Abstract
Intravascular large B-cell lymphoma (IVLBCL) is defined by the World Health Organization (WHO) Classification as one type of extranodal large B-cell lymphoma and it is characterized by the selective growth of lymphoma cells within blood vessels with minimal extravascular invasion. According to the criteria, however, several reported cases of IVLBCL with significant extravascular invasion cannot be classified as IVLBCL. The purpose of the present study was to assess the clinicopathological significance of the WHO criteria for IVLBCL. We characterized clinical, histopathological, and immunohistochemical features of 11 patients with extranodal diffuse large B-cell lymphoma (DLBCL) with significant intravascular invasion (DLBCL-IV), and statistically compared their features with those of 11 patients with IVLBCL and 15 patients with extranodal DLBCL with virtually no intravascular invasion (DLBCL-noIV). When compared with the DLBCL-noIV group, the DLBCL-IV group was characterized by significantly higher rates of splenomegaly, hemophagocytosis, advanced stage disease, and CD5 expression; higher average platelet count, serum lactate dehydrogenase level, and serum ferritin level. Progression-free survival was significantly shorter in the DLBCL-IV group than the DLBCL-noIV group. In contrast, there were no significant differences in clinicopathological features between the DLBCL-IV and the IVLBCL groups. Our study suggests that DLBCL-IV should be regarded as IVLBCL-related.
Keywords: Diffuse large B-cell lymphoma, Intravascular lymphoma, PD-L1
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) with intravascular involvement can be divided into either that with selective growth within blood vessel with or without minimal extravascular invasion or that with considerable extravascular proliferation. In the revised 4th edition of the World Health Organization (WHO) classification in 2017 (WHO-2017), the former is characterized as intravascular large B-cell lymphoma (IVLBCL) as a distinct clinicopathological entity,1 whereas the latter, DLBCL with significant intravascular invasion (DLBCL-IV), is classified into more than one DLBCL type, including DLBCL-not otherwise specified (DLBCL-NOS).
Phenotypically, IVLBCL cells have been suggested to lack adhesion molecules, such as CD29 and CD54,2,3 and to express programmed death-ligand 1 (PD-L1).4-9
As a diagnostic standpoint, several IVLBCL cases with extensive extravascular involvement or DLBCL cases with partial, but not minimal, intravascular involvement have been reported both before and after publication of the WHO-2017,7,8,10-15 but none of these lymphomas can be classified as IVLBCL if the definition of the above classification1 is applied. As a clinical standpoint, the prognostic significance of intravascular invasion of lymphoma cells and/or sinusoidal pattern in DLBCL was examined in de novo CD5+ and CD10- DLBCL, and patients with lymphoma demonstrating intravascular invasion and/or a sinusoidal pattern had a significantly shorter survival than those without these features.16 However, the clinical significance of intravascular invasion in DLBCL as a whole has not been fully elucidated. We therefore constructed a systematic study to examine the possible clinicopathological significance of intravascular invasion in DLBCL. In this study, we included autopsy and biopsy cases because of the following features of IVLBCL. 1) Although IVLBCL is known as an aggressive lymphoma with a poor prognosis, it is often a diagnostic challenge due to nonspecific clinical manifestations, including fever, malaise, and anemia. Consequently, it is not uncommon that patients with IVLBCL are diagnosed for the first time at autopsy. 2) Cases of IVLBCL diagnosed by biopsy can exhibit more than minimal extravascular involvement at autopsy as a “later event”.
MATERIALS AND METHODS
Patients
We reviewed surgical pathology files, including autopsy and biopsy specimens, at Nara Medical University and two affiliated hospitals between 2001 and 2020 to extract patients with extranodal DLBCL and IVLBCL. The number of patients with extranodal DLBCL at the three hospitals as background data was 180 in total. A diagnosis of IVLBCL was based on the WHO-2017.1 This review (See the next section for details.) revealed that all extranodal DLBCL patients had either an intravascular lymphomatous area of 50% or more in at least one organ or less than 1% of that area in all organs (none with an area between 1 and 49%). Therefore, we defined DLBCL-IV as a lymphoma having an area of intravascular invasion of 50% or more in one or more organs, whereas a diagnosis of DLBCL with no significant intravascular invasion (DLBCL-noIV) was defined as that having an area of intravascular invasion of less than 1% in all organs. From this view, surgically resected specimens and larger-sized biopsy specimens were targeted in order to more accurately examine the presence of intravascular invasion. Clinical characteristics of the corresponding patients were collected from their electronic medical records.
Hematopathological examinations
Four micron-thick sections cut from paraffin blocks were used for histology (hematoxylin-eosin staining), histochemistry (Elastica van Gieson staining), and immunohistochemistry. Primary antibodies used for the latter included those against CD20 (L26, Leica, Newcastle, UK), CD10 (56C6, Novocastra, Newcastle, UK), Bcl-2 (124, Dako, Glostrup, Denmark), Bcl-6 (LN22, Novocastra), cytoplasmic CD3 (F7.2.38, cCD3; Dako), CD5 (4C7, Novocastra), Cyclin D1 (SP4, Nichirei, Tokyo, Japan), Ki-67 (MIB-1, Dako), IRF4/MUM1 (MUM1p, Dako), CD34 (QBEnd10, Dako), CD54 (G-5, Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD29 (4B7R, Bio-Rad, Hercules, CA, USA), and PD-L1 (SP142, Spring Bioscience, CA, USA). In situ hybridization (ISH) was performed using RNA probes against the Epstein-Barr virus-encoded small non-polyadenylated RNA (EBER) (Leica).
Elastica van Gieson and CD34 stains were used to evaluate the presence of intravascular invasion of lymphoma cells. In the DLBCL-IV and the DLBCL-noIV groups, an area of intravascular invasion was estimated and presented as a percentage of the total tumor area in each organ. By this method, DLBCL-IV and DLBCL–noIV were defined as described in the previous section. Membranous expression of PD-L1 by tumor cells was evaluated using the following scoring system: rates of positive cells being less than 1% (score 0); from 1 to 50% (score 1); and more than 50% (score 2). PD-L1 scores of 1 and 2 were regarded as positive.
Statistical Analyses
Clinicopathological variables of the three groups were compared and differences in the corresponding two groups were statistically analyzed using the Tukey-Kramer test, the Steel-Dwass test, or Fisher’s exact test. Univariate survival analysis was performed using the Kaplan-Meier method and the log-rank test. Overall survival (OS) was calculated from the date of diagnosis to the date of the last follow-up or death due to lymphoma. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of relapse or death. Consequently, patients with IVLBCL diagnosed for the first time at autopsy were excluded from this analysis. P-values were adjusted by the Holm’s procedure. JMP version 14 (SAS Institute, Cary, NC, USA) was used and values less than 0.05 (two-tailed) were considered significant in all of the above tests.
RESULTS
In total, 37 patients who met the above criteria were found, and consisted of 11 with DLBCL-IV, 11 with IVLBCL, and 15 with DLBCL-noIV.
Clinicopathological features of the DLBCL-IV group
The clinical features of the patients with DLBCL-IV are summarized in Table 1. The median age was 74 years and the male-to-female ratio was 5-to-6. All patients had Ann Arbor stage IV disease. Anemia was present in 10 patients and thrombocytopenia was present in nine. Serum lactate dehydrogenase (LD) and serum ferritin levels were high in all of the patients examined, and the soluble interleukin-2 receptor level was high in 10. All of the five patients diagnosed by biopsy or surgery received rituximab with/without combined chemotherapeutic regimens: two patients had R-THP-COP (rituximab, tetrahydropyranyl adriamycin, cyclophosphamide, vincristine, and prednisolone), and three other therapies. Treatment was not performed for all of the patients diagnosed for the first time at autopsy except for one patient who received etoposide (VP-16) to treat severe hemophagocytosis.
Table 1. Clinical features of DLBCL-IV patients.
| Patient No. | Age | Sex | Disturbance of consciousness | Hepato-megaly | Spleno-megaly | Hemopha-gocytosis | Peripheral blood involvement | WBC (×102/μl) |
Hb (g/dl) |
PLT (×104/μl) |
LD (U/l) |
Ferritin (ng/ml) |
sIL-2R (U/ml) |
Treatment | Outcome from onset (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | + | - | - | - | - | 29 | 11.2 | 3.7 | 261 | 815 | 1,420 | R+CVP | Dead, 2 |
| 2 | 76 | F | + | - | - | NE | - | 61 | 11.2 | 27.1 | 590 | NE | 469 | R | Dead, 6 |
| 3 | 81 | F | - | - | - | + | - | 44 | 7.9 | 18.4 | 284 | 854 | 1,830 | R+CVP | Relapse in nasal cavity as DLBCL, 24 Alive, 52 |
| 4 | 78 | F | - | - | + | + | + | 37 | 7.0 | 7.3 | 994 | 830 | 1,540 | R-THP-COP | Relapse, 12 and 24 Dead, 25 |
| 5 | 77 | M | - | - | + | - | - | 128 | 6.3 | 4.2 | 1,385 | 1,360 | 37,583 | R-THP-COP | Alive, 20 |
| 6 | 75 | F | - | + | + | + | - | 83 | 8.4 | 1.8 | 860 | 699 | 3,870 | VP-16 | Dead, 2.5 |
| 7 | 72 | M | - | - | + | + | - | 83 | 10.1 | 5.8 | 496 | 361 | 2,160 | None | Dead, 1.5* |
| 8 | 68 | M | - | - | + | + | - | 56 | 11.0 | 4.6 | 763 | 29,070 | 11,600 | None | Dead, 1.5 |
| 9 | 69 | F | + | + | + | + | - | 49 | 10.1 | 2.0 | 496 | 1,637 | 22,250 | None | Dead, 6 |
| 10 | 77 | F | + | + | + | + | - | 39 | 11.0 | 2.8 | 2,018 | 22,932 | 13,003 | None | Dead, 0.4 |
| 11 | 83 | M | + | - | - | + | - | 45 | 12.9 | 12.4 | 440 | NE | 722 | None | Dead, 1.3 |
Abbreviations: COP, cyclophosphamide, vincristine, prednisolone; CVP, cyclophosphamide, vincristine, prednisone; DLBCL-IV, diffuse large B-cell lymphoma with significant intravascular invasion; Hb, hemoglobin; LD, lactate dehydrogenase; NE, not examined; PLT, platelet; R, rituximab; sIL-2R, soluble interleukin-2 receptor; THP, tetrahydropyranyl adriamycin; VP-16, etoposide; WBC, white blood cell.
Normal range. WBC 33-86×102/μl, Hb 13.7-16.8 g/dl (male) and 11.6-14.8 g/dl (female), PLT 15.8-34.8×104/μl, LD 124-222 U/l, Ferritin (male) 39-265 ng/ml (male) and ≤ 55 ng/ml (female), sIL-2R 145-519 U/ml.
*Patient 7 underwent breast tumor resection 17 months before his death, and a diagnosis of DLBCL with no intravascular invasion was made.
Diagnostic materials were available for six patients at autopsy, three patients at biopsy, one patient at surgery, and the remaining one patient at both biopsy and autopsy. Histologically, intravascular invasion was predominantly noted in the skin in patients diagnosed by biopsy, whereas it was observed in many organs in patients on whom autopsy was performed. Intravascular invasion in the brain was noted in one of the two patients in whom the organ was examined at autopsy. Immunohistochemically, all cases of DLBCL-IV were positive for CD20, and negative for cCD3 and EBER. Intravascular invasion was present in capillary-rich regions whose distribution differs depending on organs (Figure 1). Other immunohistochemical findings are summarized in Table 2. Both intravascular and extravascular lymphoma cells exhibited a similar immunophenotype except CD54 expression in one patient (Patient 3) in whom extravascular, but not intravascular, lymphoma cells were negative for the molecule. The clinicopathological details of Patient 2 were previously reported by us.12
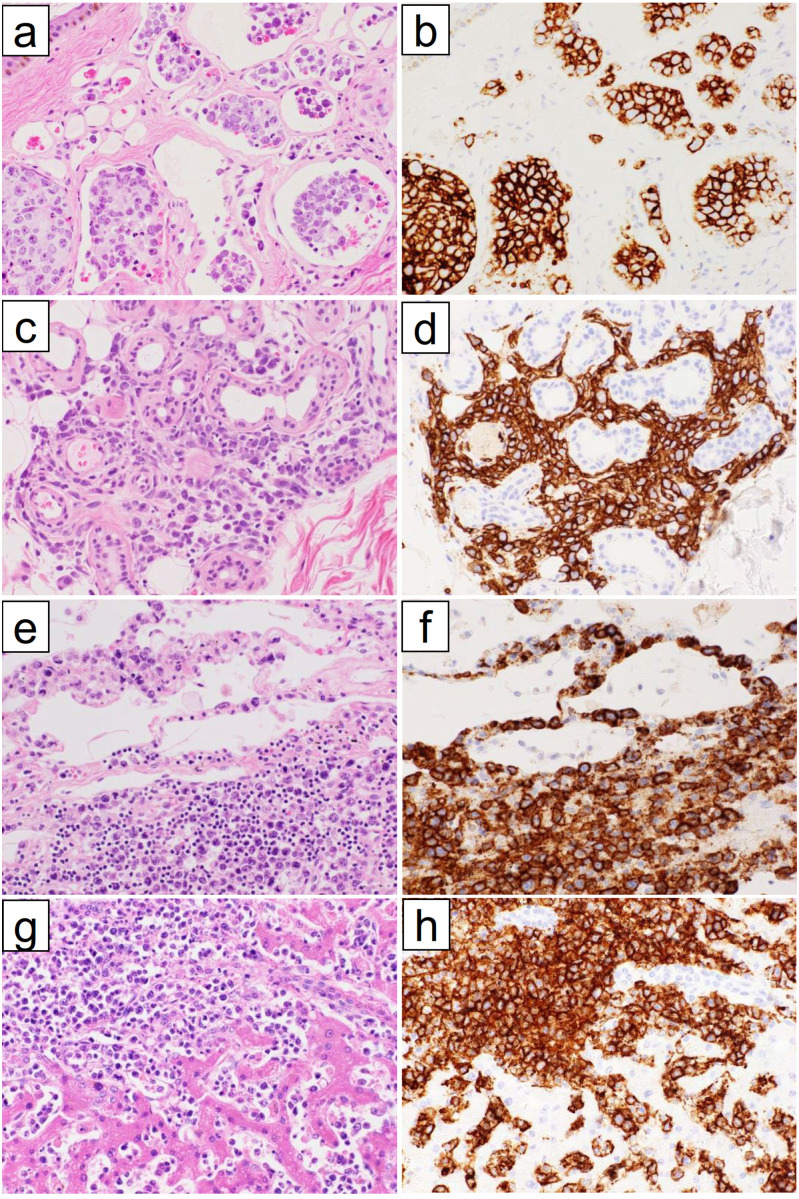
Fig. 1.
Pathological features of diffuse large B-cell lymphoma with significant intravascular invasion (DLBCL-IV). (a-d) Skin and subcutaneous adipose tissue of Patient 5. Most of the tumor cells are within blood vessels in the dermis (a), but they exhibit predominant extravascular growth in the subcutaneous adipose tissue (c). Tumor cells showing both patterns of growth are positive for CD20 (b, d). (e, f) Lung of Patient 9. The tumor cells grow both within the capillaries of alveolar walls and in the interalveolar connective tissues (e). They are positive for CD20 (f). (g, h) Liver of Patient 10. The tumor cells grow both within the sinusoids and in the extravascular portal region (g). They are positive for CD20 (h).
(a, c, e, g) Hematoxylin and eosin staining. (b, d, f, h) Immunoperoxidase staining with hematoxylin counterstaining.
Table 2. Hematopathological features of DLBCL-IV patients.
| Patient No. |
Diagnostic materials |
Diagnostic sites and rate of intravascular invasion (%) | CD5 | CD10 | Bcl-2 | Bcl-6 | IRF4/ MUM-1 | CD29 | CD54 | Ki-67 (%) | PD-L1 score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Biopsy Autopsy |
(Biopsy) Skin (40) (Autopsy) Lungs, pancreas, stomach, esophagus, large intestine, urinary bladder (100), liver, kidneys (80), spleen (50), bone marrow (<1) |
- | - | + | + | + | - | - | 85 | 0 |
| 2 | Surgery | Ovary (80) | - | - | + | - | + | - | + | 45 | 2 |
| 3 | Biopsy | Skin (70) | - | - | + | + | + | - | +/-* | 50 | 1 |
| 4 | Biopsy | Skin (100), bone marrow (<1) | + | - | + | - | + | - | - | NA | 0 |
| 5 | Biopsy | Skin (50), bone marrow (0) | + | - | - | + | + | + | - | 93 | 1 |
| 6 | Autopsy | Lungs, kidneys, adrenal glands, stomach (100), liver (85), spleen, bone marrow (0) | - | - | + | - | + | - | - | 75 | 0 |
| 7 | Autopsy | Lungs, liver, adrenal glands, stomach, pancreas, esophagus, small intestine, large intestine, lymph nodes (100), breast (10), bone marrow (0) |
- | + | + | + | + | - | - | 50 | 1 |
| 8 | Autopsy | Lungs, liver, adrenal glands, stomach, pancreas, esophagus, small intestine, large intestine, heart, gallbladder, urinary bladder, testis (100), kidney (85), lymph nodes (15), spleen, bone marrow (<1) |
+ | - | + | - | - | - | - | NA | 0 |
| 9 | Autopsy | Kidney, adrenal glands, stomach, heart, uterus (100), lungs (60), liver (20), lymph nodes (<1), spleen, bone marrow (0) |
+ | - | + | - | + | + | + | 50 | 2 |
| 10 | Autopsy | Kidney, stomach, pancreas, heart, gallbladder, small intestine, large intestine, urinary bladder, thyroid gland, uterus, fallopian tubes (100), ovaries (80), lungs, adrenal glands (70), liver (50), spleen, lymph nodes (<1), bone marrow (0) |
+ | - | + | + | + | - | + | 40 | 2 |
| 11 | Autopsy | Lungs, kidneys, stomach, urinary bladder, heart, thyroid gland, prostate, brain (100), adrenal glands (80), liver (50), lymph nodes (0) |
- | + | + | - | - | - | + | 70 | 0 |
Abbreviations: DLBCL-IV, diffuse large B-cell lymphoma with significant intravascular invasion; NA, not available; PD-L1, programmed death-ligand 1.
* The extravascular, but not intravascular, lymphoma cells were negative for CD54.
Clinicopathological features of the IVLBCL and DLBCL-noIV groups, and comparison with those of the DLBCL-IV group
In the IVLBCL group, seven patients were diagnosed by skin biopsy (either senile angioma-targeted or random) and one patient each by biopsy of both the skin and bone marrow, lung, or prostate, and one patient by surgery (gallbladder). Two patients demonstrated minimal extravascular invasion. Ten patients received rituximab with/without combined chemotherapeutic regimens: seven patients received R-THP-COP, and one patient each received R-CHOP (cyclophosphamide, hydroxy-doxorubicin, vincristine, and prednisone), THP-COP, and another treatment. The remaining one patient received no chemotherapy. Two patients who were diagnosed by skin and lung biopsy relapsed with DLBCL-noIV in the brain and inguinal lymph node, respectively. Immunohistochemically, all of the tumor cells of the IVLBCL group were positive for CD20, and negative for cCD3 and EBER. In the above two patients, tumor cells of both primary IVLBCL and relapsed DLBCL-noIV had a similar immunophenotype.
In the DLBCL-noIV group, primary sites of lymphoma included the ileum (four patients), testis (two patients), and ovary, liver, ascending colon, parotid gland, bile duct, thyroid gland, nasal cavity, skin, and brain (one patient each). Of these, the former 11 tissues were obtained by surgery, whereas the latter four were by biopsy. Twelve patients received rituximab with/without combined chemotherapeutic regimens: eight patients received R-THP-COP, one received R-CHOP+RT (radiation therapy), and six received other treatments. In our review of pathology files, no case of primary splenic DLBCL of the red pulp was found. Immunohistochemically, all of the tumor cells in this group were positive for CD20, and negative for cCD3 and EBER except for one lymphoma in which a few lymphoma cells, but not the background cells, were positive for EBER.
When compared with the DLBCL-noIV group, the DLBCL-IV group had significantly higher rates of splenomegaly, hemophagocytosis, advanced stage (III or IV) disease, and CD5 expression, and had a higher average platelet count, serum LD level, and serum ferritin level. When compared with the DLBCL-noIV group, the IVLBCL group had significantly higher rates of advanced stage disease and CD5 expression, and had a higher average serum platelet count, LD level, and serum ferritin level. There were no significant differences between the DLBCL-IV and the IVLBCL groups in any clinicopathological variables except for the average serum ferritin level, which was significantly higher in the DLBCL-IV group (Table 3). We also roughly categorized DLBCL-IV, IVLBCL, and DLBCL-noIV as one entity, extranodal large B-cell lymphoma (LBCL), and divided them into PD-L1-positive and negative extranodal LBCL groups. The clinicopathological features of the two groups were then compared, but there were no differences except for Bcl-6 expression between the groups (Table 4).
Table 3. Comparison of clinicopathological features of DLBCL-IV, IVLBCL, and DLBCL-noIV.
| DLBCL-IV (n=11) | IVLBCL (n=11) | DLBCL-noIV (n=15) | P-value | |||
|---|---|---|---|---|---|---|
| DLBCL-IV vs. IVLBCL |
DLBCL-IV vs. DLBCL-noIV | IVLBCL vs. DLBCL-noIV | ||||
| Age [years; median (range)] | 74 (62-83) | 75 (58-91) | 69 (43-86) | 0.998 | 0.495 | 0.458 |
| Sex, male [n (%)] | 5 (46) | 5 (46) | 7 (47) | 1.000 | 1.000 | 1.000 |
| Disturbance of consciousness [n (%)] | 5 (46) | 3 (27) | 1 (6.7) | 0.659 | 0.054 | 0.279 |
| Hepatomegaly [n (%)] | 3 (27) | 2 (18) | 1/12 (8.3) | 1.000 | 0.950 | 1.000 |
| Splenomegaly [n (%)] | 7 (64) | 3 (27) | 0/12 (0.0) | 0.198 | 0.004* | 0.186 |
| Hemophagocytosis [n (%)] | 8/10 (80) | 5/9 (56) | 1/7 (14) | 0.350 | 0.046* | 0.290 |
| Peripheral blood involvement [n (%)] | 1 (9.1) | 2 (18) | 0 (0.0) | 1.000 | 0.846 | 0.508 |
| Ann Arbor stage III/IV [n (%)] | 11 (100) | 10 (91) | 5 (33) | 1.000 | 0.002* | 0.010* |
| WBC [×102/μl; median (range)] | 59 (29-128) | 58 (21-110) | 66 (23-132) | 0.984 | 0.818 | 0.713 |
| Hb [g/dl; median (range)] | 9.7 (6.3-12.9) | 10 (7.5-15.6) | 11.6 (8.1-14.3) | 0.958 | 0.110 | 0.193 |
| PLT [×104/μl; median (range)] | 8.2 (1.8-27.1) | 14.1 (1.2-29.6) | 28.6 (11.1-57.9) | 0.413 | <0.001* | 0.005* |
| LD [U/l; median (range)] | 781 (261-2,018) | 867 (244-1,862) | 355 (142-953) | 0.885 | 0.046* | 0.014* |
| Ferritin [ng/ml; median (range)] | 6,506 (360-29,070) | 477 (287-759) | 159 (11.2-284) | 0.006* | 0.003* | 0.002* |
| sIL-2R [U/ml; median (range)] | 8,768 (469-37,583) | 4,633 (412-18,832) | 3,752 (225-4,180) | 0.566 | 0.396 | 0.971 |
| CD5-positive [n (%)] | 5 (46) | 6/8 (75) | 0 (0.0) | 0.352 | 0.014* | 0.001* |
| CD10-positive [n (%)] | 2 (18) | 1/8 (13) | 6 (40) | 1.000 | 0.789 | 1.000 |
| Bcl-2-positive [n (%)] | 10 (91) | 8/8 (100) | 14 (93) | 1.000 | 1.000 | 1.000 |
| Bcl-6-positive [n (%)] | 5 (46) | 5/8 (63) | 13 (87) | 0.650 | 0.115 | 0.594 |
| IRF4/MUM-1-positive [n (%)] | 9 (82) | 8/8 (100) | 12 (80) | 1.000 | 1.000 | 1.000 |
| CD29-positive [n (%)] | 2 (18) | 0/8 (0.0) | 7 (47) | 0.485 | 0.433 | 0.156 |
| CD54-positive [n (%)] | 4 (36) | 3/8 (38) | 10 (67) | 1.000 | 0.466 | 0.664 |
| Ki-67 labeling index [%; median (range)] | 62 (40-93) | 79.3 (60-100) | 67 (40-98) | 0.131 | 0.770 | 0.277 |
| PD-L1-positive [n (%)] | 6 (55) | 2/8 (25) | 10 (67) | 0.704 | 0.689 | 0.268 |
| PD-L1 score [median (range)] | 0.82 (0-2) | 0.25 (0-1) | 0.8 (0-2) | 0.211 | 0.998 | 0.195 |
Abbreviations: DLBCL-IV, diffuse large B-cell lymphoma with significant intravascular invasion; DLBCL-noIV, DLBCL with no significant intravascular invasion; Hb, hemoglobin; IVLBCL, intravascular large B-cell lymphoma; LD, lactate dehydrogenase; M, male; NE, not examined; PD-L1, programmed death-ligand 1; PLT, platelet; sIL2R, soluble interleukin 2 receptor; WBC, white blood cell; vs., versus.
*P-value < 0.05
Table 4. Comparison of clinicopathological features of PD-L1-positive extranodal LBCL and PD-L1-negative extranodal LBCL.
| PD-L1-positive extranodal LBCL (n=18) |
PD-L1-negative extranodal LBCL (n=16) |
P-value | |
|---|---|---|---|
| Age [years; median (range)] | 72 (43-86) | 72 (51-83) | 0.931 |
| Sex, male [n (%)] | 8 (44) | 9 (56) | 0.492 |
| Disturbance of consciousness [n (%)] | 4 (22) | 5 (31) | 0.703 |
| Hepatomegaly [n (%)] | 3/16 (19) | 3/15 (20) | 1.000 |
| Splenomegaly [n (%)] | 4/16 (25) | 5/15 (33) | 0.704 |
| Hemophagocytosis [n (%)] | 6/12 (50) | 6/11 (55) | 1.000 |
| Peripheral blood involvement [n (%)] | 0 (0.0) | 3 (19) | 0.094 |
| Ann Arbor stage III/IV [n (%)] | 12 (67) | 11 (69) | 1.000 |
| WBC [×102/μl; median (range)] | 63 (23-128) | 59 (21-132) | 0.671 |
| Hb [g/dl; median (range)] | 11 (6.3-14.3) | 10 (7-15.6) | 0.601 |
| PLT [×104/μl; median (range)] | 19 (2-50) | 18 (1.2-58) | 0.836 |
| LD [U/l; median (range)] | 529 (142-2,018) | 720 (172-1,862) | 0.270 |
| Ferritin [ng/ml; median (range)] | 2,415 (11.2-22,923) | 2,616 (21.2-29,070) | 0.946 |
| sIL-2R [U/ml; median (range)] | 5,284 (225-37,583) | 6,193 (340-38,000) | 0.796 |
| CD5-positive [n (%)] | 4 (22) | 7 (44) | 0.274 |
| CD10-positive [n (%)] | 5 (28) | 4 (25) | 1.000 |
| Bcl-2-positive [n (%)] | 17 (94) | 15 (94) | 1.000 |
| Bcl-6-positive [n (%)] | 15 (83) | 8 (50) | 0.066 |
| IRF4/MUM-1-positive [n (%)] | 18 (100) | 11 (69) | 0.016* |
| CD29-positive [n (%)] | 7 (39) | 2 (13) | 0.125 |
| CD54-positive [n (%)] | 11 (61) | 6 (38) | 0.169 |
| Ki-67 labeling index [%; median (range)] | 65 (30-96) | 68 (5-100) | 0.695 |
Abbreviations: LBCL, large B-cell lymphoma; Hb, hemoglobin; LD, lactate dehydrogenase; M, male; PD-L1, programmed death-ligand 1; PLT, platelet; sIL2R, soluble interleukin 2 receptor; WBC, white blood cell.
*P-value < 0.05
There were no significant differences in OS among the three groups. In PFS, there were no significant differences between the DLBCL-IV and the IVLBCL groups, or between the IVLBCL and DLBCL-noIV groups, but it was significantly shorter in patients with DLBCL-IV than in those with DLBCL-noIV (p = 0.009) (Figure 2).
Fig. 2.
Overall survival (OS) and progression-free survival (PFS). (a) OS and (b) PFS of patients with diffuse large B-cell lymphoma with significant intravascular invasion (DLBCL-IV), intravascular large B-cell lymphoma (IVLBCL), and DLBCL with no significant vascular invasion (DLBCL-noIV). There were no significant differences in OS between DLBCL-IV and DLBCL-noIV (p = 0.139), between DLBCL-IV and IVLBCL (p = 0.588), or between IVLBCL and DLBCL-noIV (p = 0.2497). There were no significant differences in PFS between DLBCL-IV and IVLBCL (p = 0.315) or between IVLBCL and DLBCL-noIV (p = 0.112), whereas it were significantly shorter in DLBCL-IV than in DLBCL-noIV (p = 0.009).
DISCUSSION
In the present study, the frequency of DLBCL-IV (n = 11) was comparable with that of IVLBCL (n = 11). However, more than half of the patients with DLBCL-IV were diagnosed at autopsy, whereas all of the patients with IVLBCL were diagnosed by biopsy except for one patient (diagnosed by the resected gallbladder). Due to the nonspecific clinical manifestations and infrequent lymphadenopathy similar to IVLBCL, the diagnosis of DLBCL-IV may be difficult without histological examination. As the rates of intravascular invasion in DLBCL-IV were different depending on organs, a diagnosis of DLBCL-IV was suggested to be difficult without autopsy unless both intravascular and extravascular invasion is found on biopsy or surgery such as in cases 1-5. However, this is not due to selection bias because both cases were extracted from the same pathology files with criteria independent of biopsy or autopsy.
Diagnostic criteria of IVLBCL, similar to those by WHO-2017, were established by the 3rd edition of the WHO classification in 2001.17 After the definition, however, some cases equivalent to DLBCL-IV were reported as intravascular lymphoma with extravascular tendencies,11 IVLBCL with concomitant extravascular central nervous system involvement,10,13 IVLBCL in bone marrow with interstitial infiltration,15 or DLBCL with intravascular pattern.8 These reports, including those recently published,8,15 suggest that the diagnostic criteria of IVLBCL by the WHO-2017 and WHO classification in 2001 are not accepted by some investigators. It is also possible that a diagnosis of IVLBCL by the WHO-2017 criteria depends on the extent of tissue examination. Thus, some IVLBCL cases diagnosed by biopsy may be DLBCL-IV when additional tissue obtained by surgery is examined.
Extravascular involvement is sometimes observed at the later stage of IVLBCL.7 In accordance with this report, we found no patients with IVLBCL diagnosed at autopsy. On the contrary, one of the two DLBCL-IV patients (Patients 3 and 4) and two IVLBCL patients exhibited no intravascular involvement at the time of relapse. In these patients, the relapsed tissues alone were characterized as DLBCL-noIV. In the third clinical setting, patients with DLBCL relapsed as IVLBCL, have also been described.18-21 In one of these reports, initial DLBCL-noIV and later developed IVLBCL exhibited a similar pattern of immunoglobulin heavy-chain gene rearrangement, suggesting that they originated from the same clone.20 Consistent with these reports, Patient 7 was characterized as having DLBCL-IV at autopsy, but he was initially diagnosed as having DLBCL-noIV by a partially resected breast sample. This suggested that DLBCL-IV and IVLBCL are similar in terms of the biological mechanism of intravascular infiltration, and that they are an identical disease with the difference being due to degree of extravascular infiltration. The above-mentioned sequential alteration of DLBCL suggests that reconsideration and discussion are required in terms of the distinctiveness of IVLBCL, criteria of IVLBCL by the WHO-2017, and significance of intravascular invasion of lymphoma cells.
There were significant differences in several clinicolaboratory parameters, including OS and PFS, between the DLBCL-IV and DLBCL-noIV groups, whereas there was a significant difference in only the serum ferritin level between the DLBCL-IV and IVLBCL groups. The average serum ferritin level was significantly higher in the DLBCL-IV group than in the IVLBCL group (Table 3), possibly due to the higher number of autopsy patients with more advanced stage disease and greater tumor burden in the DLBCL-IV group than in the IVLBCL group. Murase et al. compared the clinicopathological features of de novo CD5+/CD10- DLBCL patients with/without an intravascular or sinusoidal pattern (DLBCL-IVL/DLBCL-NOS of this immunophenotype). They found that patients with DLBCL-IVL had a significantly shorter survival than those with DLBCL-NOS, and that patients with DLBCL-IVL had significantly higher frequencies of both hepatomegaly and splenomegaly than those with DLBCL-NOS.16 The reported frequency of CD5 positivity in the IVLBCL group was 38%,16 whereas that in the DLBCL-noIV group was 5-10%.22,23 In the present study, the frequency of CD5 positivity was significantly higher in both the DLBCL-IV group (p < 0.05) and the IVLBCL group (p < 0.001) than in the DLBCL-noIV group, but the frequency was not significantly different between the DLBCL-IV and IVLBCL groups (Table 3). As 19% of de novo CD5-positive DLBCL was reported to exhibit intravascular or intrasinusoidal infiltration,24 DLBCL-IV in our study may be equivalent to the above lymphoma. In our study, there was no significant difference in the rates of PD-L1 positivity or average PD-L1 scores among the three groups (Table 3). Previous studies demonstrated that positive rates of PD-L1 in IVLBCL were 35%-45.5%,5,6,9 whereas those in DLBCL-noIV varied considerably (15%-61.7%).25-29 Therefore, it is difficult to characterize DLBCL-IV, IVLBCL, and DLBCL-noIV by PD-L1 expression, although the molecule has been reported as one of the characteristic immunophenotypic features of IVLBCL.4-9 In addition, the lack of adhesion molecules, such as CD29 and CD54, has been suggested to be a unique biological features of IVLBCL,2,3 but all of the intravascular and extravascular tumor cells in the DLBCL-IV group exhibited similar rates of CD29 and CD54 expression except for in one patient, and there were no significant differences in CD29 and CD54 expression among the three groups (Table 3).
Our study was unable to find a significant difference between DLBCL-IV and IVLBCL in terms of clinical and pathological features, although more than half of DLBCL-IV cases were diagnosed at autopsy. On the other hand, the features of DLBCL-noIV are considerably different from those of IVLBCL. In this context, it is suggested that a given DLBCL is more closely related to IVLBCL when at least 50% of the lymphomatous area exhibits intravascular involvement in one or more organs. The use of strict criteria to characterize a given lymphoma type can increase its distinctiveness, as exemplified by IVLBCL. However, features, such as intravascular invasion, which is potentially important for the prognosis, may not be paid much attention, if DLBCL-IV is included in DLBCL-NOS.
It is unclear how we should handle patients with DLBCL having an intravascular lymphomatous area between 1 to 49% because there were no such patients in the present study. Therefore, it is apparent that the present study is preliminary and further studies by other institutions with more cases and with a multivariate analysis are required for verification of our results because the number of patients in the present study was small, and their available clinicopathological and survival data were limited to a localized area (Nara prefecture) of Japan.
Lastly, it is not known whether DLBCL-IV is completely different from IVLBCL or whether they both constitute a spectrum of a single disease because of the absence of a specific genetic abnormality in the latter. However, it may be reasonable by the present study to establish an umbrella category of ‘DLBCL with intravascular involvement’ that consists of IVLBCL and DLBCL-IV.
ACKNOWLEDGMENTS
The authors thank Ms. Masako Nakata and Mr. Liu Lota (Department of Diagnostic Pathology, Nara Medical University) for their technical assistance.
Footnotes
FUNDING
No funding was received.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the ethics committee of Nara Medical University (2454).
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- 1.Nakamura S, Ponzoni M, Campo E. Intravascular large B-cell lymphoma. In : Swerdlow SH, Campo E, Harris NL, et al. (eds) : Who Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, IARC. 2017; pp. 317-318. [Google Scholar]
- 2.Ponzoni M, Arrigoni G, Gould VE, et al. Lack of CD 29 (β1 integrin) and CD 54 (ICAM-1) adhesion molecules in intravascular lymphomatosis. Hum Pathol. 2000; 31: 220-226. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita M, Izumoto S, Hashimoto N, et al. Immunohistochemical analysis of adhesion molecules and matrix metalloproteinases in malignant CNS lymphomas: a study comparing primary CNS malignant and CNS intravascular lymphomas. Brain Tumor Pathol. 2008; 25: 73-78. [DOI] [PubMed] [Google Scholar]
- 4.Sakakibara A, Inagaki Y, Imaoka E, et al. Autopsy case report of intravascular large B-cell lymphoma with neoplastic PD-L1 expression. J Clin Exp Hematop. 2018; 58: 32-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015; 126: 2193-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta GK, Jaffe ES, Pittaluga S. A study of PD-L1 expression in intravascular large B cell lymphoma: correlation with clinical and pathological features. Histopathology. 2019; 75: 282-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakakibara A, Inagaki Y, Imaoka E, et al. Divergence and heterogeneity of neoplastic PD-L1 expression: two autopsy case reports of intravascular large B-cell lymphoma. Pathol Int. 2019; 69: 148-154. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Sakakibara A, Shimada K, et al. Immune evasion-related extranodal large B-cell lymphoma: A report of six patients with neoplastic PD-L1-positive extranodal diffuse large B-cell lymphoma. Pathol Int. 2019; 69: 13-20. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Kohno K, Matsue K, et al. PD-L1 (SP142) expression in neoplastic cells predicts a poor prognosis for patients with intravascular large B-cell lymphoma treated with rituximab-based multi-agent chemotherapy. Cancer Med. 2020; 9: 4768-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai H, Kajimoto K, Taniwaki M, et al. Intravascular large B-cell lymphoma presenting with mass lesions in the central nervous system: A report of five cases. Pathol Int. 2004; 54: 231-236. [DOI] [PubMed] [Google Scholar]
- 11.Thomas CA, Guileyardo JM, Krause JR. An intravascular lymphoma with extravascular tendencies. Proc Bayl Univ Med Cent. 2014; 27: 341-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchiyama T, Nakamine H, Morita K, et al. Intravascular large B-cell lymphoma coexisting with an ovarian carcinoma. J Clin Exp Hematop. 2016; 56: 59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poropatich K, Dittmann D, Chen YH, et al. A small case series of intravascular large B-cell lymphoma with unexpected findings: subset of cases with concomitant extravascular central nervous system (CNS) involvement mimicking primary CNS lymphoma. J Pathol Transl Med. 2017; 51: 284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe Y, Usui Y, Narita K, Takeuchi M, Matsue K. Concomitant subcutaneous intravascular lymphoma lesions in a patient with cardiac diffuse large B-cell lymphoma: is intravascular lymphoma a distinct clinical entity? Int J Hematol. 2018; 108: 637-639. [DOI] [PubMed] [Google Scholar]
- 15.Matsue K, Abe Y, Narita K, et al. Diagnosis of intravascular large B cell lymphoma: novel insights into clinicopathological features from 42 patients at a single institution over 20 years. Br J Haematol. 2019; 187: 328-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007; 109: 478-485. [DOI] [PubMed] [Google Scholar]
- 17.Gatter KC, Warnke RA. Intravascular large B-cell lymphoma. In : Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) : World Health Organization: Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, IARC. 2001; pp. 177-178. [Google Scholar]
- 18.Kano R, Masaie H, Hino A, et al. Pure intravascular recurrence of CD5-positive diffuse large B-cell lymphoma primarily arising from the nasal cavities. Diagn Pathol. 2018; 13: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasuya A, Hashizume H, Takigawa M. Early diagnosis of recurrent diffuse large B-cell lymphoma showing intravascular lymphoma by random skin biopsy. J Dermatol. 2011; 38: 571-574. [DOI] [PubMed] [Google Scholar]
- 20.Zhao XF, Sands AM, Ostrow PT, et al. Recurrence of nodal diffuse large B-cell lymphoma as intravascular large B-cell lymphoma: is an intravascular component at initial diagnosis predictive? Arch Pathol Lab Med. 2005; 129: 391-394. [DOI] [PubMed] [Google Scholar]
- 21.Katz DA, Miller IJ, Gregory SA. Intravascular B-cell lymphoma following nodal diffuse large B-cell lymphoma. Clin Adv Hematol Oncol. 2010; 8: 637-641. [PubMed] [Google Scholar]
- 22.Jain P, Fayad LE, Rosenwald A, Young KH, O’Brien S. Recent advances in de novo CD5 + diffuse large B cell lymphoma. Am J Hematol. 2013; 88: 798-802. [DOI] [PubMed] [Google Scholar]
- 23.Na HY, Choe JY, Shin SA, et al. Characteristics of CD5-positive diffuse large B-cell lymphoma among Koreans: high incidence of BCL2 and MYC double-expressors. PLoS One. 2019; 14: e0224247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Seto M, Okamoto M, et al. De novo CD5+ diffuse large B-cell lymphoma: a clinicopathologic study of 109 patients. Blood. 2002; 99: 815-821. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Nong L, Liang L, et al. Comparison of PD-L1 detection assays and corresponding significance in evaluation of diffuse large B-cell lymphoma. Cancer Med. 2019; 8: 3831-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen BJ, Dashnamoorthy R, Galera P, et al. The immune checkpoint molecules PD-1, PD-L1, TIM-3 and LAG-3 in diffuse large B-cell lymphoma. Oncotarget. 2019; 10: 2030-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang X, Xiu B, Yang Z, et al. The expression and clinical relevance of PD-1, PD-L1, and TP63 in patients with diffuse large B-cell lymphoma. Medicine (Baltimore). 2017; 96: e6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon D, Kim S, Kim PJ, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016; 68: 1079-1089. [DOI] [PubMed] [Google Scholar]
- 29.Menter T, Bodmer-Haecki A, Dirnhofer S, Tzankov A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol. 2016; 54: 17-24. [DOI] [PubMed] [Google Scholar]