Abstract
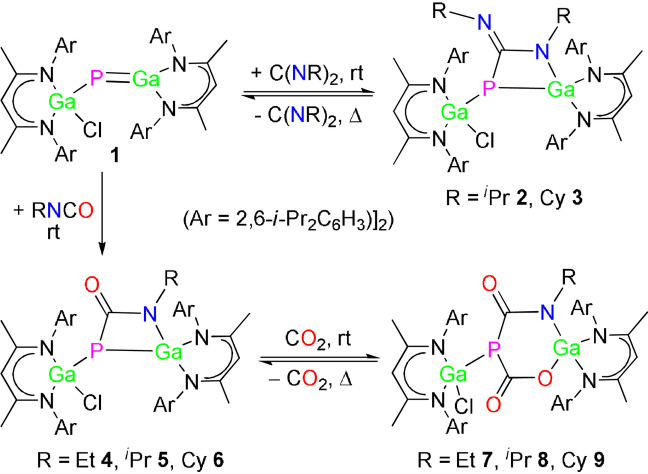
[2+2] Cycloaddition reactions of gallaphosphene L(Cl)GaPGaL 1 (L=HC[C(Me)N(2,6‐i‐Pr2C6H3)]2) with carbodiimides [C(NR)2; R=i‐Pr, Cy] and isocyanates [RNCO; R=Et, i‐Pr, Cy] yielded four‐membered metallaheterocycles LGa(Cl)P[μ‐C(X)NR]GaL (X=NR, R=i‐Pr 2, Cy 3; X=O, R=Et 4, i‐Pr 5, Cy 6). Compounds 4–6 reversibly react with CO2 via [2+2] cycloaddition at ambient temperature to the six‐membered metallaheterocycles LGa(Cl)P[μ‐C(O)O]‐μ‐C(O)N(R)GaL (R=Et 7, i‐Pr 8, Cy 9). Compounds 2–9 were characterized by IR and heteronuclear (1H, 13C{1H}, 31P{1H}) NMR spectroscopy and elemental analysis, while quantum chemical calculations provided a deeper understanding on the energetics of the reactions.
Keywords: [2+2] cycloaddition reactions, CO2 activation, gallaphosphene, heteroallenes, main group elements
[2+2] Cycloaddition reactions of gallaphosphene 1 with carbodiimides and isocyanates yield four‐membered metallaheterocyles 2–6. The carbodiimide cycloadditions are fully reversible at elevated temperature, as was also observed in reactions of compounds 4–6 with CO2.
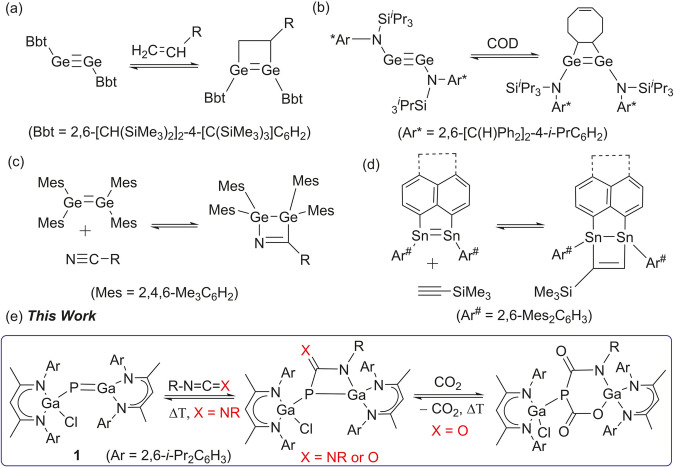
The activation of unsaturated molecules by reversible addition to reactive metal centers is a domain of transition metal chemistry and of central importance in catalytic processes. In contrast, comparable reactions at main‐group element centers are far less developed and have only recently received increasing interest, not only because of fundamental curiosities but also in order to develop transition metal‐like behavior including catalytic reactivity for main group metal complexes.[ 1 , 2 ] Among others, reversible cycloaddition of unsaturated small molecules to multiply bonded main group element compounds is of particular interest and attracted much attention due to their small, sometimes tunable HOMO–LUMO gaps and their biradical‐type bonding nature. [1] In 2009, Power et al. reported on the first reversible [2+2+2] cycloaddition of ethylene to a distannyne, [3] followed by subsequent reports on reversible [2+2] cycloaddition reactions of unsaturated molecules to digermynes (Scheme 1 a,b), [4] ditetrelenes (Scheme 1 c,d), [5] and digallene, [6] respectively. In contrast, comparable reactions at heterodiatomic multiply bonded main group element compounds have rarely been encountered. [7]
Scheme 1.
a–d) Selected examples of reversible [2+2] cycloaddition reactions of multiply bonded compounds (isoelectronic to gallaphosphene 1) containing heavier main group elements. e) [2+2] Cycloaddition reactions of gallaphosphene 1 with heteroallenes.
Metallapnictenes RMPnR (M=B–Tl; Pn=N–Bi) containing M−Pn double bonds are isovalence‐electronic to alkenes and have attracted significant interest due to their fascinating electronic structures and reactivity.[ 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 ] In remarkable contrast to compounds containing B−Pn (Pn=N, P, As) [8] and M−N double bonds (M=Al, Ga, In), [9] the heavier congeners with M−Pn (M=Al, Ga; Pn=P, As, Sb) double bonds have been reported only recently,[ 10 , 11 , 12 , 13 , 14 ] and their reactivity is virtually unexplored.[ 13 , 14 , 15 ] Since the M−Pn double bonds are expected to be highly polarized, [16] they are promising candidates for bond activation reactions of a variety of small molecules as well as unsaturated substrates.[ 13 , 14 , 15 , 16 ] However, the addition of unsaturated small molecules at the polarized Mδ+−Pnδ− double bonds is limited to a recent report by Coles et al. on the irreversible [2+2] cycloaddition of carbon dioxide to an anionic indium imide complex K[In(NONAr)(NMes)] (NONAr=[O(SiMe2NAr)2]2−, Ar=2,6‐i‐Pr2C6H3, Mes=2,4,6‐Me3C6H2), [17] while we reported on reversible [2+2+2] cycloaddition reactions of carbon dioxide to gallaphosphene LGa(Cl)PGaL 1 (L=HC[C(Me)N(Ar)]2). [14] DFT calculations revealed that the addition of the second equivalent of CO2 (ΔG =−7.5 kcal mol−1) is energetically more favored than the addition of the first CO2 molecule (ΔG =−1.8 kcal mol−1), hence only the [2+2+2] cycloaddition product was isolated. These results as well as the scarcity of π‐bonded metallapnictenes to form [2+2] cycloaddition products prompted us to investigate reactions of gallaphosphene 1 with unsaturated organic substrates (Scheme 1 e).
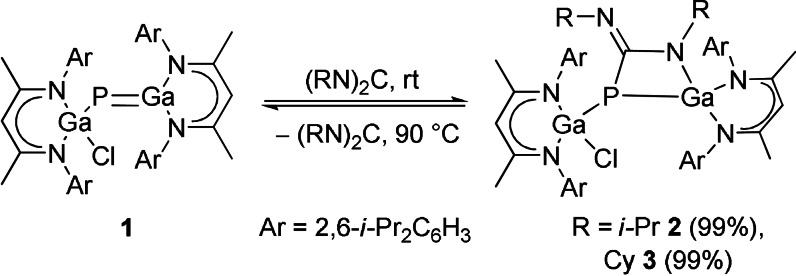
Addition of equimolar amounts of carbodiimides [i‐PrN)2C (DIC), (CyN)2C (DCC)] to red solutions of gallaphosphene 1 in toluene at ambient temperature instantaneously gave colorless solutions of the corresponding [2+2] cycloaddition products LGa(Cl)P[μ‐C(NR)NR]GaL (R=i‐Pr 2, Cy 3), which were isolated in almost quantitative (>99 %) yields (Scheme 2), whereas the sterically hindered carbodiimide (DippN)2C (Dipp=2,6‐i‐Pr2C6H3) failed to react. In contrast to reactions with CO2, [14] both reactions only yielded the [2+2] cycloaddition products 2 and 3 even with a large excess of DIC and DCC. The reactions are temperature‐dependent and fully reversible upon heating to 90 °C as shown by in situ 1H and 31P{1H} NMR spectroscopy, allowing the quantitative regeneration of gallaphosphene 1 and the corresponding carbodiimides (Figures S9–S12). To the best of our knowledge, this is the first report on a reversible, temperature‐dependent [2+2] cycloaddition of a heavier group 13/15 multiply bonded compound.
Scheme 2.
Reversible [2+2] cycloaddition reactions of carbodiimides to gallaphosphene 1.
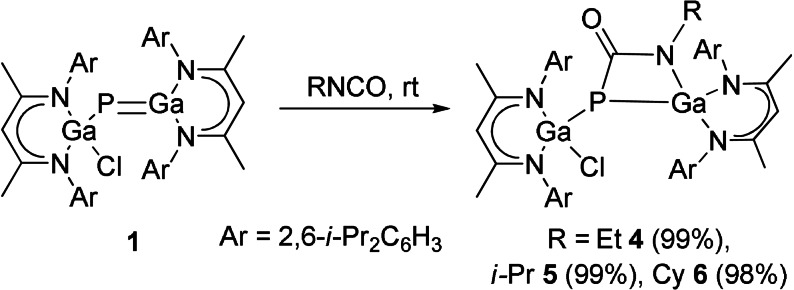
We then reacted gallaphosphene 1 with isocyanates to test whether isocyanates undergo [2+2] cycloaddition reactions with either their C−O or C−N double bonds. All reactions selectively proceeded at ambient temperature with [2+2] cycloaddition at the C−N double bonds and formation of LGa(Cl)P[μ‐C(O)NR]GaL (R=Et 4, i‐Pr 5 and Cy 6) in quantitative (>98 %) yields (Scheme 3), whereas no reaction occurred with sterically more hindered isocyanates (R=t‐Bu, Dipp), again revealing the influence of steric bulk on the reaction process. Compounds 4–6 also formed in the presence of an excess of the isocyanate, and in contrast to reactions of 1 with CO2 and carbodiimides, the isocyanate cycloaddition reactions are fully irreversible.
Scheme 3.
[2+2] cycloaddition reactions of isocyanates to gallaphosphene 1.
Compounds 2–6 are colorless crystalline solids, which can be stored without decomposition under inert gas atmosphere at ambient temperature for months, whereas they decompose upon exposure to air. The 1H and 13C NMR spectra (see Supporting Information (SI) for details) are comparable to those of LGa‐substituted gallapnictenes,[ 12 , 14 ] dipnictanes, [18] dipnictenes, [19] radicals, [20] and other complexes. [21] The 31P{1H} NMR spectra display sharp singlets (2 −108.6; 3 −105.3; 4 −112.0; 5 −114.8; 6 −115.4 ppm), which are shifted to lower field compared to 1 (−245.8 ppm), but to higher field than L(Cl)GaP[μ‐C(O)O]2GaL (−52.1 ppm). [14]
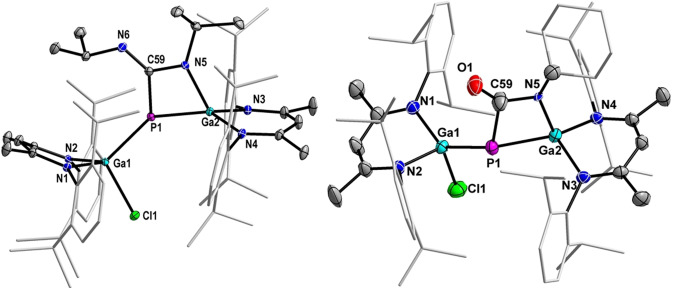
The solid‐state molecular structures of compounds 2 and 6 were determined by single‐crystal X‐ray diffraction (Figure 1). [22] Single crystals were obtained upon storage of saturated toluene/n‐hexane solutions at −30 °C (see SI for details). Compound 2 crystallizes in the monoclinic space group P21 and compound 6 in the orthorhombic space group P212121. [22]
Figure 1.
Molecular structures of compounds 2 (left) and 6 (right). Ellipsoids set at 50 % probability; hydrogen atoms, alternate positions of the minor disordered parts of 6, and solvent molecules (toluene in 2 and n‐hexane in 6) are omitted for clarity.
Both compounds exhibit planar four‐membered GaPCN heterocycles, which adopt almost perpendicular orientations to the six‐membered C3N2Ga2 ring. The gallium atoms adopt distorted tetrahedral and the phosphorous atoms trigonal pyramidal geometries. The Ga1‐P1‐Ga2 bond angles in 2 (131.1(2)°) and 6 (133.1(1)°) are significantly larger compared to that of 1 (113.87(2)°) [14] and [L(Br)Ga]2PBr (118.94(8)°). [19c] The Ga−P bond lengths in 2 (Ga1−P1 2.3084(4) Å, Ga2−P1 2.3115(4) Å) and 6 (Ga1−P1 2.296(2) Å, Ga2−P1 2.266(2) Å) agree with the sum of the calculated single‐bond radii (Ga 1.24 Å; P 1.11 Å) [23] and with Ga−P single bonds in related systems, [24] but are elongated compared to the Ga−P double bonds of neutral gallaphosphene 1 (2.16(6) Å) [14] and cationic gallaphosphene [LGaP(MecAAC)] (2.2393(6) Å). [25]
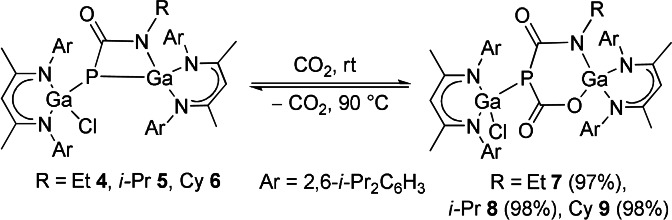
Strained ring systems are valuable synthons in organic synthesis. [26a] Introducing heteroatoms in such rings led to charge‐induced asymmetry, hence providing reactive reaction sites. [26b] The strained four‐membered metallaheterocycles in compounds 2–6 are therefore promising candidates for bond activation reactions of small molecules. Compounds 2–6 immediately react with CO2 at ambient temperature as is indicated by a slight color change of the reaction solution. Reactions of 2 and 3 yielded a mixture of single and double [14] CO2 addition products with concomitant release of the corresponding carbodiimides (Scheme S1), from which pure products could not be isolated due to their almost equal solubilities (Figures S29–S32). In contrast, compounds 4–6 cleanly reacted with CO2 to L(Cl)GaP[μ‐C(O)O][μ‐C(O)NR]GaL (R=Et 7, i‐Pr 8, and Cy 9), respectively (Scheme 4). The reactions are temperature‐dependent and fully reversible upon heating to 90 °C as shown by in situ 1H and 31P{1H} NMR spectroscopy (Figures S45–S50), allowing for the quantitative regeneration of compounds 4–6 with the release of CO2. The reversible binding of CO2 in main group chemistry was previously observed with frustrated Lewis pairs, organic Lewis bases, [27] and for gallaphosphene 1, [14] but to the best of our knowledge was only reported once for a metallaheterocycle. The calix[4]pyrrolato aluminate anion featuring a square‐planar AlIII cation was found to reversibly bind CO2 and aldehydes. [28] Moreover, temperature‐dependent ring expansion/contraction reactions are unprecedented in main group metal chemistry.
Scheme 4.
Temperature‐dependent binding/release reactions of compounds 4, 5, and 6 with CO2.
Compounds 7, 8, and 9 are colorless crystalline solids and soluble in common organic solvents. The 13C{1H} NMR spectra each exhibit two doublets of the NCO and OCO moieties, and the 31P{1H} NMR spectra show singlets at −40.0 ppm (7), −34.5 ppm (8), and −35.0 ppm (9), respectively, which are downfield shifted compared to the starting reagents 4 (−112.0 ppm), 5 (−114.8 ppm), and 6 (−115.4 ppm) as well as L(Cl)GaP−[μ‐C(O)O]2−GaL (−52.1 ppm). [14]
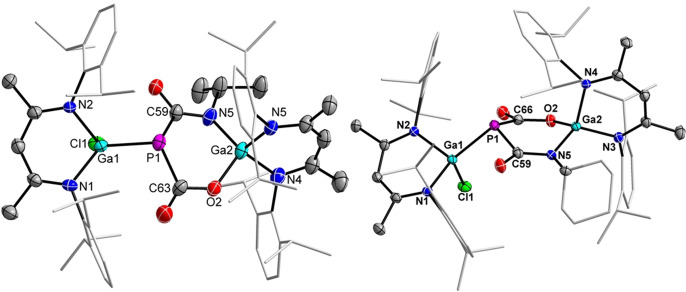
The molecular structures of compounds 8 and 9 were determined by single‐crystal X‐ray diffraction (Figure 2). [22] Crystals were obtained upon storage of saturated toluene solutions at ambient temperature. The Ga1−P1 bond lengths in 8 (2.3272(15) Å) and 9 (2.3215(5) Å) are comparable to those in compounds 2 and 6. The P−C (1.8524(18)–1.861(5) Å) and Ga2−N5 (1.887(5)–1.8963(14) Å) bond lengths of 8 and 9 as well as the Ga2−O2 bond lengths 1.840(4) Å (8) and 1.8337(12) Å (9) are almost identical.
Figure 2.
Molecular structures of 8 (left) and 9 (right). Ellipsoids set at 50 % probability; hydrogen atoms and solvent molecules (toluene in 9) are omitted for clarity.
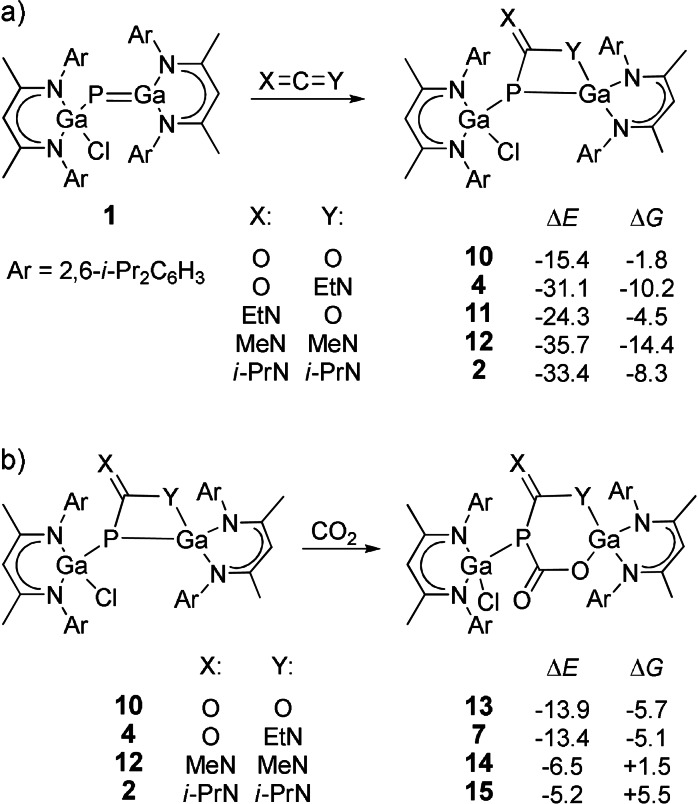
Further, we performed DFT calculations at the B3LYP‐D3BJ/def2‐TZVP level of theory [29] to determine the energetics of the [2+2] cycloaddition reactions of gallaphosphene 1 with CO2, isocyanate EtNCO, as well as carbodiimides MeNCNMe (DMC) and i‐PrNCNi‐Pr (DIC) to the corresponding four‐membered metallaheterocycles 2, 4, and 10–12 (Scheme 5 a). The two carbodiimides were chosen to investigate the influence of the alkyl group of the carbodiimide (small methyl vs. large isopropyl group) in the cycloaddition reaction. In a second step, we calculated the energetic parameters of the cycloaddition reaction of the four‐membered metallaheterocycles 2, 4, 10, and 12 with CO2 to the six‐membered metallaheterocycles 7 and 13–15 (Scheme 5 b).
Scheme 5.
a) Calculation of the [2+2] cycloaddition reactions of gallaphosphene 1 with CO2, isocyanate EtNCO and two carbodiimides DMC and DIC to the four‐membered metallaheterocycles 2, 4, and 10–12. b) Calculation of the addition of CO2 to the metallaheterocycles 2, 4, 10, and 12 yielding the six‐membered metallaheterocycles 7 and 13–15. The calculations were performed using B3LYP‐D3BJ/def2‐TZVP and the energy values are given in kcal mol−1.
The reaction energy (ΔE) for the [2+2] cycloaddition reaction of the gallaphosphenes 1 with CO2 is the most unfavorable and amounts to −15.4 kcal mol−1. The addition of EtNCO can either proceed via the C−N (4) or C−O (11) double bond. However, the calculations show that the C−N cycloaddition to 1 is energetically more favorable by 6.8 kcal mol−1 than the C−O cycloaddition. The reaction with the carbodiimides is energetically the most favorable, with values ranging from −33.4 to −35.7 kcal mol−1.
A different order is found for the calculated Gibbs free energies (ΔG). Here the C−N cycloaddition of DIC is even less favorable (−8.3 kcal mol−1) than that of EtNCO (−10.2 kcal mol−1). This means that starting from 1 the formation of 2 is less favorable than the formation of 4 due to entropic reasons. Presumably, the free rotation of the large groups in 2 is severely restricted and the entropy loss during the cycloaddition is correspondingly high. This also explains the fact that the formation of 2 and 13 is reversible, while 4 is irreversibly formed starting from 1. Note that the reversibility of the formation of 13 is due to enthalpic reasons, whereas in the case of 2 it is mainly due to entropy.
In order to examine whether the [2+2] cycloaddition reactions proceed in a concerted or stepwise fashion, the transition states for the addition of CO2 (TS1→10 ) and EtNCO (TS1→4 ) to 1 were calculated using B3LYP‐D3BJ/def2‐TZVP//B3LYP‐D3BJ/6‐31G* level of theory. The results show that both addition reactions take place in one step, whereby the Gibbs free energies amount to 14.0 (TS1→10 ) and 8.8 kcal mol−1 (TS1→4 ). Due to the highly polarized bonds, the distances between the reaction centers in the transition states differ significantly: The Ga−O and Ga−N distances (TS1→10 : 2.376 Å; TS1→4 : 2.272 Å) are by far smaller than the P−C distances (TS1→10 : 2.698 Å; TS1→4 : 3.173 Å).
We also determined the thermodynamic parameters for the addition of CO2 to the four‐membered metallaheterocycles 2, 4, 10 and 12 (Scheme 5 b). The reaction energies (ΔE) for the CO2 and EtNCO adducts 4 and 10 vary between −13.4 and −13.9 kcal mol−1, which roughly corresponds to the energy obtained by the cycloaddition of CO2 to the gallaphosphenes 1 (−15.4 kcal mol−1). The values for the Gibbs energy for the addition of CO2 to the four‐membered rings 4 and 10 are even lower than that for the addition of CO2 to the P−Ga double bond of 1; that is, the first step (addition of CO2 or EtNCO to 1) leads to an activation of the resulting system regard to a CO2 addition. The calculated Gibbs free energies (ΔG) for the formation of 7 and 13 amount to −5.1 kcal mol−1 and −5.7 kcal mol−1, respectively. Accordingly, the absolute value is quite small and explains the experimentally found reversibility of the CO2 addition to 4 and 10. The cycloaddition of CO2 to the RNCNR adducts 2 and 12 is significantly unfavored compared to the cycloaddition of CO2 to the previously mentioned systems. Both are endergonic reactions. This agrees with the experimentally found behavior of 2 in the presence of CO2, according to which products 15 and 13 are obtained. The latter is formed by the release of DIC.
In summary, we reported on the first [2+2] cycloaddition reactions of a heavier group 13/15 metallapnictene with heteroallenes. Gallaphosphene 1 selectively reacts with C−N double bonds of carbodiimides and isocyanates at ambient temperature, affording four‐membered metallaheterocycles 2–6. The reactions with carbodiimides are fully reversible, yielding gallaphosphene 1 and the corresponding carbodiimides upon heating. Compounds 2–6 are promising synthons for CO2 activation reactions. Reactions of compounds 4–6 with CO2 yielded compounds 7, 8, and 9, respectively. The reactions are fully reversible, and CO2 is released upon heating of 7–9 to 90 °C. DFT calculations show that the addition of CO2 to the four‐membered metallaheterocycles is reversible due to the low reaction energy. In contrast, the reversibility of the addition of carbodiimides showing large substituents to 1 is due to the loss of entropy in the cyclization reaction.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
Financial support from the Deutsche Forschungsgemeinschaft (grant no. SCHU 1069/27‐1) and the University of Duisburg–Essen (S.S.; G.H.) is gratefully acknowledged. Open access funding enabled and organized by Projekt DEAL.
M. K. Sharma, C. Wölper, G. Haberhauer, S. Schulz, Angew. Chem. Int. Ed. 2021, 60, 21784.
Dedicated to Professor Matthias Driess on the occasion of his 60th birthday
Contributor Information
Dr. Mahendra K. Sharma, https://www.uni‐due.de/ak_schulz/index_en.php.
Prof. Dr. Stephan Schulz, Email: stephan.schulz@uni-due.de.
References
- 1.
- 1a. Power P. P., Nature 2010, 463, 171–177; [DOI] [PubMed] [Google Scholar]
- 1b. Power P. P., Acc. Chem. Res. 2011, 44, 627–637; [DOI] [PubMed] [Google Scholar]
- 1c. Weetman C., Inoue S., ChemCatChem 2018, 10, 4213–4228; [Google Scholar]
- 1d. Melen R. L., Science 2019, 363, 479–484; [DOI] [PubMed] [Google Scholar]
- 1e. Weetman C., Chem. Eur. J. 2021, 27, 1941–1954; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1f. Hadlington T. J., Driess M., Jones C., Chem. Soc. Rev. 2018, 47, 4176–4197; [DOI] [PubMed] [Google Scholar]
- 1g. Stephan D. W., Science 2016, 354, 6317. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Rodriguez R., Gau D., Kato T., Saffon-Merceron N., De Cózar A., Cossío F. P., Baceiredo A., Angew. Chem. Int. Ed. 2011, 50, 10414–10416; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 10598–10600; [Google Scholar]
- 2b. Lips F., Fettinger J. C., Mansikkamäki A., Tuononen H. M., Power P. P., J. Am. Chem. Soc. 2014, 136, 634–637; [DOI] [PubMed] [Google Scholar]
- 2c. Lai T. Y., Gullett K. L., Chen C.-Y., Fettinger J. C., Power P. P., Organometallics 2019, 38, 1421–1424; [Google Scholar]
- 2d. Sita L. R., Bickerstaff R. D., J. Am. Chem. Soc. 1988, 110, 5208–5209; [Google Scholar]
- 2e. Boutland A. J., Carroll A., Alvarez Lamsfus C., Stasch A., Maron L., Jones C., J. Am. Chem. Soc. 2017, 139, 18190–18193; [DOI] [PubMed] [Google Scholar]
- 2f. Wu D., Ganguly R., Li Y., Hoo S. N., Hirao H., Kinjo R., Chem. Sci. 2015, 6, 7150–7155; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2g. Taylor J. W., McSkimming A., Guzman C. F., Harman W. H., J. Am. Chem. Soc. 2017, 139, 11032–11035; [DOI] [PubMed] [Google Scholar]
- 2h. Radzewich C. E., Coles M. P., Jordan R. F., J. Am. Chem. Soc. 1998, 120, 9384–9385; [Google Scholar]
- 2i. Bakewell C., White A. J. P., Crimmin M. R., Angew. Chem. Int. Ed. 2018, 57, 6638–6642; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 6748–6752; [Google Scholar]
- 2j. Bakewell C., White A. J. P., Crimmin M. R., Chem. Sci. 2019, 10, 2452–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peng Y., Ellis B. D., Wang X., Fettinger J. C., Power P. P., Science 2009, 325, 1668–1670. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Sugahara T., Guo J.-D., Sasamori T., Nagase S., Tokitoh N., Chem. Commun. 2018, 54, 519–522; [DOI] [PubMed] [Google Scholar]
- 4b. Hadlington T. J., Li J., Hermann M., Davey A., Frenking G., Jones C., Organometallics 2015, 34, 3175–3185. [Google Scholar]
- 5.
- 5a. Hardwick J. A., Baines K. M., Angew. Chem. Int. Ed. 2015, 54, 6600–6603; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 6700–6703; [Google Scholar]
- 5b. Schneider J., Henning J., Edrich J., Schubert H., Wesemann L., Inorg. Chem. 2015, 54, 6020–6027. [DOI] [PubMed] [Google Scholar]
- 6. Caputo C. A., Guo J.-D., Nagase S., Fettinger J. C., Power P. P., J. Am. Chem. Soc. 2012, 134, 7155–7164. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Nesterov V., Baierl R., Hanusch F., Ferao A. E., Inoue S., J. Am. Chem. Soc. 2019, 141, 14576–14580. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Linti G., Nöth H., Polborn K., Paine R. T., Angew. Chem. Int. Ed. Engl. 1990, 29, 682–684; [Google Scholar]; Angew. Chem. 1990, 102, 715–717; [Google Scholar]
- 8b. Rivard E., Merrill W. A., Fettinger J. C., Power P. P., Chem. Commun. 2006, 3800–3802; [DOI] [PubMed] [Google Scholar]
- 8c. Rosas-Sánchez A., Alvarado-Beltran I., Baceiredo A., Hashizume D., Saffon-Merceron N., Branchadell V., Kato T., Angew. Chem. Int. Ed. 2017, 56, 4814–4818; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 4892–4896. [Google Scholar]
- 9.
- 9a. Hardman N. J., Cui C., Roesky H. W., Fink W. H., Power P. P., Angew. Chem. Int. Ed. 2001, 40, 2172–2174; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 2230–2232; [Google Scholar]
- 9b. Wright R. J., Phillips A. D., Allen T. L., Fink W. H., Power P. P., J. Am. Chem. Soc. 2003, 125, 1694–1695; [DOI] [PubMed] [Google Scholar]
- 9c. Wright R. J., Brynda M., Fettinger J. C., Betzer A. R., Power P. P., J. Am. Chem. Soc. 2006, 128, 12498–12509; [DOI] [PubMed] [Google Scholar]
- 9d. Li J., Li X., Huang W., Hu H., Zhang J., Cui C., Chem. Eur. J. 2012, 18, 15263–15266; [DOI] [PubMed] [Google Scholar]
- 9e. Anker M. D., Lein M., Coles M. P., Chem. Sci. 2019, 10, 1212–1218; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9f. Anker M. D., Schwamm R. J., Coles M. P., Chem. Commun. 2020, 56, 2288–2291; [DOI] [PubMed] [Google Scholar]
- 9g. Heilmann A., Hicks J., Vasko P., Goicoechea J. M., Aldridge S., Angew. Chem. Int. Ed. 2020, 59, 4897–4901; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 4927–4931. [Google Scholar]
- 10. Fischer M., Nees S., Kupfer T., Goettel J. T., Braunschweig H., Hering-Junghans C., J. Am. Chem. Soc. 2021, 143, 4106–4111. [DOI] [PubMed] [Google Scholar]
- 11. von Hänisch C., Hampe O., Angew. Chem. Int. Ed. 2002, 41, 2095–2097; [PubMed] [Google Scholar]; Angew. Chem. 2002, 114, 2198–2200. [Google Scholar]
- 12.
- 12a. Ganesamoorthy C., Helling C., Wölper C., Frank W., Bill E., G. E. Cutsail III , Schulz S., Nat. Commun. 2018, 9, 87–95; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12b. Helling C., Wölper C., Schulz S., J. Am. Chem. Soc. 2018, 140, 5053–5056; [DOI] [PubMed] [Google Scholar]
- 12c. Krüger J., Ganesamoorthy C., John L., Wölper C., Schulz S., Chem. Eur. J. 2018, 24, 9157–9164; [DOI] [PubMed] [Google Scholar]
- 12d. Helling C., Wölper C., Schulte Y., G. Cutsail III , Schulz S., Inorg. Chem. 2019, 58, 10323–10332; [DOI] [PubMed] [Google Scholar]
- 12e. Schoening J., John L., Wölper C., Schulz S., Dalton Trans. 2019, 48, 17729–17734. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Wilson D. W. N., Feld J., Goicoechea J. M., Angew. Chem. Int. Ed. 2020, 59, 20914–20918; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 21100–21104; [Google Scholar]
- 13b. Wilson D., Myers W., Goicoechea J. M., Dalton Trans. 2020, 49, 15249–15255. [DOI] [PubMed] [Google Scholar]
- 14. Sharma M. K., Wölper C., Haberhauer G., Schulz S., Angew. Chem. Int. Ed. 2021, 60, 6784–6790; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 6859–6865. [Google Scholar]
- 15. Krüger J., Wölper C., Schulz S., Angew. Chem. Int. Ed. 2021, 60, 3572–3575; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 3615–3618. [Google Scholar]
- 16. Shih T.-W., Li M.-C., Su M.-D., Inorg. Chem. 2015, 54, 5154–5161. [DOI] [PubMed] [Google Scholar]
- 17. Anker M. D., Lein M., Coles M. P., Chem. Sci. 2019, 10, 1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helling C., Wölper C., Schulz S., Eur. J. Inorg. Chem. 2020, 4225–4235. [Google Scholar]
- 19.
- 19a. Song L., Schoening J., Wölper C., Schulz S., Schreiner P. R., Organometallics 2019, 38, 1640–1647; [Google Scholar]
- 19b. Krüger J., Schoening J., Ganesamoorthy C., John L., Wölper C., Schulz S., Z. Anorg. Allg. Chem. 2018, 644, 1028–1033; [Google Scholar]
- 19c. Tuscher L., Helling C., Wölper C., Frank W., Nizovtsev A. S., Schulz S., Chem. Eur. J. 2018, 24, 3241–3250. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a. Krüger J., Wölper C., Schulz S., Inorg. Chem. 2020, 59, 11142–11151; [DOI] [PubMed] [Google Scholar]
- 20b. Helling C., Wölper C., G. E. Cutsail III , Haberhauer G., Schulz S., Chem. Eur. J. 2020, 26, 13390–13399; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20c. Helling C., G. E. Cutsail III , Weinert H., Wölper C., Schulz S., Angew. Chem. Int. Ed. 2020, 59, 7561–7568; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 7631–7638. [Google Scholar]
- 21.
- 21a. Krüger J., Wölper C., John L., Song L., Schreiner P. R., Schulz S., Eur. J. Inorg. Chem. 2019, 1669–1678; [Google Scholar]
- 21b. Tuscher L., Helling C., Ganesamoorthy C., Krüger J., Wölper C., Frank W., Nizovtsev A. S., Schulz S., Chem. Eur. J. 2017, 23, 12297–12304; [DOI] [PubMed] [Google Scholar]
- 21c. Ganesamoorthy C., Krüger J., Wölper C., Nizovtsev A. S., Schulz S., Chem. Eur. J. 2017, 23, 2461–2468; [DOI] [PubMed] [Google Scholar]
- 21d. Tuscher L., Ganesamoorthy C., Bläser D., Wölper C., Schulz S., Angew. Chem. Int. Ed. 2015, 54, 10657–10661; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 10803–10807. [Google Scholar]
- 22.Full crystallographic data of all structurally characterized compounds described herein as well as central bond lengths and angles (Tables S1 and Figures S47–S51) are given in the Supporting Information. Deposition numbers 2089048 (2), 2089049 (6), 2089050 (8), and 2089051 (9) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 23. Pyykkö P., Atsumi M., Chem. Eur. J. 2009, 15, 186–197. [DOI] [PubMed] [Google Scholar]
- 24.
- 24a. Burford N., Ragogna P. J., Robertson K. N., Cameron T. S., Hardman N. J., Power P. P., J. Am. Chem. Soc. 2002, 124, 382–383; [DOI] [PubMed] [Google Scholar]
- 24b. Prabusankar G., Doddi A., Gemel C., Winter M., Fischer R. A., Inorg. Chem. 2010, 49, 7976–7980; [DOI] [PubMed] [Google Scholar]
- 24c. Seifert A., Scheid D., Linti G., Zessin T., Chem. Eur. J. 2009, 15, 12114–12120; [DOI] [PubMed] [Google Scholar]
- 24d. Li B., Bauer S., Seidl M., Scheer M., Chem. Eur. J. 2019, 25, 13714–13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.
- 25a. Li B., Wölper C., Haberhauer G., Schulz S., Angew. Chem. Int. Ed. 2021, 60, 1986–1991; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 2014–2019; [Google Scholar]
- 25b. Jancik V., Pineda L. W., Stückl A. C., Roesky H. W., Herbst-Irmer R., Organometallics 2005, 24, 1511–1515. [Google Scholar]
- 26.
- 26a. Luque A., Paternoga J., Opatz T., Chem. Eur. J. 2021, 27, 4500–4516; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26b. He G., Shynkaruk O., Lui M. W., Rivard E., Chem. Rev. 2014, 114, 7815–7880. [DOI] [PubMed] [Google Scholar]
- 27.
- 27a. Mömming C. M., Otten E., Kehr G., Fröhlich R., Grimme S., Stephan D. W., Erker G., Angew. Chem. Int. Ed. 2009, 48, 6643–6646; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 6770–6773; [Google Scholar]
- 27b. Buß F., Mehlmann P., Mück-Lichtenfeld C., Bergander K., Dielmann F., J. Am. Chem. Soc. 2016, 138, 1840–1843; [DOI] [PubMed] [Google Scholar]
- 27c. Villiers C., Dognon J.-P., Pollet R., Thury P., Ephritikhine M., Angew. Chem. Int. Ed. 2010, 49, 3465–3468; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 3543–3546; [Google Scholar]
- 27d. Murphy L. J., Robertson K. N., Kemp R. A., Tuononend H. M., Clyburne J. A. C., Chem. Commun. 2015, 51, 3942–3956. [DOI] [PubMed] [Google Scholar]
- 28. Ebner F., Sigmund L. M., Greb L., Angew. Chem. Int. Ed. 2020, 59, 17118–17124; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 17266–17272. [Google Scholar]
- 29.
- 29a. Miehlich B., Savin A., Stoll H., Preuss H., Chem. Phys. Lett. 1989, 157, 200–206; [Google Scholar]
- 29b. Becke A. D., Phys. Rev. A 1988, 38, 3098–3100; [DOI] [PubMed] [Google Scholar]
- 29c. Lee C., Yang W., Parr R. G., Phys. Rev. B 1988, 37, 785–789; [DOI] [PubMed] [Google Scholar]
- 29d. Grimme S., Ehrlich S., Goerigk L., J. Comput. Chem. 2011, 32, 1456–1465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information