Abstract
The aim of this study is to report the differences in clinicopathological features of oral tongue squamous cell carcinoma (OTSCC) and survival between adolescent and young adult (AYA) patients and elderly patients and to find the prognosticators. The medical records of 101 AYA patients and 175 control patients with OTSCC who underwent surgery were reviewed. Variables related to prognosis and their clinicopathological associations were analyzed. The 5-year overall survival (5y-OS) rates of AYA and control patients with stage I and II OTSCC were 94.4% and 89.6% (P = .353), respectively, and their 5-year disease-free survival (5y-DFS) rates were 82.0% and 76.6%, respectively (P = .476). The 5y-OS rates of patients with stages III and IV OTSCC were 83.3% and 66.7% (P = .333), respectively, and their 5y-DFS rates were 75.0% and 57.1% (P = .335), respectively. Logistic regression analysis revealed that there was no significant clinicopathological difference in AYA and control group. Furthermore, there was no significant difference in 5y-OS rates between patients who underwent elective neck dissection (END) and those who underwent therapeutic neck dissection (TND) in both group (P = 0.717 and 0.688). Overall, the present study revealed the clinicopathological features and prognosis of OTSCC were similar in AYA patients and elderly patients. Moreover, as there was no significant difference in OS between patients who underwent END and those who underwent TND in AYA and control groups, our results suggest that the indication for END in AYA patients with clinical N0 OTSCC is similar to that for elderly patients.
Keywords: adolescents and young adults, disease-free survival, elective neck dissection, oral tongue squamous cell carcinoma, overall survival, therapeutic neck dissection
1. Introduction
Over the past few decades, treatments for cancers in both adult and pediatric patients have substantially improved, thus increasing the survival rates among these patient groups.[1] Unfortunately, improvements in cancer treatment for adolescent and young adult (AYA) patients, specifically those between the ages of 15 and 39 years (the age range generally used to describe AYA patients in the United States), are lagging.[2] In this generation, cancer remains a leading cause of death behind homicide, suicide, and injuries.[3,4] The most common malignancies in AYA population are lymphoma, melanoma, testicular cancer, thyroid cancer, sarcoma, leukemia, central nervous system tumors, and breast cancer.[2] AYA patients with these malignancies have not benefited from improvements in overall survival (OS), compared to adult or pediatric patients. One possible reason for this disparity may be that malignancies among AYA patients have unique biological characteristics, resulting in differences in clinical and treatment resistance behaviors.[5] Moreover, managing cancer in AYA patients has several challenges owing to their unique clinical, psychological, and socioeconomic demands.[6–8] In addition, the participation of AYA patients in clinical trials has been inadequate for many reasons, resulting in a relative lack of progress regarding advancements in treatments in this vulnerable population.[9,10]
Oral tongue squamous cell carcinoma (OTSCC) is a rare cancer and the most common histologic type of oral cancer, accounting for approximately 90% of cases.[11] The differences in the clinicopathological features of early stage OTSCC between AYA and older adult patients were reviewed by Campbell et al.[12] However, they did not report any relationship with survival. In the present study, considering that the first choice of treatment for OTSCC is surgical resection according to National Comprehensive Cancer Network guidelines,[13] we assessed differences in clinicopathological features and survival between AYA and elderly patients in the context of OTSCC, with the aim of identifying specific prognostic factors associated with survival.
2. Materials and methods
2.1. Patients
According to the American Society of Clinical Oncology guidelines, AYAs were defined as individuals between the ages of 15 and 39 years.[6] The authors retrospectively reviewed the medical records of 107 AYA patients who underwent surgery for oral squamous cell carcinoma (OSCC) between April 2008 and March 2017 at participating hospitals. The criteria for cervical lymph node metastasis (CLNM) were as follows: using computed tomography, magnetic resonance imaging, and/or neck ultrasonography, at least below were detected: the minor diameter of the lymph node over 10 mm, the intra-lymphatic heterogeneity, and/or a round shape of node. Therapeutic neck dissection (TND) was performed in patients who were clinically diagnosed with CLNM. Also, elective neck dissection (END) was performed in patients without CLNM who required simultaneous reconstruction for vascular anastomosis. Postoperatively, negative results were observed with a wait-and-see policy.
Since the most common age for OSCC development is the 60s,[14] we excluded patients between the ages of the 40s and the 60s. Namely, the control group comprised patients in their 70s and 80s. This enabled us to clearly distinguish them from patients in the AYA group. A total of 420 patients with OSCC who met the aforementioned criteria were included in the control group. All eligible patients were capable of tolerating the surgical burden. After surgery, in patients with positive surgical margins, additional resection was performed. Postoperatively, all patients regularly underwent neck ultrasonography, computed tomography, and/or magnetic resonance imaging with or without contrast enhancement in their follow-up period. In the first year from the operation, patients visited the hospital at least once per month and underwent above imaging examinations every 3 to 6 months. In their second and third years, patients visited hospital at least once every 2 and 3 months, respectively. The follow-up period was then extended sequentially according to the duration from the operation.
2.2. Variables
The medical records, surgical procedures, clinicopathological findings, clinical courses, and prognoses were reviewed. The authors assessed age, sex, subsite of OSCC, disease stage (Union for International Cancer Control, version 7), and treatment outcomes, including local recurrence, occult CLNM (OCLNM), and distant metastasis. In addition, surgical specimens were assessed clinicopathologically for clinical type, tumor differentiation (World Health Organization grade), perineural invasion, lymphatic invasion, and vascular invasion. AYA and control groups were divided into the early (stages I and II) and late stage (stages III and IV) groups for analyses.
2.3. END and TND for AYA and control patients with OTSCC
A previous study reported that in OTSCC, one of the most important prognostic factors is the presence of neck metastasis.[15] The survival of patients who underwent END versus TND in the AYA and control groups was assessed to determine the significance of END in AYA patients with clinical N0 OTSCC. The number of AYA patients who underwent END and TND was 21 and 13, respectively, and the numbers of control patients who underwent END and TND were 9 and 45, respectively. We analyzed the 5-year OS in patients who underwent END and TND to evaluate the validity of the “wait-and-see” policy in each group.
2.4. Statistical analysis
To find clinicopathological differences between the AYA and control groups, the associations between variables and groups were assessed using Fisher exact tests and multivariate logistic regression analysis. The associations between the events (local recurrence, OCLNM, and distant metastasis) and groups were analyzed using Fisher exact test. The 5-year OS and 5-year disease-free survival (DFS) rates in the early and late stages were compared using the log-rank test. OS was assessed from the date of diagnosis to the date of death or the last follow-up date for patients who were alive. DFS was assessed from the date of diagnosis to the date of recurrence, metastasis, death, or the last follow-up date. Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Tokyo, Japan). Analysis items with two-tailed P values <.05 were considered statistically significant.
3. Results
3.1. The subsites of OSCC among AYA patients
The subsite of OSCC among almost all AYA patients was the tongue (101/107 patients, 94.4%), while the proportion of patients with OTSCC among control patients was only 41.7% (175/420 patients) (Table 1, bold value). Fisher exact test revealed a P value of <.001, indicating that the tongue is a significant OSCC subsite among AYA patients compared to that among control patients. Therefore, OTSCC patients were considered in all subsequent analyses. There was no significant bias in sex and stage among AYA and control patients.
Table 1.
Clinical characteristics and clinicopathological features of patients in the present study.
| Patients | ||
| Variable | AYA group (%) (n = 107) | Control group (%) (n = 420) |
| Age (yr) | ||
| 10s | 1 | – |
| 20s | 21 | – |
| 30s | 85 | – |
| 70s | – | 300 |
| 80s | – | 120 |
| Sex | ||
| Female | 50 | 203 |
| Male | 57 | 217 |
| Subsite | ||
| Tongue | 101 (94.4) | 175 (41.7) |
| Floor of the mouth | 4 | 20 |
| Buccal mucosa | 1 | 52 |
| Upper gingiva | 0 | 64 |
| Lower gingiva | 1 | 97 |
| Hard palate | 0 | 6 |
| Other | 0 | 6 |
| Stage | ||
| I | 61 (57.0) | 180 (42.9) |
| II | 32 (29.9) | 128 (30.5) |
| III | 8 (7.5) | 33 (7.9) |
| IV | 6 (5.6) | 77 (18.3) |
| Unknown | 0 | 2 |
3.2. Clinicopathological differences in OTSCC between the AYA and control groups
The OTSCC cohort in the AYA group included 47 women and 54 men with a median age of 33.0 (range: 21–39) years. The median follow-up period was 40.0 ± 30.6 (range: 1–132) months. In contrast, the control group included 70 females and 105 males with a median age of 76.0 years (range: 70–89). The median follow-up duration was 42.0 ± 28.8 (range: 1–130) months.
The clinical characteristics and clinicopathological features observed in the AYA and control groups during early- and late-stage OTSCC are summarized in Table 2. The differences observed in early stage OTSCC patients are as follows: the number of exophytic types in the AYA group tended to be lower, and perineural invasion, lymphatic invasion, and vascular invasion in the AYA group tended to be higher than in the control group. Univariate analysis revealed a significant difference in the clinical type of OTSCC in early stage. In contrast, multivariate analysis revealed that no variables were significantly associated with the AYA and control groups in both early and late stage. This result supports the previous study that compared the clinicopathology of early stage OTSCC between young and elderly adults.[16]
Table 2.
Analysis of clinicopathological features in AYA and control patients with oral tongue squamous cell carcinoma.
| Stage I / II | Stage III / IV | ||||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| Variables | AYA group (n = 89) | Control group (n = 154) | P value | P value | OR | 95% CI | AYA group (n = 12) | Control group (n = 21) | P value | P value | OR | 95% CI | |
| Clinical type | Superficial type | 56 | 83 | .042 | .327 | 0.835 | 0.582 to 1.198 | 1 | 1 | 1.000 | .922 | 0.926 | 0.199 to 4.310 |
| Exophytic type | 8 | 32 | 2 | 3 | |||||||||
| Endophytic type | 25 | 35 | 9 | 16 | |||||||||
| Unknown | 0 | 4 | 0 | 1 | |||||||||
| Tumor differentiation | Well | 62 | 105 | .903 | .818 | 0.948 | 0.601 to 1.495 | 7 | 9 | .722 | .470 | 0.650 | 0.202 to 2.093 |
| Moderate | 18 | 32 | 3 | 8 | |||||||||
| Poor | 8 | 11 | 2 | 4 | |||||||||
| Unknown | 1 | 6 | 0 | 0 | |||||||||
| Perineural invasion | No | 80 | 143 | .331 | .529 | 1.395 | 0.495 to 3.933 | 9 | 13 | .703 | .616 | 0.590 | 0.075 to 4.642 |
| Yes | 9 | 10 | 3 | 8 | |||||||||
| Unknown | 0 | 1 | 0 | 0 | |||||||||
| Lymphatic invasion | No | 77 | 140 | .184 | .362 | 1.551 | 0.604 to 3.983 | 7 | 14 | .716 | .555 | 1.682 | 0.299 to 9.451 |
| Yes | 12 | 12 | 5 | 7 | |||||||||
| Unknown | 0 | 2 | 0 | 0 | |||||||||
| Vascular invasion | No | 68 | 128 | .170 | .292 | 1.518 | 0.699 to 3.297 | 5 | 7 | .716 | .961 | 0.955 | 0.152 to 5.985 |
| Yes | 21 | 24 | 7 | 14 | |||||||||
| Unknown | 0 | 2 | 0 | 0 | |||||||||
3.3. Chemotherapy and radiotherapy
In the AYA group, adjuvant chemotherapy and radiotherapy were administered to 5 (4.9%) and 6 patients (5.9%), respectively, with multiple metastases with or without extranodal extension. In the control group, 10 patients (5.7%) received chemotherapy, and 14 patients (8.0%) underwent radiotherapy. According to the National Comprehensive Cancer Network guidelines,[13] concurrent chemoradiotherapy with high-dose cisplatin is recommended for patients at high risk of recurrent and/or metastatic OTSCC, which includes patients with multiple CLNM, extranodal extensions, and positive surgical margins. In both groups, the adjuvant chemotherapy regimens used were mainly high-dose cisplatin which was combined with radiotherapy. In some cases, S-1 (tegafur–gimeracil–oteracil) was administered in combination with radiotherapy. This regimen was administered to patients with close surgical margins and renal disfunction and those who refused treatment with cisplatin. Total radiation doses ranged from 50 to 63 Gy. Radiotherapy at 12 Gy was stopped in 1 patient in the control group because of rapid growth of the tumor at the other side of the neck.
Patients with inoperable recurrence or metastasis were treated with chemotherapy or chemoradiotherapy. A regimen comprising cisplatin/5-fluorouracil with or without cetuximab was mainly administered to these patients as a first-line therapy. Treatment with one of the regimens was continued until response evaluation criteria in solid tumors-defined progression disease, unacceptable toxicity, or withdrawal of consent. The second-line therapy was cetuximab plus paclitaxel or maintenance with cetuximab alone. The completion rates for chemotherapy and radiotherapy were similar in both groups.
3.4. Survival in the AYA and control groups
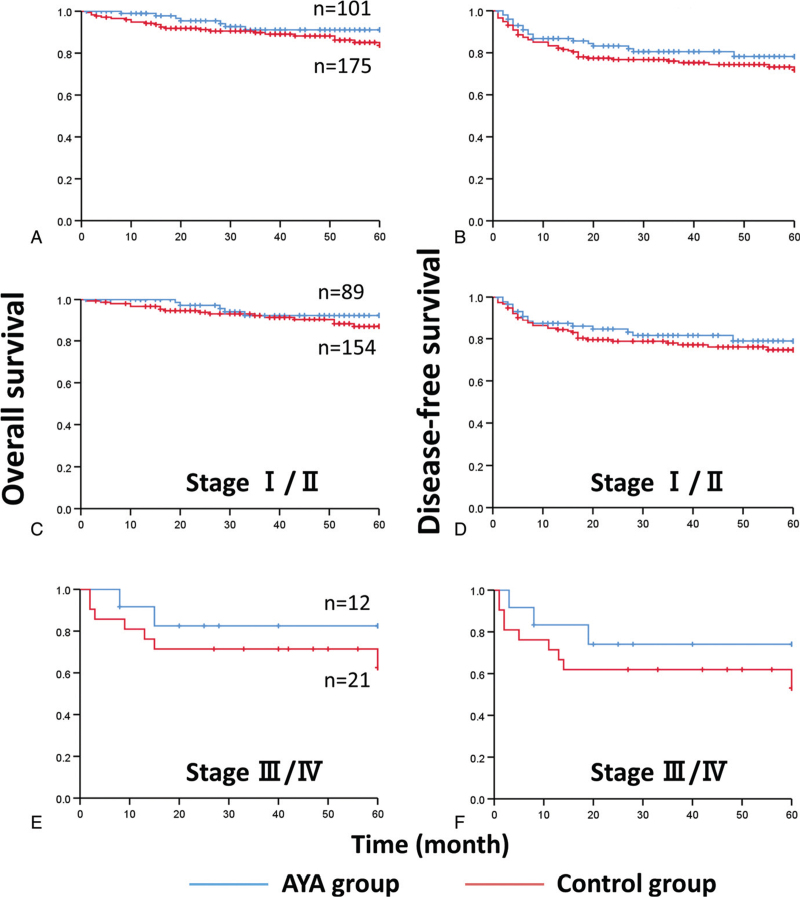
There were no significant differences in stage-specific local recurrence, OCLNM, and distant metastasis between the 2 groups (Table 3). Figure 1 shows the Kaplan–Meier curves: the 5-year OS rates of AYA and control patients with stages I and II OTSCC were 94.4 and 89.6% (total 91.4%, P = .353), respectively, and their 5-year DFS rates were 82.2 and 76.6% (total 78.6%, P = .476), respectively. The 5-year OS rates of AYA and control patients with stages III and IV OTSCC were 83.3% and 66.7% (total 72.7%, P = .333), respectively, and their 5-year DFS rates were 75.0% and 57.1% (total 63.6%, P = .335), respectively. Although there was no significant difference, there was a trend toward poorer survival outcomes, both OS and DFS, in the control group, especially in the late stage.
Table 3.
Postoperative courses among AYA and control patients with OTSCC.
| Patients n (%) | |||
| Variable | AYA group n = 101 | Control group n = 175 | P value |
| Local recurrence | |||
| No | 95 | 167 | .777 |
| Yes | 6 (5.9) | 8 (4.6) | |
| Occult cervical lymph node metastasis | |||
| No | 80 | 141 | .876 |
| Yes | 21 (20.8) | 34 (19.4) | |
| Distant metastasis | |||
| No | 95 | 169 | .366 |
| Yes | 6 (5.9) | 6 (3.4) | |
Figure 1.
(A) The 5-year overall survival (5y-OS) rates of AYA and control patients in the present study were 93.1% and 86.9% (total 89.1%, P = .215), respectively, and (B) their 5-year disease-free survival (5y-DFS) rates were 81.2% and 74.3% (total 76.8%, P = .309), respectively. (C) The 5y-OS rates for patients with stages I and II OTSCC were 94.4 and 89.6% (total 91.4%, P = .353), respectively, and (D) their 5y-DFS rates were 82.2 and 76.6% (total 78.6%, P = .476), respectively. (E) The 5y-OS rates for patients with stages III and IV OTSCC were 83.3% and 66.7% (total 72.7%, P = .333), respectively, and (F) their 5y-DFS rates were 75.0% and 57.1% (total 63.6%, P = .335), respectively. AYA = adolescent and young adult, OTSCC = oral tongue squamous cell carcinoma.
We also analyzed OS and DFS based on pathological results (p-stage). The 5-year OS rates of AYA and control patients with p-stage I and II were 98.8% (n = 84) and 89.9% (n = 149) (P = .022), respectively, and those of AYA and control patients with p-stage III and IV were 64.7% (n = 17) and 69.2% (n = 26) (P = .735), respectively. The 5-year DFS rates of AYA and control patients with p-stage I and II were 88.1% and 78.5% (P = .121), respectively, and the 5-year DFS rates for those with p-stage III and IV were 47.1% and 50.0% (P = .903), respectively.
3.5. END and TND in AYA patients with OTSCC
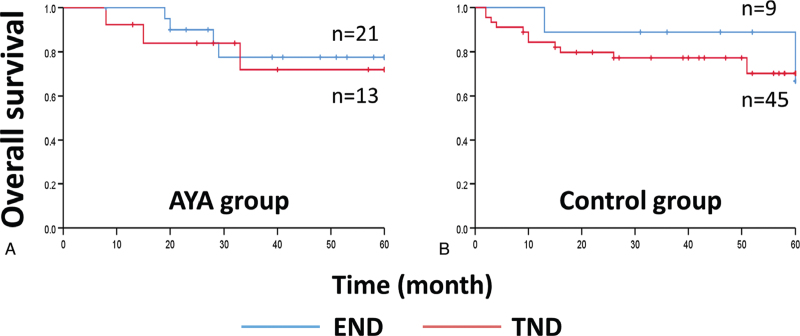
The 5-year OS rates of AYA patients who underwent END and TND were 81.0% and 76.9% (P = .717), respectively. In addition, the 5-year OS rates of control patients who underwent END and TND were 77.8% and 73.3% (P = .688), respectively (Fig. 2). Our findings suggest that the indication for END in AYA patients with clinical N0 OTSCC is similar to that for elderly patients.
Figure 2.
The 5-year overall survival rates of (A) AYA patients who underwent elective neck dissection and therapeutic neck dissection were 81.0% and 76.9% (total 79.4%, P = .717), respectively, and those of (B) control patients were 77.8% and 73.3% (total 74.1%, P = .688), respectively. AYA = adolescent and young adult, END = elective neck dissection, TND = therapeutic neck disection.
4. Discussion
In the present study, we analyzed OTSCC patients whose ages are between 15 and 39 years according to the American Society of Clinical Oncology guidelines.[6] However, this definition of the age has not been adopted worldwide.[17] In the UK, teenage and young adult patients are considered to be those between 15 and 24 years of age; groups in New Zealand and Canada reference AYAs as individuals aged 15 to 29 years; and a publication from the Shanghai Cancer Institute on cancer incidence among AYAs included persons aged from 15 to 49 years.[18–20] This discrepancy is considered as a variation of pediatric oncology practices in each country.[21] Regardless of what is effectively an administrative or academic designation, these patients are represent patients with cancer who have unique needs.[22,23]
Cancers affecting AYA patients are diverse, spanning the spectrum from pediatric to adult-type malignancies. For instance, young women between the ages of 15 and 39 years are more likely to have high-grade, locally advanced triple-negative breast cancer than elderly patients,[24] and young age appears to be a specific indicator of poor prognosis for this disease, independent of stage or histologic type.[6] Thus, identifying and characterizing genomic mutations among cancers in AYAs may help us understand the role of disease biology in determining prognosis and predicting therapeutic outcomes. In head and neck oncology, Ryu et al[25] reported that perineural invasion, PD-L1 positivity, and a higher ratio of CD163-positive tumor infiltrating macrophages to CD8-positive tumor infiltrating lymphocytes were independent factors for poor progression-free survival in young patients. However, the ability to apply such findings to routine clinical practice is limited by high costs, special techniques, and equipment associated with sequencing, and the inability to validate these findings in all hospitals.
Notably, almost all OSCCs occurred on the tongue in patients with AYA in the present study. The general causes of OTSCC have been reported to include unsuitable tooth fillings or prosthesis placement, smoking, alcohol consumption, chronic inflammation, precancerous lesions such as epithelial dysplasia, infection, endocrine disease, poor oral hygiene, heredity, mechanical trauma, galvanic phenomena, and contact allergy to metal dental restorations.[12,26–32] However, since the duration of the exposure to the aforementioned causes is apparently shorter in AYA patients than in elderly patients, those causes cannot be applied for the development of OTSCC in AYA patients. Kim et al[33] reported that the lingual position of the mandibular second molar and the narrow tongue space in young mature patients are associated with tongue cancer development. Therefore, we believe that AYA patients with OTSCC tend to have specific anatomical physical characteristics. The association between these features and the development of OTSCC among AYA patients should be prospectively investigated in the future. In the AYA population, Fanconi anemia is also a strong risk factor for the development of head and neck SCC because of the absence of DNA repair genes.[34,35] The present cohort also included 3 AYA patients with Fanconi anemia.
In the present study, the 5-year OS and DFS rates were not significantly different between the AYA and control groups, the recurrence and metastasis rates were similar in both groups, and there was no significant difference in the completion rates of chemotherapy and radiotherapy between the 2 groups. Taken together, considering that there were no significant clinicopathological and survival differences in both groups, OTSCC among AYA patients is not always characterized by increased aggressiveness compared with that in elderly patients. In contrast, Friedlander et al[36] previously reported that younger patients with OTSCC had a significantly higher rate of locoregional recurrence than older patients; however, the 5-year DFS rates in the young and older groups were not significantly different. Verschuur et al[37] also reported that young patients with head and neck SCC did not have a worse prognosis than a matched older patient group in their case-controlled study. Our data also support these results and the results of a previous meta-analysis by Pitman et al.[38] Furthermore, Oliver et al[39] reported that their propensity score-matched survival analysis in the National Cancer Database revealed that OTSCC patients aged under 40 years had a 9% higher 5-year survival rate. They concluded that younger patients with OTSCC did not have worse survival (77.1% vs 68.2%, P < .001).[39]
In the present study, the rate of OCLNM was similar between the 2 groups (20.8% and 19.4%). In addition, there was no significance in the 5-year OS rates of AYA and control patients who underwent END and TND, suggests that the indication for END for AYA patients is similar to that for elderly patients. In a systematic review and meta-analysis, Abu-Ghanem et al[40] reported that END can significantly reduce the rate of regional nodal recurrence and improve disease-specific survival rate but cannot improve OS in patients with clinical T1/2 N0 OTSCC. They reported that the “wait-and-see” policy did not decrease OS in patients with early-stage OTSCC. In contrast, D’Cruz et al[41] reported in a prospective, randomized, controlled trial that among patients with early stage OSCC, END resulted in higher OS and DFS than TND. Although they insisted on the significance of END, their study design is far different from ours. Their cohort included patients with OSCCs other than OTSCC. Their follow-up interval and the timing of their routine imaging examination were also different from ours. Therefore, their END criteria may not directly apply to the AYA cohort in our study. In addition, tumor depth is also a controversial potential factor. Otsuru et al[42] reported that END should be performed in OTSCC patients with a tumor depth of at least 4 to 5 mm, which is associated with a high rate of OCLNM. Considering that the univariate analysis in the present study revealed that the clinical type of OTSCC in the early stage is a significant difference between the AYA and control OTSCC patients, prospective studies involving large numbers of AYA patients will help to determine the efficacy of this parameter.
The present study had some limitations, mostly pertaining to its retrospective design, including cohort selection, the impact of previous exposure to risk variables (eg, smoking, alcohol consumption, and virus status), treatment approaches, follow-up, reporting (including missing data), complications (eg, diabetes mellitus and immunosuppressive conditions), and genetic mutations (eg, Fanconi anemia).
In conclusion, there were no significant differences in clinicopathological features and survival between the AYA and control group. Moreover, since there was no significant difference in OS between patients who underwent END and those who underwent TND in the AYA and control groups, our results suggest that the indication for END for AYA patients with clinical N0 OTSCC is similar to that for elderly patients.
Author contributions
Conceptualization: Kohei Okuyama.
Data curation: Kohei Okuyama, Souichi Yanamoto, Yasuyuki Michi, Eri Shibata, Maiko Tsuchiya, Misaki Yokokawa, Tomofumi Naruse, Hirofumi Tomioka, Takeshi Kuroshima, Hiroaki Shimamoto, Hiroyuki Harada.
Formal analysis: Kohei Okuyama, Souichi Yanamoto.
Investigation: Kohei Okuyama, Souichi Yanamoto, Yasuyuki Michi.
Project administration: Kohei Okuyama.
Software: Kohei Okuyama.
Supervision: Souichi Yanamoto, Tohru Ikeda, Masahiro Umeda, Tetsuya Yoda, Hiroyuki Harada.
Validation: Kohei Okuyama, Masahiro Umeda, Tetsuya Yoda, Hiroyuki Harada.
Writing – original draft: Kohei Okuyama.
Writing – review & editing: Kohei Okuyama.
Footnotes
Abbreviations: 5y-OS = 5-year overall survival, AYA = adolescent and young adult, CLNM = cervical lymph node metastasis, DFS = disease-free survival, END = elective neck dissection, OCLNM = occult cervical lymph node metastasis, OS = overall survival, OSCC = oral squamous cell carcinoma, OTSCC = oral tongue squamous cell carcinoma, SCC = squamous cell carcinoma, TND = therapeutic neck dissection.
How to cite this article: Okuyama K, Yanamoto S, Michi Y, Shibata E, Tsuchiya M, Yokokawa M, Naruse T, Tomioka H, Kuroshima T, Shimamoto H, Ikeda T, Umeda M, Yoda T, Harada H. Multicenter retrospective analysis of clinicopathological features and prognosis of oral tongue squamous cell carcinoma in adolescent and young adult patients. Medicine. 2021;100:41(e27560).
The authors have no funding and conflicts of interest to disclose.
This study conformed to the tenets of the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of each institution (reference number: 19031114).
The study protocol was approved by the institutional review boards of all participating institutions.
Due to the retrospective nature of the study, informed consent was not provided by all patients; however, they could decide to opt out at any point in the study.
The datasets generated during and/or analyzed during the current study are publicly available.
AYA = adolescent and young adult, CI = confidence interval, OR = odds ratio.
AYA = adolescent and young adult.
References
- [1].Ferreira CG, de Melo AC, Nogueira-Rodrigues A. The adolescent and young adult with cancer: state of the art—epithelial cancer. Curr Oncol Rep 2013;15:287–95. [DOI] [PubMed] [Google Scholar]
- [2].Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol 2002;38:01–10. [DOI] [PubMed] [Google Scholar]
- [3].Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin 2007;57:242–55. [DOI] [PubMed] [Google Scholar]
- [4].Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist 2006;11:590–601. [DOI] [PubMed] [Google Scholar]
- [5].Tricoli JV, Bleyer A. Adolescent and young adult cancer biology. Cancer J 2018;24:267–74. [DOI] [PubMed] [Google Scholar]
- [6].Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer 2008;8:288–98. [DOI] [PubMed] [Google Scholar]
- [7].Coccia PF, Pappo AS, Altman J, et al. Adolescent and young adult oncology, version 2.2014. J Natl Compr Canc Netw, Version 22014 2014;12:21–32. [DOI] [PubMed] [Google Scholar]
- [8].Rosenberg AR, Kroon L, Chen L, et al. Insurance status and risk of cancer mortality among adolescents and young adults. Cancer 2015;121:1279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parsons HM, Harlan LC, Seibel NL, et al. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol 2011;29:4045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bleyer A, Choi M, Fuller CD, et al. Relative lack of conditional survival improvement in young adults with cancer. Semin Oncol 2009;36:460–7. [DOI] [PubMed] [Google Scholar]
- [11].Ho AS, Kim S, Tighiouart M, et al. Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol 2017;35:3601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Campbell BR, Netterville JL, Sinard RJ, et al. Early onset oral tongue cancer in the United States: a literature review. Oral Oncol 2018;87:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colevas AD, Yom SS, Pfister DG, et al. NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw, Version 12018 2018;16:479–90. [DOI] [PubMed] [Google Scholar]
- [14].Zanoni DK, Montero PH, Migliacci JC, et al. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol 2019;90:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].El-Naaj IA, Leiser Y, Shveis M, et al. Incidence of oral cancer occult metastasis and survival of T1–T2 N0 oral cancer patients. J Oral Maxillofac Surg 2011;69:2674–9. [DOI] [PubMed] [Google Scholar]
- [16].Bello IO, Almangush A, Heikkinen I, et al. Histological characteristics of early-stage oral tongue cancer in young versus older patients: a multicenter matched-pair analysis. Oral Dis 2020;26:1081–5. [DOI] [PubMed] [Google Scholar]
- [17].Adolescent and Young Adult Oncology Progress Review Group. Closing the gap: research and care imperatives for adolescents and young adults with cancer. Office of Science Planning & Assessment (OSPA) Library. Available at: https://www.livestrong.org/content/closing-gap-research-and-care-imperatives-adolescents-and-young-adults-cancer. Updated August 2006. Accessed September 9, 2021. [Google Scholar]
- [18].Bleyers A, Albritton KH, Barr RD, et al. Trailblazers in adolescent and young adult oncology. J Adolesc Young Adult Oncol 2011;1:13–8. [DOI] [PubMed] [Google Scholar]
- [19].De P, Ellison LF, Barr RD, et al. Canadian adolescents and young adults with cancer: opportunity to improve coordination and level of care. CMAJ 2011;183:E187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu QJ, Vogtmann E, Zhang W, et al. Cancer incidence among adolescents and young adults in urban Shanghai, 1973–2005. PLoS One 2012;7:e42607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pollock BH, Birch JM. Registration and classification of adolescent and young adult cancer cases. Pediatr Blood Cancer 2008;50:1090–3. [DOI] [PubMed] [Google Scholar]
- [22].Rajani S, Young AJ, McGoldrick DA, Pearce DL, Sharaf SM. The international charter of rights for young people with cancer. J Adolesc Young Adult Oncol 2011;1:49–52. [DOI] [PubMed] [Google Scholar]
- [23].Sender L, Zabokrtsky KB. Adolescent and young adult patients with cancer: a milieu of unique features. Nat Rev Clin Oncol 2015;12:465–80. [DOI] [PubMed] [Google Scholar]
- [24].Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324–30. [DOI] [PubMed] [Google Scholar]
- [25].Ryu HJ, Kim EK, Cho BC, et al. Characterization of head and neck squamous cell carcinoma arising in young patients: Particular focus on molecular alteration and tumor immunity. Head Neck 2019;41:198–207. [DOI] [PubMed] [Google Scholar]
- [26].Lissowska J, Pilarska A, Pilarski P, et al. Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur J Cancer Prev 2003;12:25–33. [DOI] [PubMed] [Google Scholar]
- [27].Fan H, Yoon KY, Kim SM, et al. Relationship between squamous cell carcinoma of the tongue and the position of dental prosthesis. J Adv Prosthodont 2015;7:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zafereo ME, Xu L, Dahlstrom KR, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol 2016;56:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Amagasa T, Yamashiro M, Uzawa N. Oral premalignant lesions: from a clinical perspective. Int J Clin Oncol 2011;16:05–14. [DOI] [PubMed] [Google Scholar]
- [30].Hougeir FG, Yiannias JA, Hinni ML, et al. Oral metal contact allergy: a pilot study on the cause of oral squamous cell carcinoma. Int J Dermatol 2006;45:265–71. [DOI] [PubMed] [Google Scholar]
- [31].Li R, Faden DL, Fakhry C, et al. Clinical, genomic, and metagenomic characterization of oral tongue squamous cell carcinoma in patients who do not smoke. Head Neck 2015;37:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sorensen DM, Lewark TM, Haney JL, et al. Absence of p53 mutations in squamous carcinomas of the tongue in nonsmoking and nondrinking patients younger than 40 years. Arch Otolaryngol Head Neck Surg 1997;123:503–6. [DOI] [PubMed] [Google Scholar]
- [33].Kim Y, Okuyama K, Michi Y, Ohyama Y, Uzawa N, Yamaguchi S. Potential factors influencing the development of oral tongue squamous cell carcinoma in young mature patients: lingual position of the mandibular second molar and narrow tongue space. Oncol Lett 2017;14:7339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood 2003;101:1249–56. [DOI] [PubMed] [Google Scholar]
- [35].Furquim CP, Pivovar A, Amenábar JM, et al. Oral cancer in Fanconi anemia: review of 121 cases. Crit Rev Oncol Hematol 2018;125:35–40. [DOI] [PubMed] [Google Scholar]
- [36].Friedlander PL, Schantz SP, Shaha AR, et al. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck 1998;20:363–8. [DOI] [PubMed] [Google Scholar]
- [37].Verschuur HP, Irish JC, O'Sullivan B, et al. A matched control study of treatment outcome in young patients with squamous cell carcinoma of the head and neck. Laryngoscope 1999;109:249–58. [DOI] [PubMed] [Google Scholar]
- [38].Pitman KT, Johnson JT, Wagner RL, et al. Cancer of the tongue in patients less than forty. Head Neck 2000;22:297–302. [DOI] [PubMed] [Google Scholar]
- [39].Oliver JR, Wu SP, Chang CM, et al. Survival of oral tongue squamous cell carcinoma in young adults. Head Neck 2019;41:2960–8. [DOI] [PubMed] [Google Scholar]
- [40].Abu-Ghanem S, Yehuda M, Carmel NN, et al. Elective neck dissection vs observation in early-stage squamous cell carcinoma of the oral tongue with no clinically apparent lymph node metastasis in the neck: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2016;142:857–65. [DOI] [PubMed] [Google Scholar]
- [41].D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med 2015;373:521–9. [DOI] [PubMed] [Google Scholar]
- [42].Otsuru M, Ota Y, Yanamoto S, et al. A multicenter retrospective study of elective neck dissection for T1-2N0M0 tongue squamous cell carcinoma: analysis using propensity score-matching. Ann Surg Oncol 2019;26:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]