Abstract
Objective:
The purpose of our study was to investigate whether IL-10 -819C/T, -592A/C polymorphisms were associated with preeclampsia (PE) susceptibility.
Methods:
A comprehensive and systematic literature search was performed through online databases, including Web of Science, PubMed, EMBASE, and Chinese databases. Then eligible literatures were included according to inclusion criteria and exclusion criteria. Statistical data analysis was performed using Stata 10.0 software. Odds ratios (OR) and 95% confidence interval were applied to evaluated the association between IL-10 -819C/T, -592A/C polymorphisms and PE susceptibility.
Results:
According to inclusion and exclusion criteria, 9 case-control studies, including 1423 cases and 2031 controls, were included in this meta-analysis. Our meta-analysis revealed that no association was found between IL-10 -819C/T, -592A/C polymorphisms and the risk of PE in our study.
Conclusion:
Our meta-analysis suggested that IL-10 -819C/T and -592A/C polymorphisms had no association with PE susceptibility, but had a significant association with PE susceptibility in Asian and Caucasian.
Keywords: IL10 -592A/C polymorphism, IL-10 -819C/T polymorphism, meta-analysis, preeclampsia, susceptibility
1. Introduction
Preeclampsia (PE) is a pregnancycomplicated hypertensive disorder, characterized by new onset hypertension and proteinuria after 20 weeks of gestation and accompanied by renal dysfunction, liver injury, pulmonary edema and multisystem dysfunction.[1,2] Currently, 2% to 8% pregnancies throughout the world are suffered from PE, a leading cause of maternal and perinatal mortality.[3] However, the etiology and pathogenesis of PE have not yet been fully elucidated. PE is thought to be an accessory of suitable interactions among immunity, inflammation, diet, and genetic factors,[4,5] leading to a decrease in trophoblast invasiveness and abnormal remodeling of uterine spiral artery. Particularly, increasing evidence suggests that genetic factors contribute to the etiology, development and complexity of PE. Copy number variations have been reported to be associated with the risk of PE.[6–8] In addition, several studies have identified that single nucleotide polymorphisms (SNPs) have been involved in the pathogenesis of PE, such as cytokines genes SNPs.[9–11]
Inflammation has been increasingly recognized as an important factor to contribute to endothelial dysfunction and to the pathology of PE.[12–14] For example, pro-inflammatory cytokine tumor necrosis factor α, by a mechanism depending on influencing the invasiveness of trophoblast cells and remodeling of the uterine spiral artery,[15] and interleukin (IL) 6, contributing to the systemic endothelial activation and vascular damage,[16,17] play an important role in the development of PE. Contrarily, IL-10, as an important anti-inflammatory factor, maintains the function of trophoblast by regulating the balance of anti-inflammatory signals, which leads to appropriate pregnancy outcomes.[18] It has reported that dysregulation of IL-10 was involved in the pathophysiology of PE.[19] Meanwhile, IL-10 is involved in regulating secretion of matrix metalloproteinases and invasiveness of trophoblast cells.[20] Taking into account the inheritable nature of PE and crucial function of IL-10 in PE, the influence of the IL-10 gene polymorphisms on PE are not negligible.
The IL-10 gene, located on chromosome 1q31-32, exhibits high polymorphisms, such as IL-10 -1082A/G (rs1800896), IL-10 -819C/T (rs1800871), IL-10 -592A/C (rs1800872), which have been investigated to be correlated with the risk of PE, but the results remained controversial. Mehrnaz's meta-analysis identified that IL-10 -1082 G > A polymorphism was significantly associated with an increased risk of PE. However, there are several studies examining the association between IL-10 polymorphisms and PE including IL-10 -819C/T (rs1800871), IL-10 -592A/C (rs1800872), which are exhibited to be relevant to the risk of PE, characterized by deranged angiogenesis and trophoblast invasion by altering gene expression and protein production. But the results of different races and groups are contradictory and controversial.[21–29] In order to attenuate the limitations of individual study, we performed a systemic review and a meta-analysis of all eligible case control studies including 1423 pregnant women with PE and 2031 normotensive pregnant women to determine whether the IL-10 -819C/T, -592A/C polymorphisms are associated with the susceptibility to PE. However, there were unknown significance referring the relationship between IL-10 -819C/T, -592A/C polymorphisms and PE susceptibility, it had a significant association with PE susceptibility in Asian and Caucasian.
2. Methods
2.1. Search strategy
The literature, whose publication date is up to June 2020, was searched from online databases, including Web of Science, PubMed, EMBASE, and Chinese databases (Chinese National Knowledge Infrastructure and Wan Fang). The searching terms were used as following: (“preeclampsia” OR “pre-eclampsia”) AND (“polymorphism” OR “single nucleotide polymorphism” OR “SNP” OR “variant”) AND (“interleukin-10” OR “IL-10”).
2.2. Inclusion and exclusion criteria
Overall, the inclusion criteria for eligible studies in this meta-analysis were:
-
1.
case-control studies focused on associations between IL-10 -819C/T, -592A/C polymorphisms and risk of PE;
-
2.
cases were strictly included and excluded;
-
3.
the studies provided adequate original data in the case and control groups for various genotypes.
Except for English and Chinese, language constraint was applied in the selection of articles. The exclusion criteria were as follows:
-
1.
prospective study;
-
2.
meeting abstract;
-
3.
editorial;
-
4.
meta-analysis and review;
-
5.
repeated publication.
2.3. Data extraction
The relevant data was carefully extracted from all the eligible publications by 2 independent researchers for this meta-analysis. Any divergences between the 2 reviewers were resolved by consensus. The information collected from each study was as follows: first author's name, publication date, country, ethnicity, genotyping methods, case inclusion criteria, source of controls, total number of case and control group, the number of cases and controls of each studied polymorphism.
2.4. Ethical statement
Because this is a literature-based meta-analysis, ethical approval is not required.
2.5. Statistical analysis
Stata 10.0 software (Stata Corporation, USA) was carried out to perform statistical data analysis. The association between IL-10 -819C/T, -592A/C polymorphisms and PE susceptibility was evaluated by calculating odds ratios (OR) and 95% confidence interval. An allele contrast model, homozygote model, dominant and recessive model were used for evaluation of association between IL-10 -819C/T, -592A/C polymorphisms and PE susceptibility, respectively. When the heterogeneity test of P value is <.1 and the I2 is >50%, the random effect model is applied, whereas the fixed effect model is used instead. The significance of the OR was determined by the Z-test, in which P < .05 was considered that there was statistically significant correlation between each SNP and PE susceptibility. Begg funnel plot was performed to evaluate potential publication bias.
3. Results
3.1. The baselines of studies included in the meta-analysis
The flow chart of study selection shown in Figure 1 exhibits the process, of which the studies were searched and screened. Totally, 122 studies were searched online from 5 databases. And then 46 duplicate studies among databases were excluded. Fifty one irrelevant articles were removed by reviewing titles and abstracts. According to inclusion and exclusion criteria, 6 of them were prospective study, conference abstracts, reviews or meta-analysis, which were excluded. Ten records were deleted owing to duplicate publication, no data, referring to other loci polymorphism or language constraint. Finally, 9 case-control studies were included in this meta-analysis,[21–29] of which 8 studies in IL-10 -819 C/T polymorphism, 7 in IL10 -592 A/C polymorphism. A total of 1423 cases and 2031 controls, which were from 3 Caucasians, 4 Asian populations, 2 African populations, and 1 Mulatto, mixed white and black ancestry, were included in this meta-analysis. Besides, the study by Haggerty[21] included African population and Caucasian. However, because of the insufficient population of some ethnic groups, we selectively conducted ethnicity-specific meta-analysis of 2 SNPs. The general characteristics of 9 eligible studies with respect to associations between the IL-10 -819C/T, -592A/C polymorphisms and PE are summarized in Table 1.
Figure 1.
The study selection flow chart in our study.
Table 1.
General characteristics of 9 eligible studies contained in this meta-analysis.
| Study name | Country | Ethnicity | Methods | Case selection | Source of control | Sample Size Case/Control | Polymorphisms studied |
| Haggerty, 2005 | USA | EuropeanAfrican American | TaqMan | 1. SBP/DBP > 140/90 mmHg2. Proteinuria > 300 mg/24 h or protein/creatinine ratio >0.33. Hyperuricemia | 1. Normotensive2. No proteinuria or hyperuricemia during pregnancy | 130/46220/199 | IL-10 -819 C/T |
| Kamali, 2006 | Iran | Asian | ASO-PCR/PCR-RFLP | National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy National High Blood Pressure | 1. At least two successful deliveries2. Without any history of PE | 134/164 | IL-10 -819 C/T, IL-10 -592A/C |
| Mirahmadian, 2008 | Iran | Asian | PCR-SSP | National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy | 1. Normal laboratory examination2. No clinical signs and symptoms of PE | 260/100 | IL-10 -819 C/T, IL-10 -592A/C |
| de Lima, 2009 | Brazil | South American | PCR-SSP | National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy | 1. Normotensive in previous pregnancy2. At least one successful pregnancy without any maternal or fetal disorder | 92/101 | IL-10 -819 C/T, IL-10 -592A/C |
| Sowmya, 2014 | India | South-Asian | ARMS-PCR | 1. SBP/DBP >130/90 mm Hg2. New onset of proteinuria > 2 + dipstick | More than 20 weeks pregnancy with normal blood pressure, fetal growth and physiological normalities | 120/120 | IL-10 -819 C/T, IL-10 -592A/C |
| Song, 2015 | China | Asian | PCR-RFLP | 1. SBP/DBP ≥ 140/90 mm Hg2. Proteinuria ≥ 300 mg/24 h or 2+ | 1. More than 20 wks pregnant women2. No history of chronic hypertension, renal, autoimmune, metabolic or cardiovascular disease | 155/201 | IL-10 -819 C/T, IL-10 -592A/C |
| Liu, 2015 | China | Asian | PCR-RFLP | 1. SBP/DBP ≥ 140/90 mm Hg2. Proteinuria | Normal pregnant women in the same period | 177/182 | IL-10 -819 C/T |
| Fan, 2017 | China | Asian | PCR-RFLP | 1. SBP/DBP ≥140/90 mm Hg2. Proteinuria ≥300 mg/24 h | 1. More than 20 wks pregnant women2. No history of chronic hypertension, cardiovascular disease, end-stage liver or renal diseases, or diabetes | 142/260 | IL-10 -592A/C |
| Raguema, 2018 | Tunisia | African | TaqMan | 1. SBP/DBP ≥140/90 mm Hg2. Proteinuria ≥300 mg/24 h or 2+ | 1. Age-matched with cases and from the same region2. No personal or family history of hypertension and PE | 345/300 | IL-10 -819 C/T, IL-10 -592A/C |
3.2. Association between IL-10 -819C/T, -592A/C polymorphisms and PE
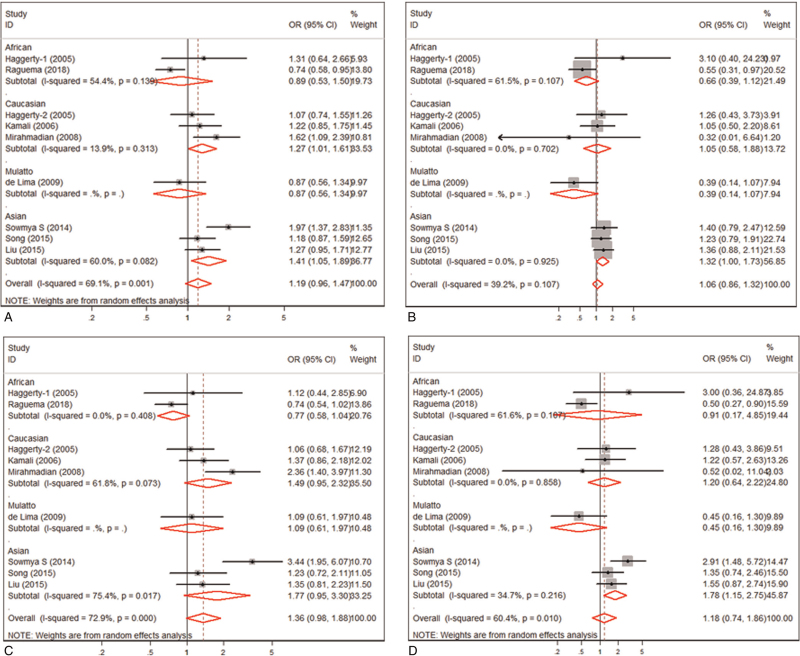
In this meta-analysis, we evaluated the association between IL-10 -819C/T, -592A/C polymorphisms and the susceptibility of PE (data shown in Table 2). Meta-analysis of IL-10 -819C/T polymorphism revealed that IL-10 -819 C allele was not significantly associated with the risk of PE (OR = 1.188, 95% CI: 0.958-1.472, P = 0.116) (Table 2 and Fig. 2A). Stratification analysis by ethnicity showed that the association between IL-10 -819 C allele and PE was significant in Caucasians (OR = 1.270, 95% CI: 1.024–1.576, P = .039) and in Asians (OR = 1.410, 95%CI: 1.054–1.886, P = .021) (Table 2). On the contrary, IL-10 -819 C allele was found to be associated with the risk of PE in Africans (OR = 0.791, 95% CI: 0.628–0.996, P = .046). The strong association between PE and the IL-10 -819C/T polymorphism was recorded using the recessive model (OR = 1.316, 95% CI: 1.002–1.728, P = .048) or a homozygote contrast in Asians (OR = 1.755, 95% CI: 1.236–2.492, P = .002), other than overall or in Caucasians and Africans (Table 2 and Fig. 2B and 2D). However, no association was found between IL-10 -819C/T polymorphism and PE under the dominant model analysis (Table 2 and Fig. 2C).
Table 2.
Meta analysis of the association of IL-10 -819C/T, -592A/C polymorphisms and PE susceptibility.
| Number | Study of association | Heterogeneity of study design | ||||||||
| Polymorphism studied | Ethnicity | Cases | Controls | OR | 95% CI | Z (P value) | P | I 2 | Model | |
| IL-10 -819 | C vs T | Overall | 1281 | 1771 | 1.188 | 0.958–1.472 | 1.57 (.116) | .001 | 69.10% | Random |
| African | 356 | 478 | 0.791 | 0.628–0.996 | 2.00 (.46) | .139 | 54.40% | Fixed | ||
| Caucasian | 385 | 695 | 1.270 | 1.024–1.576 | 2.17 (.039) | .313 | 13.90% | Fixed | ||
| Asian | 452 | 506 | 1.410 | 1.054–1.886 | 2.31 (.021) | .082 | 60.00% | Random | ||
| CC + CT vs TT (recessive) | Overall | 1281 | 1771 | 1.065 | 0.858–1.322 | 0.57 (.569) | .107 | 39.20% | Fixed | |
| African | 356 | 478 | 0.662 | 0.391–1.121 | 1.54 (.125) | .107 | 61.50% | Fixed | ||
| Caucasian | 385 | 695 | 1.046 | 0.581–1.883 | 0.15 (.880) | .702 | 0.00% | Fixed | ||
| Asian | 452 | 506 | 1.316 | 1.002–1.728 | 1.98 (.048) | .925 | 0.00% | Fixed | ||
| CC vs CT + TT (dominant) | Overall | 1281 | 1771 | 1.361 | 0.984–1.883 | 1.86 (.063) | <.001 | 72.90% | Random | |
| African | 356 | 478 | 0.774 | 0.576–1.041 | 1.69 (.091) | .408 | 0.00% | Fixed | ||
| Caucasian | 385 | 695 | 1.487 | 0.953–2.321 | 1.75 (.081) | .073 | 61.80% | Random | ||
| Asian | 452 | 506 | 1.770 | 0.948–3.303 | 1.79 (.073) | .017 | 75.40% | Random | ||
| CC vs. TT | Overall | 1281 | 1771 | 1.175 | 0.743–1.859 | 0.69 (.491) | .010 | 60.40% | Random | |
| African | 356 | 478 | 0.600 | 0.347–1.037 | 1.83 (.067) | .107 | 61.60% | Fixed | ||
| Caucasian | 385 | 695 | 1.188 | 0.645–2.189 | 0.55 (.58) | .858 | 0.00% | Fixed | ||
| Asian | 452 | 506 | 1.755 | 1.236–2.492 | 3.14 (.002) | .216 | 34.60% | Fixed | ||
| IL-10 -592 | A vs C | Overall | 1133 | 1238 | 0.786 | 0.617–1.001 | 1.95 (.051) | .001 | 73.30% | Random |
| Caucasian | 288 | 261 | 0.725 | 0.554–0.948 | 2.35 (.019) | .448 | 0.00% | Fixed | ||
| Asian | 417 | 582 | 0.635 | 0.530–0.762 | 4.88 (<.001) | .862 | 0.00% | Fixed | ||
| AA vs AC + CC (recessive) | Overall | 1133 | 1238 | 0.855 | 0.537–1.361 | 0.66 (.508) | .001 | 72.70% | Random | |
| Caucasian | 288 | 261 | 1.170 | 0.585–2.338 | 0.44 (.658) | .242 | 27.00% | Fixed | ||
| Asian | 417 | 582 | 0.536 | 0.408–0.703 | 4.50 (<.001) | .427 | 0.00% | Fixed | ||
| AA + AC vs CC (dominant) | Overall | 1133 | 1238 | 0.716 | 0.526–0.975 | 2.12 (.034) | .010 | 64.10% | Random | |
| Caucasian | 288 | 261 | 0.700 | 0.404–0.804 | 3.21 (.001) | .233 | 29.80% | Fixed | ||
| Asian | 417 | 582 | 0.609 | 0.444–0.836 | 3.07 (.002) | .638 | 0.00% | Fixed | ||
| AA vs CC | Overall | 1133 | 1238 | 0.784 | 0.459–1.341 | 0.89 (.374) | .002 | 70.90% | Random | |
| Caucasian | 288 | 261 | 0.952 | 0.463–1.959 | 0.13 (.894) | .342 | 0.00% | Fixed | ||
| Asian | 417 | 582 | 0.441 | 0.308–0.633 | 4.45 (<.001) | .935 | 0.00% | Fixed | ||
Figure 2.
Meta-analysis of the association between the IL10 -819 C/T polymorphism and PE. (A). allele control model; (B). recessive genotype model; (C). dominant genotype model; (D). homozygous genotype model.
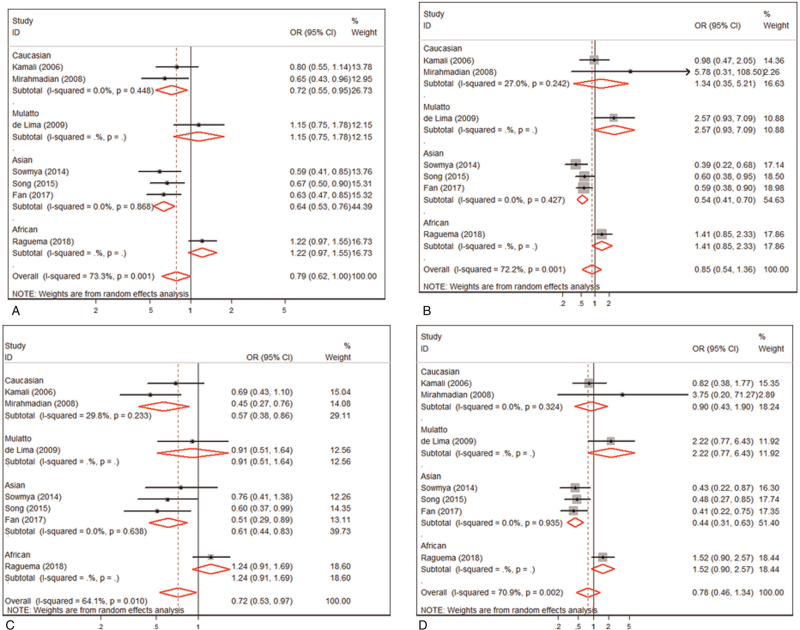
Besides, the meta-analysis indicated that no association was found between IL10 -592 A/C polymorphism and the risk of PE in overall under an allele contrast (OR = 0.786, 95% CI: 0.617–1.001, P = .051) (Fig. 3A), the recessive model contrast (OR = 0.855, 95% CI: 0.537–1.361, P = .508) (Fig. 3B) or a homozygote contrast (OR = 0.784, 95% CI: 0.459–1.341, P = .374) (Fig. 3D). Analysis by a dominant model contrast showed a significant association between the IL10 -592 A/C polymorphism and PE (OR = 0.716, 95% CI: 0.526–0.975, P = .034) (Table 2 and Fig. 3C). Stratification by ethnic divisions displayed IL-10 -592A/C polymorphism was significantly related to a risk of PE in Asians under an allele contrast (OR = 0.635, 95% CI: 0.530–0.762, P < .001) (Table 2), homozygote contrast (OR = 0.441, 95% CI: 0.308–0.633, P < .001) (Table 2), recessive (OR = 0.536, 95% CI: 0.408–0.704, P < .001) (Table 2), and dominant contrast models (OR = 0.609, 95% CI: 0.444–0.836, P = .002) (Table 2). Moreover, ethnic stratification analysis revealed a significant association between the IL-10 -592A/C polymorphism and PE in Caucasians under an allele contrast (OR = 0.725, 95% CI: 0.554–0.948, P = .019) and dominant contrast model (OR = 0.700, 95% CI: 0.404–0.836, P = .001), rather than recessive contrast model (OR = 1.170, 95% CI: 0.585–2.338, P = .658) or homozygous contrast (OR = 0.952, 95% CI: 0.463–1.959, P = .894) (Table 2).
Figure 3.
Meta-analysis of the association between the IL10 -592 A/C polymorphism and PE. (A). allele control model; (B). recessive genotype model; (C). dominant genotype model; (D). homozygous genotype model.
3.3. Publication bias
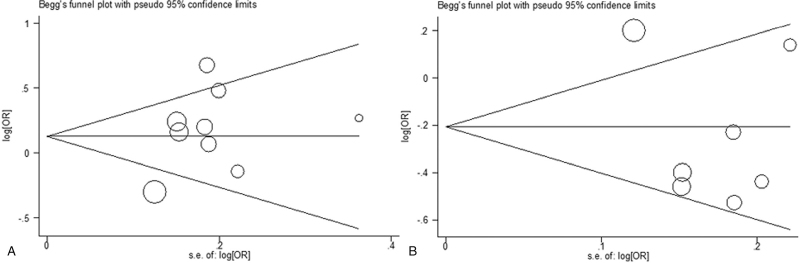
Begg funnel plot was performed to assess potential publication bias of the included studies, which revealed no significant publication bias in 2 SNPs in the allele contrast (IL-10 -819C/T, P = .271; IL-10 -592A/C, P = .368) (Fig. 4A and 4B).
Figure 4.
Begger funnel plot for publication bias among included studies. (A). IL10 -819 C/T polymorphism; (B). IL10 -592 A/C polymorphism.
4. Discussion
PE is a pregnancy-specific complication with undefined etiology and pathogenesis. Increasing evidence indicates that the occurrence and development of PE were caused by multivariate interaction including heredity, among which genetic polymorphisms have caught increasingly attention.[30,31] Meanwhile, inflammation is also an accomplice in the development of PE, in which inflammatory factors make a crucial contribution.[19,32,33] Considering the importance of inflammation and genetic polymorphisms in the etiology and progression of PE, a growing number of studies have been performed to explore the associations between inflammatory gene polymorphisms and susceptibility to PE.[21–23,34–42] More and more meta-analysis have been conducted on the relationship between inflammatory factors IL-6,[43] TNF – α[44] and anti-inflammatory factors IL-10[45] gene polymorphism and PE, which further indicates that gene polymorphism is closely related to the susceptibility of PE. In the present study, we synthesized data from published studies to explore the relationship between the risk of PE and other commonly studied polymorphisms of the IL-10 -819C/T and -592A/C.
IL-10 gene, located on chromosome 13q13, encodes an anti-inflammatory cytokine IL-10. During PE, decreased IL-10 was present in the plasma and placenta.[46,47] It was found that IL-10 treatment in PE pregnant mice significantly increasing Treg cells and decreased hypertension.[48] Hence, the decrease of IL-10 contributes to the pathogenesis of PE. So more and more studies are now investigating the relationship between IL-10 gene polymorphisms and the risk of PE. It was reported that several polymorphisms in the IL-10 gene were associated with IL-10 production, including IL-10 -819C/T,[49] and -592A/C[50,51] polymorphisms. Increasing studies are involved in investigating the relationship between IL-10 -819C/T and -592A/C polymorphisms and PE susceptibility. Fan et al have revealed that IL-10 -592A/C polymorphism was associated with PE susceptibility.[28] Moreover, it was reported that IL-10 -819C/T are associated with PE in different populations.[25,29] On the contrary, some studies have demonstrated that there was no correlation between IL-10 -819C/T and -592A/C polymorphisms and PE risk.[22] Due to the limitation of sample size and the genetic characteristics of different ethnicity, the conclusion is contradictory. In order to attenuate the analytical bias of the results depended on sample size and ethnicity, we conducted a meta-analysis.
In our meta-analysis, the IL-10 -819C/T and -592A/C polymorphisms had no correlation with the risk of PE overall under an allele contrast, but had a significantly strong relation with the risk of PE in Asian under an allele contrast, dominant contrast model, recessive contrast model or homozygous contrast. Besides, IL-10 -819 C allele and IL-10 -592 C allele were related to PE susceptibility in Caucasian. The possible reason for this discrepancy was difference in study population. Ethnicity with different genetic backgrounds has a significant heterogeneity. Therefore, our results are not consistent with the meta-analysis results of Yang et al,[52] which showed that IL-10 -819C/T and -592A/C polymorphisms were associated with PE risk under the allelic model. Due to the small number of literatures and people included for data analysis, the ethnic group analysis was not carried out in their meta-analysis. In our meta-analysis, a total of 1281cases and 1771 controls from 8 studies were included in IL-10 -819 C/T polymorphism, 1133 cases, and 1238 controls from 7 studies were included in IL10 -592 A/C polymorphism. Nevertheless, only 5 case-control studies with 631 cases and 1059 controls for IL-10 -819 C/T polymorphism and a total of 376 cases and 445 controls from 3 case-control studies were included in the 2014 meta-analysis. The inconsistent results may be that more studies containing a larger sample size and more populations, have been included in our meta-analysis, which attenuating statistical analysis results bias.
We must admit that there are some limitations in our meta-analysis. Although we updated the original meta-analysis and increased the number of included case-control studies, the number of subjects is still relatively small, which indicates that the final results may not be enough to study the real relationship statistically. It is necessary to follow up the research progress in order to update the results of system review and meta-analysis in time. Secondly, the source of control was inconsistent in each case-control study, which may lead to bias in the results. In the future investigation, the source of the control group should be unified as far as possible.
Finally, our meta-analysis results showed that there was no significant association between IL-10 -819C/T, -592A/C polymorphisms and PE susceptibility overall, but the different genotypes and alleles of IL-10 -819C/T, -592A/C polymorphisms had different effects on PE susceptibility in different ethnic groups. Therefore, according to the different genotypes and alleles, different regular follow-up are adopted for pregnant women of different ethnic groups. For example, an Asian pregnant woman with a CC genotype in the IL-10 -819C/T polymorphism should pay more attention to the monitoring of blood pressure during pregnancy, whether she has symptoms related to gestational hypertension.
5. Conclusion
Our meta-analysis suggested that IL-10 -819C/T and -592A/C polymorphisms had no correlation with the risk of PE overall, but had a significant association with PE susceptibility in Asian and Caucasian.
Acknowledgments
The authors would like to thank the Department of Science and Technology of Sichuan Province project (no. 2020YFS0100), and the Office of Science and Technology of Chengdu (no. 2019-YF05-01178-SN).
Author contributions
Conceptualization: Li Chang, Yongmei Jiang.
Data curation: Li Chang, Yongmei Jiang.
Formal analysis: Guanglu Che.
Software: Yongmei Jiang.
Writing – original draft: Guanglu Che.
Writing – review & editing: Guanglu Che, Fang Liu.
Footnotes
Abbreviations: IL = interleukin, OR = odds ratios, PE = preeclampsia, SNP = single nucleotide polymorphism.
How to cite this article: Che G, Liu F, Chang L, Jiang Y. Association of IL-10 -819C/T, -592A/C polymorphisms with the risk of preeclampsia: an updated meta-analysis. Medicine. 2021;100:41(e27437).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
ARMS = amplification refractory mutation system, ASO = antisense oligonucleotide, DBP = diastolic blood pressure, IL = interleukin, PCR = polymerase chain reaction, RFLP = restriction fragment length polymorphism, SBP = systolic blood pressure, SSP = sequence specific primer.
CI = confidence interval, OR = odds ratios.
References
- [1].Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 2010;376:631–44. [DOI] [PubMed] [Google Scholar]
- [2].von Dadelszen P, Magee LA. Pre-eclampsia: an update. Curr Hypertens Rep 2014;16:454. [DOI] [PubMed] [Google Scholar]
- [3].Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens 2015;24:131–8. [DOI] [PubMed] [Google Scholar]
- [4].Mutze S, Rudnik-Schoneborn S, Zerres K, Rath W. Genes and the preeclampsia syndrome. J Perinat Med 2008;36:38–58. [DOI] [PubMed] [Google Scholar]
- [5].Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol 2003;102:181–92. [DOI] [PubMed] [Google Scholar]
- [6].Chang CL, Chang CY, Lee DX, Cheng PJ. Characterization of human pregnancy specific glycoprotein (PSG) gene copy number variations in pre-eclampsia patients. Adv Exp Med Biol 2016;924:63–5. [DOI] [PubMed] [Google Scholar]
- [7].Qiu C, Hevner K, Enquobahrie DA, Williams MA. A case-control study of maternal blood mitochondrial DNA copy number and preeclampsia risk. Int J Mol Epidemiol Genet 2012;3:237–44. [PMC free article] [PubMed] [Google Scholar]
- [8].Zhao L, Triche EW, Walsh KM, et al. Genome-wide association study identifies a maternal copy-number deletion in PSG11 enriched among preeclampsia patients. BMC Pregnancy Childbirth 2012;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ben Ali Gannoun M, Al-Madhi SA, Zitouni H, et al. Vascular endothelial growth factor single nucleotide polymorphisms and haplotypes in pre-eclampsia: a case-control study. Cytokine 2017;97:175–80. [DOI] [PubMed] [Google Scholar]
- [10].Amosco MD, Villar VA, Naniong JM, David-Bustamante LM, Jose PA, Palmes-Saloma CP. VEGF-A and VEGFR1 SNPs associate with preeclampsia in a Philippine population. Clin Exp Hypertens 2016;38:578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu W, Yang H, Feng Y, et al. Polymorphisms in inflammatory mediator genes and risk of preeclampsia in Taiyuan, China. Reproductive Sci 2017;24:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Amaral LM, Wallace K, Owens M, LaMarca B. Pathophysiology and current clinical management of preeclampsia. Curr Hypertens Rep 2017;19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kalagiri RR, Carder T, Choudhury S, et al. Inflammation in complicated pregnancy and its outcome. Am J Perinatol 2016;33:1337–56. [DOI] [PubMed] [Google Scholar]
- [14].Harmon AC, Cornelius DC, Amaral LM, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci 2016;130:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Crocker IP, Wareing M, Ferris GR, et al. The effect of vascular origin, oxygen, and tumour necrosis factor alpha on trophoblast invasion of maternal arteries in vitro. J Pathol 2005;206:476–85. [DOI] [PubMed] [Google Scholar]
- [16].Maruo N, Morita I, Ishizaki Y, Murota S. Inhibitory effects of interleukin 6 on prostaglandin I2 production in cultured bovine vascular endothelial cells. Arch Biochem Biophy 1992;292:600–4. [DOI] [PubMed] [Google Scholar]
- [17].Desai TR, Leeper NJ, Hynes KL, Gewertz BL. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res 2002;104:118–23. [DOI] [PubMed] [Google Scholar]
- [18].Obut M, Oglak SC. Expression of CD44 and IL-10 in normotensive and preeclamptic placental tissue. Ginekol Pol 2020;91:334–41. [DOI] [PubMed] [Google Scholar]
- [19].Cubro H, Kashyap S, Nath MC, Ackerman AW, Garovic VD. The role of interleukin-10 in the pathophysiology of preeclampsia. Curr Hypertens Rep 2018;20:36. [DOI] [PubMed] [Google Scholar]
- [20].Roth I, Fisher SJ. IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev Biol 1999;205:194–204. [DOI] [PubMed] [Google Scholar]
- [21].Haggerty CL, Ferrell RE, Hubel CA, Markovic N, Harger G, Ness RB. Association between allelic variants in cytokine genes and preeclampsia. Am J Obstet Gynecol 2005;193:209–15. [DOI] [PubMed] [Google Scholar]
- [22].Kamali-Sarvestani E, Kiany S, Gharesi-Fard B, Robati M. Association study of IL-10 and IFN-gamma gene polymorphisms in Iranian women with preeclampsia. J Reprod Immunol 2006;72:118–26. [DOI] [PubMed] [Google Scholar]
- [23].Mirahmadian M, Kalantar F, Heidari G, Safdarian L, Mansouri R, Amirzargar AA. Association of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in Iranian patients with pre-eclampsia. Am J Reproductive Immunol 2008;60:179–85. [DOI] [PubMed] [Google Scholar]
- [24].de Lima TH, Sass N, Mattar R, et al. Cytokine gene polymorphisms in preeclampsia and eclampsia. Hypertens Res 2009;32:565–9. [DOI] [PubMed] [Google Scholar]
- [25].Sowmya S, Sri Manjari K, Ramaiah A, et al. Interleukin 10 gene promoter polymorphisms in women with early-onset pre-eclampsia. Clin Exp Immunol 2014;178:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Song L, Zhong M. Association between Interleukin-10 gene polymorphisms and risk of early-onset preeclampsia. Int J Clin Exp Pathol 2015;8:11659–64. [PMC free article] [PubMed] [Google Scholar]
- [27].Liu QY, Gao FY, Liu XR, et al. Investigations into the association between polymorphisms in the interleukin-10 gene and risk of early-onset preeclampsia. Genetics Mol Res 2015;14:19323–8. [DOI] [PubMed] [Google Scholar]
- [28].Fan DM, Wang Y, Liu XL, Zhang A, Xu Q. Polymorphisms in interleukin-6 and interleukin-10 may be associated with risk of preeclampsia. Genetics Mol Res 2017;16: [DOI] [PubMed] [Google Scholar]
- [29].Raguema N, Gannoun MBA, Zitouni H, et al. Interleukin-10 rs1800871 (-819C/T) and ATA haplotype are associated with preeclampsia in a Tunisian population. Pregnancy Hypertens 2018;11:105–10. [DOI] [PubMed] [Google Scholar]
- [30].Thakoordeen S, Moodley J, Naicker T. Candidate gene, genome-wide association and bioinformatic studies in pre-eclampsia: a review. Curr Hypertens Rep 2018;20:91. [DOI] [PubMed] [Google Scholar]
- [31].Valenzuela FJ, Perez-Sepulveda A, Torres MJ, Correa P, Repetto GM, Illanes SE. Pathogenesis of preeclampsia: the genetic component. J Pregnancy 2012;2012:632732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bellos I, Karageorgiou V, Kapnias D, Karamanli KE, Siristatidis C. The role of interleukins in preeclampsia: a comprehensive review. Am J Reproductive Immunol 2018;80:e13055. [DOI] [PubMed] [Google Scholar]
- [33].Armaly Z, Jadaon JE, Jabbour A, Abassi ZA. Preeclampsia: novel mechanisms and potential therapeutic approaches. Front Physiol 2018;9:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension (Dallas, Tex : 1979) 2004;44:708–14. [DOI] [PubMed] [Google Scholar]
- [35].Saarela T, Hiltunen M, Helisalmi S, Heinonen S, Laakso M. Tumour necrosis factor-alpha gene haplotype is associated with pre-eclampsia. Mol Hum Reprod 2005;11:437–40. [DOI] [PubMed] [Google Scholar]
- [36].Daher S, Sass N, Oliveira LG, Mattar R. Cytokine genotyping in preeclampsia. Am J Reproductive Immunol 2006;55:130–5. [DOI] [PubMed] [Google Scholar]
- [37].Saarela T, Hiltunen M, Helisalmi S, Heinonen S, Laakso M. Polymorphisms of interleukin-6, hepatic lipase and calpain-10 genes, and preeclampsia. Eur J Obstet Gynecol Reprod Biol 2006;128:175–9. [DOI] [PubMed] [Google Scholar]
- [38].Pazarbasi A, Kasap M, Guzel AI, et al. Polymorphisms in the tumor necrosis factor-alpha gene in Turkish women with pre-eclampsia and eclampsia. Acta Medica Okayama 2007;61:153–60. [DOI] [PubMed] [Google Scholar]
- [39].Canto-Cetina T, Canizales-Quinteros S, de la Chesnaye E, Coral-Vazquez R, Mendez JP, Canto P. Analysis of C-850T and G-308A polymorphisms of the tumor necrosis factor-alpha gene in Maya-Mestizo women with preeclampsia. Hypertens Pregnancy 2007;26:283–91. [DOI] [PubMed] [Google Scholar]
- [40].Stonek F, Hafner E, Metzenbauer M, et al. Absence of an association of tumor necrosis factor (TNF)-alpha G308A1 interleukin-6 (IL-6) G174C and interleukin-10 (IL-10) G1082A polymorphism in women with preeclampsia. J Reproductive Immunol 2008;77:85–90. [DOI] [PubMed] [Google Scholar]
- [41].Molvarec A, Jermendy A, Nagy B, et al. Association between tumor necrosis factor (TNF)-alpha G-308A gene polymorphism and preeclampsia complicated by severe fetal growth restriction. Clin Chimica Acta 2008;392:52–7. [DOI] [PubMed] [Google Scholar]
- [42].de Lima THB, Sass N, Mattar R, et al. Cytokine gene polymorphisms in preeclampsia and eclampsia. Hypertens Res 2009;32:565–9. [DOI] [PubMed] [Google Scholar]
- [43].Veisian M, Javaheri A, Amjadi N, et al. Association of IL-6-176G > C polymorphism with susceptibility to preeclampsia: a systematic review and meta-analysis. Fetal Pediatric Pathol 2019;01–12. [DOI] [PubMed] [Google Scholar]
- [44].Wang L, Qu G, Wu W, Tang X, Sun Y. Association between tumor necrosis factor-alpha-308G/A gene polymorphism and susceptibility to pre-eclampsia: an updated meta-analysis. Cytokine 2018;111:278–86. [DOI] [PubMed] [Google Scholar]
- [45].Veisian M, Tabatabaei RS, Javaheri A, et al. Association of interleukin-10-1082G > A polymorphism with susceptibility to preeclampsia: a systematic review and meta-analysis based on 21 studies. Fetal Pediatric Pathol 2019;01–15. [DOI] [PubMed] [Google Scholar]
- [46].Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J Immunol 1999;163:3491–5. [PubMed] [Google Scholar]
- [47].Arriaga-Pizano L, Jimenez-Zamudio L, Vadillo-Ortega F, Martinez-Flores A, Herrerias-Canedo T, Hernandez-Guerrero C. The predominant Th1 cytokine profile in maternal plasma of preeclamptic women is not reflected in the choriodecidual and fetal compartments. J Soci Gynecologic Investigation 2005;12:335–42. [DOI] [PubMed] [Google Scholar]
- [48].Harmon A, Cornelius D, Amaral L, et al. IL-10 supplementation increases Tregs and decreases hypertension in the RUPP rat model of preeclampsia. Hypertens Pregnancy 2015;34:291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chenjiao Y, Zili F, Haibin C, et al. IL-10 promoter polymorphisms affect IL-10 production and associate with susceptibility to acute myeloid leukemia. Die Pharmazie 2013;68:201–6. [PubMed] [Google Scholar]
- [50].Zhao N, Chen HL, Chen ZZ, Li J, Chen ZB. IL-10-592 polymorphism is associated with IL-10 expression and severity of enterovirus 71 infection in Chinese children. J Clin Virol 2017;95:42–6. [DOI] [PubMed] [Google Scholar]
- [51].Berti FCB, Pereira APL, Trugilo KP, et al. IL-10 gene polymorphism c.-592C > A increases HPV infection susceptibility and influences IL-10 levels in HPV infected women. Infection, Genetics Evolution 2017;53:128–34. [DOI] [PubMed] [Google Scholar]
- [52].Yang W, Zhu Z, Wang J, Ye W, Ding Y. Evaluation of association of maternal IL-10 polymorphisms with risk of preeclampsia by a meta-analysis. J Cellular Mol Med 2014;18:2466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]