Abstract
Background:
Prunella vulgaris (PV), a traditional Chinese medical herb, is considered beneficial for some thyroid diseases. However, the effectiveness is not consistent in different studies. This review compiles the evidence from randomized controlled trials (RCTs) and quantifies the effects of PV preparation on thyroid nodules.
Methods:
Eight databases were searched up to April 2021 to identify eligible studies. Only RCTs were included. Meta-analysis of homogeneous studies was performed by RevMan5.3 software. Cochrane risk of bias assessment tool version 2.0 was used to assess the risk of bias of each trial. The research screening, data extraction, and risk of bias assessment were employed by 2 reviewers independently, and disagreement will be decided by a third senior reviewer. The risk ratio (RR), mean difference (MD) and corresponding 95% confidence interval (CI) of each study are summarized.
Results:
Thirteen RCTs with 1468 patients were included in this study. A meta-analysis showed that the RR of the clinical efficacy of PV combined with levothyroxine sodium tablets was 1.22 (95% CI [1.11, 1.33]). The MD of thyroid nodule diameter was −0.43 (95% CI [−0.63, −0.22]). The MD of free triiodothyronine and free tetraiodothyronine levels was −1.99 (95% CI [−3.14, −0.86]) and −3.20 (95% CI [−5.50, −0.89]), respectively. The RR of the adverse reaction rate was 0.67 (95% CI [0.36, 1.22]), and the RR of the clinical efficacy of PV preparation combined with thyroxin tablets was 1.29 (95% CI [1.03, 1.62]).
Conclusions:
PV combined with levothyroxine sodium tablets or thyroxin tablets has more benefits for thyroid nodules, further improving the clinical efficiency, reducing the diameter of nodules and reducing the occurrence of adverse reactions. However, the quality of these studies is uncertain, and higher quality and more RCTs are needed to provide comprehensive evidence-based medical evidence in the future.
Keywords: efficacy, meta-analysis, Prunella vulgaris, randomized controlled trial, thyroid nodule
1. Introduction
Thyroid nodules are a common disease and are defined by the American Thyroid Association (ATA) as “a discrete lesion within the thyroid gland that is radiologically distinct from the surrounding thyroid parenchyma”.[1] Epidemiological studies have shown that the incidence of thyroid nodules after ultrasound examination in noniodine-deficient areas ranges from 19% to 67%.[2] If remedial measures are not taken promptly, the disease may have side effects on the respiratory system and endocrine system. In addition to surgical treatment, Western medicine uses conventional methods to treat thyroid nodules, whereas traditional Chinese medicine (TCM) has been treating thyroid diseases for thousands of years. Li et al[3] summarized the rule of using TCM in the clinical treatment of thyroid nodules, and Li et al[3] found that Prunella vulgaris (PV) is the most frequently used herb and that various preparations with PV are widely used in the clinic. In recent years, several clinical studies have evaluated the effect of PV preparation (PVP) in the adjuvant therapy of thyroid nodules; however, there are differences in outcome indicators, such as clinical efficiency, and the quality and conclusions of the studies lack rigorous theoretical sources. Until now, few studies have systematically examined the effectiveness and safety of PVP for treating thyroid nodules.[4] Therefore, our study aimed to evaluate the efficacy and safety of PVP in adjuvant treatment of thyroid nodules from randomized controlled trials (RCTs).
2. Materials and methods
2.1. Ethics statement
As all analyses were based on previously published studies, and no ethical approval or patient consent was required.
2.2. Study selection
Among all of the relevant studies, the ones that met the following inclusion and exclusion criteria were eligible.
The inclusion criteria were as follows: the study type was RCTs with or without blind method, and the study was written with English or Chinese language; the treatment group was treated with a preparation of PV combined with a conventional treatment, whereas the control group was treated with the same conventional treatment without the preparation of PV (different preparations of PV were administered according to the instructions); All cases with clinically confirmed thyroid nodules, including thyroid cysts and thyroid tumors, were included; and there was no restriction on age, sex or the course of disease; the main outcome indexes included the clinical effective rate and the incidence of adverse reactions, and secondary outcome indicators included the diameter of the thyroid nodule, free triiodothyronine (FT3) levels and free tetraiodothyronine (FT4) levels.
The exclusion criteria were as follows: nonclinical studies, such as basic research, animal experiments, and reviews; non-RCTs, such as before-and-after studies; the treatment group was treated only with PVP; and multiple publications reporting the same groups of participants were excluded to reduce overlapping data.
2.3. Search strategy
Eight databases, including MEDINE (PubMed), EMBASE, Cochrane Library, Web of Science, China Biomedical Database (CBM), China National Knowledge Infrastructure (CNKI), VIP Chinese Science and Technique Journals Database (VIP), and Wanfang data, were investigated from the establishment of the database to November 2020. Gray literature was retrieved, including privately published literature.
The following search terms were used: “(‘Prunella vulgaris’ OR Xiakucao) AND (‘thyroid nodule’ OR ‘thyroid cancer’ OR ‘thyroid tumor’)”.
The specific search strategy of Medline is presented as follows:
#1 Search ((“Prunella vulgaris”[Title/Abstract])) OR (Xiakucao[Title/Abstract])
#2 Search ((“thyroid nodule”[Title/Abstract])) OR (“thyroid cancer”[Title/Abstract])) OR (“thyroid tumor”[Title/Abstract])
#3 Search (#1 and #2).
2.4. Data extraction
The baseline data included study title, first author, year of publication, diagnosis criteria, sample size, sex of the patients, age of the patients, treatment duration, details of intervention, details of control, follow-up, and outcome measurement. Two researchers screened and extracted the literature independently according to predefined criteria and excluded studies that were ineligible, such as animal experiments, basic research and reviews, according to titles and abstracts. If the information could not be determined, the full text was read. If the information was still not clear, the author was contacted to acquire necessary information. The 2 researchers then cross-checked the results and submitted them to a third party for arbitration in case of disagreement.
2.5. Risk of bias and methodological quality assessment
Cochrane risk of bias assessment tool which recommended by Cochrane handbook 5.1.0 was used to assess the risk of bias of RCTs.[5] Two reviewers evaluated the methodological quality of each trial independently according to the standards advised by the tool. The key points of assessment included the generation of random sequences, allocation concealment, implementation of blindness, resulting data, selective reporting and other bias sources. The author decided “yes”, “no” or “unclear” for the above 6 items in each study with “yes” indicating low risk and “‘no” indicating high risk. Disagreement, if any, was resolved by discussion, and a consensus was reached through a third party.
2.6. Statistical methods
RevMan 5.3 software provided by the Cochrane Collaboration was used for statistical analysis (UK). The risk ratio (RR) was used to describe counting data, and continuous outcomes were expressed as the mean difference (MD) with a 95% confidence interval (CI). Heterogeneity was analyzed by means of I2 statistic. I2≤50% represented low heterogeneity, and a fixed effect model was adopted. I2 > 50% represented low heterogeneity, and a random effect model was adopted. A 2-tailed P < .05 was considered statistically significant. Descriptive analysis was performed if a meta-analysis could not be used.
3. Results
3.1. Study selection
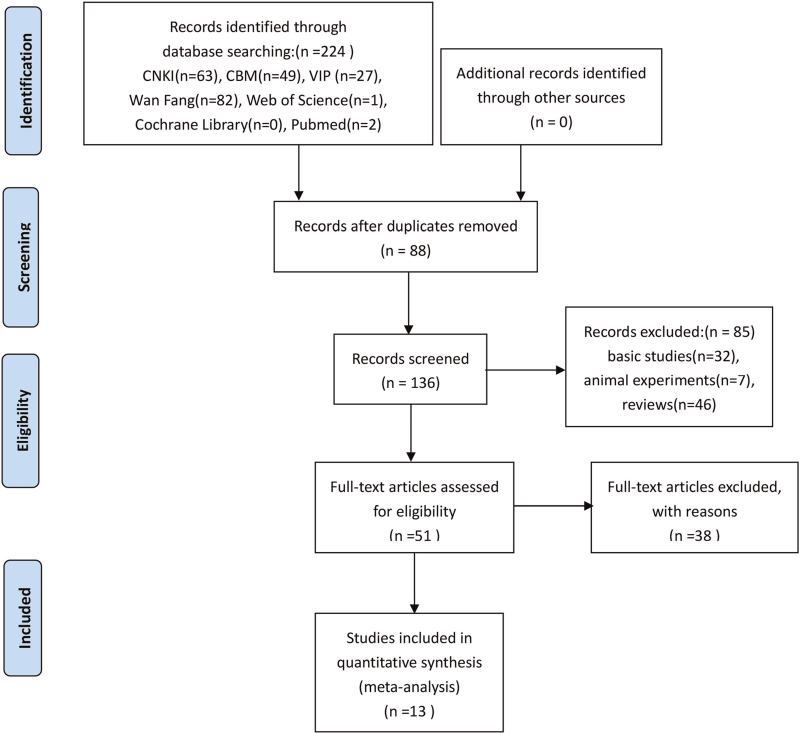
Among 224 identified studies, there were 221 in Chinese and 3 in English. Eighty-eight repeated articles were excluded by Endnote software (Thomson Corporation, headquartered in Stanford, Connecticut, USA). Thirty-two basic studies, 7 animal experiments, and 46 reviews were excluded after screening the title and abstract, and 38 unqualified studies were excluded by reading the full text. Finally, only 13 studies were eligible for data extraction according to the inclusion and exclusion criteria. The flow diagram is described in Figure 1.
Figure 1.
Flow diagram of assessment of studies identified in the systematic review. CBM = China Biomedical Database, CNKI = China National Knowledge Infrastructure, VIP = VIP Chinese Science and Technique Journals Database.
3.2. Study characteristics
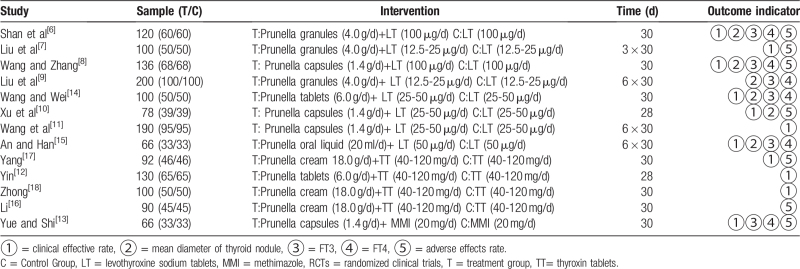
The essential characteristics of the 13 studies are described in Table 1. All the studies, including 1468 patients from the treatment group and 734 controls, were recruited into this review. According to different characteristics and different treatments, the studies were divided into the following 3 groups: PV combined with levothyroxine sodium tablets (PV+LT) vs levothyroxine sodium tablets (LT); PV combined with thyroxin tablets (PV+TT) vs thyroxin tablets (TT); and PV combined with methimazole (PV+MMI) vs methimazole (MMI). LT is the levorotatory body of TT, which is frequently used in the clinic. The preparations included Prunella capsules, granules, tablets, oral liquid, and cream.
Table 1.
Characteristics of RCTs included in meta-analysis.
3.3. Literature quality evaluation
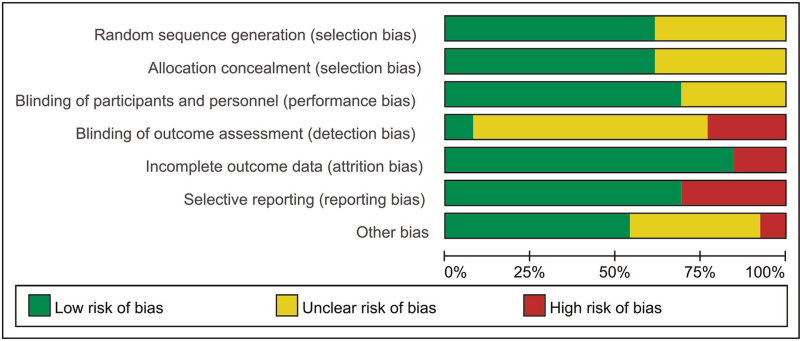
Thirteen articles met the criteria of quality evaluation, and all of them mentioned “the patients were divided randomly into treatment group and control group”; however, only 8 articles[6–13] stated “the random number table was used”, and the other trials did not provide more detail about how random sequences were generated. Eight studies[6–13] satisfied the allocation concealment, and 9 studies[6–9,11,12,14–16] mentioned how participants and personnel were blinded. However, the majority of trials did not report concrete details on allocation concealment and blinding of outcome assessors. Among the 13 studies, 11[6,8,10–18] had complete data, and 2 studies[7,9] probably lost some data, which might have affected the final result. Four studies[7,9,12,16] selectively reported results with incomplete primary outcomes that led to a high risk, and the rest of the studies could not be recognized after reading the full text. One study[17] had other bias that may lead to high risk due to the inclusion criteria for patients was no authority (Fig. 2, Table 2).
Figure 2.
Diagram of risk bias.
Table 2.
Results of quality assessment.
| Study | Generation of random sequences | Allocation concealment | Implementation of blindness (participants and personnel) | Implementation of blindness (outcome assessmet | Resulting data | Selective reporting | Other bias |
| Shan et al[6] | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Liu et al[7] | Yes | Yes | Yes | Unclear | No | No | Unclear |
| Wang and Zhang[8] | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Liu et al[9] | Yes | Yes | Yes | Unclear | No | No | Unclear |
| Wang et al[14] | Unclear | Unclear | Yes | Unclear | Yes | Yes | Yes |
| Xu et al[10] | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Wang et al[11] | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| An and Han[15] | Unclear | Unclear | Yes | Unclear | Yes | Yes | Unclear |
| Yang[17] | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No |
| Yin[12] | Yes | Yes | Yes | No | Yes | No | Unclear |
| Zhong[18] | Unclear | Unclear | Unclear | No | Yes | Yes | Yes |
| Li[16] | Unclear | Unclear | Yes | No | Yes | No | Yes |
| Yue and Shi[13] | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
3.4. Meta-analysis
3.4.1. PV+LT vs LT
3.4.1.1. Clinical effective rate
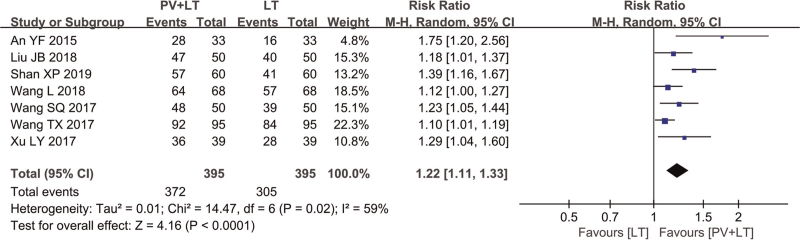
In the PV+LT vs LT group, 7 studies[6–8,10,11,14,15] (790 cases) reported clinical efficacy, and the combined results showed heterogeneity among these studies. Therefore, a randomized effect model was used (χ2 = 14.47, P1 = .02, I2 = 59%). The meta-analysis results showed that the RR was 1.22 (95% CI [1.11, 1.33]), and the difference was statistically significant (P2 < .05) (Fig. 3).
Figure 3.
Forest plot of clinical effective rate of PV+LT vs LT. CI = confidence interval, LT = levothyroxine sodium tablets, PV = Prunella vulgaris.
3.4.1.2. Mean diameter of thyroid nodule
Six studies[5,8,9,10,14,15] reported the changes in the mean diameter of thyroid nodules before and after treatment, and the analysis showed heterogeneity among studies. Therefore, a randomized effect model was adopted (χ2 = 30.62, P1 < .001, I2 = 84%). The meta-analysis results showed that the MD was −0.43 (95% CI [−0.63, −0.22]), and the difference was statistically significant (P2 < .05) (Table 3). Subgroup analysis of mean diameter of thyroid nodule showed that the heterogeneity was mainly due to the inconsistency of diagnostic criteria.
Table 3.
Meta-analysis of the secondary outcome indicators.
| Outcome indicator | The included studies | MD | 95%CI | P 1 | I2% | P 2 |
| Diameter of thyroid nodule | 4,6,7,8,9,11 | –0.43 | (–0.63,–0.22) | .00 | 84 | .00 |
| FT3 | 4,6,7,8,11 | –1.99 | (–3.14,–0.85) | .00 | 99 | .00 |
| FT4 | 4,6,7,8,11 | –3.20 | (–5.50,–0.89) | .00 | 98 | .00 |
3.4.1.3. FT3 and FT4 levels
Five studies[6,8,9,14,15] reported changes in FT3 and FT4 levels. Because the studies on FT3 showed heterogeneity, the randomized effect model was adopted (χ2 = 358.23, P1 < .001, I2 = 99%). The results showed significant difference (MD = −1.99 and 95% CI [−3.14, 0.85]) (P2 < .05). The studies on FT4 also showed heterogeneity (χ2 = 173.43, P1 < .001, I2 = 98%), and significant difference was found (MD = −3.20 and 95% CI [−5.50, 0.89]) (P < .05) (Table 3).
3.4.1.4. Adverse effects rate
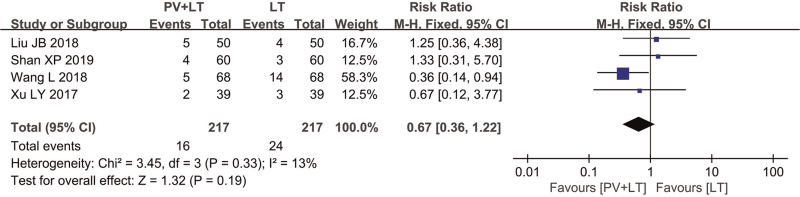
The incidence of adverse reactions was reported in 4 studies[6–8,10] with homogeneity among studies, and a fixed effect model was used (χ2 = 3.45, P = .33, I2 = 13%). The analysis results showed no significant difference between the 2 groups (RR = 0.67 and 95% CI [0.36, 1.22]) (P > .05) (Fig. 4). Subgroup analysis of adverse effects rate showed that the heterogeneity was mainly due to the inconsistency of the duration of treatment.
Figure 4.
Forest plot of adverse effects rate of PV+LT vs LT. CI = confidence interval, LT = levothyroxine sodium tablets, PV = Prunella vulgaris.
3.4.2. PV+TT vs TT
3.4.2.1. Clinical effective rate
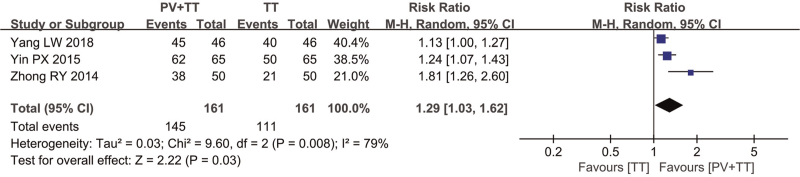
In the comparison between PVP+TT and TT, clinical effectiveness was reported in 3 studies.[12,17,18] Because the results indicated heterogeneity, a random effects model was used (χ2 = 9.60, P1 = .008, I2 = 79%). The analysis results showed that the RR was 1.29 (95% CI (1.03, 1.62]), and the difference was statistically significant (P2 < .05) (Fig. 5).
Figure 5.
Forest plot of clinical effective rate of PV+TT vs TT. CI = confidence interval, PV = Prunella vulgaris, TT = thyroxin tablets.
3.4.2.2. Adverse effects rate
The incidence of adverse reactions was reported in 2 studies.[16,17] Due to the reductive coverage, the meta-analysis could not be performed. Thus, we only performed a descriptive analysis. Li[16] showed that among 90 (45/45) patients, 31 (68.89%) patients in the control group had endocrine disorders, 26 (57.78%) patients had hyperthyroidism, and 39 (86.67%) patients had nausea and vomiting. In the treatment group, there were 9 cases (20%) of endocrine disorders, 9 cases (20%) of hyperthyroidism and 15 cases (33.33%) of nausea and vomiting (P2 < .05). Yang[17] study showed that in 92 (46/46) patients, 21 (45.65%) patients in the control group had adverse reactions, and there 7 (15.22%) patients in the treatment group (P2 < .05).
3.4.3. PV+MMI vs MMI
Because only 1 study[13] reported the clinical efficacy of PVP in combination with MMI vs MMI alone, a descriptive analysis was performed. Yue and Shi[13] showed that the total effective rate (97.0%) of the PV+MMI group was significantly higher than that of the MMI group (72.7%), and the difference was statistically significant (P < .05). After treatment, the serum levels of FT4 and FT3 in the 2 groups were significantly lower than those before treatment (P < .01), and TSH levels were significantly higher than those before treatment (P < .01). In addition, the changes were more significant in the PV+MMI group than in the MMI group (P < .01). In the PV+MMI group, there was 1 case of skin rash and 1 case of nausea, and the incidence of adverse reactions was 6.1%. In the MMI group, there was 1 case of leukopenia, 1 case of abdominal pain, 1 case of nausea and 1 case of mild liver damage, and the incidence of adverse reactions was 12.1%. There were no statistically significant differences between the 2 groups (P > .05).
3.5. Sensitivity analysis
Sensitivity analysis was performed on the excluded studies 1 by 1. Compared with the results before exclusion, there was little change after exclusion, and the results were relatively stable and reliable.
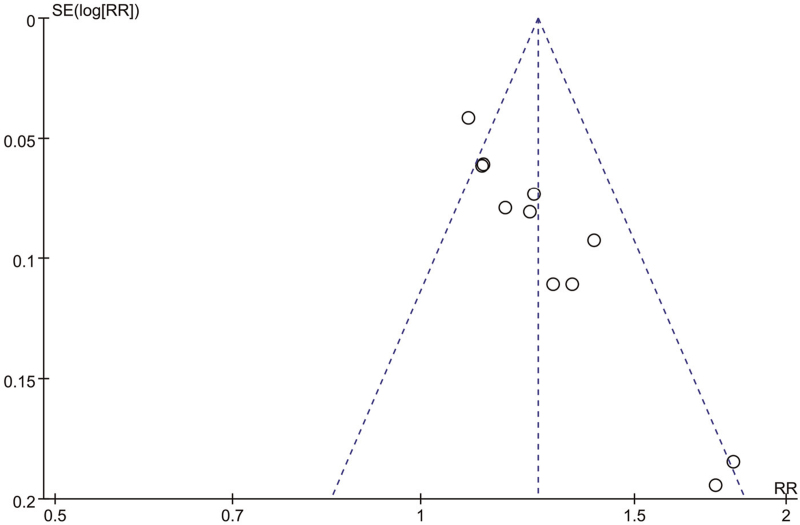
3.6. Publication bias
The inverted funnel plot analysis of the 9 clinical effective rates included in the study showed asymmetry. Because most of the statistical methodology in the studies was not strictly described, it was likely to be the main source of other bias (Fig. 6).
Figure 6.
Funnel plot of clinical effective rate. RR = risk ratio, SE = Standard Error.
4. Conclusion
Meta-analysis results showed that the conventional treatment of thyroid nodule combined with the TCM prunella preparation can effectively reduce the diameter of thyroid nodule, maybe it can avoid the pain and economic burden of the treatment caused by surgical resection. In addition, a number of results showed that the combined use of thyroid preparation can improve the clinical efficiency and reduce the risk of adverse reactions, which is instructive to clinical application.
Thyroid nodules are a common endocrine disease with complicated pathogenesis and great damage. According to pathological characteristics, thyroid nodules can be divided into benign nodules and malignant nodules. Benign nodules can be divided into nodular goiter, thyroid adenoma, Hashimoto disease, and subacute thyroiditis, and malignant nodules can be divided into follicular carcinoma, papillary carcinoma, undifferentiated carcinoma, and medullary carcinoma. Patients with benign nodules are usually asymptomatic; most patients are diagnosed by physical examinations, and a small number of patients may have local compression or discomfort in the anterior part of the neck.[19] With the application of new clinical technologies, the diagnosis of thyroid nodules is becoming more accurate. Thyroid nodules are mainly identified by means of medical history inquiry, physical examination, laboratory examination, imaging examination, and fine needle aspiration cytology (FNAC). Guidelines for the diagnosis and treatment of thyroid diseases in China describe the clinical diagnostic criteria and standardized treatment of thyroid nodules in combination with the research status of thyroid diseases in China and some guidelines published in Europe and the United States in recent years. In addition to surgical resection of thyroid nodules, Western medicine mostly adopts conventional treatment of oral drugs with slow symptom relief, and the long-term drug safety and drug resistance remain to be determined. Some thyroid nodules are treated by radioactive iodine 131 and high-frequency laser ablation. In TCM, thyroid nodules belong to the category of “Ying” with qi stagnation, blood stasis and phlegm being the basic pathogenesis. TCM is mostly treated by experience as well as basic prescriptions, Chinese patent medicines and acupuncture.[20] As an adjunctive treatment method for thyroid nodules, Chinese herbal medicine has been used in clinical practice for many years. PV has the function of clearing heat, reducing fire, dispersing nodules, and reducing swelling, and it can be used to treat thyroid diseases, such as thyroid cysts, chronic lymphocytic thyroiditis, and thyroid adenoma. To treat thyroid diseases, TCM doctors make use of PV and other medicinal materials to form a decoction, or they simply use PV as the Chinese patent medicine. Great progress has been made in this field.
Using the methods provided by the Cochrane collaboration, we objectively evaluated the efficacy and safety of PVP in adjuvant treatment of thyroid nodules. In comparison with the LT group, this review demonstrated that the PV+LT group had a more significant effect associated with clinical efficacy, the average diameter of thyroid nodules and the improvement of FT3 and FT4. However, there was no significant difference in adverse reactions between the 2 groups before and after treatment. One of the limitations of this review was that the results were based on evidence that had a high risk of bias and low quality. The Chi-square test indicated that there was high heterogeneity in the analysis results of some indicators in the group, and the reason was mainly related to the difference in inclusion criteria of patients with thyroid nodules between studies and the low sample content. Of the 8 studies, only 4[6,8,10,14] clearly stated that the included patients with thyroid nodules met the standards of the Guidelines for the diagnosis and treatment of thyroid diseases in China, whereas the remaining 4 were unclear. In the PV+TT group vs the TT group, the clinical effective rate and the incidence of adverse reactions were analyzed, indicating that the clinical effective rate was higher and the incidence of adverse reactions was lower in the treatment group. Meta-analysis revealed that the heterogeneity of the clinical effective rate was relatively high. In addition to the subjective influence of researchers, the main reason might be the difference in the criteria for determining the clinical effective rate. At present, there is a lack of relevant uniform regulations in China and abroad. By consulting the literature, we found that PV may play a therapeutic role in thyroid diseases through the several mechanisms. Yin et al[21] indicated that PV inhibits tumors by inducing apoptosis of tpc-1 and ftc-133 cell lines in differentiated thyroid cancer. Zhang et al[22] found that PV significantly promotes the apoptosis of B-CPAP thyroid cancer cells in vitro. Both studies suggested that the apoptotic effect of PV may be related to the regulation of bcl-2 protein expression.
The treatment of diseases by TCM has always been the focus of controversy among various scholars. In some clinical studies, TCM has been questioned for its uncertain efficacy compared with western medicine treatment. This study aims to explore the role of TCM in the adjuvant treatment of thyroid nodule. The objective evaluation method was adopted to analyze the effects of prunella preparation on various indicators of thyroid nodule, it affirms the efficiency of TCM and provides evidence-based medicine for clinical application. However, this study had the following limitations: after screening, only 13 studies met the inclusion criteria, and the number of studies and sample size were relatively small, which reduced the authenticity of the results; the quality of the included literature was generally low, and the methodological part of the paper was vague and unclear, leading to low value of the research results, which will hopefully be improved in future studies; and the experimental design of some basic studies was not rigorous, and various factors that may cause bias need to be considered and further improved.
Author contributions
Conceptualization: Qing Han.
Data curation: Qing Han, Bo Chen, Wei Wu.
Formal analysis: Qing Han.
Funding acquisition: Ning Xu.
Investigation: Qing Han.
Methodology: Qing Han.
Project administration: Ning Xu.
Resources: Ning Xu.
Software: Qing Han, Lei Sheng.
Visualization: Qing Han.
Writing – original draft: Qing Han.
Writing – review & editing: Qing Han, Ning Xu.
Footnotes
Abbreviations: CI = confidence interval, FT3 = free triiodothyronine, FT4 = free tetraiodothyronine, LT = levothyroxine sodium tablets, MD = mean difference, MMI = methimazole, PV = Prunella vulgaris, PVP = Prunella vulgaris preparation, RCTs = randomized controlled trials, RR = risk ratio, TCM = traditional Chinese medicine, TT = thyroxin tablets.
How to cite this article: Han Q, Xu N, Chen B, Wu W, Sheng L. Safety and efficacy of Prunella vulgaris preparation in adjuvant treatment of thyroid nodules: a meta-analysis. Medicine. 2021;100:41(e27490).
Social Science Planning and Research Project of Shandong Province (20CZXJ06).
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
No = high risk. Yes = low risk.
CI = confidence interval, FT3 = free triiodothyronine, FT4 = free tetraiodothyronine, MD = mean difference.
References
- [1].American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer.Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214. [DOI] [PubMed] [Google Scholar]
- [2].Li ZM. Investigation and analysis of thyroid nodule prevalence in healthy physical examinees. Mod Pract Med 2019;31:914–63. [Google Scholar]
- [3].Li YH, Yang WJ, Xu YS. Analysis of thyroid nodule clinical medication rule based on data mining. Shandong Med 2019;59:54–6. [Google Scholar]
- [4].Wang J, Li J. Treatment of thyroid diseases with goitre and nodule in patients with thyroid disease. Chin Herb Med 2020;51:2082–7. [Google Scholar]
- [5].Yang ZR, Sun F, Zhan SY. Risk of bias assessment series: introduction to bias assessment tool 2.0 of parallel design randomized controlled trials. Chin J Epidemiol 2017;38:1285–91. [DOI] [PubMed] [Google Scholar]
- [6].Shan XP, Song LY, Li L. Clinical study of Prunella granule combined with levothyroxine in the treatment of nodular goiter. Mod Med Clin Pract 2019;34:3708–11. [Google Scholar]
- [7].Liu JB, Yang YM, Hou N. Efficacy and safety of Prunella japonicum in the treatment of hashimoto thyroiditis with nodules. Chin Hyg Stand Manag 2018;9:115–7. [Google Scholar]
- [8].Wang L, Zhang G. Efficacy of prunella capsule combined with levothyroxine in the treatment of nodular goiter. Mod Med Clin 2018;33:2910–3. [Google Scholar]
- [9].Liu JB, Yang YM, Hou N. Effects of prunella on thyroid function and nodule size of hashimoto's thyroiditis. Chin Hyg Stand Manag 2018;9:100–1. [Google Scholar]
- [10].Xu LY, Wang YY, Huang X. Ultrasound evaluation of the clinical value of prunella capsule in the treatment of thyroid nodules. China J Pharm Econ 2017;12:71–3. [Google Scholar]
- [11].Wang TX, Huang XE, Zhou XD. Treatment of hashimoto's thyroiditis with nodules with levothyroxine sodium tablet combined with prunella capsule. New Chin Med 2017;49:60–2. [Google Scholar]
- [12].Yin PX. Efficacy of prunella tablet in the treatment of thyroid nodules. Chin J Med 2015;8:79–179. [Google Scholar]
- [13].Yue X, SHI SQ. Clinical study of methimazole combined with Xiakucao in the treatment of diffuse goiter with hyperthyroidism. J Clin Psychosom Dis 2020;26:101–4. [Google Scholar]
- [14].Wang SQ, Wei HZ, Feng BS, et al. Observation on the curative effect of prunella tablet assisted Youjiale in the treatment of qi-stagnation phlegm-blocked thyroid nodules. Mod J Integr Chin Tradit West Med 2017;26:4082–4. [Google Scholar]
- [15].An YF, Han HH. Clinical observation of prunella oral liquid in the treatment of qi-stagnation phlegm-blocked nodular goiter. Shanghai J Tradit Chin Med 2015;49:45–6. [Google Scholar]
- [16].Li T. Clinical effect analysis of comprehensive drug treatment for thyroid nodules. Chin Med Sci 2013;3:64–5. [Google Scholar]
- [17].Yang LW. Clinical effect analysis of comprehensive drug treatment for thyroid nodules. Home Med 2018;12:399. [Google Scholar]
- [18].Zhong RY. Efficacy analysis of prunella cream in adjuvant treatment of thyroid nodules. J Aerospace Med 2014;25:368–9. [Google Scholar]
- [19].Gu YM, Shao HY, Xu YS. Progress of thyroid nodules in Chinese and western medicine. Chin Med Mod Distance Educ China 2014;12:162–4. [Google Scholar]
- [20].Guo LZ, Lv X, Huang YL, et al. Thyroid nodule diagnosis and treatment of Chinese and western medicine. Hubei J Tradit Chin Med 2018;40:59–63. [Google Scholar]
- [21].Yin DT, Lei MY, Xu JH, et al. The Chinese herb Prunella vulgaris promotes apoptosis in human well-differentiated thyroid carcinoma cells via the B-cell lymphoma-2/Bcl-2-associated X protein/caspase-3 signaling pathway. Oncol Lett 2017;14:1309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang LL, Li HQ, Ma RS, et al. The pro-apoptotic effect and mechanism of Prunella vulgaris extracts on thyroid cancer cell line B-CPAP. J Xi’an Med Univ 2019;40:486–90. [Google Scholar]