Abstract
Objective:
To analyze the level of vitamin D and its influencing factors in pregnant women, and to explore the influence of vitamin D deficiency on common adverse pregnancy outcomes in pregnant women, providing evidence for prevention and intervention of vitamin D deficiency in pregnant women.
Methods:
The basic data and blood samples of pregnant women in our hospital from January 2019 to June 2020 were collected, and the 25-(OH) D levels of the serum samples were detected. Then the vitamin D levels and its influencing factors were analyzed, and the relationships between vitamin D levels and common adverse pregnancy outcomes in the pregnant women as well as the incidence of small-for-gestational-age newborns were analyzed.
Results:
The vitamin D deficiency rate, insufficiency rate and sufficiency rate of pregnant women were 83.28%, 15.36%, and 1.36% respectively, with vast majority of the pregnant women in a state of vitamin D deficiency. Analysis of the influencing factors on the vitamin D level of pregnant women showed “28 weeks ≤ gestational age ≤32 weeks, summer and autumn, high school education and above, weekly time outdoors ≥10 hours, supplement of vitamin D and trace elements during pregnancy” were protective factors for vitamin D sufficiency in pregnant women. Linear correlation analysis showed the vitamin D level of pregnant women was highly positively correlated with temperature, the higher the temperature, the higher the vitamin D level (r = 0.907, t = 6.818, P < .001). The level of vitamin D in pregnant women was related to the occurrence of spontaneous abortion and small-for-gestational age (SGA), with the incidence of spontaneous abortion and SGA in the “vitamin D deficiency group” higher than those of other groups (P = .018, P = .016).
Conclusions:
The vitamin D level of pregnant women in this area is relatively low, which is affected by multiple factors such as gestational age, season, education level of pregnant women, weekly time outdoors, vitamin D and trace element supplement during pregnancy. Low vitamin D levels can increase the risk of spontaneous abortion and SGA in pregnant women, so relevant measures should be adopted to improve the vitamin D status of pregnant women.
Keywords: influencing factors, pregnancy, small for gestational age, temperature, vitamin D
1. Introduction
Vitamin D is a ring-opening steroid hormone that can regulate calcium and phosphorus metabolism, promote bone growth and remodeling, maintain the normal development of cells, tissues and organs, and prevent the occurrence of rickets.[1] In recent years, studies have found that vitamin D also plays an important role in regulating neuroendocrine function and innate immune system function.[2] Vitamin D needed by the human body is mainly formed by the conversion of cholesterol in the body after being irradiated by ultraviolet light in sunlight, and it can also be supplemented by diet.[3] Vitamin D exists in many forms through multiple metabolisms in the body. Since 25-(OH) D is relatively stable in the human body, 25-(OH) D is usually used internationally to reflect the body's vitamin D level.[4]
Pregnant women are a special group. During pregnancy, the metabolism and endocrine functions of pregnant women will undergo subtle changes, and the nutrition intake need to meet both their own nutritional needs and the needs of fetal development.[5] With the rapid changes in modern lifestyles, vitamin D insufficiency or deficiency during pregnancy has become more common and has gradually become a global problem.[6] Clinical investigations showed that vitamin D was essential to the health of pregnant and infants. Insufficiency or deficiency of vitamin D during pregnancy had a significant bad impact on the health of pregnant women and pregnancy outcomes, such as gestational diabetes, gestational hypertension, premature rupture of membranes, premature delivery, etc.[7] Also, vitamin D insufficiency or deficiency during pregnancy had an important impact on the growth and development of the fetus, especially the development of the fetal bones, which may adversely affect the weight, head length, head circumference, and bust of the fetus.[8]
At present, although there have been studies on the vitamin D level of pregnant women and the factors affecting the serum vitamin D level in China,[9] however, the studies on the relationship between serum vitamin D levels and adverse pregnancy outcomes of pregnant women are not deep or enough,[10] and there are few studies about the impact of maternal vitamin D deficiency on the growth and development of newborns.[11] Moreover, due to the huge differences in the living environment of pregnant women in different regions, the factors affecting the serum vitamin D levels of pregnant women are also different.[12] Therefore, this study intended to analyze the overall status of serum vitamin D levels of pregnant women in this region through investigation and sample detection, and to explore the influencing factors of pregnant women's serum vitamin D levels, as well as the correlation between pregnant women's serum vitamin D levels and common adverse pregnancy outcomes, which may provide a basis for the prevention and intervention of vitamin D deficiency in pregnant women in this area.
2. Materials and methods
2.1. Participants
A total of 3080 singleton pregnant women and their newborns who had undergone a full birth check-up at Chaohu Hospital Affiliated to Anhui Medical University from January 2019 to June 2020 and gave birth normally were included in this study. Inclusion criteria: all pregnant women were not less than 8 gestational weeks at the time of enrollment; singleton pregnant women who had regular birth checkups in our hospital and gave birth normally. Exclusion criteria: pregnant women with multiple pregnancies, chronic metabolic diseases, neurological diseases, incomplete data, or dropouts.
2.2. Study design
2.2.1. Sample collection, storage and vitamin D level testing
All the blood samples of pregnant women were collected at 8 to 10 am on the sampling day. After centrifugation, the serum was separated and stored at –80°C. Roche E601 electrochemiluminescence analyzer and its supporting vitamin D detection kit were used to detect the 25-(OH) D content of all samples. All operations were carried out in strict accordance with the detection procedures, and the detection methodology was electrochemiluminescence.
2.2.2. Survey and statistics of basic information of participants
Questionnaires (electronic questionnaires and paper questionnaires) were distributed to pregnant women or their family members to fill. The content of the questionnaire included age, height, weight, gestational age, blood collection date, parity, region strata, education level, family monthly income per capita, weekly time outdoors, the supplements of vitamin D, folic acid, calcium, iron, and trace element during pregnancy, etc.
2.2.3. Criteria for vitamin D deficiency, insufficiency or sufficiency
Vitamin D deficiency: 25-(OH) D <50 nmol/L; vitamin insufficiency: 50 nmol/L ≤25-(OH) D <75 nmol/L; vitamin D sufficiency: 25-(OH) D ≥75 nmol/L).[13] According to the vitamin D level of pregnant women in the study, they were divided into “vitamin D deficiency group,” “vitamin D insufficiency group” and “vitamin D sufficiency group.”
2.2.4. Criteria for small-for-gestational age
SGA is also called small-like baby or intrauterine growth retardation baby. It refers to newborns whose birth weight is below the 10th percentile of the weight of fetuses of the same age, or whose birth weight is less than 2 standard deviations of the average weight of fetuses of the same gestational age.[14]
2.3. Statistical analysis
SPSS 25.0 software was used for the statistical analysis. Median and interquartile range were used to indicate the serum vitamin D level of pregnant women, and rate was used to indicate the occurrence of adverse pregnancy outcomes and SGA. Univariable logistic regression analysis (Kruskal–Wallis rank sum test) was used to compare the serum vitamin D levels of 2 or more groups of pregnant women. Multivariable logistic regression was used to analyze comprehensively analyze the influencing factors that may affect pregnant women's vitamin D levels. Linear correlation analysis was used to explore the influence of temperature on the serum vitamin D level of pregnant women. Chi-Squared test was used to compare the common adverse pregnancy outcomes and the incidence of SGA of pregnant women groups with different serum vitamin D levels. With 0.05 as the standard of significance level, P < .05 indicated that the difference was statistically significant.
2.4. Ethics statement
This study was approved by the Chaohu Hospital affiliated to Anhui Medical University. Informed consent was obtained from all the participants before processing the study. And this study had been reviewed by the ethics committee of Chaohu Hospital affiliated to Anhui Medical University.
3. Results
3.1. Analysis of the overall status of serum vitamin D levels in pregnant women
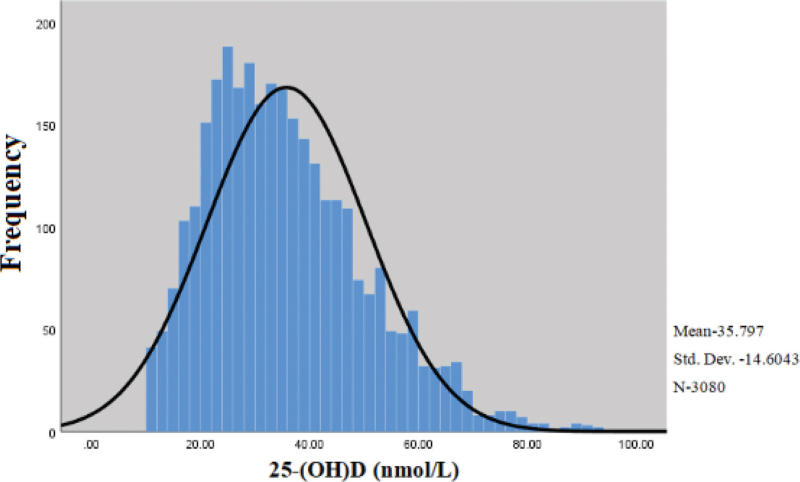
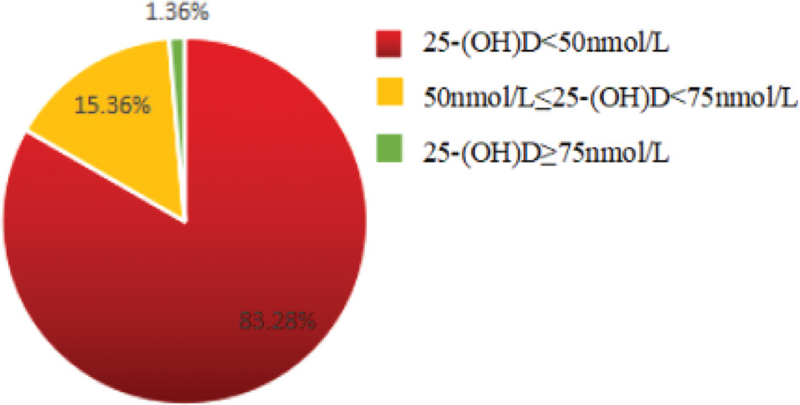
The serum 25-(OH) D levels of all pregnant women were distributed at (10.0–93.9) nmol/L, and the median and quartile levels were 33.7 (24.7, 44.7) nmol/L. Among all the participants, there were 2565 cases, 473 cases, and 42 cases of pregnant women with vitamin D deficiency, insufficiency and sufficiency levels, accounting for 83.28%, 15.36%, and 1.36%, respectively, indicating that the 25-(OH) D levels of most pregnant women were below 50 nmol/L with a state of vitamin D deficiency (Figs. 1 and 2).
Figure 1.
The frequency distribution of the 25-(OH) D measurements. The concentration of 25-(OH) D of most of the cases were less than 50 nmol/L with a state of vitamin D deficiency.
Figure 2.
The proportions of different vitamin D levels in the participants. Only 1.36% of the participants reached the sufficiency level of vitamin D 25-(OH) D ≥75 nmol/L.
3.2. Univariable logistic regression analysis of influencing factors of serum vitamin D level in pregnant women
The age range of the 3080 pregnant women in this study was (15–46) years old, and most of them were younger than 35 years old (94.19%). The gestational ages of the pregnant women ranged (10–32) weeks, and most of them were less than 28 weeks (90.75%). More than half of the blood samples were collected in summer and autumn (61.10%). And most pregnant women were in their first pregnancy (86.30%). Through Kruskal–Wallis rank sum test, there were statistically significant differences in the distribution of serum vitamin D levels among pregnant women in different ages, gestational ages, seasons, educational levels, weekly time outdoors, and vitamin D, calcium and trace element supplement during pregnancy (P < .05). The demographic characteristics and vitamin D level analysis of 3080 pregnant women were shown in Table 1.
Table 1.
Univariate analysis of influencing factors of serum vitamin D level in pregnant women.
| Influencing factor | Variable | Number | Vitamin D level (nmol/L) | Statistics Z/P value |
| Age (yr old) | 15–25 | 870 | 33.1 (24.1, 43.1) | 6.588/.021∗ |
| 25–35 | 2031 | 34.9 (24.7, 45.1) | ||
| 35–46 | 179 | 36.3 (28.7, 48.0) | ||
| BMI (kg/m2) | <18.5 | 704 | 34.6 (25.1, 44.9) | 1.624/.312 |
| 18.5–25.0 | 2248 | 37.5 (25.4, 46.6) | ||
| ≥25.0 | 128 | 35.8 (24.6, 47.2) | ||
| Gestational age (wk) | 10–13 | 1025 | 32.4 (24.2, 42.6) | 5.771/.029∗ |
| 13–28 | 1770 | 33.7 (25.0, 44.8) | ||
| 28–32 | 285 | 35.1 (26.7, 47.4) | ||
| Season | Winter and spring | 1198 | 32.3 (20.5, 40.5) | 34.628/<.001∗ |
| Summer and autumn | 1882 | 40.3 (27.7, 58.5) | ||
| Parity | First pregnancy | 2658 | 36.5 (24.9, 46.8) | 1.264/.387 |
| Non-first pregnancy | 422 | 38.3 (25.3, 47.2) | ||
| Region strata | Rural areas | 744 | 35.9 (24.7, 44.8) | 2.492/.146 |
| Cities | 2336 | 34.8 (25.3, 46.1) | ||
| Education level | Junior high school and below | 847 | 34.6 (24.2, 44.5) | 8.641/.006∗ |
| High school and above | 2233 | 36.5 (25.5, 46.8) | ||
| Family monthly income per capita (RMB) | <1500 | 381 | 35.2 (24.7, 45.2) | 1.853/.251 |
| 1500–3000 | 1384 | 34.5 (23.9, 45.8) | ||
| ≥3000 | 1315 | 35.8 (25.6, 47.1) | ||
| Weekly time outdoors (hour) | <10 | 1852 | 33.9 (23.5, 43.6) | 9.842/.004∗ |
| ≥10 | 1228 | 36.7 (25.8, 46.5) | ||
| Vitamin D supplement | Yes | 274 | 36.1 (24.9, 46.8) | 28.381/<.001∗ |
| No | 2806 | 33.5 (22.8, 44.7) | ||
| Folic acid supplement | Yes | 2188 | 35.7 (25.5, 47.2) | 3.286/.105 |
| No | 892 | 34.4 (24.6, 45.1) | ||
| Calcium supplement | Yes | 339 | 36.7 (26.2, 47.3) | 4.967/.041∗ |
| No | 2741 | 34.6 (24.3, 45.7) | ||
| Iron supplement | Yes | 135 | 35.1 (25.5, 46.7) | 2.092/.216 |
| No | 2945 | 33.8 (24.2, 45.6) | ||
| Trace element supplement | Yes | 189 | 36.2 (25.5, 47.2) | 19.856/<.001∗ |
| No | 2891 | 33.9 (23.3, 44.8) |
3.3. Multivariable logistic regression analysis of influencing factors of serum vitamin D level in pregnant women
The results of univariable analysis showed that age, gestational age, season, education level, weekly time outdoors, vitamin D supplement, calcium supplement and trace element supplement during pregnancy had an effect on the distribution of serum vitamin D levels in pregnant women (P < .05). Therefore, we used multivariable logistic regression to comprehensively analyze the influencing factors that may affect pregnant women's vitamin D levels. Taking vitamin D level as the dependent variable, the serum vitamin D level <75 nmol/L (insufficiency or deficiency) was assigned a value of 0, and the serum vitamin D level ≥75 nmol/L (sufficiency) was assigned a value of 1. The results of stepwise regression analysis showed that there was no collinearity among the independent variables. Multivariable logistic regression analysis showed that gestational age between 28 weeks and 32 weeks, summer and autumn, high school education and above, weekly time outdoors ≥10 hours, supplement of vitamin D and trace elements during pregnancy were protective factors for vitamin D sufficiency in pregnant women (Table 2).
Table 2.
Multivariate logistic regression analysis of influencing factors of serum vitamin D level in pregnant women.
| Influencing factor | Variable | OR | 95% CI of OR | P |
| Age (yr old) | 15–25 | 1.000 | --- | --- |
| 25–35 | 1.072 | 0.723∼1.285 | .322 | |
| 35–46 | 1.113 | 0.809∼1.326 | 0.253 | |
| Gestational age (wk) | 10–13 | 1.000 | --- | --- |
| 13–28 | 1.275 | 0.921∼1.632 | .086 | |
| 28–32 | 1.426 | 1.105∼1.712 | .032∗ | |
| Season | Winter and spring | 1.000 | --- | --- |
| Summer and autumn | 4.383 | 2.532∼5.967 | <.001∗ | |
| Education level | Junior high school and below | 1.000 | --- | --- |
| High school and above | 2.296 | 1.315∼3.368 | .021∗ | |
| Weekly time outdoors (hr) | <10 | 1.000 | --- | --- |
| ≥10 | 1.822 | 1.263∼2.385 | .026∗ | |
| Vitamin D supplement | Yes | 1.000 | --- | --- |
| No | 5.264 | 2.182∼8.361 | <.001∗ | |
| Calcium supplement | Yes | 1.000 | --- | --- |
| No | 1.035 | 0.812∼1.186 | .403 | |
| Trace element supplement | Yes | 1.000 | --- | --- |
| No | 3.662 | 1.864∼5.386 | <.001∗ |
3.4. The influence of air temperature on the serum vitamin D level of pregnant women
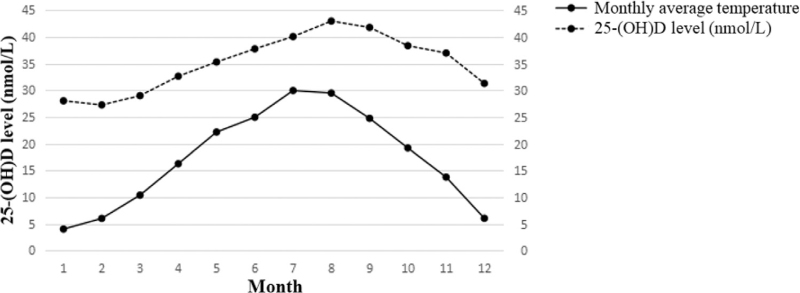
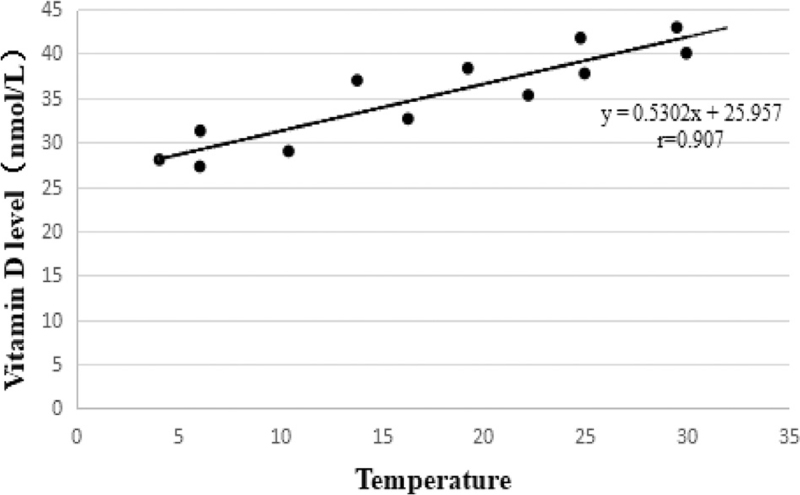
Through univariable and multivariable analysis of factors affecting the serum vitamin D level of pregnant women, it was found that the season was one of the most important factors. Considering that the main difference in different seasons was the temperature, we further analyzed the correlation between the local monthly average temperature and the monthly average concentration of pregnant women's serum vitamin D during the study period. The analysis results showed that the serum vitamin D level of pregnant women was highly positively correlated with temperature. The higher the temperature, the higher the serum 25-(OH) D level (r = 0.907, t = 6.818, P < .001) (Figs. 3 and 4).
Figure 3.
The change trends of pregnant women's serum vitamin D level and temperature with month. The serum vitamin D level of pregnant women and the temperature showed a trend of synchronous change.
Figure 4.
Correlation analysis of pregnant women's serum vitamin D level with temperature. The serum vitamin D level of pregnant women was highly positively correlated with temperature, with the higher the temperature, the higher the serum 25-(OH) D level (r = 0.907, t = 6.818, P < .001).
3.5. Analysis of the relationship between pregnant women's serum vitamin D levels and common adverse pregnancy outcomes
According to the Chi-Squared test, compared with “vitamin D sufficiency group,” the incidence of spontaneous abortion in “vitamin D deficiency group” was the higher (P = .018) with the risk of spontaneous abortion in the “vitamin D deficiency group” 7.62 times of the “vitamin D sufficiency group.” However, there was no significant difference in the incidences of gestational diabetes, hypertension-related diseases during pregnancy (including hypertension during pregnancy, preeclampsia, eclampsia, chronic hypertension with preeclampsia and chronic hypertension), premature rupture of membranes, cesarean section or premature birth in different serum vitamin D level groups (P > .05) (Table 3).
Table 3.
Univariable analysis of maternal serum vitamin D levels and common adverse pregnancy outcomes.
| Maternal vitamin D status during pregnancy | |||||
| Deficiency | Insufficiency | Sufficiency | P Deficiency VS Sufficiency | P Insufficiency VS Sufficiency | |
| Gestational diabetes | |||||
| Total number of pregnant women | 2565 | 473 | 42 | ||
| Pregnant women with gestational diabetes | 209 | 47 | 4 | ||
| RR/95% CI for gestational diabetes | 0.84 (0.298∼2.384) | 1.05 (0.358∼3.066) | 1.00 | .747 | .932 |
| Hypertension-related diseases | |||||
| Pregnant women with hypertension-related diseases | 192 | 32 | 4 | ||
| RR/95% CI for hypertension-related diseases | 0.77 (0.271∼2.176) | 0.69 (0.232∼2.052) | 1.00 | .619 | .502 |
| Premature rupture of membranes | |||||
| Pregnant women with premature rupture of membranes | 498 | 83 | 7 | ||
| RR/95% CI for premature rupture of membranes | 1.21 (0.532∼2.728) | 1.06 (0.457∼2.478) | 1.00 | .655 | .885 |
| Cesarean section | |||||
| Pregnant women with cesarean section | 1202 | 239 | 22 | ||
| RR/95% CI for cesarean section | 0.80 (0.435∼1.476) | 0.93 (0.494∼1.747) | 1.00 | .477 | .818 |
| Premature birth | |||||
| Pregnant women with premature birth | 195 | 20 | 1 | ||
| RR/95% CI for premature birth | 3.37 (0.462∼24.656) | 1.81 (0.237∼13.833) | 1.00 | .203 | .562 |
| Spontaneous abortion | |||||
| Pregnant women with spontaneous abortion | 402 | 18 | 1 | ||
| RR/95%CI for spontaneous abortion | 7.62 (1.045∼55.554) | 1.62 (0.211∼12.460) | 1.00 | .018∗ | .639 |
3.6. Analysis of the relationship between pregnant women's serum vitamin D levels and small-for-gestational age
A total of 3080 pairs of mothers and infants were included in this study, and 423 cases of SGA were born, with an incidence of 13.73%. Analysis of serum vitamin D levels and the incidence of SGA in pregnant women showed that the incidence of SGA in the “vitamin D deficiency group,” “vitamin D insufficiency group,” and “vitamin D sufficiency group” were 15.98%, 2.54%, and 2.38%, respectively. According to the Chi-Squared test, compared with “vitamin D sufficiency group,” the incidence of SGA in the “vitamin D deficiency group” was higher (P = .016), with the risk of SGA in the “vitamin D deficiency group” 7.80 times of the “vitamin D sufficiency group” (Table 4).
Table 4.
Univariate analysis of maternal serum 25-(OH)D level and incidence of SGA.
| Vitamin D level | Total number | Number of SGA | Incidence of SGA (%) | Relative hazard ratio (RR) | 95% CI of RR | P |
| Deficiency group | 2565 | 410 | 15.98 | 7.80 | 1.070∼56.867 | .016∗ |
| Insufficiency group | 473 | 12 | 2.54 | 1.07 | 0.135∼8.414 | .951 |
| Sufficiency group | 42 | 1 | 2.38 | 1.00 | --- | --- |
4. Discussion
In recent years, studies at home and abroad have shown that vitamin D deficiency during pregnancy is a very common phenomenon. According to the study of Chinese scholar Yin Wanjun et al, from 2015 to 2017, the vitamin D deficiency of pregnant women in Hefei, Anhui Province showed an upward trend year by year, and in 2017, the vitamin D deficiency rate of pregnant women was as high as 81.4%.[15] In this study, 83.28% of pregnant women in the Chaohu area of Hefei were in a state of vitamin D deficiency (<50 nmol/L), while only 1.36% of pregnant women reached sufficiency levels of vitamin D (≥75 nmol/L). This showed that the vitamin D deficiency of pregnant women in Chaohu area was already quite serious, and it was necessary to guide pregnant women to increase vitamin D intake.
Studies have shown that vitamin D is mainly synthesized by 7-dehydrocholesterol by ultraviolet light irradiation in our body, and temperature plays an important role in the adjustment process of 25(OH) D production. Within a certain range, the increase in temperature can promote the synthesis of vitamin D in the body.[16] There are many factors that affect the serum vitamin D level of pregnant women, such as the age and gestational age of pregnant woman, the season, parity, time of outdoor exercise and vitamin D supplementation, etc. Studies showed that low-age pregnant women were more likely to suffer from vitamin D deficiency or insufficiency, the serum vitamin D levels of pregnant women in summer and autumn were significantly higher than those in winter and spring, and increased outdoor exercise time and supplementation of vitamin D were protective factors for serum vitamin D sufficiency in pregnant women.[17–19] In this study, through univariable and multivariable analysis of factors affecting the serum vitamin D level of pregnant women, we found that 28≤ gestational week ≤32, summer and autumn, high school education and above, weekly time outdoors ≥10 hours, supplementation of vitamin D and trace elements during pregnancy were protective factors for vitamin D sufficiency in pregnant women, which were close to the results of related studies. Moreover, linear correlation analysis showed that the serum vitamin D level of pregnant women was highly positively correlated with temperature. The higher the temperature, the higher the serum 25-(OH) D level (r = 0.907, t = 6.818, P < .001). Regarding the season and temperature factors, the Chaohu area of Hefei City has a subtropical monsoon climate with 4 distinct seasons, and there are obvious differences in sunshine duration and temperature in different seasons. In summer and autumn, the sunshine is more abundant and the temperature is higher. Pregnant women spent more time outdoors in summer and autumn and received more sunlight than winter and spring, which may affect the serum vitamin D level of pregnant women.
The serum vitamin D level of pregnant women was closely related to the health status of the pregnant women and the growth of the babies. According to research reports, vitamin D deficiency/insufficiency during pregnancy could lead to a series of adverse pregnancy outcomes, such as gestational diabetes, gestational hypertension, premature rupture of membranes, cesarean section, premature delivery and spontaneous abortion, etc.[20] While Silvia Fogacci's researchers found that vitamin D supplementation may be useful in preventing preeclampsia and the development of hypertensive disorders in pregnancy.[21,22] Insufficiency/deficiency of vitamin D during pregnancy would seriously affect the growth and development of fetal bones, thereby affecting the breast circumference, head circumference and length of the fetus, greatly increasing the birth probability of SGA.[23] The mortality and disease risk of SGA in the perinatal period were significantly higher than those of normal-weight newborns, and they would also have cognitive impairment, low learning ability, and low adult lifelong height in adulthood.[24] In this study, the serum vitamin D level of pregnant women was related to the incidence of spontaneous abortion and SGA. The “vitamin D deficiency group” had the highest incidences of spontaneous abortion and SGA (P = .018 and P = .016).
Studies had shown that infection and inflammation were important causes of premature delivery and spontaneous abortion in pregnant women, and vitamin D had important immune regulation and infection prevention functions, which could increase serum anti-inflammatory factors and reduce the level of pro-inflammatory factors. Vitamin D deficiency during pregnancy may increase the risk of preterm birth and spontaneous abortion through infection and inflammation during pregnancy.[25] Also, vitamin D may increase the risk of iatrogenic preterm birth by increasing the occurrence of pregnancy complications.[26] In addition, vitamin D deficiency could cause a decrease in the immune tolerance of pregnant women, resulting in a relative or absolute low of regulatory T cells, which significantly weakened the body's immunosuppressive effect, and thus could not suppress the excessive immune response and increased the risk of miscarriage.[27] However, at present, the mechanism of how maternal vitamin D deficiency causes SGA during pregnancy has not been fully elucidated. Some studies believed that vitamin D played an anti-inflammatory effect in placental tissue through vitamin D receptors, thereby protecting the normal growth and development of the fetus, and low vitamin D levels would increase the risk of neonatal sepsis.[28,29]
However, this study still had some shortcomings. Firstly, this study did not investigate and analyze social factors such as work pressure, etc during pregnancy. Secondly, the number of cases in the vitamin D sufficiency group was small. In addition, when performing univariable analysis, the groups in this study were not exactly balanced. All these may cause certain biases in the research results. We will incorporate these factors into the analysis in subsequent studies in order to obtain more accurate research results.
5. Conclusion
In summary, the serum vitamin D levels and sufficiency rate of pregnant women in the Chaohu aera of Hefei city are relatively low, which are affected by multiple factors such as gestational week, season, pregnant women's education level, weekly time outdoors, and vitamin D and trace element supplementation during pregnancy. Low vitamin D levels can increase the risk of premature delivery and spontaneous abortion and neonatal SGA. Relevant effective measures should be strengthened to improve the vitamin D status of pregnant women in this region.
Acknowledgments
The authors would like to acknowledge the staff of the Department of Nuclear Medicine, Chaohu Hospital Affiliated to Anhui Medical University, for their assistance with this study.
Author contributions
Conceptualization: Yuanhong Xu.
Data curation: Bo Chen, Yongquan Chen.
Formal analysis: Bo Chen, Yongquan Chen.
Funding acquisition: Yuanhong Xu.
Methodology: Bo Chen.
Project administration: Bo Chen, Yongquan Chen.
Resources: Bo Chen, Yongquan Chen.
Supervision: Bo Chen, Yuanhong Xu.
Visualization: Yongquan Chen.
Writing – original draft: Bo Chen.
Writing – review & editing: Yongquan Chen.
Footnotes
Abbreviation: SGA = small-for-gestational age.
How to cite this article: Chen B, Chen Y, Xu Y. Vitamin D deficiency in pregnant women: influenced by multiple risk factors and increase the risks of spontaneous abortion and small-for-gestational age. Medicine. 2021;100:41(e27505).
YC and YX contributed equally to this work.
The authors have no funding and conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article.
Compared between 2 or more groups, P < .05, the difference was statistically significant.
BMI = body mass index.
Compared with the reference group, P < .05, the difference was statistically significant.
CI = confidence interval.
Comparedwith vitamin D sufficiency group, P < .05, the difference was statistically significant.
RR = relative hazard ratio.
Compared with sufficiency group, P < .05, the difference was statistically significant.
RR = relative hazard ratio.
References
- [1].Kulda V. Vitamin D metabolism. Vnitr Lek 2012;58:400–4. [PubMed] [Google Scholar]
- [2].Lisi G, Ribolsi M, Siracusano A, et al. Maternal vitamin D and its role in determining fetal origins of mental health. Curr Pharm Des 2020;26:2497–509. [DOI] [PubMed] [Google Scholar]
- [3].Prietl B, Treiber G, Pieber TR, et al. Vitamin D and immune function. Nutrients 2013;5:2502–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fan H, Hui L, Yan X, et al. Serum 25 hydroxyvitamin D levels and affecting factors among preconception fertile women. BMC Womens Health 2020;20:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kocyłowski R, Lewicka I, Grzesiak M, et al. Assessment of dietary intake and mineral status in pregnant women. Arch Gynecol Obstet 2018;297:1433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Q, Ma A, Schouten EG, Kok FJ. A double burden of tuberculosis and diabetes mellitus and the possible role of vitamin D deficiency. Clin Nutr 2021;40:350–7. [DOI] [PubMed] [Google Scholar]
- [7].Christoph P, Challande P, Raio L, et al. High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy. Swiss Med Wkly 2020;150:w20238. [DOI] [PubMed] [Google Scholar]
- [8].Larqué E, Morales E, Leis R, et al. Maternal and foetal health implications of vitamin D status during pregnancy. Ann Nutr Metab 2018;72:179–92. [DOI] [PubMed] [Google Scholar]
- [9].Wang X, Jiao X, Tian Y, et al. Associations between maternal vitamin D status during three trimesters and cord blood 25(OH) D concentrations in newborns: a prospective Shanghai birth cohort study. Eur J Nutr 2021;60:3473–83. [DOI] [PubMed] [Google Scholar]
- [10].Aji AS, Yusrawati Y, G. Malik S, et al. The association of maternal vitamin D status during pregnancy and neonatal anthropometric measurements: a longitudinal study in Minangkabau pregnant women, Indonesia. J Nutr Sci Vitaminol (Tokyo) 2020;66: (Supplement): S63–70. [DOI] [PubMed] [Google Scholar]
- [11].Liu Z, Meng T, Liu J, et al. The individual and joint effects of maternal 25-(OH) D deficiency and gestational diabetes on infant birth size. Nutr Metab Cardiovasc Dis 2020;30:2398–405. [DOI] [PubMed] [Google Scholar]
- [12].Shen Y, Pu L, Si S, et al. Vitamin D nutrient status during pregnancy and its influencing factors. Clin Nutr 2020;39:1432–9. [DOI] [PubMed] [Google Scholar]
- [13].Zhao X, Xiao J, Liao X, et al. Vitamin D status among young children aged 1–3 years: a cross-sectional study in Wuxi, China. PLoS One 2015;10:e0141595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hoseini MS, Sheibani S, Sheikhvatan M. The evaluating of pregnancy-associated plasma protein-A with the likelihood of small for gestational age. Obstet Gynecol Sci 2020;63:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yin WJ, Tao RX, Zhang Y, et al. Trends analysis of vitamin D status among pregnant women in Hefei during 2015–2017. Zhonghua Yu Fang Yi Xue Za Zhi 2019;53:947–50. [DOI] [PubMed] [Google Scholar]
- [16].Escribà-Gelonch M, Noël T, Hessel V. Microflow high-p, T intensification of vitamin D3 synthesis using an ultraviolet lamp. Org Process Res Dev 2018;22:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li H, Ma J, Huang R, et al. Prevalence of vitamin D deficiency in the pregnant women: an observational study in Shanghai, China. Arch Public Health 2020;78:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petersen SB, Strøm M, Maslova E, et al. Predicted vitamin D status during pregnancy in relation to offspring forearm fractures in childhood: a study from the Danish National Birth Cohort. Br J Nutr 2015;114:1900–8. [DOI] [PubMed] [Google Scholar]
- [19].Roth DE, Morris SK, Zlotkin S, et al. Vitamin D supplement in Pregnancy and Lactation and Infant Growth. N Engl J Med 2018;379:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dovnik A, Mujezinović F. The association of vitamin D levels with common pregnancy complications. Nutrients 2018;10:867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fogacci S, Fogacci F, Banach M, et al. Vitamin D supplementation and incident preeclampsia: a systematic review and meta-analysis of randomized clinical trials. Clin Nutr 2020;39:1742–52. [DOI] [PubMed] [Google Scholar]
- [22].Fogacci S, Fogacci F, Cicero AFG. Nutraceuticals and hypertensive disorders in pregnancy: the available clinical evidence. Nutrients 2020;12:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tao RX, Meng DH, Li JJ, et al. Current recommended vitamin D prenatal supplement and fetal growth: results from the China-Anhui Birth Cohort Study. J Clin Endocrinol Metab 2018;103:244–52. [DOI] [PubMed] [Google Scholar]
- [24].Liu C, Wang K, Guo J, et al. Small for gestational age is a risk factor for thyroid dysfunction in preterm newborns. BMC Pediatr 2020;20:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou Y, Chen YH, Fu L, et al. Vitamin D3 pretreatment protects against lipopolysaccharide- induced early embryo loss through its anti-inflammatory effects. Am J Reprod Immunol 2017;77:e12620. [DOI] [PubMed] [Google Scholar]
- [26].Shrestha D, Budhathoki S, Pokhrel S, et al. Prevalence of vitamin D deficiency in pregnant women and their babies in Bhaktapur, Nepal. BMC Nutr 2019;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sharif K, Sharif Y, Watad A, et al. Vitamin D, autoimmunity and recurrent pregnancy loss: More than an association. Am J Reprod Immunol 2018;80:e12991. [DOI] [PubMed] [Google Scholar]
- [28].Baker BC, Hayes DJ, Jones RL. Effects of micronutrients on placental function: evidence from clinical studies to animal models. Reproduction 2018;156:R69–82. [DOI] [PubMed] [Google Scholar]
- [29].Siyah Bilgin B, Gonulal D. Association between vitamin D level and community-acquired late-onset neonatal sepsis. Arch Argent Pediatr 2020;118:265–72. [DOI] [PubMed] [Google Scholar]