Abstract
Carpal tunnel syndrome (CTS) is a common neuropathy. Although CTS progression is known to be associated with thenar muscle (TM) atrophy, the diagnostic value of TM atrophy for CTS has not been established. In this research, the thenar muscle cross-sectional area (TMCSA) was evaluated to analyze the relationship between the TMCSA and CTS. We assumed that TMCSA is a major diagnostic parameter in the CTS.
Both TMCSA and thenar muscle thickness (TMT) samples were acquired from 18 CTS patients, and from 18 control subjects who underwent wrist magnetic resonance imaging with no evidence of CTS. T2-weighted transverse magnetic resonance imaging images were obtained. We measured the TMCSA and TMT at the level of first carpometacarpal joint.
The average TMCSA was 296.98 ± 49.39 mm2 in the normal group and 203.36 ± 72.13 mm2 in the CTS group. The average TMT was 8.54 ± 1.45 mm in the normal group and 7.38 ± 1.14 mm in the CTS group. CTS group had significantly lower TMCSA and TMT. Receiver operator characteristics curve analysis showed that the best cutoff point for the TMCSA was 260.18 mm2, with 77.8% sensitivity, 77.8% specificity. The best cutoff point of the TMT was 7.70 mm, with 61.1% sensitivity, 66.7% specificity.
Although the TMCSA and TMT were both significantly associated with CTS, the TMCSA was a much more sensitive measurement parameter. Thus, to evaluate CTS patients, the physician should more carefully inspect the TMCSA than TMT.
Keywords: carpal tunnel syndrome, cross-sectional area, diagnosis, thenar muscle, thickness
1. Introduction
Carpal tunnel syndrome (CTS) is a common entrapment neuropathy of the median nerve (MN). Although CTS progression is known to be associated with thenar muscle (TM) atrophy, the diagnostic value of TM atrophy for CTS has not been well established.[1–3] The TM plays an important role in the efficient hand movement. In clinical practice, assessment of TM abnormalities in CTS depends on the physical examination, history, and electrodiagnostic studies including nerve conduction studies and electromyography.[1–5] However, all of these diagnostic and clinical tests are frequently insufficient to diagnose TM dysfunction in CTS. As an alternative, use of hand-wrist magnetic resonance imaging (MRI) to evaluate the TM of hand in CTS has been demonstrated.[6–10] Recent researches have proved the merits of MRI for evaluating small anatomical lesions of the hand.[11] Therefore, MRI can be a very important diagnostic modality for characterization and detection of hand muscle anatomy, especially changes of the TM in CTS. Thus, the thenar muscle thickness (TMT) is a useful diagnostic morphological parameter for the assessment of CTS.[11,12] However, there has been limited researches investigating the anatomical exact basis of TM atrophy. Thus, to analyze relationship between TM atrophy and CTS, we measured the thenar muscle cross-sectional area (TMCSA). The TMCSA has not been studied for clinical relationship with CTS. Moreover, no studies have calculated the best optimal cutoff value about TMT and TMCSA. In this paper, we compared the accuracy of TMT and TMCSA in diagnosing CTS using MRI to obtain which parameter is a more sensitive parameter.
2. Methods
2.1. Patients
This research has been approved by the Institutional Review Board of Catholic Kwandong University (approval No. IS18RISI0013). The requirement for written informed consent was waived due to the retrospective nature of the study. We retrospectively reviewed patients who visited our orthopedic center from April 2015 to August 2020, and who were clinically diagnosed with CTS. All patients were enrolled after the diagnosis of CTS was confirmed by 2 experienced musculoskeletal radiologists. The CTS group included 18 patients (18 wrists) with an average age of 50.28 ± 13.88 years (range, 25–80 years).
The inclusion criteria of the CTS group were: positive clinical sign (Phalen test, Tilen sign, or modified Phalen test); MR image taken for review. The exclusion criteria were: any wrist surgery; history of wrist fracture; wrist infection; morbid obesity. To compare the TMT and TMCSA between individuals with and without CTS, we also enrolled a control group of individuals who underwent MRI without CTS. The healthy group consisted of 18 people (7 men and 11 women) with an average age of 42.67 ± 11.29 years (range, 24–59 years) (Table 1).
Table 1.
Comparison of the characteristics of control and CTS groups.
| Variable | Control group n = 18 | CTS group n = 18 | Statistical significance |
| Gender (male/female) | 7/11 | 5/13 | P = .724 |
| Age (yrs) | 42.67 ± 11.29 | 50.28 ± 13.88 | P = .080 |
| Body mass index (kg/m2) | 23.56 ± 2.74 | 25.89 ± 4.49 | P = .077 |
| Dominant hand (left/right) | 11/7 | 8/10 | P = .504 |
| CTS severity∗ (none/normal/mild/moderate/severe) | 8/2/2/3/3 | ||
| CTS duration (yrs) | 0.50 [0.21;2.00] | ||
| TMT (mm) | 8.54 ± 1.45 | 7.38 ± 1.14 | P = .011 |
| TMCSA (mm2) | 296.98 ± 49.39 | 203.36 ± 72.13 | P < .001 |
2.2. MRI scanning protocol
Subjects were scanned on a 1.5T Avanto (Siemens Healthineers, Erlangen, Germany) from the wrist joint to the metacarpophalangeal joint. The MRI examination was conducted with turbo spin echo transverse fat-saturated T2-weighted sequence. MRI parameters were as follows: Echo time/repetition time (TE/TR), 70/4350 ms; field of view, 120 × 120 mm; matrix size, 384 × 250; and slice thickness, 3.0 mm; number of signals averaged, 2; scan time, 192 seconds, and 3 > echo train length.
2.3. Image analysis
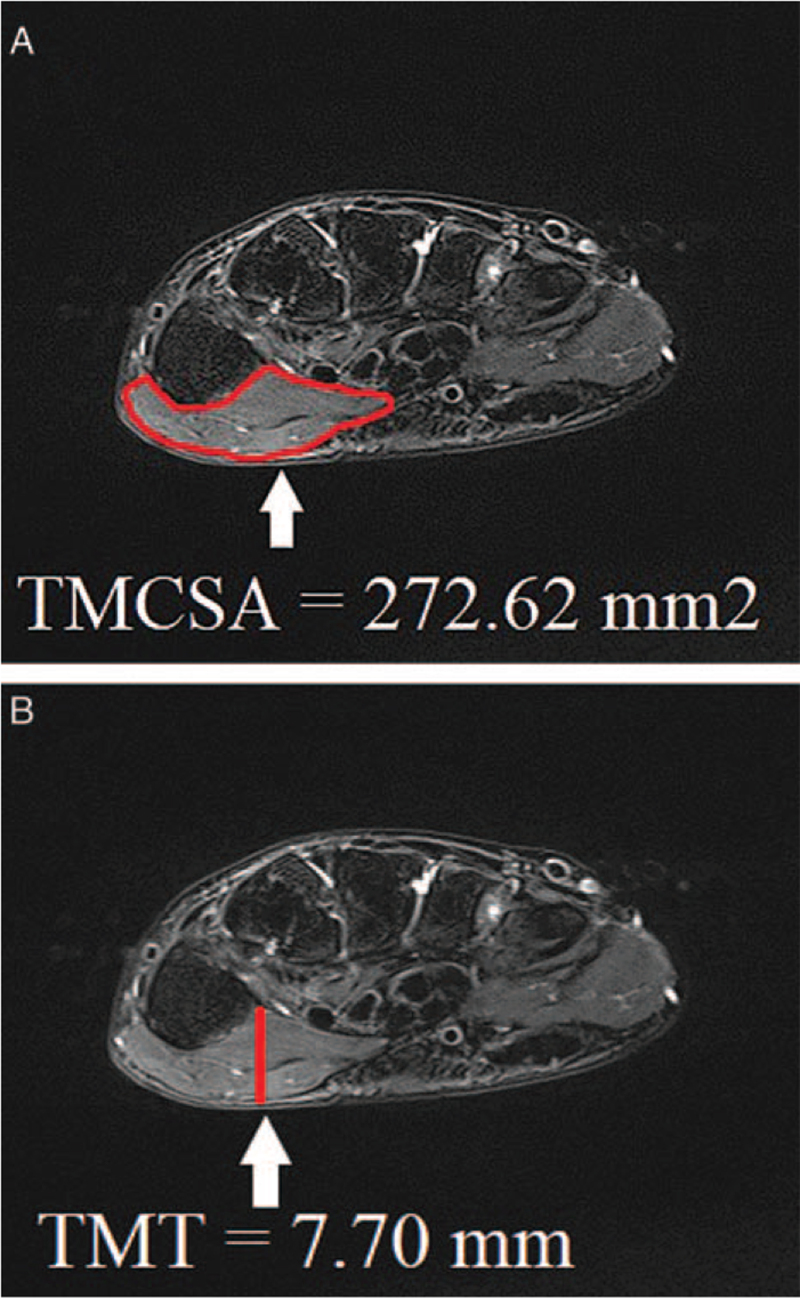
Transverse fat-saturated T2-weighted MRI image showed the tortuous shape of the TM. The TMCSA were extracted from the original images by drawing an outline. TMCSA was measured as the largest cross-sectional area of TM at the level of the first carpometacarpal joint. The TMT was measured by most hypertrophied TM (Fig. 1A, B).
Figure 1.
Transverse turbo spin echo T2-weighted fat saturated carpal tunnel magnetic resonance images of the (A) thenar muscle cross-sectional area (TMCSA) and (B) thenar muscle thickness (TMT).
2.4. Statistical analyses
Demographic variables are presented as the mean ± standard deviation. P < .05 was considered to indicate a statistically significant difference. The validity of the TMT and TMCSA for diagnosis of disease was estimated by receiver operator characteristics curves, cutoff values, area under the curve (AUC), sensitivity, and specificity with 95% confidence intervals (CIs). The AUC was also calculated independently in the results to demonstrate the value gained from the addition of each parameter. SPSS for Windows version 22 (IBM SPSS Inc., Chicago, IL) was used for the statistical analysis.
3. Results
The average TMCSA was 296.98 ± 49.39 mm2 in the normal group and 203.36 ± 72.13 mm2 in the CTS group (Table 1). The average TMT was 8.54 ± 1.45 mm in the normal group and 7.38 ± 1.14 mm in the CTS group. CTS patients had significantly lower TMCSA (P < .001) and TMT (P = .011) (Table 1). Regarding the validity of both TMCSA and TMT as predictors of CTS, receiver operator characteristics curve analysis showed that the best cutoff point for the TMCSA was 260.18 mm2, with 77.8% sensitivity, 77.8% specificity, and AUC of 0.87 (95% CI, 0.75–0.99) (Table 2, Fig. 2). The best cutoff point of the TMT was 7.70 mm, with 61.1% sensitivity, 66.7% specificity, and AUC of 0.73 (95% CI, 0.57–0.90) (Table 3, Fig. 2).
Table 2.
Sensitivity and specificity of each cutoff point of the TMCSA.
| TMCSA (mm2) | Sensitivity (%) | Specificity (%) |
| 131.40 | 11.1 | 100.0 |
| 160.56 | 33.3 | 100.0 |
| 195.59 | 55.6 | 100.0 |
| 233.33 | 66.7 | 88.9 |
| 260.18∗ | 77.8 | 77.8 |
| 277.05 | 88.9 | 66.7 |
| 297.92 | 94.4 | 50.0 |
| 321.59 | 94.4 | 27.8 |
| 381.00 | 94.4 | 5.6 |
Figure 2.
Receiver operator characteristics (ROC) curve of thenar muscle cross-sectional area (TMCSA) and thenar muscle thickness (TMT) for prediction of carpal tunnel syndrome.
Table 3.
Sensitivity and specificity of each cutoff point of the TMT.
| TMT (mm) | Sensitivity (%) | Specificity (%) |
| 5.74 | 5.6 | 100.0 |
| 6.40 | 16.7 | 94.4 |
| 6.61 | 33.3 | 94.4 |
| 7.25 | 50.0 | 88.9 |
| 7.70∗ | 61.1 | 66.7 |
| 8.23 | 72.2 | 50.0 |
| 8.52 | 83.3 | 38.9 |
| 9.28 | 94.4 | 27.8 |
| 10.45 | 100.0 | 11.1 |
4. Discussion
Our original research data demonstrate the relationship of TMCSA and CTS. CTS group had significantly lower TMCSA than control group. In our research, the most suitable cutoff value for TMCSA was 260.18 mm2, with 77.8% sensitivity, 77.8% specificity, and AUC of 0.87. And the best cutoff value of the TMT was 7.70 mm, with 61.1% sensitivity, 66.7% specificity, and AUC of 0.73. We consider this value will be the standard about both TMT and TMCSA because of there is no study about both TMT and TMCSA's optimal cutoff value. We have demonstrated that TMCSA and TMT were both significantly associated with CTS, with TMCSA being a more sensitive measurement parameter.
In clinical field, assessment of TM atrophy in CTS patients is frequently based on obtaining the past medical history and performing physical analysis and electrodiagnostic modalities.[9,13–15] However, such examination is often insufficient to identify TM atrophy and predict the clinical outcome. MRI has very important imaging modality in the detection of anatomical abnormalities of the skeletal muscles, especially changes in TM morphology due to many neurologic, inflammatory, degenerative, and traumatic conditions.[15–18] The merits of MRI for evaluation of the TM in CTS has already been demonstrated and have reported that the potential of MRI for detecting small anatomic lesions of the hand.[11,19,20] Previous researches of CTS have demonstrated that TM atrophy on MRI may mean irreversible TM damage,[2,12,15] but the prior studies did not evaluate the relationship between TM parameters and clinical studies. Thus, in the current research, we investigated to determine the correlations of TM parameters on MRI with clinical criteria. For CTS patients, TM atrophy is an evidence of severe disease based on the clinical criteria. Furthermore, there is a significant correlation between TM atrophy and the severity of median neuropathy on electrodiagnostic studies.[1] Schmid et al[21] reported that TM atrophy and impaired sensibility of the median innervation are signs of severe long-term MN compression. However, inspection of the TM is difficult and frequently fails to identify TM atrophy in CTS unlike MRI.
The TMT on MRI is an important morphological parameter for the assessment of CTS.[2,12,15] Previous studies using imaging have focused on an analysis of the TMT, and they have insisted that TMT measurements could be useful in evaluating CTS. They analyzed the TMT using 1 single measurement at the approximate “middle” of the TM.[12] However, an asymmetrical thickening or partial atrophy of the TM can occur anywhere. Therefore, measurement mistakes can frequently occur. In contrast to the TMT, the CSA of TM does not suffer from these measurement mistakes because the TMCSA measures the CSA of the TM. Thus, to assess the asymmetrical atrophy of the TM, we devised the TMCSA as a new image parameter. We assumed that the TMCSA is an important morphological parameter for atrophied TM. Therefore, we analyzed MR images to compare the TMCSA and TMT between CTS patients and healthy individuals.
In the end, our results demonstrated the association of TMCSA and CTS. CTS patients had statistically significant lower TMCSA than healthy individuals. We consider this value will be the standard about both TMT and TMCSA because there is no study about both TMT and TMCSA's optimal cutoff value. We have demonstrated that TMCSA and TMT were both significantly associated with CTS, with TMCSA being a more sensitive measurement tool. We believe that the TMCSA could be an objective, clear, precise measurement tool to assess CTS. In our current study, the TMCSA was measured from turbo spin echo transverse fat-saturated T2-weighted images.
The current research had several limitations. First of all, it included a small number of participants. Second, there might be some mistakes associated with measuring the TMCSA and TMT on MRI. Even though we tried to measure good quality of morphology in the fat saturated transverse T2-weighted wrist MR images that best showed the TM, the single MRI slice we measured the TMCSA and TMT could be not homogeneous because of differences in the cutting angle in MR images resulting from individual anatomic difference and technical issues. Third, CTS has multiple causes, including the MN flattening, transcarpal ligament, soft tissues, and flexor retinaculum.[22–24] However, we only focused on TM. Fourth, the relationship between TM atrophy and CTS was evaluated without excluding other factors that may induce TM atrophy. Fifth, it was not possible to evaluate the status of MN injury, TM function, or grip function due to the retrospective design of the study. Sixth, alternative image modalities to assess CTS has been proved to be effective at diagnosing CTS. Especially, ultrasound is a rapid, and widely available image modality.[24–27] However, the current study only evaluated the measurement of the TMCSA and TMT on MRI. In spite of these limitations, this is the very first study to report the association of TMCSA with CTS. The TMCSA is a reliable and simple measurement tool with high sensitive value to evaluate CTS.
5. Conclusion
TMCSA is an objective sensitive morphological parameter for evaluating CTS. We hope that this new adjuvant diagnostic tool will be helpful to assess patients with CTS.
Acknowledgments
The all authors thank the International St. Mary's Hospital.
Author contributions
Conceptualization: Young Uk Kim.
Data curation: Hye-Won Jeong, Sooho Lee, Young Uk Kim.
Formal analysis: Young Uk Kim.
Investigation: Hye-Won Jeong, Hyung Rae Cho, Keum Nae Kang, Jonghyuk Lee, Sooho Lee, Young Uk Kim.
Methodology: Jungmin Yi, Hye-Won Jeong, Hyung Rae Cho, Yun-Sic Bang, Young Uk Kim.
Resources: Jaeho Cho.
Supervision: Jungmin Yi, Young Uk Kim.
Validation: Young Uk Kim.
Visualization: Hye-Won Jeong, Young Uk Kim.
Writing – original draft: Hye-Won Jeong, Hyung Rae Cho, Young Uk Kim.
Writing – review & editing: Jungmin Yi, Hye-Won Jeong, Jaeho Cho, Yun-Sic Bang, Young Uk Kim.
Footnotes
Abbreviations: AUC = area under the curve, CI = confidence interval, CTS = carpal tunnel syndrome, MN = median nerve, MRI = magnetic resonance imaging, TM = thenar muscle, TMCSA = thenar muscle cross-sectional area, TMT = thenar muscle thickness.
How to cite this article: Yi J, Jeong HW, Cho HR, Kang KN, Lee J, Lee S, Cho J, Bang YS, Kim YU. Prediction of carpal tunnel syndrome using the thenar muscle cross-sectional area by magnetic resonance imaging. Medicine. 2021;100:41(e27536).
This study conforms to the Declaration of Helsinki.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data represent the mean ± standard deviation (SD) or the numbers of patients.
CTS = carpal tunnel syndrome, TMCSA = thenar muscle cross-sectional area, TMT = thenar muscle thickness.
CTS severity is presented based on the Bland scale [30] none = rating impossible due to the absence of electrodiagnostic test results.
TMCSA = thenar muscle cross-sectional area.
The best cutoff point on the receiver operator characteristics (ROC) curve.
TMT = thenar muscle thickness.
The best cutoff point on the receiver operator characteristics (ROC) curve.
References
- [1].Dilokhuttakarn T, Naito K, Kinoshita M, et al. Evaluation of thenar muscles by MRI in carpal tunnel syndrome. Exp Ther Med 2017;14:2025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Turgut MC, Saglam G, Toy S. Efficacy of extracorporeal shock wave therapy for pillar pain after open carpal tunnel release: a double-blind, randomized, sham-controlled study. Korean J Pain 2021;34:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Conforti G, Capone L, Corra S. Intradermal therapy (mesotherapy) for the treatment of acute pain in carpal tunnel syndrome: a preliminary study. Korean J Pain 2014;27:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fujita K, Kimori K, Nimura A, Okawa A, Ikuta Y. MRI analysis of carpal tunnel syndrome in hemodialysis patients versus non-hemodialysis patients: a multicenter case-control study. J Orthop Surg Res 2019;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koh SH, Kwon BC, Park C, Hwang SY, Lee JW, Kim SS. A comparison of the performance of anatomical MRI and DTI in diagnosing carpal tunnel syndrome. Eur J Radiol 2014;83:2065–73. [DOI] [PubMed] [Google Scholar]
- [6].Aoki T, Oshige T, Matsuyama A, et al. High-resolution MRI predicts steroid injection response in carpal tunnel syndrome patients. Eur Radiol 2014;24:559–65. [DOI] [PubMed] [Google Scholar]
- [7].Ng AWH, Griffith JF, Tong CSL, et al. MRI criteria for diagnosis and predicting severity of carpal tunnel syndrome. Skeletal Radiol 2020;49:397–405. [DOI] [PubMed] [Google Scholar]
- [8].Oge HK, Acu B, Gucer T, Yanik T, Savlarli S, Firat MM. Quantitative MRI analysis of idiopathic carpal tunnel syndrome. Turk Neurosurg 2012;22:763–8. [DOI] [PubMed] [Google Scholar]
- [9].Taghizadeh R, Tahir A, Stevenson S, Barnes DE, Spratt JD, Erdmann MW. The role of MRI in the diagnosis of recurrent/persistent carpal tunnel syndrome: a radiological and intra-operative correlation. J Plast Reconstr Aesthet Surg 2011;64:1250–2. [DOI] [PubMed] [Google Scholar]
- [10].Uchiyama S, Itsubo T, Nakamura K, Murakami H, Momose T, Kato H. MRI-based identification of an appropriate point of needle insertion for patients with idiopathic carpal tunnel syndrome to avoid median nerve injury. ISRN Orthop 2011;2011:528147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee S, Cho HR, Yoo JS, Kim YU. The prognostic value of median nerve thickness in diagnosing carpal tunnel syndrome using magnetic resonance imaging: a pilot study. Korean J Pain 2020;33:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Misirlioglu TO, Ozyemisci Taskiran O. Reliability of sonographic muscle thickness measurements of the thenar and hypothenar muscles. Muscle Nerve 2018;57:E14–7. [DOI] [PubMed] [Google Scholar]
- [13].Bagatur AE, Yalcinkaya M, Atca AO. Bifid median nerve causing carpal tunnel syndrome: MRI and surgical correlation. Orthopedics 2013;36:e451–6. [DOI] [PubMed] [Google Scholar]
- [14].Crnkovic T, Trkulja V, Bilic R, Gaspar D, Kolundzic R. Carpal tunnel and median nerve volume changes after tunnel release in patients with the carpal tunnel syndrome: a magnetic resonance imaging (MRI) study. Int Orthop 2016;40:981–7. [DOI] [PubMed] [Google Scholar]
- [15].Choi SC, Han S, Kwak J, Lee JY. Anaphylaxis induced by sugammadex and sugammadex-rocuronium complex - a case report. Korean J Anesthesiol 2020;73:342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Geere J, Chester R, Kale S, Jerosch-Herold C. Power grip, pinch grip, manual muscle testing or thenar atrophy - which should be assessed as a motor outcome after carpal tunnel decompression? A systematic review. BMC Musculoskelet Disord 2007;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kerasnoudis A. Ultrasound and MRI in carpal tunnel syndrome: the dilemma of simplifying the approach to a complex disease or making complex assessments of a simple problem. J Hand Surg Am 2012;37:2200–1. author reply 2201. [DOI] [PubMed] [Google Scholar]
- [18].Wang H, Ma J, Zhao L, Wang Y, Jia X. Utility of MRI diffusion tensor imaging in carpal tunnel syndrome: a meta-analysis. Med Sci Monit 2016;22:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Park JS, Won HC, Oh JY, Kim DH, Hwang SC, Yoo JI. Value of cross-sectional area of median nerve by MRI in carpal tunnel syndrome. Asian J Surg 2020;43:654–9. [DOI] [PubMed] [Google Scholar]
- [20].Mustafaoglu R, Yasaci Z, Zirek E, Griffiths MD, Ozdincler AR. The relationship between smartphone addiction and musculoskeletal pain prevalence among young population: a cross-sectional study. Korean J Pain 2021;34:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schmid AB, Elliott JM, Strudwick MW, Little M, Coppieters MW. Effect of splinting and exercise on intraneural edema of the median nerve in carpal tunnel syndrome--an MRI study to reveal therapeutic mechanisms. J Orthop Res 2012;30:1343–50. [DOI] [PubMed] [Google Scholar]
- [22].El Dassouki M, Lefaucheur JP. The correlation between muscle and nerve fiber conduction velocities in thenar muscle is lost in case of carpal tunnel syndrome. Clin Neurophysiol 2002;113:1121–4. [DOI] [PubMed] [Google Scholar]
- [23].Kim HJ, Park SH. Median nerve injuries caused by carpal tunnel injections. Korean J Pain 2014;27:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].An D, Black ND, Tierney S, Chan VWS, Niazi AU. Impact of an ultrasound-guided regional anesthesia workshop on participants’ confidence levels and clinical practice. Korean J Anesthesiol 2020;73:465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chae WS, Kim SH, Cho SH, Lee JH, Lee MS. Reduction in mechanical allodynia in complex regional pain syndrome patients with ultrasound-guided pulsed radiofrequency treatment of the superficial peroneal nerve. Korean J Pain 2016;29:266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dass RM, Kim E, Kim HK, Lee JY, Lee HJ, Rhee SJ. Alcohol neurolysis of genicular nerve for chronic knee pain. Korean J Pain 2019;32:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim SY, Cheon JH, Seo WJ, Yang GY, Choi YM, Kim KH. A pictorial review of signature patterns living in musculoskeletal ultrasonography. Korean J Pain 2016;29:217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]