Abstract
Background:
The recovery for patients after tendon repair is frequently limited by development of tendon adhesions. This scar tissue formation is dependent on immune system activation. Tacrolimus has unique properties that may contribute to the prevention of overactive scarring by inhibition of inflammatory cytokines.
Methods:
Herein, we present a case using systemic immunosuppression to prevent recurrent adhesion accumulation in a patient with a prior spaghetti wrist injury. Tacrolimus began 1 week before repeat-secondary tenolysis surgery, and it continued for 3 months postoperative. Dosing was tapered to a serum level between 5 and 8 µg/L.
Results:
The 27-year-old male patient suffered a volar wrist laceration transecting all flexor tendons and volar wrist nerves. He underwent immediate repair but had a poor outcome despite early range of motion therapy. A primary tenolysis only improved his average arc of finger motion from 72 to 95 degrees. Secondary tenolysis augmented with systemic tacrolimus improved his arc of finger motion from 95 to 202 degrees. Mechanistically, tacrolimus prevents proper function of activated T and B cells. This results in decreased proliferation, angiogenesis, and cytoskeletal organization of fibroblasts on inflammation and integrin adhesions, and it potentially explains the reduced tendon molecule adhesions seen in this patient.
Conclusions:
Tacrolimus may be effective in reducing motion, limiting tendon adhesions. The novel use of this medication resulted in the return of near-normal hand function in a patient placed on low-dose tacrolimus after primary tenolysis had failed.
INTRODUCTION
The repair of tendon lacerations has evolved in the past generation.1,2 Introduction of early therapy has improved outcomes and made acute repair standard of care.3 Despite the ubiquity of specialty guided hand therapy, it is not uncommon to develop adhesions limiting full tendon excursion. Prevention of recurrent adhesions after tenolysis is an inexact endeavor.4 Factors contributing to scar formation include tissue ischemia, infection, foreign material, exposure of denuded tendon void of paratenon, and of course, surgical intervention.5 The act of releasing scar tissue, in and of itself, recreates a new scar. Traditional approaches at improving motion after tenolysis emphasize early, next day motion. This method can produce limited results in many patients.6 Additional adjuncts have been incorporated to prevent the reaccumulation of scar tissue. These methods include corticosteroid injections and mechanical separation of the tendons by biologic and inert products, including seprafilm, tenoglide, integra, and others.7 Augmenting the immune system with anti-inflammatories to temper scar response has been used for various indications, including preventing keloid scar reformation, improving nerve regeneration, and preventing hypertrophic burn scars.8 Tacrolimus inhibits interleukin-2 gene transcription and nitric oxide synthase activation. Furthermore, it potentiates glucocorticoids’ actions, limiting inflammation and scar tissue formation.9 In conjunction with transplant immunology, we used tacrolimus to lower the T-cell response in healing after tenolysis surgery. We present our protocol and a case description of one of our patients.
CASE DESCRIPTION
A 27-year-old man presented with poor arc of motion (average 72 degrees) after repair of all nine volar finger flexor tendons, the ulnar artery, and the median and ulnar nerves following a volar wrist laceration (spaghetti wrist) from punching a glass window. The patient underwent immediate repair with compliant early, aggressive hand therapy. Initial tenolysis performed 17 weeks after injury in conjunction with hand therapy failed to significantly improve his motion (Fig. 1). This resulted in 95 degrees of average finger motion. Dense early return of adhesions prevented progress and the patient failed to improve arc of motion (98–95 degrees of average finger motion). In conjunction with transplant immunology, the patient was placed on systemic tacrolimus before and 3 months following repeat tenolysis. Near-normal range of motion was successfully achieved by 6 weeks (Fig. 2.). The average finger motion was greater than 200 degrees including independent flexor digitorum superficialis function. Eight months from his second tenolysis and nuerolysis in conjunction with tacrolimus, the patient had return of normal tendon and nerve function. Clawing had resolved and thenar opposition returned (Fig. 3). Hand therapy was instituted the day after tenolysis surgery. It consisted of controlled, aggressive active and passive range of motion by a certified hand therapist. Oral pain medications and edema garments were used to reduce discomfort and swelling during treatments.
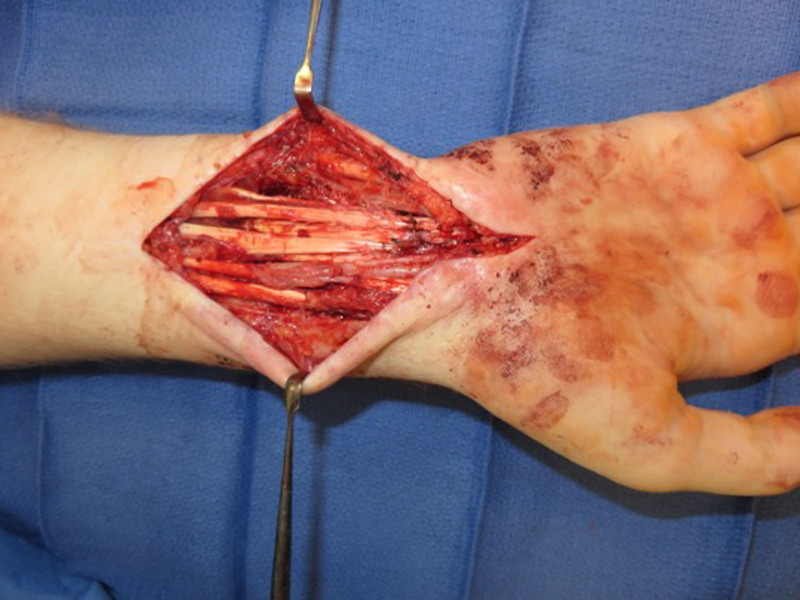
FIG. 1.
Primary tenolysis procedure releasing dense fibrotic motion limiting adhesions performed 4 months after injury. The patient quickly redeveloped thick pathologic adhesions without significant clinical benefit from scar tissue release.
FIG. 2.
The patient regained near-normal finger flexion and independent flexor digitorum superficialis function 6 weeks after secondary tenolysis treated in conjunction with systemic immunosuppressive tacrolimus therapy.
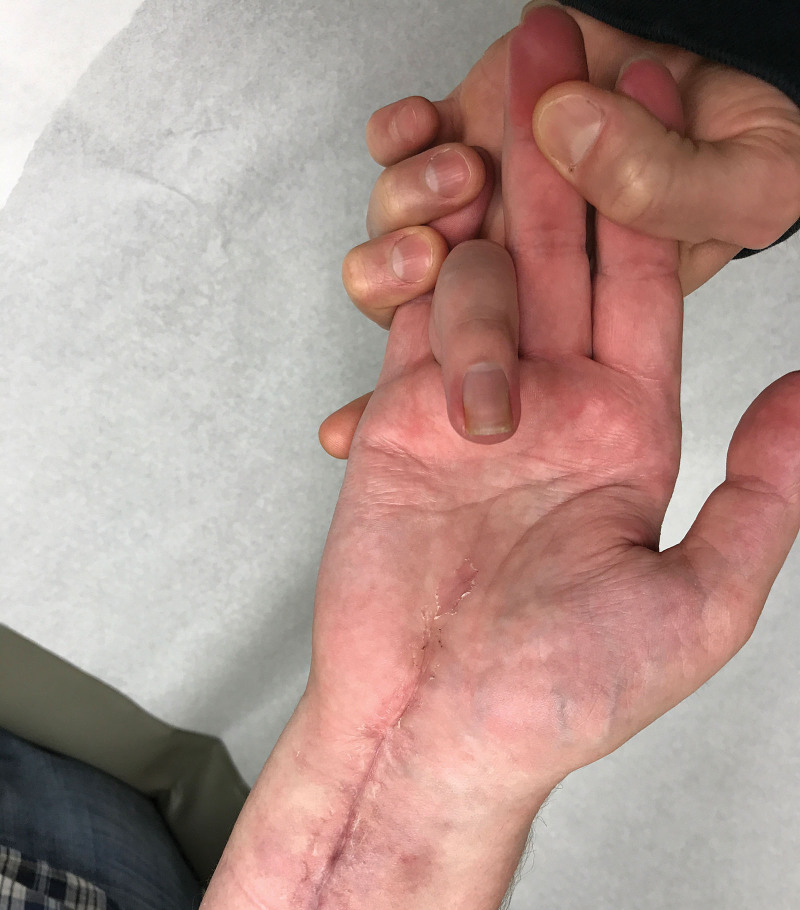
FIG. 3.
The patient regained intrinsic function, including thumb opposition and resolution of clawing by 8 months after his second surgery (13 months from injury).
Tacrolimus began 1 week before surgery and lasted 11 weeks following surgery. It was administered as an oral capsule of 1–6 mg daily, and it was tapered according to the tacrolimus trough level of 5–8 µg/L. Levels were checked every other week. If a dosing adjustment was made, the level was checked the following week. The subsequent procedure consisted of a near-identical tenolysis of dense adhesions, using sharp dissection with a 15-blade knife to separate each structure. Near-normal and independent digital range of motion returned to the hand, and it was sustained at the last follow-up at more than 1 year postoperative (Fig. 4). He was able to return to work and other daily activities. The patient was counseled pretreatment on potential side effects, specifically nephrotoxicity. The use of tacrolimus for this purpose is considered off-label; however, as it is FDA approved, IRB approval was not required.
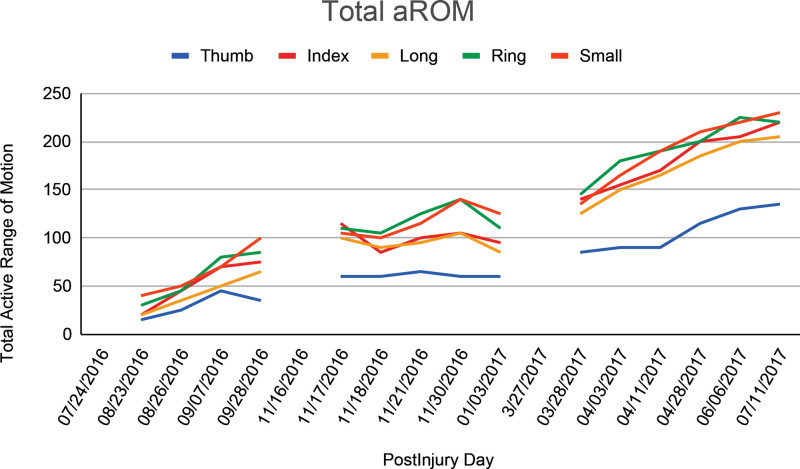
FIG. 4.
Progression of finger motion collected from hand therapy visits. The patient underwent acute repair of lacerated structures in August 2016. Postoperatively the patient plateaued in making progress with hand therapy. He underwent a standard tenolysis surgery in November 2016 and began an early aggressive therapy program without additional improvements. In April 2017, he underwent a repeat tenolysis in conjunction with tacrolimus and made dramatic improvements in finger motion. aROm, active range of motion.
Mechanistic Explanation for Tacrolimus Prevention of Excess Tendon Scarring
Mechanism for Tendon Adhesion Formation
Adhesion formation occurs from scarring between two damaged tissues when motion is restricted.10 At initial injury, granulation tissue and capillary beds fill the space between the cut tendon ends, laying the ground work for new capillary formation.11 The initial activation signal results from the secretion of lymphokines and the expression of growth factor receptors. Lymphokines bind to these receptors and generate another independent activation signal. This signal induces proliferation. Macrophages function to clear neutrophils and high levels of proinflammatory cytokines, allowing the wound to progress in the stages of healing. Wounds containing high levels of these proinflammatory cytokines, including platelet-derived growth factor, TGF-β1, epidermal growth factor, and fibroblast growth factor, are less able to control inflammation.5 In normal wound healing, TGF-β1 is suppressed as macrophages clear inflammation. This allows the breakdown of collagen, limiting scar formation, and the deposition of newly disorganized collagen follows.11,12
TGF-β1 signaling is implicated under pathological conditions of hypertrophic scarring,13,14 and it is dependent on macrophage function and quantity. At 4 weeks, fibroblast organization is present in the tendon, and there is little dense collagen present in adhesions (sparse and loosely arranged). At 5 weeks, despite an immature organization of collagen and fibers, the tendons are strong and functional. At 3 months, tendon adhesions are dense, but they are still distinguishable from the tendon. At 4 months, healed tendon and scar are indistinguishable.11
Excess collagen formation is normally prevented by fibroblastic release of matrix metalloproteinases (MMPs), which degrade collagen. However, upon injury or in pathologic scarring, fibroblasts produce IL-2, and MMP synthesis is inhibited due to the activation of TGF-β1.12,15 Excess production of TGF-β1 causes this excess scarring and, ultimately, the creation of motion-limiting tendon adhesions.
Mechanism of Action of Tacrolimus
Tacrolimus suppresses IL-2 and TGF-β1, which can prevent overaccumulation of scar tissue.9 Tacrolimus prevents the phosphatase action of calcineurin, which causes IL-2 gene suppression in T-helper lymphocytes.9 Additionally, evidence demonstrates that tacrolimus enhances MMP expression following TGF-β1 stimulation. Consequently, collagen synthesis and subsequent scar tissue formation can be attenuated.16
Tacrolimus also prevents B-cell and T-cell activation and proliferation. T cells participate in the inflammatory phase of wound healing and aid in releasing cytokines and growth factors. Fibrocytes produce collagen, and they also secrete cytokines and growth factors.17
Tacrolimus primarily targets calcineurin, a rate limiting enzyme in T-cell signal transduction. Calcineurin’s enzymatic activity is modulated by Ca2+ and calmodulin.9 Tacrolimus inhibits the transcription of proinflammatory cytokines and the proliferation of T-lymphocytes, and it suppresses the cell-mediated and humoral immune response. Specifically, it blocks lymphocyte proliferation due to its inhibition of calcium-dependent events in signal transduction that commonly follow T-lymphocyte proliferation.18
The primary documented side effect of tacrolimus is nephrotoxicity. This complication occurs through a variety of mechanisms, including vasoconstriction. This can cause acute and transient damage, leading to permanent scarring after prolonged exposure. The toxicity is dose and time-dependent, but histologically, it occurs to some extent universally at 10 years. Patients should be cautioned on use of non-steroidal anti-inflammatory drugs, diuretics, and salt depletion during use. Serum levels also need to be monitored. Additional side effects are related to alterations of the immune system, including viral infections or reactivation of herpes simplex virus. Tacrolimus can also cause hearing loss or precipitate a transient, reversible tremor. Short-term (<6 mo), low-dose use, as in this case, is associated with a low risk of complications.
DISCUSSION
Standard of care for tendon injuries includes acute repair and early controlled range of motion. This bimodality method of surgery combined with therapy has improved outcomes over the past generation.6 Despite this, many patients still require additional tenolysis surgery.2 Other investigators have described adjunctive therapies to prevent adhesions from occurring, including altering the biohealing environment.19,20 These include anti-inflammatories such as ibuprofen or aspirin.13 Tacrolimus exhibits properties specific to prevention of excess scarring, and it may be more ideal for this indication. We have had promising anecdotal experiences in the clinical environment, and we are evaluating these effects further in the laboratory. This patient represents the first reported case of using tacrolimus to augment the healing process after a tenolysis procedure. Patients considered for this treatment are evaluated by a transplant nephrologist with a discussion regarding the potential risks. Serum levels are monitored bi-weekly throughout the treatment course.
Tacrolimus might be effective in reducing tendon adhesions due to a pharmacologic mechanism that causes inhibition of certain inflammatory cytokines. This mechanism affects the cytoskeletal organization of fibroblasts, leading to inflammation and integrin adhesions. Tacrolimus also prevents the proper function of activated T cells and B cells, and this results in decreased proliferation and angiogenesis.18,22,23 Wound healing was not compromised in this patient. Tacrolimus could theoretically slow healing due to its inhibition of TNF- α in keratinocytes. However, epidermal undifferentiated cells in the basal layer heal through an independent mechanism. Tacrolimus is routinely used in patients with solid organ transplantation without significant impairment in tissue healing. These mechanisms may contribute to the reduced tendon molecule adhesions we observed in this patient, and furthermore, it can explain the mechanism of action for use of tacrolimus in preventing excessive scarring.24
Footnotes
Published online 15 October 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Kim HM, Nelson G, Thomopoulos S, et al. Technical and biological modifications for enhanced flexor tendon repair. J Hand Surg Am. 2010;35:1031–7; quiz 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lister GD, Kleinert HE, Kutz JE, et al. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg Am. 1977;2:441–451. [DOI] [PubMed] [Google Scholar]
- 3.Gelberman RH, Khabie V, Cahill CJ. The revascularization of healing flexor tendons in the digital sheath. A vascular injection study in dogs. J Bone Joint Surg Am. 1991;73:868–881. [PubMed] [Google Scholar]
- 4.Whitaker JH, Strickland JW, Ellis RK. The role of flexor tenolysis in the palm and digits. J Hand Surg Am. 1977;2:462–470. [DOI] [PubMed] [Google Scholar]
- 5.Hesketh M, Sahin KB, West ZE, et al. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18:E1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trumble TE, Vedder NB, Seiler JG, III, et al. Zone-II flexor tendon repair: a randomized prospective trial of active place-and-hold therapy compared with passive motion therapy. J Bone Joint Surg Am. 2010;92:1381–1389. [DOI] [PubMed] [Google Scholar]
- 7.Tang JB, Zhou X, Pan ZJ, et al. Strong digital flexor tendon repair, extension-flexion test, and early active flexion: Experience in 300 tendons. Hand Clin. 2017;33:455–463. [DOI] [PubMed] [Google Scholar]
- 8.Wu CS, Wu PH, Fang AH, et al. FK506 inhibits the enhancing effects of transforming growth factor (TGF)-β1 on collagen expression and TGF-β/Smad signalling in keloid fibroblasts: implication for new therapeutic approach. Br J Dermatol. 2012;167:532–541. [DOI] [PubMed] [Google Scholar]
- 9.Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17:584–591. [DOI] [PubMed] [Google Scholar]
- 10.Wong JK, Lui YH, Kapacee Z, et al. The cellular biology of flexor tendon adhesion formation: an old problem in a new paradigm. Am J Pathol. 2009;175:1938–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.POTENZA AD. Tendon healing within the flexor digital sheath in the dog. J Bone Joint Surg Am. 1962;44-A:49–64. [PubMed] [Google Scholar]
- 12.Pakyari M, Farrokhi A, Maharlooei MK, et al. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle). 2013;2:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galatz LM, Gerstenfeld L, Heber-Katz E, et al. Tendon regeneration and scar formation: The concept of scarless healing. J Orthop Res. 2015;33:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Chen J, Zhang D, et al. MicroRNA expression profiles of scar and normal tissue from patients with posterior urethral stricture caused by pelvic fracture urethral distraction defects. Int J Mol Med. 2018;41:2733–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doersch KM, DelloStritto DJ, Newell-Rogers MK. The contribution of interleukin-2 to effective wound healing. Exp Biol Med (Maywood). 2017;242:384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan CC, Fang AH, Wu PH, et al. Tacrolimus abrogates TGF-β1-induced type I collagen production in normal human fibroblasts through suppressing p38MAPK signalling pathway: implications on treatment of chronic atopic dermatitis lesions. J Eur Acad Dermatol Venereol. 2014;28:204–215. [DOI] [PubMed] [Google Scholar]
- 17.Marshall CD, Hu MS, Leavitt T, et al. Cutaneous scarring: basic science, current treatments, and future directions. Adv Wound Care (New Rochelle). 2018;7:29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooks MA. Tacrolimus, a new immunosuppressant–a review of the literature. Ann Pharmacother. 1994;28:501–511. [DOI] [PubMed] [Google Scholar]
- 19.Seiler JG, III, Gelberman RH, Williams CS, et al. Autogenous flexor-tendon grafts. A biomechanical and morphological study in dogs. J Bone Joint Surg Am. 1993;75:1004–1014. [DOI] [PubMed] [Google Scholar]
- 20.Manske PR, Lesker PA. Nutrient pathways of flexor tendons in primates. J Hand Surg Am. 1982;7:436–444. [DOI] [PubMed] [Google Scholar]
- 21.Chitturi RT, Balasubramaniam AM, Parameswar RA, et al. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J Int Oral Health. 2015;7:75–80. [PMC free article] [PubMed] [Google Scholar]
- 22.Desmoulière A, Gabbiani G. Modulation of fibroblastic cytoskeletal features during pathological situations: the role of extracellular matrix and cytokines. Cell Motil Cytoskeleton. 1994;29:195–203. [DOI] [PubMed] [Google Scholar]
- 23.Sakuma S, Higashi Y, Sato N, et al. Tacrolimus suppressed the production of cytokines involved in atopic dermatitis by direct stimulation of human PBMC system. (Comparison with steroids). Int Immunopharmacol. 2001;1:1219–1226. [DOI] [PubMed] [Google Scholar]
- 24.Thomopoulos S, Parks WC, Rifkin DB, et al. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33:832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]