. The sterilization of honey with cobalt 60 gamma radiation: a study of honey spiked with spores of clostridium botulinum and Bacillus subtilis. Experientia. 1995;51(9–10):986‐989. [DOI] [PubMed] [Google Scholar]
- 169. Allen KL, Molan PC, Reid GM. A survey of the antibacterial activity of some New Zealand honeys. J Pharm Pharmacol. 1991;43(12):817‐822. [DOI] [PubMed] [Google Scholar]
- 170. Bubb SKKC, MO, US), Zamierowski, David S ; KCI Licensing, Inc Tissue closure treatment system and method with externally‐applied patient interface. United States 2005.
- 171. Strugala V, Martin R. Meta‐analysis of comparative trials evaluating a prophylactic single‐use negative pressure wound therapy system for the prevention of surgical site complications. Surg Infect. 2017;18(7):810‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Long J, Etxeberria AE, Nand AV, Bunt CR, Ray S, Seyfoddin A. A 3D printed chitosan‐pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Korean J Couns Psychother. 2019;104:109873. [DOI] [PubMed] [Google Scholar]
- 173. Owen JA, Punt J, Stranford SA. Kuby immunology. WH Freeman; New York; 2013. [Google Scholar]
- 174. Finch RG. Antibiotic and chemotherapy : anti‐infective agents and their use in therapy. 9th ed. p 1 online resource (xii, 900 pages). [Google Scholar]
- 175. Katzung BG, Kruidering‐Hall M, Trevor AJ. Beta‐Lactam Antibiotics & Other Cell Wall‐ & Membrane‐Active Antibiotics. Katzung & Trevor's Pharmacology: Examination & Board Review, 12e. New York, NY: McGraw‐Hill Education; 2019. [Google Scholar]
- 176. Katzung BG, Kruidering‐Hall M, Trevor AJ. Tetracyclines, Macrolides, Clindamycin, Chloramphenicol, Streptogramins, & Oxazolidinones. Katzung & Trevor's Pharmacology: Examination & Board Review, 12e. New York, NY: McGraw‐Hill Education; 2019. [Google Scholar]
- 177. Katzung BG, Kruidering‐Hall M, Trevor AJ. Sulfonamides, Trimethoprim, & Fluoroquinolones. Katzung & Trevor's Pharmacology: Examination & Board Review, 12e. New York, NY: McGraw‐Hill Education; 2019. [Google Scholar]
- 178. Parker SK, Child J, Searns J, Ogle JW. Antimicrobial Therapy. In: Hay JWW, Levin MJ, Deterding RR, Abzug MJ, editors. Current Diagnosis & Treatment: Pediatrics, 24e. New York, NY: McGraw‐Hill Education; 2018. [Google Scholar]
- 179. Levinson W, Chin‐Hong P, Joyce EA, Nussbaum J, Schwartz B. Sterilization & Disinfection. Review of Medical Microbiology & Immunology: A Guide to Clinical Infectious Diseases, 16e. New York, NY: McGraw Hill; 2020. [Google Scholar]
- 180. Iserson KV. Wounds and Burns. Improvised Medicine: Providing Care in Extreme Environments, 2e. New York, NY: McGraw‐Hill Education; 2016. [Google Scholar]
- 181. Abulyazid I, Mahdy EME, Ahmed RM. Biochemical study for the effect of henna (Lawsonia inermis) on Escherichia coli. Arabian Journal of Chemistry 2013;6(3):265‐273. [Google Scholar]
- 182. Rai D, Singh JK, Roy N, Panda D. Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem J 2008;410(1):147‐155. [DOI] [PubMed] [Google Scholar]
- 183. Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J Tradit Complement Med 2015;5(1):21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Marchese A, Orhan IE, Daglia M, Barbieri R, Di Lorenzo A, Nabavi SF, Gortzi O, Izadi M, Nabavi SM. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem 2016;210:402‐414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


