Abstract
Gensini score (GS) provides valuable information on severity and prognosis of coronary artery disease (CAD).
To evaluate the relationship between the severity of CAD determined by the GS and relation to ST-elevation myocardial infarction, non-ST segment elevation myocardial infarction (NSTEMI), unstable angina pectoris, chest pain (suspected angina syndrome on admission) and risk-factors for CAD and predictors of severity.
Observational cross-sectional study.
Consecutive patients who underwent clinically-indicated coronary angiography for ST-elevation myocardial infarction, NSTEMI, unstable angina pectoris or chest pain were enrolled.
Among 600 patients, 417 (average age 67.8 ± 12.2 years) had CAD–related symptoms. Mean GS was 66.7 ± 63.8. Patients presenting with NSTEMI had the highest GS (81.3 ± 42.3; P < .001) Regression analysis of risk-factors showed the best association of GS with multivessel disease and coronary artery bypass graft. Regression analysis of medications showed that clopidogrel, had the best association with low GS.
GS correlated with the severity of CAD, multivessel disease, coronary artery bypass graft, and troponin. GS was related to the cardiovascular risk-factors of diabetes, hypertension, and high-density cholesterol.
Keywords: atherosclerosis, coronary angiography, coronary artery disease, Gensini score
1. Introduction
Coronary artery disease (CAD) is a progressive, chronic, systemic inflammatory disease. Atherosclerosis plays a major role in its etiology.[1] CAD is a leading cause of morbidity and mortality worldwide, and its incidence is gradually increasing.[1,2]
Weak evidence has been published regarding the accuracy of specific strategies, such as indirect quantitative determination of the extent and severity of coronary atherosclerosis, for follow-up and prognosis of patients treated for ST-elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infarction (NSTEMI), or unstable angina pectoris (UAP).[3]
Despite its many limitations, coronary angiography remains the gold standard for the diagnosis of CAD[2] and provides visual information about the extent of coronary atherosclerosis and of some plaque characteristics. Angiographic characteristics of patients with acute coronary syndrome (ACS) are closely associated with cardiovascular events and mortality rates.[4–6] Coronary lesions that are more stenotic have higher rates of progression to occlusion and myocardial infarction.[4–6] The number of severely diseased coronary vessels is also predictive of survival[4] and properties of stenotic lesions are 1 of the major predictive factors for cardiac events and mortality.[7]
Age, sex, high density cholesterol level (HDL), smoking, and diabetes mellitus (DM) are related to the severity of coronary lesions seen on angiograms.[8] CAD appears more often in patients with DM because the pathogenesis of DM involves direct vascular damage and endothelial dysfunction caused by hyperglycemia, hypertension (HTN), dyslipidemia and increased thrombogenesis.[9,10]
Clinically, CAD is responsible for 75% of deaths among patients with DM, and 30% of patients with ACS have DM.[9–11,12] However, other reports stated that the association between related risk-factors and angiographic findings remains controversial.[8]
Quantitative determination of atherosclerosis by Gensini score (GS) may be as important as other risk-factors for disease management.[2,4–6,13] Angiographic scoring systems are strongly correlated with each other and with atherosclerotic plaque burden. Therefore, scoring systems appear to be a valid estimate of CAD plaque burden.[2]
When properly applied, expert analysis of available data on atherosclerosis may benefit the choice of therapies and can improve the quality of care, optimize patient outcomes, and favorably affect costs by focusing resources on the most effective strategies. Most patients with CAD undergo coronary angiography and stenting, which is why it seems crucial to know their angiographic scores, to establish better stratification of follow-up and treatment.
The aim of the present study was to evaluate the relationship between the extent and severity of CAD as determined by the GS, and the CAD syndromes STEMI, ACS, UAP, chest pain (suspected for angina syndrome on admission) and major cardiovascular risk-factors among patients with indications for coronary angiography.
2. Methods
From January 2010 to July 2012, we prospectively enrolled 600 patients, who had been hospitalized in the Cardiac Care Unit at Kaplan Medical Center, Rehovot, Israel and had undergone coronary angiography. All examinations were performed at the Cardiology Clinic. The study protocol was approved by the Institutional Ethics Committee (KMC-0109-10) and each participant provided written informed consent.
All hospitalized patients underwent echocardiogram and blood tests (including Troponin) during admission. Large scale registries have concluded that Bayesian analysis, including the parameters age, sex, and type of chest pain, effectively evaluate the pretest likelihood of CAD in most cases.[4,5,13]
Consecutive patients with acute STEMI, NSTEMI, UAP or chest pain, and suspected ischemic heart disease (IHD) with positive stress tests (treadmill ergometry, stress echo or thallium test), were enrolled into 1 of the 4 groups noted above.
All participants were examined by a physician and medical history, medications, physical examination, resting blood pressure, heart rate, weight, waist circumference, NYHA classification, and echocardiography or Thalium 201 scan were obtained. IHD was defined according to previous history of proven myocardial infarction, coronary artery bypass grafting (CABG) or coronary angiography. Hyperlipidemia was defined according to treatment with hypolipidemic medicines as statins, fibrates, or ezetimibe, or total cholesterol level >200 mg/dl, LDL >100 mg/dl or triglycerides >150 mg/dl.
The patients were also evaluated for the presence or absence of DM, HTN, family history of cardiac diseases, low HDL, hyperlipidemia, hypertriglyceridemia, and cigarette smoking.
Patients were considered to have HTN if they had previously known HTN, if they were on antihypertensive therapy, or if they had a systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg, which were calculated as the mean of 2 measurements taken on each arm.
Patients were considered to have type II DM if they were previously diagnosed and treated for diabetes and/or if they had a fasting blood glucose level ≥126 mg/dl on 2 different samples.
Baseline venous blood samples for lipid profiles, liver, renal function tests, NT-Pro BNP, and hemoglobin levels were taken from patients after a 12-hour fast, low-density lipoprotein cholesterol levels were calculated using the Friedewald formula.
All patients underwent coronary angiography. The 2 senior cardiologists (M.J. and J.G.) independently examined randomly chosen angiograms, visually estimated lesion scores, and calculated GS to establish better stratification of follow-up and treatment. We chose the veteran GS over the contemporaneous Syntax score, because of its higher sensitivity.[14] The first includes lesions narrowing more than 25% of the lumen, when the second uses more developed plaques, with more than 50% vessel narrowing.
Exclusion criteria were malignant disease with diffuse metastases or severe cerebral vascular disease. The study endpoint was successful coronary angiography stenting and determination of GS.
2.1. Assessment of GS with coronary angiography
All patients underwent urgent or elective diagnostic coronary angiography. Coronary angiograms were evaluated by 2 experts and mean values were used to assess the severity of stenosis. Obstructive CAD was defined as stenosis ≥50% of the diameter of a major epicardial or branch vessel >2.0 mm in diameter. Three-vessel disease was defined as stenosis ≥50% in each of the major vessels or their major branches. GSs were calculated for each patient as defined previously.[8,15,16]
2.1.1. Gensini score
GS was used to evaluate the severity of atherosclerosis.[17] The most severe stenosis in each of the 8 coronary segments was graded from 1 to 4 (1%-49% lumen diameter reduction: 1 point; 50%-74% stenosis, 2 points; 75%-99% stenosis, 3 points; and 100% occlusion 4 points) to give a total score of 0 to 32. This score provides an index of the severity of coronary atherosclerosis. Coronary thrombus was defined as a filling defect surrounded by contrast media in the absence of calcification and dissection. Total occlusion was defined as the absence of any anterograde opacification. Coronary calcification was defined as the visualization of any coronary calcified lesion viewed by angiography. The Gensini scoring system was used to evaluate CAD severity. The GS was calculated for each patient from the coronary arteriogram by assigning a severity score to each coronary stenosis according to the degree of luminal narrowing and its geographic importance. Decreased lumen diameter and the roentgenographic appearance of concentric lesions and eccentric plaques were evaluated (reductions of 25%, 50%, 75%, 90%, 99%, and complete occlusion were given GS of 1, 2, 4, 8, 16, and 32, respectively).[14]
2.2. Statistical analysis
The relations between GS vs background and clinical factors were examined using the nonparametric Mann-Whitney and Kruskal-Wallis tests, as applicable.
In addition, multiple linear regression analysis was performed, including CABG and saphenous venous graft (SVG) or left internal mammary graft (LIMA), new multivessel stenosis, percutaneous intervention (PCI), and risk factors: diabetes, HTN, smoking, body mass index (BMI), and waist circumference to evaluate the effect of each of these factors on GS.
Various model building methods were used, including forced entry, forward selection and backward elimination. Since the results of all models were similar for raw and normalized dependent variables, we present here the results of the raw dependent variables. All statistical analyses were performed using SAS for Windows 9.2 (SAS Institute, Cary, North Carolina).
3. Results
Among 600 consecutive patients with IHD-related symptoms who met the inclusion criteria and were prospectively enrolled in this study, 183 were excluded for technical reasons, noncompliance, or lack of follow-up information. The remaining 417 patients comprised the study group. The mean left ventricular ejection fraction was 46% and mean GS was 66.7 ± 63.8.
The patients’ general characteristics, demographic, laboratory data, and distribution of selected clinical characteristics are shown in Table 1. Their mean age of total 417 patients was 67.8 ± 12.2 years (range 46-88), 282 (68%) were men, 193 (91%) had IHD before admission and 19 (9%) had a previous myocardial infarction. The mean troponin I level was 1.34 ± 4.51 (range 0-37.1). Before being referred for coronary angiography, 117 patients underwent treadmill ergonometry, stress ECHO or thallium 201 scan.
Table 1.
Patients’ general characteristics (417 patients).
| Variable | Mean | Standard deviation |
| Age (n = 417), yr | 67.8 | 12.2 |
| Male (n = 290) | 69.7% | |
| BMI | 28.9 | 6.2 |
| Waist (cm) | 104.4 | 14.8 |
| Hypertension (n = 304) | 73.6% | 13.7 |
| Dyslipidemia (297) | 71.7% | 24.6 |
| Diabetes mellitus (n = 187) | 44.2% | 28 |
| Smokers (n = 131) | 31.8% | 18.3 |
| Fam. history of early IHD | 7.0% | 4.3 |
| STEMI/NSTEMI (n = 67) | 16.0% | 4.8 |
| Total Gensini score | 66.7 | 63.8 |
| Gensini score per segment | 4.8 | 4.8 |
| CVA/TIA (n = 40) | 9.6% | 3.0 |
| CABG (n = 95) | 22.9% | 37% |
| PVD (n = 25) | 6.0% | 2.6 |
| Renal failure (n = 86) | 20.7% | 13.5 |
| Creatinine (mg/dl) | 1.8 | 0.9 |
| Hemoglobin (g%) | 15.5 | 8.8 |
| Total cholesterol (mg/dl) | 168.1 | 49.5 |
| LDL cholesterol | 98.5 | 36.5 |
| HDL cholesterol | 44.9 | 13.6 |
| Vessel disease | ||
| 1 (n = 71) | 18.3% | 10.2 |
| 2 (n = 89) | 22.9% | 8.6 |
| 3 (n = 133) | 34.3% | 13.4 |
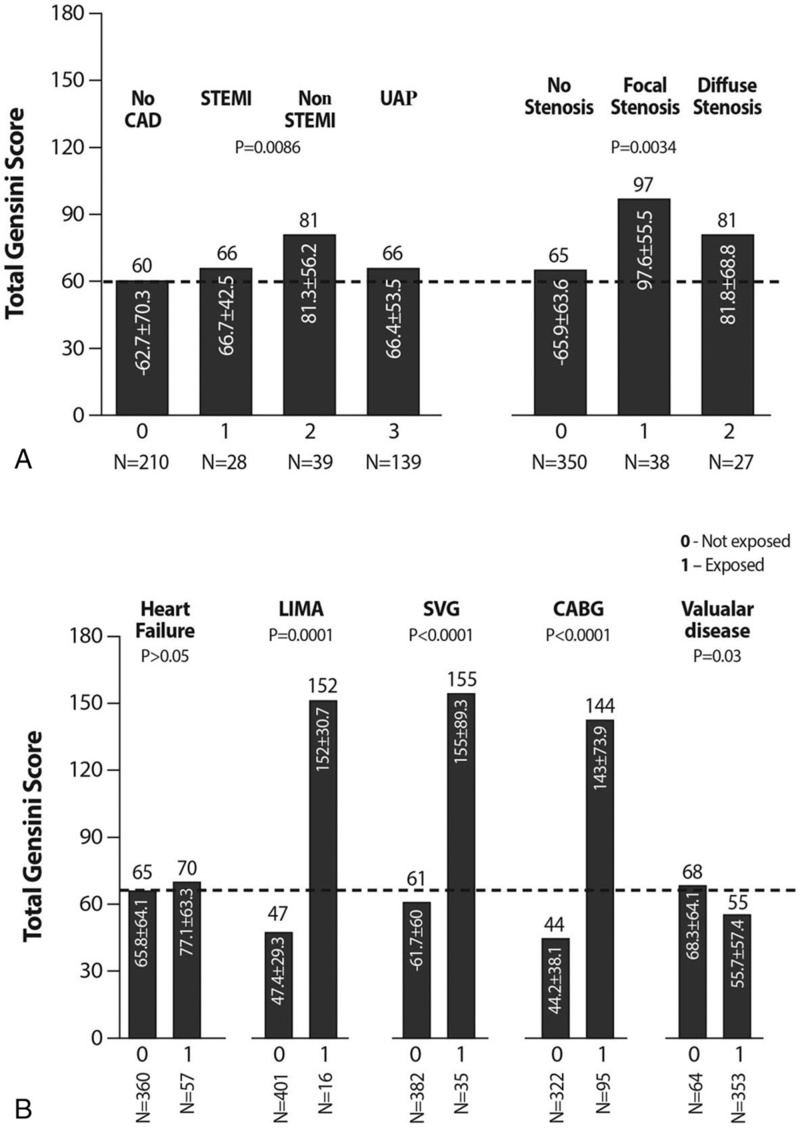
To investigate the significance of CAD and to calculate the GS in STEMI, NSTEMI, UAP and patients with chest pain, and suspected IHD, we compared the GS of coronary artery stenosis between the 4 groups of patients. The GS of patients with UAP or chest pain without CAD were similar (66.4 ± 53.5 and 62.7 ± 70.3, respectively; (P < .01).
Patients with NSTEMI had significantly higher GS 81.3 ± 56.2 as compared to patients with UAP (66.4 ± 53.5; P < .001). GS of patients with STEMI and UA were similar (Fig. 1A). Patients with valvular heart disease had GS 55.7 ± 57.4, which is lower than that of those without valvular disease (68.3 ± 64.1; P = .03; Fig. 1B).
Figure 1.
A. Differences in Gensini scores among patients with STEMI/NSTEMI, or UA and patients without active CAD. GS differences in patients with focal and diffuse stenosis and those without stenosis. B. Differences in GS in patients with HF, CABG, LIMA SVG, and valvular disease. CABG = coronary artery bypass graft, CAD = coronary artery disease, GS = Gensini score, HF = heart failure, LIMA = left internal mammary graft, NSTEMI = non-ST segment elevation myocardial infarction, STEMI = ST-elevation myocardial infarction, SVG = saphenous venous graft, UA = unstable angina.
There were no differences in GS between patients who had or did not have ecstatic or calcified vessel lesions (P = .24). A total of 72 patients (17%) needed recurrent PCI during the first follow-up year.
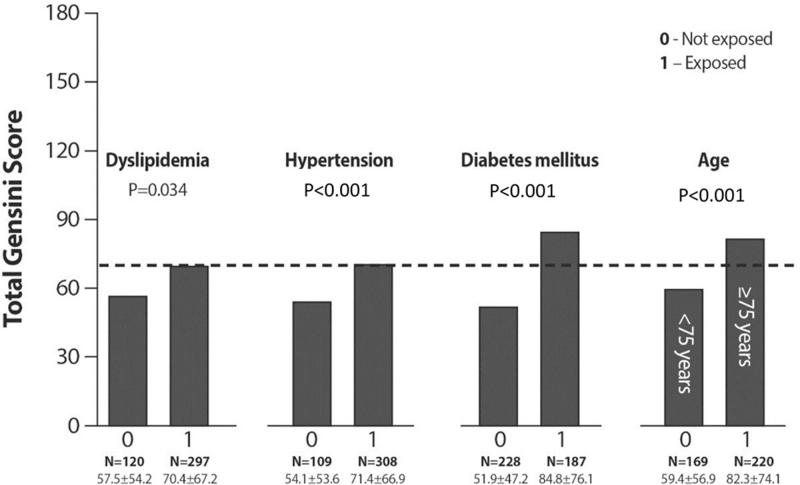
Figure 2 shows significant differences in total GS between patients with and without dyslipidemia, HTN, advanced age, and diabetes. The highest GS was found among 44% of patients with DM (84.8 ± 76.1 [P < .001]).
Figure 2.
Differences in GS between the patients who had dyslipidemia, HTN or DM and those without these risk factors. GS = Gensini score, HTN = hypertension, DM = diabetes mellitus.
Surprisingly, GS was found to be higher in patients with NSTEMI than in STEMI. Similar differences were also seen in GS calculated for each vessel.
There were no sex differences in GS based on risk-factors or on CAD syndromes.
Patients with focal stenosis had higher GS than those with diffuse lesions (P = .038). Major differences were found in patients who underwent CABG (147 patients), especially those who had LIMA implantation (65 patients). Patients who received drug eluting stents had significantly higher GS than those with bare metal stents or no stents, who had similar GS. Not surprisingly, patients with valvular disease had significantly lower GS than those without (Fig. 1B).
Table 2 describes significant Spearman correlations between different parameters in patients with and without STEMI/NSTEMI and UA. HDL and age had the highest negative correlations with GS in all patient groups (R = −0.38, P < .001, R = −0.77, P = .05). Eosinophils had good correlation only in UA patients. There were no correlations between GS and cardiac risk-factors, such as HTN, DM, smoking, troponin, BMI, and waist circumference.
Table 2.
Spearman correlations of main demographic and laboratory parameters to Gensini score in patients with UA and with STEMI/NONSTEMI.
| Parameter | Correlation index (R) unstable angina (n = 139) | P value | Correlation index (R) STEMI/NSTEMI (n = 116) | P value |
| Age | −0.3 | <.0001 | 0.48 | .04 |
| BMI | 0.07 | NS | 0.22 | .63 |
| Creatinine(mg/dl) | 0.24 | .24 | 0.02 | .72 |
| Total cholesterol(mg/dl) | −14.6 | .56 | 0.70 | .01 |
| LDL cholesterol(mg/dl) | 0.7 | .51 | 0.6 | .57 |
| HDL cholesterol(mg/dl) | −0.38 | <0.001 | 0.77 | .05 |
| Triglycerides (mg/dl) | 0.03 | .05 | 0.02 | .61 |
| Troponin(ng/dl) | 0.04 | .05 | 0.013 | .54 |
| Lymphocytes/109/L | 0.01 | .14 | 0.02 | .74 |
| Eosinophils/109/L | 0.27 | .02 | 0.01 | .65 |
| WBC/109/L | 0.02 | .32 | 0.012 | .82 |
Regression analysis of different risk-factors, angiographic anatomy data, and PCI procedures (Table 3) showed strong associations between GS and DM, l-4 vessel disease, new stenosis, new stent, and triglycerides. Strong associations of were seen in post-CABG patients with recurrent stenosis and GS. This means that stenosis in the implanted grafts is strongly correlated with accelerated atherosclerosis. DM, HTN, and HDL, but not other predisposing and treated risk-factors were associated with GS. Regression analysis of different medications in relation to atherosclerosis is expressed by GS (Table 3), which showed that aspirin, clopidogrel, ACEI, ezetrol, and ezetimide have the best association with low GS; reflecting atherosclerotic process in contrast to fibrates.
Table 3.
Regression analysis of CAD risk factors, angiography data and procedures in relation to Gensini score in a well-controlled community sample (N = 417).
| Variable | Estimated parameter | P value |
| Diabetes mellitus | 14.2 | .002 |
| Hypertension | −4.9 | .54 |
| Smoking | −2.1 | .58 |
| Body mass index | −5.7 | .72 |
| Waist circumference | −0.19 | .68 |
| 1-4 vessel disease | 21.6 | <.001 |
| CABG | 71.5 | <.001 |
| New stenosis | 1.4 | .05 |
| New stent 1 | 0.9 | .005 |
| PCI recent (de novo) | 1.2 | .001 |
| Triglycerides | 3.2 | .02 |
4. Discussion
The main finding of this study is that STEMI/NSTEMI and UAP were related to more extensive and complex coronary lesions in patients with coronary atherosclerosis. The extent of CAD was based on quantitative determination of atherosclerosis, as expressed by GS.[1,6–9,14]
Patients with NSTEMI in contrast to STEMI and UAP had higher GS of coronary arteries. This finding may be because most NSTEMI patients were older and sicker than STEMI or UAP patients were.
Marked differences in GS between patients who underwent PCI after CABG (and separately for LIMA and SVG) and those who did not have CABG and LIMA or SVG (Fig. 1B) implantation can be explained by an active atherosclerotic process and oxidative stress in non-native vessels.[18]
Patients with only chest pain and inconclusive stress test (thallium scan, stress ECHO or treadmill ergometry) had the lowest GS.
GS was well-correlated with age, total cholesterol and HDL, and DM. The highest GS was found in patients with DM because most had CAD and dyslipidemia. The question as to why Spearman correlations were not significant between GS and cardiac risk factors such as HTN, smoking, troponin, BMI, and waist circumference remains unclear. However, it may be because the patients were treated for various risk-factors, both medically and by life-style changes. A large diabetes trial did not find any relation between symptoms and disease severity for women or men with DM.[16] There were no differences in GS between smokers and nonsmokers and between the patients with BMI above or below 25 (P = .06) and in patients with and without HF or CVA. This can be explained by preventive treatment for several risk-factors. GS of patients with various risk-factors but without acute myocardial infarction (STEMI and NSTEMI) were similar by approximately 68 to 71 score (P > .05).[16]
Regression analysis showed an inverse relation between GS and several medicines, such as statins, ezetimibe, ACEI/ARB, aspirin and clopidogrel, and a slight relation with spironolactone. Fibrates, beta-blockers, warfarin, etc did not have any impact on atherosclerosis regression (in contrast to statins); however, they were shown to prevent coronary events (not shown data).
Regression analysis of different parameters related to active coronary disease showed that GS had the best relation to CABG as well as good associations with DM, multivessel disease and triglycerides and a trend to higher BMI. Surprisingly, the relation of triglycerides to GS was significant because treatment with fibrates (regression analysis) did not show any relation to GS. In addition, regression analysis did not show a relation of GS with troponin, various CAD activity predictors, such as leukocytes, lymphocytes, neutrophils, platelets,[19–22] ectatic or calcified lesions, or CVA. This can be explained by thrombosis as the main process and not due to extensive atherosclerosis. Thrombus formation is triggered by rupture or ulceration of the atherosclerotic plaque.[9,10,19–21]
Using GS to assess angiographic severity of CAD is potentially useful for predicting patient outcomes and benefit of therapies, and can improve the quality of care.
A limitation of this study was the relatively limited sample size of 417 patients and lack of follow-up. Well-controlled studies of patients according to age subgroups will provide additional, important information about the GS and disseminated atherosclerosis.
In conclusion, GS were correlated to the severity of coronary lesions, especially with multivessel disease, CABG and implanted LIMA or drug eluting stent. GS reflecting severity of atherosclerosis is related to several cardiovascular risk factors, such as age, HDL, HTN, and diabetes. GS can provide valuable information about the severity and prognosis of CAD.
Acknowledgments
Faye Schreiber, MS, the institutional medical and scientific editor, is thanked for editorial assistance. Esther Shabtay, is thanked for statistical assistance.
Author contributions
JG, GC conception, design and analysis, interpretation of data, major revisions, and final approval of the manuscript submitted.
MJ, GG, NT and DH performed the experiments and treated the patients.
AB, IG and LC drafted and revised the manuscript. All authors agree with the final version and its submission to the journal.
Conceptualization: Itamar Grosskopf, Jacob George, Gideon Charach.
Data curation: Lior Charach, Michael Jonas, Nick Teodorovitz, Dan Haberman, Itamar Grosskopf, Jacob George, Gideon Charach.
Formal analysis: Alex Blatt.
Investigation: Lior Charach, Michael Jonas, German Gendelman, Itamar Grosskopf, Gideon Charach.
Methodology: Michael Jonas, Dan Haberman, Itamar Grosskopf, Jacob George, Gideon Charach.
Project administration: Nick Teodorovitz, Jacob George.
Resources: Michael Jonas, Dan Haberman.
Software: Lior Charach, Nick Teodorovitz, Dan Haberman, Itamar Grosskopf.
Supervision: Alex Blatt, Nick Teodorovitz, Gera Gandelman, Itamar Grosskopf, Jacob George.
Validation: Alex Blatt.
Visualization: Lior Charach, Nick Teodorovitz, Dan Haberman, Gera Gandelman.
Writing – original draft: Lior Charach, Alex Blatt.
Footnotes
Abbreviations: ACS = acute coronary syndrome, BMI = body mass index, CABG = coronary artery bypass graft, CAD = coronary artery disease, DM = diabetes mellitus, GS = Gensini score, HDL = high density lipoprotein level, HTN = hypertension, IHD = ischemic heart disease, LIMA = left internal mammary graft, NSTEMI = non-ST elevation myocardial infarction, PCI = percutaneous coronary intervention, STEMI = ST-elevation myocardial infarction, SVG = saphenous venous graft, UAP = unstable angina pectoris.
How to cite this article: Charach L, Blatt A, Jonas M, Teodorovitz N, Haberman D, Gendelman G, Grosskopf I, George J, Charach G. Using the Gensini score to estimate severity of STEMI, NSTEMI, unstable angina, and anginal syndrome. Medicine. 2021;100:41(e27331).
The study was funded by departmental resources. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The study was approved by the Ethics Committee of Kaplan Medical Center (0109–10 KMC). All participants provided written informed consent prior to data collection.
The authors declare that they have no competing interests whatsoever.
The authors have no conflicts of interest to disclose.
Derived data supporting the findings of this study are available from the corresponding author [initials] on request.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
BMI = body mass index, CABG = coronary artery bypass grafting, CVA = cerebrovascular accident, HDL = high density cholesterol level, IHD = ischemic heart disease, LDL = low-density lipoprotein, LVEF = left ventricular ejection fraction, NSTEMI = non-ST segment elevation myocardial infarction, PCI = percutaneous coronary intervention, PVD = peripheral vascular disease, STEMI = ST-elevation myocardial infarction, TIA = transient ischemic attack.
BMI = body mass index, HDL = high density cholesterol level, LDL = low-density lipoprotein, NSTEMI = non-ST segment elevation myocardial infarction, STEMI = ST-elevation myocardial infarction, UA = unstable angina.
CABG = coronary artery bypass graft, CAD = coronary artery disease, PCI = percutaneous intervention.
References
- [1].Rimmerman C. Coronary Artery Disease Cleveland Clinic, Center for Continuous Education 2013. Available at: www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/cardiology/coronary-artery-disease/2013. Cleveland Clinic Center of continuing education. [Google Scholar]
- [2].Neeland IJ, Patel RS, Eshtehardi P, et al. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J 2012;164:547–52. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peppes V, Rammos G, Manios E, Koroboki E, Rokas S, Zakopoulos N. Correlation between myocardial enzyme serum levels and markers of inflammation with severity of coronary artery disease and Gensini score: a hospital-based, prospective study in Greek patients. Clin Interv Aging 2008;3:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350–8. [DOI] [PubMed] [Google Scholar]
- [5].Chaitman BR, Bourassa MG, Davis K, et al. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation 1981;64:360–7. [DOI] [PubMed] [Google Scholar]
- [6].Proudfit WL, Shirey EK, Sones FM, Jr. Selective cine coronary arteriography: correlation with clinical findings in 1,000 patients. Circulation 1966;33:901–10. [DOI] [PubMed] [Google Scholar]
- [7].Kalay N, Yarlioglues M, Ardic I, et al. The assessment of atherosclerosis on vascular structures in patients with acute coronary syndrome. Clin Invest Med 2010;33:E36–43. [DOI] [PubMed] [Google Scholar]
- [8].Mohagheghi A, Mohebi M, Hedayat D, Tabatabee A, Naseri N. The relationship of Gensini score with the cardiovascular risk of patients with indication of angiography. Tehran Univ Med J 2011;69:388–92. [Google Scholar]
- [9].Celik A, Karayakali M, Erkorkmaz U, et al. Presence of angina pectoris is related to extensive coronary artery disease in diabetic patients. Clin Cardiol 2013;36:475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cosentino F, Rydén L, Francia P. Camm AJ, Lüscher TF, Serruys PW, et al. Diabetes mellitus and metabolic syndrome. ESC Textbook of Cardiovascular Medicine 2nd ed.New York, NY: Oxford University Press; 2009. 465–96. [Google Scholar]
- [11].Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA 1979;241:2035–8. [DOI] [PubMed] [Google Scholar]
- [12].Jacoby RM, Nesto RW. Acute myocardial infarction in the diabetic patient: pathophysiology, clinical course and prognosis. J Am Coll Cardiol 1992;20:736–44. [DOI] [PubMed] [Google Scholar]
- [13].Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med 1993;118:81–90. [DOI] [PubMed] [Google Scholar]
- [14].Sinning C, Lillpopp L, Appelbaum S, et al. Angiographic score assessment improves cardiovascular risk prediction: the clinical value of SYNTAX and Gensini application. Clin Res Cardiol 2013;102:495–503. [DOI] [PubMed] [Google Scholar]
- [15].Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [DOI] [PubMed] [Google Scholar]
- [16].Tamis-Holland JE, Lu J, Bittner V, et al. BARI 2D Study Group. Sex, clinical symptoms, and angiographic findings in patients with diabetes mellitus and coronary artery disease (from the Bypass Angioplasty Revascularization Investigation [BARI] 2 Diabetes trial). Am J Cardiol 2011;107:980–5. [DOI] [PubMed] [Google Scholar]
- [17].Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACPASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation 1999;99:2829–48. [DOI] [PubMed] [Google Scholar]
- [18].George J, Afek A, Gilburd B, Harats D, Shoenfeld Y. Autoimmunity in atherosclerosis: lessons from experimental models. Lupus 2000;9:223–7. [DOI] [PubMed] [Google Scholar]
- [19].Selcuk H, Dinc L, Selcuk M, Maden O, Temizhan A. The relation between differential leukocyte count, neutrophil to lymphocyte ratio and the presence and severity of coronary artery disease. Open J Intern Med 2012;2:163–9. [Google Scholar]
- [20].Karan A, Güray Y, Güray U, et al. Mean platelet volume and the extent of coronary atherosclerosis in patients with stable coronary artery disease. Turk Kardiyol Dern Ars 2013;41:45–50. [DOI] [PubMed] [Google Scholar]
- [21].Cingoz F, Iyisoy A, Demirkol S, et al. Carotid intima-media thickness in patients with slow coronary flow and its association with neutrophil-to-lymphocyte ratio: a preliminary report. Clin Appl Thromb Hemost 2014;20:393–9. [DOI] [PubMed] [Google Scholar]
- [22].Charach G, Grosskopf I, Roth A, et al. Usefulness of total lymphocyte count as predictor of outcome in patients with chronic heart failure. Am J Cardiol 2011;107:1353–6. [DOI] [PubMed] [Google Scholar]