Abstract
This study was performed to verify whether lactate dehydrogenase to albumin (LDH/ALB) ratio could be used as an independent prognostic factor in patients with severe infection requiring intensive care.
We reviewed electronic medical records of patients hospitalized to the intensive care unit via the emergency department with a diagnosis of infection between January 2014 and December 2019. From the collected data, ALB-based ratios (LDH/ALB, blood urea nitrogen to albumin, C-reactive protein to albumin, and lactate to albumin ratios) and some severity scores (modified early warning score, mortality in emergency department sepsis score [MEDS], and Acute Physiology And Chronic Health Evaluation II [APACHE II] score) were calculated. LDH/ALB ratio for predicting the in-hospital mortality was compared with other ALB-based ratios and severity scales by univariable and receiver-operating characteristics curve analysis. Modified severity scores by LDH/ALB ratio and multivariable logistic regression were used to verify the independence and usefulness of the LDH/ALB ratio.
The median LDH/ALB ratio was higher in non-survivors than survivors (166.9 [interquartile range: 127.2–233.1] vs 214.7 [interquartile range: 160.2–309.7], P < .001). The area under the receiver-operating characteristics curve of the LDH/ALB ratio (0.642, 95% confidence interval: 0.602–0.681, P < .001) was not lower than that of other ALB-based ratios and severity scores. From multivariable logistic regression, LDH/ALB ratio was independently associated with in-hospital mortality (odds ratio = 1.001, 95% confidence interval: 1.000–1.002, P = .047). Area under the receiver-operating characteristics curves of MEDS and APACHE II scores were improved by modification with LDH/ALB ratio (MEDS: 0.643 vs 0.680, P < .001; APACHE II score: 0.675 vs 0.700, P = .003).
LDH/ALB ratio may be useful as the prognostic factor in patients with severe infection requiring intensive care.
Keywords: albumin, infection, lactate dehydrogenase, mortality
1. Introduction
Sepsis is a medical emergency and is the body's systemic immunological response to an infectious process that can lead to end-stage organ dysfunction and death.[1] Despite significant advancements in the understanding of the pathophysiology of the clinical syndrome and advancements in hemodynamic monitoring tools and resuscitation measures, sepsis remains a major cause of morbidity and mortality in infection patients.[1] Therefore, predictive prognostic factors of mortality are important for the early detection of sepsis and timely management of infection patients.
Serum lactate dehydrogenase (LDH) levels that reflect the extent of cellular damages tend to rise with an increase in infection severity. Thus some studies have mentioned that LDH level is a prognostic factor for infection.[2–7] Similarly, albumin (ALB) as a negative acute-phase proteins (APPs) can also serve as a biomarker for prognosis in sepsis patients because its levels tend to decrease with infection aggravation.[8–11] However, serum ALB levels can be affected by multiple conditions, including malnutrition and liver cirrhosis. For this reason, ALB has been used as a composite indicator such as blood urea nitrogen to albumin (BUN/ALB) ratio, lactate to albumin (LAC/ALB) ratio, C-reactive protein to albumin (CRP/ALB) ratio, and procalcitonin to ALB ratio rather than as a prognostic indicator alone.[12–20]
However, the LDH to ALB (LDH/ALB) ratio has not been assessed as a prognostic factor of infection, even though evaluated for the prognostic factor of malignancy. Thus, we hypothesized that the LDH/ALB ratio could be the prognostic factor with improved accuracy for infection patients. Moreover, we performed this study to verify whether the LDH/ALB ratio could be used as an independent prognostic factor for infection patients who needed critical care.
2. Material and methods
2.1. Study design and setting
This retrospective study was conducted by reviewing secondary data extracted from electronic medical records of patients who visited the emergency department (ED) between January 2014 and December 2019 and were hospitalized for infection management. Our hospital is a tertiary-care university hospital with 1350 beds, and the ED is visited by approximately 56,000 patients every year. This study was approved by the Institutional Review Board of our hospital (No. 2020-01-077). The extracted data included clinical data only; it did not include any personally identifiable information. Therefore, the need for informed consent was waived.
2.2. Data collection and outcome measures
To research patients who were judged to have severe sepsis, we analyzed the data of patients aged >18 years who were diagnosed with an infection and admitted via the ED for intensive care. Furthermore, the patients whose both ED and discharge diagnoses after admission were related to infection were included. Patients who met the inclusion criteria were presumed as severe sepsis patients who needed intensive care.
To extract the data of the included patients, we used the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) code; the ICD-10 codes included were A00–B99, G00–09, I00–02, I30–33, I38–41, J00–J22, J36, J37, J40–J43, J68, J69, J80, J85–J86, K11–12, K35–37, K57, K61, K63, K65, K67, K75, K77.0, K80–81, K83.0, K85, L00–08, M00–03, M86, N10, N12, N13.6, N16.0, N28.84–28.86, N30, N34, N39.0, N41, N45, N61, N70–74, O91 (Supplement 1, Supplemental Digital Content).
The extracted clinical information included age, sex, comorbid diseases, initial vital signs and laboratory data in the ED, and Acute Physiology And Chronic Health Evaluation II score (APACHE II) calculated in the intensive care unit (ICU). Moreover, we calculated the Charlson comorbidity index for each patient, categorizing patients’ comorbidities based on administrative data.[21] ALB-based ratios such as BUN/ALB, CRP/ALB, LAC/ALB, and LDH/ALB ratios were calculated using initial laboratory data. All laboratory data were measured in the emergency laboratory unit in our hospital. To verify the patient's severity, modified early warning score and mortality in the emergency department sepsis score (MEDS) were also calculated from the initial data.[22,23] Cases in which ALB-based ratio or severity scores could not be calculated due to missing data were excluded. In-hospital mortality was the primary outcome of this study.
2.3. Statistical analyses
Categorical variables were analyzed using the χ2 test or Fisher exact test, and continuous variables were analyzed using Student t test or the Mann–Whitney U test. Categorical variables are expressed by number (%), and continuous variables are expressed by median [interquartile range] or mean (standard deviation). After univariable analysis, multivariable logistic regression analysis was performed to identify independent prognostic factors. The receiver-operating characteristics curve analysis for in-hospital mortality was performed, and the areas under the receiver-operating characteristics curve (AUROC) and cutoff value (Youden index) were obtained for individual variables. Moreover, the LDH/ALB ratio was categorized using cutoff value, and adjusted odds ratios (aOR) for each category were obtained. Modification of severity scores with the categorized LDH/ALB ratio was also performed to verify whether modified severity scores were better than non-modified scores. All statistical analyses were performed using IBM SPSS Statistics, version 26 (IBM Inc., Chicago) and MedCalc 15.2.2 (MedCalc Software, Mariakerke, Belgium). P values < .05 were considered statistically significant.
3. Results
3.1. Patient characteristics
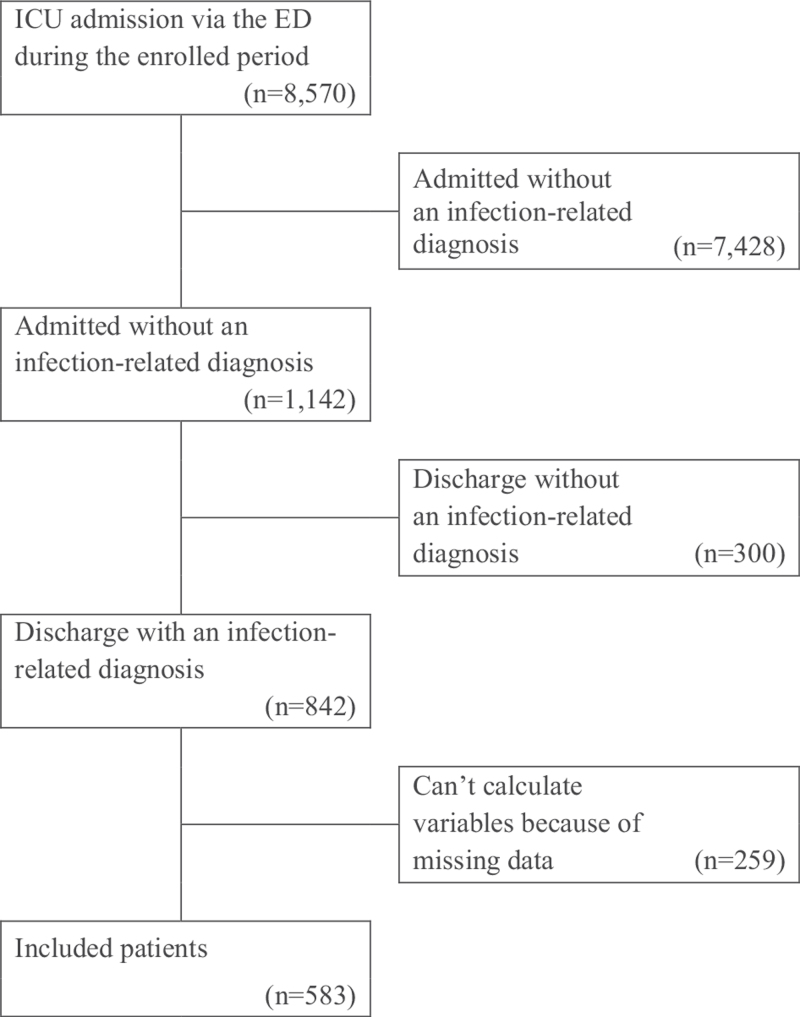
During the study period, 8570 patients were hospitalized in the ICU via the ED. Among those patients, 842 patients (9.8%) had both admission and discharge diagnosis codes related to infection. Finally, 583 patients were analyzed after excluding patients with missing data (259/842, 30.8%) (Fig. 1). The median age of the included patients was 75.0 [65.0–81.0] years, and 332 patients (56.9%) were men. The in-hospital mortality of the included patients was 20.4% (119/583). The most common infection focus was respiratory tract infection (48.5%), followed by intra-abdominal infection (23.3%) and urinary tract infection (11.8%). Among the laboratory data, ALB (2.9 [2.5–3.3] g/dL vs 2.5 [2.1–3.1] g/dL, P < .001), LAC (2.7 [1.8–4.2] mmol/L vs 3.2 [2.0–5.0] mmol/L, P = .041), LDH (486.2 [374.5–621.3] vs 542.0 [401.0–783.0], P = .006) were significantly different between survivors and non-survivors. Other demographic characteristics, comorbid diseases, vital signs, initial laboratory findings, and severity indexes are provided in Table 1.
Figure 1.
Flowchart of recruited and enrolled study participants. ED = emergency department, ICU = intensive care unit
Table 1.
Baseline characteristics.
| Overall (n = 583) | Survivors (n = 464) | Non-survivors (n = 119) | P | |
| Age, yr | 75.0 [65.0–81.0] | 74.0 [65.0–80.0] | 76.0 [68.0–82.0] | .026 |
| Sex, male | 332 (56.9) | 251 (54.1) | 81 (68.1) | .007 |
| Comorbidities | ||||
| Hypertension | 292 (50.1) | 234 (50.4) | 58 (48.7) | .821 |
| Diabetes | 195 (33.4) | 158 (34.1) | 37 (31.1) | .616 |
| Heart failure | 43 (7.4) | 33 (7.1) | 10 (8.4) | .776 |
| Chronic lung disease | 43 (7.4) | 31 (6.7) | 12 (10.1) | .284 |
| Chronic renal disease | 64 (11.0) | 46 (9.9) | 18 (15.1) | .145 |
| Chronic liver disease | 32 (5.5) | 25 (5.4) | 7 (5.9) | .822 |
| Malignancy | 71 (12.2) | 59 (12.7) | 12 (10.1) | .530 |
| Dementia | 152 (26.1) | 119 (25.6) | 33 (27.7) | .730 |
| CCI | 4.0 [3.0–6.0] | 4.0 [3.0–6.0] | 5.0 [3.0–6.0] | .072 |
| Transferred from other facility | 321 (55.1) | 246 (53.0) | 75 (63.0) | .064 |
| LTCF | 130 (22.3) | 97 (20.9) | 33 (27.7) | .141 |
| Suspected infection focus | ||||
| Respiratory tract infection | 283 (48.5) | 208 (44.8) | 75 (63.0) | <.001 |
| Urinary tract infection | 69 (11.8) | 67 (14.4) | 2 (1.7) | <.001 |
| Intra-abdominal infection | 136 (23.3) | 118 (25.4) | 18 (15.1) | .024 |
| Initial vital signs | ||||
| SBP, mm Hg | 116.0 [95.0–141.0] | 115.0 [94.0–139.8] | 117.0 [99.0–149.0] | .143 |
| DBP, mm Hg | 66.0 [55.0–79.0] | 66.0 [54.0–79.0] | 66.0 [57.0–79.0] | .441 |
| Heart rate, /min | 104.0 [89.0–122.0] | 104.0 [89.0–120.0] | 108.0 [91.0–127.0] | .114 |
| Respiration rate, /min | 24.0 [20.0–32.0] | 24.0 [20.0–30.0] | 28.0 [22.0–34.0] | .002 |
| Body temperature, °C | 37.5 [36.7–38.3] | 37.6 [36.8–38.5] | 37.1 [36.4–37.9] | <.001 |
| SpO2, % | 95.0 [89.0–97.0] | 95.0 [90.0–97.0] | 91.0 [82.0–96.0] | <.001 |
| Glasgow coma scale | 15.0 [9.0–15.0] | 15.0 [10.0–15.0] | 13.0 [8.0–15.0] | .042 |
| Initial laboratory data | ||||
| WBC, /mm3 | 10.9 [7.5–17.0] | 11.1 [7.5–16.9] | 10.9 [6.8–17.0] | .757 |
| ALB, g/dL | 2.9 [2.4–3.3] | 2.9 [2.5–3.3] | 2.5 [2.1–3.1] | <.001 |
| BUN, mg/dL | 24.0 [16.0–37.0] | 24.0 [16.0–36.8] | 25.0 [17.0–43.0] | .189 |
| CRP, mg/dL | 13.9 [5.5–20.9] | 14.0 [5.4–21.2] | 13.8 [5.5–20.1] | .764 |
| LAC, mmol/L | 2.7 [1.8–4.3] | 2.7 [1.8–4.2] | 3.2 [2.0–5.0] | .041 |
| LDH, U/L | 493.0 [378.0–658.0] | 486.5 [374.5–621.3] | 542.0 [401.0–783.0] | .006 |
| ALB-based ratio | ||||
| BUN/ALB ratio | 8.6 [5.5–14.2] | 8.3 [5.4–13.5] | 10.9 [5.9–17.3] | .008 |
| CRP/ALB ratio | 5.0 [1.7–8.6] | 4.9 [1.7–7.5] | 5.5 [1.8–9.2] | .184 |
| LAC/ALB ratio | 1.00 [0.65–1.53] | 0.93 [0.64–1.42] | 1.21 [0.84–2.00] | <.001 |
| LDH/ALB ratio | 173.4 [131.3–251.2] | 166.9 [127.2–233.1] | 214.7 [160.2–309.7] | <.001 |
| Severity scales | ||||
| MEWS | 4.0 [3.0–6.0] | 4.0 [3.0–6.0] | 4.0 [3.0–6.0] | .595 |
| MEDS | 10.0 [8.0–13.0] | 10.0 [6.0;13.0] | 12.0 [9.0–15.5] | <.001 |
| APACHE II (ICU) | 20.0 [14.0–25.0] | 19.0 [13.0–24.0] | 24.0 [18.0–30.0] | <.001 |
| Interventions | ||||
| Vasopressors | 327 (56.1) | 243 (52.4) | 84 (70.6) | <.001 |
| Mechanical ventilation | 288 (49.4) | 204 (44.0) | 84 (70.6) | <.001 |
3.2. Analysis of the AUROC: comparison of albumin-based ratios and severity indexes
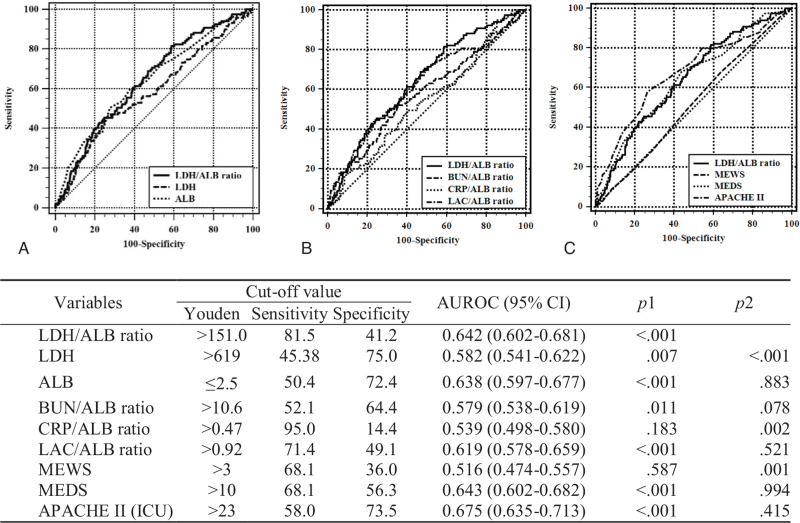
Figure 2 shows the AUROC of LDH, ALB, ALB-based ratios, and severity scores in predicting in-hospital mortality. On comparing between LDH/ALB ratio and other variables, the AUROC of the LDH/ALB ratio was higher than that of LDH, CRP/ALB ratio, and modified early warning score and was not significantly different from that of other variables (ALB, BUN/ALB ratio, LAC/ALB ratio, MEDS, and APACHE II). The ideal LDH/ALB ratio cutoff for in-hospital mortality using Youden index was 151.0 (sensitivity 81.5%, specificity 41.2%).
Figure 2.
Analysis of the receiver-operating characteristics curve for predicting the in-hospital mortality. (A) comparison of LDH and albumin, (B) comparison of the albumin-based ratios, (C) comparison of the LDH/ALB ratio and severity scores. ALB = albumin, APACHE II (ICU) = Acute Physiology And Chronic Health Evaluation II score calculated in the intensive care unit, AUROC = area under the receiver-operating characteristics curve, BUN = blood urea nitrogen, CI = confidence interval, CRP = C-reactive protein, LAC = lactate, LDH = lactate dehydrogenase, MEDS = mortality in emergency department sepsis score, MEWS = modified early warning score. The AUROCs of the models were calculated and tested mutually for significance by DeLong equality tests (p1 = P value for the AUROC of each variable; p2 = P value for equality compared to the LDH/ALB ratio).
3.3. Multivariable logistic regression analysis for in-hospital mortality
After the univariable analyses (Table 1), the selected variables, including sex, age, respiration rate, SpO2, LAC/ALB ratio, LDH/ALB ratio, infection focus, transferred from other facilities, and some comorbidities, were further analyzed using multivariable logistic regression. The results are presented in Table 2. The OR of the LDH/ALB ratio for in-hospital mortality was 1.001 (95% confidence interval [CI]: 1.000–1.002, P = .047). These results support the relevance of the LDH/ALB ratio as an independent prognostic factor for in-hospital mortality.
Table 2.
Multivariate logistic regression analysis for the in-hospital mortality.
| Variables | OR (95% CI) | P |
| Male | 1.454 (0.907–2.333) | .120 |
| Age | 1.015 (0.997–1.034) | .108 |
| Transfer | 1.524 (0.970–2.395) | .067 |
| Heart failure | 1.193 (0.535–2.662) | .667 |
| Chronic liver disease | 1.164 (0.443–3.062) | .758 |
| Chronic renal disease | 1.400 (0.710–2.758) | .331 |
| Malignancy | 0.772 (0.382–1.562) | .472 |
| RR > 30/min | 1.417 (0.858–2.341) | .174 |
| SpO2 < 90% | 1.581 (0.944–2.648) | .082 |
| BUN/ALB ratio | 1.020 (0.999–1.041) | .060 |
| LAC/ALB ratio | 1.025 (1.004–1.047) | .021 |
| LDH/ALB ratio | 1.001 (1.000–1.002) | .047 |
| Respiratory tract infection | 1.159 (0.587–2.288) | .670 |
| Intra-abdominal infection | 0.751 (0.351–1.608) | .461 |
| Urinary tract infection | 0.308 (0.094–1.006) | .051 |
3.4. Severity scores modified by LDH/ALB ratio vs non-modified severity scores
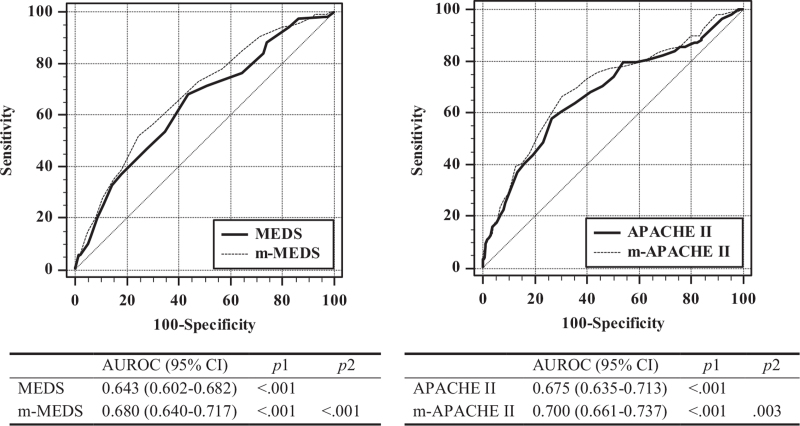
Using the cutoff value, the LDH/ALB ratio was categorized into 3 groups: <150 (LA1), between 150 and 300 (LA2), and >300 (LA3). The aOR of the categorized LDH/ALB ratio (LA) were as follows: LA1 (control): 1.000, LA2: 2.405 (95% CI: 1.382–4.185, P = .002), and LA3: 3.259 (95% CI: 1.703–6.235, P < .001) (Table 3). MEDS and APACHE II scores were modified by the categorized LDH/ALB ratio. Formulas used for modification were as follows: modified MEDS (m-MEDS) = MEDS + LA value and modified APACHE II score (m-APACHE II) = APACHE II score + 2 × LA value (LA value: LA1 = 0, LA2 = 2, LA3 = 4). AUROCs of the modified scores were improved than those of the non-modified scores (Fig. 3).
Table 3.
Adjusted odds ratio (aOR) for the in-hospital mortality of the categorized LDH/ALB ratio.
| LDH/ALB ratio | aOR (95% CI) | P |
| <150 | 1.000 | |
| 150–300 | 2.405 (1.382–4.185) | .002 |
| >300 | 3.259 (1.703–6.235) | <.001 |
Figure 3.
Comparison of the area under receiver-operating characteristics the curve for predicting the in-hospital mortality (modified severity scores vs non-modified severity scores). APACHE II = Acute Physiology And Chronic Health Evaluation II score calculated in the intensive care unit, AUROC = area under the receiver-operating characteristics curve, CI = confidence interval, m-APACHE II = APACHE II modified by the lactate dehydrogenase to albumin ratio, MEDS = mortality in emergency department sepsis score, m-MEDS = MEDS modified by the lactate dehydrogenase to albumin ratio. The AUROCs of the models were calculated and tested mutually for significance by DeLong equality tests (p1 = P value for the AUROC of each variable; p2 = P value for equality compared to the non-modified variable).
4. Discussion
Sepsis remains a major burden worldwide, with a global estimate of 31.5 million cases and 5.3 million deaths per year.[24] The most critical parameters in sepsis management are early recognition and timely broad-spectrum antibiotics administration. Thus, rapid and accurate identification of high-risk patients remains a challenge, and multiple attempts are being made to identify readily available and cost-effective biomarkers for prognostication and risk stratification of infection patients.
In this study, we assessed the association between the LDH/ALB ratio and mortality in infection patients and verified the usefulness of the LDH/ALB ratio as a prognostic biomarker that can be performed via an initial laboratory test in the ED.
In infectious diseases, the aggravation of infection induces changes in blood levels of several biomarkers such as cytokines, APPs, and biomarkers related to organ dysfunction or injury.[25] These biomarkers correlate to the prognosis and mortality of infection patients. Blood levels of many of these biomarkers, including several cytokines, positive APPs such as CRP, procalcitonin, LAC, BUN, bilirubin, and LDH increase with the aggravation of infection.[4,26–28] LDH is related to cellular energy or injuries and is present in nearly all living cells. Therefore, cell injuries owing to localized infection and organ injuries owing to systemic inflammatory response or shock increase serum LDH levels.[26] Thus, LDH has been used to evaluate patients with many types of diseases for a long time in the ED and has been assessed as a prognostic factor for infection and other diseases with cell injuries, malignant disease, hemolytic disease, infarction, some inflammatory diseases, shock, hypoxia, etc.[4,5,29,30] However, LDH has not been usually used as a single prognostic factor because of comorbid diseases that can lead to a rise in serum LDH levels.
Unlike LDH, levels of several markers decrease with the inflammatory response toward infection aggravations; ALB is a representative negative APP for inflammation. Hypoalbuminemia is a dose-dependent predictor of poor outcomes, including mortality, morbidity, and prolonged ICU and hospital stay. The association between hypoalbuminemia and poor clinical outcomes was independent of both nutritional status and inflammation.[9–11] Therefore, ALB and ALB-based ratios such as CRP/ALB, LAC/ALB, and BUN/ALB ratios have been evaluated as prognostic factors of infection and sepsis.[13–15,19,20,29,31]
As mentioned above, LDH and ALB have been usually used as a supplement to prognostic factors. However, no studies have assessed whether the LDH/ALB ratio can be used as a prognostic factor for infection patients. In this study, we found that the LDH/ALB ratio could be used as an independent prognostic factor for infection patients who needed intensive care. The AUROC of the LDH/ALB ratio was 0.642 (95% CI: 0.602–0.681, sensitivity 81.5, specificity 41.2) and was superior to that of other ALB-based predictors (LAC/ALB ratio: 0.619 [95% CI: 0.578–0.659], CRP/ALB ratio: 0.539 [95% CI: 0.498–0.580], BUN/ALB ratio: 0.579 [95% CI: 0.538–0.619]) (Fig. 2). Although the AUROC of the LDH/ALB ratio was relatively low, it was not lower than that of other ALB-based predictors previously studied in infection patients.[15,32]
Furthermore, the AUROC of the LDH/ALB ratio was not inferior to that of APACHE II and MEDS, which represent complex scoring systems (MEDS: 0.643 [95% CI: 0.602–0.682], APACHE II: 0.675 [95% CI: 0.635–0.713]) (Fig. 2). This suggests that the LDH/ALB ratio could be used to predict the prognosis of infection patients through blood tests performed in the ED rather than through complex multiple predictive scoring systems. Moreover, modified scores of MEDS and APACHE II with the LDH/ALB ratio were superior to non-modified scores (Fig. 3). Based on its cutoff value, the LDH/ALB ratio can be classified into 3 groups, and MEDS and APACHE II were modified by adding scores according to the aORs of the 3 groups.
Although MEDS and APACHE II were not the best prognostication tools in the ICU because of low AUROC for mortality in sepsis patients, most prognostication scoring systems usually used for sepsis in the ED and ICU also do not have good AUROC for severe sepsis and do not include LDH/ALB ratio.[30,33] Thus, our study result suggests that the modification of a complex prognostication scoring system with the LDH/ALB ratio could improve prognostication ability.
This above-mentioned information is clinically important because the LDH/ALB ratio obtained through a basic blood test conducted in the ED may be useful as an independent prognostic factor for in-hospital mortality in infection patients admitted to the ICU via the ED. The LDH/ALB ratio can be a prognostic factor for infection patients, making it easier for medical staff to identify patients who are more likely to develop severe sepsis and need immediate treatment.
4.1. Limitations
This study had several limitations. First, this study was retrospective in nature and thus had inherent limitations concerning selection bias. Most patients were elderly and had comorbidities such as malignant, liver disease, and chronic kidney failure. Thus, the values of LDH and ALB may vary depending on the underlying disease. The authors were aware of the biases and held multiple meetings to ensure patients were correctly identified, minimize patients missed owing to improper ICD-10 classification, and standardize the data collection protocol. Also, adjustment with comorbid diseases was made to reduce bias. Second, the data were collected from a single center. Therefore, it cannot be generalized based on our results. Third, the number of missing data was large, and we had a relatively small sample size. However, when comparing the group included in the study and the excluded group, there was no difference in age, sex, comorbidity, and mortality. Thus, it can be predicted that the risk from missing data would have been reduced. However, the relatively small sample size made it difficult to perform the subgroup analysis. Fourth, the prognosis of infection patients was predicted using the initial vital signs immediately after arriving at the ED and the initial laboratory data but not using the worst vital signs and laboratory data. Thus, the initial vital signs may appear normal upon arrival at the ED. However, changes in vital signs varied during the management in the ED, and patients with unstable vital signs and in need of organ support management were admitted to the ICU. These were reasons why the included patients were presumed as severe sepsis patients who needed intensive care. Despite these limitations, this study is meaningful as the LDH/ALB ratio could be an independent predictor for infection patients admitted to the ICU via the ED.
5. Conclusion
The LDH/ALB ratio may be useful as an independent prognostic factor for in-hospital mortality of infection patients admitted to the ICU via the ED.
Author contributions
Conceptualization: Seung Ryu, Se-Kwang Oh.
Data curation: So Young Jeon, Seung Ryu, Se-Kwang Oh.
Formal analysis: So Young Jeon, Seung Ryu, Se-Kwang Oh.
Investigation: So Young Jeon, Seung Ryu.
Methodology: So Young Jeon, Seung Ryu, Se-Kwang Oh, Jung-Soo Park, Yeon-Ho You, Won-Joon Jeong, Yong-Chul Cho, Hong-Joon Ahn, Chang-Shin Kang.
Project administration: So Young Jeon, Seung Ryu.
Supervision: Seung Ryu, Se-Kwang Oh.
Validation: So Young Jeon, Seung Ryu, Se-Kwang Oh, Jung-Soo Park, Yeon-Ho You, Won-Joon Jeong, Yong-Chul Cho, Hong-Joon Ahn, Chang-Shin Kang.
Visualization: So Young Jeon.
Writing – original draft: So Young Jeon.
Writing – review & editing: So Young Jeon, Seung Ryu, Se-Kwang Oh, Jung-Soo Park, Yeon-Ho You, Won-Joon Jeong, Yong-Chul Cho, Hong-Joon Ahn, Chang-Shin Kang.
Supplementary Material
Footnotes
Abbreviations: APACHE II = Acute Physiology And Chronic Health Evaluation II, APP = acute-phase protein, AUROC = area under the receiver-operating characteristics curve, BUN/ALB = blood urea nitrogen to albumin, CI = confidence interval, CRP/ALB = C-reactive protein to albumin, ED = emergency department, ICU = intensive care unit, LAC/ALB = lactate to albumin, LDH/ALB = lactate dehydrogenase to albumin, MEDS = mortality in emergency department sepsis score, OR = odds ratio.
How to cite this article: Jeon SY, Ryu S, Oh SK, Park JS, You YH, Jeong WJ, Cho YC, Ahn HJ, Kang CS. Lactate dehydrogenase to albumin ratio as a prognostic factor for patients with severe infection requiring intensive care. Medicine. 2021;100:41(e27538).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
Categorical variables are expressed by number (%) and continuous variables are expressed by median [interquartile range].
ALB = albumin, APACHE II (ICU) = Acute Physiology And Chronic Health Evaluation II score calculated in the intensive care unit, BUN = blood urea nitrogen, CCI = Charlson comorbidity index, CRP = C-reactive protein, DBP = diastolic blood pressure, LAC = lactate, LDH = lactate dehydrogenase, LTCF = long-term care facility, MEDS = mortality in emergency department sepsis score, MEWS = modified early warning score, SBP = systolic blood pressure, SpO2 = oxygen saturation measured by pulse oximetry, WBC = white blood cell.
ALB = albumin, BUN = blood urea nitrogen, CI = confidence interval, LAC = lactate, LDH = lactate dehydrogenase, OR = odds ratio, RR = respiration rate, SpO2 = oxygen saturation measured by pulse oximetry, Transfer = transferred from other facility.
aOR was adjusted with age, sex, transferred from other facilities, heart failure, chronic liver disease, chronic renal disease, malignancy, respiration rate, oxygen saturation measured by pulse oximetry, albumin, lactate/albumin ratio, blood urea nitrogen/albumin ratio, respiratory tract infection, urinary tract infection, intra-abdominal infection.
ALB = albumin, CI = confidence interval, LDH = lactate dehydrogenase.
References
- [1].Gyawali B, Ramakrishna K, Dhamoon A. Sepsis: the evolution in definition, pathophysiology, and management. SAGE Open Medicine 2019;7:205031211983504.doi:10.1177/2050312119835043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fernandez P, Torres A, Miró J, et al. Prognostic factors influencing the outcome in Pneumocystis carinii pneumonia in patients with AIDS. Thorax 1995;50:668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Takano Y, Sakamoto O, Suga M, Muranaka H, Ando M. Prognostic factors of nosocomial pneumonia in general wards: a prospective multivariate analysis in Japan. Respir Med 2002;96:18–23. [DOI] [PubMed] [Google Scholar]
- [4].Zein J, Lee G, Tawk M, Dabaja M, Kinasewitz G. Prognostic significance of elevated serum lactate dehydrogenase (LDH) in patients with severe sepsis. Chest J 2004;126: doi:10.1378/chest.126.4_MeetingAbstracts.873S. [Google Scholar]
- [5].Lu J, Wei Z, Jiang H, et al. Lactate dehydrogenase is associated with 28-day mortality in patients with sepsis: a retrospective observational study. J Surg Res 2018;228:314–21. [DOI] [PubMed] [Google Scholar]
- [6].Miyashita N, Horita N, Higa F, et al. Validation of a diagnostic score model for the prediction of Legionella pneumophila pneumonia. J Infect Chemother 2019;25:407–12. [DOI] [PubMed] [Google Scholar]
- [7].Pan F, Yang L, Li Y, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci 2020;17:1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yin M, Si L, Qin W, et al. Predictive value of serum albumin level for the prognosis of severe sepsis without exogenous human albumin administration: a prospective cohort study. J Intensive Care Med 2018;33:687–94. [DOI] [PubMed] [Google Scholar]
- [9].Arnau-Barrés I, Güerri-Fernández R, Luque S, Sorli L, Vázquez O, Miralles R. Serum albumin is a strong predictor of sepsis outcome in elderly patients. Eur J Clin Microbiol Infect Dis 2019;38:743–6. [DOI] [PubMed] [Google Scholar]
- [10].Godinez-Vidal AR, Correa-Montoya A, Enríquez-Santos D, Pérez-Escobedo SU, López-Romero SC, Gracida-Mancilla NI. Is albumin a predictor of severity and mortality in patients with abdominal sepsis? Cir Cir 2019;87:485–9. [DOI] [PubMed] [Google Scholar]
- [11].Takegawa R, Kabata D, Shimizu K, et al. Serum albumin as a risk factor for death in patients with prolonged sepsis: an observational study. J Crit Care 2019;51:139–44. [DOI] [PubMed] [Google Scholar]
- [12].Hwang YJ, Chung SP, Park YS, et al. Newly designed delta neutrophil index-to-serum albumin ratio prognosis of early mortality in severe sepsis. Am J Emerg Med 2015;33:1577–82. [DOI] [PubMed] [Google Scholar]
- [13].Wang B, Chen G, Cao Y, Xue J, Li J, Wu Y. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J Crit Care 2015;30:271–5. [DOI] [PubMed] [Google Scholar]
- [14].Luo X, Yang X, Li J, et al. The procalcitonin/albumin ratio as an early diagnostic predictor in discriminating urosepsis from patients with febrile urinary tract infection. Medicine (Baltimore) 2018;97:e11078.doi:10.1097/MD.0000000000011078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bou Chebl R, Jamali S, Sabra M, et al. Lactate/albumin ratio as a predictor of in-hospital mortality in septic patients presenting to the emergency department. Front Med 2020;7:550182.doi:10.3389/fmed.2020.550182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deng S, Gao J, Zhao Z, Tian M, Li Y, Gong Y. Albumin/procalcitonin ratio is a sensitive early marker of nosocomial blood stream infection in patients with intra-cerebral hemorrhage. Surg Infect (Larchmt) 2019;20:643–9. [DOI] [PubMed] [Google Scholar]
- [17].Gong Y, Li D, Cheng B, Ying B, Wang B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect 2020;148:e87.doi:10.1017/S0950268820000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Feng D-Y, Zhou Y-Q, Zou X-L, et al. Elevated blood urea nitrogen-to-serum albumin ratio as a factor that negatively affects the mortality of patients with hospital-acquired pneumonia. Can J Infect Dis Med Microbiol 2019;2019:1547405.doi:10.1155/2019/1547405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ugajin M, Yamaki K, Iwamura N, Yagi T, Asano T. Blood urea nitrogen to serum albumin ratio independently predicts mortality and severity of community-acquired pneumonia. Int J Gen Med 2012;5:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ryu S, Oh S, Cho S, et al. Utility of the blood urea nitrogen to serum albumin ratio as a prognostic factor of mortality in aspiration pneumonia patients. Am J Emerg Med 2021;43:175.doi:10.1016/j.ajem.2020.02.045. [DOI] [PubMed] [Google Scholar]
- [21].Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- [22].Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM 2001;94:521–6. [DOI] [PubMed] [Google Scholar]
- [23].Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med 2003;31:670–5. [DOI] [PubMed] [Google Scholar]
- [24].Fleischmann C, Scherag A, Adhikari N, et al. Assessment of global incidence and mortality of hospital-treated sepsis - current estimates and limitations. American journal of respiratory and critical care medicine 2015;193:259–72. [DOI] [PubMed] [Google Scholar]
- [25].Ansar W, Ghosh S. Inflammation and inflammatory diseases, markers, and mediators: role of CRP in some inflammatory diseases. Biol C React Protein Health Dis 2016;67–107. doi:10.1007/978-81-322-2680-2_4. [Google Scholar]
- [26].Higashikawa T, Okuro M, Ishigami K, et al. Procalcitonin and albumin as prognostic biomarkers in elderly patients with a risk of bacterial infection. J Int Med Res 2018;46:2606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kang HE, Park DW. Lactate as a biomarker for sepsis prognosis? Infect Chemother 2016;48:252–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miglietta F, Faneschi ML, Lobreglio G. Procalcitonin, C-reactive protein and serum lactate dehydrogenase in the diagnosis of bacterial sepsis, SIRS and systemic candidiasis. Infez Med 2015;23:230–7. [PubMed] [Google Scholar]
- [29].Aday U, Böyük A, Akkoç H. The prognostic significance of serum lactate dehydrogenase-to-albumin ratio in colorectal cancer. Ann Surg Treat Res 2020;99:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wu M, Yao L, Wang Y, et al. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir Res 2020;21:171.doi:10.1186/s12931-020-01427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gharipour A, Razavi R, Gharipour M, Mukasa D. Lactate/albumin ratio: an early prognostic marker in critically ill patients. Am J Emerg Med 2020;38:2088–95. [DOI] [PubMed] [Google Scholar]
- [32].Kim MH, Ahn JY, Song JE, et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS One 2015;10:e0132109.doi:10.1371/journal.pone.0132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nguyen HB, Banta JE, Cho TW, et al. Mortality predictions using current physiologic scoring systems in patients meeting criteria for early goal-directed therapy and the severe sepsis resuscitation bundle. Shock 2008;30:23–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.