Abstract
BACKGROUND:
Although the concept of penile rehabilitation post-radical prostatectomy (RP) has been advocated for decades, there is little definitive evidence regarding its utility or the best strategy to optimize patient outcomes.
AIM:
The goal of this study is to analyze the ability of 3 different pharmacological strategies to preserve the ability of men to achieve spontaneous (non-medication assisted) erections after bilateral nerve-sparing RP.
METHODS:
This IRB and FDA approved study studied penile rehabilitation in a three-arm fashion with a target enrollment of 200 patients. (i) Control Arm: nightly placebo with sildenafil 100mgs on demand for sexual relations (up to 6 pills/month); (ii) Nightly Sildenafil Arm: nightly sildenafil 50mgs and sildenafil 100mgs on demand for sexual relations (up to 6 pills/month); (iii) Combination Therapy Arm: nightly sildenafil 50mgs (5 nights/week) plus intracavernosal injections (ICI) twice/week. Inclusion criteria included: bilateral nerve sparing surgery, normal serum total testosterone and good preoperative baseline erectile function as measured by the erectile function domain score of the IIEF (EFD) (≥24). Patients were followed with a medication use diary and the IIEF questionnaire at 6 weeks, 3m, 6m, 12m, 18m and 24m.
OUTCOMES MEASURES:
Difference in the IIEF-EFD scores between the 3 groups at 24 months post-RP. Secondary end-points include the time to return of spontaneous functional erections, the time for patients to respond to oral erectogenic therapy and the proportion of patients who have normalization of their IIEF-EFD scores.
RESULTS:
The study was interrupted because of failure to recruit the target study population in a reasonable timeframe. A total of 76 subjects with median age of 57 (IQR: 51, 63) years, mean IIEF-EFD of 29 (IQR: 27, 30) were initially randomized, but at 24 months, the sample sizes by group were: (i) n=4; (ii) n=18; (iii) n=10, with median IIEF-EFD 24 (IQR: 18, 28), 24 (IQR: 18, 28), and 21 (IQR: 9, 26) respectively. There was no statistical difference among the groups in the final analysis.
CLINICAL IMPLICATIONS:
Definitive evidence for the ability of different pharmacological rehabilitation strategies to improve long-term EF outcomes might never be available.
STRENGTHS AND LIMITATIONS:
This was a well designed randomized and 3-arm designed trial intended to provide decisive evidence regarding the utility of penile rehabilitation. Failure to recruit the target population is the main limitation.
CONCLUSION:
The limited number of patients in the present trial precludes definitive interpretation. However, results indicate how challenging it is to conduct true rehabilitation studies.
Keywords: Rehabilitation, Radical Prostatectomy, Erectile Dysfunction, Phosphodiesterase 5 Inhibitors, Intracavernosal Injections, Penile rehabilitation, PDE-5 inhibitors
INTRODUCTION
Radical prostatectomy (RP) is a first-line therapy option for localized or locally advanced prostate cancer (1). Despite advancements in surgical technique and care, erectile dysfunction (ED) remains a common complication, with prevalence rates ranging from 20–90% (2). This great discrepancy is a result of heterogeneous patient populations and ED definitions used in the studies. However, there is still a search for an optimal strategy to improve long-term sexual outcomes in this population.
The concept of penile rehabilitation post-RP was introduced more than 20 years ago although study data are mixed with only one randomized control trial (RCT) supporting the use of regular PDE5 inhibitor for the optimization of erectile function recovery and preservation of baseline erectile function. The principle of rehabilitation is believed to revolve around utilizing strategies to maintain oxygenated blood flow to the penis, which has been suggested may minimize smooth muscle collagenization.
Of the three large industry sponsored studies only the sildenafil trial had positive results with the daily use of sildenafil 50mg or 100mg yielding a 7-fold increase in the chance of returning to preoperative erectile function (3). The vardenafil and tadalafil trials failed to demonstrate a value to daily use of drugs over on-demand use, although the study designs have been criticized as not representing a true rehabilitation trial (4, 5).
Given this controversy, the goal of this trial was to analyze the impact of 3 different pharmacological strategies (using sildenafil, and intracavernosal injections) on preserving non-medication assisted erectile function (EF) after RP.
METHODS
Study Population:
Patients scheduled for nerve sparing RP were screened for inclusion. The inclusion criteria included (i) bilateral nerve sparing surgery, (ii) normal early morning total testosterone levels (>300 ng/dL), (iii) baseline functional erections (International Index of Erectile Function, erectile function domain score ≥24). Nerve sparing was graded intraoperatively by the surgeon using a 4-point scale as follows: 1 complete preservation indicating full nerve sparing; 2 near-complete preservation representing minor nerve damage; 3 incomplete preservation indicating significant but sub-total nerve damage; 4 no preservation representing complete nerve resection. The total score (right + left sides) was calculated and only men with grade 1 or 2 on both sides were candidates for inclusion. Exclusion criteria included: preoperative or planned postoperative pelvic radiation therapy, preoperative or planned postoperative androgen deprivation, presence of Peyronie’s disease at baseline, presence of a penile prosthesis at baseline, resection of one or both nerve bundles at surgery, and any contraindications to sildenafil (nitrate use, retinitis pigmentosa, macular degeneration, MI or CVA within 3 months) or Trimix use (use of MAOI medications).
Approval & Randomization:
The study (NCT00955929; IRB approval #09–005) struggled through a 3-year process to obtain institutional IRB and US Food and Drug Administration (FDA) approval. The FDA initially had concerns about the use of phentolamine in our injection mixtures (trimix, bimix). This concern was based on the link between the use of oral phentolamine in a previous ED trial and the development of brown fat tumors in rats. After extensive discussions and demonstrating the absence of any data for such tissue changes in either animal or human intracavernosal injection models, we were granted approval to use this agent in our injection mixtures. Viagra (sildenafil citrate, Pfizer Inc Ny USA) and the placebo were supplied by Pfizer as was the cost of the research personnel. Prior to RP, patients were assigned a study number unrelated to their medical record number and these numbers were randomized into one of 3 groups (i) Control Arm: nightly placebo to be started within 48 hours of the surgical procedure with sildenafil 100mgs on demand for sexual relations (up to 6 pills/month) according to their ability to resume confortable sexual activity; (ii) Nightly Sildenafil Arm: nightly sildenafil 50mgs and sildenafil 100mgs on demand for sexual relations (up to 6 pills/month); (iii) Combination Therapy Arm: nightly sildenafil 50mgs (5 nights/week) plus intracavernosal injections (ICI) twice per week. Randomization was in a 1:2:2 ratio. The study had a target enrollment of 200 subjects. The study protocol is illustrated in Figure 1.
Figure 1:
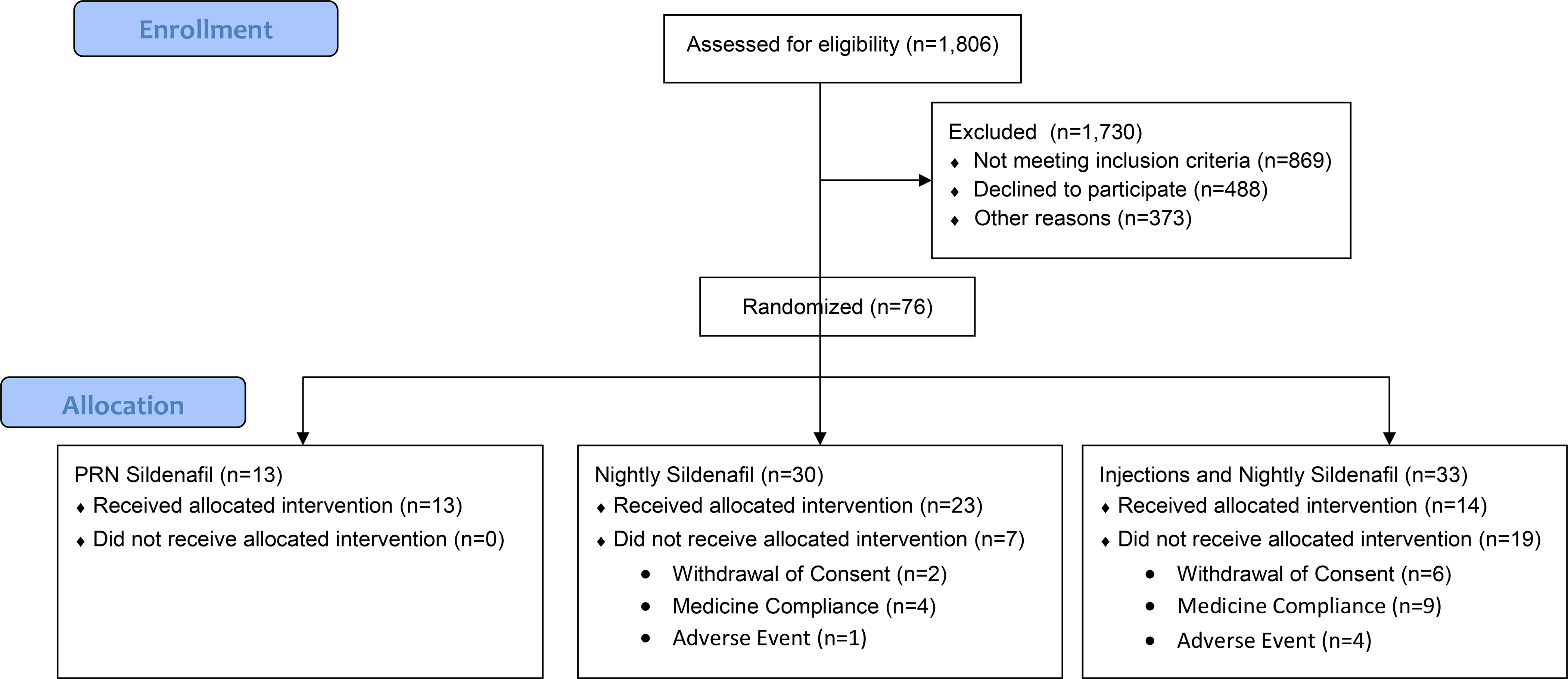
Study Protocol
CONSORT Flow Diagram
Erectile Function Assessment:
The IIEF-EFD questionnaire was administered at each clinic visit pre-RP and 6 weeks, 3, 6, 12, 18, 24 months postoperatively. Patients were evaluated for any side effects since last follow up, were given the IIEF-EFD, were asked to return their sexual function diary (recording sexual encounters, whether vaginal penetration occurred and the resultant erectile response, irrespective of whether sildenafil or ICI was used).
Statistics:
Power was calculated as follows: for each endpoint 3 analyses were conducted: 1) a comparison of nightly sildenafil to PRN sildenafil with an alpha of 2.5%; 2) a comparison of combination treatment to PRN sildenafil with an alpha of 2.5%; 3) a non-inferiority comparison between nightly sildenafil and combination treatment: in this analysis, nightly sildenafil would be considered non-inferior to combination treatment if the upper bound of a 90% confidence interval for the difference between groups does not include combination treatment being at least three points superior to nightly sildenafil. Our primary endpoint was the IIEF-EFD score at 24 months post-RP. Based on previous studies, we estimated that the SD for the IIEF-EFD was 9 points. If we could obtain data from 80 patients on each of the two active arms (nightly sildenafil and combination therapy) and 40 patients on the control arm (PRN sildenafil), we would have 89% power for the comparison between active and control and 80% power for the non-inferiority comparison between active arms. All analyses were based on the intention-to-treat principle with patients analysed in their randomized groups regardless of the treatment actually received. All comparisons between combination and PRN sildenafil, and between nightly and PRN sildenafil, were supposed to use a 2.5% alpha; all comparisons between the two treatment arms used a 90% non-inferiority bound.
RESULTS
Study Enrollment:
According to our institutional caseload, enrollment was expected to be completed within approximately 3 years. However, patient accrual was much more difficult than previous planned, as only 13% of eligible candidates were interested in taking part in the study. Of 1806 men scheduled to undergo nerve-sparing RP, 1730 (95%) were excluded: 869 (50%) not meeting inclusion criteria, 488 (28%) refusing to participate, and 373 (22%) were not approached for study participation for other reasons such as per clinician judgment, follow-up locally, etc. Many refused to participate because of the existence of the control arm, stating that they wished to pursue the ‘usual plan of care” used at the institution, which included PDE5i use early after RP and ICI if maximum dose PDE5i failed to generate penetration hardness as early as 6 weeks post-RP. Others only become interested in the study after the RP was done, when they experienced ED for the first time, but were disinterested preoperatively when their erectile function was intact. Furthermore, there was a considerable number of subjects who failed screening due to low T levels at baseline (pre-RP). The trial was reviewed by the data safety monitoring committee (DSMC) at our institution every 6 months. 3 years after the study initiation, only 39 patients having been enrolled, it was estimated that another 6 years would be required to complete patient recruitment, which led to the DSMC to prematurely close the trial.
Study Population:
A total of 76 subjects were randomized: Control Arm, n = 13; Nightly Sildenafil Arm, n = 30; Combination Therapy Arm, n = 33. Median age at prostatectomy was 57 (IQR: 51, 63) years and median baseline IIEF-EFD score was 29 (IQR: 27, 30). There were no differences among groups in intraoperative nerve sparing status, 97% had bilateral nerve sparing, 2% had unilateral nerve sparing, and 1% has no nerve sparing. In the overall study cohort, 53% of patients had hypertension; 8% had diabetes, 45% had dyslipidemia, 17% had sleep apnea, and 42% of patients had a smoking history. Patient characteristics by group can be found in Table 1.
Table 1:
Patient Demographics
| PRN Sildenafil (N=13; 17%) |
Nightly Sildenafil (N=30; 39%) |
Combination Therapy (N=33; 43%) |
|
|---|---|---|---|
| Age at surgery | 62 (57, 66) | 56 (50, 60) | 56 (51, 63) |
| Nerve sparing status | |||
| None | 0 (0%) | 0 (0%) | 1 (3.0%) |
| Bilateral | 13 (100%) | 29 (97%) | 31 (94%) |
| Unilateral | 0 (0%) | 1 (3.3%) | 1 (3.0%) |
| Pathologic Stage (N=75) | |||
| <pT3 | 9 (69%) | 19 (66%) | 18 (55%) |
| ≥pT3 | 4 (31%) | 10 (34%) | 15 (45%) |
| Hypertension | 8 (62%) | 19 (63%) | 13 (39%) |
| Hyperlipidemia | 5 (38%) | 15 (50%) | 14 (42%) |
| Diabetes | 2 (15%) | 2 (6.7%) | 2 (6.1%) |
| Sleep apnea | 1 (7.7%) | 8 (27%) | 4 (12%) |
| Smoking status (N=71) | |||
| Never | 8 (67%) | 16 (53%) | 15 (52%) |
| Current | 0 (0%) | 4 (13%) | 2 (6.9%) |
| Former | 4 (33%) | 10 (33%) | 12 (41%) |
Erectile Function Outcomes:
At 24 months, the sample sizes by group were: Control Arm =4; Nightly Sildenafil Arm =18; Combination Therapy Arm =10, totaling 32 patients in the trial. Median IIEF-scores from each individual group is demonstrated in Table 2. As there was no difference in median EFD scores between Nightly Sildenafil and Combination Therapy Arms (p=0.4), they were combined into a single Rehabilitation Group for statistical analysis. Comparisons were run between this group and the Control Arm with mean IIEF-EFD scores of 20.4 (SD=9) and 22.8 (SD=6), respectively. The mean IIEF-EFD difference at 24 months was 2.4, 95%CI −6.9 to 11.7, p = 0.6.
Table 2.
Overview of IIEF scores from each group at every time-point analysed in the study.
| PRN Sildenafil (N=13; 17%) |
Nightly Sildenafil (N=30; 39%) |
Combination Therapy (N=33; 43%) |
P-Values | |
|---|---|---|---|---|
| Baseline IIEF (N=73) | 28 (25, 30) | 30 (29, 30) | 29 (26, 30) | 0.08 |
| 3-month IIEF (N=54) | 7 (5, 11) | 16 (8, 28) | 27 (20, 29) | 0.001 |
| 6-month IIEF (N=51) | 8 (6, 15) | 20 (7, 25) | 24 (13, 28) | 0.04 |
| 9-month IIEF (N=50) | 12 (7, 21) | 21 (12, 29) | 26 (17, 28) | 0.04 |
| 12-month IIEF (N=45) | 14 (9, 22) | 23 (16, 28) | 27 (9, 29) | 0.2 |
| 18-month IIEF (N=33) | 19 (10, 23) | 23 (20, 29) | 21 (13, 24) | 0.3 |
| 24-month IIEF (N=32) | 24 (18, 28) | 24 (18, 28) | 21 (9, 26) | 0.6 |
Data are shown as medians and IQR (percentile 25, percentile 75).
DISCUSSION
RP is a well-established option for the treatment of prostate cancer. Although the procedure is relatively safe and long-term oncologic outcomes are satisfactory, post-RP ED remains the most common postoperative complication with the prevalence reaching between 20–90%(6). ED has a negative impact on patients’ quality of life and unfortunately there is no consensus regarding the best postoperative strategy to prevent or minimize it. (7, 8)
It has been suggested that ED following RP is a result of intraoperative nerve manipulation leading to neuropraxia (8), which leads to apoptotic and fibrotic changes in cavernosal smooth muscle. Penile rehabilitation involves strategies to maintain erectile tissue health, perhaps through oxygenation, thus, helping prevent penile collagenization (9). Such strategies usually consist of erectogenic pharmacotherapy including but not limited to PDE5i and/or intracavernosal injections.
Previous experimental studies in the rat cavernous nerve injury model have demonstrated that the use of PDE5i before or at the time of nerve trauma can effectively reduce smooth muscle collagenization, preserve endothelial structure and lead to improvement in erectile hemodynamics (10–12), supporting the basic concepts of rehabilitation. Observational studies in human subjects have also confirmed a possible benefit of regular medication use to protect the erectile tissue against the deleterious effect of cavernous nerve damage following RP (13–15).
However, randomized, placebo-controlled trials have not been able to confirm a clear benefit. Three such trials, all industry sponsored, have been conducted in an attempt to define the impact of PDE5i on erectile function recovery post-RP. In a Pfizer sponsored trial, 76 men were randomized to 100mg sildenafil daily vs 50mg daily vs placebo daily. Pooled data on the sildenafil arms demonstrated a 7-fold increase in the likelihood of men returning to their baseline erectile function. While statistically significant changes in IIEF-EFD score were demonstrated, the small patient number raised concerns about the validity of the data. This was followed by trials assessing vardenafil (REINVENT trial; Bayer-GSK) and tadalafil (REACCT; Lilly). In these two trials, which were almost identically designed, subjects were randomized to receive the medication daily plus placebo on-demand for sexual activity (DAILY group), or daily placebo plus medication on demand for sexual activity (ON DEMAND group), or daily placebo plus placebo on demand for sexual activity (PLACEBO group). Erectile function was assessed in response to agent administered for sexual activity (9 months), placebo (11 months) and maximum dosed medication (13 months) (4, 5). Although these multicenter RCTs were carefully designed by world experts and enrolled large numbers of participants, they failed to demonstrate a significant benefit and the authors declared that the results supported the use of PDE5i in an on-demand fashion. However, several methodological limitations (16), including failure to follow patient beyond 13 months post-RP, use of a low IIEF-EFD cut-off (22) and the use of a large number of centers without surgeon stratification, have raised concerns about the ability of these trials to answer the ‘rehabilitation question’. In addition, these studies evaluated only the utility of PDE5i alone, while there is evidence of a possible benefit of intracavernosal injection therapy (ICI) (14, 17, 18).
The use of ICI in the setting of penile rehabilitation has been poorly investigated in the literature and consists only of observational studies or small trials. In a study by Montorsi et al. in the pre-sildenafil era, 30 individuals were randomized to receiving ICI or no treatment and those in the treatment arm had better recovery of spontaneous erections 6 months after RP(14). Mulhall et al. evaluated men after RP, and in a non-randomized fashion demonstrated that rehabilitation (2) have a much greater likelihood of achieving intercourse without PDE5i, with PDE5i and with ICI (13). Thus, no previous study has adequately investigated the role of ICI in preserving long-term erectile function following RP.
The present study was carefully designed and sufficiently powered at the outset in an attempt to provide definitive evidence regarding the utility of PDE5i and ICI in the setting of penile rehabilitation post-RP. The most salient point to this analysis is that it highlights a number of critically important points in the conduct of such trials. Firstly, here were several enrollment challenges that had to be overcome. As a result of the low enrollment it is not possible to answer any of the a priori posed questions. Indeed, our institutional Data Safety Monitoring Board terminated the study after 3 years due to the slow and low enrollment. The reasons for low enrollment are several. Many patients are usually struggling with the cancer diagnosis and tend not to be focused on their long-term EF but on their oncological control. Additionally, inviting patients to start early post-RP penile injection therapy is usually not the most palatable strategy for patients to consider prior to their RP. As many rehabilitation strategies have already been used in clinical practice for years, patients were reticent to participate in a trial with a placebo arm and instead were requesting to receive the rehabilitation strategy that was in daily use in our clinical practice. Add to this, the fact that our inclusion criteria were very strict. Patients were supposed to be performing bilateral nerve-sparing surgery, have good baseline erectile function (IIEF ≥24) and normal T levels. 58% of patients initially screened were excluded from the study because of abnormal T levels alone. All these issues have contributed to the fact that in the present trial most patients failed screening or were simply not interested or were lost to follow-up.
Although different strategies to facilitate participant recruitment in randomized trials have been reported, it can still be extremely challenging even for well-designed and fully–funded studies in different medical disciplines (19). In a RCT comparing pediatric general anesthesia to spinal anesthesia, out of 4023 patients initially screened in 7 countries from 2007 through 2013, only 722 (18%) were eventually enrolled (20). The reasons for exclusion were numerous and varied among countries, but the United States (US) had the highest rate (70%) of surgeon or parental refusals. Other studies have also identified insufficient accrual as the most common reason for trial closure in a review of 694 head and neck cancer trials identified on ClinicalTrials.gov (21).
Secondly, keeping patients enrolled over a 24 month time period was very difficult. We ultimately screened 1806 patients but only 76 started the trial and only 45 and 32 completed the 12 and 24-month follow-ups assessments respectively. Previous research has also suggested that maintaining patients in clinical trials is challenging (22, 23) and might vary among different medical specialties (24). It is a fact that a substantial proportion of subjects might discontinue their participation in any given RCT (25). A review of RCTs in chronic heart failure with >400 participants reported that as much as 35% of participants are usually lost to follow-up (27). A retrospective analysis of 10 RCTs involving common eye disease leading to blindness reported that 45% failed to complete the study (22). Again it appears that these numbers may differ in different locations because of cultural or situational issues. A trial evaluating 2,447 adult trauma patients reported that participants who received treatment in the US were 3.5 times more likely to be lost to follow-up than those who received treatment in other countries (26).
Thirdly, study approval took a full 3 years with numerous discussions with the FDA and IRB approval challenges, especially pertaining to the use of ICI in this population. The FDA expressed concern regarding the use of intracavernosal phentolamine because of data linking the use of oral phentolamine in rats to the development of brown fat tumors (28), despite the fact that no study in humans has reported such findings (29–31). In contrast, an open-label study involving more than 2000 patients receiving oral phentolamine for up to 13 months reported only mild or moderate adverse events that were associated with the drug’s pharmacological properties (31), suggesting that such treatment is safe in the treatment of individuals with ED.
An interesting point to highlight is that recovery of functional erections is not the sole purpose of a penile rehabilitation program. Data from the REACTT trial and from a prospective observational study have provided evidence that rehabilitation may preserve penile length (5, 32). In the REACTT trial, patients using tadalafil once daily (n=139/423) had reduced penile length loss in comparison to placebo (n=141/423) at 9 months, with a mean difference in length preservation of 4.1 mm. The prospective non-randomized study with 118 patients demonstrated that those with regular PDE5i use had no significant length loss 6 months after RP while those without it had a mean length loss of 4.4 ± 6.6 mm.
Even though no statistical significance was found in the main outcomes measures, assessment of early postoperative IIEF scores may provide interesting insights. Patients on nightly sildenafil have demonstrated a quicker recovery of their EF in comparison to the PRN sildenafil group (Table 2). In addition, patients using ICI were better able to maintain their baseline function, highlighting the potential role of such strategy to reduce the burden of early postoperative ED. On the other hand, IIEF scores from the combination group decreased over time, which suggests that an ICI schedule might have limited adherence in the long term. Although these concepts could be used in favor of rehabilitation strategies, caution is advisable because the numbers are small and statistically nonsignificant. In fact, the overall EF recovery at 24 months in the study was outstanding as a result of strict inclusion criteria consisting of normal baseline EF and excellent nerve-sparing status. In this scenario it is possible that many patients would be able to achieve reasonable long-term EF regardless of any specific pharmacotherapy. Perhaps, penile rehabilitation could have more meaningful implications with poorer prognostic features.
Ultimately, the failure to recruit the target population number has prevented us from drawing definitive conclusions on the efficacy of the rehabilitation strategies employed. Nevertheless, this aborted trial contains an important lesson to the field of sexual medicine and the whole scientific community.
Finally, it is likely that we will have to accept the available observational and experimental studies as the basis for penile rehabilitation. It is not likely that another large scale RCT will be done in this area given the cost of the study and above methodological challenges.
CONCLUSION
The limited number of patients in the present trial precludes definitive interpretation. While the study struggled to complete enrollment, the trial had to be closed prematurely. However, results indicate how challenging it is to conduct true rehabilitation studies and consists of an important lesson to the field of sexual medicine.
REFERENCES
- 1.Fode M, Ohl DA, Ralph D, Sonksen J. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU international. 2013;112(7):998–1008. [DOI] [PubMed] [Google Scholar]
- 2.Mulhall JP. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. The Journal of urology. 2009;181(2):462–71. [DOI] [PubMed] [Google Scholar]
- 3.Padma-Nathan H, McCullough AR, Levine LA, Lipshultz LI, Siegel R, Montorsi F, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. International journal of impotence research. 2008;20(5):479–86. [DOI] [PubMed] [Google Scholar]
- 4.Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. European urology. 2008;54(4):924–31. [DOI] [PubMed] [Google Scholar]
- 5.Montorsi F, Brock G, Stolzenburg JU, Mulhall J, Moncada I, Patel HR, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT). European urology. 2014;65(3):587–96. [DOI] [PubMed] [Google Scholar]
- 6.Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. The journal of sexual medicine. 2010;7(4 Pt 2):1687–98. [DOI] [PubMed] [Google Scholar]
- 7.Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. European urology. 2012;62(2):261–72. [DOI] [PubMed] [Google Scholar]
- 8.Moskovic DJ, Miles BJ, Lipshultz LI, Khera M. Emerging concepts in erectile preservation following radical prostatectomy: a guide for clinicians. International journal of impotence research. 2011;23(5):181–92. [DOI] [PubMed] [Google Scholar]
- 9.Mulhall JP, Morgentaler A. Penile rehabilitation should become the norm for radical prostatectomy patients. The journal of sexual medicine. 2007;4(3):538–43. [DOI] [PubMed] [Google Scholar]
- 10.Mulhall JP, Muller A, Donohue JF, Mullerad M, Kobylarz K, Paduch DA, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. The journal of sexual medicine. 2008;5(5):1126–36. [DOI] [PubMed] [Google Scholar]
- 11.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68(2):429–35. [DOI] [PubMed] [Google Scholar]
- 12.Kovanecz I, Rambhatla A, Ferrini M, Vernet D, Sanchez S, Rajfer J, et al. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. International journal of impotence research. 2008;20(2):202–12. [DOI] [PubMed] [Google Scholar]
- 13.Mulhall J, Land S, Parker M, Waters WB, Flanigan RC. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. The journal of sexual medicine. 2005;2(4):532–40; discussion 40–2. [DOI] [PubMed] [Google Scholar]
- 14.Montorsi F, Guazzoni G, Strambi LF, Da Pozzo LF, Nava L, Barbieri L, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. The Journal of urology. 1997;158(4): 1408–10. [PubMed] [Google Scholar]
- 15.Raina R, Pahlajani G, Agarwal A, Zippe CD. The early use of transurethral alprostadil after radical prostatectomy potentially facilitates an earlier return of erectile function and successful sexual activity. BJU international. 2007;100(6):1317–21. [DOI] [PubMed] [Google Scholar]
- 16.Mulhall JP. Does on-demand vardenafil improve erectile function recovery after radical prostatectomy? Nature clinical practice Urology. 2009;6(1):14–5. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Lopez DS, Chen M, Wang R. Penile Rehabilitation Therapy Following Radical Prostatectomy: A Meta-Analysis. The journal of sexual medicine. 2017;14(12):1496–503. [DOI] [PubMed] [Google Scholar]
- 18.Qian SQ, Gao L, Wei Q, Yuan J. Vacuum therapy in penile rehabilitation after radical prostatectomy: review of hemodynamic and antihypoxic evidence. Asian journal of andrology. 2016;18(3):446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane database of systematic reviews (Online). 2018;2:MR000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentry KR, Arnup SJ, Disma N, Dorris L, de Graaff JC, Hunyady A, et al. Enrollment challenges in multicenter, international studies: The example of the GAS trial. Paediatric anaesthesia. 2019;29(1):51–8. [DOI] [PubMed] [Google Scholar]
- 21.Haddad RI, Chan AT, Vermorken JB. Barriers to clinical trial recruitment in head and neck cancer. Oral oncology. 2015;51(3):203–11. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B, Mitchell TC, Rusakevich AM, Brown DM, Wykoff CC. Noncompliance in Prospective Retina Clinical Trials: Analysis of Factors Predicting Loss to Follow-up. American journal of ophthalmology. 2020;210:86–96. [DOI] [PubMed] [Google Scholar]
- 23.Akl EA, Briel M, You JJ, Lamontagne F, Gangji A, Cukierman-Yaffe T, et al. LOST to follow-up Information in Trials (LOST-IT): a protocol on the potential impact. Trials. 2009;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somerson JS, Bartush KC, Shroff JB, Bhandari M, Zelle BA. Loss to follow-up in orthopaedic clinical trials: a systematic review. International orthopaedics. 2016;40(11):2213–9. [DOI] [PubMed] [Google Scholar]
- 25.Brueton VC, Tierney J, Stenning S, Harding S, Meredith S, Nazareth I, et al. Strategies to improve retention in randomised trials. Cochrane database of systematic reviews (Online). 2013;(12):MR000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madden K, Scott T, McKay P, Petrisor BA, Jeray KJ, Tanner SL, et al. Predicting and Preventing Loss to Follow-up of Adult Trauma Patients in Randomized Controlled Trials: An Example from the FLOW Trial. The Journal of bone and joint surgery American volume. 2017;99(13):1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell RT, Willox GP, Jhund PS, Hawkins NM, Huang F, Petrie MC, et al. Reporting of Lost to Follow-Up and Treatment Discontinuation in Pharmacotherapy and Device Trials in Chronic Heart Failure: A Systematic Review. Circulation Heart failure. 2016;9(5) [DOI] [PubMed] [Google Scholar]
- 28.Poulet FM, Berardi MR, Halliwell W, Hartman B, Auletta C, Bolte H. Development of hibernomas in rats dosed with phentolamine mesylate during the 24-month carcinogenicity study Toxicologic pathology. 2004;32(5):558–66. [DOI] [PubMed] [Google Scholar]
- 29.Ugarte F, Hurtado-Coll A. Comparison of the efficacy and safety of sildenafil citrate (Viagra) and oral phentolamine for the treatment of erectile dysfunction. International journal of impotence research. 2002;14 Suppl 2:S48–53. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein I Oral phentolamine: an alpha-1, alpha-2 adrenergic antagonist for the treatment of erectile dysfunction. International journal of impotence research. 2000;12 Suppl 1:S75–80. [PubMed] [Google Scholar]
- 31.Padma-Nathan H, Goldstein I, Klimberg I, Coogan C, Auerbach S, Lammers P. Long-term safety and efficacy of oral phentolamine mesylate (Vasomax) in men with mild to moderate erectile dysfunction. International journal of impotence research. 2002;14(4):266–70. [DOI] [PubMed] [Google Scholar]
- 32.Berookhim BM, Nelson CJ, Kunzel B, Mulhall JP, Narus JB. Prospective analysis of penile length changes after radical prostatectomy. BJU international. 2014;113(5b):E131–6. [DOI] [PubMed] [Google Scholar]


