Abstract
Background:
The effect of platelet-rich plasma (PRP) on patients with acute Achilles tendon rupture is still controversial. The purpose of this systematic review is to assess the efficacy of PRP injections treating acute Achilles tendon rupture.
Methods:
A comprehensive electronic literature search was performed in the PubMed, Embase, Cochrane Library, and Web of Science databases to identify relevant studies that were published prior to April 29, 2021. Randomized controlled trials evaluating the efficacy of PRP injections in treating patients with acute Achilles tendon rupture were included. Statistical analyses were conducted using RevMan software.
Results:
Five randomized controlled trials were included in this systematic review. The results of the meta-analysis showed that PRP has positive effects on ankle dorsiflexion angle, dorsal extension strength of the ankle, and calf circumference compared with that in controls. However, the current evidence failed to show that PRP effectively improves ankle plantar flexion angle, plantar flexion strength of the ankle, and pain.
Conclusions:
PRP injections for the treatment of acute Achilles tendon rupture significantly improved ankle dorsiflexion angle, dorsal extension strength of the ankle, and calf circumference compared with that in controls. Additional studies with larger sample sizes, more rigorous designs and standardized protocols are needed to draw more reliable and accurate conclusions.
Keywords: acute Achilles tendon rupture, function, pain, platelet-rich plasma, systematic review
1. Introduction
A tendon is a kind of connective tissue, in which almost no cell division occurs; however, they are the basic organ of the musculoskeletal system, and they transfer energy and connect muscles and bones.[1,2] The Achilles tendon is the strongest and largest tendon in the body and connects the plantaris muscle, soleus muscle, gastrocnemius muscle, and calcaneus bone.[3] However, tendons are prone to excessive mechanical stretching in daily life and sports, which can lead to rupture. Studies[4–7] have shown that risk factors for Achilles tendon rupture include high activity levels, weak tendon elasticity, obesity, long-term low blood supply, long-term strain, and weak muscle strength. Acute Achilles tendon rupture is one of the most commonly occurring ligament ruptures.[8,9] With population, the incidence of acute Achilles tendon rupture continues to rise. Acute Achilles tendon rupture seriously affects people's quality of life and increases the economic burden on society. Therefore, it is desirable to accelerate recovery and improve the quality of tissue repair.
A new biological treatment, a platelet-rich plasma (PRP) injection, is increasingly being used in clinical practice. PRP is a platelet concentrate that typically contains various growth factors in its α-granules. Under certain circumstances, these growth factors, including platelet-derived growth factor, epidermal growth factor, transforming growth factor-β1, insulin-like growth factor, vascular endothelial growth factor, basic fibroblast growth factor, and hepatocyte growth factor, can be released at higher than physiological levels.[10] Thus, PRP has garnered the interest of many researchers as a source of induction factors for tissue regeneration.[11–14] A number of recent studies have shown that PRP is effective in treating orthopedic injuries such as carpal tunnel syndrome,[15–17] rotator cuff injuries,[18–20] and chronic Achilles tendinopathy.[21,22] Animal models have shown that PRP promotes the histopathological recovery of the Achilles tendon, increases its structural strength, shortens the inflammatory phase, and accelerates Achilles tendon healing.[23–25]
However, the role of PRP in human Achilles tendon healing is still controversial. For example, in some studies[21–26] ankle joint function significantly improved in the PRP group compared with that in the control group. Conversely, in some trials,[27,28] there were no significant differences between the PRP and control groups in heel function. In summary, the effects of PRP on patients with acute Achilles tendon rupture need to be analyzed and explored further to guide clinical practice. Furthermore, to the best of our knowledge, no systematic reviews have evaluated the effects of PRP in patients with acute Achilles tendon rupture. Two recently published systematic reviews in this field were about the effects of PRP on chronic Achilles tendinopathy, rather than the effects of PRP on acute Achilles tendon rupture.[11,29] Thus, we performed this systematic review and meta-analysis to elucidate the efficacy of PRP in treating acute Achilles tendon rupture.
2. Methods
We conducted this systematic review and meta-analysis of randomized controlled trials (RCTs) in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.[30] In preparation for this meta-analysis, we registered the protocol in PROSPERO (CRD42020205118). Ethics approval is not required as this study is a meta-analysis based on published studies.
2.1. Search strategy
A comprehensive electronic literature search was performed in the PubMed, Embase, Cochrane Library, and Web of Science databases to identify relevant studies that were published prior to April 29, 2021. Two investigators conducted the literature search independently. The reference lists of the included studies and previous related systematic reviews were searched for additional relevant studies. The search strategy were modified specifically for each database. For example, the key search terms used for PubMed were a combination of medical subject heading (MeSH) terms and entry terms: (“Platelet-Rich Plasma”[Mesh] OR Platelet Rich Plasma[Title/Abstract] OR PRP[Title/Abstract] OR Platelet-Rich Plasma[Title/Abstract] OR thrombocyte rich plasma[Title/Abstract] OR platelet-rich plasma cell[Title/Abstract]) AND (“Achilles Tendon”[Mesh] OR Achilles tendon [Title/Abstract] OR heel tendon[Title/Abstract] OR chorda magna[Title/Abstract] OR tendo calcaneus[Title/Abstract]). More detailed information about the search strategies of each database is available in Supplemental Digital Content (Appendix A).
2.2. Inclusion criteria and exclusion criteria
Studies were included in this review if they met the following population, intervention, comparison, outcome, and study design (PICOS) criteria: P: patients diagnosed with acute Achilles tendon rupture were included; I: injections of PRP around the tendon were administered; C: placebo injections (such as normal saline or glucose injections) or no injections (such as dry needle) were administered; O: results on ankle and leg function (such as the isokinetic strength of the ankle, ankle range of motion [ROM], calf circumference), or pain were reported. The isokinetic strength of the ankle will be measured by the Multi-Joint Isokinetic Dynamometer. ROM will be measured by the goniometer. Calf circumference will be measured by a measuring tape, 10 cm below the tibial tuberosity. Pain will be measured by the Visual Analogue Scale (VAS); and S: the study design was that of an RCT. The exclusion criteria will be as follows: less than 10 samples in the intervention group or control group; no non-PRP controls were included; the article was incomplete (e.g., conference abstracts); or the article reported duplicate data (e.g., early and final papers of a clinical trial).
2.3. Literature quality evaluation
Two independent investigators evaluated the quality of the included studies by using the approach recommended by the Cochrane handbook for systematic reviews of interventions.[31] Seven recommended types of bias, namely, random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other bias, were assessed. Each item was judged as having “high risk”, “unclear risk”, or “low risk” on the basis of the data presented in the article. If the article provided sufficient and accurate information, it was considered to have “low risk”. If the article provided insufficient or missing information, it was considered to have “unclear risk”. If the article reported inaccurate data, it was considered to have “high risk”. If disagreements occurred between the 2 investigators, a third investigator joined the discussion until a consensus was reached.
2.4. Study selection and information extraction
All of the searched records were imported into EndNote X9 (Thomson Reuters, Albuquerque, United States) to eliminate duplicate studies. The 2 investigators worked independently to identify studies that met the inclusion criteria. The titles and abstracts of identified articles were screened first by 2 investigators. To further evaluate the eligibility of potential studies, we obtained full-text articles and discussed any disagreements with the third investigator. If necessary, corresponding authors of the reviewed articles were contacted to obtain missing data. Data were extracted from the included studies by 2 independent investigators using the standardized data extraction tool. From each included study, we extracted information including the author, publication year, country, sample size, participants’ mean age, participants’ sex, intervention methods, follow-up times, and outcome measurement tools.
2.5. Statistical analysis
The standardized mean difference with the 95% confidence interval (CI) was used when studies used different outcome scales, and the mean difference (MD) with the 95% CI was used when studies used the same outcome scale. The level of heterogeneity was evaluated by the I2 method, and a value of I2 > 50% was considered to indicate significant heterogeneity.[32] A fixed-effects model was used to calculate the pooled effect size if the data were not significantly heterogeneous. Otherwise, a random-effects model was used. RevMan 5.3 (Cochrane Collaboration, Oxford, England) provided by the Cochrane Collaboration was used for all statistical calculations, and a P value <.05 was considered statistically significant.
3. Results
3.1. Study Selection
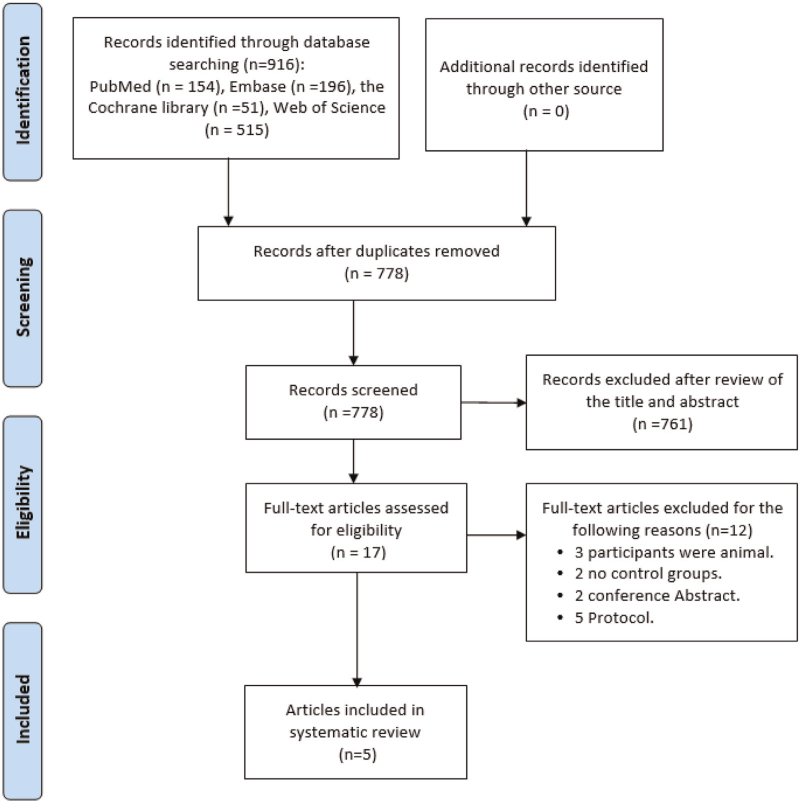
A total of 916 studies from 4 databases were identified in the final search. There were 138 duplicates, and 778 studies for screening. No additional studies were identified by manual reference list screening. The studies were first screened by the titles and abstracts, and studies were excluded according to certain parameters, such as the interventions or outcomes reported. Following this screening, 17 full texts were retrieved and evaluated according to these same parameters. Eventually, 5 studies met all the inclusion and exclusion criteria and were included. See the flowchart for a more detailed overview (Fig. 1).
Figure 1.
Flow diagram for the search and selection processes for the included studies.
3.2. Characteristics of the included publications
The main characteristics of the included studies are shown in Table 1. All 5 articles[26–28,33,34] that were included were RCTs. Two of the included trials were conducted in the UK,[28,33] and 1 study each was conducted in Sweden,[27] Denmark,[34] and China.[26] In total N = 363 patients were included in these 5 trials: 70 women (19.28%) and 293 men (80.72%). The mean age of the included patients ranged from 28.9 ± 5.7 years[26] to 45.9 ± 13.74 years.[28]
Table 1.
Characteristics of the studies included in this meta-analysis.
| Participants | |||||||||||
| Number | Age (yrs) | Male/female | |||||||||
| Study, year, country | Study design | PRP | Control | PRP | Control | PRP | Control | PRP Intervention | Control | Follow up | Outcomes |
| Zou et al, 2016, China | RCT | 16 | 20 | 30.2 ± 5.8 | 28.9 ± 5.7 | 16/0 | 19/1 | Composition: platelet concentration 6 times, leukocytes concentration 4 times.Inject volume: 3–4 mL PRP | No PRP | 3 wk, 6 wk, 12 wk, 24 wk | Isokinetic evaluation; Ankle range of motion;Calf circumference; Leppilahti score. |
| Schepull et al, 2011, Sweden | RCT | 16 | 14 | 39.8 ± 6.2 | 39.4 ± 8.3 | 13/3 | 11/3 | Composition: platelet concentration 36736 ± 1051 × 109 per mL.Inject volume: 10 mL PRP | No PRP | 7 wk, 19 wk, 59 wk | Calf circumference;Achilles tendon rupture score. |
| Keene et al, 2019, UK | RCT | 113 | 116 | 45.9 ± 13.7 | 45.2 ± 12.4 | 88/25 | 84/32 | Composition: platelet concentration 4.1-fold, leucocyte concentration 2.2-fold.Inject volume: 4 mL PRP | Placebo | 4 wk, 7 wk, 13 wk, 24 wk | Ankle range of motion;Pain visual analogue score;Achilles tendon rupture score. |
| De Carli et al, 2015, UK | RCT | 15 | 15 | No available | No available | 13/2 | 11/4 | Composition: no available.Inject volume: 4 mL PRP | No PRP | 1 mo, 3 mo, 6 mo, 24 mo | Isokinetic evaluation;Jumping evaluation. |
| Boesen et al, 2020Denmark | RCT | 19 | 19 | 39.3 ± 7.4 | 41.7 ± 8.9 | 19/0 | 19/0 | Composition: no available.Inject volume: 4 mL PRP | No PRP | 8 wk, 3 mo, 4.5 mo, 6 mo, 9 mo, 12 mo | Achilles tendon rupture scoreHeel-Rise Work and HeightAchilles Tendon LengthCalf CircumferenceAnkle Dorsiflexion ROM |
All PRPs were prepared from patients’ whole blood. In one of the studies,[26] the platelet concentration in the PRP was 6 times the normal physiological level. The concentration of leukocytes in PRP was 4 times the normal physiological level. The mean concentration of PRP platelets in one of the studies was 36,736 ± 1051 × 109 platelets per mL.[27] In 1 study, the prepared PRP had a platelet concentration that was 4.1-fold (95% CI 3.6–4.5) larger than that in whole blood and a leucocyte concentration that was 2.2-fold (95% CI 2.0–2.5) larger than that in whole blood.[28] Platelet concentration was not mentioned in the remaining study.[33,34]
Regarding the PRP injection volume and injection site, in one of the studies, 3 to 4 mL of PRP was injected into the paratenon sheath and the surrounding lacerated tissue.[26] In one of the studies, 4 mL of PRP was injected into the center of the tendon gap.[28] In one of the studies, after the tendon was sutured, 2 mL of PRP was injected in liquid form without activation near the sutured tendon. In addition, gelatinized form of PRP (2 mL), created by the addition of thrombin and 10% Ca-gluconate a few minutes before its use, was injected by suturing it to the peritoneum before skin closure. The patients received a second injection of 4 mL of PRP at 14 days postoperatively with the same preparation procedure.[33] In the remaining studies, 4 mL[34] or 6 mL[27] of PRP was injected into the rupture site.
3.3. Risk of bias
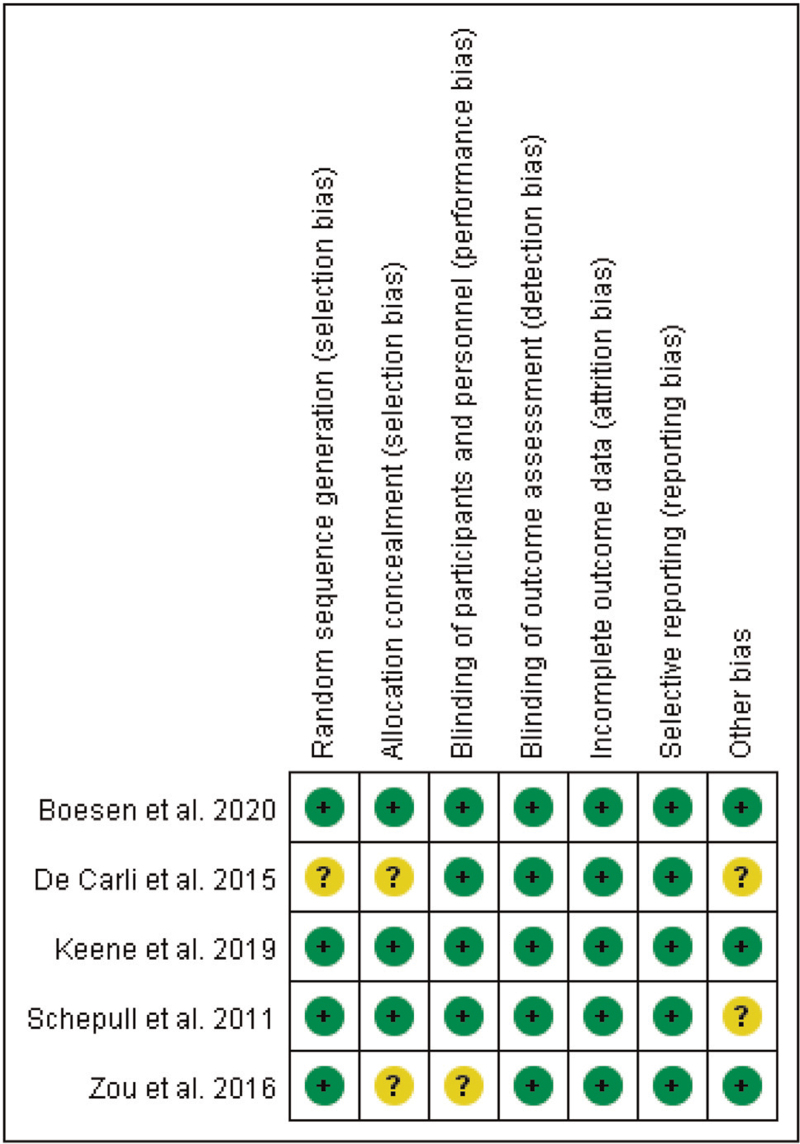
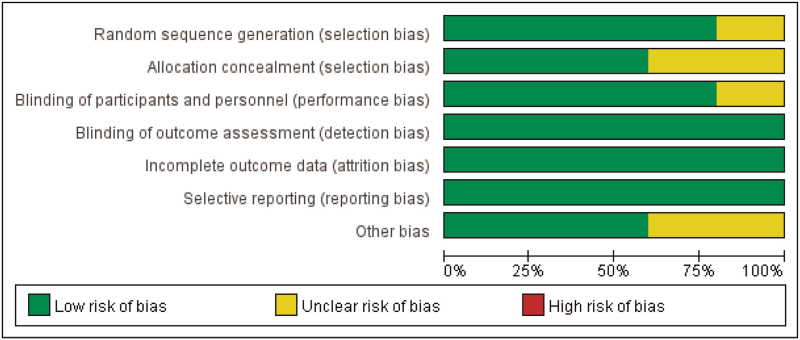
The risk of bias results for the included studies are presented in Figures 2 and 3. Four included studies reported their randomization procedures clearly,[26–28,34] so the risk of random sequence generation bias was judged determined to be low. Three studies reported allocation concealment in detail; allocation numbers were kept in “sealed envelopes”[27,34] or allocation concealment was performed on the basis of a randomization allocation system developed by the Oxford Clinical Trials Research Unit.[28] In another 2 studies,[26,33] allocation concealment was not reported adequately. Four studies[27,28,33,34] were determined to have a low risk of performance bias because the participants and personnel in the trials were blinded. There was no evidence of detection bias, attrition bias, or reporting bias in any of the included studies; therefore, the risk of bias for these items was determined to be low. The risk of other bias was categorized as unclear in 2 studies[27,33] because it was difficult to identify whether the baseline characteristics were balanced.
Figure 2.
Risk of bias summary review of the authors’ judgments about each risk of bias item for each included study.
Figure 3.
Risk of bias graph: review of the authors’ judgments about each risk of bias item, presented as percentage of included studies.
3.4. Effects of PRP among patients with acute Achilles tendon rupture
3.4.1. Ankle ROM
Three studies[26,27,34] provided detailed data on ankle ROM, which were used to evaluate the effect of PRP injections on the dorsal extension angles[26,27,34] and plantar flexion angles[26,27] at 6 months and 12 months. The dorsal extension angles and plantar flexion angles are expressed in degrees, and the differences between the healthy side and the affected side were calculated. Because the same measuring tool was used, we reported the MD as the pooled effect size.
3.4.1.1. Dorsiflexion angle
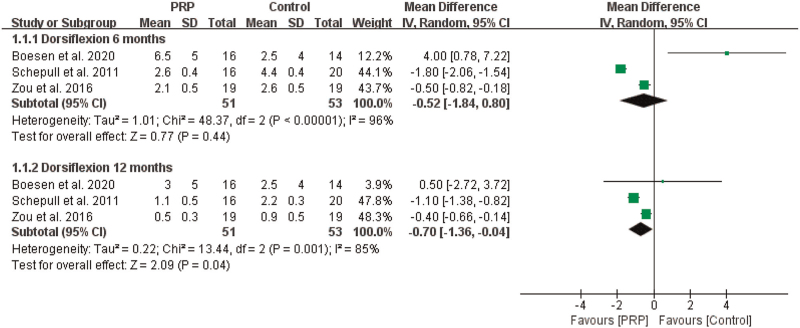
The results of the first meta-analysis showed that PRP significantly improved ankle dorsiflexion angle compared with that in controls at 12 months (n = 104, MD = –0.70, 95% CI [–1.36, –0.04], P = .04, I2 = 85%, the random-effects model; Fig. 4). However, when at 6 months, there was no obvious significant difference (n = 104, MD = –0.52, 95% CI [–1.84, 0.80], P = .44, I2 = 96%, the random-effects model; Fig. 4).
Figure 4.
The effect of PRP on ROM--- dorsiflexion angle. PRP = platelet-rich plasma, ROM = range of motion.
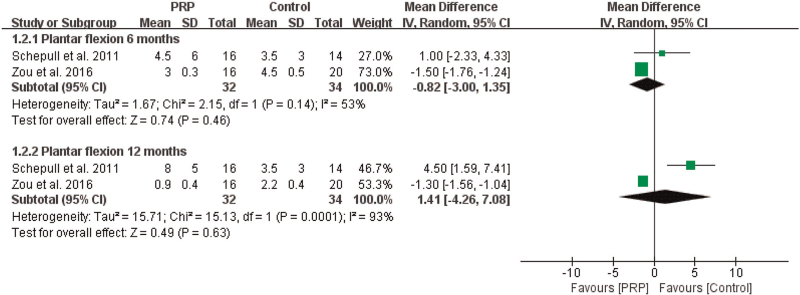
3.4.1.2. Plantar flexion angle
For the ankle plantar flexion angle, the meta-analysis indicated that there were no significant differences between the PRP and control groups at 6 months (n = 66, MD = –0.82, 95% CI [–3.00, 1.35], P = .46, I2 = 53%, the random-effects model; Fig. 5) or at 12 months (n = 66, MD = 1.41, 95% CI [–4.26, 7.08], P = .63, I2 = 93%, the random-effects model; Fig. 5).
Figure 5.
The effect of PRP on ROM--- plantar flexion angle. PRP = platelet-rich plasma, ROM = range of motion.
3.4.2. Isokinetic strength of the ankle
Two studies[26,33] provided detailed data on isokinetic strength of the ankle, in which the effect of PRP injections on dorsal extension and plantar flexion strength in the ankle were evaluated after Achilles tendon repair was performed. Because the same measuring tool was used, we reported the MD as the pooled effect size.
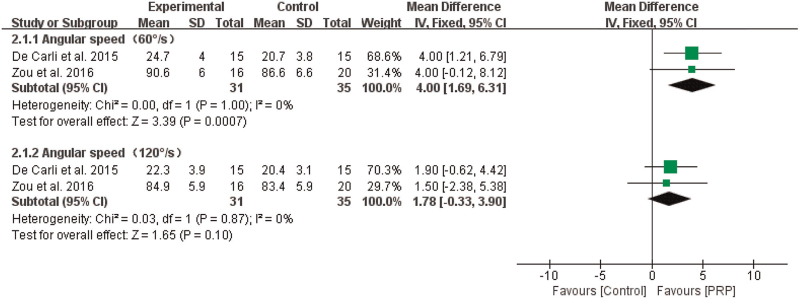
3.4.2.1. Dorsal extension strength
The results of the meta-analysis showed that PRP significantly improved dorsal extension strength compared with that in controls when the angular speed was 60°/s (n = 66, MD = 4.00, 95% CI [1.69, 6.31], P = .0007, I2 = 0%, the fixed-effects model; Fig. 6). However, when the angular speed was 120°/s, there was no obvious significant difference (n = 66, MD = 1.78, 95% CI [–0.33, 3.90], P = .10, I2 = 0%, the fixed-effects model; Fig. 6).
Figure 6.
The effect of PRP on dorsal extension strength of the ankle. PRP = platelet-rich plasma.
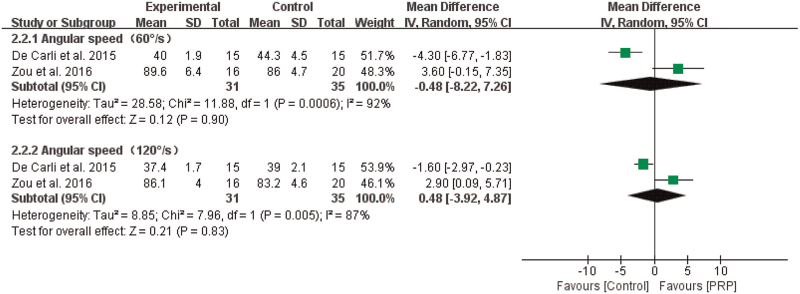
3.4.2.2. Plantar flexion strength
The results from 2 studies showed that the effect of PRP injections on plantar flexion strength was not statistically significant when the angular speed was 60°/s (n = 66, MD = –0.48, 95% CI [–8.22, 7.26], P = .90, I2 = 92%, the random-effects model; Fig. 5) or 120°/s (n = 66, MD = 0.48, 95% CI [–3.92, 4.87], P = .83, I2 = 87%, the random-effects model; Fig. 7).
Figure 7.
The effect of PRP on plantar flexion strength. PRP = platelet-rich plasma.
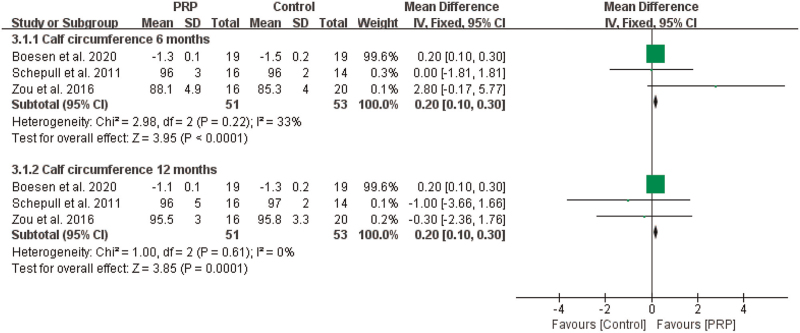
3.4.3. Calf circumference
The results from 3 studies[26,27,34] revealed that there was significant difference in calf circumference at 6 months (n = 104, MD = 0.20, 95% CI [0.10, 0.30], P < .0001, I2 = 33%, the fixed-effects model; Fig. 8) and at 12 months (n = 104, MD = 0.20, 95% CI [0.10, 0.30], P = .0001, I2 = 0%, the fixed-effects model; Fig. 8).
Figure 8.
The effect of PRP on calf circumference. PRP = platelet-rich plasma.
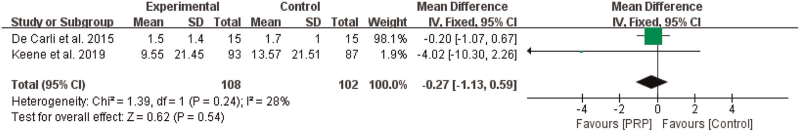
3.4.4. Pain
Two studies[28,33] reported the effects of PRP on pain, as measured by the VAS. Because the same measuring tool was used, we reported the MD as the pooled effect size. The meta-analysis results showed that PRP had no significant effect on the VAS score (n = 210, MD = –0.27, 95% CI [–1.13, 0.59], P = .54, I2 = 28%, the fixed-effects model; Fig. 9).
Figure 9.
The effect of PRP on pain. PRP = platelet-rich plasma.
4. Discussion
4.1. Summary and interpretation of results
This systematic review and meta-analysis identified 5 RCTs investigating the use of PRP injections to treat acute Achilles tendon rupture. The meta-analysis showed that PRP injections for the treatment of acute Achilles tendon rupture significantly improved ankle dorsiflexion angle (at 12 months), dorsal extension strength of the ankle (when the angular speed was 60°/s), and calf circumference compared with that in controls. However, the current evidence failed to show that PRP effectively improves ankle plantar flexion angle, plantar flexion strength of the ankle, and pain.
The meta-analysis showed that PRP injections had positive effects on the ankle dorsiflexion angle, dorsal extension strength of the ankle, and calf circumference compared with that in controls. The results were consistent with that in Sanchez et al's[35] study, which proved that injections of PRP help individuals enhance Achilles tendon healing and recover Achilles tendon function. Zou et al's[26] study showed that PRP has a positive effect on ankle joint function, as measured by the Leppilahti score, in the short to medium term. However, this result is inconsistent with that in the study by De Carli et al. In De Carli et al's[33] study, the Foot and Ankle Outcome Score and Victorian Institute of Sports Assessment Achilles score were not different between the PRP and control groups at 1, 3, 6, or 24 months after surgery for acute Achilles tendon rupture. This inconsistency in results may be caused by differences in the concentration of PRP, the content of the ingredients, the injection time, and the injection site. Rapid healing of tendons requires a rich blood supply, and the generation of blood vessels requires the supply of growth factors.[36] Lyras et al[37] found that early PRP injections for tendon injury can significantly increase angiogenesis and that PRP can reduce the tendon healing time. Some studies have shown that PRP can induce tendon cell proliferation and can also promote angiogenesis factor production.[38,39] Some in vitro experiments have also shown that platelet-rich clot release induces rabbit TSCs to differentiate into active tenocytes.[40,41] In rat experiments, it has been shown that immediate injection of PRP for tendon injury improves tendon healing in rats.[42] In contrast, Delos et al[43] found no significant differences in tendon healing between immediate injections of PRP and delayed injections. Zhou et al found that white blood cell-rich platelets may be harmful to the healing of tendon injuries in rabbits. White blood cell-rich platelets cause catabolism gene expression, increase protein production, and promote increased inflammatory mediators. This inflammatory mediator induces the differentiation of non-tendon cells.[44,45] Some studies have found that leukocyte-reduced PRP is more effective and safer than traditional PRP for the differentiation of normal cells in lesions within the joint.[46–48] Therefore, the most appropriate concentration, injection time, and injection site of PRP in the treatment of acute Achilles tendon rupture need to be explored further.
However, we found no statistically significant effects of PRP on ankle plantar flexion angle, plantar flexion strength of the ankle, or pain. Several systematic reviews have also explored the effect of PRP injection on bone and joint diseases.[49–51] Our results are consistent with the findings reported in a systematic review[52] showing that for the repair of full-sheared rotator cuffs, there was no significant difference between the clinical outcome scores of groups in which PRP was and was not used. Another systematic review[49] of the effects of PRP injections on pain and function in patients with rotator cuff tendinopathy revealed that the efficacy of PRP injections and placebos is indistinguishable in terms of pain relief and functional improvement in the short-term (3–12 weeks). Nevertheless, PRP injections led to significant long-term (>24 weeks) reduction in pain. For functional improvement in the long-term, there was no significant difference between the PRP injection and placebo groups. However, our results are inconsistent with those in a recent systematic review conducted by Catapano et al[50] who indicated that there was a statistically significant improvement in the carpal tunnel functional status and pain between PRP and control groups. Another systematic review[51] investigated the effect of PRP on postoperative failure rates following rotator cuff repair, and the results demonstrated that intra-operative PRP reduces the failure risk and has a significant protective effect on tears. In summary, the effectiveness of PRP injections among patients with bone and joint diseases remains unclear. Nevertheless, due to the insufficient evidence in the studies included in our review on the effectiveness of PRP injections in treating acute Achilles tendon rupture, additional research should be performed to determine the effects of PRP infections in patients with acute Achilles tendon rupture.
4.2. Issues that require attention and directions for future research
Several issues should be considered when the effects of PRP on the repair of acute Achilles tendon rupture is studied. First, it is important to inject PRP with containing the appropriate concentrations and amounts of relevant components, such as white blood cells.[52] Belk et al[53] found that leukocyte-poor PRP may be superior to leukocyte-rich PRP for treating knee arthritis over leukocyte-rich PRP. Therefore, additional studies are needed to directly compare the efficacy of PRP injections with varying leucocyte content in treating acute Achilles tendon rupture. Second, when PRP injections are used to treat acute Achilles tendon rupture, the injection time, injection volume, and injection site should be strictly controlled. In a study by Vilchez-Cavazos et al,[54] a single PRP injection was as effective as multiple PRP injections in relieving pain; however, multiple PRP injections seemed more effective in improving joint function than was a single PRP injection. Because the available evidence is still insufficient in this field, more research needs to be conducted in the future to verify these results. Third, there is heterogeneity among patients, and PRP injections needs to be developed with the patient's own blood to maximize the effect of PRP and guarantee the safety of patients.
4.3. Strengths and limitations
A strength of this meta-analysis is that only RCTs were included, which implies that the included studies had a rigorous study design. This study also has some limitations. First, only 5 studies were included in this systematic review, and the limited number of trials limits the strength of the evidence and generalizability of the findings. Second, the types of outcome indicators varied considerably across studies, resulting in some outcome indicators not being included in the meta-analysis. Third, the cause of acute Achilles tendon rupture and heterogeneity in the types of PRP in the included studies are factors to be considered.
5. Conclusion
Five articles on PRP injections as a treatment for acute Achilles tendon rupture were included in our systematic review. The results of the meta-analysis showed that PRP has positive effects on ankle dorsiflexion angle (at 12 months), dorsal extension strength of the ankle (when the angular speed was 60°/s), and calf circumference compared with that in controls. However, the current evidence failed to show that PRP effectively improves ankle plantar flexion angle, plantar flexion strength of the ankle, and pain. Currently, there is a relative paucity of high-quality studies in this field, and the body of evidence supporting those results is insufficient. Additional studies with larger sample sizes and more rigorous designs and standardized protocols are needed to draw more reliable and accurate conclusions.
Author contributions
Conceptualization: Qingsan Zhu.
Data curation: Chenglong Wang, Qingsan Zhu.
Formal analysis: Chenglong Wang, Hua Fan.
Funding acquisition: Zhuo Zhang.
Methodology: Chenglong Wang, Hua Fan.
Project administration: Qingsan Zhu, Zhuo Zhang.
Resources: Yuhuan Li.
Software: Zhuo Zhang, Zhihe Yun.
Validation: Qingsan Zhu, Zhuo Zhang.
Visualization: Zhuo Zhang, Zhihe Yun.
Writing – original draft: Chenglong Wang.
Writing – review & editing: Chenglong Wang, Zhuo Zhang, Qingsan Zhu.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, MD = mean difference, PRP = platelet-rich plasma, RCT = randomized controlled trial, ROM = range of motion, VAS = Visual Analogue Scale.
How to cite this article: Wang C, Fan H, Li Y, Yun Z, Zhang Z, Zhu Q. Effectiveness of platelet-rich plasma injections for the treatment of acute Achilles tendon rupture: a systematic review and meta-analysis. Medicine. 2021;100:41(e27526).
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
PRP = platelet-rich plasma, RCT = randomized controlled trial, ROM = range of motion.
References
- [1].Gaut L, Duprez D. Tendon development and diseases. Wiley Interdiscip Rev Dev Biol 2016;5:05–23. [DOI] [PubMed] [Google Scholar]
- [2].Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 2007;13:1219–27. [DOI] [PubMed] [Google Scholar]
- [3].Thevendran G, Sarraf KM, Patel NK, Sadri A, Rosenfeld P. The ruptured Achilles tendon: a current overview from biology of rupture to treatment. Musculoskelet Surg 2013;97:09–20. [DOI] [PubMed] [Google Scholar]
- [4].Noback PC, Jang ES, Cuellar DO, et al. Risk factors for achilles tendon rupture: a matched case control study. Injury 2017;48:2342–7. [DOI] [PubMed] [Google Scholar]
- [5].Chiu YH, Chang KV, Chen IJ, Wu WT, Özçakar L. Utility of sonoelastography for the evaluation of rotator cuff tendon and pertinent disorders: a systematic review and meta-analysis. Eur Radiol 2020;30:6663–72. [DOI] [PubMed] [Google Scholar]
- [6].Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec 2010;3:29–32. [DOI] [PubMed] [Google Scholar]
- [7].Chen KC, Lee TM, Wu WT, Wang TG, Han DS, Chang KV. Assessment of tongue strength in sarcopenia and sarcopenic dysphagia: a systematic review and meta-analysis. Front Nutr 2021;8:684840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matsumoto F, Trudel G, Uhthoff HK, Backman DS. Mechanical effects of immobilization on the Achilles’ tendon. Arch Phys Med Rehabil 2003;84:662–7. [DOI] [PubMed] [Google Scholar]
- [9].Zhang H, Tang H, He QY, et al. Surgical versus conservative intervention for acute achilles tendon rupture. Medicine 2015;94:e1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stanco D, Vigano M, Croiset SJ, De Girolamo L. Applications and limits of platelet-rich plasma in sports related injuries. J Biol Regul Homeost Agents 2012;26: (2 Suppl 1): 53s–61s. [PubMed] [Google Scholar]
- [11].Liu C, Yu K, Tian DH, Liu GL. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy: a meta-analysis. Medicine 2019;98:e15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wu W, Chen F, Liu Y, Ma Q, Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg 2007;65:1951–7. [DOI] [PubMed] [Google Scholar]
- [13].Chen L, Dong SW, Liu JP, Tao X, Tang KL, Xu JZ. Synergy of tendon stem cells and platelet-rich plasma in tendon healing. J Orthop Res 2012;30:991–7. [DOI] [PubMed] [Google Scholar]
- [14].Mifune Y, Matsumoto T, Takayama K, et al. The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis Cartilage 2013;21:175–85. [DOI] [PubMed] [Google Scholar]
- [15].Wu YT, Ho TY, Chou YC, et al. Six-month efficacy of platelet-rich plasma for carpal tunnel syndrome: a prospective randomized, single-blind controlled trial. Sci Rep 2017;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Malahias MA, Nikolaou VS, Johnson EO, Kaseta MK, Kazas ST, Babis GC. Platelet-rich plasma ultrasound-guided injection in the treatment of carpal tunnel syndrome: a placebo-controlled clinical study. J Tissue Eng Regen Med 2018;12:e1480–8. [DOI] [PubMed] [Google Scholar]
- [17].Uzun H, Bitik O, Uzun O, Ersoy US, Aktaş E. Platelet-rich plasma versus corticosteroid injections for carpal tunnel syndrome. J Plast Surg Hand Surg 2017;51:301–5. [DOI] [PubMed] [Google Scholar]
- [18].Sari A, Eroglu A. Comparison of ultrasound-guided platelet rich plasma, prolotherapy, and corticosteroid injections in rotator cuff lesions. J Back Musculoskelet Rehabil 2020;33:387–96. [DOI] [PubMed] [Google Scholar]
- [19].Han L, Fang WL, Jin B, Xu SC, Zheng X, Hu YG. Enhancement of tendon-bone healing after rotator cuff injuries using combined therapy with mesenchymal stem cells and platelet rich plasma. Eur Rev Med Pharmacol Sci 2019;23:9075–84. [DOI] [PubMed] [Google Scholar]
- [20].Lin MT, Chiang CF, Wu CH, Huang YT, Tu YK, Wang TG. Comparative effectiveness of injection therapies in rotator cuff tendinopathy: a systematic review, pairwise and network meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2019;100:336–49.e15. [DOI] [PubMed] [Google Scholar]
- [21].Boesen AP, Hansen R, Boesen MI, Malliaras P, Langberg H. Effect of high-volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy: a randomized double-blinded prospective study. Am J Sports Med 2017;45:2034–43. [DOI] [PubMed] [Google Scholar]
- [22].Krogh TP, Ellingsen T, Christensen R, Jensen P, Fredberg U. Ultrasound-guided injection therapy of Achilles tendinopathy with platelet-rich plasma or saline: a randomized, blinded, placebo-controlled trial. Am J Sports Med 2016;44:1990–7. [DOI] [PubMed] [Google Scholar]
- [23].Yuksel S, Gulec MA, Gultekin MZ, et al. Comparison of the early period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on the Achilles tendon ruptures in rats. Connect Tissue Res 2016;57:360–73. [DOI] [PubMed] [Google Scholar]
- [24].Yuksel S, Adanir O, Gultekin MZ, et al. Effect of platelet-rich plasma for treatment of Achilles tendons in free-moving rats after surgical incision and treatment. Acta Orthop Traumatol Turc 2015;49:544–51. [DOI] [PubMed] [Google Scholar]
- [25].Takamura M, Yasuda T, Nakano A, Shima H, Neo M. The effect of platelet-rich plasma on Achilles tendon healing in a rabbit model. Acta Orthop Traumatol Turc 2017;51:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zou J, Mo X, Shi Z, et al. A prospective study of platelet-rich plasma as biological augmentation for acute Achilles tendon rupture repair. Biomed Res Int 2016;2016:9364170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med 2011;39:38–47. [DOI] [PubMed] [Google Scholar]
- [28].Keene DJ, Alsousou J, Harrison P, et al. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ 2019;367:l6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang YJ, Xu SZ, Gu PC, et al. Is platelet-rich plasma injection effective for chronic Achilles tendinopathy? A meta-analysis. Clin Orthop Relat Res 2018;476:1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].The Cochrane Collaboration, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. [Google Scholar]
- [32].Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Carli A, Lanzetti RM, Ciompi A, et al. Can platelet-rich plasma have a role in Achilles tendon surgical repair? Knee Surg Sports Traumatol Arthrosc 2016;24:2231–7. [DOI] [PubMed] [Google Scholar]
- [34].Boesen AP, Boesen MI, Hansen R, et al. Effect of platelet-rich plasma on nonsurgically treated acute Achilles tendon ruptures: a randomized, double-blinded prospective study. Am J Sports Med 2020;48:2268–76. [DOI] [PubMed] [Google Scholar]
- [35].Sanchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med 2007;35:245–51. [DOI] [PubMed] [Google Scholar]
- [36].Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004;25:581–611. [DOI] [PubMed] [Google Scholar]
- [37].Lyras DN, Kazakos K, Verettas D, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg 2009;129:1577–82. [DOI] [PubMed] [Google Scholar]
- [38].Salamanna F, Veronesi F, Maglio M, Bella ED, Sartori M, Fini M. New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: general overview on still open questions and outlook. Biomed Res Int 2015;2015:846045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Anitua E, Andia I, Sanchez M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res 2005;23:281–6. [DOI] [PubMed] [Google Scholar]
- [40].Zhou Y, Wang JH. PRP treatment efficacy for tendinopathy: a review of basic science studies. Biomed Res Int 2016;2016:9103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med 2010;38:2477–86. [DOI] [PubMed] [Google Scholar]
- [42].Circi E, Akman YE, Sukur E, Bozkurt ER, Tüzüner T, Öztürkmen Y. Impact of platelet-rich plasma injection timing on healing of Achilles tendon injury in a rat model. Acta Orthop Traumatol Turc 2016;50:366–72. [DOI] [PubMed] [Google Scholar]
- [43].Delos D, Leineweber MJ, Chaudhury S, Alzoobaee S, Gao Y, Rodeo SA. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am J Sports Med 2014;42:2067–74. [DOI] [PubMed] [Google Scholar]
- [44].Zhang J, Wang JH. BMP-2 mediates PGE(2)-induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J Orthop Res 2012;30:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhou Y, Zhang J, Wu H, Hogan MV, Wang JHC. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther 2015;6:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Filardo G, Kon E, Pereira Ruiz MT. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc 2012;20:2082–91. [DOI] [PubMed] [Google Scholar]
- [47].Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med 2014;42:1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med 2016;44:792–800. [DOI] [PubMed] [Google Scholar]
- [49].Lin MT, Wei KC, Wu CH. Effectiveness of platelet-rich plasma injection in rotator cuff tendinopathy: a systematic review and meta-analysis of randomized controlled trials. Diagnostics (Basel) 2020;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Catapano M, Catapano J, Borschel G, et al. Effectiveness of Platelet-Rich Plasma Injections for Nonsurgical Management of Carpal Tunnel Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Arch Phys Med Rehabil 2020;101:897–906. [DOI] [PubMed] [Google Scholar]
- [51].Cavendish PA, Everhart JS, DiBartola AC, et al. The effect of perioperative platelet-rich plasma injections on postoperative failure rates following rotator cuff repair: a systematic review with meta-analysis. J Shoulder Elbow Surg 2020;29:1059–70. [DOI] [PubMed] [Google Scholar]
- [52].Cai YZ, Zhang C, Lin XJ. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J Shoulder Elbow Surg 2015;24:1852–9. [DOI] [PubMed] [Google Scholar]
- [53].Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med 2020;49:249–60. [DOI] [PubMed] [Google Scholar]
- [54].Vilchez-Cavazos F, Millan-Alanis JM, Blazquez-Saldana J, et al. Comparison of the clinical effectiveness of single versus multiple injections of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Orthop J Sports Med 2019;7:2325967119887116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.