Abstract
Background:
Comparison between endosonographic ultrasonography (EUS)-guided celiac ganglia neurolysis (CGN) and EUS-guided celiac plexus neurolysis (CPN) in pain management for pancreatic cancer has engendered controversy. To analyze the effectiveness and safety of EUS-CGN and figure out whether EUS-CGN is better than EUS-CPN, a qualitative systematic review was conducted.
Methods:
Studies were searched from Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE up to April 2020. We only included studies with full-text and in English and assessed study quality with Newcastle-Ottawa Scale or Cochrane risk-of-bias tool. We recorded details of study design, participants, procedure performed, protocol of follow-up, pain response, quality of life, survival, and adverse events. The study was conducted under Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement 2009.
Results:
Five studies involving 319 patients were included. Short-term pain response rates ranged from 65.0% to 88.46% in EUS-CGN group and most studies reported its superiority over EUS-CPN. As for adverse events, the incidence of transient hypotension and gastrointestinal symptoms seemed comparable, while results of initial pain exacerbation varied among studies. Besides, EUS-CGN might provide a shorter survival.
Conclusion:
EUS-CGN can be safely performed while it may shorten survival. In terms of short-term pain response, EUS-CGN is better than EUS-CPN while no conclusion of long-term pain control can be drawn.
Keywords: celiac ganglia neurolysis, celiac plexus neurolysis, endoscopic ultrasonography, pain management, pancreatic neoplasms
1. Introduction
Pancreatic ductal adenocarcinoma is notorious for its poor prognosis. It had already become the fourth leading cause of cancer death in the United States by the time of 2016 while the incidence and death were projected to be 56,770 and 45,750 respectively in 2019.[1] Apart from limited survival, patients with pancreatic ductal adenocarcinoma might suffer from jaundice, abdominal pain, endocrine insufficiency, exocrine insufficiency, and cachexia. Abdominal pain is one of the most common symptoms and patients are recommended to receive aggressive treatment.[2] Nowadays, World Health Organization Cancer Pain Ladder serves as the principle of analgesic management,[3] and medicine given to patients will escalate from non-opioid, mild opioid to strong opioid. Although some patients achieve pain relief with strong opioid, many others still demand larger dose to ease pain and opioid seems to be harmful to the survival of patients with pancreatic cancer.[4] In the reason of that, other therapies like celiac plexus neurolysis (CPN) and splanchnic nerve neurolysis were introduced.[5–8]
Celiac plexus is situated closely to the origin of celiac artery and consists of parasympathetic nerves, sympathetic nerves, visceral sensory afferent nerves, and ganglia.[9,10] To identify the celiac plexus, all kinds of imaging-assisted methods including fluoroscopy, computerized tomography, endosonographic ultrasonography (EUS), and magnetic resonance imaging have been introduced.[11–14] Among the methods aforementioned, EUS has become the popular one for its advantages of real-time image, cheapness, and avoidance of radiation exposure. Moreover, the visualization of celiac ganglia by EUS has also proven to be realizable.[15,16] Gerke et al[15] found celiac ganglia in 16 of 22 (72.7%) patients while most detected celiac ganglia were on the left side of the aorta. In other studies, celiac ganglia were visualized in 85.2% to 90% of patients,[16–20] except for the study of Bang et al[21] who claimed the low visualization rate ranging from 20% to 25% based on their experience. Even so, the conclusion that celiac ganglia are distinguishable in majority of cases can be drawn.
According to the classical viewpoint, celiac ganglia serve as the core of celiac plexus, so CPN should be performed adjacent to ganglia.[10] It can be inferred that direct injection of neurolytic agents into ganglia should provide the most satisfying outcomes. However, while some studies furnished evidence of the superiority of EUS-celiac ganglia neurolysis (CGN) over EUS-CPN,[18,22,23] some researchers questioned this conclusion and studies with contrary results also emerged.[19,24–26] This systematic review aimed to analyze the effectiveness and safety of EUS-CGN, explain the disparity of conclusions in those studies and answer the question of whether EUS-CGN is superior to EUS-CPN.
2. Materials and methods
2.1. Literature search
We conducted a systematic search on the Cochrane Central Register of Controlled Trials, Ovid-MEDLINE, and Ovid-EMBASE. The search strategy used the following terms: “celiac ganglia” AND “nerve block” AND “EUS” (see Text Document, Supplemental Digital Content 1 which detailed the search strategy). The last search was performed in April, 2020. The study was conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement 2009.[27]
2.2. Selection of studies
Two authors (ZW, YC) independently selected the studies to be included in the review after the literature research and disagreements were resolved through discussion with 2 other authors (BT, ZW). We included any clinical studies under the following criteria: with adult patients diagnosed with pancreatic cancer; researched on the efficacy and safety of EUS-CGN or comparing EUS-CGN to EUS-CPN; published in English and full text; included at least 10 patients in EUS-CGN group. Due to the limited number of studies, we did not restrict the study design. We excluded studies that were not full-text or not in English since we could not figure out whether EUS-CGN was performed just from the abstract. We did not contact the authors for extra data.
2.3. Data extraction and management
Two authors (ZW, YC) independently extracted data from the included studies and resolved any disagreements through discussion. Variables of interest including study design, participants, procedures performed, protocols of follow-up, pain response, quality of life, survival, and adverse events were all recorded. The demarcation between short-term or long-term was defined as 1 month.
2.4. Risk of bias
Two review authors (ZW, YC) independently assessed the risk of bias of all included studies. Randomized controlled trials (RCTs) were assessed with Cochrane risk-of-bias tool in following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcomes assessment, incomplete outcome data, selective reporting, and other bias (see Graphs and Tables, Supplemental Digital Content 2 which detailed the assessment of risk-of-bias in 2 RCTs).[28] We used Newcastle Ottawa Scale to assess the risk of bias in retrospective cohort studies (see Table, Supplemental Digital Content 3 which detailed the assessment of risk-of-bias in 3 retrospective cohort studies).[29]
3. Results
3.1. Results of search
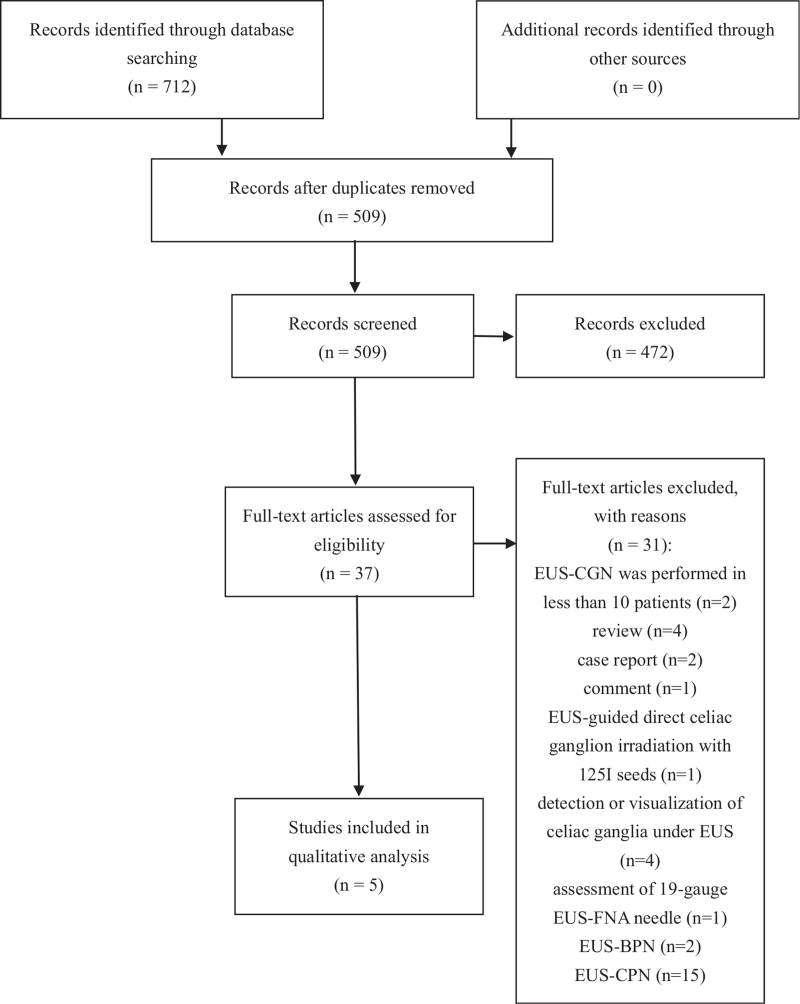
We found 712 studies by searching the database, and 509 studies were screened after removing duplicates. Thirty-seven full-text studies were assessed while 32 articles were excluded for being reviews, case reports, comments, etc (Fig. 1). We included 5 studies in this systematic review while 2 studies were RCTs,[18,19] 2 were retrospective cohort studies,[22,23] and 1 was retrospective uncontrolled study (Table 1).[30] Risk-of-bias of included studies were assessed as mentioned above (see Supplemental Digital Content 2 and Supplemental Digital Content 3).
Figure 1.
PRISMA 2009 flow diagram.
Table 1.
Characteristics of studies included.
| Study | Country | Study design | Participants | Interventions | Main outcomes |
| Levy et al[30] 2008 | USA | Uncontrolled retrospective study | 18 patients with unresectable pancreatic cancer, 18 patients with chronic pancreatitis | EUS-CGN and EUS-CGB | Pain relief, adverse events |
| Ascunce et al[22] 2011 | USA | Retrospective cohort study | 64 patients with pancreatic cancer | EUS-CGN vs EUS-CPN (bilateral) | Pain relief, narcotic usage, adverse events |
| Doi et al[18] 2013 | Japan | RCT | 68 patients with malignant tumor in the upper abdomen | EUS-CGN vs EUS-CPN (central) | Pain relief, duration of pain relief, adverse events |
| Hao et al[23] 2014 | China | Retrospective cohort study | 41 patients with unresectable pancreatic cancer | EUS-CGN vs EUS-CPN | Pain relief, duration of pain relief, adverse events |
| Levy et al[19] 2019 | USA | RCT | 110 patients with unresectable pancreatic cancer or refused surgery | EUS-CGN + CPN vs EUS-CPN (bilateral) | Pain relief, survival, QoL, performance status, morphine response, adverse events |
3.2. Procedures of EUS-CGN and EUS-CPN
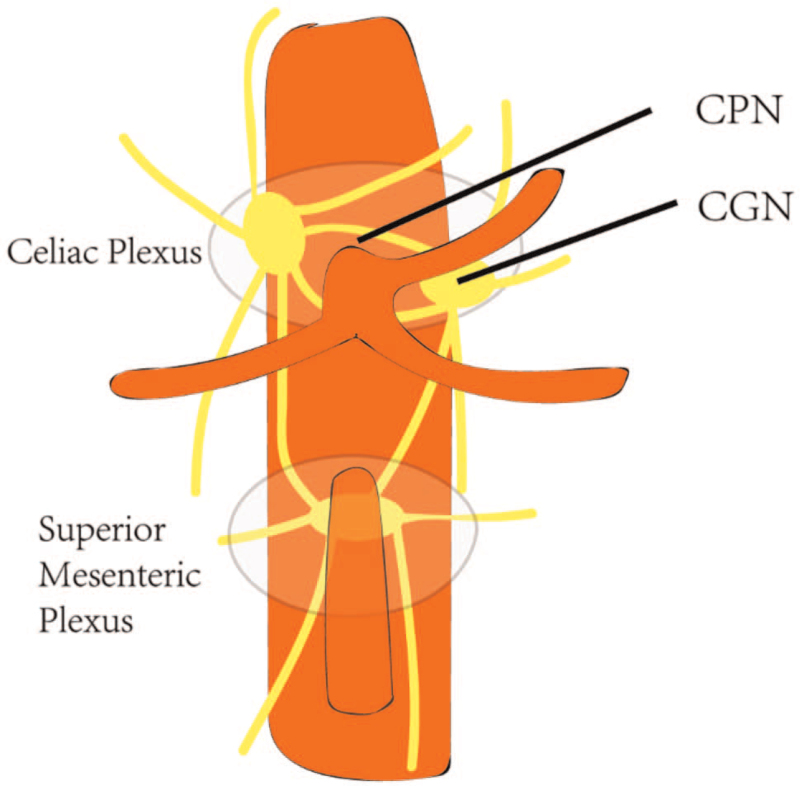
EUS-CPN was firstly proposed by Wiersema and Wiersema.[13] After the completion of sedation and examination on the feasibility of CPN, an echoendoscope would be inserted into the stomach to visualize celiac trunk. At the level of the origin of celiac artery, the needle is placed to the lateral aspect of the aorta, followed by the injection of 3 mL bupivacaine and 10 mL of dehydrated 98% absolute alcohol (Fig. 2). As a classic bilateral CPN, the same procedure would be performed on the other side of the aorta. Doi et al[18] chose the central CPN which just injects bupivacaine and absolute ethanol cephalad to the celiac trunk. The procedure of EUS-CGN is similar to EUS-CPN to a large extent.[30] Once the ganglia are identified (hypoechoic, oval, or irregular-shaped structures), a 22-G needle would be inserted into every visualized ganglion and inject neurolytic agent like ethanol.
Figure 2.
Diagram of CPN and CGN.
3.3. Short-term pain response
Ascunce et al[22] reported that EUS-CGN significantly increased pain response rate over EUS-CPN (65% vs 25%, P = .002), and the visualization of the ganglia was a significant predictor of response in a multivariate model. The decrease of the visual analog scale (VAS) was also significantly different (EUS-CGN –2.7 vs EUS-CPN –1.3, P = .014). Another study also verified the superiority of EUS-CGN.[18] Based on intention-to-treat analysis, the response rate at 1 week was significantly lower in EUS-CPN group compared to EUS-CGN group (45.5% vs 73.5%, P = .026). Furthermore, 50.0% of patients in EUS-CGN group achieved complete pain response while the number was only 18.2% for EUS-CPN (P = .010). The decrease of the numerical rating scale was 3.9 ± 2.4 in EUS-CGN group and 2.7 ± 2.4 in EUS-CPN group (P = .044). Per-protocol-based analysis also showed a significant difference in pain response (EUS-CGN vs EUS-CPN, 73.3% vs 48.7%, P = .049). Unlike the studies aforementioned, Hao et al[23] found no significant difference in pain response rates between EUS-CPN and EUS-CGN at 3 days or 1 week (80.00% vs 80.77%, P = .0983; 93.33% vs 88.46%, P = .0682).
3.4. Long-term pain response
One study reported a partial pain response of 94% (16/17) in the group using EUS-CGN.[30] In another study, the response rate was 83.3% (20/24) in EUS-CGN group and 50% (3/6) in EUS-CPN group at 1 month.[22] Differing from others, Hao et al[23] chose to assess the response rate at month 3 after the procedure, and pain response rate was significantly different between EUS-CGN and EUS-CPN (65.38% vs 53.33%, P = .0414). However, 1 study failed to find the superiority of EUS-CGN at 4 weeks.[19] Only 52.3% (23/44) of patients in EUS-CGN group and 48.1% (26/54) of patients in EUS-CPN group reported pain response. Besides, the authors found no significant difference between the 2 groups at 12 weeks (EUS-CGN 46.2% vs EUS-CPN 40.4%, P = .84).
3.5. Narcotic usage
Compared to the decrease of VAS or numerical rating scale, narcotic usage directly reflects patients’ need for analgesia. One study reported that 5% (2/40) and 8.3% (2/24) of patients in EUS-CGN group and EUS-CPN group respectively, increased narcotic use 1 week after the procedures.[22] Although the authors claimed that rate of decrease/continued not using narcotics in 2 groups differed significantly (EUS-CGN 72.5% vs EUS-CPN 33.3%, P = .002), it should be noted that patients who had been using narcotics at baseline but did not increase dosage were not included in those statistics. Doi et al[18] only reported 2 patients (1 from each group) that increased the narcotic dosage before 1 week and did not specify how many patients increased narcotic use afterward. Differing from others, Levy et al[19] reported an escalation of morphine dose from baseline to week 12 followed by a non-significant decrease in EUS-CGN group and a plateau in EUS-CPN group. It seemed like the 12th week was a turning point while morphine dose was not significantly different between the 2 groups then (EUS-CPN 93 mg/day vs EUS-CGN 105 mg/day, P = .81).
3.6. Survival
Levy et al[19] found the survival rates of EUS-CPN outnumbered EUS-CGN at 3 months (85% vs 66%), 6 months (68% vs 48%), 1 year (42% vs 26%), 2 years (15% vs 10%), and 4 years (5% vs 2%). Besides of that, EUS-CPN provided a longer survival compared to EUS-CGN (10.46 months vs 5.59 months, hazard ratios (HR) = 1.49, 95% confidence interval (CI): 1.02–2.19, P = .042). Even though no significant difference existed between the 2 groups in patients with metastatic cancer (HR = 0.89, 95% CI: 0.52–1.51), EUS-CPN seemed to be the superior one in non-metastatic pancreatic carcinoma (HR = 2.95; 95% CI: 1.61–5.45).
3.7. Quality of life (QoL)
Only 1 study researched EUS-CGN's influence on QoL.[19] The authors reported that both EUS-CGN and EUS-CPN would improve QoL but show no significant difference at week 12 no matter which scale they chose (EUS-CPN vs EUS-CGN: ΔFACT-Hep 3.5 vs 5.4, P = .72; ΔTotal MSAS –0.3 vs –0.2 P = .41; ΔBPI –0.8 vs –1.4 P = .33). Similarly, EUS-CGN failed to offer an advantage in Karnofsky score (EUS-CGN vs EUS-CPN at week 12: 65.8 ± 13.6 vs 70.5 ± 8.8, P = .088).
3.8. Adverse events
3.8.1. Initial pain exacerbation
Initial pain exacerbation is not the pain while ganglia are injected but begins in the recovery room or soon after.[30] Thirteen of 36 (36.1%) patients reported initial pain exacerbation while 7 patients were in EUS-CGN group and 6 patients were in EUS-CGB group.[30] Three patients were admitted hospitalization and 1 patient was in the emergency room. Ascunce et al[22] reported 1 case without specified whether it was in group EUS-CGN or EUS-CPN. In another study, pain exacerbation happened in 29.4% (10/34) and 21.2% (7/33) of patients treated with EUS-CPN and EUS-CGN, respectively.[18] However, the study of Levy et al[19] indicated that patients in EUS-CGN group were at a higher risk to experience initial pain exacerbation (EUS-CGN 44.9% vs EUS-CPN 8.3%, P < .001).
3.8.2. Transient hypotension
The incidence of transient hypotension varied among studies. While 1 study reported a number of 33% (12/36),[30] Hao et al[23] reported relatively low incidences of transient hypotension 4.9% (2/41). Comparison between EUS-CPN and EUS-CGN showed no significant difference in studies of Doi et al[18] (EUS-CGN 2.9% vs EUS-CPN 6.0%, P = .613) and Levy et al[19] (EUS-CGN 20.4% vs EUS-CPN 11.7%, P = .21).
3.8.3. Gastrointestinal symptoms
One study reported that 2 of 18 patients in EUS-CGB group with diarrhea, and 22% (4/18) of patients in EUS-CGN group even noted improvement of narcotic-induced constipation.[30] Self-limited loose stools happened in 23.4% of patients in the study of Ascunce et al.[22] None of those 2 RCTs found a significant difference in the incidence of diarrhea between EUS-CGN group and EUS-CPN group.[18,19] Doi et al[18] reported a case with upper gastrointestinal bleeding in EUS-CGN group.
3.8.4. Others
Paralysis was relatively rare but it was a severe complication related to EUS-CGN and EUS-CPN. Two percent (1/50) of patients developed paralysis after EUS-CGN in the study of Levy et al.[19] Doi et al[18] also reported 2 cases of inebriation (1 from each group).
4. Discussion
In this qualitative systematic review, we found EUS-CGN seemed to correlate with the better pain response at 1 week while long-term results showed a great deal of variability. As for the adverse events, severe complications like paralysis were rare, while the incidence of most mild adverse events was comparable in 2 groups. Astonishingly, combined EUS-CGN and EUS-CPN significantly shortened survival compared to EUS-CPN.
Although most studies indicated the superiority of EUS-CGN in short-term pain response, Hao et al[23] reported comparable results between EUS-CGN and EUS-CPN while response rates were significantly higher than other studies. The discrepancy of their results led to the pain response rates ranging from 65.0% to 88.46% and 25.0% to 93.33% in EUS-CGN group and EUS-CPN group, respectively. We believe the relatively broader definition of pain response might play a role in this phenomenon (Table 2). Furthermore, the study of Hao et al[23] was not designed for the comparison between EUS-CGN and EUS-CPN, so the baseline characteristics of both groups were not available for analysis while the baseline characteristics like larger tumor size, higher VAS score, localization of pancreatic head might affect the pain relief.[22] Sahai[25,26] also doubted the superiority of EUS-CGN in pain management. Based on his findings that bilateral EUS-CPN overmatched central EUS-CPN in pain management, he argued that bilateral EUS-CPN might produce the similar effect as EUS-CGN. However, recent studies reported non-significant difference between central and bilateral EUS-CPN in pain control,[31,32] and Ascunce et al[22] also indicated that EUS-CGN was superior to bilateral EUS-CPN.
Table 2.
Definition of pain response and protocol of follow-up.
| Study | Definition of pain response | Time for comparison of pain relief | Terminating follow up if non-response |
| Levy et al[30] 2008 | – | – | – |
| Ascunce et al[22] 2011 | A decrease of ≥2 points in VAS compared with baseline without an increase in oral narcotic use | 1 week and 1 months | Yes |
| Doi et al[18] 2013 | NRS decreases to ≤3 points | 1 week | Yes |
| Hao et al[23] 2014 | NRS decreases to ≤3 points or a ≥30% reduction in NRS compared with baseline without an increase in pain medication use | 3 days, 1 week, and 3 months | No |
| Levy et al[19] 2019 | A decrease of ≥3 points in NRS or a ≥50% reduction compared with baseline | 1, 2,3, 6, 9, and 12 months | No |
Results of long-term pain response varied among studies, and it could be attributed to the different pathological backgrounds, definitions of pain response, study designs, procedures performed, and follow-up protocols (Tables 1 and 2). The diverse follow-up protocols implemented significantly influenced the long-term results. To be clear, Ascunce et al[22] and Doi et al[18] did not follow up people who failed to show pain response, which means people at risk referred to those who achieved pain response at the last follow-up and survived since then. Conversely, the study of Levy et al[19] did not terminate follow-up even patients had reported treatment failure. Thus, people at risk in that study just referred to people who survived. Hao et al[23] used the number of patients at baseline as people at risk. The disparity of definition of people at risk endows the rates of pain response with different meanings. So, it is not difficult to understand the stark difference (52.3% vs 83.3%) between response rates at 1 month in EUS-CGN group in Levy's and Ascunce's studies.[19,22]
The different protocols of follow-up also shed light on many important questions. Above all, the appropriate time to evaluate the pain response, especially the long-term response, should be confirmed. While most studies chose to assess the short-term results at 1 week, the definitions of long-term varied from 1 month to 3 months.[22,23,33] Due to the gloomy prognosis of pancreatic cancer, the evaluation of long-term results would inevitably be influenced by survival. In other words, the appropriate time to assess long-term results should be decided based on the proper research on survival first. Contradicting with the viewpoints that effective analgesia correlated with better overall survival and denervation can suppress tumorigenesis,[34–36] some studies reported that CPN could not affect survival or even be harmful,[24,37–39] and some studies which performed CGN provided similar results.[19,24] Levy et al[19] explained this phenomenon with activation of inflammatory pathways or the potential relation between ganglia and other abdominal organs. All in all, the effect of EUS-CGN on survival demands more basic and clinical studies since we cannot bear the risk of reducing the limited survival in patients with pancreatic malignancy.
Alcohol-based neurolytic injectant had been the mainstay of ganglia neurolysis for decades, and new methods were introduced. Wang et al[17] inserted iodine-125 seeds into ganglia followed by standard chemotherapy (1 week after the procedure) and reported a response rate of 82.6% (19/23) at 2 weeks while VAS and MS Contin consumption increased at 1 week. It is noteworthy that all patients received chemotherapy 1 week after the procedure. The study of Bang et al[33] developed the method of radiofrequency ablation (RFA) of celiac ganglia, but ganglia were visualized in only 35.7% and 33.3% of patients in EUS-CPN group and EUS-RFA group, respectively. Notwithstanding the low detection of celiac ganglia, RFA group provided more pain relief and improved the quality of life. Unlike the alcoholic neurolytic injectant that would disperse,[40] radiotherapy might provide a more durable and concentrated effect than chemical agents, and those studies might have provided an insight into the more effective therapy.
We noted some limitations of this systematic review. Above all, we only included 5 studies with varied study designs, definitions of pain response, and follow-up protocols due to the scarcity of pertinent research. The heterogeneity hindered our attempt to pool the data, so we managed a qualitative analysis to clarify the disparity of existing results and tried to provide some valuable conclusions. Other than that, none of the included studies compared EUS-CGN with medication therapy. As a recently published article claimed that oxycodone/fentanyl might be as effective as EUS-CPN in pain relief and QoL,[39] it is reasonable to doubt the effect of EUS-CGN. In conclusion, EUS-CGN is safe to perform with a low incidence of severe adverse events while it might reduce survival in some way. Besides, the incidence of mild adverse events in both EUS-CGN and EUS-CPN groups seemed comparable, especially in terms of transient hypotension and gastrointestinal symptoms. As for the comparison of pain control between EUS-CPN and EUS-CGN, the latter showed its superiority in short-term results while no conclusion of long-term results can be drawn. Hence, more studies with an appropriate pain response definition and follow-up protocol are appealed.
Author contributions
Conceptualization: Zihe Wang, Zuowei Wu.
Investigation: Xing Huang.
Methodology: Zihe Wang, Yang Chen, Xing Huang.
Supervision: Bo-Le Tian.
Validation: Bo-Le Tian.
Visualization: Chao Wu.
Writing – original draft: Zihe Wang.
Writing – review & editing: Mao Li, Zihe Wang, Yang Chen, Zuowei Wu, Chao Wu.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CGN = celiac ganglia neurolysis, CPN = celiac plexus neurolysis, EUS = endosonographic ultrasonography, RCT = randomized controlled trial, RFA = radiofrequency ablation, VAS = visual analog scale.
How to cite this article: Li M, Wang Z, Chen Y, Wu Z, Huang X, Wu C, Tian B. EUS-CGN versus EUS-CPN in pancreatic cancer: a qualitative systematic review. Medicine. 2021;100:41(e27103).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
CGB = celiac ganglia block, CGN = celiac ganglia neurolysis, CPN = celiac plexus neurolysis, EUS = endosonographic ultrasonography, RCT = randomized controlled trial.
NRS = numerical rating scale, VAS = visual analog scale.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:07–34. [DOI] [PubMed] [Google Scholar]
- [2].Sohal DP, Mangu PB, Khorana AA, et al. Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;34:2784–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Koulouris AI, Banim P, Hart AR. Pain in patients with pancreatic cancer: prevalence, mechanisms, management and future developments. Dig Dis Sci 2017;62:861–70. [DOI] [PubMed] [Google Scholar]
- [4].Oh TK, Do S-H, Yoon Y-S, Song I-A. Association between opioid use and survival time in patients with unresectable pancreatic cancer: 10 years of clinical experience. Pancreas 2018;47:837–42. [DOI] [PubMed] [Google Scholar]
- [5].Amr YM, Makharita MY. Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer: one-year follow-up. Clin J Pain 2013;29:807–13. [DOI] [PubMed] [Google Scholar]
- [6].Koyyalagunta D, Engle MP, Yu J, Feng L, Novy DM. The effectiveness of alcohol versus phenol based splanchnic nerve neurolysis for the treatment of intra-abdominal cancer pain. Pain Physician 2016;19:281–92. [PubMed] [Google Scholar]
- [7].Bhutiani N, Cheadle GA, Bahr MH, Vitale GC. Comparative efficacy of bilateral thoracoscopic splanchnicectomy for intractable pain secondary to pancreatic cancer vs chronic pancreatitis. J Am Coll Surg 2017;224:566–71. [DOI] [PubMed] [Google Scholar]
- [8].Dobosz Ł, Stefaniak T, Dobrzycka M, et al. Invasive treatment of pain associated with pancreatic cancer on different levels of WHO analgesic ladder. BMC Surg 2016;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics 2011;31:1599–621. [DOI] [PubMed] [Google Scholar]
- [10].Ward EM, Rorie DK, Nauss LA, Bahn RC. The celiac ganglia in man: normal anatomic variations. Anesth Analg 1979;58:461–5. [DOI] [PubMed] [Google Scholar]
- [11].Hol PK, Kvarstein G, Viken O, Smedby O, Tønnessen TI. MRI-guided celiac plexus block. J Magn Reson Imaging 2000;12:562–4. [DOI] [PubMed] [Google Scholar]
- [12].Hegedüs V. Relief of pancreatic pain by radiography-guided block. AJR Am J Roentgenol 1979;133:1101–3. [DOI] [PubMed] [Google Scholar]
- [13].Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc 1996;44:656–62. [DOI] [PubMed] [Google Scholar]
- [14].Haaga JR, Kori SH, Eastwood DW, Borkowski GP. Improved technique for CT-guided celiac ganglia block. AJR Am J Roentgenol 1984;142:1201–4. [DOI] [PubMed] [Google Scholar]
- [15].Gerke H, Silva RG, Shamoun D, Johnson CJ, Jensen CS. EUS characteristics of celiac ganglia with cytologic and histologic confirmation. Gastrointest Endosc 2006;64:35–9. [DOI] [PubMed] [Google Scholar]
- [16].Levy M, Rajan E, Keeney G, Fletcher JG, Topazian M. Neural ganglia visualized by endoscopic ultrasound. Am J Gastroenterol 2006;101:1787–91. [DOI] [PubMed] [Google Scholar]
- [17].Wang KX, Jin ZD, Du YQ, et al. EUS-guided celiac ganglion irradiation with iodine-125 seeds for pain control in pancreatic carcinoma: a prospective pilot study. Gastrointest Endosc 2012;76:945–52. [DOI] [PubMed] [Google Scholar]
- [18].Doi S, Yasuda I, Kawakami H, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy 2013;45:362–9. [DOI] [PubMed] [Google Scholar]
- [19].Levy MJ, Gleeson FC, Topazian MD, et al. Combined celiac ganglia and plexus neurolysis shortens survival, without benefit, vs plexus neurolysis alone. Clin Gastroenterol Hepatol 2019;17:728–38. e729. [DOI] [PubMed] [Google Scholar]
- [20].Gleeson FC, Levy MJ, Papachristou GI, et al. Frequency of visualization of presumed celiac ganglia by endoscopic ultrasound. Endoscopy 2007;39:620–4. [DOI] [PubMed] [Google Scholar]
- [21].Bang JY, Hasan MK, Sutton B, et al. Intraprocedural increase in heart rate during EUS-guided celiac plexus neurolysis: clinically relevant or just a physiologic change? Gastrointest Endosc 2016;84:773–9. e773. [DOI] [PubMed] [Google Scholar]
- [22].Ascunce G, Ribeiro A, Reis I, et al. EUS visualization and direct celiac ganglia neurolysis predicts better pain relief in patients with pancreatic malignancy (with video). Gastrointest Endosc 2011;73:267–74. [DOI] [PubMed] [Google Scholar]
- [23].Hao S-J, Xu W-J, Di Y, et al. How to improve the efficacy of endoscopic ultrasound-guided celiac plexus neurolysis in pain management in patients with pancreatic cancer: analysis in a single center. Surg Laparosc Endosc Percutan Tech 2014;24:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fujii-Lau LL, Bamlet WR, Eldrige JS, et al. Impact of celiac neurolysis on survival in patients with pancreatic cancer. Gastrointest Endosc 2015;82:46–56. e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sahai AV. EUS-guided celiac ganglia neurolysis versus celiac plexus neurolysis: dying to know which is better. Gastrointest Endosc 2017;86:664–5. [DOI] [PubMed] [Google Scholar]
- [26].Sahai AV. The benefit of celiac ganglion injection remains unclear. Endoscopy 2013;45:854. [DOI] [PubMed] [Google Scholar]
- [27].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Higgins JPT, Thomas J, Chandler J, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available at: www.training.cochrane.org/handbook. Accessed May 01, 2020. [Google Scholar]
- [29]. GA Wells, B Shea, D O’Connell, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 04, 2020. [Google Scholar]
- [30].Levy MJ, Topazian MD, Wiersema MJ, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct ganglia neurolysis and block. Am J Gastroenterol 2008;103:98–103. [DOI] [PubMed] [Google Scholar]
- [31].Téllez-Ávila FI, Romano-Munive AF, Herrera-Esquivel JdJ, Ramírez-Luna MA. Central is as effective as bilateral endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. Endosc Ultrasound 2013;2:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lu F, Dong J, Tang Y, et al. Bilateral vs. unilateral endoscopic ultrasound-guided celiac plexus neurolysis for abdominal pain management in patients with pancreatic malignancy: a systematic review and meta-analysis. Support Care Cancer 2018;26:353–9. [DOI] [PubMed] [Google Scholar]
- [33].Bang JY, Sutton B, Hawes RH, Varadarajulu S. EUS-guided celiac ganglion radiofrequency ablation versus celiac plexus neurolysis for palliation of pain in pancreatic cancer: a randomized controlled trial (with videos). Gastrointest Endosc 2019;89:58–66. e53. [DOI] [PubMed] [Google Scholar]
- [34].Vernerey D, Huguet F, Vienot A, et al. Prognostic nomogram and score to predict overall survival in locally advanced untreated pancreatic cancer (PROLAP). Br J Cancer 2016;115:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhao C-M, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med 2014;6: 250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saloman JL, Albers KM, Li D, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A 2016;113:3078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oh TK, Lee WJ, Woo SM, Kim NW, Yim J, Kim DH. Impact of celiac plexus neurolysis on survival in patients with unresectable pancreatic cancer: a retrospective, propensity score matching analysis. Pain Physician 2017;20:E357–65. [PubMed] [Google Scholar]
- [38].Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol 2011;29:3541–6. [DOI] [PubMed] [Google Scholar]
- [39].Kanno Y, Koshita S, Masu K, et al. Efficacy of EUS-guided celiac plexus neurolysis compared with medication alone for unresectable pancreatic cancer in the oxycodone/fentanyl era: a prospective randomized control study. Gastroint Endosc 2020. [DOI] [PubMed] [Google Scholar]
- [40].Kappelle WFW, Bleys R, van Wijck AJM, Siersema PD, Vleggaar FP. EUS-guided celiac ganglia neurolysis: a clinical and human cadaver study (with video). Gastrointest Endosc 2017;86:655–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.