Abstract
Diarrhea is one of the manifestations of the novel coronavirus disease (COVID-19), but it also develops as a complication of massive antibiotic therapy in this disease. This study aimed to compare these types of diarrhea.
We included patients with COVID-19 in a cohort study and excluded patients with chronic diarrhea, laxative use, and those who died during the first day of hospitalization.
There were 89 (9.3%), 161 (16.7%), and 731 (75.7%) patients with early viral, late antibiotic-associated, and without diarrhea, respectively. Late diarrhea lasted longer (6 [4–10] vs 5 [3–7] days, P < .001) and was more severe. Clostridioides difficile was found in 70.5% of tested patients with late diarrhea and in none with early diarrhea. Presence of late diarrhea was associated with an increased risk of death after 20 days of disease (P = .009; hazard ratio = 4.7). Patients with late diarrhea had a longer hospital stay and total disease duration, and a higher proportion of these patients required intensive care unit admission. Oral amoxicillin/clavulanate (odds ratio [OR] = 2.23), oral clarithromycin (OR = 3.79), and glucocorticoids (OR = 4.41) use was a risk factor for the development of late diarrhea, while ceftriaxone use (OR = 0.35) had a protective effect. Before the development of late diarrhea, decrease in C-reactive protein levels and increase in lymphocyte count stopped but the white blood cell and neutrophil count increased. An increase in neutrophils by >0.6 × 109 cells/L predicted the development of late diarrhea in the coming days (sensitivity 82.0%, specificity 70.8%, area under the curve = 0.791 [0.710–0.872]).
Diarrhea in COVID-19 is heterogeneous, and different types of diarrhea require different management.
Keywords: antibiotic-associated diarrhea, COVID-19, diarrhea, mortality
1. Introduction
The novel coronavirus disease (COVID-19) is the first 21st century pandemic.[1] Hence, information regarding this disease continues to be relevant to physicians and researchers. It is known that COVID-19 is not limited to the respiratory system. One of the most frequent extrapulmonary manifestations is diarrhea.[2,3] Several systematic reviews have been published showing that diarrhea occurs in about 10% of patients.[4]
Patients with COVID-19 usually receive massive antibiotic therapy, which predisposes them to developing antibiotic-associated diarrhea.[5] Thus, diarrhea in COVID-19 can be divided into early viral diarrhea and late diarrhea, which is most likely antibiotic-associated.[6,7] Only 1 study describes Clostridioides difficile infection in COVID-19.[8] Viral RNA in feces was found in patients with early diarrhea, whereas feces in those with late diarrhea, as a rule, did not contain the virus.[9] Differentiating these types of diarrhea is very important, as viral diarrhea is usually benign, self-limiting, and does not require special treatment, but antibiotic-associated diarrhea without treatment can lead to death or aggravate the course of COVID-19. However, no studies have been published detailing the differences between early and late diarrhea in COVID-19. Thus, the aim of this study was to describe in detail the difference between these types of diarrhea in COVID-19.
2. Methods
This was a retrospective cohort single-center study and included all patients admitted to the Clinic of internal diseases, gastroenterology, and hepatology of Sechenov University from April to July 2020 with suspected COVID-19 according to the guidelines of World Health Organization.[10] The study excluded patients with diarrhea from other causes, those who took laxatives, and those who died during the first day of hospitalization from causes not associated with COVID-19 or diarrhea.
All patients signed informed consent authorizing the use of their medical data for scientific purposes. The study was approved by the local ethics committee of Sechenov University.
The presence of C. difficile infection was confirmed by the detection of at least one of its toxins in feces by enzyme immunoassay.
Diarrhea was defined as the presence of loose or watery stools or >3 bowel movements per day. If a patient re-developed diarrhea 3 days after stool normalization, we assumed that the patient had 2 different types of diarrhea: early and late.
Separating early viral and late antibiotic-associated diarrhea is not easy. It is known that viral diarrhea in COVID-19 usually develops during the first 10 days of the disease.[11] Therefore, if diarrhea developed while taking antibiotics ≥11 days after the onset of the disease, we considered it as late antibiotic-associated diarrhea. If diarrhea developed before antibiotics use, we considered it to be early viral diarrhea.
Difficult cases were those in whom diarrhea developed during antibiotic therapy but before the 10th day. To develop a criteria for their separation, we carried out a meta-analysis of the 4 largest studies[12–15] and found that antibiotic-associated diarrhea develops at 6.9 ± 1.6 days after the start of antibiotic therapy. Thus, we considered diarrhea as viral if it developed before 5 days after the start of antibiotic therapy and antibiotic-associated, otherwise, since this period accounts for most of the latter cases.
If the main inflammation biomarkers (white blood cells [WBC], neutrophils, lymphocytes, C-reactive protein[CRP]) were assessed at least 3 times before the development of late diarrhea, we studied their changes between the following points: point 1—upon admission, point 3—about a week before the onset of diarrhea, point 4—1 to 4 days before the onset of diarrhea, point 5—2 to 4 days after the onset of diarrhea. If data were available, 2 additional points were also analyzed: point 2—3 to 5 days after admission and point 6—at the end of diarrhea. After correlating these points with the days of hospitalization, patients without diarrhea who had these indicators measured at the same time points were categorized in Control group. Other biomarkers of inflammation (ferritin, interleukin 6, and procalcitonin) were not studied often enough to track their changes.
Results are presented as median [interquartile range]. The groups were compared out using Mann–Whitney U test for continuous data and χ2 test for categorical data. Wilcoxon test was used to assess the changes in laboratory parameters. Survival was assessed using the Kaplan-Meier estimator and Cox F test. A Cox regression model was used to assess the influence of factors on patient survival and hazard ratio. A P < .050 value was taken as the criterion for significance. Statistical calculations were performed using STATISTICA 10 (TIBCO Software, Palo Alto, CA) and SPSS 23 (IBM Corp., Armonk, NY).
3. Results
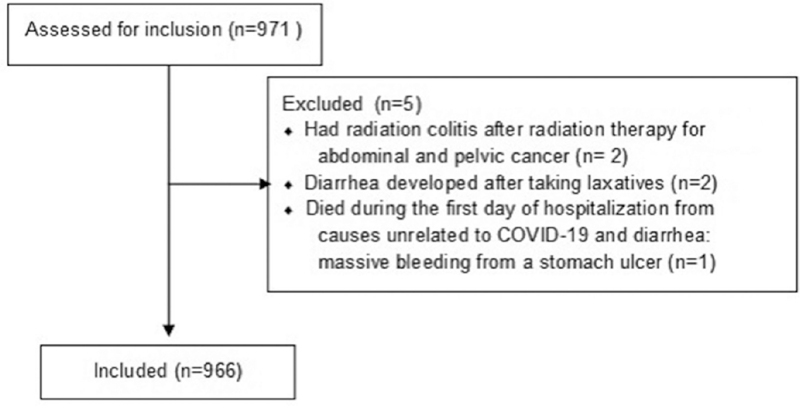
The study included 966 patients (Fig. 1). Diarrhea developed before admission in 71 (7.3%) patients and during hospitalization in 149 (15.4%), 731 (75.7%) patients had no diarrhea, 15 (1.6%) patients had 2 attacks of diarrhea (each attack was considered as a separate case): 10 had their first attack before hospitalization and the second during hospitalization, 4 patients had both attacks during hospitalization, and 1 had both attacks before hospitalization.
Figure 1.
CONSORT 2010 Flow Diagram.
3.1. Differentiating early viral and late antibiotic-associated diarrhea
Diarrhea developed in 29 (3.0%) and 221 (22.9%) patients before and after starting antibiotics, respectively. The former were considered to have early viral diarrhea. Diarrhea started later than 10 days after the onset of the disease in 145 (15.0%) patients that used antibiotics. These patients were considered to have late antibiotic-associated diarrhea.
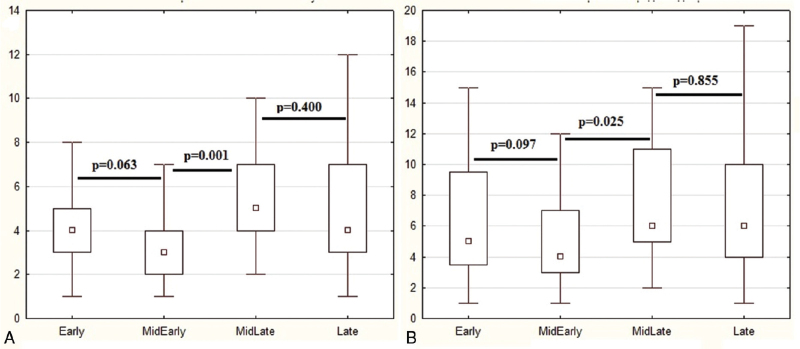
The group of patients in whom diarrhea began after the start of antibiotic therapy, but before the 10th day of the disease were divided into 2 subgroups: those in whom diarrhea began in the first 4 days of antibiotic therapy (n = 60) and those in whom diarrhea began after the 4th day of antibiotic therapy (n = 16). There was significant difference between these subgroups in terms of the main indicators of diarrhea (duration and number of bowel movements per day; Fig. 2). There was no significant difference in these indicators between patients with diarrhea before starting antibiotics and those who developed it in the first 4 days after starting antibiotics but before the 10th day of illness, as well as between patients who developed diarrhea after 10 days from the onset of the disease on antibiotic treatment and those who developed it later than the 4th day of antibiotic use, but earlier than the 10th day of the disease (Fig. 2). Thus, we considered the first subgroup of patients as having early viral diarrhea, and the second subgroup as having late antibiotic-associated diarrhea.
Figure 2.
The maximum number of bowel movements per day (a) and the duration of diarrhea (b, days) in patients with pre-antibiotic diarrhea (Early group), in patients with diarrhea developed during the first 10 days of disease in the first 1 to 4 days of antibiotics use (MidEarly group) and later 4th day of antibiotics use (MidLate group), and in patients with diarrhea developed 10 days after the onset of the disease while taking antibiotics (Late group). The point is mediana, the box contains interquartile range, the whiskers contain range without outliers.
In total, we considered that 89 (9.3%) patients had early viral diarrhea, and 161 (16.7%) had late antibiotic-associated diarrhea. Late diarrhea developed regardless of whether the patient had early diarrhea or not (16.9% vs 16.6%; P = .563).
Among the 83 cases of diarrhea before hospitalization (including cases with 2 attacks of diarrhea), 22 and 61 patients had late and early diarrhea, respectively. Diarrhea was significantly longer and associated with a greater number of bowel movements in the first patient group than in the second one (P = .003 and <.001, respectively). Among the 167 cases of diarrhea that developed during hospitalization, 139 and 28 were classified as late and early diarrhea, respectively. There was also a significant difference in the duration of diarrhea and number of bowel movements per day between these subgroups (both P < .001).
3.2. Early versus late diarrhea
The main characteristics of patient groups are presented in Table 1.
Table 1.
Main characteristics of patients with early and late diarrhea and patients without diarrhea.
| Early diarrhea (n = 89) | Late diarrhea (n = 161) | No diarrhea (n = 731) | P, early vs late | P, early vs no | P, late vs no | |
| Age, y | 56 (47 to 67) | 61 (53 to 69) | 54 (44 to 64) | .049 | .136 | <.001 |
| Male/female | 36/53 | 64/97 | 363/368 | .914 | .101 | .023 |
| Body temperature upon admission, 0C | 37.7 (37.1 to 38.0) | 37.7 (37.1 to 38.2) | 37.5 (37.0 to 38.0) | .740 | .044 | .003 |
| Body mass index, kg/m2 | 29.0 (25.1 to 30.0) | 30.0 (27.0 to 32.8) | 28.4 (25.8 to 32.3) | .464 | .713 | .084 |
| Duration of disease before admission, days | 6 (5 to 9) | 7 (5 to 11) | 7 (5 to 10) | .005 | .061 | .061 |
| Length of hospital stay, days | 15 (13 to 18) | 17 (14 to 22) | 15 (13 to 18) | <.001 | .241 | <.001 |
| Total duration of disease, days | 22 (19 to 25) | 27 (22 to 32) | 23 (20 to 28) | <.001 | .036 | <.001 |
| The day of onset of diarrhea from onset of disease | 6 (3 to 7) | 15 (12 to 20) | — | <.001 | — | — |
| The day of onset of diarrhea from day of hospitalization | −2 (−3 to 2) | 6 (4 to 11) | — | <.001 | — | — |
| The day of onset of diarrhea from day of initiation of antibiotics use | 2 (0 to 3) | 11 (8 to 17) | — | <.001 | — | — |
| The day of starting antibiotics from the onset of disease | 4 (2 to 5) | 3 (1 to 6) | 4 (2 to 7) | .204 | .143 | <.001 |
| Duration of taking antibiotics before hospitalization, days | 2 (0 to 4) | 4 (0 to 7) | 2 (0 to 5) | <.001 | .307 | <.001 |
| Taking antibiotics before hospitalization | 61 (68.5%) | 113 (70.2%) | 397 (54.3%) | .786 | .011 | .002 |
| Hospitalizations in the previous 3 mo | 6 (6.7%) | 16 (9.9%) | 36 (4.9%) | .393 | .463 | .014 |
| Duration of diarrhea, days | 5 (3 to 7) | 6 (4 to 10) | — | <.001 | — | — |
| <6 | 55 (61.8%) | 70 (43.5%) | — | .006 | — | — |
| 6–10 | 25 (28.1%) | 52 (32.3%) | — | .490 | — | — |
| 11–15 | 9 (10.1%) | 23 (14.3%) | — | .344 | — | — |
| >15 | 0 (0.0%) | 16 (9.9%) | — | .006 | — | — |
| The no. of bowel movements per day | 4 (2 to 4) | 4 (3 to 7) | — | <.001 | — | — |
| 1–3 | 26 (29.2%) | 54 (33.5%) | — | .483 | — | — |
| 4–5 | 56 (62.9%) | 51 (31.6%) | — | <.001 | — | — |
| 6–10 | 6 (6.7%) | 45 (28.0%) | — | .001 | — | — |
| 11–15 | 1 (1.1%) | 3 (1.9%) | — | .936 | — | — |
| >15 | 0 (0.0%) | 8 (5.0%) | — | .033 | — | — |
| Positive test for Clostridium difficile toxins in stool | 0/10 (0.0%) | 55/78 (70.5%) | — | .001 | — | — |
| Death | 2 (2.2%) | 9 (5.6%) | 28 (3.8%) | .078 | .156 | .194 |
| Death within the first 20 days of disease | 1 (1.1%) | 1 (0.6%) | 21 (2.9%) | — | .089 | .015 |
| Death after 20 days of disease | 1 (1.1%) | 8 (5.0%) | 7 (1.0%) | .029 | .553 | .001 |
| Admission to ICU | 2 (2.2%) | 21 (13.0%) | 44 (6.0%) | .009 | .144 | .002 |
| Day of admission to ICU from the onset of the disease | 10.5 (7 to 14) | 12 (9 to 18) | 9 (7 to 12) | .807 | .620 | .027 |
| Day of admission to ICU from the first hospitalization day | 5 (1 to 9) | 4 (0 to 7) | 2 (0 to 5) | .703 | .720 | .395 |
| Staying in ICU, days | 14.5 (7 to 22) | 6 (3 to 8) | 5 (4 to 12) | .413 | .169 | .737 |
| The need for mechanical ventilation | 2 (2.2%) | 9 (5.6%) | 24 (3.3%) | .362 | .837 | .241 |
| Colitis | 0 (0.0%) | 29 (18.1%) | — | <.001 | — | — |
Patients with late diarrhea were older than those without, and the proportion of women among them was greater.
Patients with late diarrhea had a longer hospital stay and total duration of the disease than patients without diarrhea. They started taking antibiotics earlier. Among them, there was a higher proportion of patients who were hospitalized within 3 months before the current hospitalization and who required admission to intensive care unit. Patients with early diarrhea did not differ from patients without diarrhea in the above indicators.
There was no difference between the groups in proportion of patients who required mechanical ventilation.
Late diarrhea lasted longer and was more severe than early diarrhea.
C. difficile toxins were found in the feces of 70.5% of tested patients with late diarrhea but in no patients with early diarrhea.
The incidence of various symptoms of COVID-19 and comorbidities, laboratory, and chest CT findings in patients with early and late diarrhea and without diarrhea are presented in Supplementary Tables 1 and 2.
Colitis was detected in 29 patients with late diarrhea and no patient with early diarrhea.
Stool analysis data of patients with early and late diarrhea are presented in Supplementary Table 3. Two patients with late diarrhea had green stools.
3.3. Mortality and diarrhea
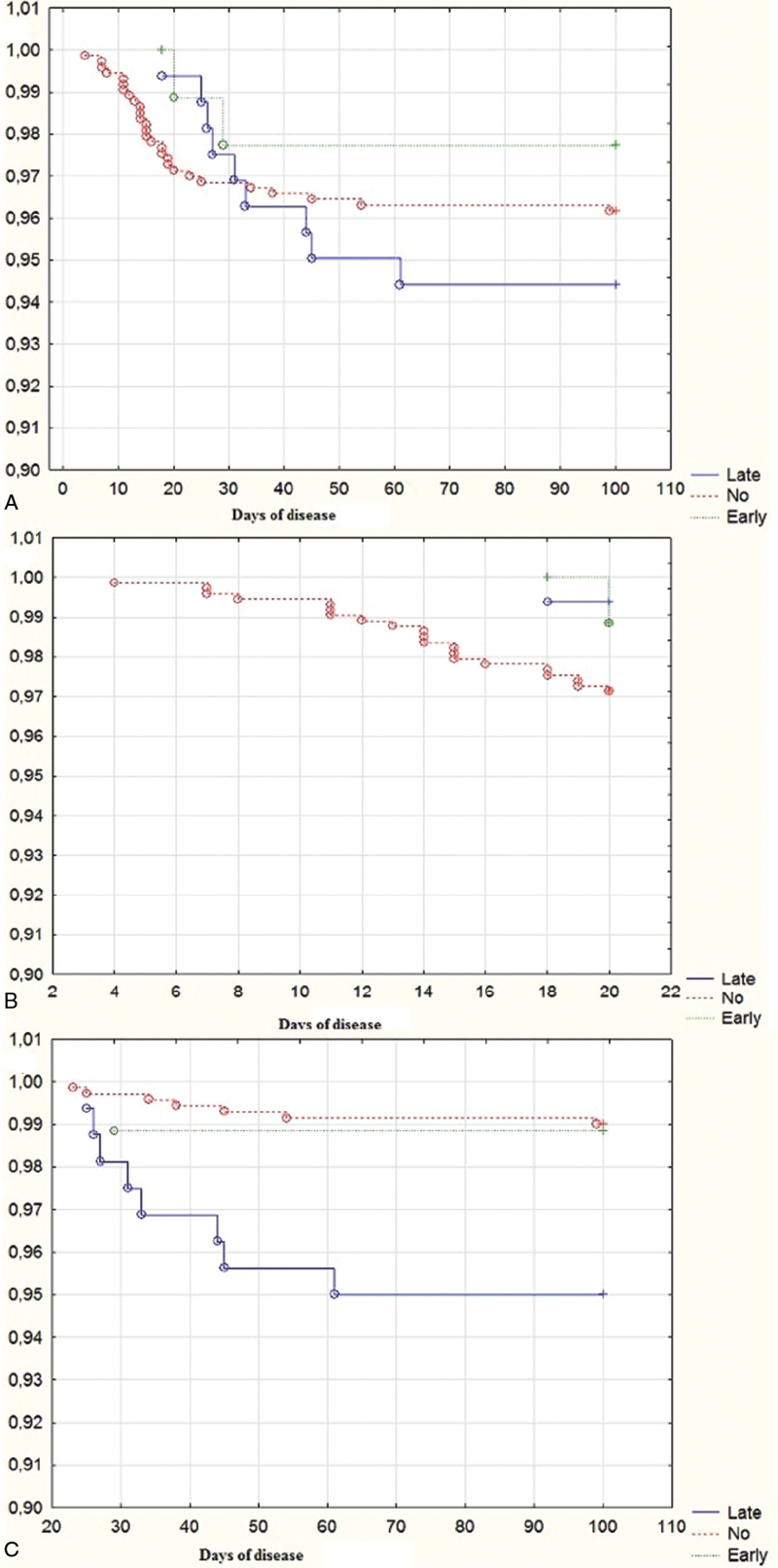
A total of 39 (4.0%) patients died, including 2 patients with early diarrhea, 9 patients with late diarrhea, and 28 patients without diarrhea. There was no significant difference in the overall mortality among patient groups, but patients with late diarrhea died later than patients without diarrhea (31 [26–44] days vs 15 [12–22] days; P < .001). If the 20th day of the disease (the upper quartile of time of onset of late diarrhea and the point at which the survival function of patients without diarrhea reaches a plateau) was taken as the cut-off point, then mortality was significantly higher in patients without diarrhea before this date (P < .001) but higher in patients with late diarrhea after it (P < .001). The mortality rate of patients with early diarrhea did not significantly differ from that of patients without diarrhea during both periods of the disease (P = .562 and P = .921). Among those who died in the first 20 days of the disease, patients with late diarrhea accounted for 4.3% of the total, but among those who died after that period, they accounted for 50% of deaths (Table 1, Fig. 3).
Figure 3.
Survival function of patients with early and late diarrhea and without diarrhea (early, late and no group, respectively): (A) general; (B) during the first 20 days of the disease; (C) After the 20th days of disease.
The presence of late diarrhea (P = .009; hazard ratio = 4.7) and older age (P = .007) were independent risk factors for death after 20 days of disease. Age (P = .002) and blood CRP upon admission (P = .023), but not the development of late diarrhea (P = .071) were the main predictors of death before the 21st day of disease. Glucocorticoids intake was not a predictor of death either before or after 20 days of disease (P = .163 and P = .266).
Four of 9 deceased patients with late diarrhea had diarrhea at the time of death. Diarrhea was replaced by paralytic ileus 1 to 5 days before death in the remaining 5. Deceased patients with late diarrhea had more often colitis detected than survivors with it (7/9 vs 22/152; P < .001). All the deceased patients with late diarrhea had C. difficile toxins in their feces.
3.4. Drug use before late diarrhea
All patients included in the study had received antibiotics. The most commonly used antibiotics were azithromycin (84.5%) and ceftriaxone (69.8%). Glucocorticoids (intravenous only) were administered in 37.1% of patients, hydroxychloroquine in 66.6% of patients, and other antiviral drugs in <1% of patients.
The maximum incidence rate of late diarrhea was observed when taking 1 antibiotic and the minimum when taking 2 antibiotics. When taking ≥3 antibiotics, the incidence rate of diarrhea was 20% to 25% (Table 2).
Table 2.
Drugs taken by patients before the onset of late diarrhea and by patients without diarrhea.
| Drugs | Late diarrhea | No diarrhea | Diarrhea rate | P |
| No. of different AB | ||||
| 1 Antibiotic | 25 | 51 | 32.9% | .001 |
| 2 Antibiotics | 49 | 345 | 12.4% | .001 |
| 3 Antibiotics | 61 | 255 | 19.3% | .471 |
| 4 Antibiotics | 16 | 56 | 22.2% | .337 |
| >4 Antibiotics | 8 | 24 | 25.0% | .300 |
| Individual AB | ||||
| Azithromycin | 130 | 622 | 17.3% | .989 |
| Levofloxacin total | 53 | 206 | 20.5% | <.001 |
| Levofloxacin oral | 35 | 117 | 23.0% | <.001 |
| Levofloxacin par. | 25 | 109 | 18.7% | .008 |
| Moxifloxacin | 30 | 103 | 22.6% | .014 |
| Moxifloxacin oral | 3 | 19 | 13.6% | .079 |
| Moxifloxacin par. | 27 | 91 | 22.9% | .037 |
| Amoxicillin/clavulanic acid | 54 | 210 | 20.5% | .130 |
| Amoxicillin/clavulanic acid oral | 44 | 136 | 24.4% | .020 |
| Amoxicillin/clavulanic acid par. | 17 | 95 | 15.2% | .062 |
| Meropenem | 15 | 46 | 24.6% | .245 |
| Ceftriaxone | 98 | 523 | 15.8% | .018 |
| GC | 85 | 245 | 25.8% | .543 |
| Clarithromycin | 13 | 72 | 15.3% | .179 |
| Clarithromycin oral | 10 | 28 | 26.3% | .146 |
| Clarithromycin par. | 3 | 48 | 5.9% | .225 |
| Josamycin oral | 6 | 12 | 33.3% | .633 |
| Cefixime oral | 3 | 18 | 14.3% | .328 |
| Antibiotic therapy regimens | ||||
| Azithromycin + ceftriaxone | 12 | 146 | 7.6% | .002 |
| Azithromycin + ceftriaxone + GC | 9 | 37 | 19.6% | .784 |
| Azithromycin + ceftriaxone + other oral AB | 14 | 87 | 13.9% | .246 |
| Azithromycin + ceftriaxone + GC + other oral AB | 13 | 36 | 26.5% | .112 |
| Azithromycin + ceftriaxone + other par. AB | 7 | 56 | 11.1% | .138 |
| Azithromycin + ceftriaxone + other par. AB + GC | 10 | 34 | 22.7% | .408 |
| Azithromycin + ceftriaxone + other par.AB + other oral AB | 5 | 26 | 16.1% | .777 |
| Azithromycin + ceftriaxone + other par.AB + other oral AB + GC | 10 | 30 | 25.0% | .242 |
| Azithromycin + other par. AB | 6 | 40 | 13.0% | .365 |
| Azithromycin + other par. AB + GC | 7 | 31 | 18.4% | .951 |
| Azithromycin + other par.AB + other oral AB | 3 | 20 | 13.0% | .527 |
| Azithromycin + other par.AB + other oral AB + GC | 12 | 21 | 36.4% | .005 |
| Other combinations of oral and par. AB ± GC | 13 | 48 | 21.3% | .286 |
| Azithromycin only | 5 | 25 | 16.7% | .841 |
| Оther oral AB ± Azithromycin | 16 | 36 | 30.8% | .014 |
| Any oral AB+GC | 8 | 7 | 53.3% | .003 |
| Other combinations of par. AB | 4 | 22 | 15.4% | .720 |
| Other combinations of par. AB +GC | 5 | 29 | 14.7% | .605 |
Logistic regression showed that the risk factors for the development of late diarrhea were the use of oral amoxicillin/clavulanic acid (odds ratio [OR] = 2.23), oral clarithromycin (OR = 3.79), and glucocorticoids (OR = 4.41), whereas the use of ceftriaxone (OR = 0.35) had a protective effect (upper half of Table 2).
The incidence rate of late diarrhea after different regimens is presented in the lower half of Table 2. Late diarrhea was least likely to develop if combination of azithromycin and ceftriaxone were used without glucocorticoids and other antibiotics (7.6%; P = .002), and most often when oral antibiotics other than azithromycin have been used (30.8%; P = .014), when azithromycin was used in combination with other oral and parenteral (not ceftriaxone) antibiotics and glucocorticoids (36.4%; P = .005), as well as when only oral antibiotics with glucocorticoids were used (53.3%; P = .003). The addition of glucocorticoids significantly increased the risk of developing late diarrhea when combined with azithromycin and ceftriaxone (OR = 3.0; P = .019) or when only oral antibiotics (OR = 3.3; P = .031) were used. In other cases, an increase in the risk of developing late diarrhea was less significant, possibly due to a smaller number of cases.
3.5. Treatment of late diarrhea
Oral metronidazole, oral vancomycin, probiotic Saccharomyces boulardii, and various combinations of these have been used for the treatment of late diarrhea. None of the regimens had significant benefits over the others (Supplementary Table 4). All patients with severe diarrhea (>10 bowel movements/day) received complex therapy (oral metronidazole + oral vancomycin + probiotics) and 35% of them received additional sulfasalazine.
Early diarrhea was mostly treated symptomatically.
3.6. Dynamics of inflammation biomarkers in patients with late diarrhea and without diarrhea
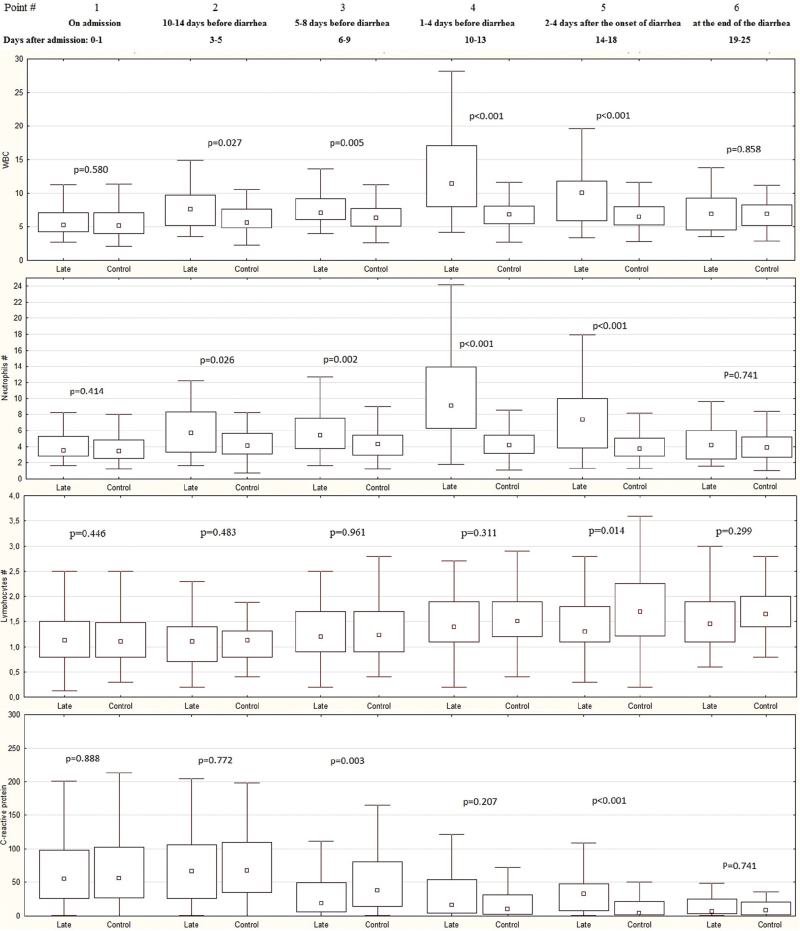
Changes in the main markers of inflammation were assessed in 48 patients with late diarrhea (Late group) and in 142 patients without diarrhea (Control group) (Fig. 4, Supplementary Table 5).
Figure 4.
Dynamics of the white blood cells (WBC), neutrophils, and lymphocytes count (109 cells/L) and blood C-reactive protein level (mg/L) during hospitalization in patients with late diarrhea (Late group) and in patients without diarrhea (Control group). The point is median, the box contains interquartile range, the whiskers contain range without outliers.
There was no significant difference between patients with late diarrhea and without diarrhea in WBC, neutrophil, and lymphocyte count and in CRP levels at admission, as well as at the time of the end of diarrhea.
In the first several days of hospitalization, there was an increase in WBC and neutrophil count in both groups of patients, which was more pronounced in patients who developed late diarrhea. Subsequently, these cell counts remained constant throughout the observation period in the control group, while in the late group, a significant increase in the content of these cells was observed at 1 to 5 days before development of diarrhea. This increase was 3.6 (1.1–6.2) × 109/L in the Late group versus 0.4 ([−0.6] to 1.5) × 109/L in the Control group (P < .001) for WBC and 3.4 (0.8–6.1) versus 0.1 ([−0.7] to 0.9) × 109/L (P < .001) for neutrophils, and did not depend on whether patients took glucocorticoids or not (P = .586; P = .615). A few days after the onset of diarrhea in the Late group, the content of these cells decreased, but remained significantly higher than in the Control group. These values leveled off only at the end of diarrhea.
Lymphocyte count did not differ significantly between groups, except at point 5. Several days after hospitalization, their levels remained unchanged in both groups. Starting from about the 5th day of hospitalization, this count started increasing, which in the Control group lasted until 14 to 18 days of hospitalization, but in the Late group it stopped when diarrhea developed. Therefore, a few days after the development of diarrhea (point 5), the lymphocyte count became higher in the Control group than in the Late group. At the end of diarrhea, this indicator was similar in both groups.
The concentration of CRP in the Control group initially increased slightly during the first several days of hospitalization, after which it decreased continuously throughout the observation period. In the Late group, CRP levels remained constant during the first few days of hospitalization, after which they dropped sharply, becoming lower than the Control group about a week before the development of diarrhea. Further, the decrease in CRP in the Late group stopped and became constant for several days before the development of diarrhea and also subsequently. This led to the fact that CRP levels were similar in the Control and Late groups 1 to 4 days before the development of diarrhea, and became higher in the Late group at 2 to 4 days after development of diarrhea. However, by the end of diarrhea, CRP levels decreased and were comparable to those in the Control group.
If a patient does not receive glucocorticoids or receives them at a constant dose for >5 days, then an increase in neutrophils by >0.6 × 109 cells/L predicts the development of late diarrhea in the next 1–5 days with a sensitivity of 82.0% and a specificity of 70.8% (area under the curve = 0.791 [0.710–0.872]; Supplementary Fig. 1a). An increase in WBC by 1 × 109 cells/L or more had a lower predictive ability (sensitivity 78.0%, specificity 64.9%; area under the curve = 0.774 [0.693–0.855]; Supplementary Fig. 1b).
4. Discussion
We divided 250 cases of diarrhea into early viral and late antibiotic-associated diarrhea. The incidence of viral diarrhea is consistent with published data,[4] and the overall incidence of diarrhea (about 25%) is consistent with the previous studies that also separated early from late diarrhea.[6,7]
Our proposed rules for the separation of these 2 types of diarrhea were verified based on the comparison of the main characteristics of diarrhea in different patient groups. We also showed that the criterion for separating early and late diarrhea cannot be based on whether patients developed diarrhea before or during hospitalization, since patients can use antibiotics for a long time before hospitalization or they can be admitted at the beginning of the disease before viral diarrhea develops.
C. difficile detection is an important marker that identifies late diarrhea, but it was not detected in 30% of these patients; therefore, a negative result cannot exclude the presence of late diarrhea, whereas a positive result indicates its presence with high probability.
Late diarrhea was more severe and had a longer duration than the more benign early diarrhea. Development of early diarrhea had no effect on the course of the disease, whereas development of late diarrhea worsened it, increasing the length of hospital stay, total disease duration, and frequency of admission to the intensive care unit. At the same time, there was no difference between groups in the proportion of patients who required mechanical ventilation, which shows that the development of late diarrhea has little effect on the respiratory complications of COVID-19. Only patients with late diarrhea had colitis detected by imaging methods.
The development of early diarrhea did not affect mortality. The relationship between late diarrhea and mortality was complex due to the fact that late diarrhea developed on average on the 15th day after the onset of the disease and many patients with severe lung damage died before it occurred. If we take patients who have lived enough time for the development of late diarrhea, then its presence determines their prognosis.
The safest regimen was the combination of azithromycin and ceftriaxone. The use of oral antibiotics (other than azithromycin) and glucocorticoids were the main risk factors for development of late diarrhea. We do not preclude the use of glucocorticoids in the treatment of COVID-19, but we think shorter courses should be favored and only used in patients with an active inflammatory process. Glucocorticoid use should be accompanied by probiotics. However, the effectiveness of these preventive measures should be confirmed by subsequent studies. We do not recommend oral antibiotics use in the inpatient management of COVID-19.
Patients with late diarrhea and without diarrhea have marked differences in the dynamics of the main markers of inflammation. A few days before the development of late diarrhea, pronounced neutrophilic leukocytosis was observed, and this also occurred in patients who did not receive glucocorticoids; therefore, it cannot be explained by their use. This parameter can be used to predict the development of late diarrhea in the coming days with high reliability.
Interestingly, in contrast to neutrophilic leukocytosis, CRP levels did not increase before the development of late diarrhea; rather, an interruption in their decrease was observed.
The limitations of our study are its retrospective nature and lack of standardization in the treatment of patients with COVID-19 and late diarrhea. This led to an abundance of different management regimens, which made it extremely difficult to compare their effectiveness and safety. Randomized trials are required to more accurately identify drugs that have an increased risk in the development of late diarrhea, to clarify the possibility of its prevention with probiotics and other drugs, to find the optimal regimen for late diarrhea treatment, and to compare antibiotic and glucocorticoid courses of different duration as risk factors of late diarrhea.
The strengths of our study are its volume (almost 1000 patients, including almost 100 patients with viral diarrhea and more than 150 with antibiotic-associated diarrhea) and the detail with which we have described the differences between the two types of diarrhea in COVID-19. The latter has no analogues among the published articles. In addition, we proposed an algorithm for separating viral and antibiotic-associated diarrhea, as well as a simple biomarker for predicting the development of the latter in the coming days with high accuracy. Also, we have indicated the safest and the riskier therapy regimens and have identified a field for future research on this subject.
In conclusion, diarrhea in COVID-19 is heterogeneous and presents as early benign viral and late dangerous antibiotic-associated ones. The development of the former appears to be impossible to prevent, but its treatment may be limited to symptomatic management. Whereas the development of the latter can be prevented and requires specific treatment, the optimal regimen of which has yet to be established. Thus, separating these types of diarrhea is possible and extremely important for determining further management.
Author contributions
Conceptualization: Roman Maslennikov, Andrey Svistunov, Vladimir Ivashkin, Elena Poluektova.
Data curation: Roman Maslennikov, Andrey Svistunov, Vladimir Ivashkin, Anna Ufimtseva, Elena Poluektova, Irina Efremova, Anatoly Ulyanin, Alexey Okhlobystin, Svetlana Kardasheva, Anastasia Kurbatova, Anna Levshina, Diana Grigoriadis, Shamil Magomedov, Natiya Dzhakhaya, Oleg Shifrin, Maria Zharkova, Elena Yuryeva, Nataliya Kokina, Manana Shirtladze, Olga Kiseleva.
Formal analysis: Roman Maslennikov, Andrey Svistunov, Vladimir Ivashkin, Anna Ufimtseva, Elena Poluektova, Irina Efremova, Anatoly Ulyanin, Alexey Okhlobystin, Svetlana Kardasheva, Anastasia Kurbatova, Anna Levshina, Diana Grigoriadis, Shamil Magomedov, Natiya Dzhakhaya, Oleg Shifrin, Maria Zharkova, Elena Yuryeva, Nataliya Kokina, Manana Shirtladze, Olga Kiseleva.
Investigation: Roman Maslennikov, Andrey Svistunov, Vladimir Ivashkin, Elena Poluektova, Irina Efremova, Anatoly Ulyanin, Alexey Okhlobystin, Svetlana Kardasheva, Anastasia Kurbatova, Anna Levshina, Diana Grigoriadis, Shamil Magomedov, Natiya Dzhakhaya, Oleg Shifrin, Maria Zharkova, Elena Yuryeva, Nataliya Kokina, Manana Shirtladze, Olga Kiseleva.
Methodology: Roman Maslennikov, Vladimir Ivashkin.
Project administration: Roman Maslennikov, Vladimir Ivashkin.
Resources: Roman Maslennikov.
Supervision: Roman Maslennikov, Andrey Svistunov, Vladimir Ivashkin.
Writing – original draft: Roman Maslennikov.
Writing – review & editing: Roman Maslennikov, Andrey Svistunov, Vladimir Ivashkin, Anna Ufimtseva, Elena Poluektova, Irina Efremova, Anatoly Ulyanin, Alexey Okhlobystin, Svetlana Kardasheva, Anastasia Kurbatova, Anna Levshina, Diana Grigoriadis, Shamil Magomedov, Natiya Dzhakhaya, Oleg Shifrin, Maria Zharkova, Elena Yuryeva, Nataliya Kokina, Manana Shirtladze, Olga Kiseleva.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: COVID-19 = novel coronavirus disease, CRP = C-reactive protein, WBC = white blood cells.
How to cite this article: Maslennikov R, Svistunov A, Ivashkin V, Ufimtseva A, Poluektova E, Efremova I, Ulyanin A, Okhlobystin A, Kardasheva S, Kurbatova A, Levshina A, Grigoriadis D, Magomedov S, Dzhakhaya N, Shifrin O, Zharkova M, Yuryeva E, Kokina N, Shirtladze M, Kiseleva O. Early viral versus late antibiotic-associated diarrhea in novel coronavirus infection. Medicine. 2021;100:41(e27528).
The authors reprot no conflicts of interest.
Funding information is not available.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
ICU = intensive care unit.
AB = antibiotics, GC = glucocorticoids; 2 patients could not name the AB they used before diarrhea developed, par. = parenterally.
References
- [1].Madabhavi I, Sarkar M, Kadakol N. COVID-19: a review. Monaldi Arch Chest Dis 2020;90: 10.4081/monaldi.2020.1298 [DOI] [PubMed] [Google Scholar]
- [2].Maslennikov R, Poluektova E, Ivashkin V, Svistunov A. Diarrhoea in adults with coronavirus disease-beyond incidence and mortality: a systematic review and meta-analysis. Infect Dis (Lond) 2021;53:348–60. [DOI] [PubMed] [Google Scholar]
- [3].Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther 2020;51:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tariq R, Saha S, Furqan F, Hassett L, Pardi D, Khanna S. Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: a systematic review and meta-analysis. Mayo Clin Proc 2020;95:1632–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mullish BH, Williams HR. Clostridium difficile infection and antibiotic-associated diarrhoea. Clin Med (Lond) 2018;18:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020;69:997–1001. [DOI] [PubMed] [Google Scholar]
- [7].Zhang L, Han C, Zhang S, et al. Diarrhea and altered inflammatory cytokine pattern in severe coronavirus disease 2019: Impact on disease course and in-hospital mortality. J Gastroenterol Hepatol 2021;36:420–9. [DOI] [PubMed] [Google Scholar]
- [8].Sandhu A, Tillotson G, Polistico J, et al. Clostridioides difficile in COVID-19 Patients, Detroit, Michigan, USA, March-April 2020. Emerging Infect Dis 2020;26:2272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shang H, Bai T, Chen Y, et al. Outcomes and implications of diarrhea in patients with SARS-CoV-2 infection. Scand J Gastroenterol 2020;55:1049–56. [DOI] [PubMed] [Google Scholar]
- [10].Clinical management of COVID-19. WHO/2019-nCoV/clinical/2020.5. Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19. [Google Scholar]
- [11].Han C, Duan C, Zhang S, et al. Digestive symptoms in covid-19 patients with mild disease severity: clinical presentation, stool viral rna testing, and outcomes. Am J Gastroenterol 2020;115:916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yapar N, Sener A, Karaca B, et al. Antibiotic-associated diarrhea in a turkish outpatient population: investigation of 288 cases. J Chemother 2005;17:77–81. [DOI] [PubMed] [Google Scholar]
- [13].Wistrom J. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother 2001;47:43–50. [DOI] [PubMed] [Google Scholar]
- [14].Duman DG, et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacterpylori eradication. Eur J Gastroenterol Hepatol 2005;17:1357–61. [DOI] [PubMed] [Google Scholar]
- [15].Kotowska M, Albrecht P, Szajewska H. Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther 2005;21:583–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.