Abstract
The key role of oxidative stress (OxSt) and inflammation for the induction of cardiovascular disease, the leading cause of excess morbidity/mortality in chronic kidney disease and dialysis patients, is known and both the activations of NADPH oxidase and RhoA/Rho kinase (ROCK) pathway are pivotal for their effects. While specific hemodialysis procedures, such as hemodiafiltration with on‐line reinfusion of ultrafiltrate and/or the use of vitamin E‐coated dialyzers, are beneficial for OxSt and inflammation, studies in peritoneal dialysis (PD) are instead scarce and results seem not favorable. In nine patients under PD OxSt in terms of mononuclear cell protein level of p22phox (Western blot), subunit of NADPH oxidase, essential for the generation of OxSt, and MYPT‐1 phosphorylation state (Western blot), a marker of ROCK activity, have been measured at the beginning and after 3 and 6 months of PD. Blood levels of interleukin 6 (IL‐6), ferritin, and albumin have been considered for evaluating the inflammatory state. p22phox protein expression, MYPT‐1‐phosphorylation, and ferritin level were increased both at baseline vs healthy subjects (P = .02, P < .0001, P = .004, respectively) and vs baseline after 3 and 6 months of peritoneal dialysis (P = .007, P < .001, P = .004, respectively). Albumin was lower after 6 months of PD (P = .0014). IL‐6 was increased at baseline vs reference values and remained unchanged at 3 and 6 months. OxSt and inflammation increase during PD confirming via molecular biology approach a report at biochemical level. To improve OxSt state in PD, a multitarget approach is necessary. It might include the use of more physiologic pH, low glucose degradation products, low lactate and iso‐osmolar PD solutions, patients’ strict glycemic control, optimal volume management, and antioxidant administration, such as N‐acetylcysteine.
Keywords: cardiovascular risk, NADPH oxidase, oxidative stress, peritoneal dialysis, Rho kinase
Oxidative stress (OxSt) and inflammation increase during peritoneal dialysis (PD) confirming via molecular biology approach a biochemical report. They may lead to structural/functional damage of the peritoneal membrane, in addition to increase cardiovascular risk, the leading cause of excess morbidity/mortality in dialysis patients.To improve OxSt in PD a multitarget approach is necessary. It might include the use of more physiologic pH, low glucose degradation products, low lactate and iso‐osmolar PD solutions, patients’ strict glycemic control, optimal volume management.Studies in our laboratory are ongoing to evaluate with a molecular biology approach in PD patients, the effect of more biocompatible solutions on OxSt and its signaling.
1. INTRODUCTION
With the increase in the life expectancy of the general population, chronic kidney disease (CKD) has become one of the most common diseases 1 and a leading cause of mortality. 2 In patients with CKD and in particular in those with end‐stage renal disease (ESRD), cardiovascular disease (CV) is the leading cause of morbidity and mortality. 3 , 4 , 5 These patients have traditional cardiovascular risk factors (hypertension, left ventricular hypertrophy, type II diabetes mellitus, and dyslipidemia), to which are added nontraditional risk factors such as oxidative stress, chronic inflammation, and endothelial dysfunction, which are further amplified with renal replacement therapy (RRT). 5 , 6 All this predisposes patients to CV complications, such as accelerated atherogenesis, CV disease, vascular calcifications, and anemia. 3 , 4 , 5 , 6 , 7 In these patients the reduction of these potentially modifiable risk factors is therefore crucial.
CKD and dialysis patients are exposed to increased oxidative stress due to activation of NADPH oxidase and to the activation of the RhoA/Rho kinase (ROCK) pathway. Both of them are deeply involved in the oxidative stress‐mediated cardiovascular risk/disease while inhibition of RhoA/ROCK signaling provides cardiovascular protection. 5 , 8 , 9
Numerous studies have considered the role of oxidative stress and ROCK signaling and the beneficial effects of their reduction in patients undergoing chronic hemodialysis, in particular using specific hemodialysis procedures, such as hemodiafiltration with on‐line reinfusion of ultrafiltrate (HFR), 10 , 11 or using vitamin E‐coated dialyzers. 12 , 13
Studies in peritoneal dialysis patients are scarce and have instead shown an increase in oxidative stress following the start of peritoneal dialysis. 14
Although a role for this increased oxidative stress with peritoneal dialysis has been generically attributed to the formation of glycosylation end products (AGEs) and other prooxidant molecules derived by glucose or lactate presents in the dialysis fluid, 14 studies with the use of other solutions are lacking. In addition, a more accurate determination of oxidative stress, for example using a molecular biology approach to the evaluation of specific and determinant markers of oxidative stress and CV remodeling is also lacking in patients under peritoneal dialysis.
The aim of this study was therefore to evaluate, using a molecular biology approach, the oxidative stress and inflammatory state in ESRD patient at the beginning and after the first 3 and 6 months from the start of peritoneal dialysis via the evaluation p22phox protein expression, subunit of NADPH oxidase, essential for the translocation of electron on the molecular oxygen to form superoxide anion 15 , 16 and the phosphorylation state of myosin‐phosphatase target protein‐1 (MYPT‐1), marker of Rho kinase (ROCK) activity, which is deeply involved in oxidative stress generation. 5 , 17
The determination of interleukin‐6 (IL‐6), ferritin, and albumin, has also been considered in order to evaluate the patients’ inflammatory state.
2. PATIENTS AND METHODS
2.1. Patients
Nine male patients with ESRD, followed at the Nephrology, Dialysis and Transplantation Unit, at Padua University‐Hospital, age range 39‐71 years, were enrolled to be studied at baseline (before the start of peritoneal dialysis) and at 3 and 6 months from the start of peritoneal dialysis (CAPD for 7 patients and APD for 2). Each patient was treated with low glucose concentration solutions (Fresenius Balance 1.5%, using lactate (35 mmol/L) as a buffer and all patient had residual diuresis at the baseline of 2194 ± 555.9 mL/day.
Patients were selected on the basis of the following criteria: nonsmokers, lack of co‐morbidity such as diabetes, chronic obstructive pulmonary diseases, cerebrovascular disease, heart failure, cancer, lack of severe infections such as peritonitis or infections of the peritoneal catheter exit site, lack of hospitalization in the last 6 months and functioning peritoneal catheter.
Three patients had benign prostatic hypertrophy, all patients were hypertensives with patients’ blood pressure ranging from 130/84 to 155/90 mm Hg under antihypertensive treatment, which included calcium channel blockers, ACE inhibitors, and α‐blockers. All patients were under epoetin treatment at the beginning of the study ranging from 6000 to 10 000 UI/week. Vitamin D was also present in the therapeutic regimen for some patients. None of the patients was under lipid lowering treatment.
The etiology of ESRD for the patients was as follows: chronic glomerulonephritis (four patients) and nephroangiosclerosis (five patients).
A group of nine male healthy normotensive subjects, age range 35‐64 years from the staff of Nephrology, Dialysis and Transplantation Unit, at Padua University‐Hospital were used as control group. All the subjects of the control group were nonsmokers and their blood pressure ranged from 130/70 to 135/85 mm Hg with no antihypertensive medications.
Blood samples for molecular biology analyses have been collected from patients and healthy subjects at the beginning of the study and from patients after 3 and 6 months from the beginning of peritoneal dialysis. Blood levels of IL‐6, ferritin, and albumin have been considered for the evaluation of the inflammatory state.
The study analyses were performed “ex vivo” on plasma and mononuclear cells obtained from a patients’ blood sample included between those for routine analyses and all the study participants gave their informed written consent to be included in the study. The study conforms the standards of the Declaration of Helsinki.
2.2. Methods
IL‐6, ferritin, and albumin were assayed at the Padova Hospital Central Laboratory. IL‐6 concentrations were determined using a fully automated chemiluminescence assay (Medical Systems, Genova, Italy), Ferritin was measured using a fully automated electrochemiluminescence immunoassay (ECLIA) (Roche Diagnostics GmbH, Mannheim, Germany) and albumin was measured using a fully automated immunoturbidimetric assay (Roche Diagnostics GmbH, Mannheim, Germany).
PD patients’ residual diuresis was considered as an index of renal residual function in addition to the evaluation of standard indexes including eGFR.
2.3. Molecular biology assays
2.3.1. p22 phox protein expression and MYPT‐1 phosphorylation state
Peripheral blood mononuclear cells (PBMCs) from 20 mL of ethylenediamine‐tetraacetic acid (EDTA) anticoagulated blood were isolated by Lympholyte‐H (Cedarlane, Burlington, Canada). MYPT‐1 phosphorylation and protein expression of p22 phox were assessed by Western blot analysis as previously reported. 10 , 17 In brief, total protein extract was obtained by lysis of mononuclear cells with lysis buffer (Tris HCl 20 mmol/L, NaCl 150 mmol/L, EDTA 5.0 mmol/L, Niaproof 1.5%, Na3VO4 1.0 mmol/L, SDS 0.1%, PMSF 0.5 mmol/L) added with Proteases Inhibitor Cocktail (Roche Molecular Biochemicals, Mannheim, Germany) and Phosphatase Inhibitor Cocktail I (Sigma‐Aldrich, St Louis, Missouri, USA). Protein concentration was evaluated by bicinchoninic acid assay (BCA ProteinAssay; Pierce). The proteins were separated by SDS‐PAGE in Tris pH 8.3. Protein transfer on nitrocellulose membranes was performed using Hoefer TE 22 Mini Tank Transfer Unit (Amersham Pharmacia Biotech, Uppsala, Sweden) with the use of the following transfer buffer: 39 mmol/L glycine, 48 mmol/L Tris base, 0.037% SDS (electrophoresis grade), and 20% methanol. The membranes were incubated overnight with a primary polyclonal antibody for the detection of a specific protein: anti‐p22phox (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), antiphospho‐MYPT (Cell Signaling technology, Danvers, Massachusetts, USA), and anti‐MYPT (Cell Signaling technology, Danvers, Massachusetts, USA). HRP‐conjugated secondary antibodies were used (Amersham Pharmacia, Uppsala, Sweden) and immunoreactive proteins were visualized with chemiluminescence using SuperSignal WestPico Chemiluminescent Substrate (Pierce). MYPT‐1 phosphorylation was evaluated using a densitometric semiquantitative analysis using NIH image software. The ratio between phospho‐MYPT‐1 and MYPT‐1 was used as an index of MYPT‐1 phosphorylation.
2.4. Statistical analysis
Statistical analysis was performed on a MacBookPro computer using GraphPad Prism 5 software (GraphPad Software Inc, La Jolla, California, USA). Data were expressed as mean ± standard error. ANOVA was used to compare the quantitative variables between groups, and the Student's t test for paired data. Values of 5% or less (P < .05) were considered significant.
3. RESULTS
As expected, patients’ residual diuresis progressively decreased from the start of PD compared with the residual diuresis at 3 and 6 months (baseline: 2194 ± 555.9 mL/day vs 3 months: 1980 ± 421.9 vs 6 months 1944 ± 480), which, however, was not statistically significant (ANOVA: 0.14, P: n.s.).
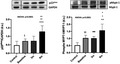
The molecular biology evaluation of markers of oxidative stress in our patients showed, as also expected, a significantly higher protein expression of p22phox at baseline (before the start of peritoneal dialysis) compared with healthy subjects: 0.72 ± 0.05 densitometric units (d.u.) vs 0.47 ± 0.08, P = .02 and significantly increases at 6 months from the start of peritoneal dialysis: 1.28 ± 0.12, P < .0001, ANOVA P < .0001, (Figure 1, panel A).
FIGURE 1.

Densitometric analysis of p22phox to GAPDH ratio (panel A) and phospho‐MYPT‐1 to MYPT ratio (panel B) in mononuclear cells of healthy subjects (C) and peritoneal dialysis patients at baseline (before the start of peritoneal dialysis), at 3 and 6 months from the start of peritoneal dialysis. The top part of each panel shows representative p22phox (panel A) and phospho‐MYPT‐1 (panel B) Western blot products from one healthy subject and one peritoneal dialysis patient. *P < .005 vs 3 mon; **P = .003 vs 3m; + P < .007 vs baseline; ++ P < .001 vs baseline; ▲▲ P < .0001 vs Control; ▲▲▲ P < .0001 vs Control; □□ P < .0001 vs Control; § P = .02 vs Control; §§ P < .0001 vs Control
p22phox protein expression was also significantly increased in peritoneal dialysis patients at 6 months compared with both baseline and 3 months (P = .003 and P = .001, respectively) ANOVA P < .0001, (Figure 1, panel A).
MYPT‐1 phosphorylation was also significantly increased in patients at baseline compared with healthy subjects: 1.03 ± 0.07 d.u. vs 0.52 ± 0.06, P < .0001 and also significantly increases compared to healthy subjects at 3 (1.02 ± 0.04, P = .0001) and 6 months from the start of peritoneal dialysis (1.57 ± 0.16, P = .0001), ANOVA P < .0001 (Figure 1).
MYPT‐1 phosphorylation also significantly increased in peritoneal dialysis patients at 6 months compared with both baseline and 3 months (P = .007 and P = .005, respectively), ANOVA P < .0001.
As shown in Figure 2, ferritin (panel A) was significantly increased (ANOVA P = .002) both at 3 (173.6 ± 11.5 μg/L) and 6 months (189.0 ± 20.3 μg/L) from the start of peritoneal dialysis compared with baseline (101.4 ± 15.3 μg/L), P = .002 and P = .004, respectively, while albumin level (panel B) reduced (ANOVA P = .007) from baseline (43.38 ± 0.10 g/L) to 3 months (40.00 ± 0.159 g/L, P = ns vs baseline) to 6 months (36.46 ± 1.43 g/L, P = .0014 vs baseline) (Figure 2).
FIGURE 2.

Plasma levels of ferritin (panel A) and albumin (panel B) in peritoneal dialysis patients at baseline, at 3 and 6 months from the start of peritoneal dialysis.+ P = .002 vs baseline; *P = .004 vs baseline (panel A); *P = .0014 vs baseline (panel B)
IL‐6 level was higher in patients at baseline (7.5 ± 1.4 ng/L) compared with reference levels (0‐5.9 ng/L). IL‐6 level remained unchanged in patients on peritoneal dialysis at 3 months (IL‐6:6.7 ± 0.94 ng/L) and at 6 month (IL‐6:7.1 ± 0.9 ng/L), (ANOVA: P = .87, n.s.).
4. DISCUSSION
While it is known that the use of RRT procedures, in particular with HFR and vitamin E‐coated dialyzers have particular efficacy in the reduction of oxidative stress and its long‐term CV complications, 10 , 13 the few studies performed in peritoneal dialysis have instead provided not favorable results showing even an increase in markers of oxidative stress. 14
The results of our study confirm that oxidative stress status in term of p22phox protein level and ROCK activity in terms of MYPT‐1 phosphorylation state are increased in ESRD patients at baseline compared to healthy subjects. 10 , 13 , 17 In addition, using a molecular biology approach, the results of this study have shown that oxidative stress further increases after 3 and 6 months from the start of peritoneal dialysis, agreeing with earlier findings provided through biochemical analyses. 14
Regarding the inflammatory status of the patients in our study, IL‐6 already elevated in the predialysis phase of the patients, did not show significant changes after the start of peritoneal dialysis, while, albumin decreased and ferritin progressively increased.
It has been shown that peritoneal albumin and protein losses do not predict outcome in peritoneal dialysis patients. 18 However, although an average of 4 grams of albumin/day is known to be lost with PD, this amount would be generally much higher and would lead to a significant loss of albumin if, for example, a leaky peritoneal membrane was present. This latter event may be determined by specific causes such as, in particular, infections or a state of high transporters which may be induced by an increased peritoneal membrane permeability. Additional cause may be represented by the presence of an inflammatory state.
We have not measured albumin concentration in the peritoneal fluid of our patients, however, both the absence of infections and their status of low‐medium transporters, established by the peritoneal equilibration test (PET), make unlikely the chance of a leaky peritoneal membrane for albumin, which may result in case of high transporter status. The increased level of IL‐6 and ferritin in addition to the increased oxidative stress markers we have found in our PD patients, instead, clearly indicates the presence of an inflammatory state, which may be able to induce the reduction of albumin level shown by our patients at 6 months from the start of PD.
A possible causative role for oxidative stress in peritoneal dialysis has been given to the glucose and lactate present in the dialysis fluids and to the accumulation in the dialysate of glucose degradation products, which would be able to induce in peritoneal cells AGEs, other prooxidants and proinflammatory substances, 14 which in the medium‐long term may induce structural and functional damage of the peritoneal membrane. The consequent chronic accumulation of prooxidant agents within the peritoneum may promote the induction of profibrotic factors that may cause peritoneal fibrosis with consequent loss and failure of ultrafiltration. 19
Improving oxidative stress in these patients is therefore crucial and needs a multitarget strategy.
Residual renal function is known to be closely associated with the survival of patients with ESRD and it should be preserved in particular in patients on peritoneal dialysis. In fact, although the cause and effect are unclear, maintenance of residual renal function in patients on peritoneal dialysis is associated with decreased oxidative status of lipids and proteins, while elevated oxidative stress is predictive of deterioration of residual renal function. 20
Dietary salt and water restriction and the use of diuretics might require less use of hypertonic solutions and, in fact, studies suggest that the use of fluids with low glucose degradation products (GDPs) 21 can protect from peritoneal fibrosis and neo‐angiogenesis. 22 In addition, more biocompatible dialysis solutions have been developed, 21 , 22 with a pH closer to physiologic, low content of GDPs and AGEs and bicarbonate as a buffer. However, the high osmolarity of solutions remains a crucial problem that contributes to the increase in oxidative stress. Fluid bags containing icodextrin, a glucose polymer, are iso‐osmolar and have a reduced concentration of GDPs. In vitro studies have shown that compared to conventional solutions, those containing icodextrin have beneficial effects on oxidative stress and inflammation, due to a reduced osmolarity. 23
Due to this higher biocompatibility, it has been, in fact, proposed that icodextrin can protect the integrity of the peritoneal membrane and reduce oxidative stress, as well as be associated with better control of lipid, glucose, and blood pressure, although this still remains to be confirmed. 23
Finally, in patients on peritoneal dialysis, oral administration of N‐acetylcysteine, a known antioxidant, has also been considered based on its effect of suppression of oxidative stress and AGEs formation, inhibition of local and systemic inflammation, support of residual renal function and diuresis and prevention of dysfunction and sclerosis of the peritoneal membrane, 14 although these beneficial effects also need to be confirmed with specific studies.
In conclusion, oxidative stress in peritoneal dialysis patients is significantly further increased compared to the already activated oxidative state of their predialysis phase, which may lead to important clinical consequences due to intra‐peritoneal and systemic oxidative stress. 20 It is still uncertain whether the use of more biocompatible dialysis solutions and various systemic therapies can improve oxidative stress in patients on peritoneal dialysis, however the first evidence from studies in this field is encouraging. Further studies are necessary and should be focused in particular on the identification of solutions capable of reducing the oxidative stress induced by peritoneal dialysis. Based on this working hypothesis, studies in our laboratory are ongoing aimed to evaluate with a molecular biology approach in patients in peritoneal dialysis, the effect of more biocompatible solutions on oxidative stress status in terms of expression and phosphorylation of proteins strictly related to oxidative stress and its signaling.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest with the contents of this article.
AUTHOR CONTRIBUTIONS
Georgie Innico and Lorenzo A. Calò were involved in concept/design. Georgie Innico, Anna Basso, and Luciana Bonfante were involved in data collection. Giovanni Bertoldi and Matteo Rigato were involved in data analysis and statistics. Georgie Innico, Laura Gobbi, Matteo Rigato, Anna Basso, Luciana Bonfante, and Lorenzo A. Calò were involved in interpretation. Georgie Innico, Laura Gobbi, Matteo Rigato, and Lorenzo A. Calò were involved in drafting article. Lorenzo A. Calò was involved in critical revision of article.
Innico G, Gobbi L, Bertoldi G, et al. Oxidative stress, inflammation, and peritoneal dialysis: A molecular biology approach. Artif Organs. 2021;45:1202–1207. 10.1111/aor.14001
Funding information
This study has been supported in part by a Grant from Department of Medicine, DIMED, University of Padova, 26/11/2020 to GI and in part by the grant DOR 2084023/2020 from University of Padova, to LAC
REFERENCES
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JU, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015 Mortality and Causes of Death Collaborators . Global regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–52. [DOI] [PubMed] [Google Scholar]
- 4. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm L, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in CVD, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–65. [DOI] [PubMed] [Google Scholar]
- 5. Ravarotto V, Simioni F, Pagnin E, Davis PA, Calò LA. Oxidative stress ‐ chronic kidney disease ‐ cardiovascular disease: a vicious circle. Life Sci. 2018;210:125–31. [DOI] [PubMed] [Google Scholar]
- 6. Yao Q, Pecoits‐Filho R, Lindholm B, Stenvinkel P. Traditional and non‐traditional risk factors as contributors to atherosclerotic cardiovascular disease in end‐stage renal disease. Scand J Urol Nephrol. 2004;38:405–16. [DOI] [PubMed] [Google Scholar]
- 7. Libetta C, Sepe V, Esposito E, Galli F, Dal Canton A. Oxidative stress and inflammation: implications in uremia and hemodialysis. Clin Biochem. 2011;44:1189–98. [DOI] [PubMed] [Google Scholar]
- 8. Calò LA, Pessina AC. RhoA/Rho‐kinase pathway: much more that just a modulation of vascular tone. Evidence from studies in humans. J Hypertens. 2007;25:259–64. [DOI] [PubMed] [Google Scholar]
- 9. Seccia TM, Rigato M, Ravarotto V, Calò LA. ROCK (RhoA/Rho Kinase) in cardiovascular‐renal pathophysiology: a review of new advancements. J Clin Med. 2020;9:1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calò LA, Naso A, Carraro G, Wratten ML, Pagnin E, Bertipaglia L, et al. Effect of haemodiafiltration with online regeneration of ultrafiltrate on oxidative stress in dialysis patients. Nephrol Dial Transplant. 2007;22:1413–9. [DOI] [PubMed] [Google Scholar]
- 11. Calò LA, Naso A, Davis PA, Pagnin E, Corradini R, Tommasi A, et al. Hemodiafiltration with online regeneration of ultrafiltrate: effect on heme‐oxygenase‐1 and inducible subunit of nitric oxide synthase and implication for oxidative stress and inflammation. Artif Organs. 2011;35:183–7. [DOI] [PubMed] [Google Scholar]
- 12. Calò LA, Naso A, Pagnin E, Davis PA, Castoro M, Corradin R, et al. Vitamin E‐coated dialyzers reduce oxidative stress related proteins and markers in hemodialysis–a molecular biological approach. Clin Nephrol. 2004;62:355–61. [DOI] [PubMed] [Google Scholar]
- 13. Calò LA, Naso A, D'Angelo A, Pagnin E, Zanardo M, Puato M, et al. Molecular biology‐based assessment of vitamin E‐coated dialyzer effects on oxidative stress, inflammation and vascular remodeling. Artif Organs. 2011;35:E33–9. [DOI] [PubMed] [Google Scholar]
- 14. Roumeliotis S, Eleftheriadis T, Liakopoulos V. Is oxidative stress an issue in peritoneal dialysis? Semin Dial. 2019;32:463–6. [DOI] [PubMed] [Google Scholar]
- 15. Griendling KK, Soresen D, Ushio‐Fukai M. NAD(P)H oxidase. Role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. [DOI] [PubMed] [Google Scholar]
- 16. Knock GA. NADPH oxidase in the vasculature: expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic Biol Med. 2019;145:385–427. [DOI] [PubMed] [Google Scholar]
- 17. Calò LA, Vertolli U, Pagnin E, Ravarotto V, Davis PA, Lupia M, et al. Increased rho‐kinase activity in mononuclear cells of dialysis and stage 3–4 chronic kidney disease patients with left ventricular hypertrophy: Cardiovascular risk implications. Life Sci. 2016;148:80–5. [DOI] [PubMed] [Google Scholar]
- 18. Balafa O, Halbesma N, Struijk DG, Dekker FW, Krediet RT. Peritoneal albumin and protein losses do not predict outcome in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2011;6:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roumeliotis S, Dounousi E, Salmas M, Eleftheriadis T, Liakopoulos V. Unfavorable effects of peritoneal dialysis solutions on the peritoneal membrane: the role of oxidative stress. Biomolecules. 2020;10:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liakopoulos V, Roumeliotis S, Gorny X, Eleftheriadis T, Mertens PR. Oxidative stress in patients undergoing peritoneal dialysis: a current review of the literature. Oxid Med Cell Longev. 2017;2017:3494867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rippe B, Simonsen O, Heimbürger O, Christensson A, Haraldsson B, Stelin G, et al. Long‐term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int. 2001;59:348–57. [DOI] [PubMed] [Google Scholar]
- 22. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro‐Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int. 2004;66:408–18. [DOI] [PubMed] [Google Scholar]
- 23. Goossen K, Becker M, Marshall MR, Bühn S, Breuing J, Firanek CA, et al. Icodextrin versus glucose solutions for the once‐daily long dwell in peritoneal dialysis: an enriched systematic review and meta‐analysis of randomized controlled trials. Am J Kidney Dis. 2020;75:830–46. [DOI] [PubMed] [Google Scholar]


