Abstract
Post-transplant lymphoproliferative disorder (PTLD) and other iatrogenic immunodeficiency-associated lymphoproliferative disorders (OIIA-LPD) are iatrogenic lymphoproliferative disorders (LPD) that develop in association with immunosuppressive treatment in the setting of organ transplantation and autoimmune disease, respectively. Each has a spectrum of pathologies ranging from lymphoid hyperplasia to lymphoma. To clarify the characteristics of the diffuse large B-cell lymphoma (DLBCL) subtype in a cohort of 25 patients with PTLD or OIIA-LPD from our institute, we selected 13 with a histological subtype of DLBCL, including 2 cases of PTLD and 11 of OIIA-LPD. The median patient age at diagnosis was 70 years, with a female predominance. Both PTLD cases developed after kidney transplant. Of the patients with OIIA-LPD, 10 had rheumatoid arthritis, 1 had mixed connective tissue disease, and 8 were treated using methotrexate. Both of the PTLD patients and 6 of the OIIA-LPD patients had extranodal manifestations. All patients except for one were classified as having the non-germinal center B-cell (non-GCB) subtype according to the Hans algorithm. Tissue samples from 8 patients were positive for CD30 and 8 were positive for Epstein–Barr virus (EBV)-encoded small RNA. Seven patients had MYC-positive tissue samples, but none had MYC translocation. Our study suggests that extranodal manifestations and the non-GCB subtype are common, that EBV is associated with the DLBCL subtype of PTLD and OIIA-LPD, and that anti-CD30 therapy is applicable. In addition, our patients with the DLBCL subtype of PTLD and OIIA-LPD exhibited MYC overexpression without MYC translocation, suggesting an alternative mechanism of MYC upregulation.
Keywords: Immunocompromised, Organ transplantation, Autoimmune disease, Germinal center, Epstein–Barr virus
INTRODUCTION
Immunodeficiency-associated lymphoproliferative disorders (LPD) are characterized by excessive lymphoid proliferation developing in the context of immunosuppression.1,2 According to the 2017 World Health Organization (WHO) classification, there are 4 categories of immunodeficiency-associated LPD: LPD associated with primary immune disorders, lymphomas associated with human immunodeficiency virus infection, post-transplant LPD (PTLD), and other iatrogenic immunodeficiency-associated LPD (OIIA-LPD).1,2 PTLD and OIIA-LPD are iatrogenic, developing in association with immunosuppressive treatment in the setting of organ transplantation and autoimmune disease, respectively. PTLD is one of the important complications after solid organ transplantation (SOT) and hematopoietic stem-cell transplantation (HSCT).1 The frequency varies depending on the organ type (10%–15% in SOT recipients and 0.5%–2.5% in HSCT recipients), with an overall frequency of 1%–3%.3 In contrast, the majority of OIID-LPD develops in patients with rheumatoid arthritis (RA) treated by methotrexate (MTX).2
PTLD encompasses heterogenous lymphoid disorders ranging from polyclonal to monoclonal proliferations.1 Monomorphic PTLD, which accounts for approximately 60%–80% of all PTLD, is characterized by monotonous proliferation of transformed lymphocytes or plasmacytic cells and fulfills the criteria for lymphoma.1 The most common histological type of monomorphic PTLD is diffuse large B-cell lymphoma (DLBCL).1 OIIA-LPD also includes a spectrum of proliferations ranging from indolent to other types of lymphoma.2 DLBCL is the most common subtype of lymphoma among OIIA-LPD.2,3
Epstein–Barr virus (EBV), a widespread human ganmma-1 herpes virus, asymptomatically infects more than 90% of the population worldwide and resides mainly in long-lived memory B-cells.4,5 Although EBV is generally controlled by EBV-specific cytotoxic T-cell activity in immunocompetent individuals, immunosuppression impairs T-cell function and results in uncontrolled proliferation of EBV-transformed B-cells in immunocompromised recipients after transplantation.4,5 Most PTLD and OIIA-LPD are of B-cell lineage, and for decades their development has been mainly attributed to EBV infection.5 However, approximately 30%–45% of PTLD cases are EBV-negative and the frequency of EBV is variable in OIIA-LPD depending on the histological subtype.2,5
The gene encoding MYC, a transcription factor that regulates cell growth, is one of the best characterized oncogenes in B-cell lymphomagenesis.6,7 The translocation of MYC to the IGH region or, less commonly the IGK or IGL locus, is the molecular hallmark of Burkitt lymphoma.8 As a result of this translocation, the MYC coding region is juxtaposed to the transcriptionally active enhancer of the immunoglobulin gene; the expression of MYC is upregulated, leading to uncontrolled cell growth. MYC translocation also occurs in DLBCL, but the frequency of this translocation is lower than the occurrence of MYC overexpression in DLBCL.6 Therefore, an alternative mechanism to gene translocation is suggested to be involved in the overexpression of MYC in DLBCL.
To clarify the characteristics of the DLBCL subtype of PTLD and OIIA-LPD, we retrospectively analyzed the case histories and tissue samples from newly diagnosed patients in our institute using histological and immunohistochemical analyses, in situ hybridization, and fluorescence in situ hybridization.
MATERIALS AND METHODS
Patients
We retrospectively reviewed 25 patients with PTLD or OIIA-LPD who were treated at our institute between 2007 and 2017. The diagnoses were made according to the 2017 WHO classification.1,2 A total of 29 cases of DLBCL, not other specified (DLBCL, NOS) that developed regardless of immunosuppressive treatment for organ transplantation or autoimmune disease diagnosed between 2017 and 2018 were used for comparison.
This study was conducted in accordance with the Helsinki Declaration and the study protocol was approved by the research ethics committee of Showa University (approval number 2479).
Histology and immunohistochemical analysis
Excised tissue specimens were fixed in 10% formalin and embedded in paraffin wax. Serial sections from the paraffin blocks were stained with hematoxylin and eosin. Immunohistochemical analysis was performed according to standard procedures. The antibodies used are as follows: CD3 (1:50, PS1; Leica Biosystems, Newcastle upon Tyne, UK), CD5 (1:50, 4C7; Leica Biosystems), CD10 (1:50, 56C6; Leica Biosystems), CD20 (1:100, L26; Leica Biosystems), CD30 (1:30, Ber-H2; Dako, Glostrup, Denmark), BCL2 oncoprotein (1:50, 124; Dako), BCL6 oncoprotein (1:100, LN22, Leica Biosystems), multiple myeloma oncogene 1 (MUM1; 1:50, MUM1p; Dako), cyclin D1 (1:100, DCS-6; Nichirei Biosciences Inc., Tokyo, Japan), Ki-67 (1:100, MIB-1; Dako), and c-MYC (1:200, Y69; Abcam plc., Cambridge, UK). Positivity was evaluated using cut-off values of 40% for MYC and 20% for others.
In situ hybridization of EBV-EBER
The presence of EBV-encoded small RNA (EBER), a marker of active EBV infection, was assessed using the Bond EBER probe (Leica Biosystems) with the Bond Polymer Refine Detection kit (Leica Biosystems). EBER positivity was evaluated using a cut-off value of 20%.
Fluorescence in situ hybridization (FISH)
MYC translocation was analyzed using a Vysis LSI MYC dual-color break-apart rearrangement probe (Abbot Laboratories, North Chicago, IL, USA) according to the manufacturer’s instructions with minor modifications. In brief, sections of formalin-fixed paraffin-embedded tissues were deparaffinized with xylene, rehydrated in graded ethanol, pretreated at 98°C, digested with pepsin, and hybridized with the FISH probes at 37°C overnight. After staining with DAPI, the slides were stored at 4°C. We examined a total of 200 cells.
Statistical analysis
Statistical analysis was performed by means of Fisher’s exact test using EZR (R version 3.2.2). P < 0.05 was considered significant.
RESULTS
Patient characteristics
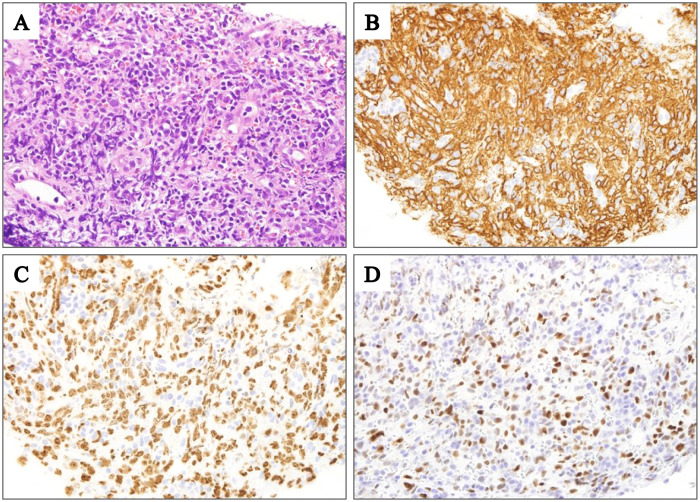
Patient characteristics are summarized in Table 1. Of the 25 patients with PTLD or OIIA-LPD, 13 (52%) were diagnosed with the DLBCL subtype: 2 with DLBCL subtype of PTLD (hereafter referred to as DLBCLPTLD) and 11 with DLBCL subtype of OIIA-LPD (DLBCLOIIA). Representative histological findings of a patient with DLBCLOIIA (Patient 13) are shown in Fig. 1A. The median patient age at diagnosis was 70 years (range, 42–84 years), with a female predominance (77%). Both DLBCLPTLD cases developed after kidney transplant. Most of the 11 DLBCLOIIA cases developed during treatment for RA (10 patients) and 1 developed during treatment for mixed connective tissue disease. Six patients were treated using MTX alone, 2 using MTX and 1 or more additional agents, and 3 using agents other than MTX. Both DLBCLPTLD patients (brain, 1 patient; pleura, 1 patient) and 6 DLBCLOIIA patients (lung, 2 patients; adrenal gland, tonsil, gum, and pharynx, 1 patient each) exhibited extranodal manifestations.
Table 1. Patient characteristics.
| Patient | Diagnosis | Age | Sex | Background | Immunosuppressive treatment | Lesion |
|---|---|---|---|---|---|---|
| 1 | PTLD | 43 | M | KT | CsA, MMF | Brain |
| 2 | PTLD | 42 | F | KT | TAC, PSL | Pleura |
| 3 | OIIA-LPD | 81 | F | RA | MTX | AG |
| 4 | OIIA-LPD | 70 | F | RA | MTX | LN |
| 5 | OIIA-LPD | 60 | F | RA | MTX | Tonsil |
| 6 | OIIA-LPD | 78 | F | RA | MTX | LN |
| 7 | OIIA-LPD | 67 | F | RA | MTX | Gum |
| 8 | OIIA-LPD | 81 | F | RA | MTX | Pharynx |
| 9 | OIIA-LPD | 84 | F | RA | MTX, SASP | Lung |
| 10 | OIIA-LPD | 82 | F | RA | PSL, SASP, BUC, MTX, TAC | Lung |
| 11 | OIIA-LPD | 56 | M | RA | BUC, SASP, PSL | LN |
| 12 | OIIA-LPD | 69 | M | RA | PEN, PSL | LN |
| 13 | OIIA-LPD | 79 | F | MCTD | PSL, TAC | LN |
PTLD, post-transplant lymphoproliferative disorder; OIIA-LPD, other iatrogenic immunodeficiency-associated lymphoproliferative disorder; KT, kidney transplantation; RA, rheumatoid arthritis; MCTD, mixed connective tissue disease CsA, cyclosporin A; MMF, mycophenolate mofetil; TAC, tacrolimus; PSL, prednisolone; MTX, methotrexate; SASP, salazosulfapyridine; BUC, bucillamine; PEN, d-penicillamine; AG, adrenal gland; LN, lymphoid node
Fig. 1.
Histological findings of a patient with DLBCLOIIA (Patient 13). (A) Diffuse infiltration of lymphoma cells (hematoxylin and eosin staining; original magnification, ×1000). (B) lymphoma cells were positive for CD20 (original magnification, ×1000). (C) Ki-67 was expressed in approximately 60% of the lymphoma cells (original magnification, ×1000). (D) MYC was expressed in approximately 60% of the lymphoma cells (original magnification, ×1000).
Immunophenotype and EBV infection
The immunohistochemical analysis results are shown in Table 2. All patients except for one with DLBCLPTLD or DLBCLOIIA (hereafter referred to as DLBCLPTLD and DLBCLOIIA) were classified as having the non-germinal center B-cell (non-GCB) subtype according to the Hans algorithm,9 demonstrating significant differences from DLBCL, NOS (p < 0.005) (Table 3). Tissue samples from all patients except one were negative for CD3 and CD5. Tissue samples from all patients were positive for CD20 (Table 2, Fig. 1B). Tissue samples from 8 patients were positive for CD30 (62%), including 4 with partial expression. The median positivity rate for Ki-67 was 75% (range, 60%–90%) (Table 2, Fig. 1C). Tissue samples from 7 patients were positive for MYC (Table 2, Fig. 1D). No significant difference was observed in the MYC positivity rate between DLBCLPTLD and DLBCLOIIA (54%) and DLBCL, NOS (31%) (p = 0.187) (Table 3).
Table 2. Results of in situ hybridization, immunohistochemistry, and fluorescence in situ hybridization.
| Patient | Immunohistological subtype | ISH | Immunohistochemistry | FISH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EBER | CD3 | CD5 | CD20 | CD10 | CD30 | BCL2 | BCL6 | MUM1 | CCND1 | Ki67(%) | MYC | MYC | ||||
| 1 | ND | + | − | − | + | − | +p | NA | NA | NA | NA | NA | − | − | ||
| 2 | non-GCB | + | − | − | + | − | +p | − | − | + | NA | 90 | + | − | ||
| 3 | non-GCB | − | − | − | + | − | − | + | − | + | − | 70 | + | − | ||
| 4 | non-GCB | − | − | − | + | − | − | + | + | + | − | 60 | − | − | ||
| 5 | non-GCB | + | − | − | + | − | + | + | − | NA | − | 80 | − | − | ||
| 6 | non-GCB | + | − | − | + | − | − | + | − | − | − | 65 | + | − | ||
| 7 | non-GCB | + | − | − | + | − | + | − | − | + | − | 90 | − | NA | ||
| 8 | non-GCB | + | − | NA | + | − | + | NA | − | + | NA | NA | + | − | ||
| 9 | non-GCB | + | − | − | + | − | +p | +p | − | + | NA | 80 | − | − | ||
| 10 | non-GCB | + | − | − | + | − | +p | +p | − | + | − | 80 | − | NA | ||
| 11 | non-GCB | +b | − | − | + | − | + | + | − | + | − | 75 | + | − | ||
| 12 | non-GCB | − | + | + | + | − | − | − | + | + | − | 70 | + | − | ||
| 13 | non-GCB | − | − | − | + | − | − | + | − | + | − | 60 | + | − | ||
+p partial positive and +b positive in background cells but not in tumor cells
ISH, in situ hybridization; FISH, fluorescent in situ hybridization; EBER, Epstein–Barr virus-encoding small RNA; CCND1, cyclin D1; GCB, germinal center B-cell; ND, not determined; NA, not available
Table 3. Comparison between the diffuse large B-cell lymphoma subtype of iatrogenic immunodeficiency-associated lymphoproliferative disorder and diffuse large B-cell lymphoma.
| DLBCLPTLD DLBCLOIIA (n=13) |
DLBCL, NOS (n=29) |
p | |
|---|---|---|---|
| Immunohistological subtype* | |||
| GCB | 0 | 13 | <0.005 |
| non-GCB | 12 | 16 | |
| EBER | |||
| positive | 8 | 0 | <0.001 |
| negative | 5 | 29 | |
| MYC expression | |||
| positive | 7 | 9 | NS |
| negative | 6 | 20 | |
| MYC translocation** | |||
| positive | 0 | 4 | <0.05 |
| negative | 11 | 5 |
*The immunological subtype of Patient 1 was not determined. **Fluorescent in situ hybridization for MYC translocation was not performed for 2 patients with the diffuse large B-cell lymphoma subtype of other iatrogenic immunodeficiency-associated lymphoproliferative disorder or 20 patients with diffuse large B-cell lymphoma. DLBCLPTLD, diffuse large B-cell lymphoma subtype of post-transplant lymphoproliferative disorder; DLBCLOIIA, diffuse large B-cell lymphoma subtype of other iatrogenic immunodeficiency-associated lymphoproliferative disorder; DLBCL, NOS, diffuse large B-cell lymphoma, not other specified; GCB, germinal center B-cell; EBER, Epstein–Barr virus-encoding small RNA; NS, not significant
In situ hybridization revealed EBER expression in 8 patients. The EBER positivity rate was significantly higher in DLBCLPTLD and DLBCLOIIA (62%) than in DLBCL, NOS (0%) (p < 0.001) (Table 3).
MYC translocation
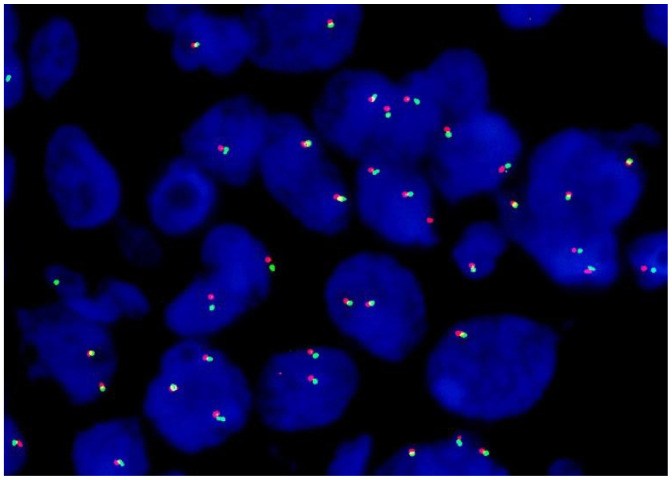
FISH revealed that MYC translocation was not present in all of the patients (Table 2, Fig. 2). The rate of MYC translocation differed between DLBCLPTLD and DLBCLOIIA (0%) and DLBCL, NOS (44%) (p < 0.05) (Table 3).
Fig. 2.
Fluorescence in situ hybridization for MYC (Patient 13). Splitting of red (5’MYC) and green (3’MYC) signals was not detected, indicating the absence of MYC translocation.
DISCUSSION
In this investigation of the characteristics of DLBCLPTLD and DLBCLOIIA, we evaluated the case histories and tissue samples from 2 patients with PTLD that developed after kidney transplant and 11 patients with OIIA-LPD, 8 of whom received methotrexate. Both PTLD patients and 6 OIIA-LPD patients exhibited extranodal manifestations. All patients except for one had the non-GCB subtype according to the Hans’ algorithm. Tissue samples from 8 patients were positive for CD30 and 8 were positive for EBV-encoded small RNA. Seven patients had MYC-positive tissue samples, but none had MYC translocation. Our study suggests that extranodal manifestations and the non-GCB subtype are common, and that EBV is associated with DLBCLPTLD and DLBCLOIIA. In addition, our DLBCLPTLD and DLBCLOIIA patients exhibited MYC overexpression without MYC gene translocation, suggesting an alternative mechanism of MYC upregulation.
Previous studies reported that the frequency of PTLD correlates with a recipient age of younger than 10 or older than 60 years3 and does not differ between sexes. The risk of PTLD depends on allograft type, with the highest risk in multiorgan and intestinal transplants (<20%), followed by transplants of the lung (3.0%–10.0%), heart (2.0%–8.0%), liver (1.0%–5.5%), pancreas (0.5%–5.0%), and kidney (0.8%–2.5%), depending on the amount of donor lymphoid tissue and the degree of immunosuppressive treatment.10 In the present study, both of the DLBCLPTLD patients were kidney transplant recipients in the fifth decade of age, one male and one female. OIIA-LPD is prevalent in females and RA patients treated using MTX.3 Nine of 11 DLBCLOIIA patients (82%) were female and all but one (91%) were RA patients. Extranodal manifestations are common in DLBCLPTLD and DLBCLOIIA.3 Consistent with this, both of our DLBCLPTLD patients (100%) and 6 of the DLBCLOIIA patients (55%) exhibited extranodal manifestations.
Gene expression profiling (GEP) enables the classification of DLBCL into 3 prognostically important subtypes: GCB-like, activated B-cell–like, and unclassified.11 Hans et al. proposed a widely applicable and practical immunohistochemical algorithm in which DLBCL is divided into GCB and non-GCB subtypes based on antibody reactivity against CD10, MUM1, and BCL6.9 The Hans algorithm was demonstrated to function as a surrogate for GEP classification in providing useful prognostic information.12 In our series, all cases of DLBCLPTLD and DLBCLOIIA except for one were classified as the non-GCB subtype, and a significant difference in the frequency of non-GCB was observed between DLBCLPTLD and DLBCLOIIA and DLBCL, NOS. Although the sample size of our control group was small, a larger series reported a ratio of GCB to the non-GCB subtype of 46%:54% in 730 patients with DLBCL.13 The frequency of non-GCB is high in lymphoma originating from extranodal sites. In the present study, the significant difference in the frequency of non-GCB may have resulted from the high frequency of extranodal involvement in DLBCLPTLD and DLBCLOIIA.
CD30, a member of the tumor necrosis factor receptor superfamily, affects cell proliferation through a number of diverse signaling pathways.14 In hematopoietic malignancies, including classic Hodgkin lymphoma and anaplastic large cell lymphoma, CD30 is expressed on tumor cells and has been used as a target for immunotherapy.14 CD30 is commonly expressed in DLBCLPTLD and MTX-associated DLBCLOIIA,1,2 but CD30 was positive in the 3 DLBCLOIIA patients, and partially positive in the 2 DLBCLPTLD patients and 3 DLBCLOIIA patients in our cohort. However, a phase 2 study reported no significant correlation between the level of CD30 expression and response to anti-CD30 therapy using brentuximab vedotin in DLBCL.15 Several hypotheses were proposed to account for this discrepancy, including heterogenous CD30 expression within the tumor and some minimal threshold of CD30 being required for response.15 These studies promote the potential of anti-CD30 therapy for treating DLBCLPTLD and DLBCLOIIA.
EBV preferentially infects B-cells and induces their expression of several viral proteins that predispose cells to transformation. Thus, EBV is implicated in the pathogenesis of several lymphoid malignancies, including Burkitt lymphoma, classic Hodgkin lymphoma, and DLBCL.16 EBV infection is present in 55%–70% of PTLD patients3 and approximately 40% of LPD patients with RA.3 The positivity rate of EBER was significantly higher in DLBCLPTLD and DLBCLOIIA than in DLBCL, NOS, suggesting a strong association between EBV and DLBCLPTLD and DLBCLOIIA.
MYC regulates a large number of genes involved in cell proliferation, survival, and differentiation.7 Although MYC expression is strictly controlled in normal tissues, its deregulation is common in numerous cancers.7 Based on previous reports, the frequency of MYC translocation is 19% in monomorphic B-cell PTLD17 and 15% in MTX-associated DLBCLOIIA.18 However, in the present study, MYC translocation was not observed in all patients with DLBCLPTLD and DLBCLOIIA, whereas MYC expression was detected in 54%. The frequency of MYC translocation in our control group was higher than that previously reported in DLBCL (5%–15%).19 A larger series detected MYC translocation and MYC expression in DLBCL in 11.7% and 32.7%, respectively.20 Excess MYC expression can be induced by retroviral promoter insertion, activation of super-enhancers within MYC, and mutation of upstream signaling pathways that affect the stability of MYC expression, in addition to chromosomal translocation or amplification.21 Although the patient cohort in the present study was small, it suggests that a mechanism other than MYC gene translocation is involved in the overexpression of MYC in DLBCL subtype of iatrogenic LPD.
ACKNOWLEDGMENTS
We express our gratitude to Yosuke Sasaki for technical assistance with immunohistochemical staining and FISH analyses.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Swerdlow SH, Webber SA, Chadobum A, Ferry JA. Post-transplant lymphoproliferative disorders. In : Swerdlow SH, Campo E, Harris NL, et al. (eds) : WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th ed, Lyon, International Agency for Research on Cancer. 2017; pp. 453-462. [Google Scholar]
- 2.Gaulard P, Swerdlow SH, Harris NL, Sundström C, Jaffe ES. Other iatrogenic immunodeficiency associated lymphoproliferative disorders. In : Swerdlow SH, Campo E, Harris NL, et al. (eds) : WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th ed, Lyon, International Agency for Research on Cancer. 2017; pp. 462-464. [Google Scholar]
- 3.Marques-Piubelli ML, Salas YI, Pachas C, et al. Epstein–Barr virus-associated B-cell lymphoproliferative disorders and lymphomas: a review. Pathology. 2020; 52: 40-52. [DOI] [PubMed] [Google Scholar]
- 4.Ru Y, Chen J, Wu D. Epstein-Barr virus post-transplant lymphoproliferative disease (PTLD) after hematopoietic stem cell transplantation. Eur J Haematol. 2018; 101: 283-290. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mansour Z, Nelson BP, Evens AM. Post-transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013; 8: 173-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spevak CC, Park CY. Novel mechanism regulates c-Myc expression in diffuse large B-cell lymphoma. J Natl Cancer Inst. 2020; 112: 7-9. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Liu H, Qing G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct Target Ther. 2018; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leoncini L, Campo E, Stein H, et al. Burkit lymphoma. In : Swerdlow SH, Campo E, Harris NL, et al. (eds) : WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th ed, Lyon, International Agency for Research on Cancer. 2017; pp. 330-334. [Google Scholar]
- 9.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103: 275-282. [DOI] [PubMed] [Google Scholar]
- 10.Abbas F, El Kossi M, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020; 10: 29-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000; 403: 503-511. [DOI] [PubMed] [Google Scholar]
- 12.Choi WWL, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009; 15: 5494-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seki R, Ohshima K, Fujisaki T, et al. Prognostic impact of immunohistochemical biomarkers in diffuse large B-cell lymphoma in the rituximab era. Cancer Sci. 2009; 100: 1842-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Weyden CA, Pileri SA, Feldman AL, Whisstock J, Prince HM. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J. 2017; 7: e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015; 125: 1394-1402. [DOI] [PubMed] [Google Scholar]
- 16.Crombie JL, LaCasce AS. Epstein Barr virus associated B-cell lymphomas and iatrogenic lymphoproliferative disorders. Front Oncol. 2019; 9: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djokic M, Le Beau MM, Swinnen LJ, et al. Post-transplant lymphoproliferative disorder subtypes correlate with different recurring chromosomal abnormalities. Genes Chromosomes Cancer. 2006; 45: 313-318. [DOI] [PubMed] [Google Scholar]
- 18.Carreras J, Yukie Kikuti Y, Miyaoka M, et al. Genomic profile and pathologic features of diffuse large B-cell lymphoma subtype of methotrexate-associated lymphoproliferative disorder in rheumatoid arthritis patients. Am J Surg Pathol. 2018; 42: 936-950. [DOI] [PubMed] [Google Scholar]
- 19.Kramer MHH, Hermans J, Wijburg E, et al. Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood. 1998; 92: 3152-3162. [PubMed] [Google Scholar]
- 20.Xu-Monette ZY, Dabaja BS, Wang X, et al. Clinical features, tumor biology, and prognosis associated with MYC rearrangement and Myc overexpression in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol. 2015; 28: 1555-1573. [DOI] [PubMed] [Google Scholar]
- 21.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008; 8: 976-990. [DOI] [PubMed] [Google Scholar]