Abstract
Background:
Guillain-Barre syndrome (GBS) is a disease with the features of acuteness, paralysis, inflammation, and in peripheral nerves. There are many current treatment options with varying efficacy, and to assess their effectiveness, we performed a network meta-analysis (NMA). The study protocol was registered at PROSPERO (CRD: 42019119178). Posted history: this manuscript was previously posted to medRxiv: doi: https://doi.org/10.1101/2020.06.03.20121780.
Methods:
The literature search database includes Web of Science, PubMed, Embase, and the Cochrane library that meets the requirements. We performed the NMA using controlled trials with 2 kinds of outcomes. We used the gemtc R package to perform the NMA to evaluate different GBS treatments’ relative results. The consistency of direct and indirect evidence was also assessed by R software with gemtc package.
Results:
This NMA study included a total of 2474 subjects from 28 trials with 15 kinds of therapies. No improvement was observed in methylprednisolone and prednisolone compared with placebo. Conversely, plasma exchange (PE) and intravenous immunoglobulin (IVIg) were more effective than placebo. There was no significant difference between different doses and courses of PE and IVIg. For combination treatment, such as IVIg+eculizumab, immunoadsorption followed by IVIg and PE followed by IVIg, they didn’t show significant advantages than IVIg and PE in NMA. On the consistency examination between direct and indirect evidence, there was no apparent heterogeneity between them. Funnel plots indicated there was little possibility of publication bias in this study.
Conclusion:
PE or IVIg has significant efficacy for GBS patients. The effects of several kinds of therapies should be further explored. Corticosteroids have no considerable impact on GBS.
Keywords: efficacy, Guillain-Barre syndrome, network meta-analysis
1. Introduction
Guillain-Barre syndrome (GBS) is a disease with the features of acuteness, paralysis, inflammation, and in peripheral nerves. GBS often has monophasic disease course, symmetrical limb weakness, and areflexia.[1,2] GBS incidence rates vary between 0.66 and 1.79 cases per 100,000 persons in the general population.[2,3] It is an autoimmune disease caused by an immune reaction against an infectious agent that shares an antigen with nerves.[4] This pathological mechanism has led to the use of immune therapies for GBS. The standard treatments for GBS include plasma exchange (PE),[5] intravenous immunoglobulin (IVIg).[6] Other effective GBS therapies include corticosteroids,[7] unconventional treatments, and non-routine doses of IVIg and PE.[8] Many clinical trials and meta-analyses have investigated their therapeutic effects on GBS.[5–8] Hughes et al[6] conducted a meta-analysis on intravenous immunoglobulin and found that IVIg started within 2 weeks from the onset of GBS hastens recovery as much as PE. In another meta-analysis on corticosteroids, Hughes et al[7] found that corticosteroids given alone could not significantly hasten recovery from GBS or affect the long-term outcome. Hughes et al[8] found no significant effect of interferon beta-1α, brain-derived neurotrophic factor, and tripterygium polyglycoside for GBS in a meta-analysis study. To assess all kinds of therapeutic effects integrally and compare the efficacy of therapies on GBS from previous trials, we conducted a network meta-analysis (NMA) with 2 kinds of endpoints (disability scale grade change after 4 weeks, rates of improvement by ≥1 grades of disability scale after 4 weeks). NMA is a statistical technique that allows the comparison of multiple treatments in the same meta-analysis simultaneously. It can be performed under a frequentist or a Bayesian framework.[9] We carried on R software (version:3.6.1) with a gemtc package to calculate the therapeutic effects and rank probabilities between different therapies under a Bayesian framework. The protocol has been registered at PROSPERO (CRD: 42019119178). Posted history: This manuscript was previously posted to medRxiv: doi: https://doi.org/10.1101/2020.06.03.20121780.
2. Material and methods
2.1. Search strategy
We searched Web of Science, PubMed, Embase, and the Cochrane library for related articles concerning the therapeutic effects of GBS therapies. All kinds of therapies were enrolled, including PE,[10] IVIg with different doses and courses,[11–14] corticosteroids,[15] cerebrospinal fluid (CSF) filtration,[16] the combination of more than one therapy, etc. Articles published between January 1, 1980, and January 1, 2019, were retrieved in the search. The following Mesh terms and their synonyms and abbreviations were used to find relevant studies: “Guillain-Barre syndrome,” “polyradiculoneuropathy,” “polyneuropathies,” “methylprednisolone,” “prednisolone (Pred),” “IVIg or intravenous immunoglobin,” “plasma exchange,” etc. Two authors independently screened titles and abstracts of retrieved articles to evaluate their qualifications according to the inclusion criteria. The reference list of enrolled articles was also reviewed manually to improve the integrity of this study. This analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[17] All comments were based on previously published studies; thus, no ethical approval and patient consent are required.
2.2. Evaluated outcomes and inclusion criteria
In this study, disability scale grade change after 4 weeks (outcome ①) and rates of improvement by ≥1 grades of disability scale after 4 weeks (outcome ②) were applied to evaluate patients. There’re several versions of the disability scale, and all versions are approximately composed of 7 layers: 0. Healthy. 1. Minor symptoms or signs of neuropathy but capable of manual work. 2. Able to walk without the support of a stick but incapable of manual labor. 3. Able to walk with a stick, appliance, or support. 4. Confined to bed or chair bound. 5. Requiring assisted ventilation. 6. Dead. We included all randomized controlled trials (RCTs) and unrandomized controlled trials, and children or adults with GBS of all degrees of severity. According to internationally accepted diagnostic criteria,[4] we defined GBS as acute polyradiculoneuropathy, causing progressive weakness of ≥2 limbs. The onset phase is not >4 weeks, reduced or absent tendon reflexes, and lacking alternative causes.
2.3. Data extraction
Two authors extracted relevant data from eligible articles independently. Extracted information was as follows: the last name of the first author, year of publication, origin country, type of clinical trial, number of subjects, treatments, and outcomes. A 3rd author would resolve discrepancies after discussion. Disability grade change after 4 weeks (outcome ①) was considered as the primary outcome in this study.
2.4. Statistical analysis
We used NMA to evaluate the efficacy of different treatments on GBS. Bayesian NMA was performed in R software with the getmc[18] package to compare direct and indirect therapies. Moreover, the forest graph, ranking probability graph, and heterogeneity test between direct and indirect evidence would also be painted or assessed by R software with the getmc package. Besides the getmc package, we also adopted netmeta[19] packages to creating a funnel plot. We set the parameter of Bayes iterations as n.adapt = 5000, n.iter = 20,000 to ensure the convergence.
3. Results
3.1. Study characteristics
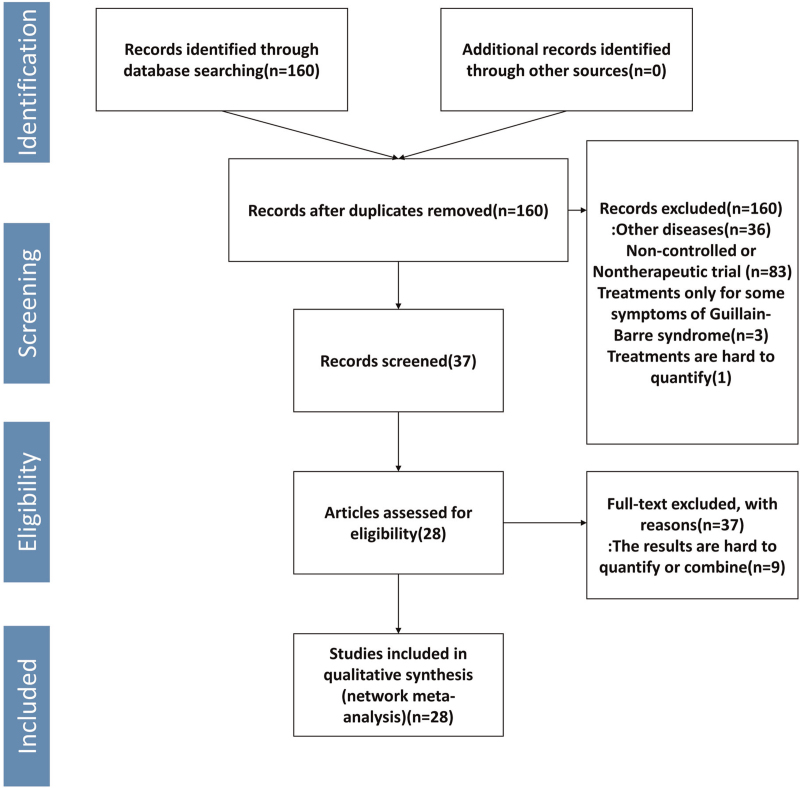
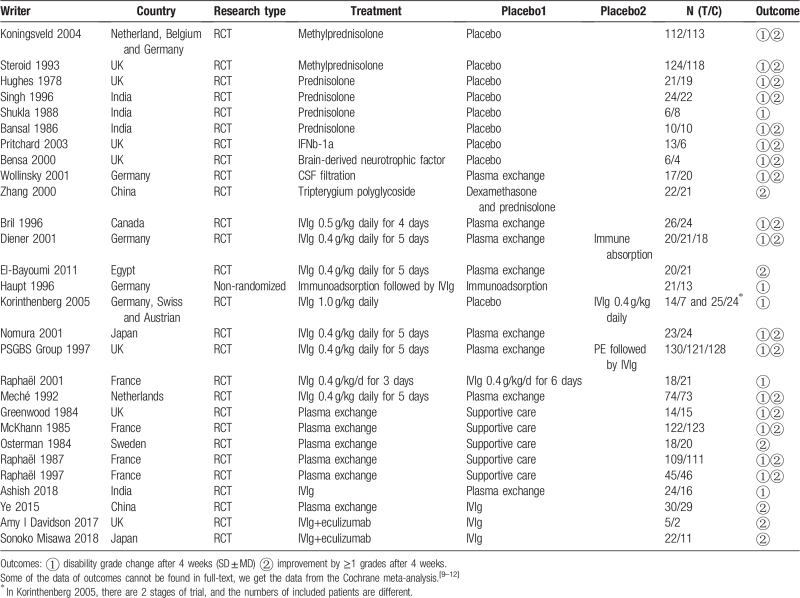
A total of 2474 subjects from 28 trials were involved in investigating the efficacy of 15 kinds of therapies for GBS. The filtration process was shown in Fig. 1, and the characteristics of included researches were shown in Table 1.[10–16,20–39] Therapies involved in this meta-analysis were plasma exchange (PE), intravenous immunoglobulin (IVIg), methylprednisolone (MTP), Pred, immunoadsorption plasmapheresis, interferon beta-1α (IFNb-1α), brain-derived neurotrophic factor (BDNF), CSF filtration, tripterygium wilfordii polyglycoside (TWP), PE followed by IVIg (PE+IVIg), immunoadsorption followed by IVIg, IVIg 0.4 g/kg/d for 3 days, IVIg 1 g/kg daily (1 g/kg for 2 days), half-course of treatment of PE, and IVIg+eculizumab.
Figure 1.
Flow chart of the research for eligible studies.
Table 1.
Characteristics of enrolled trials.
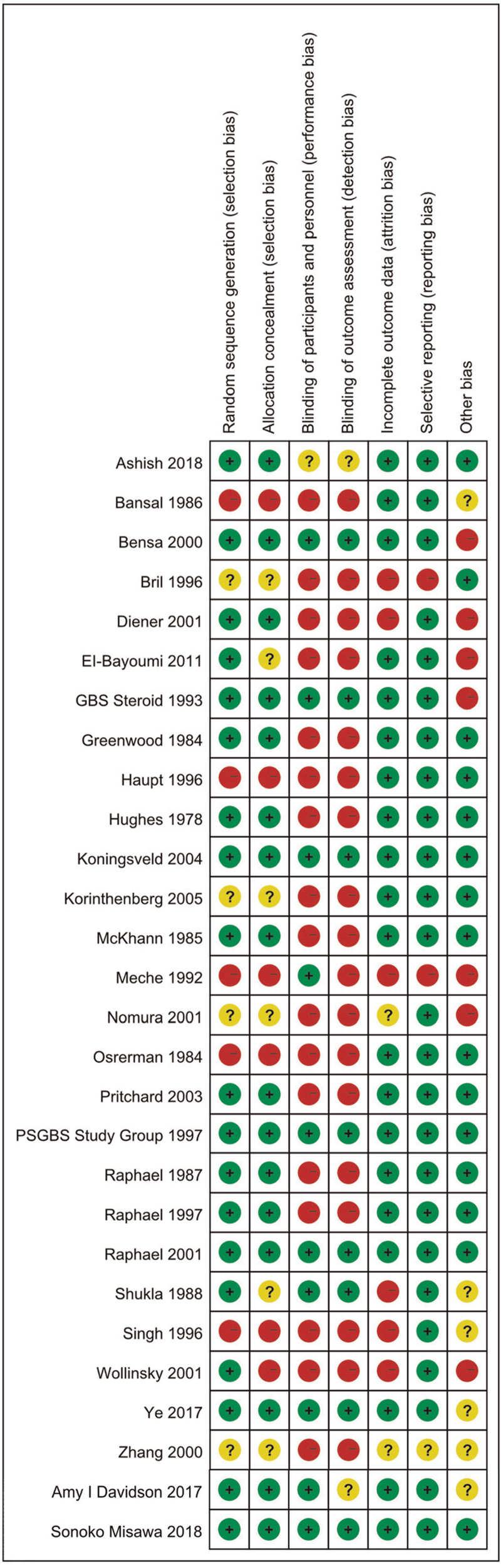
3.2. Quality assessment
Of 27 trials (total 28 trials) mentioned randomization, 19 RCTs (68%) used a specific random sequence generation method. Most of these RCTs (16 RCTs) mentioned allocation concealment, and only 10 RCTs (36%) used the way of blinding. Most trials (20 RCTs) did not select outcome reporting or had incomplete outcome data. Besides, most of the included trials (27 RCTs) described the inclusion criteria or diagnosis criteria. The risk of biases graph was shown in Fig. 2.
Figure 2.
Risk of biases graph. Note: Red: high risk of bias. Green: low risk of bias. Yellow: uncertain risk of bias.
3.3. Network meta-analysis results
3.3.1. NMA results
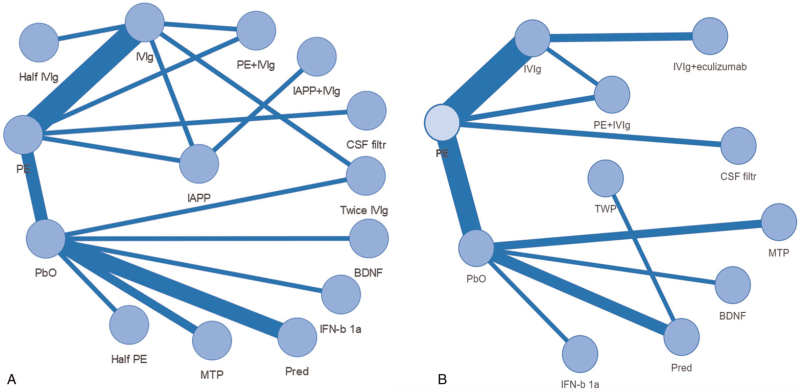
We depicted a network graph of 15 kinds of therapies for GBS. The graph was made by R 3.6.1 software with a visNetwork package[40] (Fig. 3). Of 22 studies, including 13 kinds of therapies, disability grade changes after 4 weeks (outcome ①) as the outcome measure. Meanwhile, 23 studies, including 10 kinds of therapies, all used the improvement rate of grade 1 or more after 4 weeks (result ②) as the outcome indicator.
Figure 3.
Network graph for therapies of GBS. Note: Each endpoint represented a kind of therapy. Each line connecting with 2 endpoints represented there were one or more comparisons between 2 therapies. Line thicknesses correspond to the number of trials used for comparisons. (A) Trials with outcome ① of disability grade change after 4 weeks. (B) Trials with outcome ② of the rates of improvement by ≥1 grades after 4 weeks. BDNF = brain-derived neurotrophic factor, CSF filter = cerebrospinal fluid filtration, Half IVIg = 0.4 g/kg for 3 days, Half PE = half course of PE, IFNb-1α = interferon beta-1α, IVIg = intravenous immunoglobulin 0.4–0.5 g/kg daily for 4–6 days, IAPP+IVIg = immunoadsorption followed by IVIg, IAPP = immunoadsorption plasmapheresis, IVIg+eculizumab = intravenous immunoglobulin 0.4 g/kg daily for 5 days+eculizumab 900 mg once a week for 4 weeks, MTP = methylprednisolone, PbO = placebo, Pred = prednisolone, PE = plasma exchange, PE+IVIg = PE followed by IVIg, twice IVIg = 1 g/kg for 2 days, TWP = tripterygium wilfordii polyglycoside.
3.3.2. Forest plots
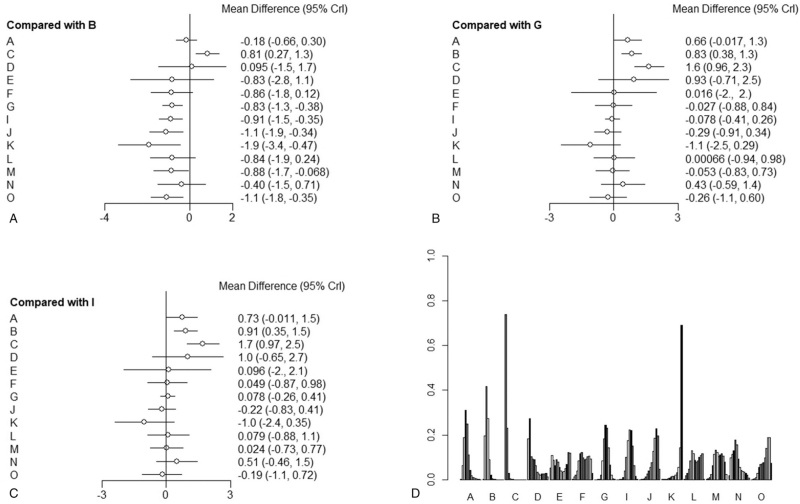
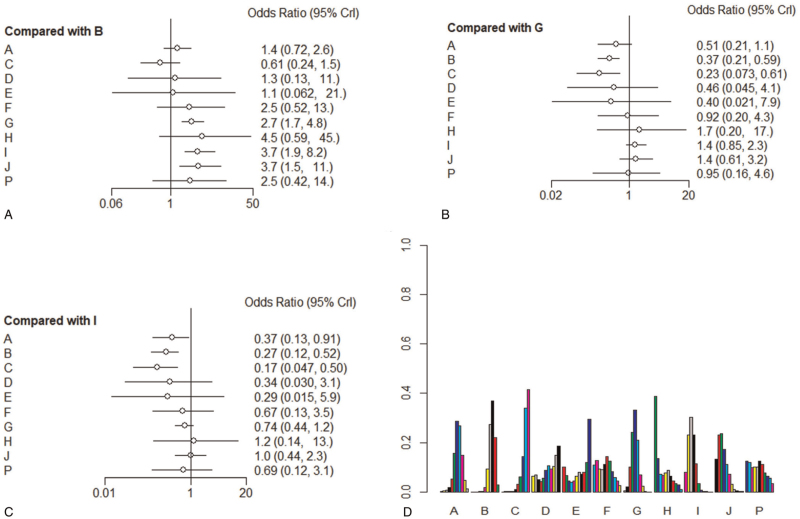
The forest plots showed the results of NMA on different therapies. Their calculated ranking probabilities were also shown in Figs. 4 and 5. First, results of forest plots on efficacy of treatments for GBS (Figs. 4A and 5A) suggested that PE (4–5 times of PE), IVIg (IVIg 0.4–0.5 g/kg daily for 4–5 days), PE+IVIg, immunoadsorption followed by IVIg, IVIg 1 g/kg daily for 2 days, 2 times of PE were significantly effective in treating GBS (compared with placebo [PbO], outcome ①: PE MD = –0.83, 95% Crl [–1.3, –0.38]; IVIg MD = –0.91, 95% Crl [–1.5, –0.35]; PE+IVIg MD = –1.1, 95% Crl [–1.9, –0.34], immunoadsorption followed by IVIg MD = –1.9, 95% Crl [–3.4, –0.47], IVIg 1 g/kg daily MD = –0.88, 95% Crl [–1.7, –0.068], 2 times of PEs MD = –1.1, 95% Crl [–1.8, –0.35]; outcome ②: PE OR = 2.7, 95% Crl [1.7,4.8], IVIg OR = 3.7, 95% Crl [1.9, 8.2], PE+IVIg OR = 3.7, 95% Crl [1.5, 11.0]).
Figure 4.
Forest graph and ranking probability graph on outcome ①. A: Methylprednisolone, B: placebo, C: prednisolone, D: IFNb-1α, E: brain-derived neurotrophic factor, F: CSF filtration, G: PE, I: IVIg 0.4–0.5 g/kg daily for 4–5 days, J: PE followed by IVIg, K: immunoadsorption followed by IVIg, L: immunoadsorption, M: IVIg 1 g/kg daily for 2 days, N: IVIg 0.4 g/kg/d for 3 days, O: Half-times of PE. (A–C): Forest plots (references were placebo, PE, and IVIg, respectively), indicate relative effect results compared with placebo group, PE, and IVIg. (D): Ranking probability graph of outcome ①. The heights of bars represent the probability to be a certain rank; for every therapy, bars from left to right represent ranks from worst to best.
Figure 5.
Forest graph and ranking probability graph on outcome ②: A–G, I–J: Same with Fig. 4. H: Tripterygium wilfordii polyglycoside, P: IVIg+eculizumab. (A–C): Forest plots (References were Placebo, PE, and IVIg, respectively), indicate relative effect results compared with placebo group, PE, and IVIg. (D): Ranking probability graph of outcome ②. The heights of bars represent the probability to be a certain rank; for every therapy, bars from left to right represent ranks from best to worst.
Second, the results also showed that although PE and IVIg were available for GBS, all kinds of corticosteroids had no significant efficacy for GBS (outcome ①: MTP MD = –0.18, 95% Crl [–0.66, 0.30]; Pred MD = 0.81, 95% Crl [0.27, 1.3]; outcome ②: MTP OR = 1.4, 95% Crl [0.72, 2.6], Pred OR = 0.61, 95% Crl [0.24, 1.5]).
Then, we transferred the base reference of forest graph to PE or IVIg (Figs. 4B, C and 5B, C), we could find no other therapies being more effective with significant difference than PE IVIg. We compared different doses of PE and IVIg (IVIg 0.4–0.5 g/kg daily for 4–5 days, 4–5 times of PE, IVIg 1 g/kg daily for 2 days, IVIg 0.4 g/kg/d for 3 days, 2 times of PE) and found no significant difference between them.
Furthermore, for other kinds of therapies, such as IFNb-1a, brain-derived neurotrophic factor, CSF filtration, TWP, IVIg +eculizumab (900 mg once a week), they had no significant-difference efficacy compared with PbO (outcome ①: IFNb-1α MD = 0.095, 95% Crl [–1.5, 1.7]; BDNF MD = –0.83, 95% Crl [–2.8, 1.1]; CSF filtration MD = –0.86, 95% Crl [–1.8, 0.12]; IVIg 0.4 g/kg/d for 3 days MD = –0.4, 95% Crl [–1.5, 0.71]; outcome ②: IFNb-1α OR = 1.1, 95% Crl [0.13, 11.0]; BDNF OR = 1.1, 95% Crl [0.056, 19.0]; CSF filtration OR = 2.5, 95% Crl [0.49,12.0]; TWP OR = 4.6, 95% Crl [0.6, 47.0]; IVIg+eculizumab OR = 2.5, 95% Crl [0.42, 14.0]).
Finally, regarding to the effect for GBS among PE, IVIg, and corticosteroid, the 3 most conventional treatments, IVIg was the most effective one (compared with PE: MD = 0.073, 95% Crl [–0.26, 0.41]; methylprednisolone MD = 0.72, 95% Crl [–0.01, 1.5]; Pred MD = 1.7, 95% Crl [0.96, 2.5]), but there was no significant difference between PE and IVIg. The effect of the 2 hormones were worse than that of PE and IVIg (outcome ①: MTP vs PE MD = 0.66, 95% Crl [–0.017, 1.30]; Pred vs PE MD = 1.6, 95% Crl [0.96,2.3]; MTP vs IVIg MD = 0.73, 95% Crl [–0.011, 1.5]; Pred vs IVIg MD = 1.7, 95% Crl [0.97, 2.5]; PE vs IVIg MD = 0.078, 95% Crl [–0.26, 0.41]; outcome ②: MTP vs PE OR = 0.51, 95% Crl [0.22, 1.1]; Pred vs PE OR = 0.22, 95% Crl [0.075, 0.62]; MTP vs IVIg OR = 0.38, 95% Crl [0.13, 0.92]; Pred vs IVIg OR = 0.17, 95% Crl [0.048,0.50]; PE vs IVIg OR = 0.74, 95% Crl [0.44, 1.2]).
3.3.3. Ranking probability
We generated a clustered ranking plot to present NMA results visually. The ranking plot was to evaluate probabilities to be the best therapy in all medications. Results were presented in Figs. 4D and 5D. As suggested by ranking probabilities of outcome ①, immunoadsorption+IVIg had the most significant possibility of being the best treatment. PE+IVIg also had strong possibility to be the best treatment. For outcome ②, IVIg 0.4 to 0.5 g/kg daily for 4 to 5 days, PE+IVIg had superior results. For the least probability in the ranking plot, in outcome ① was prednisone, IFNb-1α followed; in outcome ② was prednisone, IFNb-1α and BDNF followed.
3.3.4. Consistency analysis and heterogeneity test
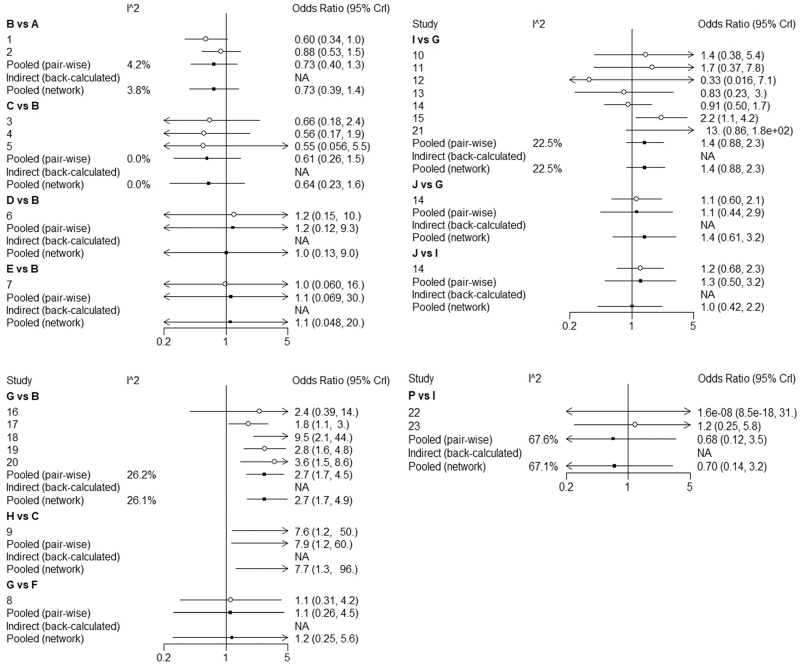
We used I2 to test the consistency of direct-evidence results (Figs. 6 and 7). Most of the I2 was under 50%, which means the heterogeneity of direct evidence was in a low range, and the direct evidence was reliable. We used a fixed-effect model for this NMA.
Figure 6.
Consistency analysis of outcome ① A–G, I–J: Same with Fig. 4.
Figure 7.
Consistency analysis of outcome ② A–J, P: Same with Fig. 5.
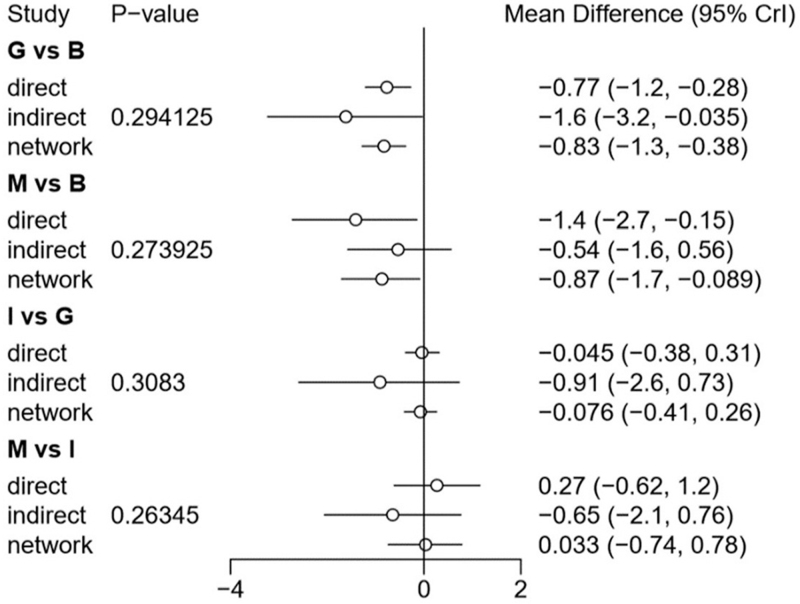
We used the node-splitting method[41] and its Bayesian P-value to assess our results’ inconsistency between direct and indirect results (Fig. 8). We found that there was some heterogeneity between the 4 groups in outcome ① (G vs B, M vs B, I vs G, M vs I, B: PbO, G: PE, I: IVIg, M: IVIg 1 g/kg daily for 2 days), while there was no apparent heterogeneity in outcome ②. The results showed P > .1, indicated that the heterogeneity was in an acceptable range. Based on the above results, we believed in there was no significant heterogeneity in the network meta-analysis. Finally, we could find that most of the comparison results in this study are obtained through indirect comparison. Since the indirect results of the Meta were calculated based on the Bayesian algorithm, they still needed to be verified by a large number of direct comparisons (from RCTs).
Figure 8.
Heterogeneity of direct and indirect evidence of outcome ① A–G, I–J: Same with Fig. 4.
3.3.5. Publication bias
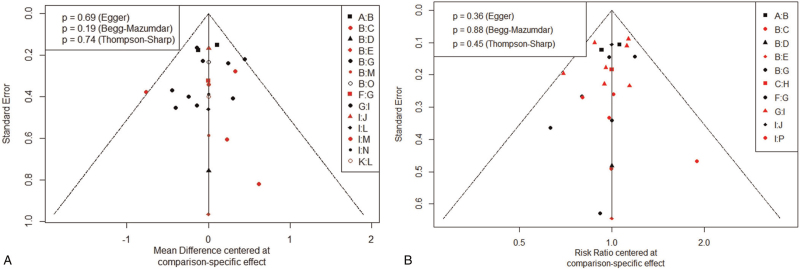
Funnel plots were used to measure the publication bias. The funnel plot of outcome ① and outcome ② showed potential publication bias of the included RCTs (Fig. 9). It can see from the funnel plot that almost all the studies fall within the Funnel, and the 2 sides of the funnel were symmetrical, so there was little publication bias in this study.
Figure 9.
Funnel plots for outcome ① and outcome ②.
4. Discussion
GBS is an acute inflammatory immune-mediated polyradiculoneuropathy that could cause progressive weakness in limbs, even resulting in difficult breathing.[42] It has practical significance to carry out NMA to compare the efficacy between different GBS therapies, which could also give some references to clinical work.
PE and IVIg have been widely used in clinical practice to alleviate GBS[5,6] and recommended by the European guidelines.[43,44] However, some evidence indicated that corticosteroids had no significant efficacy for GBS.[7] In our studies, PE and IVIg as GBS therapies were equal or better compared with other therapies. PE and IVIg are conventional treatments for GBS, and our NMA helps to confirm their effectiveness in clinical practice.[31–33,45,46] Immunoadsorption+IVIg had the highest possibility of 1st rank for the outcome ①, which might infer it had the best efficacy on GBS treatment. However, the number of patients included in the clinical trial using immunoadsorption+IVIg was only 21, and the sample size was insufficient.
Moreover, the comparisons between immunoadsorption+IVIg and PE, immunoadsorption+IVIg and IVIg were indirect results, so the efficacy of immunoadsorption+IVIg needs large-sample research verification. Corticosteroids were not sufficient, as reported before.[21,24] IVIg+eculizumab was a relatively new option for GBS, but it didn’t show advantages than IVIg and PE in NMA. The sample size of IVIg+eculizumab was also insufficient.
The results of outcome ① and outcome ② were almost the same, which enhanced the reliability of the study. On the other hand, this study involved many therapies, only one of the included studies reporting the efficacy, so most comparison results were obtained through indirect comparison. Therefore, whether the conclusion is accurate or not, more direct comparison (randomized controlled trials) and in-depth study are still needed.
For the immunoadsorption, in 2 articles,[28,30] we found it showed equally favorable GBS results compared with PE or IVIg. Treatment-related adverse reactions were fewer in the immunoglobulin group compared with other groups.[28] Immunoadsorption has also been used successfully to treat myasthenia gravis,[47,48] which is also an autoimmunity disease.
Combination therapies have been somewhat controversial for GBS.[43,44] The result of combined treatment of PE and IVIg, conducted by PSGBS Group 1997, had no significant difference compared with PE or IVIg only. In this NMA study, we found PE+IVIg got a higher rank than IVIg, but the advantage was slight. As for immunoadsorption followed by IVIg, we found it reached the highest rank in outcome ①, although there were no significant differences between it and PE or IVIg. Osterman et al[34] showed that immunoadsorption combined with IVIg have rapid recovery than immunoadsorption or PE alone. Consequently, although dual therapy for GBS didn’t have been recommended in reviews or guidelines,[43,44] we thought the effect of combination therapy, especially for PE+IVIg and immunoadsorption followed by IVIg, needed further research.
We adopted the fixed effect model in the meta-analysis because most of I2 was <50% indirect evidence. In terms of the heterogeneity analysis of indirect and direct evidence through the node-splitting method (Fig. 8), we can see P > .1, which indicated heterogeneity was at a low level. As the heterogeneity is under control, the conclusion obtained through indirect comparison in this study was believable.
Previous clinical studies have confirmed the excellent efficacy of PE and IVIg,[42] and this meta-analysis further confirmed and elucidated the usefulness of them. We demonstrated the effectiveness of these 2 approaches from a more extensive range of treatment options. According to previous studies, the results also showed the limitation of corticosteroids in treating GBS.[42] Compared with a single RCT trial, our NMA results indicated a more integral conclusion.
This analysis's primary limitation was the limited sample size of involved therapies and subjects, especially for some treatments with good results, such as combination treatments of IVIg+PE and IVIg+immumoadsorption. Also, there were some different age groups in the included researches. In Korinthenberg et al,[11] the primary patients of GBS were children; others were adults. Simultaneously, since most GBS therapies are non-drug treatments, the implementation of blind methods in GBS research was not easy. So, the number of blind ways (36% using blind) used in the included literature is relatively small. Besides, the clinical research we included often set inclusion criteria with GBS diagnose criteria and did not specify which subtype, so we didn’t assess efficacy of the treatments with GBS variants. Lastly, the inclusion criteria and disability scale were not identical. It also suggested that we establish more unified GBS diagnostic criteria and a more unified evaluation of the curative effect.
5. Conclusion
In conclusion, in our meta-analysis, we observed that PE and IVIg had a significant efficacy for GBS patients. Different doses of IVIg or PE, a combination of PE and IVIg, had no significant difference with PE (4–5 times of PE) or IVIg (IVIg 0.4–0.5 g/kg daily for 4–5 days) alone. The effects of IVIg+PE, IVIg+immunoadsorption, IVIg+eculizumab need further exploration. Corticosteroids have no significant impact on GBS. It also requires more extensive clinical trials on the efficacy of therapies with GBS for further investigation.
Acknowledgments
Thanks to Monkey Brother in Zhihu, who shared his NMA experience. Thanks to Richard A.C. Hughes and his team's meta-analysis results published in Cochrane Library. They are also grateful to Xueqin Hu for English polish, who worked as a visiting scholar in Harvard Medical School for 2 years.
Author contributions
Jingfeng Lin and Qiang Gao had fully assessed to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the meta-analysis. Jingfeng Lin, Kang Xiao, and Qiang Gao designed the study and determined the content. Jingfeng Lin determined the retrieval scheme, collected the data, performed the statistical analysis, and drafted the manuscript. Qiang Gao supervised the study, doublechecked the statistical analysis. Danfeng Tian and Wenyue Hu screened articles. Zhenyun Han revised the manuscript. All authors have read and approved the manuscript.
Conceptualization: Jingfeng Lin, Qiang Gao, Kang Xiao, Zhenyun Han.
Data curation: Jingfeng Lin, Kang Xiao, Wenyue Hu, Zhenyun Han.
Formal analysis: Jingfeng Lin, Qiang Gao, Kang Xiao, Zhenyun Han.
Methodology: Jingfeng Lin, Wenyue Hu.
Software: Jingfeng Lin, Wenyue Hu.
Visualization: Danfeng Tian.
Writing – original draft: Jingfeng Lin, Qiang Gao.
Writing – review & editing: Jingfeng Lin, Qiang Gao, Danfeng Tian, Zhenyun Han.
Footnotes
Abbreviations: BDNF = brain-derived neurotrophic factor, CSF filter = cerebrospinal fluid filtration, GBS = Guillain-Barre Syndrome, IVIg = intravenous immunoglobulin 0.4–0.5 g/kg daily for 4–6 days, MTP = methylprednisolone, NMA = network meta-analysis, PbO = placebo, Pred = prednisolone, PE = plasma exchange, PE+IVIg = PE followed by IVIg, RCT = randomized controlled trail, TWP = tripterygium wilfordii polyglycoside.
How to cite this article: Lin J, Gao Q, Xiao K, Tian D, Hu W, Han Z. Efficacy of therapies in the treatment of Guillain-Barre syndrome: A network meta-analysis. Medicine. 2021;100:41(e27351).
JL and QG contributed equally to this work.
This work was funded by National Natural Science Foundation of China (No.81673910). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Sciacca G, Nicoletti A, Fermo SL, et al. Looks can be deceiving: three cases of neurological diseases mimicking Guillain–Barrè syndrome. Neurol Sci 2016;37:541–5. [DOI] [PubMed] [Google Scholar]
- [2].Chiò A, Cocito D, Leone M, et al. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology 2003;60:1146–50. [DOI] [PubMed] [Google Scholar]
- [3].Rees JH, Thompson RD, Smeeton NC, et al. Epidemiological study of Guillain-Barre syndrome in south east England. J Neurol Neurosurg Psychiatry 1998;64:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Paterson BJ, Durrheim DN, Siemieniuk RA, et al. Guillain–Barré syndrome. N Engl J Med 2012;367:973–4. [DOI] [PubMed] [Google Scholar]
- [5].Raphaël JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev 2012;CD001798.doi: 10.1002/14651858.CD001798.pub2. [DOI] [PubMed] [Google Scholar]
- [6].Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev 2014;2014:CD002063. [DOI] [PubMed] [Google Scholar]
- [7].Hughes RA, Brassington R, Gunn AA, et al. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev 2016;CD001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hughes RA, Pritchard J, Hadden RD. Pharmacological treatment other than corticosteroids, intravenous immunoglobulin and plasma exchange for Guillain Barré syndrome. Cochrane Database Syst Rev 2020;CD008630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Neupane B, Richer D, Bonner AJ, et al. Network meta-analysis using r: a review of currently available automated packages. PLoS ONE 2014;9:e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bril V, Ilse WK, Pearce R, et al. Pilot trial of immunoglobulin versus plasma exchange in patients with Guillain-Barre syndrome. Neurology 1996;46:100–3. [DOI] [PubMed] [Google Scholar]
- [11].Korinthenberg R, Schessl J, Kirschner J, et al. Intravenously administered immunoglobulin in the treatment of childhood Guillain-Barre syndrome: a randomized trial. Pediatrics 2005;116:08–14. [DOI] [PubMed] [Google Scholar]
- [12].Nomura T, Hamaguchi K, Hattori T, et al. A randomized controlled trial comparing intravenous immunoglobulin and plasmapheresis in Guillain-Barré syndrome. Neurol Ther 2001;18:69–81. [Google Scholar]
- [13].Hughes RAC, Swan AV, Cornblath DR, et al. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet 1997;349:225–30. [PubMed] [Google Scholar]
- [14].Raphael J, Chevret S, Harboun M, et al. Intravenous immune globulins in patients with Guillain-Barré syndrome and contraindications to plasma exchange: 3 days versus 6 days. J Neurol Neurosurg Psychiatry 2001;71:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bansal BC, Sood AK, Gupta AK, et al. Role of steroids in the treatment of Guillain-Barré syndrome-a controlled trial. Neurol India 1986;34:329–35. [Google Scholar]
- [16].Wollinsky KH, Hulser PJ, Brinkmeier H, et al. CSF filtration is an effective treatment of Guillain-Barre syndrome: a randomized clinical trial. Neurology 2001;57:774–80. [DOI] [PubMed] [Google Scholar]
- [17].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2010;39:91–2. [DOI] [PubMed] [Google Scholar]
- [18].van Valkenhoef G, Lu G, de Brock EO, et al. Automating network meta-analysis. Res Synth Methods 2012;3:285–99. [DOI] [PubMed] [Google Scholar]
- [19].Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol 2013;13:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Koningsveld R, Schmitz P, van der Meché F, et al. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain-Barré syndrome: randomised trial. Lancet 2004;363:192–6. [DOI] [PubMed] [Google Scholar]
- [21].Steroid G. Double-blind trial of intravenous methylprednisolone in Guillain-Barre syndrome. Guillain-Barre Syndrome Steroid Trial Group. Lancet 1993;341:586–90. [PubMed] [Google Scholar]
- [22].Hughes RA, Newsom-Davis JM, Perkin GD, et al. Controlled trial prednisolone in acute polyneuropathy. Lancet 1978;2:750–3. [DOI] [PubMed] [Google Scholar]
- [23].Singh NK, Gupta A. Do corticosteroids influence the disease course or mortality in Guillain-Barre'syndrome? J Assoc Physicians India 1996;44:22–4. [PubMed] [Google Scholar]
- [24].Shukla SK, Agarwal R, Gupta OP, et al. Double blind control trial of prednisolone in Guillain-Barré syndrome: a clinical study. Clin India 1988;52:128–34. [Google Scholar]
- [25].Pritchard J, Gray IA, Idrissova ZR, et al. A randomized controlled trial of recombinant interferon-beta 1a in Guillain-Barré syndrome. Neurology 2003;61:1282–4. [DOI] [PubMed] [Google Scholar]
- [26].Bensa S, Hadden R, Hahn A, et al. Randomized controlled trial of brain-derived neurotrophic factor in Guillain–Barré syndrome: a pilot study. Eur J Neurol 2000;7:423–6. [DOI] [PubMed] [Google Scholar]
- [27].Zhang X, Xia J, Ye H. Effect of Tripterygium polyglycoside on interleukin-6 in patients with Guillain-Barre syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000;20:332–4. [PubMed] [Google Scholar]
- [28].Philipp T, Koeppen S, Vietorisz A. Study Group. A preliminary, randomized, multicenter study comparing intravenous immunoglobulin, plasma exchange, and immune adsorption in Guillain-Barré syndrome. Eur Neurol 2001;46:107–9. [DOI] [PubMed] [Google Scholar]
- [29].El-Bayoumi MA, El-Refaey AM, Abdelkader AM, et al. Comparison of intravenous immunoglobulin and plasma exchange in treatment of mechanically ventilated children with Guillain Barré syndrome: a randomized study. Crit Care 2011;15:R164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Haupt WF, Rosenow F, van der Ven C, et al. Sequential treatment of Guillain-Barré syndrome with extracorporeal elimination and intravenous immunoglobulin. J Neurol Sci 1996;137:145–9. [DOI] [PubMed] [Google Scholar]
- [31].Van der Meché F, Schmitz P, Dutch GBSG. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. N Engl J Med 1992;326:1123–9. [DOI] [PubMed] [Google Scholar]
- [32].Greenwood RJ, Hughes R, Bowden AN, et al. Controlled trial of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet 1984;323:877–9. [DOI] [PubMed] [Google Scholar]
- [33].Gbs SG. Plasmapheresis and acute Guillain-Barre syndrome. Neurology 1985;35:1096–104. [PubMed] [Google Scholar]
- [34].Osterman PO, Lundemo G, Pirskanen R, et al. Beneficial effects of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet 1984;324:1296–9. [DOI] [PubMed] [Google Scholar]
- [35].French CGOP. Efficiency of plasma exchange in Guillain-Barré syndrome: role of replacement fluids. Ann Neurol 1987;22:753–61. [DOI] [PubMed] [Google Scholar]
- [36].Maheshwari A, Sharma RR, Prinja S, et al. Cost-minimization analysis in the Indian subcontinent for treating Guillain Barre Syndrome patients with therapeutic plasma exchange as compared to intravenous immunoglobulin. J Clin Apher 2018;33:631–7. [DOI] [PubMed] [Google Scholar]
- [37].Ye Y, Li SL, Li YJ. Comparison on therapeutic effect of plasma exchange and intravenous immunoglobulin for Guillian-Barre syndrome. Transfus Med 2015;25:79–84. [DOI] [PubMed] [Google Scholar]
- [38].Davidson AI, Halstead S, Goodfellow JA, et al. Inhibition of complement in Guillain-Barré syndrome: the ICA-GBS study. J Peripher Nerv Syst 2017;22:04–12. [DOI] [PubMed] [Google Scholar]
- [39].Misawa S, Kuwabara S, Sato Y, et al. Safety and efficacy of eculizumab in Guillain-Barré syndrome: a multicentre, double-blind, randomised phase 2 trial. Lancet Neurol 2018;17:519–29. [DOI] [PubMed] [Google Scholar]
- [40].Almende BV, Thieurmel B, Robert T. visNetwork: Network Visualization using vis. js Library R package version 2.0. 4[Z]; 2018. [Google Scholar]
- [41].van Valkenhoef G, Dias S, Ades AE, et al. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods 2016;7:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hughes RA, Cornblath DR. Guillain-barre syndrome. Lancet 2005;366:1653–66. [DOI] [PubMed] [Google Scholar]
- [43]. van Doorn PA. Current treatment in Guillain-Barré Syndrome and myasthenia gravis. 2018. 4th congress of the European Academy of Neurology, Lisbon-Portugal. [Google Scholar]
- [44].Willison HJP, Jacobs BCP, van Doorn PAP. Guillain-Barré syndrome. Lancet 2016;388:717–27. [DOI] [PubMed] [Google Scholar]
- [45].Castro LH, Ropper AH. Human immune globulin infusion in Guillain-Barré syndrome: worsening during and after treatment. Neurology 1993;43:1034–6. [DOI] [PubMed] [Google Scholar]
- [46].Bussel JB, Saal S, Gordon B. Combined plasma exchange and intravenous gammaglobulin in the treatment of patients with refractory immune thrombocytopenic purpura. Transfusion 1988;28:38–41. [DOI] [PubMed] [Google Scholar]
- [47].Shibuya N, Sato T, Osame M, et al. Immunoadsorption therapy for myasthenia gravis. J Neurol Neurosurg Psychiatry 1994;57:578–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Grob D, Simpson D, Mitsumoto H, et al. Treatment of myasthenia gravis by immunoadsorption of plasma. Neurology 1995;45:338–44. [DOI] [PubMed] [Google Scholar]