ABSTRACT
Birds are some of the most diverse organisms on Earth, with species inhabiting a wide variety of niches across every major biome. As such, birds are vital to our understanding of modern ecosystems. Unfortunately, our understanding of the evolutionary history of modern ecosystems is hampered by knowledge gaps in the origin of modern bird diversity and ecosystem ecology. A crucial part of addressing these shortcomings is improving our understanding of the earliest birds, the non‐avian avialans (i.e. non‐crown birds), particularly of their diet. The diet of non‐avian avialans has been a matter of debate, in large part because of the ambiguous qualitative approaches that have been used to reconstruct it. Here we review methods for determining diet in modern and fossil avians (i.e. crown birds) as well as non‐avian theropods, and comment on their usefulness when applied to non‐avian avialans. We use this to propose a set of comparable, quantitative approaches to ascertain fossil bird diet and on this basis provide a consensus of what we currently know about fossil bird diet. While no single approach can precisely predict diet in birds, each can exclude some diets and narrow the dietary possibilities. We recommend combining (i) dental microwear, (ii) landmark‐based muscular reconstruction, (iii) stable isotope geochemistry, (iv) body mass estimations, (v) traditional and/or geometric morphometric analysis, (vi) lever modelling, and (vii) finite element analysis to reconstruct fossil bird diet accurately. Our review provides specific methodologies to implement each approach and discusses complications future researchers should keep in mind. We note that current forms of assessment of dental mesowear, skull traditional morphometrics, geometric morphometrics, and certain stable isotope systems have yet to be proven effective at discerning fossil bird diet. On this basis we report the current state of knowledge of non‐avian avialan diet which remains very incomplete. The ancestral dietary condition in non‐avian avialans remains unclear due to scarce data and contradictory evidence in Archaeopteryx. Among early non‐avian pygostylians, Confuciusornis has finite element analysis and mechanical advantage evidence pointing to herbivory, whilst Sapeornis only has mechanical advantage evidence indicating granivory, agreeing with fossilised ingested material known for this taxon. The enantiornithine ornithothoracine Shenqiornis has mechanical advantage and pedal morphometric evidence pointing to carnivory. In the hongshanornithid ornithuromorph Hongshanornis only mechanical advantage evidence indicates granivory, but this agrees with evidence of gastrolith ingestion in this taxon. Mechanical advantage and ingested fish support carnivory in the songlingornithid ornithuromorph Yanornis. Due to the sparsity of robust dietary assignments, no clear trends in non‐avian avialan dietary evolution have yet emerged. Dietary diversity seems to increase through time, but this is a preservational bias associated with a predominance of data from the Early Cretaceous Jehol Lagerstätte. With this new framework and our synthesis of the current knowledge of non‐avian avialan diet, we expect dietary knowledge and evolutionary trends to become much clearer in the coming years, especially as fossils from other locations and climates are found. This will allow for a deeper and more robust understanding of the role birds played in Mesozoic ecosystems and how this developed into their pivotal role in modern ecosystems.
Keywords: Avialae, birds, dental microwear, diet, theropods, dinosaurs, finite element analysis, fossil, mechanical advantage, morphometrics, stable isotopes
Short abstract
I. INTRODUCTION
(1). Modern and ancient bird diet
Living birds [Aves: used herein to refer to crown birds (see Pittman et al., 2020a )] have been studied more than almost any other organisms, and are at the forefront of human efforts to understand global ecology (Tietze, 2018). In large part this is because birds display some of the most varied diets in the animal kingdom. Many people are familiar with their neighbourhood songbirds which feed on worms and seeds. However, birds are able to thrive in aquatic, terrestrial, and aerial environments around the world (Rico‐Guevara et al., 2019) and consume nearly every source of nutrition imaginable therein. Rodents, fruit, fish, leaves, plankton, blood, beeswax, and organic mud are just a few of the food sources living birds may utilise (Lopes et al., 2016). This rich diversity has also evolved in incredible ways. The ancestral avian has been proposed as an aquatic predator (Brusatte, O'Connor & Jarvis, 2015), granivore (Larson, Brown & Evans, 2016), or omnivore (Felice & Goswami, 2018) with myriad dietary radiations occurring during avian evolution including at least three origins of nectarivory, seven origins of aquatic predation, and 18 origins of frugivory (Felice et al., 2019a ).
Dietary diversification outside of Aves among the non‐avian avialan birds [Avialae: used herein to refer to crown birds plus relatives as distant as Archaeopteryx (Gauthier, 1986; and see Pittman et al., 2020a )] is much less well understood. Two species of early‐diverging avialans preserve evidence of granivory, a single enantiornithine preserves an ingested invertebrate, and 10 ornithuromorph species preserve evidence of granivory or piscivory (O'Connor, 2019). Beyond these, the diet of non‐avian avialans is virtually unknown, and accordingly an ancestral avialan diet has not been proposed. It remains unclear if the vast dietary breath of living birds is unique or has deeper roots in the avialan tree, and if birds played the same unique ecological roles during the Late Jurassic and Cretaceous periods as they do in extant ecosystems.
(2). Diet and morphology in Aves
While avian diet itself has been well reported, few correlations between diet and morphology are known in living birds, and fewer still from more than a single quantitative study. We provide a convenient glossary for the various descriptors of diet used herein in Table 1. Invertivorous birds possess skulls with a low mechanical advantage (Corbin, Lowenberger & Gray, 2015; Olsen, 2017), while probing feeders [e.g. sandpipers (Pettigrew & Frost, 1985), ibises (Frederick & Bildstein, 1992), kiwis (Cunningham et al., 2013), and some songbirds (Lockie, 1956; Adamík & Kornan, 2004)] have particularly elongate rostra (Barbosa & Moreno, 1999; Kulemeyer et al., 2009). Granivorous birds tend to have ventrodorsally tall beaks (van der Meij, 2004; Soons et al., 2010) exhibiting high mechanical advantage (Corbin et al., 2015; Navalón et al., 2018a ) and a high strength (Soons et al., 2010, 2015) [i.e. low peak Von Mises stresses when loaded (Dumont, Grosse & Slater, 2009)]. Raptorial birds possess talons that, on average, are hypertrophied at digit I (Fowler, Freedman & Scannella, 2009; Csermely, Rossi & Nasi, 2012) and are more recurved (Csermely & Rossi, 2006; Tsang et al., 2019) than in non‐raptorial birds. Among raptors, specialists in hunting other birds have longer toes (Csermely & Rossi, 2006; Tsang et al., 2019) and a wider skull (Hertel, 1995; Sun et al., 2018) [presumably for housing a larger cerebellum, the part of the brain that processes spatial orientation (Sun et al., 2018)] while those that specialise in hunting fish tend to have all four talons enlarged (Einoder & Richardson, 2007; Fowler et al., 2009). Scavenging raptors appear to be the most morphologically diagnostic group, characterised by large body size (Einoder & Richardson, 2007; Fowler et al., 2009) and a narrow, shallow (Bright et al., 2016; Navalón et al., 2018a ; Sun et al., 2018) and long (Hertel, 1995; Kulemeyer et al., 2009; Si et al., 2015; Sun et al., 2018; Pecsics et al., 2019) skull with a highly recurved rostrum (Hertel, 1995; Kulemeyer et al., 2009).
Table 1.
Glossary of dietary categories used in this review. Note that these are general classifications that may or may not be mutually exclusive and may be employed differently by different studies. A reference providing additional detail for each classification is provided. The prefix ‘hyper‐’ is occasionally applied to diet categories, indicating a particularly high percentage of the animal's diet consists of that food source
| Term | Definition | Source |
|---|---|---|
| Carnivorous | Energy acquired primarily by consuming animal tissue. | Ullrey (2018) |
| Durophagous | Consuming hard parts of organisms, or otherwise breaking their hard parts before consumption. | Crofts & Summers (2014) |
| Frugivorous | Consuming the nutritive tissue (‘flesh’) of fruits. | Jordano (2000) |
| Granivorous | Consuming plant seeds, before or after dispersal. | Hulme & Benkman (2002) |
| Herbivorous | Energy acquired primarily by consuming plant tissue. | Karban & Agrawal (2002) |
| Invertivorous | Consuming invertebrate animal tissue. | Thomas (2014) |
| Molliphagous | Consuming food that is soft, i.e. requiring relatively little energy to fracture. | Present study |
| Nectarivorous | Consuming nectar, a sugary liquid exuded by flowers. | Nicolson & Fleming (2014) |
| Omnivorous | Consuming a variety of foods, with no one source providing the majority of energy. | Thompson et al. (2007) |
| Osteophagous | Consuming bone or bone marrow. | Wroe et al. (2005) |
| Piscivorous | Consuming ‘fish’ (non‐tetrapod gnathostome) tissue. | Eklöv & Diehl (1994) |
| Predatory | Consuming tissue of animals killed by the consumer. | Taylor (2013) |
| Raptorial | Predation in which the pes plays a major role in killing and/or restraining the prey. | Fowler et al. (2009) |
| Scavenging | Consuming tissue of animals not killed by the consumer. | Turner et al. (2017) |
| Vertivorous | Consuming vertebrate animal tissue. | Garrard et al. (2012) |
Beyond these, diet/morphology correlations are at best known from a single study [e.g. small body size as indicative of nectarivory (Pigot et al., 2020)] and at worst contradicted among studies [e.g. bill curvature has been positively (Kulemeyer et al., 2009), negatively (Navalón et al., 2018a ), or not (Barbosa & Moreno, 1999) correlated with probing behaviour]. Characters known from a single study may be awaiting corroboration by future studies, but contradictions among studies suggest that we do not yet understand some aspects of avian diet/morphology relations fully. If living birds are to be used as proxies for fossil birds (i.e. bird taxa known only from fossils), then further work on understanding what they eat and why is imperative.
(3). Techniques for determining diet
When an ornithologist wants to know what a bird eats, the most straightforward technique is to directly observe them feeding and record what they ate [reviewed in Rosenberg & Cooper (1990) alongside most of the following methods]. Proxies for direct dietary observation include remote observation (detailed in Sullivan et al., 2009; Zhang et al., 2019) and examination of faeces [critiqued in Carlisle & Holberton (2006); augmented in Jarman et al. (2013); detailed in Ralph, Nagata & Ralph (1985)], pellets (critiqued in Votier et al., 2001), uneaten prey remains near the nest (critiqued in Tornberg & Reif, 2007), or stomach contents [from dead birds (reviewed in Duffy & Jackson, 1986), those captured and forced to regurgitate (critiqued in Gales, 1987; Carlisle & Holberton, 2006), or collected natural non‐pellet regurgitations (detailed in Oro et al., 1997)]. These ‘direct evidence’ data provide an unambiguous association between an organism and a certain diet. Unfortunately, these techniques require an animal to be alive or recently deceased, and the closest fossil equivalents to these forms of direct evidence, preserved meals, rarely fossilise and are prone to a variety of preservational biases. This makes them insufficient to reconstruct the diet of non‐avian avialans without other lines of evidence. Chemical analysis of stable isotopes in the soft tissues of living birds is commonly used to reconstruct trophic webs (detailed in Kloskowski, Trembaczowski & Filipiuk, 2019), with similar methods applied to bioapatite and amino acids preserved in bird fossils (see Section III.2). However, the wide variety of factors affecting stable isotope ratios make them more ambiguous lines of evidence.
While not used to determine diet per se, several physical approaches have been used to study extant birds to explain observed dietary trends. These approaches, in turn, have been applied to extinct organisms with unknown diets. Body mass has recently been found to predict large amounts of dietary variance in extant birds (Navalón et al., 2018a ; Pigot et al., 2020). Body mass estimation, then, may represent a key metric that has been relatively unexplored in fossil birds. With that said, most past studies have adopted ‘physical approaches’, those grounded in morphology and/or mechanics, to the study of avian diet. Dietary studies tend to focus on the skull, the most important tool for feeding in living birds (Rico‐Guevara et al., 2019), and the pes, their most important tool for manipulation of food prior to feeding (Clark, 1973; Sustaita et al., 2013) and the primary tool for killing prey in raptorial birds (Fowler et al., 2009). Traditional (detailed in Hertel, 1994) and geometric (detailed in Bright et al., 2016) morphometrics both seek to quantify the shape of body parts, under the assumption that form will reflect function. Other studies investigate functional capacity directly. Lever model simplifications of skulls (detailed in Corbin et al., 2015) describe the efficiency of force production and speed of the jaw, while finite element analysis (detailed in Soons et al., 2010) models the response of body parts to loading in order to compare their relative strength among organisms. Each of these techniques have been applied to living birds and non‐avialan theropods, but only the two forms of morphometrics have included non‐avian avialan taxa. While physical approaches have some of the broadest applications to fossil organisms, they also introduce a variety of complications (see Section VI.4).
There are also a select few lines of direct evidence that have been applied to fossil taxa but never to living or fossil birds. Dental wear analysis is commonly applied to living and fossil mammals (Green & Croft, 2018) with a recent application in theropods (Bestwick et al., 2018; Torices et al., 2018). While inapplicable to avians in its current form because they are all toothless, it is of potential value in the study of toothed non‐avian avialans. Neck musculature has been proposed to inform food disassembly behaviours in non‐avialan theropods (Snively & Russell, 2007b ), although the study of food disassembly in living birds has focused on the head and pes exclusively (Sustaita, 2008; Fowler et al., 2009; Sustaita et al., 2019). Additionally, ultrastructure of dentine (Brink et al., 2016) and enamel (Li et al., 2020) have been put forth as dietary proxies applicable across Dinosauria. Each of these techniques is also addressed in this review due to their potential application in fossil birds.
(4). Fossil birds and the focus of this review
Avialans appear in the fossil record as early as the Late Jurassic, and by the Middle Cretaceous inhabited tropical to polar latitudes and were present on every continent (Pittman et al., 2020b ). While most Mesozoic birds are considered to fall somewhere along a continuum of arboreal and terrestrial lifestyles (Mayr, 2017; Serrano et al., 2017; Cobb & Sellers, 2020), Ichthyornis and Hesperornithiformes are undisputedly aquatic (Rees & Lindgren, 2005; Hinić‐Frlog & Motani, 2010). Enantiornithes are the most widespread and speciose Mesozoic birds. They comprise roughly 60% of all non‐avian avialan genera followed by non‐avian ornithuromorph avialans (~25%) with the remainder made up by Archaeopteryx, Jeholornithiformes, Confuciusornithidae, Jinguofortisidae, and incertae sedis taxa [see Table 5 in Pittman et al. (2020b ); uncertainty based on their notes of taxa possibly referable to non‐avialan clades]. Anchiornithinae and Scansoriopterygidae have been placed in Avialae previously, but their inclusion is controversial (Pittman et al., 2020a ). This review will err on the side of inclusivity and discuss these two clades as ‘avialans’ in addition to well‐established Avialae [although personally we only see anchiornithines as avialans (see Pittman et al., 2020a )].
A variety of dietary habits have been proposed for non‐avian avialans. The vast majority are based on qualitative methodologies vulnerable to individual interpretation (Thulborn & Hamley, 1985; Zinoviev, 2009; Martyniuk, 2012; O'Connor et al., 2013b ; Dumont et al., 2016) or a few preserved meals which provide a definite but limited glimpse of diet (O'Connor, 2019; O'Connor & Zhou, 2019). More comparable, quantitative approaches to fossil avialan diet have been made (Navalón, 2014; Wang et al., 2014b ; Attard et al., 2016) but these are few and far between. We seek in this review to establish techniques that have proved effective at discriminating diet in living birds as well as fossil dinosaurs and to construct a framework for studying non‐avian avialan diet. Parts of this framework not relying on teeth can also be applied to fossil avians, which have a narrower and better‐constrained extant phylogenetic bracket (Witmer, 1995). On this basis, we then present a consensus of what we currently know about non‐avian avialan diet and how this can be improved moving forward. With this, future studies could make strides both in understanding Mesozoic ecosystems and in tracing the evolution of one of the most important groups of living organisms.
II. DIRECT EVIDENCE
(1). Preserved meals
(a). Introduction
Preserved meals may take the form of food preserved in the digestive system (consumulites) or as closely associated excretions (coprolites) or egestions (regurgitilites) (all sensu Hunt et al., 2012). Identification of consumulites may be problematic as accessing them often requires destroying the overlying remains, and misdiagnosis of ingested material may lead to erroneous dietary inferences (e.g. Nesbitt et al., 2006). Coprolites, while ostensibly simpler to analyse, are often taxonomically indeterminate (e.g. Chin et al., 1998; Hollocher et al., 2001; Hunt et al., 2012; Qvarnström et al., 2019) and of questionable association with surrounding body fossils (e.g. James & Burney, 1997; Wood et al., 2008; Hunt et al., 2012; Wang, Zhou & Sullivan, 2016a ). Regurgitilites are sparse in the fossil record, possibly due to collection biases and/or misdiagnosis as coprolites (Myhrvold, 2012), but otherwise provide similar information to coprolites with similar referral issues. Hunt et al. (2012) provide a detailed review of the study of coprolites, and Myhrvold (2012) provides one for regurgitilites (which he refers to as emetolites). There appears to be no comprehensive review of consumulites across fossil taxa other than the two discussed in the following section. Reports are typically centred on individual specimens. Smith & Scanferla (2016) provide an example of a single specimen with both strong and weak candidates for being true consumulites (lizard and insect, respectively).
A preserved meal sheds light on only a single meal in an organism's life. Extant organisms are known to consume a wide variety of food (Cortés, 1997; Vitt & Pianka, 2005; Wilman et al., 2014), so any preserved meal should be viewed as a single data point in reconstructing diet. Taphonomic effects should also be taken into account, as meals with elements that fossilise easier are more likely to be preserved inside of their consumer (O'Connor, 2019). As the remains of deceased individuals, the meals associated with a fossil may be the thing that killed them rather than a normal food source. This is more likely in the context of consumulites or minimally processed regurgitilites than coprolites, given that coprolites require time and digestion to produce. In short, the possibility of a preserved meal being atypical cannot be ruled out by a single specimen.
(b). Avialan consumulites
O'Connor (2019) reviews the consumulites known from traditional Cretaceous avialans which consist of seeds, fish, and invertebrate exoskeletons. O'Connor & Zhou (2019) expand this review to cover all paravians, attributing lizard and fish consumulites to anchiornithines. A coprolite containing fish bones has been associated with a specimen of Baptornis (Martin & Tate, 1976), and an indistinct coprolite is known from a Sapeornis specimen (Zheng et al., 2011). Four specimens record evidence of avialans themselves as prey of other organisms [ichthyosaurs (Kear, Boles & Smith, 2003), non‐avialan theropods (O'Connor, Zhou & Xu, 2011; Xing et al., 2012), and an indeterminate pellet‐producing animal (Sanz et al., 2001)].
There is particular controversy around enantiornithine consumulites, which merits discussion. Only a single uncontroversial consumulite is known from an enantiornithine: small (<5 mm) sections of crustacean exoskeleton in the abdomen of the holotype of Eoalulavis hoyasi (Sanz et al., 1996). The fish bones associated with the holotype of Piscivorenantiornis inusitatus (Wang et al., 2016a ; Wang & Zhou, 2017) and the amber in the abdomen of the holotype of Enantiophoenix electrophyla (Dalla Vecchia & Chiappe, 2002; Cau & Arduini, 2008) were both rejected as consumulites by O'Connor (2019). The former is interpreted as a fish coprolite (but see Xu et al., 2020) and the latter as elements reworked from surrounding soil (O'Connor, 2019). Spherical inclusions in two incertae sedis enantiornithines (and specimens of Jeholornis and Eoconfuciusornis) have been proposed as consumed plant matter (Mayr & Manegold, 2013; Mayr, 2016; O'Connor & Zhou, 2019; Mayr et al., 2020b ), although other studies have suggested that these inclusions are fossilised ovarian follicles (Zheng et al., 2013; O'Connor et al., 2014b ; Wang et al., 2016b ; Bailleul et al., 2019, 2020; O'Connor, 2019; O'Connor & Zhou, 2019).
Consumulite preservation is known from at least four specimens of Jeholornis, eight specimens of Sapeornis, and 14 specimens of ornithuromorph birds (Eogranivora edentulata, Piscivoravis lii, and 12 specimens of Yanornis martini) (see Table 1 in O'Connor, 2019). The absence or scarcity of consumulites in confuciusornithid and enantiornithine specimens (none in the former, one in the latter) has been used to suggest that they were generally molliphagous (feeding on soft things; opposite of durophagous) (O'Connor, 2019, p. 191). However, several other factors may be at play. Preservation of a consumulite is dependent on the meal being inside the animal's body (i.e. retained in the gut) and for parts hard enough to fossilise not to be dissolved at time of death. Thus, a longer gut retention time and a lower gut acidity favour the preservation of consumulites. Gut retention times vary among extant birds due both to long‐term lifestyle differences and short‐term events. Lifestyle differences include locomotor habits (Barton & Houston, 1992; Jackson, 1992; Hilton et al., 1999; Caviedes‐Vidal et al., 2007; Frei et al., 2014) and nutrient density of diets (Hilton, Houston & Furness, 1998; Hilton, Furness & Houston, 2000a ; McWhorter & Martínez del Rio, 2000; Levey & Del Rio, 2001). Events include coincidental dietary switching (Hilton, Furness & Houston, 2000b ), migration (McWilliams, Caviedes‐Vidal & Karasov, 1999), and rearing young (Thouzeau et al., 2004). Stomach acidities are also known to vary among extant raptors, with less bone remaining in the pellets of raptors with lower stomach pH (Duke, 1997). To our knowledge explanations of differing stomach pH have not been explored. Of these complicating factors, locomotor habits are of particular note as enantiornithines have been viewed as more arboreally inclined than contemporary ornithuromorphs (Field et al., 2018a ). In living birds, fully terrestrial species tend to have much longer gut retention times than flighted species (Frei et al., 2014). Among flighted species, those that are more active in flight tend to have shorter gut retention times (Jackson, 1992; Hilton et al., 1999; Caviedes‐Vidal et al., 2007). If enantiornithines were more active fliers than contemporary ornithuromorphs they likely also had shorter gut retention times. This would contribute to a lower incidence of consumulite preservation. Other possibilities, such as diets with higher nutrient density or a generally higher stomach pH than contemporary ornithuromorphs, can only be tested by future discoveries of consumulites or potentially corroborated by the other methods explored below.
(2). Dental wear
(a). Introduction
Teeth are dynamic systems worn continuously both by contact with ingested material and other occluding teeth (Green & Croft, 2018). As such, the way teeth are worn provides direct evidence of the diet of an animal. Dental wear analysis traditionally occurs at two distinct scales: mesowear, visible to the naked eye and reflecting periods of months to years (Green & Croft, 2018); and microwear, visible only under magnification and reflecting the animal's final days before death (Ungar, 2019). Green & Croft (2018) provide a review of both scales while Ungar (2015, 2018, 2019) provides more in‐depth reviews of microwear in particular. Studies of larger‐scale wear (e.g. fracture and erasure of denticles) have been used to examine patterns of occlusion (Lambe, 1917; Farlow & Brinkman, 1994; Sankey et al., 2002), penetration angle (Farlow & Brinkman, 1994; D'Amore, 2009), and potentially grooming (Currie & Evans, 2019) in dinosaurs. All of these studies have remained purely qualitative and do not address diet.
(b). Mesowear
Dental mesowear has only been analysed in herbivorous mammals in order to distinguish between browsers and grazers (Green & Croft, 2018). Its underlying principle is worth unpacking for potential broader use. Teeth experience two distinct types of mesowear: attrition, from contact with occluding teeth; and abrasion, from contact with ingested materials (Fortelius & Solounias, 2000). In mammalian herbivores, these sharpen and dull the teeth respectively (Fortelius & Solounias, 2000; Green & Croft, 2018). Dental mesowear can be seen as the interaction between these two forces: in softer food diets attrition dominates and cusps are sharper, in tougher food diets abrasion dominates and cusps are rounder and are eventually completely flattened (Fortelius & Solounias, 2000; Green & Croft, 2018; see supporting online material of Mihlbachler et al., 2011).
This approach is likely applicable to hadrosaurian dinosaurs whose dentition is reminiscent of equid mammals (Carrano, Janis & Sepkoski, 1999) and possibly to ceratopsian dinosaurs where attrition is believed to play a more complicated role (Erickson et al., 2015). Both possess teeth with tight occlusion upon which interactions between attrition and abrasion similar to those of extant herbivorous mammals may have occurred. The teeth of known theropods occlude only slightly, although to a greater extent than the unoccluding teeth of extant saurians (Schubert & Ungar, 2005). Because of this, the interpretation of mesowear in theropods is obscure. Schubert & Ungar (2005) propose “wear facets” on tyrannosaurid teeth to be the product of attrition while “enamel spalling” is the product of abrasion; the former completely obliterates the latter over time. Candeiro et al. (2017) identify two additional attritional features (vertical and perpendicular attritional surfaces) and one additional abrasional feature (apical grooves). They also broaden the phylogenetic bracket of these features to Theropoda. All attritional/abrasional features may or may not overwrite one another based on chance, and so cannot be quantified as simply as the antagonistic mesowear seen in herbivorous mammals. Mesowear analysis as it currently exists, then, is inapplicable to the currently known fossil birds. Instead, a new system would need to be constructed. Because theropod teeth occlude on only one surface (the lingual surface of upper teeth and labial surface of lower teeth), the difference in wear between the two surfaces may provide similar information to mesowear analysis. The mesowear paradigms of attrition and abrasion balance can, instead of being quantified from cusp shape, be quantified as a ratio between the number and depth of marks on occluding and non‐occluding surfaces. Assuming Schubert & Ungar (2005) are correct and that attritional wear would overwrite abrasional wear, the non‐occluding surface would provide a baseline for abrasion while the occluding surface would provide information on attrition. However, a lack of extant analogues with theropod‐like occlusion renders the validity of such an approach dubious; digital or practical modelling of theropod tooth occlusion may provide a baseline for study.
(c). Microwear
(i). Introduction
Dental microwear has been studied across vertebrates (Purnell, 1995; Purnell et al., 2006; Nevatte et al., 2017; Bestwick, Unwin & Purnell, 2019; Ungar, 2019; Winkler et al., 2019). Dental microwear describes the surface scarring of tooth enamel at a microscopic level, which can provide insight into the hardness (resistance to fracture) and toughness (resistance to tearing) of an animal's last meals, typically within the last few days of its life (Ungar, 2019).
Traditionally, microwear analysis involves directly counting surface features under light microscopy or from electron micrographs, where greater numbers of pits are considered indicative of consuming harder foods while greater numbers of scratches are indicative of consuming tougher foods (Ungar, 2019). This technique is inconsistent, with counting errors regularly reaching 10% among trained professionals (Grine, Ungar & Teaford, 2002; Mihlbachler et al., 2012). In order to remove measurement noise, wear surfaces have more recently been imported as point clouds and analysed as fractal surfaces (Ungar, 2019) using techniques and software common in micro‐scale manufacturing (e.g. Ţălu et al., 2014). An increased area‐scale fractal complexity is associated with harder foods in the diet, while an increased surface texture anisotropy is associated with tougher foods (Ungar, 2015). The only known source of error exclusive to fractal surface quantification is inter‐microscope variability, which can be minimised by incorporating consistent automated treatments (Arman et al., 2016). Purnell, Seehausen & Galis (2012) propose an alternative surface quantification method involving multivariate analysis of International Organization for Standardization (ISO) standard measures. While this alternative method captures more objective data about the surface and aspects of this can be correlated with diet (Purnell et al., 2012; Bestwick et al., 2019), we do not recommend this method at present. This is because the dietary significance of any given measurement is not well understood.
While microwear has been viewed as phylogenetically independent, this may be because it was studied among closely related taxa. A recent study comparing disparate clades of herbivorous mammals found microwear to describe phylogeny better than diet, but to distinguish diet successfully within each phylogenetic group (Mihlbachler et al., 2016). It seems, then, that dental microwear comparisons should be restricted in taxonomic scope out of caution in order to avoid potential biases. This restriction may be less important outside of Mammalia though as dental microwear trends have proved consistent within percomorph fishes (Purnell & Darras, 2015) and across Lepidosauria (Winkler et al., 2019). Because the temporal coverage of dental microwear is so short, analyses also require a large sample size properly to encompass the full breadth of an animal's diet (Ungar, 2019). Green & Croft (2018) imply that a minimum of 10 individuals should be sampled. Individual tooth sets can still act as individual examples of diet similar to preserved meals, and differently worn teeth within a single jaw have been proposed to give insight into non‐dietary behaviours such as grooming (see Section VI.4f.i).
(ii). Rhamphotheca microwear
The possibility of applying techniques like those used in dental microwear to bird rhamphothecae (the horny covering of the beak) is interesting, but remains uncertain in viability. Sload (2014) is the only researcher to apply microwear techniques to biological structures other than teeth: claws of Florida stone crabs, Menippe mercenaria. He notes that the lower hardness of the carapace [average microhardness of 1.33 GPa in melanised Florida stone crab carapace (Melnick, Chen & Mecholsky, 1996) versus 3.56 GPa in human enamel (Eimar et al., 2012)] leads to atypical patterns of microwear, with many surfaces worn completely away (Sload, 2014, p. 11). Reported averages for rhamphotheca hardness range from 1/4 [woodpecker Melanerpes carolinus (Lee et al., 2014)] to 1/11 [starling Sturnus vulgaris (Bonser & Witter, 1993)] that of Florida stone crab carapace, and so may experience even more extreme destruction of surface features. Rhamphotheca microwear is expected to reflect only very short periods of dietary input, requiring accordingly large sample sizes in order to acquire meaningful data about diet. However, hardness alone cannot predict wear resistance or patterns. Material behaviour and contact angle with abrading particles also play major roles (Zum Gahr, 1998). Enamel and crab carapace are both brittle ceramics for whom wear resistance increases near‐linearly with hardness, but more flexible materials like metals and, potentially, keratin can display neutral or even negative correlations between hardness and wear resistance (see Fig. 4 in Zum Gahr, 1998). The combined differences in material type and contact angles of food particles (with a rhamphotheca likely straighter and smoother than any dental battery) means rhamphothecal microwear patterns will not resemble any known dental microwear patterns. A ground‐up approach will be necessary to make this application feasible: just as early mammal researchers identified scratches and pits to reflect tough and hard foods, diagnostic features of rhamphotheca wear have to be identified. The most straightforward method for identifying features would be laboratory experiments feeding birds known diets with differing mechanical properties. Such features could provide additional insight into living birds with obscure dietary habits as well as potentially being applicable to fossilised rhamphothecae (see Section II.3).
(iii). Microwear in fossil theropods
Owing to their lack of teeth, dental microwear studies have not been performed on any extant avians (see above). There are two studies of dental microwear in fossil theropods, both of which address diet. Candeiro et al. (2017) provide little detail of their methodology but stated that analysis was “undertaken with the support of a scanning electron microscope” (p. 230). They observed the presence of an elongated groove worn into select teeth and proposed it as evidence of osteophagy (Candeiro et al., 2017). Torices et al. (2018) combine qualitative analysis of dental microwear with finite element analysis (see Section VI.3c.iii). They found all theropod teeth from the area of study to be worn with only scratches and no pits (Torices et al., 2018), indicating a diet of tough but soft material (Ungar, 2019). Torices et al. (2018) interpret this as a lack of bone‐crushing behaviour, where either flesh was removed selectively or prey was swallowed whole. They also cite the bimodal distribution of scratch directions as evidence of a puncture‐and‐pull feeding style. They propose scratches parallel to the tooth margin are formed while biting down while those oblique to the margin form when pulling back to disassemble prey (Torices et al., 2018). Finally, they comment on possible omnivory in the troodontid Troodon based on finite element analysis. In their example micrographs though, the scratches on the Troodon teeth appear longer, more numerous, and less parallel to the tooth margin than those in cf. Pyroraptor or Gorgosaurus (Fig. 2 in Torices et al., 2018). This may reflect Troodon incorporating more abrasive foods into its diet than the contemporary theropods studied. If not an artefact of the small number of examples provided, quantification of microwear may provide additional insight into dietary differences among toothed theropods. Also of note is a conference abstract finding the dental microwear of Archaeopteryx to resemble most closely that of invertivorous saurians (Bestwick et al., 2018). These results are planned for full publication, but dietary conclusions for Archaeopteryx are tentative due to a small sample size (Jordan Bestwick, personal communication 2020).
(iv). Application to fossil avialans
Fractal quantification of microwear (Ungar, 2015) has not been applied to any theropod taxon, and may be of particular interest in testing the proposed durophagy of certain enantiornithines including Shenqiornis (Wang et al., 2010a ; O'Connor & Chiappe, 2011) and Sulcavis (O'Connor et al., 2013b ). Successful application of the technique to fossil lepidosaurians of a similar size (Bestwick et al., 2019) shows promise for success in toothed avialans. Should dental microwear of toothed avialans prove exclusively scratch‐dominated as in non‐avialan theropods (Torices et al., 2018), only durophagy can be effectively ruled out. Other techniques are necessary to refine a dietary niche further. It is worth noting that, while complications from swallowing prey whole have been raised (Torices et al., 2018; O'Connor & Zhou, 2019), microwear in particular has been observed to reflect diet in extant lepidosaurs and archosaurs despite their limited use of the teeth in prey processing (Bestwick et al., 2019; Winkler et al., 2019). Purely tooth‐based approaches avoid many of the issues of reconstructing skull material. However, the possibility of anterior or posterior rhamphothecae in toothed avialans (Wang et al., 2020a ) acting as an additional feeding surface may complicate conclusions drawn from dental analysis only.
Application of microwear to beaked fossil avialans is contingent on the preservation of the rhamphotheca and on validation studies in extant birds. Microwear represents only a short window of time in tooth enamel usage (Green & Croft, 2018), and appears to turn over even faster in crab carapace (Sload, 2014). Thus rhamphothecae, which are even softer, will likely require large sample sizes for meaningful data. With less than a dozen rhamphothecae reported in the entire vertebrate fossil record (see Section II.3) this avenue requires the discovery of more specimens before it can be attempted. Procedures may also need to be devised to account for alterations to the microstructure of the keratin during burial, as in feathers (Fig. 3 in Saitta, Kaye & Vinther, 2019).
(3). Soft tissue
While not direct evidence of diet, the preservation of muscular tissue can aid in determining the inputs for functional models of extinct animal feeding. Unfortunately, fossilised jaw musculature has only been reported in placoderm fish (Trinajstic et al., 2007) and fossilised gular musculature in an ornithomimosaurian theropod (Briggs et al., 1997). The more commonly preserved postcranial musculature (e.g. Schultze, 1989; Kellner, 1996; Dal Sasso & Signore, 1998) may become useful in reconstructing dietary habits as the alliance between cranial and postcranial systems in feeding becomes better understood (Montuelle & Kane, 2019). Similar can be said for body outlines (Wang et al., 2017a ) for corroborating landmark‐based muscular reconstructions (see Section II.3a).
Fossilised rhamphothecae are invaluable when studying the diet of edentulous fossil taxa. Fossilised rhamphotheca impressions are known from a pterosaur (Frey, Martill & Buchy, 2003), hadrosaurid (Sternberg, 1935; Morris, 1970; Farke et al., 2013) and ceratopsian (Lingham‐Soliar, 2008) ornithischians, and ornithomimosaurid (Norell, Makovicky & Currie, 2001; Barrett, 2005) and confuciusornithid (Hou et al., 1999b ; Zhang, Zhou & Benton, 2008a ; Chiappe & Meng, 2016, p. 156; Falk et al., 2019; Miller et al., 2020; Zheng et al., 2020) theropods. While preservation of rhamphothecae appears to be rare from this small sample size, the fact that half of known confuciusornithid rhamphothecae are only visible with the use of UV or laser‐stimulated fluorescence (LSF) imaging (Chiappe & Meng, 2016, p. 156; Falk et al., 2019; Miller et al., 2020) shows promise for modern imaging techniques revealing previously unknown rhamphothecae. These fossils allow construction of more accurate models of beaked organisms in the fossil record. They also narrow the phylogenetic bracket for studying taxa with rhamphothecae that were not preserved.
(a). Landmark‐based cervical reconstructions in fossil theropods
While the skull is often the first point of contact and/or the primary tool used in feeding, it functions only with the aid of postcranial systems (Montuelle & Kane, 2019). Once the jaws have bitten down, the neck powers further disassembly of food by using the teeth and/or beak to tear material into a swallowable size [except in cases of chewing, which is not known in theropods (Zanno & Makovicky, 2011)]. In an animal that uses its neck for disassembly, selection is expected to favour an increase in size of those muscles that power disassembly. While not studied at length in living birds, comparisons of radiographs of Gallus and Anas appear to show greater muscle volume spanning the areas of most intense flexion during feeding (Figs 3 and 4 in van der Leeuw, Bout & Zweers, 2001) and one study (Marek et al., 2021) found the cervical vertebrae of insectivorous and tetrapod‐eating birds to have distinct overall shapes. Thus, reconstruction of neck musculature can elucidate what way, if any, non‐avian avialans disassembled their food before swallowing.
Among theropods, neck muscles have been reconstructed in therizinosaurians (Smith, 2015), ceratosaurids (Snively & Russell, 2007b ), allosaurids (Bakker, 1998; Snively & Russell, 2007b ; Snively et al., 2013), and tyrannosaurids (Bakker & Williams, 1988; Snively & Russell, 2007a , b ; Tsuihiji, 2010) based primarily on the occipital region of the skull. The general consensus of these studies is that ceratosaurids and allosaurids could exhibit greater force in dorsiflexion while therizinosaurians and tyrannosaurids could exhibit greater lateroflexive force. This leads to reconstruction of ceratosaurids and allosaurids pulling their heads back to disassemble prey and tyrannosaurids shaking their heads side‐to‐side (Snively & Russell, 2007b ). Such reconstructions have yet to be attempted in paravian theropods.
(i). Application to fossil avialans
Tsuihiji (2005, 2007) compiled homologies of cervical muscles across extant diapsids, including crocodilians and birds. This provides an extant phylogenetic bracket for reconstruction of non‐avian avialan cervical muscles, although typically two‐dimensional fossil preservation may prove to be an obstacle to the reconstruction process. Aside from the possibility of digital reconstruction (see Section VI.1), only Archaeopteryx (Alonso et al., 2004; Rauhut, 2014), Neuquenornis (Chiappe & Calvo, 1994), Piscivorenantiornis (Wang & Zhou, 2017), Enaliornis (Elzanowski & Galton, 1991), Hesperornis (Elzanowski, 1991), and Ichthyornis (Field et al., 2018b ) preserve the occipital region well enough to potentially identify muscular insertions. Of these, only Neuquenornis, Hesperornis, and Ichthyornis preserve any other regions of the skull. However, cervical muscles can be mapped onto cervical vertebrae in lateral view (Snively & Russell, 2007b ; Tsuihiji, 2010), and the relative size of muscle insertions on the skull are consistent with those on the vertebrae (Snively & Russell, 2007b ). Muscles that in both crocodilians and birds contribute to dorsoventral flexion (e.g. m. spinalis capitis) and lateral flexion (e.g. m. obliquus capitis) can be identified from the cervical columns of avialan compression fossils preserved in lateral view [e.g. IVPP V13313 (Dalsätt et al., 2006), IVPP V13558 (Zheng et al., 2014), STM 2–15 (O'Connor et al., 2018), STM 29–11 (O'Connor et al., 2016c )]. Once identified, their relative areas can be compared following the methodology of Snively & Russell (2007b ) to determine predispositions in cervical flexion and, in turn, methods of prey disassembly. We propose that prey disassembly method, in turn, can be extrapolated to inform the typical loading of the jaw. Their work implies that dorsiflexion shifts the muscular load vector cranially, ventroflexion shifts it rostrally, and lateroflexion shifts it laterally although they did not explicitly state this.
(4). Discussion
Lines of direct evidence are the most powerful and unequivocal data that we can obtain about avialan diet but have a small scope for application. The study of dental mesowear is not recommended to investigate toothed avialan diet due to the lack of occlusion in the clade. While the information they provide is vital, the paucity of avialan consumulites in the fossil record prevents reliance on them for understanding diet in most specimens. The lack of fossilised avialan musculature means that reconstructions must currently rely on landmarks for their attachment. Landmarks on the cervical vertebrae in particular can inform habits of prey disassembly by presenting adaptations for cervical flexion (see also Section VI.3b.iv) in both toothed and beaked avialans. The most promising line of direct evidence of avialan diet is dental microwear due to its broad applicability. In particular, its utility in detecting the input of hard foods into animals’ diets makes it ideal for investigations of possible durophagy in enantiornithines. The main drawback of this technique is the requirement for a large sample size, and so referral of unidentified specimens to known taxa may be necessary before such studies can be undertaken at phylogenetically meaningful levels. Rhamphotheca microwear requires a foundation in extant taxa before any application to beaked avialans can be attempted.
III. STABLE ISOTOPES
(1). Introduction
Natural abundances of stable isotopes (i.e. those not known to show radioactive decay) vary both geographically and in the way they are preferentially incorporated into biomolecules. With knowledge of these variations observed in living communities, the abundance of stable isotopes in the tissues of extinct animals can be used to reconstruct various aspects of palaeobiology (Clementz, 2012). The preservation window of stable isotope systems cited in all publications post‐2010 can be traced to those listed in Table 5.2 of Koch's (2007) review of the stable isotope chemistry in fossil vertebrates. Koch, however, does little to justify the ranges of these windows. He justifies the preservation window of bone, enamel, and soft tissues, but the provided temporal limits of individual isotope systems in these tissues are not justified. As such, the exclusion of an isotope system from analysis because the specimen is ‘too old’ is unfounded. Therefore, in addition to the traditional systems of carbon, oxygen and calcium isotopes commonly analysed in Mesozoic enamel and bone (bioapatite), we will also address hydrogen, nitrogen, and sulphur systems found in collagen [convincing evidence of preservation dating to the Early Jurassic (Lee et al., 2017)] as well as heavy metal (strontium, neodymium, lead, iron, copper, magnesium, and zinc) systems found in bioapatite. Keratin can theoretically preserve all isotope systems collagen does (Koch, 2007), although preservation of keratin chemistry in deep time is currently debated (Moyer, Zheng & Schweitzer, 2016; Schweitzer et al., 2018; Saitta et al., 2019).
(a). Carbon isotopes
13C is enriched relative to 12C in plants utilising a C4 photosynthetic pathway relative to those using the C3 pathway (Park & Epstein, 1960). The isotopic ratio of the carbon contained in the CO3 components of bioapatite and within the amino acids of collagen can be used to determine what photosynthetic source(s) the nutrition in question ultimately came from. DNA and palynological evidence agree on an Oligocene origin of the C4 pathway (Sage, Sage & Kocacinar, 2012), and so this aspect of the isotope system is uninformative of diet in specimens older than roughly 30 million years.
Marine ecosystems are known to be enriched in 13C relative to terrestrial ecosystems, although upper extremes of terrestrial species tend to overlap with those of marine species (Schoeninger & DeNiro, 1984) likely due to terrestrial input from C4 plants. Thus, prior to the emergence of C4 plants, we may expect a more bimodal distribution of 13C enrichment, with high enrichment of 13C indicating marine input into an organism's diet.
(b). Oxygen isotopes
18O is enriched relative to 16O in the leaves of plants relative to their other tissues, with increasing enrichment for leaves higher in the canopy (Koch, 2007). So, an enrichment in 18O in the CO3 and PO4 of bioapatite or the amino acids of collagen may indicate a higher proportion of leaves in the diet or feeding on leaves higher in the canopy. While not directly indicative of foraging height, 18O could potentially be used as a proxy. However, this enrichment must be evaluated based on comparisons with specimens from the same locality, as atmospheric temperature and water temperature are stronger controls on the enrichment of 18O.
More frequently, 18O enrichment is used to discern metabolic activity, which can confound the use of this isotope system in dietary studies. Body fluids are known to fractionate 18O with temperature (Koch, 2007). Specifically homeothermy can be identified by comparisons of 18O enrichment among different bones within an organism (Barrick & Showers, 1994; Barrick, Showers & Fischer, 1996; but see Kolodny et al., 1996) and comparing global trends of 18O enrichment to that of known ectotherms (Fricke & Rogers, 2000; Amiot et al., 2006). So, if used in dietary reconstruction, metabolic rate must be kept constant when making comparisons.
(c). Calcium isotopes
44Ca is known to deplete relative to all other calcium isotopes with increasing trophic level (Clementz, 2012), and so ratios of 44Ca/Ca in bioapatite across a locality may allow for rough approximation of the trophic pyramid. However, carnivorous taxa that do not consume the mineralised tissues of prey (e.g. early hominids) appear to be at a lower trophic level from these ratios (Reynard, Henderson & Hedges, 2010). Osteophagous herbivorous taxa (Esque & Peters, 1994; Hutson, Burke & Haynes, 2013) will presumably appear to be at a higher trophic level.
(d). Hydrogen isotopes
2H is enriched relative to 1H with similar trends in plant tissues to 18O/16O, but with less contribution from evaporative conditions and greater contribution from differences in plant tissues (Koch, 2007). If collagen can be recovered, then this system appears more appropriate than oxygen for comparing between different localities and between organisms with differing metabolic rates.
(e). Nitrogen isotopes
15N is enriched relative to 14N with increasing trophic level, and is used widely in analyses of extant food webs (e.g. Rau et al., 1992; Gu, Schelske & Hoyer, 1996; Davenport & Bax, 2002). The baseline enrichment of 15N varies based on locality, and so comparisons must be made within a given locality (Koch, 2007). Nitrogen is sourced in the amino acids of an organism which are more difficult to avoid consuming than bone, and so if collagen can be recovered nitrogen isotopes may provide a superior reflection of trophic level to calcium isotopes.
15N is also known to be enriched in marine ecosystems relative to terrestrial ecosystems, and with significant separation in levels between the two except in reef fish (Schoeninger & DeNiro, 1984). As such, an ecosystem with organisms feeding on exclusively marine or terrestrial organisms should display a bimodal distribution and not affect the signal for trophic level, but organisms taking from both sources may muddy the waters.
(f). Sulphur isotopes
Sulphur isotopes are known to vary among plants in extant ecosystems, but not in any predictable manner (Connolly et al., 2004; Koch, 2007). Sulphur extracted from collagen may provide evidence of consumers with different producers contributing to their diet. Without fossilised plant proteins, which taphonomic studies rule unlikely (Fogel & Tuross, 1999), greater precision appears impossible.
(g). Heavy metal isotopes
87Sr/86Sr (Koch, 2007), 144Nd/143Nd (van de Flierdt et al., 2006), and 207Pb/206Pb (Scheuhammer & Templeton, 1998) are not known to fractionate in biological systems, and so are typically used as indicators of location. In fossil terms, differences in these systems in the bioapatite of organisms from a given locality would represent different migratory patterns of those organisms in life. These could be used to identify distinct populations, potentially drawing on different food sources.
In extant mammals, 56Fe/54Fe is higher in females than males while the reverse is true for 65Cu/63Cu (Jaouen et al., 2012; Martin, Tacail & Balter, 2017). This is potentially useful for identifying sexual dimorphism from bioapatite, and in turn identifying sexually dimorphic diets (Shine, 1989). However, the isotopic trend is hypothesised to be linked to menstrual cycles (Martin et al., 2017) and thus may not be applicable outside of Eutheria.
Enrichment of both 26Mg and 66Zn in bioapatite with increasing trophic level have also been reported, but these isotopes appear more vulnerable to small regional variations than calcium or nitrogen (Martin et al., 2017). They may prove to be effective as secondary systems to confirm predictions based on calcium and/or nitrogen.
(2). Stable isotopes in extant birds
The first record of stable isotopes analysed in extant birds comes from a study across vertebrates by Schoeninger & DeNiro (1984), followed by Hobson (1987) as the first to focus specifically on birds. Both studies focused on determining marine or terrestrial input to the diet via 13C and/or 15N. Hobson (1990) was the first to apply 13C towards determining trophic level, and proposed 15N as a superior alternative. His following paper (Hobson, 1993) codified the role of 15N in determining trophic level and served as a basis for all subsequent avian studies. 18O (Farmer et al., 2003; Hobson et al., 2004), 2H (Chamberlain et al., 1996; Farmer et al., 2003; Lott, Meehan & Heath, 2003; Hobson et al., 2004; Norris et al., 2006), 34S (Farmer et al., 2003; Lott et al., 2003; Sanpera et al., 2007), 87Sr (Chamberlain et al., 1996; Blum, Taliaferro & Holmes, 2001), and 207Pb (Scheuhammer & Templeton, 1998; Scheuhammer et al., 2003; Svanberg et al., 2006) have all only been used as indicators of locality in extant birds, typically by comparing sets of feathers from known localities to determine an isotopic signature without regard to the ecological drivers described above. To our knowledge no studies of calcium, neodymium, iron, copper, magnesium, or zinc stable isotopes in birds have been performed.
(3). Stable isotopes in fossil theropods
Among fossil avians, stable isotopes have been used in dietary reconstructions in recent (Hobson & Montevecchi, 1991; Miller et al., 2005) and Palaeocene–Eocene (Angst et al., 2014, 2015) taxa. Dietary studies older than this are restricted to non‐avialan dinosaurs, primarily analyses of 13C determining that ecosystems were based on C3 plants (Ghosh et al., 2003; Amiot et al., 2010, 2015; Montanari & Norell, 2011; van Baal et al., 2013). Two studies (Frederickson, Engel & Cifelli, 2018, 2020) do incorporate paravian teeth, and also show evidence for niche partitioning through differences in carbon isotopes. The latter proposes a direct predator–prey relationship between two taxa based on carbon isotope signatures (Frederickson et al., 2020), although we are dubious of this being broadly useful as many herbivores in extant ecosystems have similar carbon isotope ratios to one another (Ambrose & DeNiro, 1986). There are two studies that have investigated diet in fossil theropods specifically using stable isotopes. The first is Ostrom et al. (1993) who tabulated 15N enrichment in bulk bones and teeth of vertebrates from the Judith River Formation, and recovered expected trends of greater enrichment in proposed hypercarnivorous taxa (e.g. tyrannosaurs, plesiosaurs) than proposed herbivorous/omnivorous taxa (e.g. hadrosaurs, sturgeon). The second is Hassler et al. (2018), who manage to separate carnivores and herbivores as well as terrestrial and aquatic predators in the Late Cretaceous of Northern Africa by measuring 44Ca depletion in tooth enamel and fish scale ganoine. To our knowledge no stable isotopes of any non‐avian avialan tissue have been analysed.
(4). Discussion
Stable isotopes promise information with validity akin to that of direct evidence, but the variety of influences on their ratios complicates the signals they provide. As such, the range of tissues and elements used in dietary inference is inherently small. Any attempts at bioapatite stable isotope analysis should be performed on teeth rather than bone if possible to ensure the highest level of accuracy (Hollund et al., 2015). The only locality with a large number of published avialan teeth is the Jehol Group (O'Connor & Chiappe, 2011; Chiappe & Meng, 2016). All evidence points to the Jehol Group being entirely terrestrial (Zhou, Barrett & Hilton, 2003) so distinction of marine and terrestrial producer input via carbon isotopes is irrelevant. Use of oxygen isotopes to determine foraging height may be effective in the earliest‐diverging avialans. However the variability of histological character in enantiornithines (Cambra‐Moo et al., 2006; O'Connor et al., 2014a ) and early‐diverging ornithuromorphs (Wang et al., 2019) would predict variation in metabolic rates acting as a confounding factor. The only isotope systems of interest in all avialan bioapatite, then, would be calcium isotopes with potential secondary confirmation with magnesium and zinc. While calcium isotopes could reliably be recovered (Hassler et al., 2018), the aforementioned exceptions to their trends make them less desirable than nitrogen [although paravian theropods are known to swallow prey whole (O'Connor & Zhou, 2019) which requires consumption of mineralised tissues and negates this issue in at least some taxa]. Reconstructing trophic levels via nitrogen isotopes after Ostrom et al. (1993) seems ideal, but collagen in bone is easily contaminated and enamel preserves only small amounts of protein (Hollund et al., 2015). Leichliter et al. (2020a ) display a promising preliminary replication of Ostrom et al. (1993)'s results using oxidation‐denitrification methods (Leichliter et al., 2020b ) to extract nitrogen from enamel more efficiently. Because these results are still preliminary, we recommend that nitrogen isotope studies of bird fossils use specimens that are incomplete or otherwise of low scientific value so that priceless fossils are not damaged unnecessarily. Exploratory studies should compare calcium and nitrogen isotope ratios across a given fossil site to ensure that trends agree between the two.
IV. BODY MASS
(1). Introduction
While not traditionally used to determine diet in fossil organisms, recent studies of extant birds (Bright et al., 2016; Navalón et al., 2018a ; Pigot et al., 2020) have found body mass to explain more of the variance in diet than physical approaches. Invertebrate feeders tend to be smaller than those that scavenge or hunt vertebrates (separation near 300 g) (Navalón et al., 2018a ), and among raptorial birds scavengers are distinctly larger than active hunters or omnivores (Bright et al., 2016). It is worth noting that this trend appears to apply only to feeding on animals, as herbivorous diet types are spread across the range of measured body masses (Fig. 6 in Navalón et al., 2018a ). However, body mass alone can only consistently predict nectarivory in extant birds (Pigot et al., 2020). Body mass has a major influence on feeding strategy in extant birds, but can be used only as a component in analysis [e.g. coupled with traditional morphometrics as in Pigot et al. (2020)] or as a secondary determinant. For instance, if other methods within the framework provide evidence of general carnivory, mass may help specify prey to vertebrates or invertebrates.
(2). Discussion
Body mass is a universal metric among animals, but the reasons behind its observed effects on bird diet remain unclear. Thus, its application to fossil avialans is questionable. The previously proposed reason for size having such influence in raptorial birds is the tight integration of the rostrum and cranium, disallowing significant change of one without the other (Bright et al., 2016). If this is true, then this means that the control of size on diet is developmental, not mechanical. Bird skulls have undergone extreme changes from the early‐diverging avialan condition, thought to be brought about by radical shifts in developmental controls (Bhullar et al., 2016). Certain features of non‐ornithuromorph skulls [small premaxilla (except in confuciusornithids), large maxilla (except in confuciusornithids), robust nasal and lacrimal, prominent postorbital (O'Connor & Chiappe, 2011; Rauhut, 2014; Hu et al., 2020a )] more strongly resemble that of early‐diverging theropods than extant birds. As such, developmental constraints on extant bird skulls are unlikely to be acting upon groups diverging earlier than Ornithuromorpha.
However, size explaining the largest portion of dietary variance persists across extant birds (Navalón et al., 2018a ; Pigot et al., 2020) despite differences in modularity between avian clades and a general decoupling of the rostrum and braincase shape across living birds as a whole (Felice & Goswami, 2018). The relationship between diet and body mass, we propose, may be more under mechanical control rather than developmental control. For instance, invertebrate taxa tend to be smaller than vertebrate taxa, so larger birds are less likely to feed on them. With that assumption, it is recommended that mass calculations be factored into dietary reconstructions of fossil birds if possible.
Serrano, Palmqvist & Sanz (2015) provide mass estimates of 43 Mesozoic birds based on extant bird skeletons (see their Table 8). Table 2 expands on their work, using their equations to provide mass estimates for 71 additional specimens of non‐avian avialans representing 61 species and based on scaled photographs in the literature. Combined with the estimates of Serrano et al. (2015), ~65% of non‐avian avialan species likely fell below the 300 g dietary transition observed by Navalón et al. (2018a ). However, this is largely driven by ornithothoracine species. Among non‐ornithothoracine taxa only three specimens of Archaeopteryx have an estimated mass range below 300 g (Table 8 in Serrano et al., 2015). There may be some taphonomic bias against preservation of large ornithothoracines, as the largest enantiornithine (Atterholt, Hutchison & O'Connor, 2018) and non‐avian ornithuromorph (Buffetaut & Angst, 2016) taxa are only known from highly fragmentary material. Of taxa known from more‐complete material, it appears that predatory ornithothoracines would be more likely to prey on invertebrates. Conversely, non‐ornithothoracine avialans lacking distinct evidence of herbivory (Archaeopteryx and Confuciusornithidae) are more likely to have taken vertebrate prey. Some ornithothoracine groups [Bohaiornithidae (e.g. Bohaiornis), Pengornithidae (e.g. Pengornis), Songlingornithidae (e.g. Yanornis)] also tend to have body masses above 300 g (Table 2) and may represent a secondary adaptation to take vertebrate prey. Thirteen specimens of Yanornis (a songlingornithid) preserving ingested fish (O'Connor, 2019) support this premise.
Table 2.
Mass estimates of avialan individuals. Input measurements are taken from scaled images in the literature. When scales of specimen photographs were in conflict (i.e. when scaled elements were larger or smaller than in the whole body photograph) we scaled all measurements so that humeral length was equal to measurements provided in the publication. Estimates are made using the equations of Serrano et al. (2015). The ORNnl equation is less precise and used in cases where a key component in the ENAN or ORPH equations is not preserved. The source text has a typographical error in four of the equations (Serrano, 2020). The corrected equations are: See Serrano et al. (2015) for measurement details. Body mass correction factors were not included in the original paper and so were back‐calculated from the reported values; all were very close to 1. Equation abbreviations are as follows: bcL, length of bicipital crest; DCmW, midshaft width from the cranial edge of major metacarpal to the caudal edge of minor metacarpal; dFWcc, craniocaudal width at femoral midshaft; dFWml, mediolateral width at femoral midshaft; deHW, dorsoventral width of distal humerus; deUW, dorsoventral width of distal ulna; dHW, dorsoventral width at midshaft of humerus; dUW, craniocaudal width at midshaft of ulna; FL, length of femur; HL, length of humerus; peUW, dorsoventral width of proximal ulna; RL, length of radius; TL, length of tibiotarsus; TmL, length of tarsometatarsus from the crista medial to the trochlea of metatarsus III; UL, length of ulna
| Taxon | Specimen | Mean mass estimate (kg) | Lower mass estimate (kg) | Upper mass estimate (kg) | Equation used |
|---|---|---|---|---|---|
| Jeholornithiformes | |||||
| Jeholornithiformes indet. | DLNM D2139 | 1.418 | 1.173 | 1.664 | JEHO |
| Jeholornis curvipes | YFGP‐yb2 | 1.504 | 1.244 | 1.764 | JEHO |
| Jeholornis prima | STM 2‐15 | 1.442 | 1.193 | 1.692 | JEHO |
| Kompsornis longicaudus | AGB‐6997 | 0.952 | 0.787 | 1.117 | JEHO |
| Shenzhouraptor sinensis | LPM 00193 | 0.883 | 0.730 | 1.036 | JEHO |
| Confuciusornithidae | |||||
| Confuciusornis sanctus | IVPP V13313 | 0.598 | 0.497 | 0.700 | CONF |
| Yangavis confucii | IVPP V18929 | 0.564 | 0.468 | 0.659 | CONF |
| Sapeornithiformes | |||||
| Omnivoropteryx sinousaorum | CAGS 02‐IG‐gausa‐3 | 1.429 | 1.193 | 1.665 | SAPE |
| Enantiornithes | |||||
| Alethoalaornis agitornis | LPM 00038 | 0.158 | 0.127 | 0.189 | ENAN |
| Bohaiornis guoi | IVPP V17963 | 0.300 | 0.242 | 0.358 | ENAN |
| B. guoi | LPM B00167 | 0.249 | 0.201 | 0.298 | ENAN |
| Cathayornis yandica | IVPP V9769a/b | 0.062 | 0.050 | 0.074 | ENAN |
| Chiappeavis magnapremaxillo | STM 29‐11 | 0.465 | 0.375 | 0.556 | ENAN |
| Dalingheornis liweii | CNU VB2005001 | 0.008 | 0.007 | 0.010 | ENAN |
| Dapingfangornis sentisorhinus | LPM 00039 | 0.204 | 0.164 | 0.243 | ENAN |
| Dunhuangia cuii | GSGM‐05‐CM‐030 | 0.124 | 0.099 | 0.149 | ORNnl |
| Elsornis keni | MPD‐b 100/201 | 1.512 | 1.206 | 1.817 | ORNnl |
| Eopengornis martini | STM 24‐1 | 0.193 | 0.155 | 0.230 | ENAN |
| Fortunguavis xiaotaizicus | IVPP V18631 | 0.296 | 0.236 | 0.356 | ORNnl |
| Grabauornis lingyuanensis | IVPP V14595 | 0.127 | 0.102 | 0.151 | ENAN |
| Gracilornis jiufotangensis | PMOL‐AB00170 | 0.027 | 0.021 | 0.032 | ENAN |
| Gretcheniao sinensis | BMNHC Ph‐829 | 0.455 | 0.367 | 0.543 | ENAN |
| Houornis caudatus | IVPP V10917/1, IVPP V10917/2 | 0.107 | 0.086 | 0.129 | ORNnl |
| Huoshanornis huji | DNM D2126 | 0.071 | 0.057 | 0.085 | ENAN |
| Jibeinia luanhera | Drawing in Hou (1997), holotype lost | 0.065 | 0.053 | 0.078 | ENAN |
| Junornis houi | BMNHC‐PH 919a/b | 0.074 | 0.059 | 0.088 | ENAN |
| Liaoningornis longidigitris | IVPP V11303 | 0.180 | 0.145 | 0.215 | ENAN |
| Linyiornis amoena | STM 11‐80 | 0.215 | 0.173 | 0.256 | ENAN |
| Longipteryx chaoyangensis | DNHM D2889 | 0.154 | 0.124 | 0.184 | ENAN |
| Longusunguis kurochkini | IVPP V17964 | 0.171 | 0.137 | 0.204 | ENAN |
| L. kurochkini | IVPP V18693 | 0.237 | 0.191 | 0.283 | ENAN |
| Microenantiornis vulgaris | PMOL AB00171 | 0.067 | 0.054 | 0.080 | ENAN |
| Monoenantiornis sihedangia | IVPP V20289 | 0.355 | 0.286 | 0.424 | ENAN |
| Noguerornis gonzalezi | LP.1702.P | 0.020 | 0.016 | 0.024 | ORNnl |
| Orienantius ritteri | BMNHC Ph‐1154a/b | 0.071 | 0.057 | 0.085 | ENAN |
| O. ritteri | BMNHC Ph‐1156a/b | 0.083 | 0.067 | 0.100 | ENAN |
| Parabohaiornis martini | IVPP V18691 | 0.221 | 0.178 | 0.263 | ENAN |
| Parapengornis eurycaudatus | IVPP V18687 | 0.429 | 0.345 | 0.512 | ENAN |
| Paraprotopteryx gracilis | STM V001 | 0.046 | 0.037 | 0.055 | ENAN |
| Parvavis chuxiongensis | IVPP V18586/1, IVPP V18586/2 | 0.024 | 0.020 | 0.029 | ENAN |
| Piscivorenantiornis inusitatus | IVPP V22582 | 0.281 | 0.227 | 0.336 | ENAN |
| Protopteryx fengningensis | BMNHC Ph‐1060a/b | 0.109 | 0.088 | 0.130 | ENAN |
| P. fengningensis | BMNHC Ph‐1158a/b | 0.088 | 0.071 | 0.105 | ENAN |
| Pterygornis dapingfangensis | IVPP V20729 | 0.080 | 0.064 | 0.095 | ENAN |
| Shangyang graciles | IVPP V25033 | 0.108 | 0.087 | 0.129 | ENAN |
| Shanweiniao cooperorum | DNHM D1878/1, DNHM D1878/2 | 0.057 | 0.046 | 0.068 | ENAN |
| Shengjingornis yangi | PMOL AB00179 | 0.340 | 0.274 | 0.406 | ENAN |
| Shenqiornis mengi | DNHM D2950/1 | 0.340 | 0.274 | 0.406 | ENAN |
| Sulcavis geeorum | BMNH Ph‐000805 | 0.333 | 0.268 | 0.397 | ENAN |
| Yuanjiawaornis viriosus | PMOL AB00032 | 0.418 | 0.337 | 0.499 | ENAN |
| Zhouornis hani (subadult) | BMNHCPh 756 | 0.253 | 0.204 | 0.303 | ENAN |
| Z. hani | CNUVB‐0903 | 0.758 | 0.611 | 0.905 | ENAN |
| Non‐avian Ornithuromorpha | |||||
| Abitusavis lii | IVPP V14606 | 0.326 | 0.263 | 0.389 | ORPH |
| Archaeorhynchus spathula | IVPP V17075 | 0.282 | 0.227 | 0.336 | ORPH |
| A. spathula | IVPP V17091 | 0.153 | 0.123 | 0.183 | ORPH |
| Archaeornithura meemannae | STM 7‐145 | 0.136 | 0.109 | 0.162 | ORPH |
| Bellulia rectusunguis | IVPP V17970 | 0.778 | 0.627 | 0.928 | ORPH |
| Changzuiornis ahgm | AGB5840 | 0.240 | 0.193 | 0.286 | ORPH |
| Dingavis longimaxilla | IVPP V20284 | 0.526 | 0.424 | 0.629 | ORPH |
| Eogranivora edentulata | STM 35‐3 | 0.291 | 0.235 | 0.348 | ORPH |
| Gansus yumenensis | GSGM‐05‐CM‐014 | 0.142 | 0.114 | 0.169 | ORPH |
| Hongshanornis longicresta | DNHM D2945 | 0.075 | 0.061 | 0.090 | ORPH |
| Mengciusornis dentatus | IVPP V26275 | 0.452 | 0.364 | 0.540 | ORPH |
| Patagopteryx deferrariisi | MACN‐N‐11 | 1.130 | 0.911 | 1.349 | ORPH |
| Piscivoravis lii | IVPP V17078 | 0.849 | 0.684 | 1.013 | ORPH |
| Schizooura lii | IVPP V16861 | 0.377 | 0.304 | 0.450 | ORPH |
| Similiyanornis brevipectus | IVPP V13278 | 0.634 | 0.511 | 0.757 | ORPH |
| Tianyuornis cheni | STM 7‐53 | 0.112 | 0.090 | 0.133 | ORPH |
| Xinghaiornis lini | XHPM 1121 | 0.539 | 0.434 | 0.643 | ORPH |
| Yanornis martini (juvenile?) | IVPP V13358 | 0.117 | 0.094 | 0.140 | ORPH |
| Yanornis sp. | STM 9‐15 | 0.577 | 0.465 | 0.689 | ORPH |
| Yanornis sp. | STM 9‐46 | 0.984 | 0.793 | 1.175 | ORPH |
| Yumenornis huangi | GSGM‐06‐CM‐013 | 0.321 | 0.256 | 0.386 | ORNnl |
V. DENTAL ULTRASTRUCTURE
(1). Dentine ultrastructure
Brink et al. (2016) report that the tubule density of dentine in archosaur teeth (imaged via multiple harmonic generation microscopy) is able to discriminate between taxa proposed as hypercarnivorous and hyperherbivorous. Hypercarnivorous taxa, according to their study, possess a higher density of tubules within the dentine. While potentially promising in the future, the authors provide an incomplete explanation for the functional significance of this difference. The taxonomic breadth of the study leaves room for the differences observed at least partially to reflect phylogeny (as proposed by Wang et al., 2015a ) rather than function.
(2). Enamel ultrastructure
Li et al. (2020) performed similar investigations into theropod enamel. They found loss of interglobular porous spaces and thinning of enamel at the avialan transition. These are proposed as reductions in tooth strength coincident with a dietary shift away from hard foods. However, while the authors propose interglobular porous spaces as restricting crack propagation, the mechanical differences between enamel and dentine are sufficient for this purpose in extant species (Bechtle et al., 2010). Enamel thinning has alternatively been proposed as a by‐product of selection for rapid incubation (Yang & Sander, 2018). This does not preclude enamel thinning from coincidentally causing a dietary transition as another by‐product. The thickening of enamel in enantiornithines proposed as durophagous (Li et al., 2020, p. 6) would seem to imply an ecological effect. This hypothesis will be worth examining against the overall jaw strength using finite element models of non‐avian avialans (see Section VI.3c).
(3). Discussion
As stated above, future studies of dentine ultrastructure require better sampling to support it reflecting diet rather than other factors like phylogeny. One clear way to test for phylogenetic influence would be to study dentine ultrastructure of herbivorous theropods like therizinosaurians or Sapeornis. Enamel ultrastructure has shown promising preliminary results (Li et al., 2020), but the researchers’ proposals of mechanical significance are yet to be supported. Physical approaches (Section VI) and phylogenetic controls are necessary to corroborate their findings. Should future studies validate the dietary signal of these techniques they would be applicable to any specimens in which dental wear analysis can be used.
VI. PHYSICAL APPROACHES
(1). Skull reconstruction
(a). Existing reconstructions
All of the physical approaches described below, when applied to skulls, require reconstruction of the skull. A listing of all reported avialan skulls is provided in Table S1. No complete non‐avian avialan skull is preserved in three dimensions. A composite reconstruction of the skull of the non‐avian ornithuran Ichthyornis dispar has been created in 3D (Field et al., 2018b ). The available elements of Falcatakely forsterae have been reconstructed in 3D (O'Connor et al., 2020), but this only represents roughly three‐quarters of the upper jaw. A team has also assembled a full 3D model of Archaeopteryx, but it is unreleased and intended for public education so its accuracy is unclear (Carney et al., 2018). All other non‐avian avialan skull reconstructions to date are 2D, owing to the flattened preservation of most avialans.
Over a dozen 2D skull reconstructions of Archaeopteryx exist (Elzanowski, 2001b ; Rauhut, 2014). These tend to agree with one another, differing mostly in how bones contact at the antorbital fenestra and in the dorsocranial region. The same is true of Hesperornis, with some variation in the structure of the orbit (compare Gingerich, 1973; Bühler, Martin & Witmer, 1988) and the addition of the predentary to later restorations (Martin & Naples, 2008). Reconstructions of Sapeornis are less consistent, with the skull generally seen as more robust with a more downturned rostrum and mandible over time (compare Zhou & Zhang, 2003; Hu et al., 2020a ). Skull reconstructions of Confuciusornis (Chiappe et al., 1999; Zhou & Hou, 2002; Navalón, 2014; Elzanowski, Peters & Mayr, 2018) have no clear trend in their variation. Most differences between reconstructions are in the length of the rostrum and height of the frontal, possibly representing intraspecific variation. Anchiornis sees the most variability in skull reconstruction, with noticeable differences in the size of fenestrae, shape of the mandible, and placement of sutures in all reconstructions (Xu et al., 2011; Xu et al., 2014; Wang et al., 2017a ). In each case the skull was merely illustrative with no record of reconstruction methods, and so a more intentional reconstruction of the skull of Anchiornis is necessary. For quite a few avialans there is only a single skull reconstruction. These include Yi (Xu et al., 2015) [probably a non‐avialan pennaraptoran (Pittman et al., 2020a )], Xiaotingia (Xu et al., 2011), Jeholornis (O'Connor et al., 2013a ), Gobipteryx (Elzanowski, 1977), Cathayornis (Martin & Zhou, 1997), Eoenantiornis (Hou et al., 1999a ), Shenqiornis, Rapaxavis, Pengornis (O'Connor & Chiappe, 2011), Piscivorenantiornis (Wang et al., 2016a ), Falcatakely forsterae (O'Connor et al., 2020), an indeterminate enantiornithine hatchling (Sanz et al., 1997), Patagopteryx (Chiappe, 2002), Yanornis (Huang et al., 2016), and Yixianornis (Clarke, Zhou & Zhang, 2006).
(b). Avenues for improvement
Due to most avialan specimens being compression fossils, relatively little material lends itself to construction of 3D models. A 3D reconstruction of Parahesperornis, or at least a general hesperornithiform, should be possible with reported material. A nearly complete 3D skull of Parahesperornis is known, alongside well‐preserved 3D pieces of the skull of Hesperornis (Bell & Chiappe, 2020) and Pasquiaornis (Sanchez, 2010). Excellent skulls of Gobipteryx minuta (Elzanowski, 1974, 1977; Chiappe, Norell & Clark, 2001) are nearly complete and preserved in three dimensions, and represent the most complete picture of the enantiornithine skull. The skulls of Gobipipus reshetovi (Kurochkin, Chatterjee & Mikhailov, 2013) and an unnamed gobipterygid (Lu et al., 2011) are more fragmentary, but are similar enough to Gobipteryx that complete reconstruction is possible. Unfortunately, the skulls of these taxa are highly derived (Hu et al., 2019) and are of dubious use in reconstructing the skulls of other enantiornithines (O'Connor & Chiappe, 2011). One complete enantiornithine skull is preserved in amber (Xing et al., 2017), but is of an extremely early ontogenetic stage, damaged from preparation, and extremely difficult to image (Xing et al., 2017, p. 266). The remainder of three‐dimensionally preserved non‐avian avialan skull material (Table S1) is too fragmentary for reconstruction. While an avian, the holotype of the Mesozoic bird Asteriornis includes a well‐preserved and nearly complete skull (Field et al., 2020) which can also offer insight into avialan roles in ecosystems of the Late Cretaceous.
As the vast majority of avialan skulls are preserved two‐dimensionally (Table S1), 2D reconstructions will likely remain standard in the coming years. As such, valid approaches to 2D reconstruction are imperative for applying physical approaches to avialans. In an ideal scenario of a near‐perfect skull (Sanz et al., 1997) reconstruction is often just a matter of retrodeformation. When no one skull can supply all the information needed, multiple skulls must be combined into a chimera. O'Connor & Chiappe (2011) set the standard for enantiornithines by restricting the phylogenetic bracket to only other members of the clade. Within Enantiornithes, however, there is still ample variation in skull morphology (Zhang, Ericson & Zhou, 2004; Morschhauser et al., 2009; O'Connor et al., 2013b ). We therefore recommend a refinement of current 2D avialan reconstructions by narrowing the phylogenetic bracket of reference taxa further. Four distinct families are generally recognised within Enantiornithes [Avisauridae (Brett‐Surman & Paul, 1985; Atterholt et al., 2018), Bohaiornithidae (Wang et al., 2014b ; O'Connor, 2019; Shi & Li, 2019; contra Chiappe et al., 2019b ), Longipterygidae (O'Connor et al., 2009; O'Connor, 2019; Shi & Li, 2019; Pittman et al., 2020a ), and Pengornithidae (Wang et al., 2014b ; O'Connor, 2019; Pittman et al., 2020a )], and so missing information can be preferentially filled by family members. A similar situation is true for the three widely recognised families of non‐avian ornithuromorphs [Hesperornithiformes (Clarke, 2004; Bell & Chiappe, 2016), Hongshanornithidae (O'Connor, Gao & Chiappe, 2010a ; Wang et al., 2015b ; Pittman et al., 2020a ), and Songlingornithidae (Hou, 1997; Clarke et al., 2006; Pittman et al., 2020a )]. In addition, the level of error introduced by chimerisation can be estimated by creating similar chimeras of extant bird skulls and comparing results of individuals to that of the composite. Skulls or individual teeth of lizards can help estimate the effects of chimeric dentition for toothed avialans. This will provide information on the accuracy of any analyses performed on inevitably chimeric avialan skull reconstructions.
Alternatively, technological advances may allow for 3D reconstruction from 2D sources. 3D images of specimens can be taken using computed tomography (CT) (Abel, Laurini & Richter, 2012) or augmented laminography (AL) (Zuber et al., 2017). Subsequently, broken parts can be segmented and reconstructed according to techniques described by Lautenschlager (2016). Unfortunately, in addition to the man hours necessary to segment and manipulate the numerous shards of a shattered skull, there are significant obstacles to collecting these data. Typically, avialan skulls are part of slab specimens. These slabs do not yield good results when imaged with standard scanners on site and are often too large for higher‐resolution scanners (Michael Pittman, personal observations). Preparation can resolve the size issue, but most museums are hesitant to approve preparation work, especially as slab specimens are generally beautifully articulated (Michael Pittman, personal observations). This means that to obtain CT data, whole slabs must be sent to distant specialist scanners (large‐chamber μCT scanner and synchrotron scanner) which involves time‐consuming permit‐ and logistics‐related paperwork, high transport costs (usually personal courier via air travel) and an elevated risk of damage to the specimen during its transport. Thus, 3D data have been difficult to collect from avialan skulls and will continue to be a challenge whilst these obstacles remain. The 2D approach described above is recommended to circumvent these issues and preserve fossils for future study, although fossils whose skulls are already disarticulated [e.g. Longusunguis (Wang et al., 2014b ; Hu et al., 2020b ) or Eogranivora (Zheng et al., 2018a )] (see also Table S1) would be ideal for pilot studies of 3D reconstruction of avialan skulls.
(2). Morphometrics
(a). Introduction
Morphometrics is the study of shape and the quantification of shape change, typically in a biological context (Rohlf, 1990). Shapes are quantified by defining landmarks, “(i) homologous anatomical loci that (ii) do not alter their topological positions relative to other landmarks, (iii) provide adequate coverage of the morphology, [and] (iv) can be found repeatedly and reliably…” (Zelditch et al., 2004, p. 24). In studies that that are concerned with only two dimensions of geometry, all landmarks must also lie in the same plane (Zelditch et al., 2004, p. 24). Once landmarks are placed on the structure in question, the methods with which they are analysed differentiate traditional morphometrics (TM), based on pre‐selected distances and angles between landmarks (Marcus, 1990), and geometric morphometrics (GM), where the differences between landmark positions in all models are quantified and significant variables are identified a posteriori (Zelditch et al., 2004, p. 24). Guillerme et al. (2020) review the collection, analysis, and interpretation of all morphometric data.
(b). Traditional morphometrics
(i). Introduction
Comparing linear measurements of animals likely predates the formalisation of science itself. TM are differentiated by rigorous statistical considerations of measurements taken, and selection of measurements believed to be relevant to the topic of study. The full range of statistical techniques used in TM are detailed by Marcus (1990).
The traditional appeal of TM has been the low computing power required. Measurements can be taken by hand and compiled into a spreadsheet for analysis of relatively low complexity. Now that computing power is no longer a limiting factor, the main appeals of TM are the lower investment of time and money into the project. TM does not necessitate creating two‐ or three‐dimensional models nor placement of landmarks and semi‐landmarks onto models (Rohlf, 1990). TM also allows for a priori selection of measures believed to have functional significance [although this may lead to arguments of cherry picking or p‐hacking (e.g. Warton & Hui, 2011)]. TM has been recently applied in studies of diet with positive results (Hertel, 1994, 1995; McBrayer & Corbin, 2007; Surkov & Benton, 2008; Fraser & Theodor, 2011).
(ii). Traditional morphometrics in extant bird skulls
Hertel (1994, 1995) defines 21 linear and four angular measurements to describe the avian skull, with all skull measurements in subsequent studies representing some subset of these. However, subsequent studies of the skull tend to focus exclusively on linear (Ladyguin, 2000; Li & Clarke, 2016) or angular (Button, 2018) morphometrics. These studies, as well as those incorporating postcranial morphometrics (Barbosa & Moreno, 1999; Corbin, 2008; Herrel et al., 2010b ; Pigot et al., 2020) and mechanical properties of the skull (Herrel et al., 2010b ; Corbin et al., 2015), have all been successful in predicting diet in extant birds. However, as is always true with TM, the a priori selection of measurements may limit their broader applicability. Beak curvature, for instance, is a major axis of the morphospace in vultures (Hertel, 1994) but considered irrelevant in shorebirds (Barbosa & Moreno, 1999) and flycatchers (Corbin, 2008). Pigot et al. (2020) successfully applied their morphometrics to 99.7% of extant bird species, but their reliance on soft tissue landmarks means they are inapplicable to most fossil taxa.
(iii). Traditional morphometrics in fossil theropod skulls
While TM has been applied to non‐avialan theropods across a broad range of taxa, the diets of all extinct theropods are necessarily speculative. So TM use has mostly been restricted to detecting inter‐population differences (Smith, 1998) or niche partitioning (Henderson, 1998; Van Valkenburgh & Molnar, 2002; Holtz, 2008) in fossil theropods. Holtz (2008) frames his study of tyrannosaurids in terms of hunting style, but the section therein focusing on skull morphometrics only shows a lack of separation of tyrannosaurid and non‐tyrannosaurid morphospaces with no comments on diet. Button & Zanno (2020) incorporate many skull measurements typical of TM in a study of theropod diet, such as skull length or premaxillary angle. However, because they investigate them in context of function rather than shape the study is covered in Section VI.3b.iii.
While most rigorously defined for phylogenetic use (Hendrickx & Mateus, 2014), TM has been applied twice to non‐avialan theropod teeth as an exploration of diet. Holtz, Brinkman & Chandler (1998) found a trend of generally coarser denticles in herbivorous vertebrates than carnivorous ones, with therizinosauroids and some troodontids plotting near the former. This technique is inapplicable to currently known avialans as all reported avialan teeth are unserrated [O'Connor (2019); but see Dumont et al. (2016) and Wang et al. (2015a )]. Holtz (2008) examined the height, length, and width of theropod tooth crowns, which can be applied to unserrated teeth. While tyrannosaurid teeth weakly separated in the morphospace from non‐tyrannosaurids, they were only effectively distinguished when examining functional indices (with an implication of increased strength indicating osteophagy). No comparisons to taxa with known diets were made.
(iv). Traditional morphometrics in extant and extinct theropod feet
Every TM study of the fossil theropod pes has included extant taxa for comparison, and few studies of extant avians exclude fossil taxa. It is, therefore, unhelpful to divide these studies by their subject, and so instead they are divided by their focus on angular or linear measurements. In addition to studies concerned directly with dietary cues (Csermely & Rossi, 2006; Einoder & Richardson, 2007; Fowler et al., 2009, 2011; Csermely et al., 2012; Mosto & Tambussi, 2014; M. Wang et al., 2014b ) and grasping ability (Kambic, 2008), we also include studies concerned purely with locomotion (Peters & Görgner, 1992; Feduccia, 1993; Clark et al., 1998; Zhou, 1999; Hopson, 2001; Pike & Maitland, 2004; Glen & Bennett, 2007; Morschhauser et al., 2009; Dececchi & Larsson, 2011; Tinius & Russell, 2017; Cobb & Sellers, 2020). Locomotion can provide insight into what resources would be available to individuals to feed on and how free the hind limbs were to manipulate food while feeding.
(A). Angular measures
Feduccia (1993) and Pike & Maitland (2004) define the major forms of angular TM in theropod claws. Both model the arc of the claw as a circle, with Feduccia (1993) concerned with the ventral arc (Fig. 1A) and Pike & Maitland (2004) measuring the dorsal arc (Fig. 1C). Both proposed that a greater degree of curvature (i.e. larger central angle measure) of said arc indicated a greater degree of arboreal behaviour within avians. Csermely & Rossi (2006) utilised the latter to investigate feeding behaviour. They investigated if it could distinguish between raptorial and non‐raptorial birds, as the former are known to utilise their feet extensively in prey restraint and manipulation (e.g. Goslow, 1972; Csermely & Gaibani, 1998; Ward, Weigl & Conroy, 2002; Sustaita et al., 2013). Subsequent studies measuring curvature either measured the dorsal arc exclusively (Glen & Bennett, 2007; Csermely et al., 2012; Mosto & Tambussi, 2014) or both the dorsal and ventral arcs (Fowler et al., 2009, 2011; Birn‐Jeffery et al., 2012; Tinius & Russell, 2017; Cobb & Sellers, 2020) [although note that Fowler et al. (2009, 2011) and Tinius & Russell (2017) use an alternative ventral arc proximal landmark (Fig. 1B)]. Dececchi & Larsson (2011) code claws as either “straight”, “recurved”, or “highly recurved” without clarification of how these codings are determined. Of these methods, the angle from the dorsal arc as defined by Pike & Maitland (2004) or the ventral arc as defined by Fowler et al. (2009) appear to be the most informative as both utilise unambiguous landmarks that represent the proximal and distal extents of the keratinous covering of the talon (Hedrick et al., 2019a ). Because the majority of studies incorporate other measurements in addition to curvature it is impossible to pinpoint which measure most effectively discriminates between groups.
Fig 1.

Methods employed in measuring the angle of bird claw curvature. Methods are diagrammed onto the digit I ungual of the enantiornithine Mirarce eatoni, as depicted by Atterholt et al. (2018). (A, B) Inner arc angle (A) as codified by Feduccia (1993) and (B) Fowler et al. (2009). (C) Outer arc angle as codified by Pike & Maitland (2004). In all cases, an initial chord (red) is drawn between the tip of the claw and the (A) proximal walking surface of the ungual, or the (B) ventroproximal or (C) dorsoproximal extent of the horny sheath of the claw (visible as inflection points in bony cores). A perpendicular line (yellow) is drawn from the midpoint of this chord to the ventral (A and B) or dorsal (C) arc of the ungual. Lines connecting the endpoints (green) are drawn, as well as lines perpendicular to the midpoint of these green lines (blue). The intersection of the blue lines defines the centre of a circle approximated by the arc. From this centre, lines (magenta) are drawn to the endpoints of the initial chord, and the angle between the magenta lines is the angle of curvature of the claw arc.
(B). Linear measures
Zhou (1999) included a chapter which systematised linear pedal TM in birds, although the paradigm was popularised by and is commonly attributed to Hopson (2001). Both studies followed similar methodologies to determine locomotor behaviour in extinct birds. Csermely & Rossi (2006) used these to investigate dietary signal (specifically predatory behaviour), while Kambic (2008) searched for signals specific to grasping ability. Unlike angular measurements, linear measurements of the avian pes are far from standardised. Non‐ungual phalanges may have measurements of length [whole toe (Clark et al., 1998; Zhou, 1999; Hopson, 2001; Kambic, 2008; Morschhauser et al., 2009; Dececchi & Larsson, 2011; Wang et al., 2014b ; Abourachid et al., 2017; Falk, Lamsdell & Gong, 2020) and individual phalanges (Clark et al., 1998; Zhou, 1999; Hopson, 2001; Kambic, 2008; Morschhauser et al., 2009; Dececchi & Larsson, 2011; Wang et al., 2014b ; Abourachid et al., 2017)], width [whole toe (Csermely & Rossi, 2006; Csermely et al., 2012) and individual phalanges (Kambic, 2008; Abourachid et al., 2017)], and height [whole toe (Csermely & Rossi, 2006; Csermely et al., 2012) and individual phalanges (Kambic, 2008; Abourachid et al., 2017)] on record (Fig. 2A–C). Ungual phalanges may have measures of length [chord (Csermely & Rossi, 2006; Fowler et al., 2009, 2011; Csermely et al., 2012; Hedrick et al., 2019a ), arc length (Mosto & Tambussi, 2014; Abourachid et al., 2017), and flexor tubercle chord (Mosto & Tambussi, 2014; Abourachid et al., 2017)], width [at the base of the claw (Csermely & Rossi, 2006; Csermely et al., 2012) and of the flexor tubercle (Mosto & Tambussi, 2014)], and height [at the base of the claw (Fowler et al., 2009; Fowler et al., 2011), at the midpoint of the arc (Fowler et al., 2009, 2011), and of the flexor tubercle (Mosto & Tambussi, 2014)] on record (Fig. 2D, E). The only linear measurements comparable among the majority of studies of linear phalangeal measurements are ungual chord length and individual phalanx length [which closely approximates total toe length (Falk, 2014)]. Arguments have been made for the inclusion of certain measurements, e.g. the ungual chord length, as a proxy for body size (Pike & Maitland, 2004) or phalangeal lengths indicating flexibility of the toes [Zhou (1999) citing Fisher (1946)]. However, the inconsistency of measures attests to how little justification there is for any given set of linear measurements to quantify properly the shape of avian feet.
Fig 2.

Length, width, and height measurements of the avian pes. Length (red), width (green) and height (blue), diagrammed onto the digit I phalanx I and digit I ungual of Mirarce eatoni as depicted by Atterholt et al. (2018). (A–C) Measurements of the non‐ungual phalanges in (A) lateral, (B) dorsal, and (C) distal views. (D–E) Measures of the ungual phalanx in (D) lateral and (E) proximal views. Note that most measures refer to the greatest distance measurable in a given dimension, but some such as wft are shown as being less than the greatest measurable distance without explanation in the works in which they are used. Abbreviations: la, arc length of ungual; lc, chord length of ungual; lft, proximodistal length of flexor tubercle; lp, proximodistal length of phalanx; hb, height at the base of the ungual; hft, dorsoventral height of the flexor tubercle; hm, height at the mid‐arc of the ungual; hp, dorsoventral height of phalanx; wft, mediolateral width of flexor tubercle; wp, mediolateral width of phalanx.
This category includes two of the three quantitative studies focused on avialan diet, although both were directly examining locomotion and only refer to diet as a secondary result. Hou et al. (2004) hypothesised that Longirostravis was a probing feeder, and Morschhauser et al. (2009) tested this hypothesis through the closely related Rapaxavis. Morschhauser et al. (2009) incorporated digit III phalanx measurements of Rapaxavis into the data set of Hopson (2001) and found it to fall into the arboreal morphospace. From this they propose that, instead of being ground probers comparable to charadriiforms, they may have instead been bark probers (Morschhauser et al., 2009, p. 553). Wang et al. (2014b ) took a similar approach to Bohaiornithidae, adding their phalangeal measurements to the data set of Hopson (2001) and adding claw curvature measurements of Pike & Maitland (2004); it appears comparisons to the latter are merely qualitative, referring only to “high” and “low” curvature. Narrowing the data set to raptorial birds due to high claw curvature and lack of specialised limb proportions associated with climbing, they found the closest phalangeal proportions to be those of Pandion. While the researchers propose a piscivorous diet in bohaiornithids because of this similarity, they attenuate the diagnosis with qualitative assertions of their teeth seeming to be more adapted for durophagy (Wang et al., 2014b , p. 68).
(v). Discussion
Pilot studies are necessary to examine the application of TM to non‐avian avialan skulls to predict diet. All TM frameworks that rely on the curvature of the premaxilla (Hertel, 1994, 1995; Barbosa & Moreno, 1999; Button, 2018) cannot be applied to toothed avialans as nearly all possess straight premaxillae. The exceptions are for Sapeornis, Ichthyornis and the Hesperornithiformes which are interpreted as possessing rhamphothecae which replaced the rostralmost teeth (Wang et al., 2020b ); premaxillary curvature is likely a product of edentulism with teeth no longer serving as a gripping surface for food. Whether the straight beaks of all known fully edentulous avialans (Chiappe et al., 2001; Zhou & Li, 2010; Zhou, Zhou & O'Connor, 2012, 2013; Elzanowski et al., 2018; O'Connor, 2019) represents a dietary or developmental signal is unclear. Frameworks reliant on the width of the skull (Corbin, 2008; Corbin et al., 2015; Li & Clarke, 2016; Pigot et al., 2020) would require referral of more specimens with skulls preserved in dorsal or ventral views. Skull width is unknown in all described non‐avian avialan genera except Anchiornis, Confuciusornis, Sapeornis, Dalingheornis, Eopengornis, Fortunguavis, Longusunguis, Monoenantiornis, Archaeorhynchus, Yixianornis, Ichthyornis, Hesperornis, and Parahesperornis (Table S1). Of these all but Ichthyornis, Hesperornis, and Parahesperornis are crushed skulls whose widths we consider dubious. TM seems more likely to prove useful when applied to dentition. Dental TM is already known to determine effectively the phylogenetic placement of most theropods (Hendrickx & Mateus, 2014; Hendrickx, Tschopp & d'Ezcurra, 2020), with universal characters already defined in the literature (Hendrickx, Mateus & Araújo, 2015). While preliminary studies of lizard teeth have shown only a tenuous link with diet (Estes & Williams, 1984; Melstrom, 2017), they remain the best extant group to compare with due to the much more narrow range of tooth form in crocodilians (Erickson et al., 2012). We therefore recommend a study of extant lizard teeth using the existing TM framework (Hendrickx et al., 2015) as a baseline dietary morphospace. Otherwise, the lack of effective application of skull TM to non‐avialan theropod diet does not bode well for applying this technique to non‐avian avialans.
TM studies of the theropod pes have both a more consistent focus on dietary analogues and a more consistently applied set of measurements, and so a more thorough evaluation of methods is possible. Most TM studies of the pes concerned themselves only with digit III (DIII) (Peters & Görgner, 1992; Feduccia, 1993; Clark et al., 1998; Pike & Maitland, 2004; Glen & Bennett, 2007; Kambic, 2008; Morschhauser et al., 2009; Dececchi & Larsson, 2011; Wang et al., 2014b ; Tinius & Russell, 2017; Cobb & Sellers, 2020), two studies with digit I (DI) and DIII (Csermely & Rossi, 2006; Csermely et al., 2012), two studies with digit II (DII)–digit IV (DIV) (Zhou, 1999; Hopson, 2001), and the remainder with all four digits (Einoder & Richardson, 2007; Fowler et al., 2009, 2011; Mosto & Tambussi, 2014; Falk et al., 2020). Studies that rely solely on DIII justify this choice by DIII being the weight‐bearing toe (Glen & Bennett, 2007; Hedrick et al., 2019a ), and all but (Kambic, 2008) are concerned with locomotion. Studies on DII–DIV (Zhou, 1999; Hopson, 2001) are similar in concept, disregarding DI as it rarely bears weight during forward motion. Zhou (1999) compared the phalangeal proportions of DII–IV individually and found that each digit discriminated between locomotor categories equally well (his Figs 38–42). Backus et al. (2015) provide a strong argument for the efficacy of the two‐dimensional simplification of avian grasping (i.e. the action of DI and DIII) as such simplification is adequate to create functioning artificial ‘hands’ for robots (Dollar & Howe, 2011; Backus, Odhner & Dollar, 2014). Abourachid et al. (2017), however, note that in non‐anisodactyl pedal arrangements DII and DIV supplement or supplant the role of DI and DIII in grasping. The subdivisions of anisodactyl arrangements recently proposed by Falk et al. (2020) may prove to have similar influence on the grasping role of digits. Measuring all digits of the pes acquires the most data possible about its structure but imposes additional complexity in the analysis and time for data collection. Significant interdigital variation is often cited as a reason to avoid measurements of the whole pes (Hedrick et al., 2019a ; Cobb & Sellers, 2020), but Fowler et al. (2009, 2011) argue that interdigital variation itself is a diagnostic that separates ecological niches. Given that all studies of all four digits were able to discriminate ecological groups it seems unlikely that measuring four digits is detrimental to TM analysis, but any benefit from analysing all digits over the analysis of a single digit is equivocal.
It is recommended in TM (and GM) studies of ungual phalanges to compare the bony cores of claws rather than the keratin sheaths. Studies measuring both sheath and core found both to give similar information (Hedrick et al., 2019a ; Cobb & Sellers, 2020), and many fossils do not preserve the keratin sheath. In those that do, the keratin stain is likely more deformed than the bone (Saitta et al., 2019). Therefore empirical conversion factors (e.g. Glen & Bennett, 2007; Hedrick et al., 2019a ) or direct measurements of the stain give less valid data than measuring the core. Extant avian ungual bones can be found in many museum skeletal collections, and remaining keratin can easily be removed from the claws of macerated skeletons by soaking the claws in dilute ammonia (Stephen Rogers, Carnegie Museum of Natural History, personal communication 2019). Among styles of applying TM, Fowler et al. (2009, 2011) produced the finest scale of discrimination. Their framework delineated not only arboreal/ground and raptor/non‐raptor groups, but also the individual hunting guilds among raptors. However, these studies did not employ phylogenetic correction. Thus, some authors have proposed that they simply group talons based on familial relations (Hedrick et al., 2019a ), which happen to correlate with predatory style in extant raptors (Goslow, 1972; Csermely & Gaibani, 1998; Sustaita, 2008; Fowler et al., 2009). More fundamentally, the Fowler et al. (2009, 2011) studies appear to utilise improper statistical analysis. They apply correspondence analysis, designed to utilise categorical data. Numerical data can be binned and analysed as categorical data (Kim, 2011), but Fowler et al. (2009, 2011) do not indicate doing so in either study. When the more appropriate principal component analysis is applied to these data (see Fig. S1) groups inhabit less distinct regions of the morphospace. With that said, they still show more distinction than that of the next most discriminating study, Csermely et al. (2012). It is recommended that any pes‐based TM investigation of non‐avian avialan diet be based on the analytical procedures of Fowler et al. (2009) with an appropriate phylogenetic correction (see Section VI.4a).
One study of avialan ecology which should be mentioned here is that of Mitchell & Makovicky (2014). This study translates traditional morphometric indices into discrete characters rather than using the measurements themselves. This brought considerable success both in discriminating ecology among extant birds and in matching preserved meals and depositional environments of fossil birds [although we are sceptical of their prediction of Bohaiornis as herbivorous and insectivorous, as the “gastroliths” in its stomach are believed to be preservational artefacts (O'Connor, 2019) and its body mass is greater than expected for an invertivore (Section IV; Table 2)]. This approach necessarily removes data without an intuitive explanation. For instance, there is no reason given why birds with skulls whose length/width ratio is below, near, or above one should be expected to be ecologically distinct. This approach is appealing, though, in its ability to combine form with qualitative traits (e.g. known preserved meals) as in Zanno & Makovicky (2011). So for taxa with dietary evidence not easily converted into morphometrics, this combination approach may yield superior results. When this approach is used we suggest taking the extant morphometric data and performing some form of cluster analysis (Jain, 2010) to determine where the appropriate points are to discretise characters. For most non‐avian avialans, however, we expect TM approaches to be superior at determining diet than conversion into discrete characters.
(c). Geometric morphometrics
(i). Introduction
Geometric morphometrics (GM) uses digital models, either full 3D models or 2D dorsal/lateral silhouettes, with landmarks placed just as one would with TM (Zelditch et al., 2004). Landmarks are typically supplemented by sliding semi‐landmarks, a digitisation of the curve(s) between landmarks (Perez, Bernal & Gonzalez, 2006). The shifts in the relative position of landmarks and semi‐landmarks between models can be quantified and analysed digitally (Adams & Otarola‐Castillo, 2013).
While GM can be significantly more time‐consuming than TM (Rohlf, 1990), GM provides at least as much information about the shape of the element in question as every possible measurement taken between every pair of landmarks (Zelditch et al., 2004, p. 2–7). With the introduction of semi‐landmarks the information increases beyond that possible in TM (Zelditch et al., 2004, p. 396). Perez et al. (2006) detail different methods of creating semi‐landmarks. Note that at an inter‐species or higher level, such as that typically employed in palaeontology, the differences between methods should be negligible. Because GM creates an unbiased representation of form it has proved effective in tracing evolutionary trends (Figueirido et al., 2011; Miyashita, 2013; Openshaw et al., 2016; Polly et al., 2016; Fernandez Blanco, Cassini & Bona, 2018) but has returned only mixed results in feeding studies (Samuels, 2009; Meloro, Hudson & Rook, 2015; Gailer et al., 2016; Klaczko, Sherratt & Setz, 2016; Tarquini et al., 2019).
Another form of GM, outline‐based GM, has been introduced as an alternative method of quantifying 2D shape. This method interprets and compares the outlines of bodies as composites of trigonometric curves (Fourier analysis). The primary advantage over landmark‐based methods is applicability to bodies with few homologous landmarks (Bonhomme et al., 2014). While the concept dates back several decades (Kaesler & Waters, 1972) this method has been used very little in vertebrate palaeontology to date (Navarro, Martin‐Silverstone & Stubbs, 2018; Schaeffer et al., 2019) and so by default ‘GM’ will be used herein to refer to landmark‐based GM. In phylogenetically broad studies in which homology is unclear this method may prove useful in the future.
(ii). Geometric morphometrics in extant bird skulls
GM studies of extant avian diet can be divided into those that include only the bill (van der Meij, 2004; Sustaita & Rubega, 2014; Matsui et al., 2016; Cooney et al., 2017; Olsen, 2017; Button, 2018; Navalón et al., 2018a ), those that treat the beak and cranium as a single unit (Navalón, 2014; Si et al., 2015; Bright et al., 2016, 2019; Tokita et al., 2017; Sun et al., 2018; Felice et al., 2019a ; Pecsics et al., 2019; Chávez‐Hoffmeister, 2020), and those that examine each individually (Kulemeyer et al., 2009; Bright et al., 2016; Felice et al., 2019a ). Landmarks for the bill tend to be consistent. The bill tip and the base of the frontal are used in nearly every study (van der Meij, 2004; Kulemeyer et al., 2009; Navalón, 2014; Si et al., 2015; Bright et al., 2016, 2019; Matsui et al., 2016; Tokita et al., 2017; Button, 2018; Navalón et al., 2018a ; Sun et al., 2018; Chávez‐Hoffmeister, 2020) alongside either the rostral extreme of the jugal (van der Meij, 2004; Navalón, 2014; Matsui et al., 2016; Tokita et al., 2017; Button, 2018; Navalón et al., 2018a ; Bright et al., 2019; Chávez‐Hoffmeister, 2020) or the ventrocranial extreme of the palatine (Kulemeyer et al., 2009; Si et al., 2015; Tokita et al., 2017; Sun et al., 2018; Pecsics et al., 2019). Landmarks of the cranium are less consistent, with only the border of the orbit commonly mapped (Kulemeyer et al., 2009; Si et al., 2015; Tokita et al., 2017; Sun et al., 2018; Felice et al., 2019a ). Discrimination of diet is inconsistent. GM can often discriminate diet in restricted phylogenetic groups (family to superfamily level) (van der Meij, 2004; Kulemeyer et al., 2009; Sustaita & Rubega, 2014; Si et al., 2015; Olsen, 2017; Sun et al., 2018; Pecsics et al., 2019; Chávez‐Hoffmeister, 2020), but dietary morphospace tends to overlap heavily between groups (Bright et al., 2016, 2019; Tokita et al., 2017) and across birds overall (Navalón, 2014; Button, 2018; Navalón et al., 2018a ; Felice et al., 2019a ). It seems likely that more distantly related clades of birds face developmental constraints that either prevent them from attaining converging morphotypes or predisposes them towards differing solutions under similar environmental pressures (Gould, 2002). Similar levels of craniofacial integration within most families of land birds (Telluraves) (Navalón et al., 2020) implies similar developmental patterns among them. Constraints preventing shape/diet correlation, then, are less expected within Telluraves.
(iii). Geometric morphometrics in fossil theropod skulls
Marugán‐Lobón & Buscalioni (2004) laid the groundwork for non‐avialan theropod GM, focusing purely on disparity (quantified difference in shape). Shychoski & Snively (2008a ) published an abstract that reported juvenile tyrannosaur mandibles to be closer in shape to non‐tyrannosaurid morphotypes than their adult forms, but full publication of the data is not planned (Eric Snively, personal communication 2020). Brusatte et al. (2012) found oviraptorids and, to a lesser extent, ornithomimosaurians and alvarezsaurians [all proposed as herbivorous (Zanno & Makovicky, 2011)] to cluster outside the ‘carnivorous’ morphospace. The lack of any confirmed diets in the data set limits confidence in these results. Foth & Rauhut (2013) reported similar results with a significant correlation between purported diet and shape. They saw more overlap in dietary morphospace and also did not include taxa with known diets. Recoding taxa based on more explicit evidence of diet (Fig. S2; see Table S2 for recoding rationale) provides a division between herbivorous and carnivorous morphospace if Anchiornis and Bambiraptor are excluded (which inhabit a unique region of the morphospace overlapping with herbivores). This study was the first to include early‐diverging avialan taxa in GM analysis (Table S1 in Foth & Rauhut, 2013). Schaeffer et al. (2019) were the first to incorporate outline‐based GM to study theropod diet, and compare it to both landmark‐based GM and discrete characters. The authors found all three approaches to be of similar effectiveness, although it is noteworthy that separation of morphospace via discrete characters appears more vulnerable to changes in sample size (compare their Figs 3C and 7C). When diets are more conservatively assigned (Fig. S3; see Table S3 for recoding rationale) resolution between herbivores/omnivores and small carnivores increases. Their graphs show landmark‐based GM as less effective at discriminating between large and small carnivores than outline‐based GM (their Figs 3 and 7), although this appears to be due to principal components defining different shapes between the two (their Fig. 4). When plotted as PC1 versus PC3 a similar trend is seen in landmark‐based GM to that shown for outline‐based (their Fig. S7 and supplemental files; our Fig. S3E). Button (2018) and Navalón (2014) are the only researchers to combine GM analysis of extant avian taxa with known diets and early‐diverging theropods. The dietary morphospace of most extant avians in these studies overlap and are often completely enveloped in another morphospace [Fig. 33 in Button (2018); Fig. 8 in Navalón (2014)]. In both studies almost all fossil taxa either plot in regions of heavy overlap or fall completely outside the dietary morphospace. The exception is two unidentified taxa in Button (2018), which plot in a region inhabited only by terrestrial herbivores.
All GM studies of early‐diverging theropod diet are performed in two dimensions, all are concerned with the upper jaw except for Schaeffer et al. (2019) and Shychoski & Snively (2008a ) (who investigated the lower jaw), and all landmarks are placed in only lateral view except for in Button (2018) where they are placed in lateral and dorsal view. Landmarks of theropod skulls are variable. The only landmarks shared between the four studies of the upper jaw are the rostroventral extreme of the premaxilla and the contact between the jugal and maxilla (Brusatte et al., 2012; Foth & Rauhut, 2013; Navalón, 2014; Button, 2018). Landmarks used in both studies of the lower jaw (Shychoski & Snively, 2008b ; Schaeffer et al., 2019) are the anterodorsal and anteroventral corners of the dentary, the dentary–surangular suture, the dentary–angular suture, and the articular glenoid. None of the studies include any landmarks on teeth, allowing toothed organisms to be examined using the same landmarks as those with varying extents of edentulism (Button, 2018; Wang et al., 2020a ). The importance of tooth morphology in lepidosaur (Smith, 1993) and mammal (Bergqvist, 2003; Pineda‐Munoz et al., 2017) diet raises questions of error introduced in this practice. A single study (D'Amore, 2009) studied theropod teeth in isolation using GM, but focused on penetration angle independent of diet.
(iv). Geometric morphometrics in extant bird feet
Tinius & Russell (2017) introduced the concept of using GM on bird claws, finding GM to be the only one of six claw angle measures capable of discriminating locomotor groups in the taxa studied [GM, the aforementioned methods of Feduccia (1993) and Peters & Görgner (1992) – although note their execution of Feduccia (1993) more closely resembles that of Fowler et al. (2009) –, one method applied to insects (Petie & Muller, 2007), one to lizards (Zani, 2000), and one theorised in but never applied to amniotes (Thompson, 1942)]. Hedrick et al. (2019a ) were the first group to investigate dietary signal with geometric morphometrics. They found neither TM nor GM could discriminate between ecological groups. Abourachid et al. (2017) and Tsang et al. (2019) incorporate 3D models and landmarks into their studies, but Abourachid et al. (2017) uses them primarily to automate the collection of linear measurements for TM. Tsang et al. (2019) were able to discriminate both between predatory and non‐predatory taxa and between sizes of prey that predators fed on. This level of precision presents a promising outlook for the future. While theoretically 3D GM provides the most accurate representation of claw shape, the merits of this technique have not yet been proved consistent in discrimination of avian ecology.
(v). Discussion
GM studies of theropod taxa both extant and extinct have been highly variable in both methodology and results. 3D GM studies of skulls, presumably capturing the largest amount of data about shape, tend to find strong relationships between form and diet at the family to superfamily level (Kulemeyer et al., 2009; Olsen, 2017) that disappear when comparing larger‐scale trends (Bright et al., 2016; Navalón et al., 2018a ; Felice et al., 2019a ). GM analysis of non‐avian theropods is inhibited by the lack of known diets in the group. Certain groups, in particular oviraptorids, tend to cluster distinctly from other theropods (Brusatte et al., 2012; Foth & Rauhut, 2013). A study incorporating both oviraptorids and caenagnathids [both beaked but respectively hypothesised to be herbivorous and carnivorous (Ma et al., 2020)] may clarify if the segregation in these studies stems from edentulism or a dietary shift. Schaeffer et al. (2019) found considerable overlap of dentary morphospace in herbivorous and small carnivorous taxa. However, they coded all oviraptorosaurians as herbivorous (including caenagnathids) and all troodontids as small carnivorous [including those proposed as omnivorous (Holtz et al., 1998; Torices et al., 2018)] (Table S5 in Schaeffer et al., 2019). This study highlights the necessity for calibrating dietary morphospaces with organisms whose diet is not controversial. When assumptions of herbivory in oviraptorosaurians and carnivory in avialans are discarded, the morphospaces become more distinct (Fig. S3C–E). The study also shows that landmark‐ and outline‐based GM can provide similar information about skull shape, although comparisons between the two should be preceded by comparing thin spline plates to ensure the same shape changes are being modelled on each axis (Fig. S3E). While Button (2018) successfully combined extant and extinct theropods in skull GM analysis, he reported that only landmarks placed in dorsal/ventral view are effective at discriminating diet categories. Even then, his model appears to separate terrestrial feeders from other groups rather than any particular diet categories (his Fig. 33). Regardless, dorsal/ventral placement of skull landmarks excludes most published non‐avian avialan specimens (Table S1). Navalón (2014) managed to assign non‐avian avialans to a diet category using GM, but only in conjunction with mechanical advantage (see Section VI.3b.iii). In sum, skull shape tends to be a poor predictor of diet in extant avians. Studies of non‐avian theropod morphospaces are limited by a lack of knowledge about the diet of taxa included, and still provide only mixed results. An attempt to combine avian and non‐avian theropods shows poor resolution in the dietary morphospace and has limited application to fossil avialans. Therefore, GM of the non‐avian avialan skull is recommended as at most a complement to a functional study (e.g. Navalón et al., 2018a ) if it is utilised at all.
Tsang et al. (2019) were able to distinguish non‐predatory taxa (both non‐raptors and scavenging raptors) from predatory taxa, and to partially distinguish between predators hunting large prey and small prey. Unfortunately, there are few fossil bird claws preserved in three dimensions. Large‐scale 2D geometric morphometric analysis of bird claws has only been undertaken by Hedrick et al. (2019a ) who failed to discriminate between any ecological groups. By their own admission, their ecological categories may have been too broad to allow for delineation (Hedrick et al., 2019a , p. 9), and the ratio between the lengths of keratin sheaths and bone cores was a major element of the first principal component in their analysis (Hedrick et al., 2019a , p. 6). It is possible that a similar study landmarking solely bone cores and with a more diverse set of ecological groupings [e.g. the ecological groups of Fowler et al. (2009), Glen & Bennett (2007) and Pigot et al. (2020)] may produce more useful results. Therefore, we recommend utilising the techniques of Tsang et al. (2019) where possible and an improved 2D GM framework where not possible.
(3). Functional studies
(a). Introduction
Of the two styles of functional study described herein, finite element analysis (FEA) is typically preferred for fossil dietary inference. Lever models, which provide measures of mechanical advantage (MA), are more commonly used as broad approximations that can be calculated easily and quickly. However, recent questions of the validity of FEA results in small animals (see Section VI.3c.ii) may apply to non‐avian avialans [in which the largest taxa other than Hesperornis (Martin & Naples, 2008) and possibly Gargantuavis (Mayr et al., 2020a ) have a skull length less than 9 cm (Field et al., 2018b )]. Both techniques, then, should be taken into consideration.
Relevant to both techniques are concerns of dimensionality and comparison taxa. In both cases, researchers will likely be restricted to 2D analysis due to reconstruction issues related to incomplete skull preservation (detailed in Section VI.1). But, if possible, validation studies with smaller sample sizes using both 2D and 3D techniques should be performed. A 3D model of the skull of Ichthyornis dispar is already published (Field et al., 2018b ), and the phalanges of Mirarce eatoni are three‐dimensionally preserved (Atterholt et al., 2018). Both are prime candidates for such studies.
Both FEA and lever models of fossil taxa are only interpretable in comparison with other models. The choice of comparative taxa then is of vital importance. To create a phylogenetic bracket, beyond the obvious extant avians, it is recommended that early‐diverging non‐avialan paravians with preserved meals (O'Connor & Zhou, 2019) are included. Certain lepidosaurian taxa that share similarities in dentition (Smith, 1993) with toothed avialans may be necessary to include as well.
(b). Lever modelling
(i). Introduction
The jaw of most animals acts as a third class lever: the joint acts as a fulcrum about which a load – the distal bone – is rotated by the effort – a muscle – attached in between (Fig. 3). Because jaws act as levers we can use mathematical principles to predict their behaviour. Examples include models utilising known muscle vectors to calculate bite vectors (Santana, Dumont & Davis, 2010) or to compare torque generation (Kiltie, 1982). However, when studying fossils, muscle size can often only be very roughly approximated. Instead, they tend to rely on the concept of MA.
Fig 3.

Third‐class lever nature of animal jaws. Here a reconstruction of Shenqiornis after O'Connor & Chiappe (2011) is used as an example. The load (green) is represented as a square of theoretical foodstuff between the teeth. The effort (blue) is represented as a simplification of the m. adductor mandibulae complex (see Fig. 4A for full reconstruction of attachments). The fulcrum (red) is represented as the circled articulation between the upper and lower jaws.
The basic principle of MA was outlined in antiquity by Archimedes of Syracuse (1897, p. 192–194): the force exerted on a lever will be multiplied by the distance from it to the fulcrum (the inlever) and divided by the distance from the fulcrum to the load (the outlever). MA, then, is the useful ratio defined as the inlever divided by the outlever. In practical terms, it can be viewed as the ratio of the output force to the input force of a system, or as the factor by which the input force is multiplied to determine the output force. When mechanical advantage is lowered, the load is moved across a greater distance in the same amount of time and thus at a higher speed. As a trade‐off, the output force is reduced.
In animal jaws the inlever is the distance from the site of adductor muscle attachment to the joint between the cranium and mandible. The outlever is the distance from that joint to the point at which the animal is biting down. The bite point is typically operationalised as the rostralmost or cranialmost point of the occlusal surface (either of the rhamphotheca or apices of teeth) (Ma et al., 2020; Navalón et al., 2018a ). More rarely MA will be calculated for each tooth in the row (Sakamoto, 2010; Therrien et al., 2016; Cox, 2017). Functionally, MA is seen as the trade‐off between speed and power in skull architecture (Stayton, 2006; Dumont et al., 2014; Corbin et al., 2015; Adams et al., 2019), although this trade‐off is a simplification and can be circumvented (McHenry & Summers, 2011; Corbin et al., 2015). Organisms feeding on immobile sources, such as plant matter, experience selective pressure for high jaw forces (high MA) that can efficiently process food. By contrast, those hunting mobile prey, such as insects or small vertebrates, experience selective pressure for jaws able to open and close quickly (low MA) in order to capture prey (Stayton, 2006; Corbin et al., 2015; Adams et al., 2019). Some trophic specialisations involving elongation of the snout such as nectarivory (Dumont et al., 2014) or probe‐feeding (Navalón et al., 2018a ) necessarily also reduce MA.
(ii). Lever models of extant birds
Small‐scope studies of avian MA include those on cormorant (Phalacrocorax) species (Burger, 1978) and suliform birds (Carlos, Alvarenga & Mazzochi, 2017). Both found higher MA to correlate with capturing large prey and lower MA to correlate with hunting small, fast‐moving prey. Corbin et al. (2015) examined trends in bite force across extant avians (especially among passerines) and in the process laid the groundwork for modern avian MA measurements. Corbin et al. (2015) found that bite force and velocity correlate positively and negatively with MA, respectively. They briefly and qualitatively comment on diet by predicting low MA in insectivorous birds and high MA in granivorous birds (Corbin et al., 2015, p. 813). When analysed quantitatively the data they provide show only a weak correlation (r 2 ≈ 0.05) between MA and diet. The correlation increases to moderate strength (r 2 ≈ 0.40) when restricted to passerine taxa (Fig. S4). Olsen (2017) was the first to examine the relationship between MA and diet quantitatively, finding that increased MA correlates with increased consumption of leaves in Anseriformes. Navalón et al. (2018a ) broadened the scope of this technique by applying it across extant avians, but found MA to explain dietary components only weakly. Their data show that MA better predicts the use of the beak during feeding (UBF), particularly when combined with beak curvature (Navalón et al., 2018a ). Further research may translate UBF into a set of possible dietary categories: taxa with tearing UBFs are often raptorial, cracking UBFs at least partly granivorous, etc. It is of note that all quantitative dietary analyses of avian skull MA have focused on the upper jaw, with those regarding the lower jaw (Burger, 1978; Carlos et al., 2017) being purely qualitative (but see Fig. S4).
(iii). Lever models of fossil theropods
Sakamoto (2010) and Brusatte et al. (2012) calculated MA across the tooth row for a variety of non‐ornithothoracine tetanurans and found lower values of MA in smaller taxa. This was tentatively interpreted as smaller taxa being adapted for hunting more agile prey. Sakamoto (2010, p. 3330), however, proposes the potential for saw‐motion biting in dromaeosaurids feeding on large prey. Noticeable in their figures [Fig. S6 in Brusatte et al. (2012); Fig. 2b in Sakamoto (2010)] but not commented on in the text is the high MA of oviraptorosaurians, studied in depth by Ma et al. (2017, 2019, 2020). Oviraptorosaurians consist of two distinct groups, Oviraptoridae and Caenagnathidae (Funston et al., 2018). Only the Oviraptoridae was included in previous studies (Sakamoto, 2010; Brusatte et al., 2012). Ma et al. (2017) in passing, and later with statistical rigour (Ma et al., 2019), noted that caenagnathids tend to have a lower MA than oviraptorids. This was proposed to reflect herbivory in oviraptorids and carnivory in caenagnathids. Caenagnathid MA indeed falls within ranges of other theropods, while oviraptorid MA is significantly higher (Ma et al., 2020). Ma et al. (2020) introduce MA measurements of Jeholornis as well as anchiornithines and scansoriopterygids. Button & Zanno (2020) include six measures of MA, five additional mechanical indices, and 22 traditional morphometric ratios believed to have functional implications in diet. While the study is incredibly effective at delineating herbivory and carnivory as well as distinct herbivorous strategies, the lack of variance explained by the principal components used is problematic for use in diagnosing diet (discussed further in the next section). Navalón (2014) is the only MA study to focus chiefly on non‐avian avialans. Combining MA with GM, it recovers Confuciusornis and Sapeornis as herbivorous and Eoconfuciusornis as omnivorous. While it presents initial promise, additional MA measurements of the fossil taxa should be taken before broader comparisons are made (Guillermo Navalón, personal communication 2020), and a subsequent study discussed above that expanded the avian taxa included (Navalón et al., 2018a ) resolved diets much less effectively. This highlights the importance of including not only extant taxa but a wide range of extant taxa for ecological comparisons.
(iv). Discussion
While MA has proved an effective predictor of diet in lepidosaurs (Stayton, 2006) and small mammals (Dumont et al., 2014; Adams et al., 2019), Navalón et al. (2018a ) report minimal association between diet and MA across extant birds. This is likely due to the fact that, as they point out, similar diets can often be associated with radically different foraging strategies (Navalón et al., 2018a , p. 423). In particular, only some granivorous birds crack open the hard outer coating of seeds (Prosser & Hart, 2005). Those that do not de‐husk in this way instead crush them in the muscular gizzard (Janzen, 1981). Confuciusornithids and enantiornithines are not believed to possess a gizzard (O'Connor & Zhou, 2019) meaning they would not be susceptible to this source of signal interference. With this trend accounted for, mechanical advantages above 0.15 in Navalón et al. (2018a )'s data set appear to correspond to herbivory while those below 0.15 correspond to carnivory, nectarivory, and frugivory, although the latter is complicated by the inclusion of nuts as fruits rather than with seeds. For instance, Anodorhynchus has an MA near 0.35 (Fig. 6 in Navalón et al., 2018a ) and is coded as 70–100% frugivorous depending on species (Wilman et al., 2014). However these ‘fruits’ are palm nuts (Faria et al., 2008) which are hard enough that a close relative has been used as a model for construction materials (Staufenberg, Graupner & Müssig, 2015). Together, these factors make MA promisingly informative of diet in confuciusornithids and enantiornithines. MA may still provide insight into other non‐avian avialans, but low MA cannot definitely rule out herbivory in these other groups.
The broad scope of the study by Button & Zanno (2020), combining MA with TM and some less commonly used functional indices, is highly effective at delineating diet. Taxa such as ornithopods and sauropods that are well established as herbivorous occupy a region of the morphospace distant from theropods well established as carnivorous (e.g. tyrannosauroids). Their methods, at first, appear promising to diagnose the diet of non‐avian avialans. However, this study was intended to track stepwise evolutionary changes, not to determine diet, so several issues arise when adapting it for that purpose. While Button and Zanno cite their resolution as a reason to incorporate large complexes of characters in dietary analyses (Button & Zanno, 2020, p. 163), the small amount of variance explained by their graphs makes this less than ideal. The authors perform statistical operations within the first three principal components of morphospace which only explains 45.7% of the total variance observed; nine principal components are required to pass 70% explanation (their Data S2) as is the common standard (Jolliffe & Cadima, 2016, p. 4). PCA functions to reduce the number of dimensions worked within an analysis by creating axes that explain large amounts of the variance. But once data encompasses a large number of dimensions it is unlikely that principal components will be able to explain adequate amounts of variance in the three dimensions humans can easily work in (Brown, 2009). It is therefore recommended here that the number of variables investigated are reduced to reduce the dimensionality of the data [but see Guillerme et al. (2020) for alternatives to dimension reduction]. Of the 34 measurements taken by Button & Zanno (2020), only nine (C2–8, 22, 23) can be applied to compression fossils and have proved effective to our knowledge at discriminating diet among extant groups other than ungulates. We found that when only these measurements are used, the first three principal components explain more than 70% of the variance while maintaining the same general structure of the morphospace (Fig. S5). Data can be further reduced as the MAs for each individual muscle group have a similar influence on the principal components (Fig. S2A in Button & Zanno, 2020) and represent redundant information. They can be merged into anterior and posterior jaw‐closing MA. Ma et al. (2020) additionally define jaw‐opening MA and two other functional indices which have proved discriminative of diet across a wide range extant taxa. We recommend combining these studies to, in total, three forms of MA and five accompanying functional indices (Fig. 4). However, unlike these two studies which measure MA based on the lower jaw, we recommend measurements of the upper jaw after the sensitivity analysis of Brusatte et al. (2012). They found the upper jaw, not the lower jaw, has the greatest effect on the overall MA of the jaw system (their Fig. S27). Transition from the lower to upper jaw will necessarily require adaptation of landmarks from which measurements are taken. Landmarks at the anterior and posterior of the occlusal margin, attachment sites of the adductor muscles, and those for m. depressor mandibulae will refer to their locations on the upper jaw rather than the lower jaw. The articular glenoid of the surangular will be replaced with the articular condyle of the quadratojugal (Fig. 4B–D). All other landmarks remain unchanged from those in Button & Zanno (2020) and Ma et al. (2020).
Fig 4.

Illustration of the functional measurements used by Ma et al. (2020) as well as characters 22 and 23 from Button & Zanno (2020). The example is a reconstruction of Shenqiornis after O'Connor & Chiappe (2011). C2–7 of Button & Zanno (2020) are combined into anterior and posterior jaw‐closing MA as defined by Ma et al. (2020). Articular offset is identical between the two. (A) Reconstruction of skull muscle attachments after Holliday (2009). Reconstructions of m. adductor mandibulae externus (purple) and m. adductor mandibulae posterior (green) are more certain while reconstruction of m. pterygoideus (orange) is tentative due to the uncertain nature of the pterygoid in enantiornithines (Chiappe et al., 2001; O'Connor & Chiappe, 2011). The yellow star is the centroid of the irregular shape bounding all attachments, treated as the centre of force for adduction. Uncertainty of attachment area size precludes more precise weighting of the attachment centroid. The abductor muscle for the jaw, the m. depressor mandibulae (cyan), attaches perpendicular to the viewing plane (Lautenschlager et al., 2014a ) and so is represented as a line along the back of the skull. (B–D) Diagrams illustrating inlevers (red) and outlevers (blue) for calculating (B) anterior jaw‐closing mechanical advantage, (C) posterior jaw‐closing mechanical advantage, and (D) jaw‐opening mechanical advantage. (E–G) Illustrations of measurements to calculate: (E) relative articular offset, (F) relative maximum rostral height, and (G) relative average rostral height. See Ma et al. (2020) for an explanation of calculations. (H, I) Close‐up of the premaxilla indicating measurements of (H) C22 tooth angle and (I) C23 tooth slenderness index. See Data S4 in Button & Zanno (2020) for an explanation of calculations.
(c). Finite element analysis
(i). Introduction
Finite element analysis (FEA) is a technique originally applied to structural engineering. In it, irregular bodies are partitioned into a mesh of simple shapes, the ‘elements’, in order to predict the response of the body to a given load (Bathe, 2014). Applications in palaeontology began with structural predictions of depth adaptations in cephalopods (Daniel et al., 1997). Soon after it was used in its most common palaeontological application today: vertebrate jaws (Rayfield et al., 2001). Bright (2014) provides an effective review of techniques up until 2014, but several notable advances have taken place since. The rise of formalised digital reconstruction (Lautenschlager, 2016) augmented by quantification of skeletal asymmetry (Hedrick et al., 2019c ) has enabled analysis of specimens previously considered hopeless cases. These techniques may allow for a greater number of studies incorporating both the upper and lower jaw. Studies taking both into account (Wroe et al., 2007; Moreno et al., 2008; Wroe, 2008; Lautenschlager et al., 2013; Attard et al., 2014, 2016; Adams et al., 2019) find differing peak Von Mises (VM) stress [a summary statistic of distortion energy in a body (Ugural & Fenster, 2012, p. 189–190)] and differing distribution of VM stresses between the upper and lower jaw. These differences could in theory cause overestimation of jaw strength by only using the upper or lower jaw if the unmeasured element is the limiting factor in jaw strength. To our knowledge no study has been undertaken to explore the potential significance of this error, but no study of the full jaw has reported differing dietary signals between the upper and lower jaws.
Formalisation of applying 2D FEA techniques to organisms (Marcé‐Nogué et al., 2013; Neenan et al., 2014) allows FE models to be constructed and analysed much faster with a lower prerequisite of computing power. It also allows for analysis of compression fossils. Simplifying 3D bodies into 2D outlines inherently requires assumptions that will induce error. Most commonly in palaeontology these assumptions are of planar strain, where strain in the excluded dimension is assumed to be negligible, or planar stress, where the stress exerted on the excluded dimension is assumed to be negligible. Planar strain is known to introduce error into sheer strain predictions in bone (Verner et al., 2016). Planar stress requires a known thickness of material (Marcé‐Nogué et al., 2013) that will likely vary over models. When thickness is known, creating 3D extrusion models from outlines has proved more valid than 2D assumptions (Morales‐García et al., 2019). Plane stress assumptions, then, have little reason for use. For compression fossils, in which thickness is unknown, it is recommended to use planar strain assumptions so that error will be similar across studies. The exception to this recommendation is in groups like ornithopod dinosaurs where significant sheer strain is theorised to be involved in feeding (Rybczynski et al., 2008).
Finally, the comparison of FEA outputs has undergone a major paradigm shift. In the past, FE models have been compared qualitatively (Rayfield, 2005), or their peak (Rayfield et al., 2007) and/or average (Rayfield, 2011b ) strength criteria compared quantitatively. Both quantitative methods, however, are sensitive to the ways the FE model is constructed and require considerable mathematical correction (Marcé‐Nogué et al., 2016). The amount of data required for correction renders comparison across studies nearly impossible. Marcé‐Nogué et al. (2017) introduced the intervals method as a quantitative comparison robust to model construction. Ultimately, it determines the percentage of the model area/volume which experiences a given interval of stress. These percentages can then be compared directly in a histogram or plotted into principal component space. The intervals method has proved effective in subsequent feeding studies (Zhou et al., 2019; Coatham et al., 2020; Miller et al., 2020), and is recommended here.
(ii). The strength criterion
A strength criterion is the measure of a model by which a researcher judges it. Typically, the lower the value of a strength criterion, the stronger the model. The strength criterion for biological FEA has traditionally been the peak VM stress (Fig. 5A), after the recommendation of Dumont et al. (2009). Dumont et al. (2009) justify the use of VM stress to predict failure with a textbook on machine component design (Juvinall & Marshek, 2011). By contrast, studies comparing failure criteria in models of human femora found that maximum principal strain, not VM stress, best predicted the location of, and load required for, fracture (Schileo et al., 2008; Yosibash, Tal & Trabelsi, 2010). This criterion remains in use in the medical community as the most effective method of predicting fracture risk in patients (Dahan et al., 2019). This discrepancy likely originates in the use of a failure criterion for machine components, abiotic metallic objects, to describe the behaviour of bones, living composite ceramic (sensu Carter & Norton, 2007) objects. The textbook takes into account two forms of failure in machine parts, plastic distortion and fracture (Juvinall & Marshek, 2011, p. 250). Unlike metal, when bones deform plastically they are often capable of rapid repair and light use during rest (Vogel, 2013, p. 342). Therefore, fracture is the more critical component of bone failure. The work of fracture of commercial steel (Tattersall & Tappin, 1966), as one might find in machine components, is nearly 20 times that of bovine femora and still an order of magnitude beyond that of even impact‐resistant antler (Currey, 1999). This means that once cracks form in bone they propagate much more readily than in steel. Here it is proposed that principal strain of finite element models best predicts failure in bone because surficial cracks in bone, indicated by extreme principal strain at the surface, propagate readily to the point of failure under normal loading conditions. This allows principal strain‐based FEA (Fig. 5B) to predict areas of weakness more precisely (Schileo et al., 2008) and with greater validity (Yosibash et al., 2010) in bones. For these reasons we suggest that future studies should evaluate the strength of models based on differences in maximum principal strain, not VM stress. The FEA software Abaqus (Dassault Systèmes, France), Ansys (Ansys, Inc., USA), COSMOSWorks (Dassault Systèmes, France), Optistruct (Altair Engineering, Inc., USA), Strand7 (Strand7 Pty. Ltd., Australia), and VOX‐FE2 (Banglawala et al., 2015) are already capable of this.
Fig 5.

Comparison of finite element analysis (FEA) failure criteria. Comparisons are made using a reconstruction of the upper jaw of Shenqiornis after O'Connor & Chiappe (2011). All models use isotropic material properties of ostrich mandible (Rayfield, 2011a ), make plane strain assumptions, constrain the articular condyle in all directions, constrain the first premaxillary tooth in dorsoventral translation, and load the skull with an equivalent amount of force. Force was applied using the macro in Morales‐García et al. (2019) which replicates muscle fibres, with attachments based on those pictured in Fig. 4. Legends are scaled to make the models look as similar as possible. (A) Von Mises (VM) stress. (B) Principal strain. Note that while both map very similarly onto the model, the region of high distortion at the occipital condyle is smaller in B than A. In human studies, this smaller region represents a narrower margin of error for the location of failure (Schileo et al., 2008; Yosibash et al., 2010). This implies greater validity for principal strain as a strength criterion.
It is worth noting that the maximum principal strain criterion may decrease in validity with decreasing body mass, due to the decreasing importance of fracture in smaller organisms. Work of fracture is a relationship between work (energy) and surface area. The effective work done by an animal and the surface area created by breaks scale differently with size. In an isometric scenario one would expect work output to increase linearly with mass (m 1). It is the product of distance (i.e. length), which scales with m 0.33, and force, which is known to scale with muscle cross‐sectional area at m 0.66 (Froese, 2006). The surface area of a crack in bone, like any other surface (Froese, 2006), should scale with m 0.66. As work scales at a higher rate than the surface area it creates in cracks, one would expect cracks in bones to propagate more easily at higher body masses. Conversely at low body masses cracks propagate less readily relative to loading. Thus, in smaller organisms, the formation of cracks via principal strain of the surface may impose only a weak selective pressure. McIntosh & Cox (2016, p. 8) point out a similar trend in VM stress. Small animals working far from the yield strength of bone tend to experience selection towards mechanical efficiency of biting over minimising VM stress in the skull. Mechanical advantage analysis of lever models (see Section VI.3b) may be more appropriate for dietary inferences in small animals where these pressures hold sway. What size range(s) this shift in selective pressure affects, potential allometric complications [e.g. phylogenetic (Wroe, McHenry & Thomason, 2005) or dietary (van der Meij & Bout, 2004) influences on scaling], and what other measures of feeding efficiency may be applicable [e.g. total strain energy as proposed by Dumont et al. (2009)] all warrant further study.
(iii). Finite element analysis in fossil theropod skulls
Rayfield et al. (2001) performed FEA on a 3D model of the upper jaw of Allosaurus fragilis. Seeing the skull could withstand stresses greater than predicted bite forces, they proposed the animal fed by slashing its jaws at high velocity into prey. Rayfield et al. (2007) combine FEA of extinct and extant taxa, comparing spinosaurid theropods and extant crocodilians. From their analysis, they predict piscivory in Baryonyx walkeri. Shychoski & Snively (2008a ) found the mandibles of adult tyrannosaurids to be more resistant to stress than that of juvenile tyrannosaurids or non‐tyrannosaurids, proposing ontogenetic niche partitioning. These results are not currently in preparation for full publication (Eric Snively, personal communication 2020). Torices et al. (2018) performed FEA on individual theropod teeth. They found Troodon teeth experienced higher stresses than other taxa when loaded non‐optimally (i.e. at an angle other than the scratches observed in dental microwear). This is interpreted as its teeth being poorly adapted for struggling prey, and thus Troodon more likely fed on plant matter and/or small animals (Torices et al., 2018). Lautenschlager et al. (2013) study both the upper and lower jaws of a theropod, finding the lower jaw to display higher VM stress in all loading conditions than the upper jaw. They also apply postcranial forces to the skull, proposing that deconstruction powered by the cervical muscles compensated for low bite forces. This was expanded on in the follow‐up study (Lautenschlager, 2017) in which loadings were varied in orientation to compare skulls’ adaptation for specific feeding styles. This technique forgoes direct modelling of cervical action and instead simply investigates the resultant forces the jaw would experience in contact with food. Miller et al. (2020) incorporate a true fossilised rhamphotheca into an FE model. They found Confuciusornis sanctus to be most similar to an extant sally striking predator or general herbivore, the only FEA results from a non‐avian avialan so far. Cost et al. (2019) present the most complex dinosaur FE models to date, with beams given ligament properties to connect bones. These were used to compare the skull of T. rex to that of an extant avian and lepidosaur. This study was concerned with the presence or absence of cranial kinesis, but their increased accuracy of reconstruction used in a manner like Lautenschlager (2017) and Rayfield et al. (2007) could show promise for illuminating dietary preferences and feeding strategies.
Two FEA studies on the skulls of fossil avians have been undertaken. Degrange et al. (2010) compared the stress distribution in the upper jaw of Andalgalornis steulleti to those of Haliaeetus albicilla and Cariama cristata. They found Andalgalornis to experience the lowest stresses in models of pull‐back feeding, analogous to extant accipiters. Attard et al. (2016) compared five genera of moa (Dinornithiformes) to two extant ratites in a variety of loading conditions to see which they were best suited to. They found that loadings with the lowest stress reflected observed feeding styles of the extant ratites, and that those of the moa match consumulite evidence (Figs S6 and S7 in Attard et al., 2016).
(iv). Finite element analysis in extant bird skulls
Herrel et al. (2010b ) and Soons et al. (2010) performed similar FE analyses on the upper jaws of Darwin's finches. Both found that finches that ate harder foods experienced lower peak stresses, and even that taxa known to feed using the tip or base of the beak experienced lower peak stresses when loaded there. Subsequent studies of Darwin's finches (Soons et al., 2015) support these initial results and emphasise the role of keratin in stress dissipation and the necessity of including it in FE models. All other applications of FEA to extant birds have been validation studies performed on a Java finch Lonchura oryzivora (Soons et al., 2012b ), toco toucan Ramphastos toco (Seki, Mackey & Meyers, 2012), and ostrich Struthio camelus (Rayfield, 2011a ; Cuff, Bright & Rayfield, 2015). The Java finch study was qualitatively evaluated as showing a “fairly good correspondence” between the model and physical specimen (Soons et al., 2012b , p. 190). The same was said of the toucan study despite the ex vivo stress/strain curves provided appearing disjointed from those predicted by the model (Fig. 7 in Seki et al., 2012). The ostrich studies (Rayfield, 2011a ; Cuff et al., 2015) were more thorough in their criticism. They determined that strain patterns (e.g. areas of lower and higher strain and the range of strains experienced) were reflected in FEA but absolute strain and strain angles were not, particularly in the cranial region of the skull. The conclusion these studies support, whether directly or by post hoc interpretation of their data, is that FEA is effective at showing stress/strain distributions in bird skulls and thus reflecting dietary habits. However, modelling limitations prevent them from providing any absolute information about in vivo strain states. Similar conclusions when testing extant crocodilians (Porro et al., 2011; Reed et al., 2011; Sellers et al., 2017) and mammals (Kupczik et al., 2007; Bright & Rayfield, 2011; Godinho et al., 2017) imply this is true across amniotes.
(v). Finite element analysis in extant bird claws
Birn‐Jeffery & Rayfield (2009) were the first to apply FEA to bird claws, and reported preliminary success discriminating between both locomotor and predator/non‐predator categories with 2D FEA. A full study has not yet been published. Tsang et al. (2019) were the first to analyse 3D FE models of bird claws. FEA revealed differences in strength that, while not easily identified as variables in GM analysis, were diagnostic of the behaviours associated with raptorial predation (Goslow, 1972; Csermely & Gaibani, 1998; Ward et al., 2002). The broad scope and robust results of this study are promising for detection of raptorial behaviour in fossil species. Unfortunately, its application may be limited by the large number of fossil birds known only from compression fossils.
(A). Robotic modelling
While not a form of FEA, the modelling techniques of Backus et al. (2015) more strongly resemble FEA than any other techniques commonly applied in palaeontology. Their technique is herein dubbed ‘robotic modelling’, due to its original application in the construction of robotic hands (Dollar & Howe, 2011; Backus et al., 2014). Their approach models non‐ungual phalanges as beams and ungual phalanges as semicircles, with actuators acting as the digital flexor tendons. They evaluate models based on the tensional force exerted in order to maintain a grip with given parameters. Backus et al. (2015) were primarily concerned with the differences in actuation between passerine and non‐passerine feet: passerine birds have digital flexors inserted distally and proximally while non‐passerines have only distal digital flexors. They found both on average to be equally well equipped for grasping but passerine actuation to be uniquely well adapted for minimising forces required to perch. While used to compare perching and grasping behaviours in this study, this technique could easily be expanded for use in more granular studies. Future avenues include comparing adaptations for raptorial behaviour in which prey is completely encircled in the toes (e.g. owls) and held in an open grip (e.g. accipiters) (Fowler et al., 2009), with these results compared to those of fossil taxa.
(vi). Finite element analysis in fossil theropod claws
Birn‐Jeffery & Rayfield (2009) incorporated maniraptorans – including the early‐diverging avialan Archaeopteryx – into their 2D FEA analysis of bird claws. They only report results for Archaeopteryx, with stress regimes aligning with those of arboreal perching taxa. Manning et al. (2009) were the first full study to apply 3D FEA to a theropod claw. They tested an earlier hypothesis based on a practical model (Manning et al., 2006) that dromaeosaurids utilised their recurved second digit unguals for climbing rather than tearing through flesh. Creating fixed points in a model of a Velociraptor claw to replicate use during climbing, the team found the claw to experience levels of stress well below the yield strength of bone under a loading of the estimated body mass of Velociraptor. Thus, they proposed the animal could have supported its body weight on the claw during climbing (Manning et al., 2009). Unfortunately, validation studies of FEA in animals find that only patterns of stress/strain distribution, not absolute values, are predicted by FEA (Bright & Rayfield, 2011; Rayfield, 2011a ; Cuff et al., 2015; Stansfield, Parker & O'Higgins, 2018). As such, this interpretation is called into doubt. Furthermore, it is unclear how their climbing load simulation differs from a theoretical simulation of slashing (both would be loaded at a point slightly proximal to the claw apex and in a direction subparallel to the chord of the claw arc). This work, then, is regarded as inconclusive in its palaeobiological reconstruction. The work of Lautenschlager (2014) on therizinosaurian unguals provides a superior framework for modelling claw use. Manual claws were distinctly loaded as if digging, hooking and pulling, or piercing a substrate. The lowest VM stresses were seen in piercing simulations in all but Alxasaurus elesitaiensis. While not linked directly to diet in the study, behavioural optimisation of claws may provide information regarding niche and food available [e.g. fossorial adaptations likely indicate consumption of arthropods (Smith, 1982) or tubers (Andersen, 1987)].
(vii). Discussion
Reconstructions of non‐avian avialan skulls are recommended to remain in 2D for the time being (see Section VI.1), and so FE models will have to remain 2D as well. However, indications of significant lateroflexion in the neck (see Section II.3a) would suggest that 2D models will not fully capture typical loading. Analysis of these models should, for reasons described above, incorporate planar strain assumptions and examine principal strain as a failure criterion using the intervals method (Marcé‐Nogué et al., 2017). However, strength may be less strongly selected for than efficiency in smaller animals (Dumont et al., 2014; McIntosh & Cox, 2016). If true, total strain energy may better reflect the efficiency of jaws (Dumont et al., 2009) and thus selection for a given diet. With that said, strength‐based FEA of Darwin's finches, smaller than many non‐avian avialans, was able to provide a clear dietary signal (Herrel et al., 2010b ; Soons et al., 2010). Size concerns then may be irrelevant in avialans. It is therefore suggested here that both principal strain and total strain energy be compared to determine which best explains diet among small extant avian taxa. The superior metric can then be applied to fossil avialans. Finally, images of extant avian skulls should ideally be taken as radiographs and modelled with their original keratin thickness so as to model the effects of the rhamphotheca (Soons et al., 2012a ). However the precise thickness of the rhamphotheca seems to have little effect on stress/strain distributions or magnitudes in bird mandibles in our simulations (Fig. 6). So, in lieu of radiographs, surface photographs may be used for model construction as well (Miller et al., 2020). When possible, fossilised rhamphothecae (see Section II.3) should be modelled directly in FE models of extinct taxa (Miller et al., 2020). Otherwise, hypothetical rhamphothecae can be crafted for extinct taxa as in Erlikosaurus (Lautenschlager et al., 2014b ) with refinement by subsequent studies of beak shape in relation to the underlying bone (Button, 2018; Urano et al., 2018; Miller et al., 2020). Note the sensitivity analyses of Lautenschlager (2017) and Soons et al. (2012a ) find keratin inclusion to affect stress and strain magnitudes but not patterns. Thus, comparisons within a skull, e.g. comparing various theoretical loadings, should not need to incorporate rhamphothecae. They may also then be unnecessary when comparing beaked avialans to one another, and only needed when toothed avialans are examined.
Fig 6.

Sensitivity analysis of rhamphotheca thickness in the red‐tailed hawk Buteo jamaicensis. Comparisons are made using a radiograph of the lower jaw of Buteo jamaicensis after Smith & Smith (1990). All models use isotropic material properties of ostrich mandible and rhamphotheca (Rayfield, 2011a ), make plane strain assumptions, constrain the articular glenoid in all directions, constrain the rostral tip of the rhamphotheca in dorsoventral translation, and load the mandible with an equivalent amount of force. Force was applied using the macro in Morales‐García et al. (2019) which replicates muscle fibres, with attachments based on Lautenschlager et al. (2014a ). A bite force of 9.0 N was calculated using the regressions of Sustaita & Hertel (2010) assuming a body mass of 1 kg. The rhamphotheca is highlighted with a red outline. Length of the jaw overall is kept constant with the bone underneath modelled (A) realistically (i.e. as in the radiograph), (B) with a greatly thinned rhamphotheca, and (C) with a greatly thickened rhamphotheca. Note that the strain magnitude and distribution in each is nearly identical. Thus, the precise thickness of the rhamphotheca appears unimportant when constructing 2D FE models of avian lower jaws.
Cranial kinesis plays a major role in feeding in Neognathae (Zusi, 1984; Bout & Zweers, 2001; Bhullar et al., 2016; absent in palaeognaths Gussekloo & Bout, 2005) and thus excluding it will undoubtedly alter the principal strain or total strain energy modelled from the in vivo conditions. Conversely, levels of kinesis similar to those in Neognathae are believed never to have been reached in non‐neognathous avialans (Bhullar et al., 2016; Hu et al., 2019) with the possible exception of Gobipteryx and Ichthyornis (Hu et al., 2019, p. 19576). Incorporating connective structures to allow kinesis to present itself as in Cost et al. (2019) will in theory increase the validity of models, although our attempts to incorporate them into the skull of an enantiornithine have proved troublesome. Jointing of the skull imposes unreasonable dislocation of the jugal, quadrate, and quadratojugal (Fig. 7A, B). This may be an inherent flaw either with 2D simplification of the skull or the reconstruction itself, although sensitivity analyses showed a considerable influence of the cross section and Young's modulus used for connective tissue in the model (Fig. 7C). This issue can only be addressed with further research of suture and ligament physical properties. Avoiding kinesis by loading models of the upper jaw posterior to the bending zone of extant birds would mean loading at the maxilla/jugal contact in many taxa (Fig. 1 and Table 1 in Zusi, 1984), well cranial to the tooth row in most toothed avialans. The exclusion of kinesis in Darwin's finches seemed to have no major repercussions on FEA reflecting diet (Herrel et al., 2010b ; Soons et al., 2010) but this likely stems from similar levels of cranial kinesis in all studied groups. To avoid issues in modelling kinesis, comparison between the akinetic lower jaws of taxa is recommended here as a simple solution. While studies including both jaws in FEA (Wroe et al., 2007; Moreno et al., 2008; Wroe, 2008; Lautenschlager et al., 2013; Attard et al., 2014, 2016; Adams et al., 2019) have recovered differing peak VM stresses between the jaws and different stress distributions (thus making models of both jaws ideal) the information has not conflicted in terms of dietary interpretation.
Fig 7.

Comparison of construction of finite element models with flexible connective tissue using a reconstruction. Comparisons are made using the upper jaw of Shenqiornis after O'Connor & Chiappe (2011). Base models were constructed as in Fig. 5. Breaks were created in the model and filled with beam elements to replicate connective tissue, after the techniques of Cost et al. (2019). A bite force of 9.3 N was chosen to be similar to those recorded by Corbin et al. (2015). (A) Beams using the cross‐sectional area and Young's modulus of rat cranial sutures, as detailed in Chien et al. (2008). These properties were used by Cost et al. (2019) to model the flexible components of the skull in Gekko and Psittacus, animals of similar size to Shenqiornis. (B) Beams using the cross‐sectional area and Young's modulus of canine patellar tendon, as detailed in Haut et al. (1992). These properties were used by Cost et al. (2019) to model flexible components in the skull of Tyrannosaurus. Note that in A the quadrate (i) and jugal (ii) are dislocated to a biologically unreasonable degree and in B the quadrate (iii) is dislocated cranially to a lesser but still unreasonable degree. (C) Beams assigned properties extrapolated from those of a variety of connective tissues reported in the literature and normalized to body mass (Table S4). Note both the lower degree of dislocation of the quadrate (iv) and jugal (v) and the lower principal strains experienced (vi). However, it is unclear how valid this model is. Only three data points are available for calculating cross‐section trends. For Young's modulus, even after excluding outliers, r 2 values for a trend line could not be increased above 0.25. Sensitivity analyses show cross section of beams has a stronger control on excursion; rat suture models can only achieve similar excursion to the scaled property model if tendon cross sections are 45 times greater or if their Young's modulus increases 60‐fold. In all three models the overall strain experienced is reduced relative to those in Fig. 5B, but artificially inflated at the locations of beam attachment (clearest at the jugal/cranium contact) due to singularities.
Application of FEA to determining raptorial use of the pes is promising after the results of Tsang et al. (2019). However, because their predatory groupings differed markedly on dorsal and lateral curvatures (their Fig. S2), which is not accounted for in 2D FEA and not known in compression fossils, larger 2D FEA studies should be preceded by a comparative analysis of 2D and 3D FE models (see Section VI.3a). The reported success of Birn‐Jeffery & Rayfield (2009) shows promise for their correspondence, but a lack of published data renders a full proof‐of‐concept necessary. Refinement of raptorial use type to more precise grips could be achieved by modifying the work of Backus et al. (2015). A pilot study will be necessary to determine in extant raptors if their method works using the curvature of ungual bones rather than claw sheaths. If not, reconstructions of keratin sheaths will be necessary [from avialans preserving impressions of the sheath, e.g. AGB‐6997 (Wang et al., 2020b ), DNHM D2945/6 (Chiappe et al., 2014), GMV‐2130 (Chiappe et al., 1999), GSGM‐05‐CM‐004 (O'Connor et al., 2016a ), IVPP V18687 (Hu, O'Connor & Zhou, 2015)]. While Lautenschlager (2014) provides an outline for modelling the effectiveness of varying claw uses, the use of claws directly in acquiring food is rare among extant avians. Piscivorous raptors are known to pierce fish with their talons in order to maintain grip (Fowler et al., 2009, p. 7), so claw FEA may prove useful to test the hypothesis of similar habits in bohaiornithids (Wang et al., 2014b ). Digging with the claws plays a major role in foraging in Megapodidae (Friedmann, 1931) and a more minor role in shorebirds (Jacobs, 1982). Beyond these cases, claws tend to play a minor role in prey manipulation compared to the whole toes (Clark, 1973; Csermely & Gaibani, 1998; Sustaita et al., 2013), at most increasing traction (Ramos & Walker, 1998; Fowler et al., 2009; Backus et al., 2015) or elongating the toes to increase grasp reach (Csermely, Bertè & Camoni, 1998; Fowler et al., 2009). While general strength trends appear useful in parsing raptorial behaviour, the lack of functional importance of claws outside specialised groups renders more specific loading comparisons a lower priority in lieu of established hypotheses to test.
(4). Complications applicable across physical approaches
While eating is essential to an organism's survival, myriad unrelated factors play into the form and function of body parts. These factors are confounding variables in any palaeodietary reconstruction. Some factors can be corrected for, theoretically negating their influence, but all must be kept in mind when interpreting data from methods described herein. Table 3 provides a convenient reference for when such complications must be accounted for.
Table 3.
Quick reference for complications that significantly affect the physical approaches discussed. Rationale is provided within subsections of Section VI.4. Complications with an asterisk (*) are those for which mathematical corrections are available
| Physical approaches | Complications | ||||||
|---|---|---|---|---|---|---|---|
| Phylogenetic signal* | Allometric signal* | Many‐to‐one‐mapping | Liem's paradox | Integration | Modularity | Behavioural signals | |
| Traditional morphometrics | X | X | X | X | X | X | |
| Geometric morphometrics | X | X | X | X | X | X | |
| Lever modelling | X | X | X | X | |||
| Finite element analysis | X | X | X | X | |||
(a). Phylogenetic signal
It is easy to see how phylogeny can affect shape. Genetics is one of the principal factors controlling shape, and thus in the absence of selection one would assume that the more similar the genetics of two organisms the more similar they will appear (Blomberg, Garland & Ives, 2003). At large enough phylogenetic scales, developmental pathways may even completely prohibit an optimised form, or predispose two organisms to find different functionally optimised forms (Gould, 2002). Thus, morphometric comparisons at any timescale over which evolution is a factor should undergo phylogenetic corrections (de Bello et al., 2015). Uyeda et al. (2018) review how to craft hypotheses effectively so that phylogenetic corrections can be made. Adams & Collyer (2018) review the mathematical methods and assumptions required for these corrections. Guillerme et al. (2020) provide general guidance for the timing of corrections and potential pitfalls of certain evolutionary models.
Over broad enough phylogenetic scales, homologous structures may eventually become functionally incomparable. This is particularly important in the theropod pes due to the evolution of flight. Among coelurosaurian theropods, grasping ability of the manus generally decreases the later a taxon diverges (Hutchinson & Allen, 2008). The proximally fused metacarpals of Sapeornis and later‐diverging avialans (Fig. 4 in Pittman et al., 2020a ) likely placed similar constraints on manipulation as in living avians, meaning the two groups would have relied similarly on the pes for grasping. The more refined manual manipulation of some early‐diverging paravians (Senter, 2006) means their pes was probably subjected to weaker selective pressures as a device for manipulation. In this example a comparison of pedal proportions of function among Jeholornithiformes and pygostylians should provide meaningful data, but a comparison of all paravians may not.
(b). Allometric signal
Different measures of organisms, such as length, surface area, and volume, have different dimensionality. This means they grow at different rates relative to one another. Most organisms would cease to function if they grew isometrically (i.e. with every part scaling the same way as every other part) both through ontogeny and evolution (Schmidt‐Nielsen, 1984). Thus, nearly all organisms display some form of allometry, i.e. certain components scaling at different rates than others (Froese, 2006). In other words, body size has an inherent effect on body shape. Klingenberg (2016) reviews methods to quantify and correct for this effect in morphometric studies. Functional effects of allometry have almost exclusively been derived empirically and are known to vary phylogenetically (van der Meij & Bout, 2004; Wroe et al., 2005). Thus, allometry presents a more confounding influence in functional studies. In addition to influencing the strength or efficiency of structures inherently, size may also determine if strength or efficiency is more strongly selected for (see Section VI.3c.ii).
(c). Many‐to‐one mapping
Coined by Wainwright et al. (2005), many‐to‐one mapping describes the ability of systems with different forms to perform the same mechanical function. This means that elements that morphometrics classifies as very different may be operating essentially the same in practice. This aligns with the observation above that GM tends to provide mixed results in feeding studies. GM creates a level of abstraction between the data and how the animal interacts with the world. Identifying a case of many‐to‐one mapping via comparison of morphometric and functional studies can be useful. It can formulate hypotheses of evolutionary constraints on form (Ungar & Hlusko, 2016; Button & Zanno, 2020) or illuminate interference of common behavioural signals (e.g. herbivorous and carnivorous taxa engaging in similar bouts of intrasexual competition for mates, or diving predators and foragers facing similar pressures from long‐term submersion).
(d). Liem's paradox
Occasionally referred to as one‐to‐many mapping, Liem's paradox was originally coined to describe the peculiarity that a set of cichlid fishes with highly specialised jaws seemed to have no particular dietary speciality (Liem, 1980). The prevailing explanation has been that some specialist morphologies minimally inhibit acquiring ‘easy’ resources while aiding in obtaining ‘difficult’ resources (commonly referred to as ‘fallback foods’) when others are scarce (Robinson & Wilson, 1998). Recent ecological evidence supports this theory (Lambert et al., 2004; Golcher‐Benavides & Wagner, 2019; Wiseman et al., 2019). Essentially, this means that organisms that appear morphologically specialised for a certain diet cannot be precluded from being generalists (or specialists feeding on a different easy‐to‐acquire food source) overall. This is also the reason dental microwear studies emphasise large sample sizes for analysis, to capture signs of rare but important resource use (Ungar, 2018).
(e). Integration and modularity
Put simply, integration is when otherwise distinct parts of an organism function and/or evolve as one unit. Modularity is when an organism displays distinct regions (modules) within which integration is high and between which integration is low (Klingenberg, 2014). Farina, Kane & Hernandez (2019) provide more rigorous definitions and a review of the concepts.
Both extreme modularity and extreme integration can encourage diversification (Hedrick et al., 2019b ). In highly modular systems parts are free to evolve independently from one another. This should, in theory, increase adaptation to environmental changes and thus speciation. Highly integrated systems, on the other hand, restrict parts to evolving as a single unit. While limiting form to a single spectrum, it can allow for rapid diversification and speciation along that spectrum (Hedrick et al., 2019b ). However, each does not encourage diversification on the same scale. Modularity is associated with diversification overall in high‐level clades (class to subclass level) (Marroig et al., 2009; Hu et al., 2016; Felice & Goswami, 2018; Felice et al., 2019b ) and at smaller scales in several evolutionary circumstances (Tokita, Kiyoshi & Armstrong, 2007; Drake & Klingenberg, 2008; Young, Wagner & Hallgrímsson, 2010; Collar et al., 2014). Integration, by contrast, is known to lead to diversification when lineages invade habitats with a preponderance of unoccupied niches Hu et al., 2016; (Hedrick et al., 2019b ; Navalón et al., 2020).
Integration and modularity are of most concern in a morphometric context. Integration limits disparity to a single continuum (Hedrick et al., 2019b ; Navalón et al., 2020). So highly integrated structures are likely to cluster or spread on a single axis of shape. Modularity also can help prioritise functional studies. More modular structures, with more ability to create unique geometries, are more likely to exhibit many‐to‐one mapping. Thus, they should be checked for functional convergence with higher priority. Adams & Felice (2014) and Adams (2016) provide techniques for quantifying integration and modularity. Because of the complicated relationship they have with evolutionary trajectories no universal correction for their effects has yet been proposed.
(f). Behavioural signals
(i). Grooming
The influence of grooming behaviour on morphology has been studied more thoroughly in birds than any other group (Bush & Clayton, 2018). Some anatomical work has been done on specialised dental (Gingerich & Rose, 1979; Rose, Walker & Jacobs, 1981; Asher, 1998) and manual (Bishop, 1962; Koenigswald, Habersetzer & Gingerich, 2011; Maiolino, Boyer & Rosenberger, 2011; Dunkel, 2019) grooming in primates. In birds, destruction of ectoparasites is known to be aided by a short bill (Clayton & Cotgreave, 1994) with a rostral hook (Clayton & Walther, 2001; Clayton et al., 2005; Bush et al., 2012). Intraspecific differences in bill shape are known to reflect ectoparasite load in communities (Bardwell, Benkman & Gould, 2001; Moyer, Peterson & Clayton, 2002). Some birds also have a pectinate (‘comb’) claw with distinct serrations on the lateral surface thought to play a role in grooming. Studies of its effect, however, have yielded mixed results (Clayton et al., 2010; Bush et al., 2012). It is worth noting that, of these variables, only bill length is always reflected in the animal's skeletal morphology. The same is not true of mammalian toothcombs. Toothcombs convergently evolved in lemurs, flying lemurs, tree shrews, and the arctocyonid Thryptacodon (Rose et al., 1981). In toothcombs the mandibular incisors and, variably, canines are deflected rostrally. During grooming they are brushed perpendicular to hair shafts to aid in ectoparasite removal (Schwartz, 1978; Gingerich & Rose, 1979; Rose et al., 1981). This role is augmented or replaced by a specialised, more robust and recurved grooming claw (‘toilet claw’) in non‐simian primates and several polyphyletic simians (Koenigswald et al., 2011; Maiolino et al., 2011). Manual grooming has been reported thoroughly in simian primates, but almost exclusively in social (Schino, 2006; Xia et al., 2019) or spatial (Freeland, 1981; Dunbar, 1991) contexts, rather than its effect on morphology. Opposable thumbs have been proposed as the product of selective pressures related to manual grooming (Bishop, 1962), although this theory remains only one of many (Dunkel, 2019).
From these trends in birds and mammals, oral grooming may be expected to select for perpendicularly oriented structures in the rostrum (beak hook, tooth comb) while manual grooming can be accomplished with more diverse structures (pectinate claw, grooming claw, possibly opposable thumbs). Each of these structures accompanies grooming behaviour with stresses distinct from that of feeding. In turn, structures experience selective pressure to resist those stresses. To our knowledge only circumstantial notes have been made on how significant these stresses may be. There are notes of beak overhangs breaking (Bush et al., 2012) and enamel showing microscopic wear (Rose et al., 1981) from grooming activities. Because wear patterns on tooth combs differ from those of the surrounding teeth, unique wear in teeth has been proposed as indicative of a grooming function in the dromaeosaurid Saurornitholestes (Currie & Evans, 2019).
(ii). Thermoregulation
The size of structures is commonly assumed to relate to their strength or speed, but thermoregulation is known to influence the scale of elements. Joel Asaph Allen famously noted that animals living in warmer climates tend to have larger extremities and vice versa (Allen, 1877, p. 112–119), a trend now referred to as Allen's rule. The rule is supported by several quantitative studies (Nudds & Oswald, 2007; Tilkens et al., 2007; Symonds & Tattersall, 2010; Alho et al., 2011; Greenberg et al., 2012; contra Stevenson, 1986). More dramatic structures such as goat horns (Taylor, 1966), elephant ears (Phillips & Heath, 1992), and toucan bills (Tattersall, Andrade & Abe, 2009) have all been proposed as tools for active thermoregulation. The size of body parts should then be interpreted with the climate inhabited by the organism in mind, and hypertrophy of body parts, particularly when not accompanied by significant increases in structural strength, may be a sign of active thermoregulation rather than dietary pressure.
(iii). Sensation
Sensory systems are paramount in both feeding (Montuelle & Kane, 2019) and reproduction (Ptacek, 2000) in living animals. Thus, selective pressure on sensation can be expected to dramatically shape organisms. For instance, enhanced mechanoreception is often associated with elongation and extensive pitting of the skull in amniotes in general (Morhardt, 2009) and birds in particular (Cunningham et al., 2013). In the same vein, an increase in size of amniote eyes often creates a corresponding reduction in bite force (Henderson, 2002; Fortuny et al., 2011). Sensory specialisations that parallel those in extant taxa can be understood and tested for by understanding the biology of those taxa. The possibility of novel forms of sensory augmentation present only in extinct taxa, however, renders sensation a true confounding variable.
(iv). Sexual display
Animals have a variety of tools for communication and competition that improve survival and reproductive success. All of these may alter the body in unpredictable ways. Protuberances may serve as intersexual signals (Mayr, 2018) or as bases for intrasexual combat (Clutton‐Brock, 1982; Rico‐Guevara & Araya‐Salas, 2014). Changes in skull morphology can lead to changes in vocalisation (Huber & Podos, 2006; Herrel et al., 2009; Giraudeau et al., 2014) or altered ability to detect chemical messages (Rouquier & Giorgi, 2007). Sexual dimorphism itself can lead to functional differences on small (Verwaijen, Van Damme & Herrel, 2002) or large (Pietsch, 2005) scales. Sexual dimorphism may be able to be detected in the fossil record given an adequate number of samples (Plavcan, 1994), but other forms of sexual display can drive shape and affect function with little evidence left in the fossil record. Thus, sexual display also remains a true confounding variable.
(5). Discussion
The broad application of physical approaches across fossil taxa makes them ideal for comparative frameworks, but not all frameworks are appropriate approaches to dietary reconstruction. Neither TM nor GM of theropod skulls appears particularly effective at isolating features of diet across phylogenetically diverse groups. In addition, the lack of consistent landmarks in studies highlights how few measurements there are of the theropod skull that would have an intuitive effect on dietary choice. Both forms of morphometrics are more effective at revealing diet when applied to the pes. This is likely because the pes performs a smaller variety of roles in the organism (Montuelle & Kane, 2019) and those other roles are often in service of obtaining food (Fowler et al., 2009; Sustaita et al., 2013). Results have been obtained with both TM and GM analysis of extant avian feet, and so neither is recommended over the other by this review. TM may also prove useful to find dietary signals in teeth due to the large disparity seen across theropods as a whole (Hendrickx & Mateus, 2014; Hendrickx et al., 2020) and Avialae in particular (O'Connor & Chiappe, 2011; Huang et al., 2016; O'Connor, 2019). A lack of prior study in this field leaves this recommendation only tentative. Lizards are the closest related extant taxon with similarly disparate teeth, and quantitative analyses of lizard teeth have already identified characters indicative of durophagy (Estes & Williams, 1984) and herbivory (Melstrom, 2017) that could be applied to toothed avialans.
In general, this review recommends functional studies over morphometrics due to the fewer complications that influence them (see Table 3). 2D simplifications of functional models appear necessary for the time being, but if possible, comparisons of 2D and 3D models should be undertaken to confirm the former's validity. Whether more valid dietary signal can be gleaned from lever models of the upper jaw combined with functional indices from Button & Zanno (2020) and Ma et al. (2020) (Fig. 4) or principal strain‐based FEA of the lower jaw is still unclear. The size at which selection for strength transitions to selection for mechanical efficiency (Adams et al., 2019), if such a transition truly exists, is not yet established. Ideally, a study including both approaches could directly compare the two. Separate studies, each focusing on one functional approach, should have results that can be compared nearly as effectively. Incorporating connective tissue into the skull to create kinetic structures is not recommended pending more precise understanding of the physical properties of those connective tissues. Discovery of fossilised rhamphothecae allows for them to be included in models, as is suggested here.
VII. THE FRAMEWORK AND CURRENT KNOWLEDGE OF NON‐AVIAN AVIALAN DIET
As individual techniques described in Sections II., VI. provide only limited insight into bird diet, taking the consensus of multiple lines of evidence is essential to gaining useful information. However, from our experience, differences in fossil taxa studied, extant reference taxa, and dietary categories make studies using single techniques difficult or impossible to combine. Thus, we combine the recommended techniques into a framework for narrowing possible fossil avialan diets. Fig. 8 provides a summary of these techniques, the body parts which need to be preserved for their use, expected results from their application, and interpretation of the results. A general workflow for applying the framework is provided in Fig. 9, from determining specimens of interest to synthesising test results into a dietary assignment. All seven of the recommended approaches are not necessary for results to be valid, but agreement of a greater number of techniques should increase the validity of findings.
Fig 8.
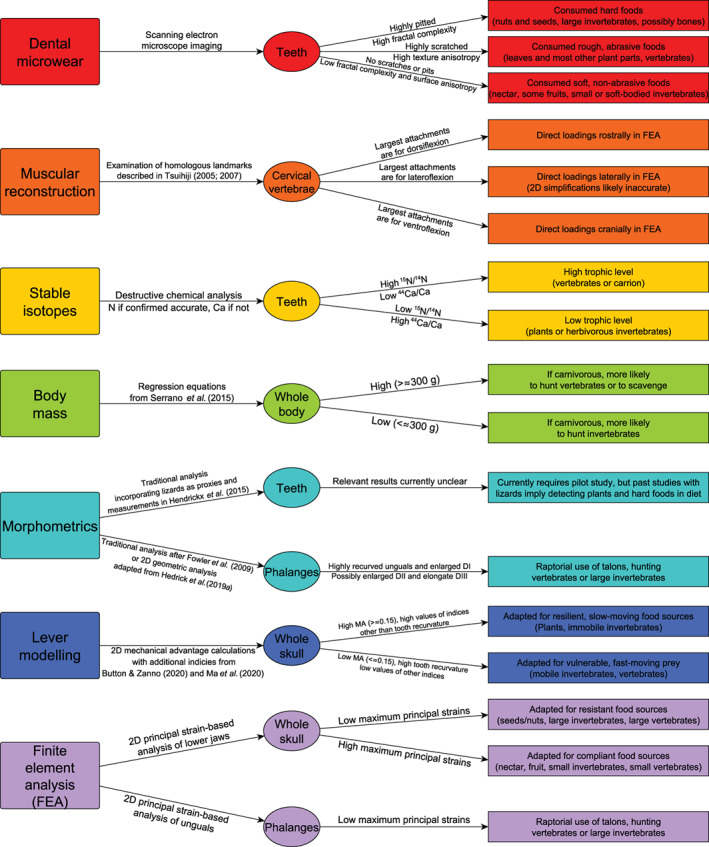
Summary chart of our recommended framework for the study of non‐avian avialan diet. Approaches are followed by a brief description of specific prescribed techniques, the body part it would be performed on, relevant results, and recommended dietary and/or modelling interpretations of the results.
Fig 9.

Summary workflow for using the framework described in this paper. (1) Identify fossil specimen(s): Section VII suggests several taxa of particular interest, and Application/Discussion sections throughout this work list published specimens with particular promise. (2) Determine tests: the second column of Fig. 8 lists which preserved body parts are necessary for which tests. Note that while stable isotope analysis can be performed on bones in addition to teeth, this is not recommended as the signatures they give are less reliable. (3) Test: perform one of the seven listed tests on the specimen of interest. The sections in this paper for each technique provide references for methodology. Dashed lines pointing to the test types indicate that not all tests may be possible. The solid lines pointing away from them indicate that any tests which can be performed should have their interpretations contribute to the final synthesis. (4) Interpret test results: the final column of Fig. 8 provides idealised interpretations for test results into a set of potential diets. Section VI.4 may also be pertinent to this workflow stage. (5) Synthesise into diet assignment: combine test results with those of other tests performed. In the simplest terms, this means finding the common elements between each test's interpretations, although the possibility of feeding styles not seen in any extant group may require more creativity at this stage.
We provide an example of applying our framework to the extant golden eagle (Aquila chrysaetos), a raptorial vertebrate predator. It was chosen as it appears in more studies compatible with our framework than any other bird. Fig. 10 provides a graphical summary of this example. While Aquila chrysaetos does not have teeth, if it did, they should have high surface anisotropy from the repeated tearing of tough meat. Its pull‐back method of prey disassembly should select for enlarged attachment sites for dorsiflexive muscles, and finite element models of its lower jaw should have muscular loadings deflected cranially. Its bones should be depleted in 44Ca and its proteins enriched in 15N due to its high trophic level. Its body mass should be above 300 g due to vertebrate predation; this is true even in unusually small subspecies (Watson, 2010, p. 33). Its talons should be highly recurved with a hypertrophied DI due to their raptorial use; this has been reported (Fowler et al., 2009). Its jaws should have a low MA, high tooth recurvature if it had teeth, and high values of the other recommended indices as it hunts agile prey; its jaw MA is low among avians (Navalón et al., 2018a ). Its lower jaw should experience relatively high principal strains when loaded in FEA due to the compliance of animal flesh. Finally, its talons should experience relatively low principal strains when loaded in FEA due to their raptorial use; this has been supported (Tsang et al., 2019). Given the current studies that fit into our framework, there are four lines of evidence which would lead to classifying the golden eagle as a raptorial vertebrate predator if it was extinct (Fig. 10).
Fig 10.

Summary of our recommended study framework, as applied to the extant golden eagle Aquila chrysaetos. Morphometrics and lever modelling are transposed from Fig. 8 for clarity. Lever modelling shows adaptation for vulnerable and possibly mobile prey, pointing to either predation or molliphagous herbivory. Morphometrics and FEA of the pes provide evidence of raptorial use, and thus refine the lever modelling findings to predation. Given that the animal is carnivorous, then, body mass makes vertebrate consumption much more likely than invertebrate consumption. Thus. these four lines of evidence point to Aquila chrysaetos being a raptorial vertebrate predator, which indeed it is (Watson, 2010).
While limited by a dearth of quantitative studies, our framework can be used to establish what we currently know of non‐avian avialan diet (Fig. 11). The scansoriopterygids [possibly early‐diverging avialans or non‐avialan pennaraptorans (Pittman et al., 2020a )] Epidexipteryx and Yi appear adapted for carnivory due to low values of mechanical advantage and most of the functional indices recommended herein (Ma et al., 2020). Body mass estimates above 300 g in Yi (Dececchi et al., 2020) specifically point to it being a vertivore. To our knowledge, no quantitative study of anchiornithine [possibly early‐diverging avialans or troodontids (Pittman et al., 2020a )] diet has been performed. Preserved fish and lizard meals are known in Anchiornis (Zheng et al., 2018b ), but it is unclear if this represents a typical part of its diet. Evidence of diet in the earliest‐diverging unequivocal avialan Archaeopteryx appears contradictory. Studies show dental microwear reminiscent of invertivores (Bestwick et al., 2018), a body mass in the range of vertivores (Serrano et al., 2015), and relatively high jaw MA (Navalón, 2014) expected in herbivores or durophages. The most likely source of this contradiction is the specimens studied. Bestwick et al. (2018) studied microwear of the Munich specimen (Jordan Bestwick, personal communication 2020); Serrano et al. (2015) measured the London, Berlin, Eichstätt, and Thermopolis specimens; and the work of Navalón (2014) was based on the reconstruction of Rauhut (2014), which was in turn based primarily on the Eichstätt specimen. Considerable morphological disparity has been noted previously within Archaeopteryx (Rauhut, Foth & Tischlinger, 2018) which may yet indicate diverse diets within the genus. Alternatively, the contradictory evidence may be indicative of Liem's paradox at work, with fallback food(s) not captured in the small sample size of dental microwear [see Lambert et al. (2004) for an extant equivalent]. Five preserved seed meals are currently known in the early‐diverging avialan Jeholornis (Zhou & Zhang, 2002; O'Connor & Zhou, 2019), but it is unknown if other major parts of its diet remain unaccounted for. Due to a sparsity of quantitative data, especially the seemingly contradictory evidence in Archaeopteryx, the ancestral dietary condition in avialans remains unclear.
Fig 11.

Currently known diets of non‐avian avialans labelled onto an avialan phylogeny. The tree topology is taken from Fig. 3A in Pittman et al. (2020a ), with Scansoriopterygidae and Anchiornithinae grafted on from Fig. 1 in the same chapter. Eogranivora is placed in a polytomy with Bellulornis and Schizooura after O'Connor (2019) grouping it with the former. A taxon is assigned a diet if specimens of it have preserved meals and/or if two lines of evidence agree on a diet. Even with these low bars for assignment, less than 15% of taxa in the tree are assigned a diet, and the tree itself includes less than half of the named non‐avian avialan genera.
The early‐diverging pygostylian Confuciusornis has jaws with both strength (Miller et al., 2020) and MA (Navalón, 2014) consistent with herbivorous avians (Navalón, 2014; Miller et al., 2020). One study applying pedal morphometrics (Cobb & Sellers, 2020) recovered Confuciusornis as raptorial, and its body mass estimates are consistent with vertivory (Serrano et al., 2015; Table 2). However, because the morphometric study measured only curvature of unguals and not their relative sizes, we consider that raptorial behaviour is not ruled out, but is not confirmed. This discrepancy can be directly addressed with stable isotope analysis, for which Confuciusornis is a prime candidate due to the large number of specimens known. Its close relative Eoconfuciusornis has only had MA measurements taken (Navalón, 2014), which we consider inadequate for dietary assignment. No dietary study has been conducted on any member of Jinguofortisidae to our knowledge. We also consider the single line of quantitative MA evidence (Navalón, 2014) favouring granivory in the early pygostylian Sapeornis to be inadequate for diet assignment, although it does agree with previously reported ingested material (Zheng et al., 2011; O'Connor, 2019; O'Connor & Zhou, 2019).
Among the enantiornithine ornithothoracines, Shenqiornis is tentatively proposed here as predatory due to low jaw MA (Navalón, 2014) and raptorial pes morphometrics in its close relatives (Wang et al., 2014b ). Its MA is of particular interest for future studies to attempt to replicate due to qualitative assertions of durophagy in the taxon (Wang et al., 2010b ). Navalón (2014) additionally reports intermediate values of MA for Pengornis and an indeterminate hatchling and low MA for Rapaxavis. Again, we consider this single line of evidence inadequate for dietary assignment. One specimen of Zhouornis (BMNHC Ph 756) has been reported as having claws as straight as extant ground birds (Cobb & Sellers, 2020), which would rule out raptorial behaviour in the taxon. However the claw measured, DIII, is aberrantly straight in this genus with the other claws highly recurved (Zhang et al., 2013; Zhang et al., 2014) so we do not consider raptorial behaviour ruled out. The holotype of Eoalulavis preserves part of a crustacean in its stomach (Sanz et al., 1996), but the lack of a skull or feet in the specimen inhibit investigation of the typical diet of this taxon within our framework.
Finally among non‐avian ornithuromorphs, MA values reported in Navalón (2014) are congruent with ingested gastroliths in Hongshanornis (Chiappe et al., 2014) indicating herbivory and fish in Yanornis (Zhou et al., 2004; Zheng et al., 2014) indicating carnivory. Additionally, Eogranivora and Piscivoravis have preserved meals that provide evidence of granivory (Zheng et al., 2018a ) and piscivory (Zhou, Zhou & O'Connor, 2014) respectively, but determining whether these were normal parts of their diet requires further study.
The paucity of dietary assignments renders trends in avialan dietary evolution murky. Our framework supports a mixture of carnivory and herbivory/omnivory among both early‐diverging non‐avian avialans (Archaeopteryx, Confusciusornis, Sapeornis) and later‐diverging ones (Shenqiornis, Hongshanornis, Yanornis) (Fig. 11). Therefore, no particular macroevolutionary trends are currently apparent. Dietary diversity seems to increase through time, but this is a preservational bias associated with the predominance of data from the Early Cretaceous Jehol Lagerstätte (all of these taxa except Archaeopteryx). This also means that relatively little geographic and climatic range is accounted for among currently known non‐avian avialan diets. Thus, non‐avian avialan material from a wider range of localities should also be prioritised for future study.
VIII. CONCLUSIONS
Our aim was to build a framework for studying non‐avian avialan diet by reviewing techniques that have proved effective in both avians and non‐avian theropods and the use this to summarise our current state of knowledge. With this framework in place, we expect it will generate progress in the reconstruction of Mesozoic ecosystems and in our understanding of the ecological history of birds.
Fig. 8 provides a convenient summary of the techniques discussed in this review and our recommendations for applying them. Expected outcomes and their general interpretations are also provided. We recommend combining direct evidence of diet with dental microwear, stable isotope geochemistry, body mass estimation, pes morphometrics, and functional analysis to obtain multiple lines of evidence relevant to diet.
Due to a dearth of quantitative studies, current knowledge of non‐avian avialan diet is sparse. The ancestral avialan diet remains obscure, in large part due to contradictory evidence concerning the diet of Archaeopteryx. Both carnivory and herbivory/omnivory are present in early‐diverging (Archaeopteryx, Confuciusornis, Sapeornis) and later‐diverging (Shenqiornis, Hongshanornis, Yanornis) avialans, but no trends in the dietary evolution of non‐avian avialans have presented themselves. We believe that new avialan specimens from a wider range of localities covering different geographies and climates will be instrumental to elucidating these trends in the future.
Our review demonstrates the need for establishing links between diet and morphology in avians, reconstructing the often‐crushed remains of non‐avian avialans, and combining the two in robust quantitative frameworks. Combined with a growing understanding of extant ecology, these will provide a new and exciting picture of earth during some of the most ground‐breaking evolutionary transitions known.
Supporting information
Table S1. Listing of published non‐avian avialan skulls.
Fig. S1. Principal component analysis (PCA) of avian pedal measurements from Fowler et al. (2009, 2011).
Fig. S2. Principal component analysis of theropod skulls from Foth & Rauhut (2013).
Table S2. Table summarising all dietary recoding used in Fig. S2.
Fig. S3. Principal component analysis of theropod skulls from Schaeffer et al. (2019).
Fig. S4. Relationship between mechanical advantage and plant consumption in passerines.
Fig. S5. Biomechanical morphospace of Dinosauria from Button & Zanno (2020).
Table S4. Calculation of cranial connective properties of Shenqiornis.
IX. ACKNOWLEDGEMENTS
We wish to thank our two anonymous reviewers for their helpful comments that improved the quality of our review. C.V.M. is supported by a Postgraduate Scholarship from The University of Hong Kong (HKU PGS). M.P. is supported by the Research Grant Council of Hong Kong's General Research Fund (17120920; 17103315; 17105221) and the RAE Improvement Fund of the Faculty of Science, The University of Hong Kong.
Contributor Information
Case Vincent Miller, Email: case.miller@connect.hku.hk.
Michael Pittman, Email: mpittman@hku.hk.
REFERENCES
- Abel, R. L. , Laurini, C. R. & Richter, M. (2012). A palaeobiologist's guide to ‘virtual’ micro‐CT preparation. Palaeontologia Electronica 15, 6T. [Google Scholar]
- Abourachid, A. , Fabre, A. C. , Cornette, R. & Hofling, E. (2017). Foot shape in arboreal birds: two morphological patterns for the same pincer‐like tool. Journal of Anatomy 231, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamík, P. & Kornan, M. (2004). Foraging ecology of two bark foraging passerine birds in an old‐growth temperate forest. Ornis Fennica 81, 13–22. [Google Scholar]
- Adams, D. C. (2016). Evaluating modularity in morphometric data: challenges with the RV coefficient and a new test measure. Methods in Ecology and Evolution 7, 565–572. [Google Scholar]
- Adams, D. C. & Collyer, M. L. (2018). Multivariate phylogenetic comparative methods: evaluations, comparisons, and recommendations. Systematic Biology 67, 14–31. [DOI] [PubMed] [Google Scholar]
- Adams, D. C. & Felice, R. N. (2014). Assessing trait covariation and morphological integration on phylogenies using evolutionary covariance matrices. PLoS One 9, e94335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, D. C. & Otarola‐Castillo, E. (2013). Geomorph: an r package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution 4, 393–399. [Google Scholar]
- Adams, N. F. , Rayfield, E. J. , Cox, P. G. , Cobb, S. N. & Corfe, I. J. (2019). Functional tests of the competitive exclusion hypothesis for multituberculate extinction. Royal Society Open Science 6, 181536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho, J. S. , Herczeg, G. , Laugen, A. T. , Räsänen, K. , Laurila, A. & Merilä, J. (2011). Allen's rule revisited: quantitative genetics of extremity length in the common frog along a latitudinal gradient. Journal of Evolutionary Biology 24, 59–70. [DOI] [PubMed] [Google Scholar]
- Allen, J. A. (1877). The influence of physical conditions in the genesis of species. Radical Review 1, 108–140. [Google Scholar]
- Alonso, P. D. , Milner, A. C. , Ketcham, R. A. , Cookson, M. J. & Rowe, T. B. (2004). The avian nature of the brain and inner ear of Archaeopteryx . Nature 430, 666–669. [DOI] [PubMed] [Google Scholar]
- Ambrose, S. H. & DeNiro, M. J. (1986). The isotopic ecology of east African mammals. Oecologia 69, 395–406. [DOI] [PubMed] [Google Scholar]
- Amiot, R. , Lécuyer, C. , Buffetaut, E. , Escarguel, G. , Fluteau, F. & Martineau, F. (2006). Oxygen isotopes from biogenic apatites suggest widespread endothermy in cretaceous dinosaurs. Earth and Planetary Science Letters 246, 41–54. [Google Scholar]
- Amiot, R. , Wang, X. , Lecuyer, C. , Buffetaut, E. , Boudad, L. , Cavin, L. , Ding, Z. L. , Fluteau, F. , Kellner, A. W. A. , Tong, H. Y. & Zhang, F. S. (2010). Oxygen and carbon isotope compositions of middle cretaceous vertebrates from North Africa and Brazil: ecological and environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology 297, 439–451. [Google Scholar]
- Amiot, R. , Wang, X. , Zhou, Z. , Wang, X. , Lécuyer, C. , Buffetaut, E. , Fluteau, F. , Ding, Z. , Kusuhashi, N. & Mo, J. (2015). Environment and ecology of east Asian dinosaurs during the early cretaceous inferred from stable oxygen and carbon isotopes in apatite. Journal of Asian Earth Sciences 98, 358–370. [Google Scholar]
- Andersen, D. C. (1987). Below‐ground herbivory in natural communities: a review emphasizing fossorial animals. The Quarterly Review of Biology 62, 261–286. [Google Scholar]
- Angst, D. , Amiot, R. , Buffetaut, E. , Fourel, F. , Martineau, F. , Lazzerini, N. & Lécuyer, C. (2015). Diet and climatic context of giant birds inferred from δ13Cc and δ18Oc values of late Palaeocene and early Eocene eggshells from southern France. Palaeogeography, Palaeoclimatology, Palaeoecology 435, 210–221. [Google Scholar]
- Angst, D. , Lécuyer, C. , Amiot, R. , Buffetaut, E. , Fourel, F. , Martineau, F. , Legendre, S. , Abourachid, A. & Herrel, A. (2014). Isotopic and anatomical evidence of an herbivorous diet in the early tertiary giant bird Gastornis. Implications for the structure of Paleocene terrestrial ecosystems. Naturwissenschaften 101, 313–322. [DOI] [PubMed] [Google Scholar]
- Archimedes of Syracuse (1897). On the equilibrium of planes, books I and II. In The Works of Archimedes (ed. Heath T. L.), pp. 189–220. Cambridge University Press, London. [Google Scholar]
- Arman, S. D. , Ungar, P. S. , Brown, C. A. , DeSantis, L. R. G. , Schmidt, C. & Prideaux, G. J. (2016). Minimizing inter‐microscope variability in dental microwear texture analysis. Surface Topography: Metrology and Properties 4, 024007. [Google Scholar]
- Asher, R. J. (1998). Morphological diversity of anatomical strepsirrhinism and the evolution of the lemuriform toothcomb. American Journal of Physical Anthropology 105, 355–367. [DOI] [PubMed] [Google Scholar]
- Attard, M. R. G. , Parr, W. C. H. , Wilson, L. A. B. , Archer, M. , Hand, S. J. , Rogers, T. L. & Wroe, S. (2014). Virtual reconstruction and prey size preference in the mid Cenozoic thylacinid, Nimbacinus dicksoni (Thylacinidae, Marsupialia). PLoS One 9, e93088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard, M. R. G. , Wilson, L. A. B. , Worthy, T. H. , Scofield, P. , Johnston, P. , Parr, W. C. H. & Wroe, S. (2016). Moa diet fits the bill: virtual reconstruction incorporating mummified remains and prediction of biomechanical performance in avian giants. Proceedings of the Royal Society B: Biological Sciences 283, 20152043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atterholt, J. , Hutchison, J. H. & O'Connor, J. K. (2018). The most complete enantiornithine from North America and a phylogenetic analysis of the Avisauridae. PeerJ 6, e5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus, S. B. , Odhner, L. U. & Dollar, A. M. (2014). Design of hands for aerial manipulation: actuator number and routing for grasping and perching. In 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, pp. 34–40. IEEE.
- Backus, S. B. , Sustaita, D. , Odhner, L. U. & Dollar, A. M. (2015). Mechanical analysis of avian feet: multiarticular muscles in grasping and perching. Royal Society Open Science 2, 140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul, A. M. , O'Connor, J. , Zhang, S. K. , Li, Z. H. , Wang, Q. , Lamanna, M. C. , Zhu, X. F. & Zhou, Z. H. (2019). An early cretaceous enantiornithine (Aves) preserving an unlaid egg and probable medullary bone. Nature Communications 10, 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul, A. M. , O'Connor, J. , Li, Z. , Wu, Q. , Zhao, T. , Monleon, M. A. M. , Wang, M. & Zheng, X. (2020). Confirmation of ovarian follicles in an enantiornithine (Aves) from the Jehol biota using soft tissue analyses. Communications Biology 3, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker, R. T. (1998). Brontosaur killers: late Jurassic allosaurids as sabre‐tooth cat analogues. Gaia 15, 145–158. [Google Scholar]
- Bakker, R. T. & Williams, M. (1988). Nanotyrannus, a new genus of pygmy tyrannosaur, from the latest cretaceous of Montana. Hunteria 1, 1–30. [Google Scholar]
- Banglawala, N. , Bethunel, I. , Fagan, M. & Holbrey, R. (2015). Voxel‐based finite element modelling with VOX‐FE2. ARCHER White Papers .
- Barbosa, A. & Moreno, E. (1999). Evolution of foraging strategies in shorebirds: an ecomorphological approach. The Auk: Ornithological Advances 116, 712–725. [Google Scholar]
- Bardwell, E. , Benkman, C. W. & Gould, W. R. (2001). Adaptive geographic variation in Western scrub‐jays. Ecology 82, 2617–2627. [Google Scholar]
- Barrett, P. M. (2000). Prosauropod dinosaurs and iguanas: speculations on the diets of extinct reptiles. In Evolution of Herbivory in Terrestrial Vertebrates: Perspectives from the Fossil Record (ed. Sues H.‐D.), pp. 42–78. Cambridge University Press, Cambridge. [Google Scholar]
- Barrett, P. M. (2005). The diet of ostrich dinosaurs (Theropoda: Ornithomimosauria). Palaeontology 48, 347–358. [Google Scholar]
- Barrick, R. E. & Showers, W. J. (1994). Thermophysiology of Tyrannosaurus rex: evidence from oxygen isotopes. Science 265, 222–224. [DOI] [PubMed] [Google Scholar]
- Barrick, R. E. , Showers, W. J. & Fischer, A. G. (1996). Comparison of thermoregulation of four ornithischian dinosaurs and a varanid lizard from the cretaceous two medicine formation: evidence from oxygen isotopes. Palaios 11, 295–305. [Google Scholar]
- Barton, N. W. H. & Houston, D. C. (1992). The Influence of Gut Morphology on Digestion Time in Raptors. University of Glasgow, Glasgow. [Google Scholar]
- Bathe, K. J. (2014). Finite Element Procedures, 2nd Edition. Bathe, Klaus‐Jürgen, Watertown. [Google Scholar]
- Bechtle, S. , Fett, T. , Rizzi, G. , Habelitz, S. , Klocke, A. & Schneider, G. A. (2010). Crack arrest within teeth at the dentinoenamel junction caused by elastic modulus mismatch. Biomaterials 31, 4238–4247. [DOI] [PubMed] [Google Scholar]
- Bell, A. & Chiappe, L. M. (2016). A species‐level phylogeny of the cretaceous Hesperornithiformes (Aves: Ornithuromorpha): implications for body size evolution amongst the earliest diving birds. Journal of Systematic Palaeontology 14, 239–251. [Google Scholar]
- Bell, A. & Chiappe, L. M. (2020). Anatomy of Parahesperornis: evolutionary mosaicism in the cretaceous Hesperornithiformes (Aves). Life 10, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist, L. P. (2003). The role of teeth in mammal history. Brazilian Journal of Oral Sciences 2, 249–257. [Google Scholar]
- Bestwick, J. , Unwin, D. M. , Butler, R. J. , Henderson, D. H. & Purnell, M. A. (2018). Dental microwear textural analysis: reconstructing diets of non‐mammalian fossil taxa from the Solnhofen archipelago. In The Palaeontological Association 62nd Annual Meeting, pp. 68–69. University of Bristol, Bristol. [Google Scholar]
- Bestwick, J. , Unwin, D. M. & Purnell, M. A. (2019). Dietary differences in archosaur and lepidosaur reptiles revealed by dental microwear textural analysis. Scientific Reports 9, 11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar, B. A. S. , Hanson, M. , Fabbri, M. , Pritchard, A. , Bever, G. S. & Hoffman, E. (2016). How to make a bird skull: major transitions in the evolution of the avian cranium, paedomorphosis, and the beak as a surrogate hand. Integrative and Comparative Biology 56, 389–403. [DOI] [PubMed] [Google Scholar]
- Birn‐Jeffery, A. V. , Miller, C. E. , Naish, D. , Rayfield, E. J. & Hone, D. W. E. (2012). Pedal claw curvature in birds, lizards and mesozoic dinosaurs ‐ complicated categories and compensating for mass‐specific and phylogenetic control. PLoS One 7, e50555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn‐Jeffery, A. V. & Rayfield, E. (2009). Finite element analysis of pedal claws to determine mode of life in birds, lizards and maniraptoran theropods. In Sixty‐Ninth Annual Meeting Society of Vertebrate Paleontology and the Fifty‐Seventh Symposium of Vertebrate Palaeontology and Comparative Anatomy (SVPCA) vol. 29, pp. 64A. Society of Vertebrate Paleontology.
- Bishop, A. (1962). Control of the hand in lower primates. Annals of the New York Academy of Sciences 102, 316–337. [DOI] [PubMed] [Google Scholar]
- Blomberg, S. P. , Garland, T. & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. [DOI] [PubMed] [Google Scholar]
- Blum, J. D. , Taliaferro, E. H. & Holmes, R. T. (2001). Determining the sources of calcium for migratory songbirds using stable strontium isotopes. Oecologia 126, 569–574. [DOI] [PubMed] [Google Scholar]
- Bonhomme, V. , Picq, S. , Gaucherel, C. & Claude, J. (2014). Momocs: outline analysis using R. Journal of Statistical Software 56, 1–24. [Google Scholar]
- Bonser, R. H. C. & Witter, M. S. (1993). Indentation hardness of the bill keratin of the European starling. The Condor: Ornithological Applications 95, 736–738. [Google Scholar]
- Bout, R. G. & Zweers, G. A. (2001). The role of cranial kinesis in birds. Comparative Biochemistry and Physiology ‐ Part A: Molecular & Integrative Physiology 131, 197–205. [DOI] [PubMed] [Google Scholar]
- Brett‐Surman, M. K. & Paul, G. S. (1985). A new family of bird‐like dinosaurs linking Laurasia and Gondwanaland. Journal of Vertebrate Paleontology 5, 133–138. [Google Scholar]
- Briggs, D. E. G. , Wilby, P. R. , Perez‐Moreno, B. P. , Sanz, J. L. & Fregenal‐Martínez, M. (1997). The mineralization of dinosaur soft tissue in the lower cretaceous of Las Hoyas, Spain. Journal of the Geological Society 154, 587–588. [Google Scholar]
- Bright, J. A. (2014). A review of paleontological finite element models and their validity. Journal of Paleontology 88, 760–769. [Google Scholar]
- Bright, J. A. , Marugán‐Lobón, J. , Cobbe, S. N. & Rayfield, E. J. (2016). The shapes of bird beaks are highly controlled by nondietary factors. Proceedings of the National Academy of Sciences of the United States of America 113, 5352–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright, J. A. , Marugán‐Lobón, J. , Rayfield, E. J. & Cobb, S. N. (2019). The multifactorial nature of beak and skull shape evolution in parrots and cockatoos (Psittaciformes). BMC Evolutionary Biology 19, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright, J. A. & Rayfield, E. J. (2011). Sensitivity and ex vivo validation of finite element models of the domestic pig cranium. Journal of Anatomy 219, 456–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink, K. S. , Chen, Y. C. , Wu, Y. N. , Liu, W. M. , Shieh, D. B. , Huang, T. D. , Sun, C. K. & Reisz, R. R. (2016). Dietary adaptions in the ultrastructure of dinosaur dentine. Journal of the Royal Society Interface 13, 20160626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. (2009). Choosing the right number of components or factors in PCA and EFA. JALT Testing & Evaluation SIG Newsletter 13, 19–23. [Google Scholar]
- Brusatte, S. L. , O'Connor, J. K. & Jarvis, E. D. (2015). The origin and diversification of birds. Current Biology 25, R888–R898. [DOI] [PubMed] [Google Scholar]
- Brusatte, S. L. , Sakamoto, M. , Montanari, S. & Harcourt Smith, W. E. H. (2012). The evolution of cranial form and function in theropod dinosaurs: insights from geometric morphometrics. Journal of Evolutionary Biology 25, 365–377. [DOI] [PubMed] [Google Scholar]
- Buffetaut, E. & Angst, D. (2016). The giant flightless bird Gargantuavis philoinos from the late cretaceous of southwestern Europe: a review. In Cretaceous Period: Biotic Diversity and Biogeography New Mexico Museum of Natural History and Science Bulletin (eds Khosla A. and Lucas S. G.), pp. 45–50. New Mexico Museum of Natural History and Science, Albuquerque. [Google Scholar]
- Bühler, P. , Martin, L. D. & Witmer, L. M. (1988). Cranial kinesis in the late cretaceous birds Hesperornis and Parahesperornis . The Auk: Ornithological Advances 105, 111–122. [Google Scholar]
- Burger, A. E. A. (1978). Functional anatomy of the feeding apparatus of four south African cormorants. African Zoology 13, 81–102. [Google Scholar]
- Bush, S. E. & Clayton, D. H. (2018). Anti‐parasite behaviour of birds. Philosophical Transactions of the Royal Society B: Biological Sciences 373, 20170196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, S. E. , Villa, S. M. , Boves, T. J. , Brewer, D. & Belthoff, J. R. (2012). Influence of bill and foot morphology on the ectoparasites of barn owls. Journal of Parasitology 98, 256–261. [DOI] [PubMed] [Google Scholar]
- Button, D. J. & Zanno, L. E. (2020). Repeated evolution of divergent modes of herbivory in non‐avian dinosaurs. Current Biology 30, 158–168. [DOI] [PubMed] [Google Scholar]
- Button, K. A. (2018). Soft Tissue Reconstruction and Ecomorphology of Beaks in Extant and Extinct Theropod Dinosaurs. North Carolina State University, Raleigh. [Google Scholar]
- Cambra‐Moo, O. , Buscalioni, Á. D. , Cubo, J. , Castanet, J. , Loth, M.‐M. , de Margerie, E. & de Ricqlès, A. (2006). Histological observations of enantiornithine bone (Saurischia, Aves) from the lower cretaceous of Las Hoyas (Spain). Comptes Rendus Palevol 5, 685–691. [Google Scholar]
- Candeiro, C. R. A. , Currie, P. J. , Candeiro, C. L. & Bergqvist, L. P. (2017). Tooth wear and microwear of theropods from the late Maastrichtian Marília formation (Bauru group), Minas Gerais state, Brazil. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 106, 229–233. [Google Scholar]
- Carlisle, J. D. & Holberton, R. L. (2006). Relative efficiency of fecal versus regurgitated samples for assessing diet and the deleterious effects of a tartar emetic on migratory birds. Journal of Field Ornithology 77, 126–135. [Google Scholar]
- Carlos, C. J. , Alvarenga, J. G. & Mazzochi, M. S. (2017). Osteology of the feeding apparatus of magnificent Frigatebird Fregata magnificens and Brown booby Sula leucogaster (Aves: Suliformes). Papéis Avulsos de Zoologia 57, 265–274. [Google Scholar]
- Carney, R. , Kaplan, H. , Kirk, A. , Baines, A. & Mason, S. (2018). Archaeopteryx holographica: bringing the urvogel back to life with scientific animation and VR/AR. In Society of Vertebrate Paleontology 78th Annual Meeting, pp. 102, Albuquerque.
- Carpenter, K. (1998). Evidence of predatory behavior by carnivorous dinosaurs. Gaia 15, 135–144. [Google Scholar]
- Carrano, M. T. , Janis, C. M. & Sepkoski, J. J. (1999). Hadrosaurs as ungulate parallels: lost life styles and deficient data. Acta Palaeontologica Polonica 44, 237–261. [Google Scholar]
- Carter, C. B. & Norton, M. G. (2007). Ceramics in biology and medicine. In Ceramic Materials: Science and Engineering (Volume 716, eds Carter C. B. and Norton M. G.), pp. 659–676. Springer‐Verlag, New York City. [Google Scholar]
- Carvalho, I. D. S. , Novas, F. E. , Agnolín, F. L. , Isasi, M. P. , Freitas, F. I. & Andrade, J. A. (2015). A new genus and species of enantiornithine bird from the early cretaceous of Brazil. Brazilian Journal of Geology 45, 161–171. [Google Scholar]
- Cau, A. & Arduini, P. (2008). Enantiophoenix electrophyla gen. Et sp. nov.(Aves, Enantiomithes) from the upper cretaceous (Cenomanian) of Lebanon and its phylogenetic relationships. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 149, 293–324. [Google Scholar]
- Caviedes‐Vidal, E. , McWhorter, T. J. , Lavin, S. R. , Chediack, J. G. , Tracy, C. R. & Karasov, W. H. (2007). The digestive adaptation of flying vertebrates: high intestinal paracellular absorption compensates for smaller guts. Proceedings of the National Academy of Sciences of the United States of America 104, 19132–19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain, C. , Blum, J. , Holmes, R. T. , Feng, X. , Sherry, T. & Graves, G. R. (1996). The use of isotope tracers for identifying populations of migratory birds. Oecologia 109, 132–141. [DOI] [PubMed] [Google Scholar]
- Chávez‐Hoffmeister, M. (2020). Bill disparity and feeding strategies among fossil and modern penguins. Paleobiology 46, 176–192. [Google Scholar]
- Chiappe, L. M. (2002). Osteology of the flightless Patagopteryx deferrariisi from the late cretaceous of Patagonia (Argentina). In Mesozoic Birds: Above the Heads of Dinosaurs (eds Chiappe L. M. and Witmer L. M.), pp. 281–316. University of California Press, Berkeley and Los Angeles. [Google Scholar]
- Chiappe, L. M. & Calvo, J. O. (1994). Neuquenornis volans, a new late cretaceous bird (Enantiornithes: Avisauridae) from Patagonia, Argentina. Journal of Vertebrate Paleontology 14, 230–246. [Google Scholar]
- Chiappe, L. M. , Di, L. , Serrano, F. J. , Yuguang, Z. & Meng, Q. (2019a). Anatomy and flight performance of the early enantiornithine bird Protopteryx fengningensis: information from new specimens of the early cretaceous Huajiying formation of China. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 303, 716–731. [DOI] [PubMed] [Google Scholar]
- Chiappe, L. M. & Meng, Q. (2016). Birds of Stone: Chinese Avian Fossils from the Age of Dinosaurs. John Hopkins University Press, Baltimore. [Google Scholar]
- Chiappe, L. M. , Norell, M. & Clark, J. (2001). A new skull of Gobipteryx minuta (Aves: Enantiornithes) from the cretaceous of the Gobi Desert. American Museum Novitates 3346, 1–16. [Google Scholar]
- Chiappe, L. M. , Qingjin, M. , Serrano, F. , Sigurdsen, T. , Min, W. , Bell, A. & Di, L. (2019b). New Bohaiornis‐like bird from the early cretaceous of China: enantiornithine interrelationships and flight performance. PeerJ 7, e7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe, L. M. , Shu'an, J. , Qiang, J. & Norell, M. A. (1999). Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the late mesozoic of northeastern China. Bulletin of the American Museum of Natural History 242, 3–89. [Google Scholar]
- Chiappe, L. M. & Walker, C. A. (2002). Skeletal morphology and systematics of the cretaceous Euenantiornithes (Ornithothoraces: Enantiornithes). In Mesozoic Birds: Above the Heads of Dinosaurs (eds Chiappe L. M. and Witmer L. M.), pp. 240–267. University of California Press, Berkeley and Los Angeles. [Google Scholar]
- Chiappe, L. M. , Zhao, B. , O'Connor, J. K. , Gao, C. L. , Wang, X. R. , Habib, M. , Marugán‐Lobón, J. , Meng, Q. J. & Cheng, X. D. (2014). A new specimen of the early cretaceous bird Hongshanornis longicresta: insights into the aerodynamics and diet of a basal ornithuromorph. PeerJ 2, e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien, C. H. , Wu, Y. D. , Chao, Y.‐J. , Chen, T. , Chen, W. F. , Yu, J. C. & Li, X. (2008). The effects of different cranial modules on mechanical properties of cranial suture in Lewis rats and same‐aged C57BL/6 mice. Strain 44, 272–277. [Google Scholar]
- Chin, K. , Tokaryk, T. T. , Erickson, G. M. & Calk, L. C. (1998). A king‐sized theropod coprolite. Nature 393, 680–682. [Google Scholar]
- Chinsamy‐Turan, A. , Chiappe, L. M. , Marugán‐Lobón, J. , Gao, C. L. & Zhang, F. J. (2013). Gender identification of the Mesozoic bird Confuciusornis sanctus . Nature Communications 4, 1381. [DOI] [PubMed] [Google Scholar]
- Christiansen, P. & Bonde, N. (2004). Body plumage in Archaeopteryx: a review, and new evidence from the Berlin specimen. Comptes Rendus Palevol 3, 99–118. [Google Scholar]
- Clark, G. A. (1973). Holding food with the feet in passerines. Bird‐Banding 44, 91–99. [Google Scholar]
- Clark, J. , Hopson, J. , Fastovsky, D. & Montellano, M. (1998). Foot posture in a primitive pterosaur. Nature 391, 886–889. [Google Scholar]
- Clarke, J. A. (2004). Morphology, phylogenetic taxonomy, and systematics of Ichthyornis and Apatornis (Avialae: Ornithurae). Bulletin of the American Museum of Natural History 2004, 1–179. [Google Scholar]
- Clarke, J. A. & Norell, M. A. (2002). The morphology and phylogenetic position of Apsaravis ukhaana from the late cretaceous of Mongolia. American Museum Novitates 3387, 1–46. [Google Scholar]
- Clarke, J. A. , Zhou, Z. & Zhang, F. (2006). Insight into the evolution of avian flight from a new clade of early cretaceous ornithurines from China and the morphology of Yixianornis grabaui . Journal of Anatomy 208, 287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, D. H. & Cotgreave, P. (1994). Relationship of bill morphology to grooming behavior in birds. Animal Behaviour 47, 195–201. [Google Scholar]
- Clayton, D. H. , Koop, J. A. , Harbison, C. W. , Moyer, B. R. & Bush, S. E. (2010). How birds combat ectoparasites. Open Ornithology Journal 3, 41–71. [Google Scholar]
- Clayton, D. H. , Moyer, B. R. , Bush, S. E. , Jones, T. G. , Gardiner, D. W. , Rhodes, B. B. & Goller, F. (2005). Adaptive significance of avian beak morphology for ectoparasite control. Proceedings of the Royal Society B: Biological Sciences 272, 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, D. H. & Walther, B. A. (2001). Influence of host ecology and morphology on the diversity of neotropical bird lice. Oikos 94, 455–467. [Google Scholar]
- Clementz, M. T. (2012). New insight from old bones: stable isotope analysis of fossil mammals. Journal of Mammalogy 93, 368–380. [Google Scholar]
- Clutton‐Brock, T. H. (1982). The functions of antlers. Behaviour 79, 108–124. [Google Scholar]
- Coatham, S. J. , Vinther, J. , Rayfield, E. J. & Klug, C. (2020). Was the Devonian placoderm Titanichthys a suspension feeder? Royal Society Open Science 7, 200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb, S. E. & Sellers, W. I. (2020). Inferring lifestyle for Aves and Theropoda: a model based on curvatures of extant avian ungual bones. PLoS One 15, e0211173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collar, D. C. , Wainwright, P. C. , Alfaro, M. E. , Revell, L. J. & Mehta, R. S. (2014). Biting disrupts integration to spur skull evolution in eels. Nature Communications 5, 5505. [DOI] [PubMed] [Google Scholar]
- Connolly, R. M. , Guest, M. A. , Melville, A. J. & Oakes, J. M. (2004). Sulfur stable isotopes separate producers in marine food‐web analysis. Oecologia 138, 161–167. [DOI] [PubMed] [Google Scholar]
- Cooney, C. R. , Bright, J. A. , Capp, E. J. R. , Chira, A. M. , Hughes, E. C. , Moody, C. J. A. , Nouri, L. O. , Varley, Z. K. & Thomas, G. H. (2017). Mega‐evolutionary dynamics of the adaptive radiation of birds. Nature 542, 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin, C. E. (2008). Foraging ecomorphology within north American flycatchers and a test of concordance with southern African species. Journal of Ornithology 149, 83–95. [Google Scholar]
- Corbin, C. E. , Lowenberger, L. K. & Gray, B. L. (2015). Linkage and trade‐off in trophic morphology and behavioural performance of birds. Functional Ecology 29, 808–815. [Google Scholar]
- Cortés, E. (1997). A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences 54, 726–738. [Google Scholar]
- Cost, I. N. , Middleton, K. M. , Sellers, K. C. , Echols, M. S. , Witmer, L. M. , Davis, J. L. & Holliday, C. M. (2019). Palatal biomechanics and its significance for cranial kinesis in Tyrannosaurus rex . The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 303, 999–1017. [DOI] [PubMed] [Google Scholar]
- Cox, P. G. (2017). The jaw is a second‐class lever in Pedetes capensis (Rodentia: Pedetidae). PeerJ 5, e3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, S. B. & Summers, A. P. (2014). How to best smash a snail: the effect of tooth shape on crushing load. Journal of the Royal Society Interface 11, 20131053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csermely, D. , Bertè, L. & Camoni, R. (1998). Prey killing by Eurasian kestrels: the role of the foot and the significance of bill and talons. Journal of Avian Biology 29, 10–16. [Google Scholar]
- Csermely, D. & Gaibani, G. (1998). Is foot squeezing pressure by two raptor species sufficient to subdue their prey? The Condor: Ornithological Applications 100, 757–763. [Google Scholar]
- Csermely, D. & Rossi, O. (2006). Bird claws and bird of prey talons: where is the difference? Italian Journal of Zoology 73, 43–53. [Google Scholar]
- Csermely, D. , Rossi, O. & Nasi, F. (2012). Comparison of claw geometrical characteristics among birds of prey and non‐raptorial birds. Italian Journal of Zoology 79, 410–433. [Google Scholar]
- Cuff, A. R. , Bright, J. A. & Rayfield, E. J. (2015). Validation experiments on finite element models of an ostrich (Struthio camelus) cranium. PeerJ 3, e1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, S. J. , Corfield, J. R. , Iwaniuk, A. N. , Castro, I. , Alley, M. R. , Birkhead, T. R. & Parsons, S. (2013). The anatomy of the bill tip of kiwi and associated somatosensory regions of the brain: comparisons with shorebirds. PLoS One 8, e80036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey, J. D. (1999). The design of mineralised hard tissues for their mechanical functions. Journal of Experimental Biology 202, 3285–3294. [DOI] [PubMed] [Google Scholar]
- Currie, P. J. & Chen, P. J. (2001). Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Canadian Journal of Earth Sciences 38, 1705–1727. [Google Scholar]
- Currie, P. J. & Evans, D. C. (2019). Cranial anatomy of new specimens of Saurornitholestes langstoni (Dinosauria, Theropoda, Dromaeosauridae) from the Dinosaur Park formation (Campanian) of Alberta. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 303, 691–715. [DOI] [PubMed] [Google Scholar]
- Curtis, N. , Jones, M. E. H. , Evans, S. E. , O'higgins, P. & Fagan, M. J. (2013). Cranial sutures work collectively to distribute strain throughout the reptile skull. Journal of the Royal Society Interface 10, 20130442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkas, S. A. & Feduccia, A. (2014). Jurassic archosaur is a non‐dinosaurian bird. Journal of Ornithology 155, 841–851. [Google Scholar]
- D'Amore, D. C. (2009). A functional explanation for denticulation in theropod dinosaur teeth. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 292, 1297–1314. [DOI] [PubMed] [Google Scholar]
- Dahan, G. , Trabelsi, N. , Safran, O. & Yosibash, Z. (2019). Finite element analyses for predicting anatomical neck fractures in the proximal humerus. Clinical biomechanics 68, 114–121. [DOI] [PubMed] [Google Scholar]
- Dal Sasso, C. & Signore, M. (1998). Exceptional soft‐tissue preservation in a theropod dinosaur from Italy. Nature 392, 383–387. [Google Scholar]
- Dalla Vecchia, F. M. & Chiappe, L. M. (2002). First avian skeleton from the mesozoic of northern Gondwana. Journal of Vertebrate Paleontology 22, 856–860. [Google Scholar]
- Dalsätt, J. , Ericson, P. G. P. & Zhou, Z. G. (2014). A new Enantiornithes (Aves) from the early cretaceous of China. Acta Geologica Sinica (English Edition) 88, 1034–1040. [Google Scholar]
- Dalsätt, J. , Zhou, Z. , Zhang, F. & Ericson, P. G. P. (2006). Food remains in Confuciusornis sanctus suggest a fish diet. Naturwissenschaften 93, 444–446. [DOI] [PubMed] [Google Scholar]
- Daniel, T. L. , Helmuth, B. S. , Saunders, W. B. & Ward, P. D. (1997). Septal complexity in ammonoid cephalopods increased mechanical risk and limited depth. Paleobiology 23, 470–481. [Google Scholar]
- Davenport, S. R. & Bax, N. J. (2002). A trophic study of a marine ecosystem off southeastern Australia using stable isotopes of carbon and nitrogen. Canadian Journal of Fisheries and Aquatic Sciences 59, 514–530. [Google Scholar]
- Davis, M. T. , Loyd, A. M. , Shen, H. Y. H. , Mulroy, M. H. , Nightingale, R. W. , Myers, B. S. & Bass, C. D. (2012). The mechanical and morphological properties of 6 year‐old cranial bone. Journal of Biomechanics 45, 2493–2498. [DOI] [PubMed] [Google Scholar]
- de Bello, F. , Berg, M. P. , Dias, A. T. C. , Diniz, J. A. F. , Gotzenberger, L. , Hortal, J. , Ladle, R. J. & Leps, J. (2015). On the need for phylogenetic ‘corrections’ in functional trait‐based approaches. Folia Geobotanica 50, 349–357. [Google Scholar]
- Dececchi, T. A. & Larsson, H. C. (2011). Assessing arboreal adaptations of bird antecedents: testing the ecological setting of the origin of the avian flight stroke. PLoS One 6, e22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dececchi, T. A. , Roy, A. , Pittman, M. , Kaye, T. G. , Xu, X. , Habib, M. B. , Larsson, H. C. E. , Wang, X. & Zheng, X. (2020). Aerodynamics show membranous‐winged theropods were a poor gliding dead‐end. iScience 23, 101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrange, F. J. , Tambussi, C. P. , Moreno, K. , Witmer, L. M. & Wroe, S. (2010). Mechanical analysis of feeding behavior in the extinct “terror bird” Andalgalornis steulleti (Gruiformes: Phorusrhacidae). PLoS One 5, e11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimery, N. J. , Alexander, R. M. & Deyst, K. A. (1985). Mechanics of the ligamentum nuchae of some artiodactyls. Journal of Zoology 206, 341–351. [Google Scholar]
- Dollar, A. M. & Howe, R. D. (2011). Joint coupling design of underactuated hands for unstructured environments. The International Journal of Robotics Research 30, 1157–1169. [Google Scholar]
- Drake, A. G. & Klingenberg, C. P. (2008). The pace of morphological change: historical transformation of skull shape in St Bernard dogs. Proceedings of the Royal Society B: Biological Sciences 275, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumheller, S. K. , McHugh, J. B. , Kane, M. , Riedel, A. & D'Amore, D. C. (2020). High frequencies of theropod bite marks provide evidence for feeding, scavenging, and possible cannibalism in a stressed late Jurassic ecosystem. PLoS One 15, e0233115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, D. C. & Jackson, S. (1986). Diet studies of seabirds: a review of methods. Colonial Waterbirds 9, 1–17. [Google Scholar]
- Duke, G. E. (1997). Gastrointestinal physiology and nutrition in wild birds. Proceedings of the Nutrition Society 56, 1049–1056. [DOI] [PubMed] [Google Scholar]
- Dumont, E. R. , Grosse, I. R. & Slater, G. J. (2009). Requirements for comparing the performance of finite element models of biological structures. Journal of Theoretical Biology 256, 96–103. [DOI] [PubMed] [Google Scholar]
- Dumont, E. R. , Samadevam, K. , Grosse, I. , Warsi, O. M. , Baird, B. & Davalos, L. M. (2014). Selection for mechanical advantage underlies multiple cranial optima in new world leaf‐nosed bats. Evolution 68, 1436–1449. [DOI] [PubMed] [Google Scholar]
- Dumont, M. , Tafforeau, P. , Bertin, T. , Bhullar, B. A. , Field, D. , Schulp, A. , Strilisky, B. , Thivichon‐Prince, B. , Viriot, L. & Louchart, A. (2016). Synchrotron imaging of dentition provides insights into the biology of Hesperornis and Ichthyornis, the “last” toothed birds. BMC Evolutionary Biology 16, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, R. I. M. (1991). Functional significance of social grooming in primates. Folia Primatologica 57, 121–131. [Google Scholar]
- Dunkel, A. (2019). The evolution of grooming and hand use in primates: an interdisciplinary perspective, pp. 1–34, unpublished preprint.
- Eimar, H. , Ghadimi, E. , Marelli, B. , Vali, H. , Nazhat, S. N. , Amin, W. M. , Torres, J. , Ciobanu, O. , Junior, R. F. A. & Tamimi, F. (2012). Regulation of enamel hardness by its crystallographic dimensions. Acta Biomaterialia 8, 3400–3410. [DOI] [PubMed] [Google Scholar]
- Einoder, L. D. & Richardson, A. M. M. (2007). Aspects of the hindlimb morphology of some Australian birds of prey: a comparative and quantitative study. The Auk: Ornithological Advances 124, 773–788. [Google Scholar]
- Eklöv, P. & Diehl, S. (1994). Piscivore efficiency and refuging prey: the importance of predator search mode. Oecologia 98, 344–353. [DOI] [PubMed] [Google Scholar]
- Elzanowski, A. (1974). Preliminary note on the palaeognathous bird from the upper cretaceous of Mongolia. Acta Palaeontologia Polonica 30, 103–109. [Google Scholar]
- Elzanowski, A. (1977). Skulls of Gobipteryx (Aves) from the upper cretaceous of Mongolia. Palaeontologica Polonica 37, 153–165. [Google Scholar]
- Elzanowski, A. (1981). Embryonic bird skeletons from the late cretaceous of Mongolia. Palaeontologia Polonica 42, 147–176. [Google Scholar]
- Elzanowski, A. (1991). New observations on the skull of Hesperornis with reconctructions of the bony palate and otic region. Postilla 207, 1–20. [Google Scholar]
- Elzanowski, A. (2001a). A new genus and species for the largest specimen of Archaeopteryx . Acta Palaeontologica Polonica 46, 519–532. [Google Scholar]
- Elzanowski, A. (2001b). A novel reconstruction of the skull of Archaeopteryx . Netherlands Journal of Zoology 51, 207–215. [Google Scholar]
- Elzanowski, A. & Galton, P. M. (1991). Braincase of Enaliornis, an early cretaceous bird from England. Journal of Vertebrate Paleontology 11, 90–107. [Google Scholar]
- Elzanowski, A. , Peters, D. S. & Mayr, G. (2018). Cranial morphology of the early cretaceous bird Confuciusornis . Journal of Vertebrate Paleontology 38, e1439832. [Google Scholar]
- Erickson, G. M. , Gignac, P. M. , Steppan, S. J. , Lappin, A. K. , Vliet, K. A. , Brueggen, J. D. , Inouye, B. D. , Kledzik, D. & Webb, G. J. W. (2012). Insights into the ecology and evolutionary success of crocodilians revealed through bite‐force and tooth‐pressure experimentation. PLoS One 7, e31781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, G. M. , Sidebottom, M. A. , Kay, D. I. , Turner, K. T. , Ip, N. , Norell, M. A. , Sawyer, W. G. & Krick, B. A. (2015). Wear biomechanics in the slicing dentition of the giant horned dinosaur Triceratops . Science Advances 1, e1500055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, G. M. , van Kirk, S. D. , Su, J. , Levenston, M. E. , Caler, W. E. & Carter, D. R. (1996). Bite‐force estimation for Tyrannosaurus rex from tooth‐marked bones. Nature 382, 706–708. [Google Scholar]
- Esque, T. C. & Peters, E. L. (1994). Ingestion of bones, stones, and soil by desert tortoises. Fish and Wildlife Research 13, 73–84. [Google Scholar]
- Estes, R. & Williams, E. E. (1984). Ontogenetic variation in the molariform teeth of lizards. Journal of Vertebrate Paleontology 4, 96–107. [Google Scholar]
- Ewer, R. F. (1965). The anatomy of the thecodont reptile Euparkeria capensis broom. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 248, 379–435. [Google Scholar]
- Falk, A. R. (2014). Foot and Hindlimb Morphology, Soft Tissues, and Tracemaking Behaviors of Early Cretaceous Birds from China and the Republic of Korea with a Comparison to Modern Avian Morphology and Behavior. University of Kansas, Lawrence. [Google Scholar]
- Falk, A. R. , Kaye, T. G. , Zhou, Z. H. & Burnham, D. A. (2016). Laser fluorescence illuminates the soft tissue and life habits of the early cretaceous bird Confuciusornis . PLoS One 11, e0167284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk, A. R. , Lamsdell, J. C. & Gong, E. (2020). Principal component analysis of avian hind limb and foot morphometrics and the relationship between ecology and phylogeny. Paleobiology 42, 314–346. [Google Scholar]
- Falk, A. R. , O'Connor, J. , Wang, M. & Zhou, Z. (2019). On the preservation of the beak in Confuciusornis (Aves: Pygostylia). Diversity 11, 212. [Google Scholar]
- Faria, P. J. , Guedes, N. M. R. , Yamashita, C. , Martuscelli, P. & Miyaki, C. Y. (2008). Genetic variation and population structure of the endangered hyacinth macaw (Anodorhynchus hyacinthinus): implications for conservation. Biodiversity and Conservation 17, 765–779. [Google Scholar]
- Farina, S. C. , Kane, E. A. & Hernandez, L. P. (2019). Multifunctional structures and multistructural functions: integration in the evolution of biomechanical systems. Integrative and Comparative Biology 59, 338–345. [DOI] [PubMed] [Google Scholar]
- Farke, A. A. (2008). Frontal sinuses and head‐butting in goats: a finite element analysis. Journal of Experimental Biology 211, 3085–3094. [DOI] [PubMed] [Google Scholar]
- Farke, A. A. , Chok, D. J. , Herrero, A. , Scolieri, B. & Werning, S. (2013). Ontogeny in the tube‐crested dinosaur Parasaurolophus (Hadrosauridae) and heterochrony in hadrosaurids. PeerJ 1, e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow, J. O. & Brinkman, D. L. (1994). Wear surfaces on the teeth of tyrannosaurs. The Paleontological Society Special Publications 7, 165–176. [Google Scholar]
- Farmer, A. , Rye, R. , Landis, G. , Bern, C. , Kester, C. & Ridley, I. (2003). Tracing the pathways of neotropical migratory shorebirds using stable isotopes: a pilot study. Isotopes in Environmental and Health Studies 39, 169–177. [DOI] [PubMed] [Google Scholar]
- Feduccia, A. (1993). Evidence from claw geometry indicating arboreal habits of Archaeopteryx . Science 259, 790–793. [DOI] [PubMed] [Google Scholar]
- Felice, R. N. & Goswami, A. (2018). Developmental origins of mosaic evolution in the avian cranium. Proceedings of the National Academy of Sciences of the United States of America 115, 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice, R. N. , Tobias, J. A. , Pigot, A. L. & Goswami, A. (2019a). Dietary niche and the evolution of cranial morphology in birds. Proceedings of the Royal Society B: Biological Sciences 286, 20182677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice, R. N. , Watanabe, A. , Cuff, A. R. , Noirault, E. , Pol, D. , Witmer, L. M. , Norell, M. A. , O'Connor, P. M. & Goswami, A. (2019b). Evolutionary integration and modularity in the archosaur cranium. Integrative and Comparative Biology 59, 371–382. [DOI] [PubMed] [Google Scholar]
- Fernandez Blanco, M. V. , Cassini, G. H. & Bona, P. (2018). Skull ontogeny of extant caimans: a three‐dimensional geometric morphometric approach. Zoology 129, 69–81. [DOI] [PubMed] [Google Scholar]
- Field, D. J. , Benito, J. , Chen, A. , Jagt, J. W. M. & Ksepka, D. T. (2020). Late cretaceous neornithine from Europe illuminates the origins of crown birds. Nature 579, 397–401. [DOI] [PubMed] [Google Scholar]
- Field, D. J. , Bercovici, A. , Berv, J. S. , Dunn, R. , Fastovsky, D. E. , Lyson, T. R. , Vajda, V. & Gauthier, J. A. (2018a). Early evolution of modern birds structured by global forest collapse at the end‐cretaceous mass extinction. Current Biology 28, 1825–1831. [DOI] [PubMed] [Google Scholar]
- Field, D. J. , Hanson, M. , Burnham, D. , Wilson, L. E. , Super, K. , Ehret, D. , Ebersole, J. A. & Bhullar, B. A. S. (2018b). Complete Ichthyornis skull illuminates mosaic assembly of the avian head. Nature 557, 96–100. [DOI] [PubMed] [Google Scholar]
- Figueirido, B. , MacLeod, N. , Krieger, J. , De Renzi, M. , Perez‐Claros, J. A. & Palmqvist, P. (2011). Constraint and adaptation in the evolution of carnivoran skull shape. Paleobiology 37, 490–518. [Google Scholar]
- Fisher, H. I. (1946). Adaptations and comparative anatomy of the locomotor apparatus of New World vultures. The American Midland Naturalist 35, 545–727. [Google Scholar]
- Fogel, M. L. & Tuross, N. (1999). Transformation of plant biochemicals to geological macromolecules during early diagenesis. Oecologia 120, 336–346. [DOI] [PubMed] [Google Scholar]
- Fortelius, M. & Solounias, N. (2000). Functional characterization of ungulate molars using the abrasion‐attrition wear gradient: a new method for reconstructing paleodiets. American Museum Novitates 3301, 1–36. [Google Scholar]
- Fortuny, J. , Marcé‐Nogué, J. , de Esteban‐Trivigno, S. , Gil, L. & Galobart, A. (2011). Temnospondyli bite club: ecomorphological patterns of the most diverse group of early tetrapods. Journal of Evolutionary Biology 24, 2040–2054. [DOI] [PubMed] [Google Scholar]
- Foth, C. & Rauhut, O. W. M. (2013). Macroevolutionary and morphofunctional patterns in theropod skulls: a morphometric approach. Acta Palaeontologica Polonica 58, 1–16. [Google Scholar]
- Foth, C. , Tischlinger, H. & Rauhut, O. W. M. (2014). New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature 511, 79–82. [DOI] [PubMed] [Google Scholar]
- Fowler, D. W. , Freedman, E. A. & Scannella, J. B. (2009). Predatory functional morphology in raptors: interdigital variation in talon size is related to prey restraint and immobilisation technique. PLoS One 4, e7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, D. W. , Freedman, E. A. , Scannella, J. B. & Kambic, R. E. (2011). The predatory ecology of Deinonychus and the origin of flapping in birds. PLoS One 6, e28964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, D. & Theodor, J. M. (2011). Anterior dentary shape as an indicator of diet in ruminant artiodactyls. Journal of Vertebrate Paleontology 31, 1366–1375. [Google Scholar]
- Frederick, P. C. & Bildstein, K. L. (1992). Foraging ecology of seven species of neotropical ibises (Threskiornithidae) during the dry season in the llanos of Venezuela. Wilson Bulletin 104, 1–21. [Google Scholar]
- Frederickson, J. A. , Engel, M. H. & Cifelli, R. L. (2018). Niche partitioning in theropod dinosaurs: diet and habitat preference in predators from the uppermost Cedar Mountain formation (Utah, USA). Scientific Reports 8, 17872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson, J. A. , Engel, M. H. & Cifelli, R. L. (2020). Ontogenetic dietary shifts in Deinonychus antirrhopus (Theropoda; Dromaeosauridae): insights into the ecology and social behavior of raptorial dinosaurs through stable isotope analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 552, 109780. [Google Scholar]
- Freeland, W. J. (1981). Functional aspects of primate grooming. The Ohio Journal of Science 81, 173–177. [Google Scholar]
- Frei, S. , Ortmann, S. , Reutlinger, C. , Kreuzer, M. , Hatt, J. M. & Clauss, M. (2014). Comparative digesta retention patterns in ratites. The Auk: Ornithological Advances 132, 119–131. [Google Scholar]
- Frey, E. , Martill, D. M. & Buchy, M. (2003). A new species of Tapejarid pterosaur with soft‐tissue head crest. In Evolution and Palaeobiology of Pterosaurs. Geological Society, London, Special Publications (eds Buffetaut E. and Mazin J. M.), pp. 1–3. The Geological Society London, London. [Google Scholar]
- Fricke, H. C. & Rogers, R. R. (2000). Multiple taxon–multiple locality approach to providing oxygen isotope evidence for warm‐blooded theropod dinosaurs. Geology 28, 799–802. [Google Scholar]
- Friedmann, H. (1931). Observations on the growth rate of the foot in the mound birds of the genus Megapodius . Proceedings of the United States National Museum 80, 1–4. [Google Scholar]
- Froese, R. (2006). Cube law, condition factor and weight–length relationships: history, meta‐analysis and recommendations. Journal of Applied Ichthyology 22, 241–253. [Google Scholar]
- Funston, G. F. , Mendonca, S. E. , Currie, P. J. & Barsbold, R. (2018). Oviraptorosaur anatomy, diversity and ecology in the Nemegt Basin. Palaeogeography, Palaeoclimatology, Palaeoecology 494, 101–120. [Google Scholar]
- Gailer, J. P. , Calandra, I. , Schulz‐Kornas, E. & Kaiser, T. M. (2016). Morphology is not destiny: discrepancy between form, function and dietary adaptation in bovid cheek teeth. Journal of Mammalian Evolution 23, 369–383. [Google Scholar]
- Gales, R. P. (1987). Validation of the stomach‐flushing technique for obtaining stomach contents of penguins. Ibis 129, 335–343. [Google Scholar]
- Gao, C. , Chiappe, L. M. , Zhang, F. , Pomeroy, D. L. , Shen, C. , Chinsamy, A. & Walsh, M. O. (2012). A subadult specimen of the early cretaceous bird Sapeornis chaoyangensis and a taxonomic reassessment of sapeornithids. Journal of Vertebrate Paleontology 32, 1103–1112. [Google Scholar]
- Garrard, G. E. , McCarthy, M. A. , Vesk, P. A. , Radford, J. Q. & Bennett, A. F. (2012). A predictive model of avian natal dispersal distance provides prior information for investigating response to landscape change. Journal of Animal Ecology 81, 14–23. [DOI] [PubMed] [Google Scholar]
- Gauthier, J. (1986). Saurischian monophyly and the origin of birds. Memoirs of the California Academy of sciences 8, 1–55. [Google Scholar]
- Ghosh, P. , Bhattacharya, S. K. , Sahni, A. , Kar, R. K. , Mohabey, D. M. & Ambwani, K. (2003). Dinosaur coprolites from the late cretaceous (Maastrichtian) Lameta formation of India: isotopic and other markers suggesting a C‐3 plant diet. Cretaceous Research 24, 743–750. [Google Scholar]
- Gingerich, P. D. (1973). Skull of Hesperornis and early evolution of birds. Nature 243, 70–73. [Google Scholar]
- Gingerich, P. D. & Rose, K. D. (1979). Anterior dentition of the eocene condylarth Thryptacodon ‐ convergence with the tooth comb of lemurs. Journal of Mammalogy 60, 16–22. [Google Scholar]
- Giraudeau, M. , Nolan, P. M. , Black, C. E. , Earl, S. R. , Hasegawa, M. & McGraw, K. J. (2014). Song characteristics track bill morphology along a gradient of urbanization in house finches (Haemorhous mexicanus). Frontiers in Zoology 11, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glen, C. L. & Bennett, M. B. (2007). Foraging modes of Mesozoic birds and non‐avian theropods. Current Biology 17, R911–R912. [DOI] [PubMed] [Google Scholar]
- Godefroit, P. , Cau, A. , Dong‐Yu, H. , Escuillié, F. , Wenhao, W. & Dyke, G. (2013a). A Jurassic avialan dinosaur from China resolves the early phylogenetic history of birds. Nature 498, 359–362. [DOI] [PubMed] [Google Scholar]
- Godefroit, P. , Demuynck, H. , Dyke, G. , Hu, D. , Escuillié, F. & Claeys, P. (2013b). Reduced plumage and flight ability of a new Jurassic paravian theropod from China. Nature Communications 4, 1394. [DOI] [PubMed] [Google Scholar]
- Godinho, R. M. , Toro‐Ibacache, V. , Fitton, L. C. & O'Higgins, P. (2017). Finite element analysis of the cranium: validity, sensitivity and future directions. Comptes Rendus Palevol 16, 600–612. [Google Scholar]
- Golcher‐Benavides, J. & Wagner, C. E. (2019). Playing out Liem's paradox: opportunistic piscivory across Lake Tanganyikan cichlids. American Naturalist 194, 260–267. [DOI] [PubMed] [Google Scholar]
- Goslow, G. E. (1972). Adaptive mechanisms of the raptor pelvic limb. The Auk: Ornithological Advances 89, 47–64. [Google Scholar]
- Gould, S. J. (2002). The integration and adaptation (structure and function) in ontogeny and phylogeny: historical constraints and the evolution of development. In The Structure of Evolutionary Theory, pp. 1025–1177. Harvard University Press, Cambridge. [Google Scholar]
- Grau, N. , Daw, J. L. , Patel, R. , Evans, C. , Lewis, N. & Mao, J. J. (2006). Nanostructural and nanomechanical properties of synostosed postnatal human cranial sutures. Journal of Craniofacial Surgery 17, 91–98. [DOI] [PubMed] [Google Scholar]
- Green, J. L. & Croft, D. A. (2018). Using dental Mesowear and microwear for dietary inference: a review of current techniques and applications. In Methods in Paleoecology: Reconstructing Cenozoic Terrestrial Environments and Ecological Communities (eds Croft D. A., Su D. F. and Simpson S. W.), pp. 53–73. Springer International Publishing, Cham. [Google Scholar]
- Greenberg, R. , Cadena, V. , Danner, R. M. & Tattersall, G. (2012). Heat loss may explain bill size differences between birds occupying different habitats. PLoS One 7, e40933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grine, F. E. , Ungar, P. S. & Teaford, M. F. (2002). Error rates in dental microwear quantification using scanning electron microscopy. Scanning: The Journal of Scanning Microscopies 24, 144–153. [DOI] [PubMed] [Google Scholar]
- Gu, B. , Schelske, C. L. & Hoyer, M. V. (1996). Stable isotopes of carbon and nitrogen as indicators of diet and trophic structure of the fish community in a shallow hypereutrophic lake. Journal of Fish Biology 49, 1233–1243. [Google Scholar]
- Guillerme, T. , Jones, M. , Lloyd, G. , O'Reilly, J. , Pate, A. , Puttick, M. , Rayfield, E. , Saupe, E. , Sherratt, E. & Slater, G. (2020). Disparities in the analysis of morphological disparity. Biology Letters 16, 20200199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X. , Xu, L. & Jia, S. (2018). Morphological and phylogenetic study based on new materials of Anchiornis huxleyi (Dinosauria, Theropoda) from Jianchang, Western Liaoning, China. Acta Geologica Sinica (English Edition) 92, 1–15. [Google Scholar]
- Gussekloo, S. W. & Bout, R. G. (2005). Cranial kinesis in palaeognathous birds. Journal of Experimental Biology 208, 3409–3419. [DOI] [PubMed] [Google Scholar]
- Hassler, A. , Martin, J. , Amiot, R. , Tacail, T. , Godet, F. A. , Allain, R. & Balter, V. (2018). Calcium isotopes offer clues on resource partitioning among cretaceous predatory dinosaurs. Proceedings of the Royal Society B: Biological Sciences 285, 20180197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut, R. C. , Lancaster, R. L. & DeCamp, C. E. (1992). Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. Journal of Biomechanics 25, 163–173. [DOI] [PubMed] [Google Scholar]
- Hedrick, B. P. , Cordero, S. A. , Zanno, L. E. , Noto, C. & Dodson, P. (2019a). Quantifying shape and ecology in avian pedal claws: the relationship between the bony core and keratinous sheath. Ecology and Evolution 9, 11545–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick, B. P. , Mutumi, G. L. , Munteanu, V. D. , Sadier, A. , Davies, K. T. , Rossiter, S. J. , Sears, K. E. , Dávalos, L. M. & Dumont, E. (2019b). Morphological diversification under high integration in a hyper diverse mammal clade. Journal of Mammalian Evolution 27, 563–575. [Google Scholar]
- Hedrick, B. P. , Schachner, E. R. , Rivera, G. , Dodson, P. & Pierce, S. E. (2019c). The effects of skeletal asymmetry on interpreting biologic variation and taphonomy in the fossil record. Paleobiology 45, 154–166. [Google Scholar]
- Henderson, D. M. (1998). Skull and tooth morphology as indicators of niche partitioning in sympatric Morrison formation theropods. Gaia 15, 219–226. [Google Scholar]
- Henderson, D. M. (2002). The eyes have it: the sizes, shapes, and orientations of theropod orbits as indicators of skull strength and bite force. Journal of Vertebrate Paleontology 22, 766–778. [Google Scholar]
- Hendrickx, C. & Mateus, O. (2014). Abelisauridae (Dinosauria: Theropoda) from the late Jurassic of Portugal and dentition‐based phylogeny as a contribution for the identification of isolated theropod teeth. Zootaxa 3759, 1–74. [DOI] [PubMed] [Google Scholar]
- Hendrickx, C. , Mateus, O. & Araújo, R. (2015). A proposed terminology of theropod teeth (Dinosauria, Saurischia). Journal of Vertebrate Paleontology 35, e982797. [Google Scholar]
- Hendrickx, C. , Tschopp, E. & d'Ezcurra, M. (2020). Taxonomic identification of isolated theropod teeth: the case of the shed tooth crown associated with Aerosteon (Theropoda: Megaraptora) and the dentition of Abelisauridae. Cretaceous Research 108, 104312. [Google Scholar]
- Herrel, A. , Moore, J. A. , Bredeweg, E. M. & Nelson, N. J. (2010a). Sexual dimorphism, body size, bite force and male mating success in tuatara. Biological Journal of the Linnean Society 100, 287–292. [Google Scholar]
- Herrel, A. , Podos, J. , Vanhooydonck, B. & Hendry, A. P. (2009). Force‐velocity trade‐off in Darwin's finch jaw function: a biomechanical basis for ecological speciation? Functional Ecology 23, 119–125. [Google Scholar]
- Herrel, A. , Soons, J. , Aerts, P. , Dirckx, J. , Boone, M. , Jacobs, P. , Adriaens, D. & Podos, J. (2010b). Adaptation and function of the bills of Darwin's finches: divergence by feeding type and sex. Emu ‐ Austral Ornithology 110, 39–47. [Google Scholar]
- Hertel, F. (1994). Diversity in body size and feeding morphology within past and present vulture assemblages. Ecology 75, 1074–1084. [Google Scholar]
- Hertel, F. (1995). Ecomorphological indicators of feeding behavior in recent and fossil raptors. The Auk: Ornithological Advances 112, 890–903. [Google Scholar]
- Hilton, G. M. , Furness, R. W. & Houston, D. C. (2000a). A comparative study of digestion in North Atlantic seabirds. Journal of Avian Biology 31, 36–46. [Google Scholar]
- Hilton, G. M. , Furness, R. W. & Houston, D. C. (2000b). The effects of diet switching and mixing on digestion in seabirds. Functional Ecology 14, 145–154. [Google Scholar]
- Hilton, G. M. , Houston, D. C. , Barton, N. W. H. , Furness, R. W. & Ruxton, G. D. (1999). Ecological constraints on digestive physiology in carnivorous and piscivorous birds. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 283, 365–376. [Google Scholar]
- Hilton, G. M. , Houston, D. C. & Furness, R. W. (1998). Which components of diet quality affect retention time of digesta in seabirds? Functional Ecology 12, 929–939. [Google Scholar]
- Hinić‐Frlog, S. & Motani, R. (2010). Relationship between osteology and aquatic locomotion in birds: determining modes of locomotion in extinct Ornithurae. Journal of Evolutionary Biology 23, 372–385. [DOI] [PubMed] [Google Scholar]
- Hobson, K. A. (1987). Use of stable‐carbon isotope analysis to estimate marine and terrestrial protein content in gull diets. Canadian Journal of Zoology 65, 1210–1213. [Google Scholar]
- Hobson, K. A. (1990). Stable isotope analysis of marbled murrelets: evidence for freshwater feeding and determination of trophic level. The Condor: Ornithological Applications 92, 897–903. [Google Scholar]
- Hobson, K. A. (1993). Trophic relationships among high arctic seabirds ‐ insights from tissue‐dependent stable‐isotope models. Marine Ecology Progress Series 95, 7–18. [Google Scholar]
- Hobson, K. A. , Bowen, G. J. , Wassenaar, L. I. , Ferrand, Y. & Lormee, H. (2004). Using stable hydrogen and oxygen isotope measurements of feathers to infer geographical origins of migrating European birds. Oecologia 141, 477–488. [DOI] [PubMed] [Google Scholar]
- Hobson, K. A. & Montevecchi, W. A. (1991). Stable isotopic determinations of trophic relationships of great auks. Oecologia 87, 528–531. [DOI] [PubMed] [Google Scholar]
- Holliday, C. M. (2009). New insights into dinosaur jaw muscle anatomy. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 292, 1246–1265. [DOI] [PubMed] [Google Scholar]
- Hollocher, T. C. , Chin, K. , Hollocher, K. T. & Kruge, M. A. (2001). Bacterial residues in coprolite of herbivorous dinosaurs: role of bacteria in mineralization of feces. Palaios 16, 547–565. [Google Scholar]
- Hollund, H. I. , Arts, N. , Jans, M. M. E. & Kars, H. (2015). Are teeth better? Histological characterization of diagenesis in archaeological bone–tooth pairs and a discussion of the consequences for archaeometric sample selection and analyses. International Journal of Osteoarchaeology 25, 901–911. [Google Scholar]
- Holtz, T. R. Jr. (2008). A critical reappraisal of the obligate scavenging hypothesis for Tyrannosaurus rex and other tyrant dinosaurs. In Tyrannosaurus Rex the Tyrant King (eds Larson P. L. and Carpenter K.), pp. 371–396. Indiana University Press, Bloomington. [Google Scholar]
- Holtz, T. R. Jr. , Brinkman, D. L. & Chandler, C. L. (1998). Denticle morphometrics and a possibly omnivorous feeding habit for the theropod dinosaur Troodon . Gaia 15, 159–166. [Google Scholar]
- Hopson, J. A. (2001). Ecomorphology of avian and nonavian Theropod phalangeal proportions: implications for the arboreal versus terrestrial origin of bird flight. In New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom (eds Ostrom J. H., Gall L. F. and Gauthier J.), pp. 211–235. Peabody Museum of Natural History, New Haven. [Google Scholar]
- Hou, L. (1997). Mesozoic Birds of China. Taiwan: Phoenix Valley Provincial Aviary of Taiwan. [Google Scholar]
- Hou, L. & Chen, P. (1999). Liaoxiornis delicatus gen. Et sp. nov., the smallest Mesozoic bird. Chinese Science Bulletin 44, 834–838. [Google Scholar]
- Hou, L. , Martin, L. D. , Zhou, Z. & Feduccia, A. (1999a). Archaeopteryx to opposite birds—missing link from the Mesozoic of China. Vertebrata PalAsiatica 37, 88–95. [Google Scholar]
- Hou, L. , Martin, L. D. , Zhou, Z. , Feduccia, A. & Zhang, F. (1999b). A diapsid skull in a new species of the primitive bird Confuciusornis . Nature 399, 679–682. [Google Scholar]
- Hou, L. H. , Chiappe, L. M. , Zhang, F. C. & Chuong, C. M. (2004). New early cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften 91, 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D. , Li, L. , Hou, L. & Xu, X. (2010). A new sapeornithid bird from China and its implication for early avian evolution. Acta Geologica Sinica (English Edition) 84, 472–482. [Google Scholar]
- Hu, D. , Li, L. , Hou, L. & Xu, X. (2011). A new enantiornithine bird from the lower cretaceous of western Liaoning, China. Journal of Vertebrate Paleontology 31, 154–161. [Google Scholar]
- Hu, H. & O'Connor, J. K. (2017). First species of Enantiornithes from Sihedang elucidates skeletal development in early cretaceous enantiornithines. Journal of Systematic Palaeontology 15, 909–926. [Google Scholar]
- Hu, H. , O'Connor, J. K. & Zhou, Z. H. (2015). A new species of Pengornithidae (Aves: Enantiornithes) from the lower cretaceous of China suggests a specialized scansorial habitat previously unknown in early birds. PLoS One 10, e0126791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, H. , O'Connor, J. K. , McDonald, P. G. & Wroe, S. (2020a). Cranial osteology of the early cretaceous Sapeornis chaoyangensis (Aves: Pygostylia). Cretaceous Research 113, 104496. [Google Scholar]
- Hu, H. , O'Connor, J. K. , Wang, M. , Wroe, S. & McDonald, P. G. (2020b). New anatomical information on the bohaiornithid Longusunguis and the presence of a plesiomorphic diapsid skull in Enantiornithes. Journal of Systematic Palaeontology 18, 1481–1495. [Google Scholar]
- Hu, H. , Sansalone, G. , Wroe, S. , McDonald, P. G. , O'Connor, J. K. , Li, Z. H. , Xu, X. & Zhou, Z. H. (2019). Evolution of the vomer and its implications for cranial kinesis in Paraves. Proceedings of the National Academy of Sciences of the United States of America 116, 19571–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y. , Ghigliotti, L. , Vacchi, M. , Pisano, E. , Detrich, H. W. & Albertson, R. C. (2016). Evolution in an extreme environment: developmental biases and phenotypic integration in the adaptive radiation of antarctic notothenioids. BMC Evolutionary Biology 16, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Wang, X. , Hu, Y. , Liu, J. , Peteya, J. A. & Clarke, J. A. (2016). A new ornithurine from the early cretaceous of China sheds light on the evolution of early ecological and cranial diversity in birds. PeerJ 4, e1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, S. K. & Podos, J. (2006). Beak morphology and song features covary in a population of Darwin's finches (Geospiza fortis). Biological Journal of the Linnean Society 88, 489–498. [Google Scholar]
- Hulme, P. E. & Benkman, C. W. (2002). Granivory. In Plant–Animal Interactions: An Evolutionary Approach (eds Pellmyr O. and Herra C. M.), pp. 185–208. Blackwell Science Ltd, Malden. [Google Scholar]
- Hunt, A. P. , Lucas, S. G. , Milan, J. & Spielmann, J. A. (2012). Vertebrate coprolite studies: status and prospectus. New Mexico Museum of Natural History Science Bulletin 57, 5–24. [Google Scholar]
- Hutchinson, J. R. & Allen, V. (2008). The evolutionary continuum of limb function from early theropods to birds. Naturwissenschaften 96, 423–448. [DOI] [PubMed] [Google Scholar]
- Hutson, J. M. , Burke, C. C. & Haynes, G. (2013). Osteophagia and bone modifications by giraffe and other large ungulates. Journal of Archaeological Science 40, 4139–4149. [Google Scholar]
- Jackson, S. (1992). Do seabird gut sizes and mean retention times reflect adaptation to diet and foraging method? Physiological Zoology 65, 674–697. [Google Scholar]
- Jacobs, R. P. W. M. (1982). Component Studies in Seagrass Ecosystems along West European Coasts. Radboud University Nijmegen, Nijmegen. [Google Scholar]
- Jain, A. K. (2010). Data clustering: 50 years beyond K‐means. Pattern Recognition Letters 31, 651–666. [Google Scholar]
- James, H. F. & Burney, D. A. (1997). The diet and ecology of Hawaii's extinct flightless waterfowl: evidence from coprolites. Biological Journal of the Linnean Society 62, 279–297. [Google Scholar]
- Janzen, D. H. (1981). Ficus ovalis seed predation by an orange‐chinned parakeet (Brotogeris jugularis) in Costa Rica. The Auk: Ornithological Advances 98, 841–844. [Google Scholar]
- Jaouen, K. , Balter, V. , Herrscher, E. , Lamboux, A. , Telouk, P. & Albarede, F. (2012). Fe and cu stable isotopes in archeological human bones and their relationship to sex. American Journal of Physical Anthropology 148, 334–340. [DOI] [PubMed] [Google Scholar]
- Jarman, S. N. , McInnes, J. C. , Faux, C. , Polanowski, A. M. , Marthick, J. , Deagle, B. E. , Southwell, C. & Emmerson, L. (2013). Adélie penguin population diet monitoring by analysis of food DNA in scats. PLoS One 8, e82227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, Q. , Ji, S. , Zhang, H. , You, H. , Zhang, J. , Wang, L. , Yuan, C. & Ji, X. (2002). A new avialian bird ‐ Jixiangornis orientalis gen. Et sp. nov. ‐ from the lower cretaceous of western Liaoning, NE China. Journal of Nanjing University Natural Sciences Edition 38, 723–736. [Google Scholar]
- Ji, Q. , Ji, S. A. , You, H. , Zhang, J. , Zhang, H. , Zhang, N. , Yuan, C. & Ji, X. (2003). An early cretaceous avialian bird, Shenzhouraptor sinensis from western Liaoning, China. Acta Geologica Sinica (English Edition) 77, 21–27. [Google Scholar]
- Jolliffe, I. T. & Cadima, J. (2016). Principal component analysis: a review and recent developments. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 374, 20150202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. E. , Bielby, J. , Cardillo, M. , Fritz, S. A. , O'Dell, J. , Orme, C. D. L. , Safi, K. , Sechrest, W. , Boakes, E. H. , Carbone, C. , Connolly, C. , Cutts, M. J. , Foster, J. K. , Grenyer, R. , Habib, M. , et al. (2009). PanTHERIA: a species‐level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648–2648. [Google Scholar]
- Jordano, P. (2000). Fruits and Frugivory. In Seeds: The Ecology of Regeneration in Plant Communities (ed. Fenner M.), pp. 125–166. CABI Publishing, Wallingford. [Google Scholar]
- Juvinall, R. C. & Marshek, K. M. (2011). Failure theories, safety factors, and reliability. In Fundamentals of Machine Component Design, pp. 248–282. Wiley, Hoboken. [Google Scholar]
- Kaesler, R. L. & Waters, J. A. (1972). Fourier analysis of the ostracode margin. Geological Society of America Bulletin 83, 1169–1178. [Google Scholar]
- Kambic, R. E. (2008). Multivariate Analysis of Avian and Non‐Avian Theropod Pedal Phalanges. Bozeman, Montana: Montana State University‐Bozeman, College of Letters & Science. [Google Scholar]
- Karban, R. & Agrawal, A. A. (2002). Herbivore offense. Annual Review of Ecology and Systematics 33, 641–664. [Google Scholar]
- Kaye, T. G. , Pittman, M. , Marugán‐Lobón, J. , Martín‐Abad, H. , Sanz, J. L. & Buscalioni, A. D. (2019). Fully fledged enantiornithine hatchling revealed by laser‐stimulated fluorescence supports precocial nesting behavior. Scientific Reports 9, 5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kear, B. P. , Boles, W. E. & Smith, E. T. (2003). Unusual gut contents in a cretaceous ichthyosaur. Proceedings of the Royal Society B: Biological Sciences 270, S206–S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner, A. W. (1996). Fossilized theropod soft tissue. Nature 379, 32. [Google Scholar]
- Kiltie, R. A. (1982). Bite force as a basis for niche differentiation between rain forest peccaries (Tayassu tajacu and T. pecari). Biotropica 14, 188–195. [Google Scholar]
- Kim, S.‐K. (2011). The Effect of Discretization of Continuous Data: Principal Component Analysis Vs. Correspondence Analysis (Dual Scaling). American Educational Research Association, New Orleans. [Google Scholar]
- Klaczko, J. , Sherratt, E. & Setz, E. Z. F. (2016). Are diet preferences associated to skulls shape diversification in xenodontine snakes? PLoS One 11, e0148375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg, C. P. (2014). Studying morphological integration and modularity at multiple levels: concepts and analysis. Philosophical Transactions of the Royal Society B: Biological Sciences 369, 20130249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg, C. P. (2016). Size, shape, and form: concepts of allometry in geometric morphometrics. Development Genes and Evolution 226, 113–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloskowski, J. , Trembaczowski, A. & Filipiuk, M. (2019). Stable isotope tracing of links between marine wintering and freshwater breeding habitats of red‐necked grebes. Journal of Ornithology 160, 593–605. [Google Scholar]
- Koch, P. L. (2007). Isotopic study of the biology of modern and fossil vertebrates. In Stable Isotopes in Ecology and Environmental Science (Volume 2, eds Michener R. H. and Lajtha K.), pp. 99–154. Blackwell Publishing, Hoboken. [Google Scholar]
- Koenigswald, W. V. , Habersetzer, J. & Gingerich, P. D. (2011). Morphology and evolution of the distal phalanges in primates. In The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates (eds Lehmann T. and Schaal S.), pp. 91–94. International Senckenberg Conference, Frankfurt am Main. [Google Scholar]
- Kolodny, Y. , Luz, B. , Sander, M. & Clemens, W. (1996). Dinosaur bones: fossils or pseudomorphs? The pitfalls of physiology reconstruction from apatitic fossils. Palaeogeography, Palaeoclimatology, Palaeoecology 126, 161–171. [Google Scholar]
- Kulemeyer, C. , Asbahr, K. , Gunz, P. , Frahnert, S. & Bairlein, F. (2009). Functional morphology and integration of corvid skulls ‐ a 3D geometric morphometric approach. Frontiers in Zoology 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundrát, M. , Nudds, J. , Kear, B. P. , Lü, J. & Ahlberg, P. (2019). The first specimen of Archaeopteryx from the upper Jurassic Mörnsheim formation of Germany. Historical Biology 31, 3–63. [Google Scholar]
- Kupczik, K. , Dobson, C. A. , Fagan, M. J. , Crompton, R. H. , Oxnard, C. E. & O'Higgins, P. (2007). Assessing mechanical function of the zygomatic region in macaques: validation and sensitivity testing of finite element models. Journal of Anatomy 210, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurochkin, E. N. , Chatterjee, S. & Mikhailov, K. E. (2013). An embryonic enantiornithine bird and associated eggs from the cretaceous of Mongolia. Paleontological Journal 47, 1252–1269. [Google Scholar]
- Ladyguin, A. (2000). The morphology of the bill apparatus in the Steller's sea eagle. In First Symposium on Steller's and White‐Tailed Sea Eagles in East Asia (eds Ueta M. and McGrady M. J.), pp. 1–10. Wild Bird Society of Japan, Tokyo. [Google Scholar]
- Lambe, L. M. (1917). The Cretaceous Theropodus Dinosaur Gorgosaurus. Government Printing Bureau, Ottawa. [Google Scholar]
- Lambert, J. E. , Chapman, C. A. , Wrangham, R. W. & Conklin‐Brittain, N. L. (2004). Hardness of cercopithecine foods: implications for the critical function of enamel thickness in exploiting fallback foods. American Journal of Physical Anthropology: The Official Publication of the American Association of Physical Anthropologists 125, 363–368. [DOI] [PubMed] [Google Scholar]
- Larson, D. W. , Brown, C. M. & Evans, D. C. (2016). Dental disparity and ecological stability in bird‐like dinosaurs prior to the end‐cretaceous mass extinction. Current Biology 26, 1325–1333. [DOI] [PubMed] [Google Scholar]
- Lautenschlager, S. (2014). Morphological and functional diversity in therizinosaur claws and the implications for theropod claw evolution. Proceedings of the Royal Society B: Biological Sciences 281, 20140497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager, S. (2016). Reconstructing the past: methods and techniques for the digital restoration of fossils. Royal Society Open Science 3, 160342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager, S. (2017). Functional niche partitioning in Therizinosauria provides new insights into the evolution of theropod herbivory. Palaeontology 60, 375–387. [Google Scholar]
- Lautenschlager, S. , Bright, J. A. & Rayfield, E. J. (2014a). Digital dissection–using contrast‐enhanced computed tomography scanning to elucidate hard‐and soft‐tissue anatomy in the common buzzard Buteo buteo . Journal of Anatomy 224, 412–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager, S. , Witmer, L. M. , Altangerel, P. & Rayfield, E. J. (2013). Edentulism, beaks, and biomechanical innovations in the evolution of theropod dinosaurs. Proceedings of the National Academy of Sciences of the United States of America 110, 20657–20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager, S. , Witmer, L. M. , Altangerel, P. , Zanno, L. E. & Rayfield, E. J. (2014b). Cranial anatomy of Erlikosaurus andrewsi (Dinosauria, Therizinosauria): new insights based on digital reconstruction. Journal of Vertebrate Paleontology 34, 1263–1291. [Google Scholar]
- Lee, N. , Horstemeyer, M. , Rhee, H. , Nabors, B. , Liao, J. & Williams, L. N. (2014). Hierarchical multiscale structure–property relationships of the red‐bellied woodpecker (Melanerpes carolinus) beak. Journal of the Royal Society Interface 11, 20140274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. C. , Chiang, C. C. , Huang, P. Y. , Chung, C. Y. , Huang, T. D. , Wang, C. C. , Chen, C. L. , Chang, R. S. , Liao, C. H. & Reisz, R. R. (2017). Evidence of preserved collagen in an early Jurassic sauropodomorph dinosaur revealed by synchrotron FTIR microspectroscopy. Nature Communications 8, 14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre, U. , Cau, A. , Cincotta, A. , Hu, D. , Chinsamy, A. , Escuillié, F. & Godefroit, P. (2017). A new Jurassic theropod from China documents a transitional step in the macrostructure of feathers. The Science of Nature 104, 74. [DOI] [PubMed] [Google Scholar]
- Lefèvre, U. , Hu, D. , Escuillié, F. , Dyke, G. & Godefroit, P. (2014). A new long‐tailed basal bird from the lower cretaceous of North‐Eastern China. Biological Journal of the Linnean Society 113, 790–804. [Google Scholar]
- Leichliter, J. , Lüdecke, T. , Duprey, N. , Winkler, D. E. , Clauss, M. , Tütken, T. & Martínez‐García, A. (2020a). Measuring nitrogen isotopes in tooth enamel: a novel method for characterizing trophic position in fossil ecosystems. Journal of Vertebrate Paleontology 40, 117–118. [Google Scholar]
- Leichliter, J. N. , Lüdecke, T. , Foreman, A. D. , Duprey, N. N. , Winkler, D. E. , Kast, E. R. , Vonhof, H. , Sigman, D. M. , Haug, G. H. , Clauss, M. , Tütken, T. & Martínez‐García, A. (2020b). Nitrogen isotopes in tooth enamel record diet and trophic level enrichment: results from a controlled feeding experiment. Chemical Geology 563, 120047. [Google Scholar]
- Levey, D. J. & del Rio, C. M. (2001). It takes guts (and more) to eat fruit: lessons from avian nutritional ecology. The Auk: Ornithological Advances 118, 819–831. [Google Scholar]
- Li, L. , Duan, Y. , Hu, D. Y. , Wang, L. , Cheng, S. L. & Hou, L. H. (2006). New eoenantiornithid bird from the early cretaceous Jiufotang formation of western Liaoning, China. Acta Geologica Sinica (English Edition) 80, 38–41. [Google Scholar]
- Li, L. , Gong, E. , Zhang, L. , Yang, Y. & Hou, L. (2010). A new enantiornithine bird (Aves) from the early cretaceous of Liaoning, China. Acta Palaeontologica Sinica 49, 524–531. [Google Scholar]
- Li, L. & Hou, S.‐L. (2011). Discovery of a new bird (Enantiornithines) from lower cretaceous in western Liaoning, China. Journal of Jilin University (Earth Science Edition) 41, 759–763. [Google Scholar]
- Li, L. , Hu, D. , Duan, Y. , Gong, E. & Hou, L. (2007). Alethoalaornithidae fam. Nov.: a new family of enantiornithine bird from the lower cretaceous of western Liaoning. Acta Palaeontologica Sinica 46, 371. [Google Scholar]
- Li, L. , Wang, J. , Zhang, X. & Hou, S. (2012). A new enantiornithine bird from the lower cretaceous Jiufotang formation in Jinzhou area, Western Liaoning Province, China. Acta Geologica Sinica (English Edition) 86, 1039–1044. [Google Scholar]
- Li, Q. G. , Clarke, J. A. , Gao, K. Q. , Peteye, J. A. & Shawkey, M. D. (2018). Elaborate plumage patterning in a cretaceous bird. PeerJ 6, e5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Wang, C. C. , Wang, M. , Chiang, C. C. , Wang, Y. , Zheng, X. , Huang, E. W. , Hsiao, K. & Zhou, Z. (2020). Ultramicrostructural reductions in teeth: implications for dietary transition from non‐avian dinosaurs to birds. BMC Evolutionary Biology 20, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. H. & Clarke, J. A. (2016). The craniolingual morphology of waterfowl (Aves, Anseriformes) and its relationship with feeding mode revealed through contrast‐enhanced x‐ray computed tomography and 2D morphometrics. Evolutionary Biology 43, 12–25. [Google Scholar]
- Li, Z. H. , Zhou, Z. H. , Wang, M. & Clarke, J. A. (2014). A new specimen of large‐bodied basal enantiornithine Bohaiornis from the early cretaceous of China and the inference of feeding ecology in Mesozoic birds. Journal of Paleontology 88, 99–108. [Google Scholar]
- Liem, K. F. (1980). Adaptive significance of intra‐and interspecific differences in the feeding repertoires of cichlid fishes. American Zoologist 20, 295–314. [Google Scholar]
- Lingham‐Soliar, T. (2008). A unique cross section through the skin of the dinosaur Psittacosaurus from China showing a complex fibre architecture. Proceedings of the Royal Society B: Biological Sciences 275, 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. , Chiappe, L. M. , Serrano, F. , Habib, M. , Zhang, Y. G. & Meng, Q. J. (2017). Flight aerodynamics in enantiornithines: information from a new Chinese early cretaceous bird. PLoS One 12, e0184637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. , Chiappe, L. M. , Zhang, Y. G. , Serrano, F. J. & Meng, Q. J. (2019). Soft tissue preservation in two new enantiornithine specimens (Aves) from the lower cretaceous Huajiying formation of Hebei Province, China. Cretaceous Research 95, 191–207. [Google Scholar]
- Lockie, J. D. (1956). The food and feeding behaviour of the jackdaw, rook and carrion crow. The Journal of Animal Ecology 25, 421–428. [Google Scholar]
- Lopes, L. E. , Fernandes, A. M. , Medeiros, M. C. I. & Marini, M. A. (2016). A classification scheme for avian diet types. Journal of Field Ornithology 87, 309–322. [Google Scholar]
- Lott, C. A. , Meehan, T. D. & Heath, J. A. (2003). Estimating the latitudinal origins of migratory birds using hydrogen and sulfur stable isotopes in feathers: influence of marine prey base. Oecologia 134, 505–510. [DOI] [PubMed] [Google Scholar]
- Lu, J. , Xu, L. , Zhang, X. , Jia, S. & Chang, H. (2011). A new Gobipterygid bird from the late cretaceous Central China and its biogeographic implications. Journal of Vertebrate Paleontology 31, 147. [Google Scholar]
- Ma, W. , Brusatte, S. L. , Lü, J. & Sakamoto, M. (2019). The skull evolution of oviraptorosaurian dinosaurs: the role of niche‐partitioning in diversification. Journal of Evolutionary Biology 33, 178–188. [DOI] [PubMed] [Google Scholar]
- Ma, W. , Pittman, M. , Lautenschlager, S. , Meade, L. E. & Xu, X. (2020). Functional morphology of the oviraptorosaur and scansoriopterygid skull. In Pennaraptoran Theropod Dinosaurs: Past Progress and New Frontiers (eds Pittman M. and Xu X.), pp. 229–249. New York, NY: Bulletin of the American Museum of Natural History. [Google Scholar]
- Ma, W. , Wang, J. , Pittman, M. , Tan, Q. , Tan, L. , Guo, B. & Xu, X. (2017). Functional anatomy of a giant toothless mandible from a bird‐like dinosaur: Gigantoraptor and the evolution of the oviraptorosaurian jaw. Scientific Reports 7, 16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiolino, S. , Boyer, D. M. & Rosenberger, A. (2011). Morphological correlates of the grooming claw in distal phalanges of platyrrhines and other primates: a preliminary study. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 294, 1975–1990. [DOI] [PubMed] [Google Scholar]
- Manning, P. L. , Margetts, L. , Johnson, M. R. , Withers, P. J. , Sellers, W. I. , Falkingham, P. L. , Mummery, P. M. , Barrett, P. M. & Raymont, D. R. (2009). Biomechanics of dromaeosaurid dinosaur claws: application of X‐ray microtomography, nanoindentation, and finite element analysis. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 292, 1397–1405. [DOI] [PubMed] [Google Scholar]
- Manning, P. L. , Payne, D. , Pennicott, J. , Barrett, P. M. & Ennos, R. A. (2006). Dinosaur killer claws or climbing crampons? Biology Letters 2, 110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcé‐Nogué, J. , de Esteban‐Trivigno, S. , Escrig Pérez, C. & Gil Espert, L. (2016). Accounting for differences in element size and homogeneity when comparing finite element models: armadillos as a case study. Palaeontologia Electronica 19, 2T. [Google Scholar]
- Marcé‐Nogué, J. , de Esteban‐Trivigno, S. , Püschel, T. A. & Fortuny, J. (2017). The intervals method: a new approach to analyse finite element outputs using multivariate statistics. PeerJ 5, e3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcé‐Nogué, J. , DeMiguel, D. , Fortuny, J. , de Esteban‐Trivigno, S. & Gil Espert, L. (2013). Quasi‐homothetic transformation for comparing the mechanical performance of planar models in biological research. Palaeontologia Electronica 16, 6T. [Google Scholar]
- Marcus, L. F. (1990). Traditional morphometrics. In Proceedings of the Michigan Morphometrics Workshop (eds Rohlf F. J. and Bookstein F. L.), pp. 77–122. University of Michigan Museum of Zoology, Ann Arbor. [Google Scholar]
- Marek, R. D. , Falkingham, P. L. , Benson, R. B. J. , Gardiner, J. D. , Maddox, T. W. & Bates, K. T. (2021). Evolutionary versatility of the avian neck. Proceedings of the Royal Society B: Biological Sciences 288, 20203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroig, G. , Shirai, L. T. , Porto, A. , de Oliveira, F. B. & De Conto, V. (2009). The evolution of modularity in the mammalian skull II: evolutionary consequences. Evolutionary Biology 36, 136–148. [Google Scholar]
- Martin, J. E. , Tacail, T. & Balter, V. (2017). Non‐traditional isotope perspectives in vertebrate palaeobiology. Palaeontology 60, 485–502. [Google Scholar]
- Martin, L. D. & Naples, V. L. (2008). Mandibular kinesis in Hesperornis . Oryctos 7, 61–65. [Google Scholar]
- Martin, L. D. & Tate, J. (1976). The skeleton of Baptornis advenus (Aves: Hesperornithiformes). Smithsonian Contributions to Paleobiology 27, 35–66. [Google Scholar]
- Martin, L. D. & Zhou, Z. H. (1997). Archaeopteryx‐like skull in Enantiornithine bird. Nature 389, 556–556. [Google Scholar]
- Martinez, R. N. , Sereno, P. C. , Alcober, O. A. , Colombi, C. E. , Renne, P. R. , Montañez, I. P. & Currie, B. S. (2011). A basal dinosaur from the dawn of the dinosaur era in southwestern Pangaea. Science 331, 206–210. [DOI] [PubMed] [Google Scholar]
- Martyniuk, M. P. (2012). A Field Guide to Mesozoic Birds and Other Winged Dinosaurs. Pan Aves, Vernon. [Google Scholar]
- Marugán‐Lobón, J. & Buscalioni, Á. D. (2004). Geometric morphometrics in macroevolution: morphological diversity of the skull in modern avian forms in contrast to some theropod dinosaurs. In Morphometrics (ed. Elewa A. M. T.), pp. 157–173. Springer‐Verlag, Berlin. [Google Scholar]
- Matsui, H. , Hunt, G. R. , Oberhofer, K. , Ogihara, N. , McGowan, K. J. , Mithraratne, K. , Yamasaki, T. , Gray, R. D. & Izawa, E. I. (2016). Adaptive bill morphology for enhanced tool manipulation in new Caledonian crows. Scientific Reports 6, 22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, G. (2016). Avian Evolution: The Fossil Record of Birds and its Paleobiological Significance. John Wiley & Sons, Oxford. [Google Scholar]
- Mayr, G. (2017). Evolution of avian breeding strategies and its relation to the habitat preferences of Mesozoic birds. Evolutionary Ecology 31, 131–141. [Google Scholar]
- Mayr, G. (2018). A survey of casques, frontal humps, and other extravagant bony cranial protuberances in birds. Zoomorphology 137, 457–472. [Google Scholar]
- Mayr, G. , Codrea, V. , Solomon, A. , Bordeianu, M. & Smith, T. (2020a). Reply to comments on “a well‐preserved pelvis from the Maastrichtian of Romania suggests that the enigmatic Gargantuavis is neither an ornithurine bird nor an insular endemic”. Cretaceous Research 112, 104465. [Google Scholar]
- Mayr, G. , Kaye, T. G. , Pittman, M. , Saitta, E. T. & Pott, C. (2020b). Reanalysis of putative ovarian follicles suggests that early cretaceous birds were feeding not breeding. Scientific Reports 10, 19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr, G. & Manegold, A. (2013). Can ovarian follicles fossilize? Nature 499, E1. [DOI] [PubMed] [Google Scholar]
- McBrayer, L. D. & Corbin, C. E. (2007). Patterns of head shape variation in lizards: morphological correlates of foraging mode. In Lizard Ecology (ed. Lance D.), pp. 271–301. Cambridge University Press, Cambridge. [Google Scholar]
- McHenry, M. & Summers, A. (2011). A force–speed trade‐off is not absolute. Biology Letters 7, 880–881. [Google Scholar]
- McIntosh, A. F. & Cox, P. G. (2016). The impact of gape on the performance of the skull in chisel‐tooth digging and scratch digging mole‐rats (Rodentia: Bathyergidae). Royal Society Open Science 3, 160568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, E. , Zhang, Y. G. , Pashley, D. , Borke, J. & Yu, J. C. (2000). The load‐displacement characteristics of neonatal rat cranial sutures. The Cleft Palate‐Craniofacial Journal 37, 590–595. [DOI] [PubMed] [Google Scholar]
- McWhorter, T. J. & Martínez del Rio, C. (2000). Does gut function limit hummingbird food intake? Physiological and Biochemical Zoology 73, 313–324. [DOI] [PubMed] [Google Scholar]
- McWilliams, S. R. , Caviedes‐Vidal, E. & Karasov, W. H. (1999). Digestive adjustments in cedar waxwings to high feeding rate. Journal of Experimental Zoology 283, 394–407. [Google Scholar]
- Melnick, C. A. , Chen, Z. & Mecholsky, J. J. (1996). Hardness and toughness of exoskeleton material in the stone crab, Menippe mercenaria . Journal of Materials Research 11, 2903–2907. [Google Scholar]
- Meloro, C. , Hudson, A. & Rook, L. (2015). Feeding habits of extant and fossil canids as determined by their skull geometry. Journal of Zoology 295, 178–188. [Google Scholar]
- Melstrom, K. M. (2017). The relationship between diet and tooth complexity in living dentigerous saurians. Journal of Morphology 278, 500–522. [DOI] [PubMed] [Google Scholar]
- Mihlbachler, M. C. , Beatty, B. L. , Caldera‐Siu, A. , Chan, D. & Lee, R. (2012). Error rates and observer bias in dental microwear analysis using light microscopy. Palaeontologia Electronica 15, 12A. [Google Scholar]
- Mihlbachler, M. C. , Campbell, D. , Ayoub, M. , Chen, C. & Ghani, I. (2016). Comparative dental microwear of ruminant and perissodactyl molars: implications for paleodietary analysis of rare and extinct ungulate clades. Paleobiology 42, 98–116. [Google Scholar]
- Mihlbachler, M. C. , Rivals, F. , Solounias, N. & Semprebon, G. M. (2011). Dietary change and evolution of horses in North America. Science 331, 1178–1181. [DOI] [PubMed] [Google Scholar]
- Miller, C. V. , Pittman, M. , Kaye, T. G. , Wang, X. , Bright, J. A. & Zheng, X. (2020). Disassociated rhamphotheca of fossil bird Confuciusornis informs early beak reconstruction, stress regime, and developmental patterns. Communications Biology 3, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. H. , Fogel, M. L. , Magee, J. W. , Gagan, M. K. , Clarke, S. J. & Johnson, B. J. (2005). Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science 309, 287–290. [DOI] [PubMed] [Google Scholar]
- Mitchell, J. S. & Makovicky, P. J. (2014). Low ecological disparity in early cretaceous birds. Proceedings of the Royal Society B: Biological Sciences 281, 20140608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita, T. (2013). Geometric and developmental perspectives on the evolution of the skull and internal carotid circulation in turtles. In Morphology and Evolution of Turtles (eds Brinkman D. B., Holroyd P. A. and Gardner J. D.), pp. 71–101. Springer Netherlands, Dordrecht. [Google Scholar]
- Montanari, S. & Norell, M. (2011). Dietary inferences of protoceratopsian dinosaurs from the late cretaceous of Mongolia based on stable isotope geochemistry. Journal of Vertebrate Paleontology 31, 160–161. [Google Scholar]
- Montuelle, S. J. & Kane, E. A. (2019). Food capture in vertebrates: a complex integrative performance of the cranial and postcranial systems. In Feeding in Vertebrates: Evolution, Morphology, Behavior, Biomechanics (ed. Bels V.), pp. 71–137. Springer International Publishing, Cham. [Google Scholar]
- Morales‐García, N. M. , Burgess, T. D. , Hill, J. J. , Gill, P. G. & Rayfield, E. J. (2019). The use of extruded finite‐element models as a novel alternative to tomography‐based models: a case study using early mammal jaws. Journal of the Royal Society Interface 16, 20190674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, K. , Wroe, S. , Clausen, P. , McHenry, C. , D'Amore, D. C. , Rayfield, E. J. & Cunningham, E. (2008). Cranial performance in the komodo dragon (Varanus komodoensis) as revealed by high‐resolution 3‐D finite element analysis. Journal of Anatomy 212, 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morhardt, A. C. (2009). Dinosaur Smiles: Do the Texture and Morphology of the Premaxilla, Maxilla, and Dentary Bones of Sauropsids Provide Osteological Correlates for Inferring Extra‐Oral Structures Reliably in Dinosaurs?. Western Illinois University, Macomb. [Google Scholar]
- Morris, W. J. (1970). Hadrosaurian Dinosaur Bills: Morphology and Function. Los Angeles County Museum of Natural History, Los Angleles. [Google Scholar]
- Morschhauser, E. M. , Varricchio, D. J. , Chunling, G. , Liu, J. , Wang, X. , Cheng, X. & Meng, Q. (2009). Anatomy of the early cretaceous bird Rapaxavis pani, a new species from Liaoning Province, China. Journal of Vertebrate Paleontology 29, 545–554. [Google Scholar]
- Mosto, M. C. & Tambussi, C. P. (2014). Qualitative and quantitative analysis of talons of diurnal bird of prey. Anatomia Histologia Embryologia 43, 6–15. [DOI] [PubMed] [Google Scholar]
- Moyer, A. E. , Zheng, W. & Schweitzer, M. H. (2016). Keratin durability has implications for the fossil record: results from a 10 year feather degradation experiment. PLoS One 11, e0157699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer, B. R. , Peterson, A. T. & Clayton, D. H. (2002). Influence of bill shape on ectoparasite load in Western scrub‐jays. The Condor: Ornithological Applications 104, 675–678. [Google Scholar]
- Myhrvold, N. P. (2012). A call to search for fossilised gastric pellets. Historical Biology 24, 505–517. [Google Scholar]
- Navalón, G. (2014). Reconstructing the Palaeobiology of Confuciusornis and Other Confuciusornithiformes. University of Bristol, Bristol. [Google Scholar]
- Navalón, G. , Bright, J. A. , Marugán‐Lobón, J. & Rayfield, E. J. (2018a). The evolutionary relationship among beak shape, mechanical advantage, and feeding ecology in modern birds. Evolution 73, 422–435. [DOI] [PubMed] [Google Scholar]
- Navalón, G. , Marugán‐Lobón, J. , Bright, J. A. , Cooney, C. R. & Rayfield, E. J. (2020). The consequences of craniofacial integration for the adaptive radiations of Darwin's finches and Hawaiian honeycreepers. Nature Ecology & Evolution 4, 270–278. [DOI] [PubMed] [Google Scholar]
- Navalón, G. , Meng, Q. , Marugán‐Lobón, J. , Zhang, Y. , Wang, B. , Xing, H. , Liu, D. & Chiappe, L. M. (2018b). Diversity and evolution of the Confuciusornithidae: evidence from a new 131‐million‐year‐old specimen from the Huajiying formation in NE China. Journal of Asian Earth Sciences 152, 12–22. [Google Scholar]
- Navarro, C. A. , Martin‐Silverstone, E. & Stubbs, T. L. (2018). Morphometric assessment of pterosaur jaw disparity. Royal Society Open Science 5, 172130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neenan, J. M. , Ruta, M. , Clack, J. A. & Rayfield, E. J. (2014). Feeding biomechanics in Acanthostega and across the fish–tetrapod transition. Proceedings of the Royal Society B: Biological Sciences 281, 20132689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt, S. J. , Turner, A. H. , Erickson, G. M. & Norell, M. A. (2006). Prey choice and cannibalistic behaviour in the theropod Coelophysis . Biology Letters 2, 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevatte, R. J. , Wueringer, B. E. , Jacob, D. E. , Park, J. M. & Williamson, J. E. (2017). First insights into the function of the sawshark rostrum through examination of rostral tooth microwear. Journal of Fish Biology 91, 1582–1602. [DOI] [PubMed] [Google Scholar]
- Nicolson, S. W. & Fleming, P. A. (2014). Drinking problems on a ‘simple’ diet: physiological convergence in nectar‐feeding birds. Journal of Experimental Biology 217, 1015–1023. [DOI] [PubMed] [Google Scholar]
- Norell, M. A. , Makovicky, P. J. & Currie, P. J. (2001). The beaks of ostrich dinosaurs. Nature 412, 873–874. [DOI] [PubMed] [Google Scholar]
- Norris, D. R. , Marra, P. P. , Bowen, G. J. , Ratcliffe, L. M. , Royle, J. A. & Kyser, T. K. (2006). Migratory connectivity of a widely distributed songbird, the American redstart (Setophaga ruticilla). Ornithological Monographs 61, 14–28. [Google Scholar]
- Novelli, E. L. B. , Diniz, Y. S. , Galhardi, C. M. , Ebaid, G. M. X. , Rodrigues, H. G. , Mani, F. , Fernandes, A. A. H. , Cicogna, A. C. & Novelli Filho, J. L. V. B. (2007). Anthropometrical parameters and markers of obesity in rats. Laboratory Animals 41, 111–119. [DOI] [PubMed] [Google Scholar]
- Nudds, R. L. & Dyke, G. J. (2010). Narrow primary feather rachises in Confuciusornis and Archaeopteryx suggest poor flight ability. Science 328, 887–889. [DOI] [PubMed] [Google Scholar]
- Nudds, R. L. & Oswald, S. A. (2007). An interspecific test of Allen's rule: evolutionary implications for endothermic species. Evolution: International Journal of Organic Evolution 61, 2839–2848. [DOI] [PubMed] [Google Scholar]
- O'Connor, J. K. (2019). The trophic habits of early birds. Palaeogeography, Palaeoclimatology, Palaeoecology 513, 178–195. [Google Scholar]
- O'Connor, J. K. & Chiappe, L. M. (2011). A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. Journal of Systematic Palaeontology 9, 135–157. [Google Scholar]
- O'Connor, J. K. & Dyke, G. (2010). A reassessment of Sinornis santensis and Cathayornis yandica (Aves: Enantiornithes). Records of the Australian Museum 62, 7–20. [Google Scholar]
- O'Connor, J. K. , Gao, K. Q. & Chiappe, L. M. (2010a). A new ornithuromorph (Aves: Ornithothoraces) bird from the Jehol group indicative of higher‐level diversity. Journal of Vertebrate Paleontology 30, 311–321. [Google Scholar]
- O'Connor, J. K. , Li, D. Q. , Lamanna, M. C. , Wang, M. , Harris, J. D. , Atterholt, J. & You, H. L. (2016a). A new early cretaceous enantiornithine (Aves, Ornithothoraces) from northwestern China with elaborate tail ornamentation. Journal of Vertebrate Paleontology 36, e1054035. [Google Scholar]
- O'Connor, J. K. , Sun, C. , Xu, X. , Wang, X. & Zhou, Z. (2012). A new species of Jeholornis with complete caudal integument. Historical Biology 24, 29–41. [Google Scholar]
- O'Connor, J. K. , Wang, M. & Hu, H. (2016b). A new ornithuromorph (Aves) with an elongate rostrum from the Jehol biota, and the early evolution of rostralization in birds. Journal of Systematic Palaeontology 14, 939–948. [Google Scholar]
- O'Connor, J. K. , Wang, M. , Zheng, X. T. , Wang, X. L. & Zhou, Z. H. (2014a). The histology of two female early cretaceous birds. Vertebrata PalAsiatica 52, 112–128. [Google Scholar]
- O'Connor, J. K. , Wang, X. L. , Sullivan, C. , Wang, Y. , Zheng, X. T. , Hu, H. , Zhang, X. M. & Zhou, Z. H. (2018). First report of gastroliths in the early cretaceous basal bird Jeholornis . Cretaceous Research 84, 200–208. [Google Scholar]
- O'Connor, J. K. , Wang, X. L. , Sullivan, C. , Zheng, X. , Tubaro, P. , Zhang, X. & Zhou, Z. (2013a). Unique caudal plumage of Jeholornis and complex tail evolution in early birds. Proceedings of the National Academy of Sciences of the United States of America 110, 17404–17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, J. K. , Wang, X. L. , Zheng, X. T. , Hu, H. , Zhang, X. M. & Zhou, Z. H. (2016c). An enantiornithine with a fan‐shaped tail, and the evolution of the rectricial complex in early birds. Current Biology 26, 114–119. [DOI] [PubMed] [Google Scholar]
- O'Connor, J. K. , Wang, X. R. , Chiappe, L. M. , Gao, C. L. , Meng, Q. J. , Cheng, X. D. & Liu, J. Y. (2009). Phylogenetic support for a specialized clade of cretaceous enantiornithine birds with information from a new species. Journal of Vertebrate Paleontology 29, 188–204. [Google Scholar]
- O'Connor, J. K. , Zhang, Y. G. , Chiappe, L. M. , Meng, Q. J. , Li, Q. G. & Di, L. (2013b). A new enantiornithine from the Yixian formation with the first recognized avian enamel specialization. Journal of Vertebrate Paleontology 33, 1–12. [Google Scholar]
- O'Connor, J. K. , Zheng, X. , Wang, X. , Wang, Y. & Zhou, Z. (2014b). Ovarian follicles shed new light on dinosaur reproduction during the transition towards birds. National Science Review 1, 15–17. [Google Scholar]
- O'Connor, J. K. & Zhou, Z. (2019). The evolution of the modern avian digestive system: insights from paravian fossils from the Yanliao and Jehol biotas. Palaeontology 63, 13–27. [Google Scholar]
- O'Connor, J. K. , Zhou, Z. H. & Zhang, F. C. (2010b). A reappraisal of Boluochia zhengi (Aves: Enantiornithes) and a discussion of intraclade diversity in the Jehol avifauna, China. Journal of Systematic Palaeontology 9, 51–63. [Google Scholar]
- O'Connor, J. K. , Zhou, Z. & Xu, X. (2011). Additional specimen of Microraptor provides unique evidence of dinosaurs preying on birds. Proceedings of the National Academy of Sciences of the United States of America 108, 19662–19665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, P. M. , Turner, A. H. , Groenke, J. R. , Felice, R. N. , Rogers, R. R. , Krause, D. W. & Rahantarisoa, L. J. (2020). Late cretaceous bird from Madagascar reveals unique development of beaks. Nature 588, 272–276. [DOI] [PubMed] [Google Scholar]
- Olsen, A. M. (2017). Feeding ecology is the primary driver of beak shape diversification in waterfowl. Functional Ecology 31, 1985–1995. [Google Scholar]
- Openshaw, G. H. , D'Amore, D. C. , Vidal‐García, M. & Keogh, J. S. (2016). Combining geometric morphometric analyses of multiple 2D observation views improves interpretation of evolutionary allometry and shape diversification in monitor lizard (Varanus) crania. Biological Journal of the Linnean Society 120, 539–552. [Google Scholar]
- Oro, D. , Ruiz, X. , Jover, L. , Pedrocchi, V. & González‐Solís, J. (1997). Diet and adult time budgets of Audouin's gull Larus audouinii in response to changes in commercial fisheries. Ibis 139, 631–637. [Google Scholar]
- Ostrom, J. H. (1976). Archaeopteryx and the origin of birds. Biological Journal of the Linnean Society 8, 91–182. [Google Scholar]
- Ostrom, J. H. (1978). The osteology of Compsognathus longipes Wagner. Zitteliana 4, 73–118. [Google Scholar]
- Ostrom, P. H. , Macko, S. A. , Engel, M. H. & Russell, D. A. (1993). Assessment of trophic structure of cretaceous communities based on stable nitrogen isotope analyses. Geology 21, 491–494. [Google Scholar]
- Park, R. & Epstein, S. (1960). Carbon isotope fractionation during photosynthesis. Geochimica et Cosmochimica Acta 21, 110–126. [Google Scholar]
- Pecsics, T. , Laczi, M. , Nagy, G. , KoNdor, T. & Csörgő, T. (2019). Analysis of skull morphometric characters in diurnal raptors (Accipitriformes and Falconiformes). Ornis Hungarica 27, 117–131. [Google Scholar]
- Pei, R. , Li, Q. , Meng, Q. , Norell, M. A. & Gao, K.‐Q. (2017). New specimens of Anchiornis huxleyi (Theropoda: Paraves) from the late Jurassic of northeastern China. Bulletin of the American Museum of Natural History 2017, 1–67. [Google Scholar]
- Perez, S. I. , Bernal, V. & Gonzalez, P. N. (2006). Differences between sliding semi‐landmark methods in geometric morphometrics, with an application to human craniofacial and dental variation. Journal of Anatomy 208, 769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, D. S. & Görgner, E. (1992). A comparative study on the claws of Archaeopteryx . In Papers in Avian Paleontology Honoring Pierce Brodkorb, Vol. 36. Natural History Museum of Los Angeles County Science Series (ed. Campbell K. C. Jr.), pp. 29–37. Natural History Museum of Los Angeles County, Los Angeles. [Google Scholar]
- Peteya, J. A. , Clarke, J. A. , Li, Q. G. , Gao, K. Q. & Shawkey, M. D. (2017). The plumage and colouration of an enantiornithine bird from the early cretaceous of China. Palaeontology 60, 55–71. [Google Scholar]
- Petie, R. & Muller, M. (2007). Curvature facilitates prey fixation in predatory insect claws. Journal of Theoretical Biology 244, 565–575. [DOI] [PubMed] [Google Scholar]
- Pettigrew, J. D. & Frost, B. J. (1985). A tactile fovea in the Scolopacidae? Brain, Behavior and Evolution 26, 185–195. [PubMed] [Google Scholar]
- Phillips, P. K. & Heath, J. E. (1992). Heat exchange by the pinna of the African elephant (Loxodonta africana). Comparative Biochemistry and Physiology ‐ Part A: Physiology 101, 693–699. [DOI] [PubMed] [Google Scholar]
- Pietsch, T. W. (2005). Dimorphism, parasitism, and sex revisited: modes of reproduction among deep‐sea ceratioid anglerfishes (Teleostei: Lophiiformes). Ichthyological Research 52, 207–236. [Google Scholar]
- Pigot, A. L. , Sheard, C. , Miller, E. T. , Bregman, T. P. , Freeman, B. G. , Roll, U. , Seddon, N. , Trisos, C. H. , Weeks, B. C. & Tobias, J. A. (2020). Macroevolutionary convergence connects morphological form to ecological function in birds. Nature Ecology & Evolution 4, 230–239. [DOI] [PubMed] [Google Scholar]
- Pike, A. V. L. & Maitland, D. P. (2004). Scaling of bird claws. Journal of Zoology 262, 73–81. [Google Scholar]
- Pineda‐Munoz, S. , Lazagabaster, I. A. , Alroy, J. & Evans, A. R. (2017). Inferring diet from dental morphology in terrestrial mammals. Methods in Ecology and Evolution 8, 481–491. [Google Scholar]
- Pittman, M. , O'Connor, J. , Field, D. J. , Turner, A. H. , Ma, W. , Makovicky, P. J. & Xu, X. (2020a). Pennaraptoran systematics. In Pennaraptoran Theropod Dinosaurs: Past Progress and New Frontiers (eds Pittman M. and Xu X.), pp. 7–36. New York, NY: Bulletin of the American Museum of Natural History. [Google Scholar]
- Pittman, M. , O'Connor, J. , Tse, E. , Makovicky, P. J. , Field, D. J. , Ma, W. , Turner, A. H. , Norell, M. A. & Xu, X. (2020b). The fossil record of Mesozoic and Paleocene pennaraptorans. In Pennaraptoran Theropod Dinosaurs: Past Progress and New Frontiers (eds Pittman M. and Xu X.), pp. 37–96. New York, NY: Bulletin of the American Museum of Natural History. [Google Scholar]
- Plavcan, J. M. (1994). Comparison of four simple methods for estimating sexual dimorphism in fossils. American Journal of Physical Anthropology 94, 465–476. [DOI] [PubMed] [Google Scholar]
- Polly, P. D. , Stayton, C. T. , Dumont, E. R. , Pierce, S. E. , Rayfield, E. J. & Angielczyk, K. D. (2016). Combining geometric morphometrics and finite element analysis with evolutionary modeling: towards a synthesis. Journal of Vertebrate Paleontology 36, e1111225. [Google Scholar]
- Pomeroy, D. L. (2013). A Morphological and Taxonomic Revision of the Early Cretaceous Sapeornithidae (Aves: Pygostylia) of Liaoning Province, China. California State University, Long Beach. [Google Scholar]
- Porro, L. B. , Holliday, C. M. , Anapol, F. , Ontiveros, L. C. , Ontiveros, L. T. & Ross, C. F. (2011). Free body analysis, beam mechanics, and finite element modeling of the mandible of Alligator mississippiensis . Journal of Morphology 272, 910–937. [DOI] [PubMed] [Google Scholar]
- Prosser, P. & Hart, A. D. M. (2005). Assessing potential exposure of birds to pesticide‐treated seeds. Ecotoxicology 14, 679–691. [DOI] [PubMed] [Google Scholar]
- Provini, P. , Zhou, Z. & Zhang, F. (2009). A new species of the basal bird Sapeornis from the early cretaceous of Liaoning, China. Vertebrata PalAsiatica 47, 194–207. [Google Scholar]
- Ptacek, M. B. (2000). The role of mating preferences in shaping interspecific divergence in mating signals in vertebrates. Behavioural Processes 51, 111–134. [DOI] [PubMed] [Google Scholar]
- Purnell, M. , Seehausen, O. & Galis, F. (2012). Quantitative three‐dimensional microtextural analyses of tooth wear as a tool for dietary discrimination in fishes. Journal of the Royal Society Interface 9, 2225–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell, M. A. (1995). Microwear on conodont elements and macrophagy in the first vertebrates. Nature 374, 798–800. [Google Scholar]
- Purnell, M. A. & Darras, L. P. G. (2015). 3D tooth microwear texture analysis in fishes as a test of dietary hypotheses of durophagy. Surface Topography: Metrology and Properties 4, 014006. [Google Scholar]
- Purnell, M. A. , Hart, P. J. B. , Baines, D. C. & Bell, M. A. (2006). Quantitative analysis of dental microwear in threespine stickleback: a new approach to analysis of trophic ecology in aquatic vertebrates. Journal of Animal Ecology 75, 967–977. [DOI] [PubMed] [Google Scholar]
- Qvarnström, M. , Wernstrom, J. V. , Piechowski, R. , Talanda, M. , Ahlberg, P. E. & Niedzwiedzki, G. (2019). Beetle‐bearing coprolites possibly reveal the diet of a late Triassic dinosauriform. Royal Society Open Science 6, 181042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2019). prcomp function | R Documentation Version 3.6.2.
- Radhakrishnan, P. & Mao, J. J. (2004). Nanomechanical properties of facial sutures and sutural mineralization front. Journal of Dental Research 83, 470–475. [DOI] [PubMed] [Google Scholar]
- Ralph, C. P. , Nagata, S. E. & Ralph, C. J. (1985). Analysis of droppings to describe diets of small birds. Journal of Field Ornithology 56, 165–174. [Google Scholar]
- Ramos, A. M. & Walker, I. D. (1998). Raptors—Inroads to multifingered grasping. In Proceedings. 1998 IEEE/RSJ International Conference on Intelligent Robots and Systems. Innovations in Theory, Practice and Applications (Cat. No. 98CH36190), vol. 1, pp. 467–475. IEEE.
- Rau, G. H. , Ainley, D. G. , Bengtson, J. L. , Torres, J. J. & Hopkins, T. L. (1992). 15N/14N and 13C/12C in Weddell Sea birds, seals, and fish ‐ implications for diet and trophic structure. Marine Ecology Progress Series 84, 1–8. [Google Scholar]
- Rauhut, O. W. M. (2014). New observations on the skull of Archaeopteryx . Paläontologische Zeitschrift 88, 211–221. [Google Scholar]
- Rauhut, O. W. M. , Foth, C. & Tischlinger, H. (2018). The oldest Archaeopteryx (Theropoda: Avialiae): a new specimen from the Kimmeridgian/Tithonian boundary of Schamhaupten, Bavaria. PeerJ 6, e4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayfield, E. J. (2005). Aspects of comparative cranial mechanics in the theropod dinosaurs Coelophysis, Allosaurus and Tyrannosaurus . Zoological Journal of the Linnean Society 144, 309–316. [Google Scholar]
- Rayfield, E. J. (2011a). Strain in the ostrich mandible during simulated pecking and validation of specimen‐specific finite element models. Journal of Anatomy 218, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayfield, E. J. (2011b). Structural performance of tetanuran theropod skulls, with emphasis on the Megalosauridae, Spinosauridae and Carcharodontosauridae. Special Papers in Palaeontology 86, 241–253. [Google Scholar]
- Rayfield, E. J. , Milner, A. C. , Xuan, V. B. & Young, P. G. (2007). Functional morphology of spinosaur ‘crocodile‐mimic’ dinosaurs. Journal of Vertebrate Paleontology 27, 892–901. [Google Scholar]
- Rayfield, E. J. , Norman, D. B. , Horner, C. C. , Horner, J. R. , Smith, P. M. , Thomason, J. J. & Upchurch, P. (2001). Cranial design and function in a large theropod dinosaur. Nature 409, 1033–1037. [DOI] [PubMed] [Google Scholar]
- Reed, D. A. , Porro, L. B. , Iriarte‐Diaz, J. , Lemberg, J. B. , Holliday, C. M. , Anapol, F. & Ross, C. F. (2011). The impact of bone and suture material properties on mandibular function in Alligator mississippiensis: testing theoretical phenotypes with finite element analysis. Journal of Anatomy 218, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, J. & Lindgren, J. (2005). Aquatic birds from the upper cretaceous (lower Campanian) of Sweden and the biology and distribution of hesperornithiforms. Palaeontology 48, 1321–1329. [Google Scholar]
- Reynard, L. M. , Henderson, G. M. & Hedges, R. E. M. (2010). Calcium isotope ratios in animal and human bone. Geochimica et Cosmochimica Acta 74, 3735–3750. [Google Scholar]
- Rico‐Guevara, A. & Araya‐Salas, M. (2014). Bills as daggers? A test for sexually dimorphic weapons in a lekking hummingbird. Behavioral Ecology 26, 21–29. [Google Scholar]
- Rico‐Guevara, A. , Sustaita, D. , Gussekloo, S. , Olsen, A. , Bright, J. , Corbin, C. & Dudley, R. (2019). Feeding in birds: thriving in terrestrial, aquatic, and aerial niches. In Feeding in Vertebrates: Evolution, Morphology, Behavior, Biomechanics (ed. Bels V.), pp. 643–693. Springer International Publishing, Cham. [Google Scholar]
- Robinson, B. W. & Wilson, D. S. (1998). Optimal foraging, specialization, and a solution to Liem's paradox. American Naturalist 151, 223–235. [DOI] [PubMed] [Google Scholar]
- Rogers, R. R. , Krause, D. W. , Rogers, K. C. , Rasoamiaramanana, A. H. & Rahantarisoa, L. (2007). Paleoenvironment and paleoecology of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the late cretaceous of Madagascar. Journal of Vertebrate Paleontology 27, 21–31. [Google Scholar]
- Rohlf, F. J. (1990). Morphometrics. Annual Review of Ecology and Systematics 21, 299–316. [Google Scholar]
- Rose, K. D. , Walker, A. & Jacobs, L. L. (1981). Function of the mandibular tooth comb in living and extinct mammals. Nature 289, 583–585. [DOI] [PubMed] [Google Scholar]
- Rosenberg, K. V. & Cooper, R. J. (1990). Approaches to avian diet analysis. Studies in Avian Biology 13, 80–90. [Google Scholar]
- Rouquier, S. & Giorgi, D. (2007). Olfactory receptor gene repertoires in mammals. Mutation Research ‐ Fundamental and Molecular Mechanisms of Mutagenesis 616, 95–102. [DOI] [PubMed] [Google Scholar]
- Rybczynski, N. , Tirabasso, A. , Bloskie, P. , Cuthbertson, R. & Holliday, C. (2008). A three‐dimensional animation model of Edmontosaurus (Hadrosauridae) for testing chewing hypotheses. Palaeontologia Electronica 11, 9A. [Google Scholar]
- Sage, R. F. , Sage, T. L. & Kocacinar, F. (2012). Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Saitta, E. T. , Kaye, T. G. & Vinther, J. (2019). Sediment‐encased maturation: a novel method for simulating diagenesis in organic fossil preservation. Palaeontology 62, 135–150. [Google Scholar]
- Sakamoto, M. (2010). Jaw biomechanics and the evolution of biting performance in theropod dinosaurs. Proceedings of the Royal Society B: Biological Sciences 277, 3327–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels, J. X. (2009). Cranial morphology and dietary habits of rodents. Zoological Journal of the Linnean Society 156, 864–888. [Google Scholar]
- Sanchez, J. (2010). Late Cretaceous (Cenomanian) Hesperornithiformes from the Pasquia Hills. Carleton University, Ottawa. [Google Scholar]
- Sankey, J. T. , Brinkman, D. B. , Guenther, M. & Currie, P. J. (2002). Small theropod and bird teeth from the Judith River group (late Campanian), Alberta. Journal of Paleontology 76, 751–763. [Google Scholar]
- Sanpera, C. , Ruiz, X. , Moreno, R. , Jover, L. & Waldron, S. (2007). Mercury and stable isotopes in feathers of Audouin's gulls as indicators of feeding habits and migratory connectivity. The Condor: Ornithological Applications 109, 268–275. [Google Scholar]
- Santana, S. E. , Dumont, E. R. & Davis, J. L. (2010). Mechanics of bite force production and its relationship to diet in bats. Functional Ecology 24, 776–784. [Google Scholar]
- Sanz, J. L. , Chiappe, L. M. , Fernádez‐Jalvo, Y. , Ortega, F. , Sánchez‐Chillón, B. , Poyato‐Ariza, F. J. & Pérez‐Moreno, B. P. (2001). An early cretaceous pellet. Nature 409, 998–1000. [DOI] [PubMed] [Google Scholar]
- Sanz, J. L. , Chiappe, L. M. , Pérez‐Moreno, B. P. , Buscalioni, A. D. , Moratalla, J. J. , Ortega, F. & Poyato‐Ariza, F. J. (1996). An early cretaceous bird from Spain and its implications for the evolution of avian flight. Nature 382, 442–445. [Google Scholar]
- Sanz, J. L. , Chiappe, L. M. , Pérez‐Moreno, B. P. , Moratalla, J. J. , Hernández‐Carrasquilla, F. , Buscalioni, A. D. , Ortega, F. , Poyato‐Ariza, F. J. , Rasskin‐Gutman, D. & Martínez‐Delclòs, X. (1997). A nestling bird from the lower cretaceous of Spain: implications for avian skull and neck evolution. Science 276, 1543–1546. [Google Scholar]
- Savoldi, F. , Xu, B. , Tsoi, J. K. H. , Paganelli, C. & Matinlinna, J. P. (2018). Anatomical and mechanical properties of swine midpalatal suture in the premaxillary, maxillary, and palatine region. Scientific Reports 8, 7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer, J. , Benton, M. J. , Rayfield, E. J. & Stubbs, T. L. (2019). Morphological disparity in theropod jaws: comparing discrete characters and geometric morphometrics. Palaeontology 63, 283–299. [Google Scholar]
- Scheuhammer, A. M. , Bond, D. E. , Burgess, N. M. & Rodrigue, J. (2003). Lead and stable lead isotope ratios in soil, earthworms, and bones of American woodcock (Scolopax minor) from eastern Canada. Environmental Toxicology and Chemistry: An International Journal 22, 2585–2591. [DOI] [PubMed] [Google Scholar]
- Scheuhammer, A. M. & Templeton, D. M. (1998). Use of stable isotope ratios to distinguish sources of lead exposure in wild birds. Ecotoxicology 7, 37–42. [Google Scholar]
- Schileo, E. , Taddei, F. , Cristofolini, L. & Viceconti, M. (2008). Subject‐specific finite element models implementing a maximum principal strain criterion are able to estimate failure risk and fracture location on human femurs tested in vitro . Journal of Biomechanics 41, 356–367. [DOI] [PubMed] [Google Scholar]
- Schino, G. (2006). Grooming and agonistic support: a meta‐analysis of primate reciprocal altruism. Behavioral Ecology 18, 115–120. [Google Scholar]
- Schmidt‐Nielsen, K. (1984). Scaling: Why Is Animal Size So Important?. Cambridge University Press, Cambridge. [Google Scholar]
- Schoeninger, M. J. & DeNiro, M. J. (1984). Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochimica et Cosmochimica Acta 48, 625–639. [Google Scholar]
- Schubert, B. W. & Ungar, P. S. (2005). Wear facets and enamel spalling in tyrannosaurid dinosaurs. Acta Palaeontologica Polonica 50, 93–99. [Google Scholar]
- Schultze, H. P. (1989). Three‐dimensional muscle preservation in Jurassic fishes of Chile. Andean Geology 16, 183–215. [Google Scholar]
- Schwartz, J. H. (1978). Homologies of the toothcomb. American Journal of Physical Anthropology 49, 23–29. [DOI] [PubMed] [Google Scholar]
- Schweitzer, M. H. , Zheng, W. X. , Moyer, A. E. , Sjovall, P. & Lindgren, J. (2018). Preservation potential of keratin in deep time. PLoS One 13, e0206569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciscio, L. , Knoll, F. , Bordy, E. M. , de Kock, M. O. & Redelstorff, R. (2017). Digital reconstruction of the mandible of an adult Lesothosaurus diagnosticus with insight into the tooth replacement process and diet. PeerJ 5, e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki, Y. , Mackey, M. & Meyers, M. A. (2012). Structure and micro‐computed tomography‐based finite element modeling of toucan beak. Journal of the Mechanical Behavior of Biomedical Materials 9, 1–8. [DOI] [PubMed] [Google Scholar]
- Sellers, K. C. , Middleton, K. M. , Davis, J. L. & Holliday, C. M. (2017). Ontogeny of bite force in a validated biomechanical model of the American alligator. Journal of Experimental Biology 220, 2036–2046. [DOI] [PubMed] [Google Scholar]
- Senter, P. (2006). Comparison of forelimb function between Deinonychus and Bambiraptor (Theropoda: Dromaeosauridae). Journal of Vertebrate Paleontology 26, 897–906. [Google Scholar]
- Sereno, P. C. , Martínez, R. N. & Alcober, O. A. (2013). Osteology of Eoraptor lunensis (Dinosauria, Sauropodomorpha). Journal of Vertebrate Paleontology 32, 83–179. [Google Scholar]
- Serrano, F. J. (2020). ERRATUM. https://www.researchgate.net/publication/271965760_Multivariate_analysis_of_neognath_skeletal_measurements_Implications_for_body_mass_estimation_in_Mesozoic_birds/comments?focusedCommentId=5e8b253e3843b064c1c343a8&sldffc=0
- Serrano, F. J. , Palmqvist, P. , Chiappe, L. M. & Sanz, J. L. (2017). Inferring flight parameters of Mesozoic avians through multivariate analyses of forelimb elements in their living relatives. Paleobiology 43, 144–169. [Google Scholar]
- Serrano, F. J. , Palmqvist, P. & Sanz, J. L. (2015). Multivariate analysis of neognath skeletal measurements: implications for body mass estimation in Mesozoic birds. Zoological Journal of the Linnean Society 173, 929–955. [Google Scholar]
- Shi, H. & Li, L. (2019). Advances in cladistic analysis of Enantiornithes from China. Open Journal of Nature Science 7, 387–397. [Google Scholar]
- Shine, R. (1989). Ecological causes for the evolution of sexual dimorphism: a review of the evidence. The Quarterly Review of Biology 64, 419–461. [DOI] [PubMed] [Google Scholar]
- Shychoski, L. & Snively, E. (2008a). Ecological implications of tyrannosaurid lower jaw ontogeny, biomechanical scaling and bite function. Journal of Vertebrate Paleontology 28, 142A–142A. [Google Scholar]
- Shychoski, L. & Snively, E. (2008b). Ecological implications of tyrannosaurid lower jaw ontogeny, biomechanical scaling and bite function. In Sixty‐Eighth Annual Meeting of the Society of Vertebrate Paleontology. Poster IV 53, Cleveland, OH.
- Si, G. , Dong, Y. , Ma, Y. & Zhang, Z. (2015). Shape similarities and differences in the skulls of scavenging raptors. Zoological Science 32, 171–178. [DOI] [PubMed] [Google Scholar]
- Sload, E. J. (2014). Microwear Analysis of Crab Claw Fingers: A Functional Morphological Approach. Kent State University, Kent. [Google Scholar]
- Smith, C. R. (1982). Food resource partitioning of fossorial Florida reptiles. Wildlife Research Report 13, 173–178. [Google Scholar]
- Smith, D. K. (1998). A morphometric analysis of Allosaurus . Journal of Vertebrate Paleontology 18, 126–142. [Google Scholar]
- Smith, D. K. (2015). Craniocervical myology and functional morphology of the small‐headed therizinosaurian theropods Falcarius utahensis and Nothronychus mckinleyi . PLoS One 10, e0117281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. K. (1993). The form of the feeding apparatus in terrestrial vertebrates: studies of adaptation and constraint. In The Skull, Volume 3: Functional and Evolutionary Mechanisms (eds Hanken J. and Hall B. K.), pp. 150–196. The University of Chicago Press, Chicago. [Google Scholar]
- Smith, K. T. & Scanferla, A. (2016). Fossil snake preserving three trophic levels and evidence for an ontogenetic dietary shift. Palaeobiodiversity and Palaeoenvironments 96, 589–599. [Google Scholar]
- Smith, R. J. & Jungers, W. L. (1997). Body mass in comparative primatology. Journal of Human Evolution 32, 523–559. [DOI] [PubMed] [Google Scholar]
- Smith, S. A. & Smith, B. J. (1990). Normal xeroradiographic and radiographic anatomy of the red‐tailed hawk (Buteo jamaicencis), with reference to other diurnal raptors. Veterinary Radiology 31, 301–312. [Google Scholar]
- Snively, E. , Cotton, J. R. , Ridgely, R. & Witmer, L. M. (2013). Multibody dynamics model of head and neck function in Allosaurus (Dinosauria, Theropoda). Palaeontologia Electronica 16, 11A. [Google Scholar]
- Snively, E. & Russell, A. P. (2007a). Functional morphology of neck musculature in the Tyrannosauridae (Dinosauria, Theropoda) as determined via a hierarchical inferential approach. Zoological Journal of the Linnean Society 151, 759–808. [Google Scholar]
- Snively, E. & Russell, A. P. (2007b). Functional variation of neck muscles and their relation to feeding style in Tyrannosauridae and other large theropod dinosaurs. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 290, 934–957. [DOI] [PubMed] [Google Scholar]
- Soons, J. , Genbrugge, A. , Podos, J. , Adriaens, D. , Aerts, P. , Dirckx, J. & Herrel, A. (2015). Is beak morphology in Darwin's finches tuned to loading demands? PLoS One 10, e0129479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons, J. , Herrel, A. , Genbrugge, A. , Adriaens, D. , Aerts, P. & Dirckx, J. (2012a). Multi‐layered bird beaks: a finite‐element approach towards the role of keratin in stress dissipation. Journal of the Royal Society Interface 9, 1787–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons, J. , Herrel, A. , Genbrugge, A. , Aerts, P. , Podos, J. , Adriaens, D. , de Witte, Y. , Jacobs, P. & Dirckx, J. (2010). Mechanical stress, fracture risk and beak evolution in Darwin's ground finches (Geospiza). Philosophical Transactions of the Royal Society B: Biological Sciences 365, 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons, J. , Lava, P. , Debruyne, D. & Dirckx, J. (2012b). Full‐field optical deformation measurement in biomechanics: digital speckle pattern interferometry and 3D digital image correlation applied to bird beaks. Journal of the Mechanical Behavior of Biomedical Materials 14, 186–191. [DOI] [PubMed] [Google Scholar]
- Stansfield, E. , Parker, J. & O'Higgins, P. (2018). A sensitivity study of human mandibular biting simulations using finite element analysis. Journal of Archaeological Science‐Reports 22, 420–432. [Google Scholar]
- Staufenberg, G. , Graupner, N. & Müssig, J. (2015). Impact and hardness optimisation of composite materials inspired by the babassu nut (Orbignya speciosa). Bioinspiration & Biomimetics 10, 056006. [DOI] [PubMed] [Google Scholar]
- Stayton, C. T. (2006). Testing hypotheses of convergence with multivariate data: morphological and functional convergence among herbivorous lizards. Evolution 60, 824–841. [PubMed] [Google Scholar]
- Sternberg, C. M. (1935). Hooded Hadrosaurs of the Belly River series of the upper cretaceous: a comparison, with descriptions of new species. In National Museum of Canada (ed. Collins W. H.). Patenaude, J. O., I.S.O., Ottawa. [Google Scholar]
- Stevenson, R. D. (1986). Allen's rule in north American rabbits (Sylvilagus) and hares (Lepus) is an exception, not a rule. Journal of Mammalogy 67, 312–316. [Google Scholar]
- Sues, H.‐D. , Nesbitt, S. J. , Berman, D. S. & Henrici, A. C. (2011). A late‐surviving basal theropod dinosaur from the latest Triassic of North America. Proceedings of the Royal Society B: Biological Sciences 278, 3459–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, B. L. , Wood, C. L. , Iliff, M. J. , Bonney, R. E. , Fink, D. & Kelling, S. (2009). eBird: a citizen‐based bird observation network in the biological sciences. Biological Conservation 142, 2282–2292. [Google Scholar]
- Sun, Y. , Si, G. , Wang, X. , Wang, K. & Zhang, Z. (2018). Geometric morphometric analysis of skull shape in the Accipitridae. Zoomorphology 137, 445–456. [Google Scholar]
- Surkov, M. V. & Benton, M. J. (2008). Head kinematics and feeding adaptations of the Permian and Triassic dicynodonts. Journal of Vertebrate Paleontology 28, 1120–1129. [Google Scholar]
- Sustaita, D. (2008). Musculoskeletal underpinnings to differences in killing behavior between north American accipiters (Falconiformes: Accipitridae) and falcons (Falconidae). Journal of Morphology 269, 283–301. [DOI] [PubMed] [Google Scholar]
- Sustaita, D. , Gloumakov, Y. , Tsang, L. R. & Dollar, A. M. (2019). Behavioral correlates of semi‐zygodactyly in ospreys (Pandion haliaetus) based on analysis of internet images. PeerJ 7, e6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustaita, D. & Hertel, F. (2010). In vivo bite and grip forces, morphology and prey‐killing behavior of north American accipiters (Accipitridae) and falcons (Falconidae). Journal of Experimental Biology 213, 2617–2628. [DOI] [PubMed] [Google Scholar]
- Sustaita, D. , Pouydebat, E. , Manzano, A. , Abdala, V. , Hertel, F. & Herrel, A. (2013). Getting a grip on tetrapod grasping: form, function, and evolution. Biological Reviews 88, 380–405. [DOI] [PubMed] [Google Scholar]
- Sustaita, D. & Rubega, M. A. (2014). The anatomy of a shrike bite: bill shape and bite performance in loggerhead shrikes. Biological Journal of the Linnean Society 112, 485–498. [Google Scholar]
- Svanberg, F. , Mateo, R. , Hillström, L. , Green, A. J. , Taggart, M. A. , Raab, A. & Meharg, A. A. (2006). Lead isotopes and lead shot ingestion in the globally threatened marbled teal (Marmaronetta angustirostris) and white‐headed duck (Oxyura leucocephala). Science of the Total Environment 370, 416–424. [DOI] [PubMed] [Google Scholar]
- Symonds, M. R. E. & Tattersall, G. J. (2010). Geographical variation in bill size across bird species provides evidence for Allen's rule. American Naturalist 176, 188–197. [DOI] [PubMed] [Google Scholar]
- Ţălu, Ş. , Stach, S. , Méndez, A. , Trejo, G. & Ţălu, M. (2014). Multifractal characterization of nanostructure surfaces of electrodeposited Ni‐P coatings. Journal of the Electrochemical Society 161, D44–D47. [Google Scholar]
- Tarquini, S. D. , Chemisquy, M. A. , Ladevèze, S. & Prevosti, F. J. (2019). The scope of traditional and geometric morphometrics for inferences of diet in carnivorous fossil mammals. Ameghiniana 56, 307–318. [Google Scholar]
- Tattersall, G. J. , Andrade, D. V. & Abe, A. S. (2009). Heat exchange from the toucan bill reveals a controllable vascular thermal radiator. Science 325, 468–470. [DOI] [PubMed] [Google Scholar]
- Tattersall, H. G. & Tappin, G. (1966). The work of fracture and its measurement in metals, ceramics and other materials. Journal of Materials Science 1, 296–301. [Google Scholar]
- Taylor, C. R. (1966). The vascularity and possible thermoregulatory function of the horns in goats. Physiological Zoology 39, 127–139. [Google Scholar]
- Taylor, R. J. (2013). Predators and predation. In Predation, pp. 1–5. Springer Science & Business Media, Berlin. [Google Scholar]
- Therrien, F. , Quinney, A. , Tanaka, K. & Zelenitsky, D. K. (2016). Accuracy of mandibular force profiles for bite force estimation and feeding behavior reconstruction in extant and extinct carnivorans. Journal of Experimental Biology 219, 3738–3749. [DOI] [PubMed] [Google Scholar]
- Thomas, M. A. (2014). Aspects of the Trophic Ecology of an Invertivorous Snake Community. Eastern Illinois University, Charleston. [Google Scholar]
- Thompson, D. W. (1942). On Growth and Form, 2nd Edition. Macmillan, Cambridge University Press, Oxford. [Google Scholar]
- Thompson, R. M. , Hemberg, M. , Starzomski, B. M. & Shurin, J. B. (2007). Trophic levels and trophic tangles: the prevalence of omnivory in real food webs. Ecology 88, 612–617. [DOI] [PubMed] [Google Scholar]
- Thouzeau, C. , Peters, G. , Le Bohec, C. & Le Maho, Y. (2004). Adjustments of gastric pH, motility and temperature during long‐term preservation of stomach contents in free‐ranging incubating king penguins. Journal of Experimental Biology 207, 2715–2724. [DOI] [PubMed] [Google Scholar]
- Thulborn, R. A. & Hamley, T. L. (1985). A new Palaeoecological role for Archaeopteryx . In The Beginnings of Birds: Proceedings of the International Archaeopteryx Conference, Eichstatt 1984 (eds Hecht M. K., Ostrom J. H., Viohl G. and Wellnhofer P.), pp. 81–89. Freunde des Jura‐Museums, Eichstätt. [Google Scholar]
- Tietze, D. T. (2018). Introduction: studying birds in time and space. In Bird Species: How They Arise, Modify and Vanish (ed. Tietze D. T.), pp. 1–7. Springer International Publishing, Cham. [Google Scholar]
- Tilkens, M. J. , Wall‐Scheffler, C. , Weaver, T. D. & Steudel‐Numbers, K. (2007). The effects of body proportions on thermoregulation: an experimental assessment of Allen's rule. Journal of Human Evolution 53, 286–291. [DOI] [PubMed] [Google Scholar]
- Tinius, A. & Russell, A. P. (2017). Points on the curve: an analysis of methods for assessing the shape of vertebrate claws. Journal of Morphology 278, 150–169. [DOI] [PubMed] [Google Scholar]
- Tokita, M. , Kiyoshi, T. & Armstrong, K. N. (2007). Evolution of craniofacial novelty in parrots through developmental modularity and heterochrony. Evolution & Development 9, 590–601. [DOI] [PubMed] [Google Scholar]
- Tokita, M. , Yano, W. , James, H. F. & Abzhanov, A. (2017). Cranial shape evolution in adaptive radiations of birds: comparative morphometrics of Darwin's finches and Hawaiian honeycreepers. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torices, A. , Wilkinson, R. , Arbour, V. M. , Ruiz‐Omenaca, J. I. & Currie, P. J. (2018). Puncture‐and‐pull biomechanics in the teeth of predatory coelurosaurian dinosaurs. Current Biology 28, 1467–1474. [DOI] [PubMed] [Google Scholar]
- Tornberg, R. & Reif, V. (2007). Assessing the diet of birds of prey: a comparison of prey items found in nests and images. Ornis Fennica 84, 21. [Google Scholar]
- Trinajstic, K. , Marshall, C. , Long, J. & Bifield, K. (2007). Exceptional preservation of nerve and muscle tissues in late Devonian placoderm fish and their evolutionary implications. Biology Letters 3, 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, L. R. , Wilson, L. A. B. , Ledogar, J. , Wroe, S. , Attard, M. & Sansalone, G. (2019). Raptor talon shape and biomechanical performance are controlled by relative prey size but not by allometry. Scientific Reports 9, 7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuihiji, T. (2005). Homologies of the transversospinalis muscles in the anterior presacral region of Sauria (crown Diapsida). Journal of Morphology 263, 151–178. [DOI] [PubMed] [Google Scholar]
- Tsuihiji, T. (2007). Homologies of the longissimus, iliocostalis, and hypaxial muscles in the anterior presacral region of extant Diapsida. Journal of Morphology 268, 986–1020. [DOI] [PubMed] [Google Scholar]
- Tsuihiji, T. (2010). Reconstructions of the axial muscle insertions in the occipital region of dinosaurs: evaluations of past hypotheses on Marginocephalia and Tyrannosauridae using the extant phylogenetic bracket approach. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 293, 1360–1386. [DOI] [PubMed] [Google Scholar]
- Turner, K. L. , Abernethy, E. F. , Conner, L. M. , Rhodes, O. E. Jr. & Beasley, J. C. (2017). Abiotic and biotic factors modulate carrion fate and vertebrate scavenging communities. Ecology 98, 2413–2424. [DOI] [PubMed] [Google Scholar]
- Ugural, A. C. & Fenster, S. K. (2012). Advanced Mechanics of Materials and Applied Elasticity, 5th Edition. Pearson Education, London. [Google Scholar]
- Ullrey, D. E. (2018). Nutrient requirements: carnivores. In Encyclopedia of Animal Science (eds Pond W. G., Ullrey D. E. and Baer C. K.), pp. 670–673. CRC Press, Boca Raton. [Google Scholar]
- Ungar, P. S. (2015). Mammalian dental function and wear: a review. Biosurface and Biotribology 1, 25–41. [Google Scholar]
- Ungar, P. S. (2018). Tooth surface topography: a scale‐sensitive approach with implications for inferring dental adaptation and diet. In New Geospatial Approaches to the Anthropological Sciences (eds Anemone R. L. and Conroy G. C.), pp. 101–120. University of New Mexico Press, Albuquerque. [Google Scholar]
- Ungar, P. S. (2019). Inference of diets of early hominins from primate molar form and microwear. Journal of Dental Research 98, 398–405. [DOI] [PubMed] [Google Scholar]
- Ungar, P. S. & Hlusko, L. J. (2016). The evolutionary path of least resistance. Science 353, 29–30. [DOI] [PubMed] [Google Scholar]
- Urano, Y. , Tanoue, K. , Matsumoto, R. , Kawabe, S. , Ohashi, T. & Fujiwara, S. (2018). How does the curvature of the upper beak bone reflect the overlying rhinotheca morphology? Journal of Morphology 279, 636–647. [DOI] [PubMed] [Google Scholar]
- Uyeda, J. C. , Zenil‐Ferguson, R. & Pennell, M. W. (2018). Rethinking phylogenetic comparative methods. Systematic Biology 67, 1091–1109. [DOI] [PubMed] [Google Scholar]
- van Baal, R. R. , Janssen, R. , van der Lubbe, H. J. L. , Schulp, A. S. , Jagt, J. W. M. & Vonhof, H. B. (2013). Oxygen and carbon stable isotope records of marine vertebrates from the type Maastrichtian, The Netherlands and Northeast Belgium (late cretaceous). Palaeogeography, Palaeoclimatology, Palaeoecology 392, 71–78. [Google Scholar]
- van de Flierdt, T. , Robinson, L. F. , Adkins, J. F. , Hemming, S. R. & Goldstein, S. L. (2006). Temporal stability of the neodymium isotope signature of the Holocene to glacial North Atlantic. Paleoceanography 21, PA4102. [Google Scholar]
- van der Leeuw, A. H. J. , Bout, R. G. & Zweers, G. A. (2001). Control of the cranio‐cervical system during feeding in birds. American Zoologist 41, 1352–1363. [Google Scholar]
- van der Meij, M. A. A. (2004). A Tough Nut to Crack. Adaptations to Seed Cracking in Finches. Leiden University, Leiden. [Google Scholar]
- van der Meij, M. A. A. & Bout, R. G. (2004). Scaling of jaw muscle size and maximal bite force in finches. Journal of Experimental Biology 207, 2745–2753. [DOI] [PubMed] [Google Scholar]
- van Valkenburgh, B. & Molnar, R. E. (2002). Dinosaurian and mammalian predators compared. Paleobiology 28, 527–543. [Google Scholar]
- Verner, K. A. , Lehner, M. , Lamas, L. P. & Main, R. P. (2016). Experimental tests of planar strain theory for predicting bone cross‐sectional longitudinal and shear strains. Journal of Experimental Biology 219, 3082–3090. [DOI] [PubMed] [Google Scholar]
- Verwaijen, D. , van Damme, R. & Herrel, A. (2002). Relationships between head size, bite force, prey handling efficiency and diet in two sympatric lacertid lizards. Functional Ecology 16, 842–850. [Google Scholar]
- Vitt, L. J. & Pianka, E. R. (2005). Deep history impacts present‐day ecology and biodiversity. Proceedings of the National Academy of Sciences of the United States of America 102, 7877–7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, S. (2013). Biological materials: cracks and composites. In Comparative Biomechanics: Life's Physical World, pp. 329–346. Princeton University Press, Princeton. [Google Scholar]
- Votier, S. C. , Bearhop, S. , Ratcliffe, N. & Furness, R. W. (2001). Pellets as indicators of diet in great Skuas Catharacta skua . Bird Study 48, 373–376. [Google Scholar]
- Wainwright, P. C. , Alfaro, M. E. , Bolnick, D. I. & Hulsey, C. D. (2005). Many‐to‐one mapping of form to function: a general principle in organismal design? Integrative and Comparative Biology 45, 256–262. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Song, Y. , Song, S. , Ji, Q. , Chiang, C. , Meng, Q. , Li, H. , Hsiao, K. , Lu, Y. & Shew, B. (2015a). Evolution and function of dinosaur teeth at ultramicrostructural level revealed using synchrotron transmission X‐ray microscopy. Scientific Reports 5, 15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Hao, X. , Kundrát, M. , Liu, Z. , Uesugi, K. , Jurašeková, Z. , Guo, B. , Hoshino, M. , Li, Y. & Monfroy, Q. (2019). Bone tissue histology of the early cretaceous bird Yanornis: evidence for a diphyletic origin of modern avian growth strategies within Ornithuromorpha. Historical Biology 32, 1422–1434. [Google Scholar]
- Wang, J. , Zou, D. , Li, Z. , Huang, P. , Li, D. , Shao, Y. , Wang, H. & Chen, Y. (2014a). Mechanical properties of cranial bones and sutures in 1–2‐year‐old infants. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 20, 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Li, Z. , Liu, Q. & Zhou, Z. (2020b). Two new early cretaceous ornithuromorph birds provide insights into the taxonomy and divergence of Yanornithidae (Aves: Ornithothoraces). Journal of Systematic Palaeontology 18, 1805–1827. [Google Scholar]
- Wang, M. , Li, Z. & Zhou, Z. (2017c). Insight into the growth pattern and bone fusion of basal birds from an early cretaceous enantiornithine bird. Proceedings of the National Academy of Sciences of the United States of America 114, 11470–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. & Liu, D. (2016). Taxonomical reappraisal of Cathayornithidae (Aves: Enantiornithes). Journal of Systematic Palaeontology 14, 29–47. [Google Scholar]
- Wang, M. , O'Connor, J. K. & Zhou, Z. H. (2014c). A new robust enantiornithine bird from the lower cretaceous of China with scansorial adaptations. Journal of Vertebrate Paleontology 34, 657–671. [Google Scholar]
- Wang, M. , O'Connor, J. & Zhou, Z. (2019a). A taxonomical revision of the Confuciusornithiformes (Aves: Pygostylia). Vertebrata PalAsiatica 10, 1–37. [Google Scholar]
- Wang, M. , O'Connor, J. K. , Pan, Y. & Zhou, Z. (2017d). A bizarre early cretaceous enantiornithine bird with unique crural feathers and an ornithuromorph plough‐shaped pygostyle. Nature Communications 8, 14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , O'Connor, J. K. , Xu, X. & Zhou, Z. (2019b). A new Jurassic scansoriopterygid and the loss of membranous wings in theropod dinosaurs. Nature 569, 256–259. [DOI] [PubMed] [Google Scholar]
- Wang, M. , O'Connor, J. K. , Zhou, S. & Zhou, Z. (2020d). New toothed early cretaceous ornithuromorph bird reveals intraclade diversity in pattern of tooth loss. Journal of Systematic Palaeontology 18, 631–645. [Google Scholar]
- Wang, M. , Stidham, T. A. & Zhou, Z. (2018a). A new clade of basal early cretaceous pygostylian birds and developmental plasticity of the avian shoulder girdle. Proceedings of the National Academy of Sciences of the United States of America 115, 10708–10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Zheng, X. , O'Connor, J. K. , Lloyd, G. T. , Wang, X. , Wang, Y. , Zhang, X. & Zhou, Z. (2015b). The oldest record of Ornithuromorpha from the early cretaceous of China. Nature Communications 6, 6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. & Zhou, Z. (2016). A new adult specimen of the basalmost ornithuromorph bird Archaeorhynchus spathula (Aves: Ornithuromorpha) and its implications for early avian ontogeny. Journal of Systematic Palaeontology 15, 1–18. [Google Scholar]
- Wang, M. & Zhou, Z. (2017). A morphological study of the first known piscivorous enantiornithine bird from the early cretaceous of China. Journal of Vertebrate Paleontology 37, e1278702. [Google Scholar]
- Wang, M. & Zhou, Z. (2019a). A new confuciusornithid (Aves: Pygostylia) from the early cretaceous increases the morphological disparity of the Confuciusornithidae. Zoological Journal of the Linnean Society 185, 417–430. [Google Scholar]
- Wang, M. & Zhou, Z. (2020). Anatomy of a new specimen of Piscivorenantiornis inusitatus (Aves: Enantiornithes) from the lower cretaceous Jehol biota. Journal of Vertebrate Paleontology 40, e1783278. [Google Scholar]
- Wang, M. , Zhou, Z. , O'Connor, J. K. & Zelenkov, N. V. (2014d). A new diverse enantiornithine family (Bohaiornithidae fam. Nov.) from the lower cretaceous of China with information from two new species. Vertebrata PalAsiatica 52, 31–76. [Google Scholar]
- Wang, M. & Zhou, Z. H. (2019b). A new enantiornithine (Aves: Ornithothoraces) with completely fused premaxillae from the early cretaceous of China. Journal of Systematic Palaeontology 17, 1079–1092. [Google Scholar]
- Wang, M. , Zhou, Z. H. & Sullivan, C. (2016a). A fish‐eating enantiornithine bird from the early cretaceous of China provides evidence of modern avian digestive features. Current Biology 26, 1170–1176. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Zhou, Z. H. & Xu, G. H. (2014e). The first enantiornithine bird from the upper cretaceous of China. Journal of Vertebrate Paleontology 34, 135–145. [Google Scholar]
- Wang, S. , Stiegler, J. , Wu, P. & Chuong, C.‐M. (2020a). Tooth vs beak: the evolutionary developmental control of the avian feeding apparatus. In Pennaraptoran Theropod Dinosaurs: Past Progress and New Frontiers (eds Pittman M. and Xu X.), pp. 205–228. New York, NY: Bulletin of the American Museum of Natural History. [Google Scholar]
- Wang, X. , Zhang, Z. , Gao, C. , Hou, L. , Meng, Q. & Liu, J. (2010a). A new enantiornithine bird from the early cretaceous of Western Liaoning, China. The Condor: Ornithological Applications 112, 432–437. [Google Scholar]
- Wang, X. L. , O'Connor, J. K. , Maina, J. N. , Pan, Y. , Wang, M. , Wang, Y. , Zheng, X. & Zhou, Z. (2018b). Archaeorhynchus preserving significant soft tissue including probable fossilized lungs. Proceedings of the National Academy of Sciences of the United States of America 115, 11555–11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. L. , O'Connor, J. K. , Zheng, X. T. , Wang, M. , Hu, H. & Zhou, Z. H. (2014b). Insights into the evolution of rachis dominated tail feathers from a new basal enantiornithine (Aves: Ornithothoraces). Biological Journal of the Linnean Society 113, 805–819. [Google Scholar]
- Wang, X. L. , Pittman, M. , Zheng, X. , Kaye, T. G. , Falk, A. R. , Hartman, S. A. & Xu, X. (2017a). Basal paravian functional anatomy illuminated by high‐detail body outline. Nature Communications 8, 14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. R. , Cau, A. , Kundrát, M. , Chiappe, L. M. , Ji, Q. , Wang, Y. , Li, T. & Wu, W. (2020c). A new advanced ornithuromorph bird from Inner Mongolia documents the northernmost geographic distribution of the Jehol paleornithofauna in China. Historical Biology 1–13. [Google Scholar]
- Wang, X. R. , Chiappe, L. M. , Teng, F. & Ji, Q. (2013). Xinghaiornis lini (Aves: Ornithothoraces) from the early cretaceous of Liaoning: an example of evolutionary mosaic in early birds. Acta Geologica Sinica (English Edition) 87, 686–689. [Google Scholar]
- Wang, X. R. , Huang, J. , Kundrát, M. , Cau, A. , Liu, X. , Wang, Y. & Ju, S. (2020e). A new jeholornithiform exhibits the earliest appearance of the fused sternum and pelvis in the evolution of avialan dinosaurs. Journal of Asian Earth Sciences 199, 104401. [Google Scholar]
- Wang, X. R. , O'Connor, J. K. , Zhao, B. , Chiappe, L. M. , Gao, C. L. & Cheng, X. D. (2010b). New species of Enantiornithes (Aves: Ornithothoraces) from the Qiaotou formation in northern Hebei, China. Acta Geologica Sinica (English Edition) 84, 247–256. [Google Scholar]
- Wang, X. R. , Zhao, B. , Shen, C. Z. , Liu, S. Z. , Gao, C. L. , Cheng, X. D. & Zhang, F. J. (2015c). New material of Longipteryx (Aves: Enantiornithes) from the lower cretaceous Yixian formation of China with the first recognized avian tooth crenulations. Zootaxa 3941, 565–578. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Hu, H. , O'Connor, J. K. , Wang, M. , Xu, X. , Zhou, Z. , Wang, X. & Zheng, X. (2017b). A previously undescribed specimen reveals new information on the dentition of Sapeornis chaoyangensis . Cretaceous Research 74, 1–10. [Google Scholar]
- Wang, Y. , Wang, M. , O'Connor, J. K. , Wang, X. L. , Zheng, X. T. & Zhang, X. M. (2016b). A new Jehol enantiornithine bird with three‐dimensional preservation and ovarian follicles. Journal of Vertebrate Paleontology 36, e1054496. [Google Scholar]
- Ward, A. B. , Weigl, P. D. & Conroy, R. M. (2002). Functional morphology of raptor hindlimbs: implications for resource partitioning. The Auk: Ornithological Advances 119, 1052–1063. [Google Scholar]
- Warton, D. I. & Hui, F. K. C. (2011). The arcsine is asinine: the analysis of proportions in ecology. Ecology 92, 3–10. [DOI] [PubMed] [Google Scholar]
- Watson, J. (2010). The Golden Eagle. Bloomsbury Publishing, London. [Google Scholar]
- Wei, Z.‐Y. & Li, L. (2011). Discovery of a new enantiornithine bird from lower cretaceous of western Liaoning, China. Global Geology 36, 655–662. [Google Scholar]
- Wellnhofer, P. (2010). A short history of research on Archaeopteryx and its relationship with dinosaurs. Geological Society, London, Special Publications 343, 237–250. [Google Scholar]
- Whetstone, K. N. (1983). Braincase of Mesozoic birds: I. new preparation of the “London” Archaeopteryx . Journal of Vertebrate Paleontology 2, 439–452. [Google Scholar]
- Wilman, H. , Belmaker, J. , Simpson, J. , de la Rosa, C. , Rivadeneira, M. M. & Jetz, W. (2014). EltonTraits 1.0: species‐level foraging attributes of the world's birds and mammals. Ecology 95, 2027. [Google Scholar]
- Wilson, L. E. , Chin, K. & Cumbaa, S. L. (2016). A new hesperornithiform (Aves) specimen from the late cretaceous Canadian high Arctic with comments on high‐latitude hesperornithiform diet. Canadian Journal of Earth Sciences 53, 1476–1483. [Google Scholar]
- Winkler, D. E. , Schulz‐Kornas, E. , Kaiser, T. M. & Tütken, T. (2019). Dental microwear texture reflects dietary tendencies in extant Lepidosauria despite their limited use of oral food processing. Proceedings of the Royal Society B: Biological Sciences 286, 20190544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman, K. D. , Greene, H. W. , Koo, M. S. & Long, D. J. (2019). Feeding ecology of a generalist predator, the California kingsnake (Lampropeltis californiae): why rare prey matter. Herpetological Conservation and Biology 14, 1–30. [Google Scholar]
- Witmer, L. M. (1995). The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In Functional Morphology in Vertebrate Paleontology (ed. Thomason J.), pp. 19–33. Cambridge University Press, Cambridge. [Google Scholar]
- Wood, J. R. , Rawlence, N. J. , Rogers, G. M. , Austin, J. J. , Worthy, T. H. & Cooper, A. (2008). Coprolite deposits reveal the diet and ecology of the extinct New Zealand megaherbivore moa (Aves, Dinornithiformes). Quaternary Science Reviews 27, 2593–2602. [Google Scholar]
- Wroe, S. (2008). Cranial mechanics compared in extinct marsupial and extant African lions using a finite‐element approach. Journal of Zoology 274, 332–339. [Google Scholar]
- Wroe, S. , Clausen, P. , McHenry, C. , Moreno, K. & Cunningham, E. (2007). Computer simulation of feeding behaviour in the thylacine and dingo as a novel test for convergence and niche overlap. Proceedings of the Royal Society B: Biological Sciences 274, 2819–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroe, S. , McHenry, C. & Thomason, J. (2005). Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa. Proceedings of the Royal Society B: Biological Sciences 272, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Chiappe, L. M. , Bottjer, D. J. & Bailleul, A. (2020). Tooth cycling and replacement patterns of Cretaceous ornithuromorph birds. In The Society of Vertebrate Paleontology 80th Annual Meeting, pp. 354–355, Virtual Conference.
- Xia, D. , Kyes, R. C. , Wang, X. , Sun, B. , Sun, L. & Li, J.‐H. (2019). Grooming networks reveal intra‐ and intersexual social relationships in Macaca thibetana . Primates 60, 223–232. [DOI] [PubMed] [Google Scholar]
- Xing, L. , Bell, P. R. , Persons, W. S. , Ji, S. A. , Miyashita, T. , Burns, M. E. , Ji, Q. & Currie, P. J. (2012). Abdominal contents from two large early cretaceous compsognathids (Dinosauria: Theropoda) demonstrate feeding on confuciusornithids and dromaeosaurids. PLoS One 7, e44012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, L. , O'Connor, J. K. , McKellar, R. C. , Chiappe, L. M. , Tseng, K. , Li, G. & Bai, M. (2017). A mid‐cretaceous enantiornithine (Aves) hatchling preserved in Burmese amber with unusual plumage. Gondwana Research 49, 264–277. [Google Scholar]
- Xing, L. , Persons, W. S. IV , Bell, P. R. , Xu, X. , Zhang, J. , Miyashita, T. , Wang, F. & Currie, P. J. (2013). Piscivory in the feathered dinosaur Microraptor . Evolution 67, 2441–2445. [DOI] [PubMed] [Google Scholar]
- Xu, X. , You, H. , Du, K. & Han, F. (2011). An Archaeopteryx‐like theropod from China and the origin of Avialae. Nature 475, 465–470. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Zheng, X. , Sullivan, C. , Wang, X. , Xing, L. , Wang, Y. , Zhang, X. , O'Connor, J. K. , Zhang, F. & Pan, Y. (2015). A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature 521, 70–73. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Zhou, Z. , Wang, Y. & Wang, M. (2020). Study on the Jehol biota: recent advances and future prospects. Science China Earth Sciences 63, 757–773. [Google Scholar]
- Xu, X. , Zhou, Z. H. , Dudley, R. , Mackem, S. , Chuong, C. M. , Erickson, G. M. & Varricchio, D. J. (2014). An integrative approach to understanding bird origins. Science 346, 1253293. [DOI] [PubMed] [Google Scholar]
- Yang, T. R. & Sander, P. M. (2018). The origin of the bird's beak: new insights from dinosaur incubation periods. Biology Letters 14, 20180090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosibash, Z. , Tal, D. & Trabelsi, N. (2010). Predicting the yield of the proximal femur using high‐order finite‐element analysis with inhomogeneous orthotropic material properties. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 368, 2707–2723. [DOI] [PubMed] [Google Scholar]
- Young, N. M. , Wagner, G. P. & Hallgrímsson, B. (2010). Development and the evolvability of human limbs. Proceedings of the National Academy of Sciences of the United States of America 107, 3400–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, C. (2008). A new genus and species of Sapeornithidae from lower cretaceous in western Liaoning, China. Acta Geologica Sinica (English Edition) 82, 48–55. [Google Scholar]
- Zani, P. A. (2000). The comparative evolution of lizard claw and toe morphology and clinging performance. Journal of Evolutionary Biology 13, 316–325. [Google Scholar]
- Zanno, L. E. & Makovicky, P. J. (2011). Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. Proceedings of the National Academy of Sciences of the United States of America 108, 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelditch, M. L. , Swiderski, D. L. , Sheets, H. D. & Fink, W. L. (2004). Geometric Morphometrics for Biologists: A Primer, 1st Edition. Academic Press, San Diego. [Google Scholar]
- Zelenkov, N. V. & Averianov, A. O. (2016). A historical specimen of enantiornithine bird from the early cretaceous of Mongolia representing a new taxon with a specialized neck morphology. Journal of Systematic Palaeontology 14, 319–338. [Google Scholar]
- Zhang, F. , Ericson, P. G. P. & Zhou, Z. (2004). Description of a new enantiornithine bird from the early cretaceous of Hebei, northern China. Canadian Journal of Earth Sciences 41, 1097–1107. [Google Scholar]
- Zhang, F. , Zhou, Z. & Benton, M. J. (2008a). A primitive confuciusornithid bird from China and its implications for early avian flight. Science in China Series D: Earth Sciences 51, 625–639. [Google Scholar]
- Zhang, F. , Zhou, Z. , Xu, X. & Wang, X. (2002). A juvenile coelurosaurian theropod from China indicates arboreal habits. Naturwissenschaften 89, 394–398. [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Zhou, Z. , Xu, X. , Wang, X. & Sullivan, C. (2008b). A bizarre Jurassic maniraptoran from China with elongate ribbon‐like feathers. Nature 455, 1105–1108. [DOI] [PubMed] [Google Scholar]
- Zhang, J. J. , Rayner, M. , Vickers, S. , Landers, T. , Sagar, R. , Stewart, J. & Dunphy, B. (2019). GPS telemetry for small seabirds: using hidden Markov models to infer foraging behaviour of common diving petrels (Pelecanoides urinatrix urinatrix). Emu ‐ Austral Ornithology 119, 126–137. [Google Scholar]
- Zhang, Y. G. , O'Connor, J. , Di, L. , Meng, Q. J. , Sigurdsen, T. & Chiappe, L. M. (2014). New information on the anatomy of the Chinese early cretaceous Bohaiornithidae (Aves: Enantiornithes) from a subadult specimen of Zhouornis hani . PeerJ 2, e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. H. , Chiappe, L. M. , Han, G. & Chinsamy‐Turan, A. (2013). A large bird from the early cretaceous of China: new information on the skull of enantiornithines. Journal of Vertebrate Paleontology 33, 1176–1189. [Google Scholar]
- Zhang, Z. H. , Hou, L. H. , Yoshikasu, H. , O'Connor, J. , Martin, L. D. & Chiappe, L. M. (2006). The first Mesozoic heterodactyl bird from China. Acta Geologica Sinica (English Edition) 80, 631–635. [Google Scholar]
- Zheng, X. , Martin, L. D. , Zhou, Z. , Burnham, D. A. , Zhang, F. & Miao, D. (2011). Fossil evidence of avian crops from the early cretaceous of China. Proceedings of the National Academy of Sciences of the United States of America 108, 15904–15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , O'Connor, J. K. , Huchzermeyer, F. , Wang, X. , Wang, Y. , Zhang, X. & Zhou, Z. (2014). New specimens of Yanornis indicate a piscivorous diet and modern alimentary canal. PLoS One 9, e95036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , O'Connor, J. K. , Wang, X. , Wang, Y. & Zhou, Z. (2018a). Reinterpretation of a previously described Jehol bird clarifies early trophic evolution in the Ornithuromorpha. Proceedings of the Royal Society B: Biological Sciences 285, 20172494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , O'Connor, J. , Huchzermeyer, F. , Wang, X. , Wang, Y. , Wang, M. & Zhou, Z. (2013). Preservation of ovarian follicles reveals early evolution of avian reproductive behaviour. Nature 495, 507–511. [DOI] [PubMed] [Google Scholar]
- Zheng, X. , O'Connor, J. K. , Wang, Y. , Wang, X. , Xuwei, Y. , Zhang, X. & Zhou, Z. (2020). New information on the keratinous beak of Confuciusornis (Aves: Pygostylia) from two new specimens. Frontiers in Earth Science 8, 367. [Google Scholar]
- Zheng, X. , O'Connor, J. K. , Wang, X. , Pan, Y. , Wang, Y. , Wang, M. & Zhou, Z. (2017). Exceptional preservation of soft tissue in a new specimen of Eoconfuciusornis and its biological implications. National Science Review 4, 441–452. [Google Scholar]
- Zheng, X. , Wang, X. , Sullivan, C. , Zhang, X. , Zhang, F. , Wang, Y. , Li, F. & Xu, X. (2018b). Exceptional dinosaur fossils reveal early origin of avian‐style digestion. Scientific Reports 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X. , Zhang, Z. & Hou, L. (2007). A new enantiornitine bird with four long rectrices from the early cretaceous of northern Hebei, China. Acta Geologica Sinica (English Edition) 81, 703–708. [Google Scholar]
- Zhou, S. , Zhou, Z. & O'Connor, J. (2014). A new piscivorous ornithuromorph from the Jehol biota. Historical Biology 26, 608–618. [Google Scholar]
- Zhou, S. , Zhou, Z. & O'Connor, J. K. (2012). A new basal beaked ornithurine bird from the lower cretaceous of western Liaoning, China. Vertebrata PalAsiatica 50, 9–24. [Google Scholar]
- Zhou, S. , Zhou, Z. & O'Connor, J. K. (2013). Anatomy of the basal ornithuromorph bird Archaeorhynchus spathula from the early cretaceous of Liaoning, China. Journal of Vertebrate Paleontology 33, 141–152. [Google Scholar]
- Zhou, Z. (1999). Early Evolution of Birds and Avian Flight: Evidence from Mesozoic Fossils and Modern Birds. University of Kansas, Lawrence. [Google Scholar]
- Zhou, Z. , Barrett, P. M. & Hilton, J. (2003). An exceptionally preserved lower cretaceous ecosystem. Nature 421, 807–814. [DOI] [PubMed] [Google Scholar]
- Zhou, Z. & Hou, L. (2002). The discovery and study of Mesozoic birds in China. In Mesozoic Birds: Above the Heads of Dinosaurs (eds Chiappe L. M. and Witmer L. M.), pp. 160–183. University of California Press, Berkeley and Los Angeles. [Google Scholar]
- Zhou, Z. & Li, F. Z. Z. (2010). A new lower cretaceous bird from China and tooth reduction in early avian evolution. Proceedings of the Royal Society B: Biological Sciences 277, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. , Winkler, D. E. , Fortuny, J. , Kaiser, T. M. & Marcé‐Nogué, J. (2019). Why ruminating ungulates chew sloppily: biomechanics discern a phylogenetic pattern. PLoS One 14, e0214510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. & Zhang, F. (2002). A long‐tailed, seed‐eating bird from the early cretaceous of China. Nature 418, 405–409. [DOI] [PubMed] [Google Scholar]
- Zhou, Z. & Zhang, F. (2003). Anatomy of the primitive bird Sapeornis chaoyangensis from the early cretaceous of Liaoning, China. Canadian Journal of Earth Sciences 40, 731–747. [Google Scholar]
- Zhou, Z. & Zhang, F. (2005). Discovery of an ornithurine bird and its implication for early cretaceous avian radiation. Proceedings of the National Academy of Sciences of the United States of America 102, 18998–19002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. & Zhang, F. (2006). A beaked basal ornithurine bird (Aves, Ornithurae) from the lower cretaceous of China. Zoologica Scripta 35, 363–373. [Google Scholar]
- Zhou, Z. , Zhang, F. & Li, Z. (2009). A new basal ornithurine bird (Jianchangornis microdonta gen. Et sp. nov.) from the lower cretaceous of China. Vertebrata PalAsiatica 47, 299–310. [Google Scholar]
- Zhou, Z. H. , Chiappe, L. M. & Zhang, F. C. (2005). Anatomy of the early cretaceous bird Eoenantiornis buhleri (Aves: Enantiornithes) from China. Canadian Journal of Earth Sciences 42, 1331–1338. [Google Scholar]
- Zhou, Z. H. , Clarke, J. & Zhang, F. C. (2008). Insight into diversity, body size and morphological evolution from the largest early cretaceous enantiornithine bird. Journal of Anatomy 212, 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. H. , Clarke, J. , Zhang, F. C. & Wings, O. (2004). Gastroliths in Yanornis: an indication of the earliest radical diet‐switching and gizzard plasticity in the lineage leading to living birds? Naturwissenschaften 91, 571–574. [DOI] [PubMed] [Google Scholar]
- Zinoviev, A. V. (2009). An attempt to reconstruct the lifestyle of confuciusornithids (Aves, Confuciusornithiformes). Paleontological Journal 43, 444–452. [Google Scholar]
- Zuber, M. , Laaß, M. , Hamann, E. , Kretschmer, S. , Hauschke, N. , van de Kamp, T. , Baumbach, T. & Koenig, T. (2017). Augmented laminography, a correlative 3D imaging method for revealing the inner structure of compressed fossils. Scientific Reports 7, 41413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zum Gahr, K.‐H. (1998). Wear by hard particles. Tribology International 31, 587–596. [Google Scholar]
- Zusi, R. L. (1984). A functional and evolutionary analysis of rhynchokinesis in birds. Smithsonian Contributions to Zoology 395, 1–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Listing of published non‐avian avialan skulls.
Fig. S1. Principal component analysis (PCA) of avian pedal measurements from Fowler et al. (2009, 2011).
Fig. S2. Principal component analysis of theropod skulls from Foth & Rauhut (2013).
Table S2. Table summarising all dietary recoding used in Fig. S2.
Fig. S3. Principal component analysis of theropod skulls from Schaeffer et al. (2019).
Fig. S4. Relationship between mechanical advantage and plant consumption in passerines.
Fig. S5. Biomechanical morphospace of Dinosauria from Button & Zanno (2020).
Table S4. Calculation of cranial connective properties of Shenqiornis.


