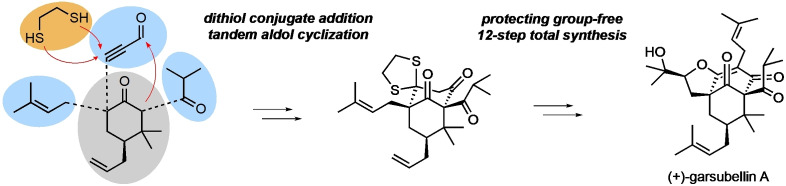
Abstract
Garsubellin A is a meroterpene capable of enhancing the enzyme choline acetyltransferase whose decreased level is believed to play a central role in the symptoms of Alzheimer's disease. Due to the potentially useful biological activity together with the novel bridged and fused cyclic molecular architecture, garsubellin A has garnered substantial synthetic interest, but its absolute stereostructure has been undetermined. We report here the first enantioselective total synthesis of (+)‐garsubellin A. Our synthesis relies on stereoselective fashioning of a cyclohexanone framework and double conjugate addition of 1,2‐ethanedithiol that promotes aldol cyclization to build the bicyclic [3.3.1] skeleton. The twelve‐step, protecting group‐free synthetic route has enabled the syntheses of both the natural (−)‐garsubellin A and its unnatural (+)‐antipode for biological evaluations.
Keywords: carbocycles, carbonylation, natural products, PPAP, total synthesis
The first enantioselective total synthesis of garsubellin A establishes the absolute stereostructure of the plant‐derived meroterpene that enhances the choline acetyltransferase enzyme responsible for the biosynthesis of the neurotransmitter acetylcholine. The 12‐step, protecting group‐free synthesis features use of a dithiolane for efficient construction of the bridged and fused ring system.
Garsubellin A (1) is a polycyclic polyprenylated acylphloroglucinol (PPAP) isolated from the wood of Garcinia subelliptica. [1] It is a potent inducer of choline acetyltransferase (ChAT), the enzyme responsible for the biosynthesis of the neurotransmitter acetylcholine (ACh). As neurodegenerative pathologies are associated with an atrophy of cholinergic neurons and attenuation in ACh levels, this meroterpenoid could, in principle, function as a nonpeptidyl neuromodulatory agent for the treatment of Alzheimer's disease. [2] Structurally, 1 features a highly congested [3.3.1] bicyclic skeleton, in which both of the bridgehead positions are quaternary stereocenters. This core motif, characteristically conserved among the natural products of the PPAP family, [3] is further adorned with a fused tetrahydrofuran ring, posing formidable challenges to synthetic undertaking. Not surprisingly, the novel molecular architecture along with the potentially useful biological activity has rendered garsubellin A an attractive target of chemical synthesis investigations. [4] Thus far, the groups of Shibasaki, Danishefsky, Nakada, and Maimone have accomplished total syntheses, each providing an ingenious synthetic road map to 1. [5] These feats, however, have been performed in racemic settings, and the absolute stereostructure of 1 remains to be established. In this regard, it is well to note that the C9 carbonyl bridge and the C7 prenyl or geranyl chain may be configured to be of both α‐ and β‐orientations in the PPAP biosynthesis (2 to 1 in Scheme 1). [6] Therefore, it is difficult to infer the absolute stereochemistry of any PPAP despite the high degree of structural homology existing within the family, [7] as exemplified by the elegant chemical synthesis investigations on clusianone and nemorosone which have revealed the absolute configurational sense of these compounds to be antipodal to that of hyperforin. [8] Reported here is the first enantioselective total synthesis of garsubellin A (1) that enabled determination of its absolute stereochemistry.
Scheme 1.
Structure and retrosynthesis of Garsubellin A.
The major synthetic challenge of 1 resides in the C1 region where a quaternary stereocenter is placed at the bridgehead position, surrounded by three ketones and an additional quaternary center of the gem‐dimethylated C8. Our strategy evolved from the notion that the C1−C2 connection constituting a bridgehead stereocenter might be forged via bicyclic ring closure, [9] also establishing the novel confluence of the three carbonyls (Scheme 1). Hence, the challenge of constructing the decorated [3.3.1] system could be reduced to positioning suitable C1 and C2 units in close proximity for bond formation, which would resolve most of the synthetic issues in the northern half of 1. We envisioned that such a scenario could be implemented with a carbonyl intermediate of seco‐tricycle 3 or seco‐bicycle 4, arising each from an intra‐ or intermolecular alkyne fashioning process. The requisite alkyne 5 was then expected to be assembled by stereocontrolled incorporation of isobutylidene, prenyl, and alkynyl units into cyclohexenone 6, which in turn could be prepared in an enantiomeric form from readily available building blocks.
Our synthetic studies were first focused on enantio‐defined preparation of cyclohexenone 6 which would serve as an initial staging post (Scheme 2). Starting with the known enol ether 7, prepared from dimedone and L‐menthol, [10] the route employed the Stork–Danheiser protocol for the allylation and reductive ketone transposition. [11] While the allylation of 7 furnished 8 as a 1:1 diastereomeric mixture, the β‐allylated enone 8 b could be accessed in reliable yield (>70 %, dr>100:1) on multi‐decagram scales after a few rounds of recycling of 8 a through base promoted epimerization. The configuration of the allyl‐attached C7 center was established by X‐ray crystallographic analysis of iodide 8 c, [12] which correlated with 8 b upon reductive deiodination.
Scheme 2.
Alkoxycarbonylation approach to Garsubellin A. LDA=lithium diisopropylamide, LAH=lithium aluminum hydride, HMDS=hexamethyldisilazide, TMSCl=trimethylsilyl chloride, DMAP=4‐dimethylaminopyridine, L‐selectride=lithium tri‐sec‐butylborohydride, m‐CPBA=3‐chloroperbenzoic acid, TFA=trifluoroacetic acid, 2,6‐DtBpyr=2,6‐di‐tert‐butylpyridine.
Having procured 6 in high enantiopurity, we turned to the synthesis of the proposed key intermediate 5 (cf. 12) and evaluation of the tandem cyclization via intermediate 3 (Z=Pd). Thus, enone 6 was subjected to a series of reactions that introduced isobutylidene, prenyl and alkynyl groups to the cyclohexanone framework. The Mukaiyama aldol reaction of 6 with isobutyraldehyde followed by elimination of the aldol adduct produced dienone 9 with high (Z)‐selectivity.[ 13 , 14 ] The installation of a prenyl group was then carried out through the conjugate reduction‐enolate trapping process employing L‐selectride and prenyl bromide, [15] which took place only at the endocyclic alkene with complete stereoselectivity to afford 10 as a single isomer. The strong α‐facial preference of the enolate was also manifested in the subsequent alkynylation, [16] thus establishing the C5 quaternary stereogenic center that would become a bridgehead position. After decarboxylation of 11 under palladium catalysis to effect allylation (cf. 12 a) [17] as well as removal of the Alloc group (cf. 12 b), [18] oxidation of the prenyl chain with m‐CPBA and hydrolysis of the resulting epoxide furnished, albeit with low diastereoselectivity, diols 13 a and 13 b poised for alkoxycarbonylation.
With the requisite diols 13 in hand, the cascade carbonylative cyclization was probed for its potential to construct the bridged ring system. Subjection of the internal alkyne 13 a to the PdII‐catalyzed conditions for alkoxycarbonylation, [19] however, did not give rise to the desired [3.3.1] skeleton but led instead to the formation of tricyclic ketal 16 via 5‐endo cyclization of hemi ketal 15 which might exist in equilibrium with ketone 14. [20] While the intended 5‐exo cyclization was feasible with the terminal alkyne substrate 13 b, the reaction induced only monocyclization to give the spirocyclic methyl ester 17 without generating the desired carbonyl bridged ring system 18. A series of attempts were further made to transform 17 into 19 making use of exogenous cyanide and thiolate nucleophiles. Unfortunately, the projected tandem Michael–Dieckmann approach, notwithstanding considerable experimentation, proved none too promising, as the exo‐alkene of 17 was recalcitrant toward conjugate addition while the methyl enoate was prone to facile E to Z isomerization. [21]
In light of the difficulty encountered in the oxycarbonylation approach based on a migratory insertion event of metal acyl 3, we sought to explore an alternative strategy using carbonyl addition of 4 (Z=H). In pursuing this line of thought, it was anticipated that the formation of the critical C1−C2 bond would be facilitated by engaging a C2 carbonyl, devoid of the conjugation and spirocyclic scaffold, with an activated C1 alkene (formally R′=OH in 4). Hence, our revised synthetic campaign commenced with the sequence comprising isobutyrylation of 6, reductive prenylation of 20 and alkynylation of 21 that uneventfully produced diketone 22 a (Scheme 3). Similar to the previous route to 11, all of these C−C bond‐forming events occurred with exclusive diastereoselectivity owing again to the α‐facial preference of the enolate presumably exerted by the C7 β‐allyl chain in concert with the C8 gem‐dimethyl group. [22] Notably, the alkynylation of 21 using t‐BuOK proceeded smoothly, despite the presence of two more potential reaction sites at C1 and C28, to place the alkynoate group only at C5. In contrast, the use of LiTMP as a base for the same reaction brought about exclusive alkynylation at C28 (Table S4 in the Supporting Information). Noteworthy in these alkynylation reactions was that neither condition affected the C1 methine flanked by two ketones, [23] although 22 a having an α‐isobutyryl group was found to isomerize easily to the more stable β‐epimer 22 b upon warming prior to workup. As observed in the reductive prenylation of 20 using L‐selectride that gave 21 without implicating carbonyl reduction, treatment of 22 b with Dibal‐H at −78 °C led to clean reduction of the ethyl ester to produce aldehyde 23, leaving the two ketones intact. [24]
Scheme 3.
Enantioselective total synthesis of (+)‐Garsubellin A. TBAI=tetrabutylammonium iodide, Dibal‐H=diisobutylaluminum hydride, TMG=1,1,3,3‐tetramethylguanidine, DMP=Dess–Martin periodinane, Grubbs’ 2nd=Grubbs second‐generation catalyst.
Having established a robust and efficient route to 23, wherein was the C2 unit in place as an aldehyde, we set out to examine the alkyne addition process that would impart a C4 functionality suitable for the construction of the THF ring while disposing the three‐carbon aldehyde chain amenable for aldol cyclization. Since a β‐ketoaldehyde which would derive from hydration of the ynal was deemed unapt for ring closure due to enolization, we resorted to dithioketal formation to effect formal hydration. [25] Pleasingly, the conjugate addition of 1,2‐ethanedithiol to 23 under Ley's condition (NaOMe, MeOH‐CH2Cl2, −10 °C) [25c] occurred smoothly to give rise directly to a mixture of lactol 25 (dr=1:1) and alcohol 26 (dr=1:0), rather than aldehyde 24. The remarkable facility with which the aldol cyclization took place appeared to be the consequence of the steric buttressing effect of the 1,3‐dithiolane juxtaposed with the bridgehead quaternary center. [26] While exposure of the purified 25 to a catalytic amount of a base resulted in redistribution into a 1.2:1 mixture of 25 and 26, interestingly, this presumable thermodynamic ratio could be reoriented to 1:3 favoring aldol 26 in the presence of a stoichiometric amine base (Table S7 in the Supporting Information). From a set of screening experiments, TMG was identified to be an effective base that optimally promoted both the double conjugate addition and aldol cyclization to give 26, which could be in situ oxidized with DMP to triketone 27.
In advancing the [3.3.1] bicyclic intermediate 27 to the very end of the synthetic campaign, we first directed our efforts toward oxidation of the prenyl chain. Given the diastereoselectivity noted in the formation of 13 in favor, albeit slight (dr=1.2–2.3:1), of the desired C18 β‐epimer, we anticipated that this stereopreference might be enhanced by the presence of the 1,3‐dithiolane segment. Treatment of 27 with m‐CPBA, however, brought about rapid oxidation at a sulfur center, leading to the formation of a mixture of the dimeric thiosulfinate 28 a and thiosulfonate 28 b probably via disproportionation reactions of a ring‐opened sulfenic acid intermediate (Scheme S5 in the Supporting Information). Resubjection of 28 a to the reaction with excess m‐CPBA indeed induced oxidation at the prenyl group to give, following workup, the THF‐fused enone 30 as the major product. [27] The beneficial effect of the sulfur substituent, in terms of the stereoselectivity of the epoxidation and the facility of the subsequent THF ring formation, was evident when compared with the result of the corresponding reaction of methyl ether 29 which gave 30 with a lower diastereoselectivity following treatment with a strong acid. In the event, epoxidation and concomitant removal of the dithiolane ring could be accomplished by exposing 27 to 4.5 equivalents of m‐CPBA at −10 °C to produce 30 in 77 % yield with 4:1 diastereoselectivity. The final stage of the total synthesis involved installation of the C3 and C7 prenyl groups, which was achieved in two steps. As the direct alkenyl C−H allylation of 30 proved challenging without protection of the C19 alcohol, an in situ protocol was developed in which the copper mediated allylation was conducted, [28] with the C19 alcohol transiently masked as an alkoxyzincate, [29] to yield the penultimate bis‐allylated intermediate 31. Finally, ruthenium‐catalyzed cross‐metathesis with 2‐methyl‐2‐butene delivered 1, [30] whose (+)‐sign of optical rotation revealed our synthetic compound to be the enantiomer of the natural garsubellin A. This result shows that the absolute stereostructure of the natural (−)‐garsubellin A is in line with those of (+)‐clusianone and (+)‐nemorosone, in which the C9 carbonyl bridge and C7 prenyl chain are both α‐oriented.
We have reported the first enantioselective total synthesis of garsubellin A (1). Our synthesis features high stereocontrol in fashioning a dimedone‐derived cyclohexane in the early phase and the late‐stage construction of the bicyclic core. Whereas the cascade oxycarbonylation approach was unsuccessful, the strategy based on the double conjugate addition of 1,2‐ethanedithiol proved effective to build the bicyclo[3.3.1]nonane framework. Notably, the 1,3‐dithiolane installed by the conjugate addition served to facilitate aldol cyclization, stereoselective epoxidation and THF ring fusion. Also noteworthy in this twelve‐step, protecting group‐free synthetic route is that the single stereocenter established at C7 in the initial stage controlled the configurations of the rest of the stereogenic centers. We have also completed the total synthesis of the natural (−)‐garsubellin A (Scheme S7 in the Supporting Information). Studies are underway to evaluate the biological performance of the unnatural antipode with an aim to identify a therapeutically relevant target and mode of action. The results will be reported in due course.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
Support for this work was provided by the National Research Foundation (NRF) funded by the Ministry of Science and ICT of Korea (2017R1A2B3002869 and 2020R1A2B5B3002271). We also thank the NIH of USA for the support (R01GM073065) in the early phase of this work.
D. Jang, M. Choi, J. Chen, C. Lee, Angew. Chem. Int. Ed. 2021, 60, 22735.
This paper is dedicated to Professor Eun Lee on the occasion of his 75th birthday.
References
- 1. Fukuyama Y., Kuwayama A., Minami H., Chem. Pharm. Bull. 1997, 45, 947–949. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. MacMillan K. S., Naidoo J., Liang J., Melito L., Williams N. S., Morlock L., Huntington P. J., Estill S. J., Longgood J., Becker G. L., McKnight S. L., Pieper A. A., De Brabander J. K., Ready J. M., J. Am. Chem. Soc. 2011, 133, 1428–1437; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b. Akagi M., Matsui N., Akae H., Hirashima N., Fukuishi N., Fukuyama Y., Akagi R., J. Pharmacol. Sci. 2015, 127, 155–163. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Ciochina R., Grossman R. B., Chem. Rev. 2006, 106, 3963–3986; [DOI] [PubMed] [Google Scholar]
- 3b. Richard J.-A., Pouwer R. H., Chen D. Y.-K., Angew. Chem. Int. Ed. 2012, 51, 4536–4561; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 4612–4638; [Google Scholar]
- 3c. Yang X.-W., Grossman R. B., Xu G., Chem. Rev. 2018, 118, 3508–3558. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Nicolaou K. C., Pfefferkorn J. A., Kim S., Wei H. X., J. Am. Chem. Soc. 1999, 121, 4724–4725; [Google Scholar]
- 4b. Usuda H., Kanai M., Shibasaki M., Org. Lett. 2002, 4, 859–862; [DOI] [PubMed] [Google Scholar]
- 4c. Usuda H., Kanai M., Shibasaki M., Tetrahedron Lett. 2002, 43, 3621–3624; [Google Scholar]
- 4d. Mehta G., Bera M. K., Tetrahedron 2013, 69, 1815–1821; [Google Scholar]
- 4e. Ciochina R., Grossman R. B., Org. Lett. 2003, 5, 4619–4621; [DOI] [PubMed] [Google Scholar]
- 4f. Uetake Y., Uwamori M., Nakada M., J. Org. Chem. 2015, 80, 1735–1745; [DOI] [PubMed] [Google Scholar]
- 4g. Kraus G. A., Dneprovskaia E., Nguyen T. H., Jeon I., Tetrahedron 2003, 59, 8975–8978; [Google Scholar]
- 4h. Klein A., Miesch M., Tetrahedron Lett. 2003, 44, 4483–4485; [Google Scholar]
- 4i. Ahmad N. M., Rodeschini V., Simpkins N. S., Ward S. E., Wilson C., Org. Biomol. Chem. 2007, 5, 1924–1934; [DOI] [PubMed] [Google Scholar]
- 4j. Nuhant P., David M., Pouplin T., Delpech B., Marazano C., Org. Lett. 2007, 9, 287–289; [DOI] [PubMed] [Google Scholar]
- 4k. Takagi R., Nerio T., Miwa Y., Matsumura S., Ohkata K., Tetrahedron Lett. 2004, 45, 7401–7405; [Google Scholar]
- 4l. Kuninobu Y., Morita J., Nishi M., Kawata A., Takai K., Org. Lett. 2009, 11, 2535–2537; [DOI] [PubMed] [Google Scholar]
- 4m. Vidali V. P., Mitsopoulou K. P., Dakanali M., Demadis K. D., Odysseos A. D., Christou Y. A., Couladouros E., Org. Lett. 2013, 15, 5404–5407; [DOI] [PubMed] [Google Scholar]
- 4n. Schmitt S., Feidt E., Hartmann D., Huch V., Jauch J., Synlett 2014, 25, 2025–2029; [Google Scholar]
- 4o. Kallepu S., Gollapelli K. K., Nanubolu J. B., Chegondi R., Chem. Commun. 2015, 51, 16840–16843; [DOI] [PubMed] [Google Scholar]
- 4p. Wang L., Sun L., Wang X., Wu R., Zhou H., Zheng C., Xu H., Org. Lett. 2019, 21, 8075–8079. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Kuramochi A., Usuda H., Yamatsugu K., Kanai M., Shibasaki M., J. Am. Chem. Soc. 2005, 127, 14200–14201; [DOI] [PubMed] [Google Scholar]
- 5b. Siegel D. R., Danishefsky S. J., J. Am. Chem. Soc. 2006, 128, 1048–1049; [DOI] [PubMed] [Google Scholar]
- 5c. Uwamori M., Nakada M., J. Antibiot. 2013, 66, 141–145; [DOI] [PubMed] [Google Scholar]
- 5d. Shen X., Ting C. P., Xu G., Maimone T. J., Nat. Commun. 2020, 11, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.
- 6a. Adam P., Arigoni D., Bacher A., Eisenreich W., J. Med. Chem. 2002, 45, 4786–4793; [DOI] [PubMed] [Google Scholar]
- 6b. Horeischi F., Biber N., Plietker B., J. Am. Chem. Soc. 2014, 136, 4026–4030. [DOI] [PubMed] [Google Scholar]
- 7.For example, see: Wang X., Phang Y., Feng J., Liu S., Zhang H., Fu W., Zhou H., Xu G., Xu H., Zheng C., Org. Lett. 2021, 23, 4203–4208. [DOI] [PubMed] [Google Scholar]
- 8.For clusianone, see:
- 8a. Rodeschini V., Simpkins N. S., Wilson C., J. Org. Chem. 2007, 72, 4265–4267; [DOI] [PubMed] [Google Scholar]
- 8b. Garnsey M. R., Lim D., Yost J. M., Coltart D. M., Org. Lett. 2010, 12, 5234–5237; [DOI] [PubMed] [Google Scholar]
- 8c. Boyce J. H., J. A. Porco, Jr. , Angew. Chem. Int. Ed. 2014, 53, 7832–7837; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 7966–7971; [Google Scholar]
- 8d. Horeischi F., Guttroff C., Plietker B., Chem. Commun. 2015, 51, 2259–2261. For nemorosone, see: [DOI] [PubMed] [Google Scholar]
- 8e. Sparling B. A., Tucker J. K., Moebius D. C., Shair M. D., Org. Lett. 2015, 17, 3398–3401; [DOI] [PubMed] [Google Scholar]
- 8f. Wen S., Boyce J. H., Kandappa S. K., Sivaguru J., J. A. Porco, Jr. , J. Am. Chem. Soc. 2019, 141, 11315–11321. For hyperforin, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8g. Shimizu Y., Shi S.-L., Usuda H., Kanai M., Shibasaki M., Angew. Chem. Int. Ed. 2010, 49, 1103–1106; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 1121–1124; [Google Scholar]
- 8h. Shimizu Y., Shi S.-L., Usuda H., Kanai M., Shibasaki M., Tetrahedron 2010, 66, 6569–6584; [Google Scholar]
- 8i. Sparling B. A., Moebius D. C., Shair M. D., J. Am. Chem. Soc. 2013, 135, 644–647. [DOI] [PubMed] [Google Scholar]
- 9.For the disconnection of C1−C2 in related synthetic studies, see:
- 9a. Spessard S. J., Stoltz B. M., Org. Lett. 2002, 4, 1943–1946; [DOI] [PubMed] [Google Scholar]
- 9b. Young D. G. J., Zeng D., J. Org. Chem. 2002, 67, 3134–3137; [DOI] [PubMed] [Google Scholar]
- 9c. Mehta G., Bera M. K., Tetrahedron Lett. 2006, 47, 689–692; [Google Scholar]
- 9d. Shimizu Y., Kuramochi A., Usuda H., Kanai M., Shibasaki M., Tetrahedron Lett. 2007, 48, 4173–4177; [Google Scholar]
- 9e. Pouplin T., Tolon B., Nuhant P., Delpech B., Marazano C., Eur. J. Org. Chem. 2007, 5117–5125; [Google Scholar]
- 9f. Bellavance G., Barriault L., Angew. Chem. Int. Ed. 2014, 53, 6701–6704; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 6819–6822. [Google Scholar]
- 10. Hadjiarapoglou L., de Meijere A., Seitz H.-J., Klein I., Spitzner D., Tetrahedron Lett. 1994, 35, 3269–3272. [Google Scholar]
- 11.
- 11a. Stork G., Danheiser R. L., J. Org. Chem. 1973, 38, 1775–1776; [Google Scholar]
- 11b. Dudley G. B., Takaki K. S., Cha D. D., Danheiser R. L., Org. Lett. 2000, 2, 3407–3410. [DOI] [PubMed] [Google Scholar]
- 12. Deposition Number 2089439 (for 8 c) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 13.
- 13a. Barner B. A., Liu Y., Rahman A., Tetrahedron 1989, 45, 6101–6112; [Google Scholar]
- 13b. Ganiu M. O., Cleveland A. H., Paul J. L., Kartika R., Org. Lett. 2019, 21, 5611–5615. [DOI] [PubMed] [Google Scholar]
- 14.The high (Z)-selectivity appears to be a consequence of syn-selective aldol and syn-elimination, likely due to the steric congestion from the gem-dimethyl group. See Table S1 in the Supporting Information.
- 15. Ganem B., J. Org. Chem. 1975, 40, 146–147. [Google Scholar]
- 16. Poulsen T. B., Bernardi L., Aleman J., Overgaard J., Jørgensen K. A., J. Am. Chem. Soc. 2007, 129, 441–449. [DOI] [PubMed] [Google Scholar]
- 17. Rayabarapu D. K., Tunge J. A., J. Am. Chem. Soc. 2005, 127, 13510–13511. [DOI] [PubMed] [Google Scholar]
- 18. Okamoto S., Ono N., Tani K., Yoshida Y., Sato F., J. Chem. Soc. Chem. Commun. 1994, 279–280. [Google Scholar]
- 19.
- 19a. Kato K., Nishimura A., Yamamoto Y., Akita H., Tetrahedron Lett. 2001, 42, 4203–4205; [Google Scholar]
- 19b. Kato K., Motodate S., Mochida T., Kobayashi T., Akita H., Angew. Chem. Int. Ed. 2009, 48, 3326–3328; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 3376–3378; [Google Scholar]
- 19c. Yasuhara S., Sasa M., Kusakabe T., Takayama H., Kimura M., Mochida T., Kato K., Angew. Chem. Int. Ed. 2011, 50, 3912–3915; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 3998–4001. [Google Scholar]
- 20.
- 20a. Fukuda Y., Shiragami H., Utimoto K., Nozaki H., J. Org. Chem. 1991, 56, 5816–5819; [Google Scholar]
- 20b. Asao N., Nogami T., Takahashi K., Yamamoto Y., J. Am. Chem. Soc. 2002, 124, 764–765. [DOI] [PubMed] [Google Scholar]
- 21.The Nagata hydrocyanation could be carried out but did not lead to the desired Dieckmann reaction. Nagata W., Yoshioka M., Hirai S., J. Am. Chem. Soc. 1972, 94, 4635–4643. [Google Scholar]
- 22.In the sequences from 6 to 11 (Scheme 2) and to 22 (Scheme 3) through consecutive reactions with carbonyl, prenyl and alkynyl electrophiles, the stereochemical outcomes appear to follow the control of the C7 stereocenter. Both the stereoelectronic preference of the axial trajectory and the stereosteric effect of the C8 axial methyl group in the transition state synergistically favor the α-approach of an electrophile. See Scheme S4 in the Supporting Information.
- 23.For a similar observation regarding the kinetic inertness of the C1 methine toward a hindered base, see ref. [4b].
- 24.The addition of more than 2.5 equivalents of Dibal-H began to reduce the C21 ketone. See Table S5 in the Supporting Information.
- 25.
- 25a. Ranu B. C., Bhar S., Chakraborti R., J. Org. Chem. 1992, 57, 7349–7352; [Google Scholar]
- 25b. Kuroda H., Tomita I., Endo T., Synth. Commun. 1996, 26, 1539–1543; [Google Scholar]
- 25c. Sneddon H. F., van den Heuvel A., Hirsch A., Booth R. A., Shaw D. M., Gaunt M. J., Ley S. V., J. Org. Chem. 2006, 71, 2715–2725; [DOI] [PubMed] [Google Scholar]
- 25d. Worch J. C., Stubbs C. J., Price M. J., Dove A. P., Chem. Rev. 2021, 121, 6744–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.
- 26a. Choony N., Dadabhoy A., Sammes P. G., J. Chem. Soc. Perkin Trans. 1 1998, 2017–2021; [Google Scholar]
- 26b. Kim H., Park Y., Hong J., Angew. Chem. Int. Ed. 2009, 48, 7577–7581; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 7713–7717. [Google Scholar]
- 27.Thioethers 27 and 28, vis-à-vis ether 29, were more easily converted into THF 30 upon formation and hydrolysis of the prenyl epoxide likely due to the intermediacy of a β-sulfinylenone. See Scheme S5 in the Supporting Information. Also see: Freeman F., Angeletakis C. N., J. Am. Chem. Soc. 1983, 105, 4039–4049. [Google Scholar]
- 28. Ahmad N. M., Rodeschini V., Simpkins N. S., Ward S. E., Blake A. J., J. Org. Chem. 2007, 72, 4803–4815. [DOI] [PubMed] [Google Scholar]
- 29.
- 29a. Fabicon R. M., Parvez M., H. G. Richey, Jr. , J. Am. Chem. Soc. 1991, 113, 1412–1414; [Google Scholar]
- 29b. Jaric M., Haag B. A., Manolikakes S. M., Knochel P., Org. Lett. 2011, 13, 2306–2309. [DOI] [PubMed] [Google Scholar]
- 30. Chatterjee A. K., Sanders D. P., Grubbs R. H., Org. Lett. 2002, 4, 1939–1942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information