ABSTRACT
All clinical Clostridioides difficile strains identified to date express a surface capsule-like polysaccharide structure known as polysaccharide II (PSII). The PSII antigen is immunogenic and, when conjugated to a protein carrier, induces a protective antibody response in animal models. Given that CD1d-restricted natural killer T (NKT) cells promote antibody responses, including those against carbohydrates, we tested the hypothesis that immunization with PSII and a CD1d-binding glycolipid adjuvant could lead to enhanced protection against a live C. difficile challenge. We purified PSII from a clinical isolate of C. difficile and immunized B6 mice with PSII alone or PSII plus the CD1d-binding glycolipid α-galactosylceramide (α-GC). PSII-specific IgM and IgG titers were evident in sera from immunized mice. The inclusion of α-GC had a modest influence on isotype switch but increased the IgG1/IgG2c ratio. Enhanced protection against C. difficile disease was achieved by inclusion of the α-GC ligand and was associated with reduced bacterial numbers in fecal pellets. In contrast, NKT-deficient Traj18−/− mice were not protected by the PSII/α-GC immunization modality. Absence of NKT cells similarly had a modest effect on isotype switch, but ratios of IgG1/IgG2c decreased. These results indicate that α-GC-driven NKT cells move the humoral immune response against C. difficile PSII antigen toward Th2-driven IgG1 and may contribute to augmented protection. This study suggests that NKT activation represents a pathway for additional B-cell help that could be used to supplement existing efforts to develop vaccines against polysaccharides derived from C. difficile and other pathogens.
KEYWORDS: humoral immunity, carbohydrate, NKT cell, Clostridium difficile, Clostridioides difficile, humoral immunity
INTRODUCTION
The systemic pathology associated with fulminant Clostridioides difficile infection (CDI) is largely attributable to two secreted toxins, known as toxin A (TcdA) and toxin B (TcdB), with the latter playing the dominant role in vivo (1, 2). Recurrent infection is a particular problem in CDI and is associated with progressive toxicity and, ultimately, death (3). Therefore, stimulating protective toxin-specific immune memory is a current focus of efforts to generate a first-generation vaccine.
However, targeting secreted C. difficile toxins by vaccination may run the risk of creating a pool of asymptomatic but infectious individuals capable of transmitting toxigenic strains to others. For these reasons, vaccine researchers are also taking into consideration antigens that allow direct targeting of the pathogen. C. difficile expresses three major polysaccharide antigens, knowns as PSI, PSII, and PSIII. PSII is expressed by all clinical C. difficile strains, albeit in various amounts (4). PSII intrinsically leads to T-independent humoral responses with poor B-cell memory but can be conjugated to protein carriers to induce antibody (Ab) class switch and B-cell memory (5–7). PSII therefore has proven immunogenicity and is protective in animal models of immunization and challenge (8).
It is well established that CD1d-restricted natural killer T (NKT) cells intersect with the cellular machinery that governs humoral and cellular immunity (9). The inclusion of NKT cell-activating, CD1d-binding glycolipid antigens in experimental vaccine formulations leads to enhanced Th and cytotoxic T-lymphocyte (CTL) responses (10). Furthermore, primary antibody titers are boosted as a result of NKT activation, as are memory B-cell-driven recall responses (11–14). NKT cells also contribute to the longevity of antibody-secreting plasma cells (15, 16).
In this study, we used a murine immunization and infectious challenge model to test the ability of the NKT-activating α-galactosylceramide (α-GC) adjuvant to alter the immune response to C. difficile-derived PSII. We report that α-GC led to enhanced production of PSII-specific IgG1, which was associated with disease mitigation in challenged B6 mice. This work suggests that PSII and α-GC could be incorporated into a vaccine and adjuvant platform to provide protection targeted at toxins as well as the pathogen.
RESULTS
Enrichment of PSII for immunization studies.
All clinical strains of C. difficile express PSII, a polyhexasaccharide containing a repeating immunogenic phosphate moiety (4, 8), which can stimulate protective Ab responses when conjugated to a T-dependent protein carrier (7). Here, we used a modified version of the method by Monteiro to isolate PSII (8). A C. difficile-derived phenol/hot water-extracted crude carbohydrate preparation was applied to a Sephadex size exclusion gravity column (Fig. 1A). This resulted in elution of two major peaks from fractions 5 to 30 and 50 to 75, with the former consisting largely of PSIII, nucleic acids, and proteins. The latter peak did not contain detectable protein or nucleic acid, and a 31P nuclear magnetic resonance (NMR) analysis revealed three major peaks, with the most abundant (at −1.27 ppm) representing PSII (Fig. 1B). The less abundant peaks at 1.88 and 1.75 ppm represent PSIII. Further resolution of the PSII/PSIII peak was possible by pooling fractions into four batches and by selecting the highest fraction numbers (Fig. 1C). An enzyme-linked immunosorbent assay (ELISA) in which plates were coated with purified PSII or oxygen-killed vegetative C. difficile was also optimized for this study (Fig. 1D). Sera from PSII-immunized and naive rabbits were able to detect PSII in isolation and in the context of whole bacteria, with good correlation between the two assays. PSII detection was also confirmed by dot blot of sera against membrane-spotted PSII (Fig. 1E). The isolated PSII was used as the immunogen for the studies described here.
FIG 1.
Purification and characterization of the C. difficile PSII antigen. As described in Materials and Methods, a crude carbohydrate fraction was extracted from C. difficile using hot water and phenol. After dialysis, the crude carbohydrate preparation was applied to a GS50 size exclusion column. (A) Total carbohydrate profile of eluted fractions as determined by the sulfuric acid/phenol assay. PSII was concentrated in the peak indicated and as demonstrated in panel B, whereby 31P NMR analysis was used to confirm the predominance of PSII in the pooled column fractions. The image on the left depicts the entire PSII peak. (C) Four sequential subpools of those fractions, whereby the largest fraction numbers, or tail end of the peak (purple), have the PSIII removed. (D) Detection of PSII by ELISA using plates coated with PSII (left) or oxygen-killed C. difficile (center) as well as the correlation between the two assays (right). (E) Rabbit anti-PSII but not a control antiserum could detect PSII by dot blotting. Neither proteins nor nucleic acids could be detected in the column fractions where PSII was concentrated. Data are representative of at least 4 experiments.
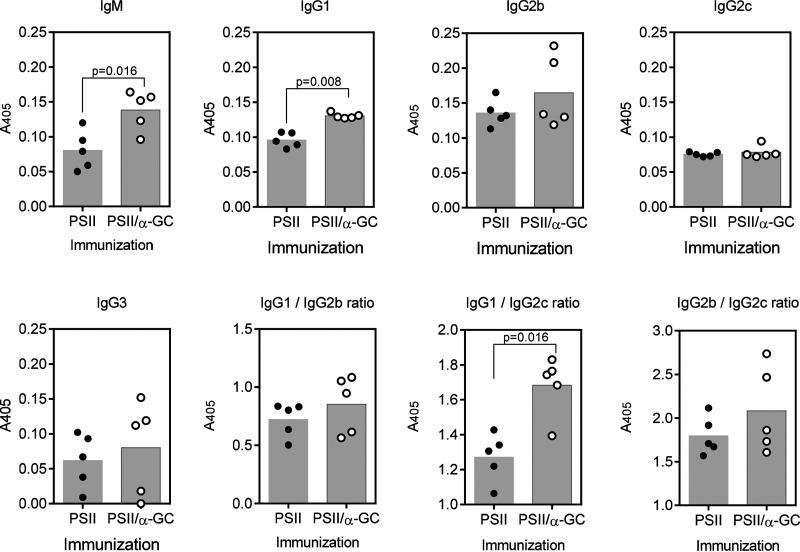
The antibody response to PSII is altered by an NKT cell-activating glycolipid adjuvant.
B6 mice were immunized with PSII alone or PSII plus the CD1d-binding glycolipid adjuvant α-GC. In response to PSII alone, PSII-specific IgM, IgG1, IgG2b, IgG2c, and IgG3 were produced (Fig. 2). Inclusion of α-GC in the vaccine significantly but modestly increased IgM and IgG1 production but did not affect IgG2b, IgG2c, or IgG3. The IgG1/IgG2b ratios were unaltered, as was IgG2b/IgG2c. However, the IgG1/IgG2c ratio increased significantly. These results show that the NKT-activating glycolipid adjuvant exerts a small but significant influence on isotype switch against PSII, increasing Th2-driven IgG1. Anti-PSII titers could not be detected in mice immunized with α-GC alone (not depicted).
FIG 2.
NKT-influenced IgM and IgG production against PSII. B6 mice were immunized s.c. with 15 μg PSII or PSII plus 4 μg α-GC. After 1 week, mice were boosted with 7.5 μg PSII. Sera were collected after 28 days, and PSII-specific IgM, IgG1, IgG2b, IgG2c, and IgG3 were detected by ELISA. Each data point represents an individual mouse. A two-tailed t test was used to detect significant differences between groups.
PSII vaccine-induced protection against C. difficile is enhanced by α-GC.
Naïve B6 mice were pretreated for 10 days with cefoperazone, followed by oral gavage with water (naive uninfected) or with water containing 104 C. difficile VPI 10463 spores (naive infected). The uninfected mice made marginal weight gains over the 6-day follow-up period, demonstrating that the gavage method had no adverse effects related to eating or drinking (Fig. 3A). In contrast, the naive infected mice demonstrated a small (5 to 10%) but significant loss in total body weight that took 5 additional days to return to the preinfection state (Fig. 3B). B6 mice were also immunized prior to cefoperazone treatment and oral gavage (Fig. 3C to G). Mice that were given PSII alone (followed by the PSII booster) (Fig. 3C) or just the booster (Fig. 3D) demonstrated a pattern similar to that of naive infected mice, suggesting that PSII alone did not lead to protection. When mice were treated with α-GC to activate NKT cells followed 1 week later with PSII, no weight loss was observed (Fig. 3E). When mice were immunized with PSII plus α-GC followed by the booster, no weight loss occurred (Fig. 3F). A marginal, nonsignificant weight gain was observed similar to that of the naive uninfected group. Immunization with α-GC alone did not protect against weight loss (Fig. 3G). These data demonstrate that inclusion of α-GC in the PSII vaccine leads to enhanced protection upon challenge with live C. difficile spores, while α-GC alone did not lead to protection. C. difficile shed into the fecal pellets was also determined (Fig. 3H). The large number of C. difficile CFU observed after culture in selection medium was not significantly impacted by the various vaccination regimens used, with the exception of administration of PSII plus α-GC, where CFU numbers were reduced by more than 2 orders of magnitude. These data show that PSII/α-GC immunization was able to cause substantial reductions in bacterial burden. To confirm results shown in Fig. 2, sera collected before C. difficile challenge were assessed for C. difficile-specific IgG1 (Fig. 3i). Administration of PSII plus α-GC followed by a PSII booster resulted in IgG1 production that was significantly higher than that in all other experimental groups. Therefore, the serum C. difficile-specific IgG1 induced by PSII/α-GC immunization was associated with the best protection against disease and reduction in bacterial load.
FIG 3.
PSII/α-GC vaccination leads to enhanced protection against C. difficile disease. (A to G) Age- and sex-matched naive B6 mice were cefoperazone treated and then mock infected or infected with 104 live C. difficile spores. Mice previously immunized with PSII alone, PSII plus α-GC, or α-GC and PSII 1 week apart were also cefoperazone treated and infected. Graphs show the percentage of original weight, which was obtained daily and for the duration indicated. Data show the results of two pooled experiments with n = 5 for each group except for the naive-infected, α-GC- and then vehicle-treated, and PSII/α-GC groups (n = 10). Statistical significance was determined by repeated-measure ANOVA and Tukey’s multiple-comparison test (*, P < 0.05; **, P < 0.01). (H) Fecal pellets collected 2 days after infection were used to determine bacterial load. In group A, zero counts were detected (limit of detection in assay, 102). (I) ELISA measurement of C. difficile-specific IgG1 in response to the various vaccination modalities used. In panels H and I, significance was determined by one-way ANOVA with Tukey’s multiple-comparison test (*, P < 0.05; **, P < 0.01). (J and K) Naïve (n = 4) and PSII/α-GC-immunized (n = 5) mice, antibiotic treated and infected with 5 × 104 spores. Weight loss and survival are depicted. Numbers above bars on the left are the numbers of remaining live animals in the group. A Mantel-Cox log rank test was used to detect differences between groups subject to Kaplan-Meier survival analysis.
The challenge experiments in Fig. 3a to g involved a low infectious dose of C. difficile (104 spores per animal). In our other ongoing projects, challenge with 5 × 104 spores routinely causes a weight loss of approximately 10 to 15% (data not shown). In subsequent experiments here, the higher spore number was used for challenges. Given that PSII/α-GC protected against a mild course of disease, naive and PSII/α-GC-immunized mice were also infected with 5 × 104 spores, such that a more serious course of disease ensued (Fig. 3j and k). In that experiment, 50% of naive infected mice lost 20% of their weight and were euthanized. In the immunized infected group, 80% of mice showed no weight loss, and there was 100% survival in this group. This shows that the PSII/α-GC immunization modality was able to protect against a more serious course of disease.
NKT cells are required for α-GC-enhanced protection but do not affect disease susceptibility of naive mice to C. difficile.
To determine whether the protective response was NKT cell dependent, control mice and littermate (Traj18−/−) mice lacking α-GC-reactive NKT cells (17) were immunized before antibiotic treatment and infection with 5 × 104 spores per animal (Fig. 4a). All PSII/α-GC-immunized Traj18+/+ and Traj18+/− control mice were protected against weight loss. Two of three Traj18−/− mice lost 20% of overall body weight and were euthanized, while the third mouse lost 9% of body weight before recovery. While this experiment evaluated only 3 Traj18−/− mice, these data suggest that NKT cells were required for protection of PSII/α-GC-immunized mice. This observation is consistent with results in B6 mice whereby immunization with PSII alone was not protective.
FIG 4.
NKT cells are required for α-GC-enhanced protection but do not affect disease susceptibility of naive mice to C. difficile. Mice depicted were immunized with PSII plus α-GC and boosted with PSII before treatment with cefoperazone and infection with 5 × 104 C. difficile spores per mouse. (A) Mean weight (± standard deviations) in NKT-sufficient controls (Traj18+/+, Traj18+/−, n = 7) and in 3 individual NKT-deficient Traj18−/− littermate mice. (B) PSII-specific IgM, IgG1, IgG2b, IgG2c, and IgG3 with samples pooled from experiment shown in panel A and the Traj18−/− samples shown in panel C. In panels A and B, significance was determined by two-tailed t test. (C) Weights in PSII/α-GC-immunized B6 (n = 5) and Traj18−/− (n = 6) mice following challenge with 3 × 105 spores per mouse. (D) Weights in naive B6 (n = 5) and Traj18−/− (n = 6) mice following challenge with 5 × 104 spores per mouse.
Serum IgM, IgG1, IgG2b, IgG2c, and IgG3 titers were measured (Fig. 4b). This analysis pooled samples from Fig. 4a and the Traj18−/− mice in Fig. 4c. As expected, each subclass was detected in control Traj18+/+ mice, consistent with that observed in B6 mice. IgM, IgG1, IgG2b, IgG2c, and IgG3 did not differ significantly in NKT sufficient (Traj18+/+, Traj18+/−) and NKT-deficient (Traj18−/−) mice. Subclass ratios were altered by the absence of NKT cells such that IgG1/IgG2c ratios were significantly decreased. This showed a decrease in the Th2/Th1 ratio in NKT-deficient mice, the opposite effect of using α-GC to activate NKT cells in B6 mice. These results confirmed that NKT cells were required for protection induced by the PSII/α-GC vaccination modality and associated with a reduced Th2/Th1 ratio.
The limits of the protective response were also probed in this study, wherein PSII/α-GC-immunized B6 and Traj18−/− mice were challenged with 3 × 105 C. difficile spores (Fig. 4c). Both groups succumbed to infection within 2 days, losing 20% of body weight, necessitating euthanasia. These data show that the NKT-dependent component of protection can be overcome by an overwhelming infectious dose.
To discern between intrinsic- and vaccine-activated NKT cell-mediated protection, nonimmunized B6 and Traj18−/− mice were antibiotic treated and challenged with C. difficile spores. B6 and Traj18−/− mice displayed similar weight loss (Fig. 4d). This shows that mice lacking NKT cells were as susceptible to C. difficile disease as NKT-sufficient controls, implicating α-GC-activated NKT cells in protection.
DISCUSSION
The results of this study show that NKT cell activation with CD1d-binding glycolipid adjuvants influences the immune response to C. difficile-derived polysaccharides. There is some precedent for the concept, with prior studies showing that α-GC resulted in IgG1 class switch against Ficoll, the model T-independent carbohydrate antigen (18). Ficoll immunization stimulated IgM production and a limited IgG3 class switch. However, the addition of α-GC to the immunization led to additional IgG1 production. The Bendelac laboratory has also reported that NKT cells can similarly influence Ab responses to polysaccharide antigens from Streptococcus pneumoniae with increases in IgM and IgG1 subclasses (19). Here, we observed that inclusion of α-GC in a vaccine to C. difficile PSII increased PSII-specific IgG1 and the IgG1/IgG2c ratio, reflective of a more Th2-driven response. The inclusion of α-GC led to protection, as evidenced by maintenance of body weight and a lower bacterial burden.
Administration of α-GC alone was not protective against C. difficile, showing that the effects were dependent on coimmunization with PSII. Administration of α-GC 1 week before PSII resulted in protection, suggesting that the α-GC-activated NKT cells were still able to provide B-cell help at this later stage after they encountered the PSII. This timing is consistent with α-GC-stimulated expansion of NKT follicular helper (NKTfh) cell expansion (20–22). We recently used Cre-Lox technology to block expression of the transcription factor Bcl6 and, thus, development of T and NKT follicular helper (NKTfh) cells. Using this system, we determined that NKTfh cells were necessary for the α-GC effect on anti-Ficoll responses (23). However, modest NKTfh expansion following immunization with pneumococcal polysaccharide and α-GC has also been reported (19). It is therefore possible that NKTfh cells could contribute to the effects of α-GC on the anti-PSII response. In an initial experiment, we observed superior survival of PSII/α-GC-immunized mice following Tfh and NKTfh cell ablation (Lang et al., unpublished observations). This suggests that further investigation of NKTfh cells in relation to protection is warranted.
The lack of α-GC-reactive NKT cells in Traj18−/− mice resulted in a subtle shift in IgG1 and IgG2c production, resulting in a more Th1-driven response and a lack of protection. This suggests that vaccination-stimulated Th2-driven IgG subclasses are contributory to protection. C. difficile is the most common diarrheal disease in patients presenting with HIV, implicating CD4+ T cells, but this could be a function of reduced Th2, Th1, or both subsets (24). These results must be considered in the context of the incompletely understood relationship between the microbiome and the NKT cellular compartment (reviewed in references 25 and 26). While NKT cells develop normally in the absence of a microbiome (germfree mice) and there are effects on NKT tissue distribution and function, that information is less germane to this study, as the extremes of a germfree environment were not applied to the present study. However, the NKT compartment and the absence of it can affect the microbiome. CD1d−/− mice lacking all NKT cells tend to have a greater and more inflammatory commensal burden in the small intestine than their wild-type counterparts (27, 28). The mechanism is postulated to be dependent on NKT-derived IFN-γ stimulation of antimicrobial peptides by Paneth cells keeping the microbiota in check (27). Arguably, a lack of NKT cells could render a mouse less susceptible to C. difficile infection by increasing the preantibiotic commensal burden or more susceptible by increasing inflammation. However, the absence of α-GC-reactive NKT cells may be less consequential in the colon than in the small intestine. In NKT-sufficient mice, numbers are lower in the colon than the small intestine and correlate inversely with commensal numbers (29). The absence of NKT cells in CD1d−/− pigs (30) and administration of α-GC to B6 mice (31) were observed to have no substantial effect on the fecal microbiota. Traj18−/− mice selectively lack α-GC-reactive NKT cells, and the background effect on the microbiome may be less than it is in CD1d−/− mice lacking all NKT cells. While the evidence favors the lack of NKT cells in Traj18−/− mice being disruptive to the colon microbiota, the differences in disease outcome in this study do not preclude the possibility of a microbiome in Traj18−/− mice that is more inflammatory than in littermate controls.
Differences in amounts of PSII-specific Ab between experimental groups appeared more nuanced than outcomes (weight loss and bacterial counts). This suggests that other effects of α-GC could contribute to protection. Some studies have shown the existence of polysaccharide-specific T-cell receptors, but activation relies on binding of an associated peptide with major histocompatability complex class II (MHC II) (32, 33), so this is not a likely mechanism in the present study. We observed that α-GC administered alone was not protective, so a direct NKT cell effect, or a downstream effect such as NK cell activation, also seems unlikely. It is feasible that the small amounts of Ab detected and small shifts upon α-GC administration or NKT deficiency are due to technical limitations, with ELISAs for polysaccharides underestimating the amount of Ab present. However, Ab responses may be quite low and remain highly protective. The Kasper laboratory reported very low Ab responses to group B Streptococcus-derived polysaccharides yet observed strong protection against pathogenic challenges (33). In those studies, protection was attributed to higher-affinity and/or -avidity Ab being produced, but arguably small changes in specific IgG subclass could also contribute to protection.
Several studies show that when α-GC is coadministered with T-dependent protein antigens, it leads to enhance primary and recall responses and stimulates Ig class switch, good B-cell memory, and plasma cell longevity, the major hallmarks of a successful vaccine (reviewed in references 9, 34, and 35). Formation of glycoconjugates whereby proteins or MHC II-loading peptides are linked to bacterial polysaccharides can induce class switch, memory, and plasma cell longevity. We have previously addressed whether α-GC can influence memory to polysaccharides by showing that immunization with α-GC and Ficoll did not lead to Ab recall responses (23). In contrast, the Bendelac group reported recall IgG1 responses to pneumococcal polysaccharides when α-GC was included in the vaccine (19). Interestingly, those studies differed by the former admixing α-GC and the polysaccharide and the latter by incorporating both into liposome-type particles. Therefore, particulate formulations may better stimulate NKT cell-enhanced B cell memory. These studies may provide valuable information on the immune response to the pathogen versus a polysaccharide vaccine, because shedding of small polysaccharides could exert a decoy effect and limit development of B-cell memory, whereas particulates may stimulate appropriate B-cell antigen receptor signaling for memory differentiation.
The methods by which α-GC is included as a vaccine adjuvant may therefore depend very much upon the formulation when polysaccharides serve as the antigen. However, the inclusion of α-GC in existing adjuvant platforms and addition to glycoconjugate vaccines could selectively affect class switch in a manner coordinate with classical T-cell help that leads to B-cell memory and plasma cell longevity. Indeed, our prior studies have shown a coordinated action of Th, Tfh, and NKTfh in T-dependent responses (14). Further examination of the intersection of NKT cell and Th cell help for glycoprotein vaccines (glycoconjugates) may be warranted.
MATERIALS AND METHODS
Ethics.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (36). All animal procedures were approved by the OUHSC Institutional Animal Care and Use Committee (protocols 16–037 and 17–078-CHI).
Mice.
Female C57BL/6 (B6) mice were purchased from Charles River (Bethesda, MD, USA). Heterozygous Traj18+/− mice were purchased from Jax.org and bred to generate Traj18−/−, Traj18+/−, and Traj18+/+ offspring. Experiments were conducted on mice that were littermates and cage mates. Disposable sterile caging, bedding, and water were used for housing. Genotyping and phenotyping was performed according to protocols provided by Jax.org. Additional mice (used for experiments shown in Fig. 4d) were provided by J. Alberola-Ila (Oklahoma Medical Research Foundation). Traj18−/− mice were obtained under a Material Transfer Agreement with the La Jolla Institute for Allergy and Immunology (LIAI; La Jolla, CA), originally generated by the Kronenberg laboratory (LIAI), and first reported in reference 17.
Reagents.
α-GC was purchased from Enzo Life Sciences (Farmingdale, NY). Horseradish peroxidase-conjugated Abs for ELISA were purchased from Southern Biotech (Birmingham, AL).
PSII purification.
PSII was prepared by the method described by Monteiro (8). In brief, a wet paste of C. difficile (strain VPI 10463) was subject to phenol/hot water extraction. After dialysis of the partitioned aqueous phase, carbohydrate-enriched material was freeze-dried under vacuum and then reconstituted in double-distilled H2O. Samples were run on a Sephadex G25 gravity column, and fractions were analyzed by 31P NMR (Oklahoma State University NMR Facility, Stillwater, OK). Nucleic acids and proteins could not be detected in the PSII-containing fractions.
Immunizations.
Mice were immunized subcutaneously (s.c.) in both flanks with a total of 15 μg PSII or PSII plus 4 μg α-GC in a 200-μl total volume of sterile low-endotoxin phosphate-buffered saline (PBS). The amount of α-GC administered has been determined in previous studies to exert a strong CD1d- and NKT-dependent effect on Ab responses (15, 37–39). The booster vaccine consisted of 7.5 μg PSII in PBS administered s.c. over both flanks. The booster was administered 1 week after the primary immunization.
C. difficile spore preparation.
C. difficile VPI 10463 spores were prepared and isolated as follows. A single colony was used to inoculate 2 ml of prereduced Columbia broth (BD) and incubated overnight at 37°C in an anaerobic chamber. This was followed by addition of 40 ml prereduced Clospore medium (39) and 5 to 7 days of anaerobic growth at 37°C. Spores were harvested via centrifugation and washed at least 3 times in ice-cold sterile water. Spores were stored at 4°C in sterile water. Before infection of mice, C. difficile spores were heated for 20 min at 65°C, and CFU were quantified by plating on TCCFA (taurocholate cycloserine cefoxitin fructose agar) (40).
Challenge assays.
Mice were transferred to new sterile cages and given sterilized feed. Mice were also given 0.5 mg/ml cefoperazone in drinking water for 10 days, which was changed every 2 days. Sterile drinking water was then provided for a further 2 days. The mice were then challenged by oral gavage with a 20-μl inoculum of water containing 2 × 104, 5 × 104, or 3 × 105 VPI 10463 spores. Animal weight was then measured daily.
Quantification of fecal C. difficile CFU numbers.
Bacteria shed were quantified on day 3 postgavage, unless otherwise indicated. Fecal pellets were homogenized with 1× PBS, serially diluted, plated on TCCFA, and cultured under anaerobic conditions at 37°C. CFU were counted after 48 h (40).
ELISA.
To assess PSII-specific antibodies, 96-well ELISA plates (Nunc, Thermo Scientific, Rochester, NY) were coated with 10 μg/ml of PSII in carbonate coating buffer overnight at 4°C. For bacterium-specific antibodies, plates were coated with oxygen-killed C. difficile bacteria diluted in carbonate coating buffer. Wells were blocked with 1% bovine serum albumin in PBS-T (1× TBS) for 2 h at room temperature and incubated overnight at 4°C with mouse sera diluted 200-fold in TBS. Wells were washed with TBS and then incubated for 1 h with horseradish peroxidase-conjugated IgM, IgG1, IgG2b, IgG2c, or IgG3 at a 2,500-fold dilution of the stock. Wells were washed and developed for 5 min at room temperature with ABTS substrate (KPL, Gaithersburg, MD). A 10%, wt/vol, SDS solution was used to stop the reaction. Endpoint Ab titers were determined by measuring the optical density at 405 nm.
Statistics.
Data were analyzed using GraphPad Prism 8.1 (La Jolla, CA, USA). A two-tailed t test and one-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test was used for statistical analysis between two and multiple experimental groups, respectively. A two-way repeated-measure ANOVA with Tukey’s multiple-comparison test was used to determine statistical significance in weight loss over time. A Mantel-Cox log rank test was used to detect differences between groups subject to Kaplan-Meier survival analysis.
ACKNOWLEDGMENTS
We thank Rodney Tweten (OUHSC) for assistance with column chromatography. We thank Gayatri Vedantam (Arizona State University) for the kind gift of the rabbit PSII antisera and Margaret Eastman (Oklahoma State University) for running NMR. We thank Jose Alberola-Ila (Oklahoma Medical Research Foundation) for providing Traj18−/− mice.
This work was supported by NIH grants AI125708 and AI134719 to M.L.L. and AI119048 to J.D.B.
Contributor Information
Mark L. Lang, Email: mark-lang@ouhsc.edu.
Victor J. Torres, New York University School of Medicine
REFERENCES
- 1.Ballard JD. 2010. Medical microbiology: a toxin contest. Nature 467:665–666. 10.1038/467665a. [DOI] [PubMed] [Google Scholar]
- 2.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713. 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JG. 2010. Clostridium difficile: progress and challenges. Ann N Y Acad Sci 1213:62–69. 10.1111/j.1749-6632.2010.05863.x. [DOI] [PubMed] [Google Scholar]
- 4.Chu M, Mallozzi MJ, Roxas BP, Bertolo L, Monteiro MA, Agellon A, Viswanathan VK, Vedantam G. 2016. A Clostridium difficile cell wall glycopolymer locus influences bacterial shape, polysaccharide production and virulence. PLoS Pathog 12:e1005946. 10.1371/journal.ppat.1005946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamo R, Romano MR, Berti F, Leuzzi R, Tontini M, Danieli E, Cappelletti E, Cakici OS, Swennen E, Pinto V, Brogioni B, Proietti D, Galeotti CL, Lay L, Monteiro MA, Scarselli M, Costantino P. 2012. Phosphorylation of the synthetic hexasaccharide repeating unit is essential for the induction of antibodies to Clostridium difficile PSII cell wall polysaccharide. ACS Chem Biol 7:1420–1428. 10.1021/cb300221f. [DOI] [PubMed] [Google Scholar]
- 6.Bertolo L, Boncheff AG, Ma Z, Chen YH, Wakeford T, Friendship RM, Rosseau J, Weese JS, Chu M, Mallozzi M, Vedantam G, Monteiro MA. 2012. Clostridium difficile carbohydrates: glucan in spores, PSII common antigen in cells, immunogenicity of PSII in swine and synthesis of a dual C. difficile-ETEC conjugate vaccine. Carbohydrate Res 354:79–86. 10.1016/j.carres.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro MA, Ma Z, Bertolo L, Jiao Y, Arroyo L, Hodgins D, Mallozzi M, Vedantam G, Sagermann M, Sundsmo J, Chow H. 2013. Carbohydrate-based Clostridium difficile vaccines. Expert Rev Vaccines 12:421–431. 10.1586/erv.13.9. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro MA. 2016. The design of a Clostridium difficile carbohydrate-based vaccine. Methods Mol Biol 1403:397–408. 10.1007/978-1-4939-3387-7_21. [DOI] [PubMed] [Google Scholar]
- 9.Lang ML. 2018. The influence of invariant natural killer T cells on humoral immunity to T-dependent and -independent antigens. Front Immunol 9:305. 10.3389/fimmu.2018.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. 2003. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol 171:5140–5147. 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 11.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. 2007. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA 104:3984–3989. 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang GA, Johnson AM, Devera TS, Joshi SK, Lang ML. 2011. Reduction of CD1d expression in vivo minimally affects NKT-enhanced antibody production but boosts B-cell memory. Int Immunol 23:251–260. 10.1093/intimm/dxq477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devera TS, Joshi SK, Aye LM, Lang GA, Ballard JD, Lang ML. 2011. Regulation of anthrax toxin-specific antibody titers by natural killer T cell-derived IL-4 and IFNgamma. PLoS One 6:e23817. 10.1371/journal.pone.0023817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampuria P, Lang GA, Devera TS, Gilmore C, Ballard JD, Lang ML. 2017. Coordination between T helper cells, iNKT cells, and their follicular helper subsets in the humoral immune response against Clostridium difficile toxin B. J Leukoc Biol 101:567–576. 10.1189/jlb.4A0616-271R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devera TS, Shah HB, Lang GA, Lang ML. 2008. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur J Immunol 38:1001–1011. 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah HB, Joshi SK, Rampuria P, Devera TS, Lang GA, Stohl W, Lang ML. 2013. BAFF- and APRIL-dependent maintenance of antibody titers after immunization with T-dependent antigen and CD1d-binding ligand. J Immunol 191:1154–1163. 10.4049/jimmunol.1300263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra S, Zhao M, Budelsky A, de Mingo Pulido A, Day J, Fu Z, Siegel L, Smith D, Kronenberg M. 2015. A new mouse strain for the analysis of invariant NKT cell function. Nat Immunol 16:799–800. 10.1038/ni.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang GA, Exley MA, Lang ML. 2006. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology 119:116–125. 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai L, Deng S, Reboulet R, Mathew R, Teyton L, Savage PB, Bendelac A. 2013. Natural killer T (NKT)-B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc Natl Acad Sci USA 110:16097–16102. 10.1073/pnas.1303218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, Nutt SL, Brink R, Godfrey DI, Batista FD, Vinuesa CG. 2011. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol 13:35–43. 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 21.King IL, Fortier A, Tighe M, Dibble J, Watts GF, Veerapen N, Haberman AM, Besra GS, Mohrs M, Brenner MB, Leadbetter EA. 2011. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol 13:44–50. 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonti E, Fedeli M, Napolitano A, Iannacone M, von Andrian UH, Guidotti LG, Abrignani S, Casorati G, Dellabona P. 2012. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J Immunol 188:3217–3222. 10.4049/jimmunol.1103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang GA, Amadou Amani S, Quinn JL, Axtell RC, Lang ML. 2019. Immunization-expanded NKT follicular helper cells drive IgG1 isotype switch against an exogenous T-independent polysaccharide but do not promote recall responses. Immunohorizons 3:88–93. 10.4049/immunohorizons.1800081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collini PJ, Bauer M, Kuijper E, Dockrell DH. 2012. Clostridium difficile infection in HIV-seropositive individuals and transplant recipients. J Infect 64:131–147. 10.1016/j.jinf.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Constantinides MG. 2018. Interactions between the microbiota and innate and innate-like lymphocytes. J Leukoc Biol 103:409–419. 10.1002/JLB.3RI0917-378R. [DOI] [PubMed] [Google Scholar]
- 26.Hapil FZ, Wingender G. 2018. The interaction between invariant Natural Killer T cells and the mucosal microbiota. Immunology 155:164–175. 10.1111/imm.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, Brugman S, Yamaguchi K, Ishikawa H, Aiba Y, Koga Y, Samsom JN, Oshima K, Kikuchi M, Escher JC, Hattori M, Onderdonk AB, Blumberg RS. 2009. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest 119:1241–1250. 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvanantham T, Lin Q, Guo CX, Surendra A, Fieve S, Escalante NK, Guttman DS, Streutker CJ, Robertson SJ, Philpott DJ, Mallevaey T. 2016. NKt cell-deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J Immunol 197:4464–4472. 10.4049/jimmunol.1601410. [DOI] [PubMed] [Google Scholar]
- 29.Wingender G, Stepniak D, Krebs P, Lin L, McBride S, Wei B, Braun J, Mazmanian SK, Kronenberg M. 2012. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology 143:418–428. 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang G, Artiaga BL, Hackmann TJ, Samuel MS, Walters EM, Salek-Ardakani S, Driver JP. 2015. Targeted disruption of CD1d prevents NKT cell development in pigs. Mamm Genome 26:264–270. 10.1007/s00335-015-9564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saez de Guinoa J, Jimeno R, Gaya M, Kipling D, Garzon MJ, Dunn-Walters D, Ubeda C, Barral P. 2018. CD1d-mediated lipid presentation by CD11c(+) cells regulates intestinal homeostasis. EMBO J 37:e97537. 10.15252/embj.201797537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avci FY, Li X, Tsuji M, Kasper DL. 2011. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 17:1602–1609. 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanetti G, Okan N, Fink A, Gardner E, Kasper DL. 2019. Glycoconjugate vaccine using a genetically modified O antigen induces protective antibodies to Francisella tularensis. Proc Natl Acad Sci USA 116:7062–7070. 10.1073/pnas.1900144116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vomhof-DeKrey EE, Yates J, Leadbetter EA. 2014. Invariant NKT cells provide innate and adaptive help for B cells. Curr Opin Immunol 28:12–17. 10.1016/j.coi.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doherty DG, Melo AM, Moreno-Olivera A, Solomos AC. 2018. Activation and regulation of B cell responses by invariant natural killer T cells. Front Immunol 9:1360. 10.3389/fimmu.2018.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 37.Lang GA, Lang ML. 2006. Protein kinase Balpha is required for vesicle trafficking and class II presentation of IgA Fc receptor (CD89)-targeted antigen. J Immunol 176:3987–3994. 10.4049/jimmunol.176.7.3987. [DOI] [PubMed] [Google Scholar]
- 38.Lang GA, Devera TS, Lang ML. 2008. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood 111:2158–2162. 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devera TS, Aye LM, Lang GA, Joshi SK, Ballard JD, Lang ML. 2010. CD1d-dependent B-cell help by NK-like T cells leads to enhanced and sustained production of Bacillus anthracis lethal toxin-neutralizing antibodies. Infect Immun 78:1610–1617. 10.1128/IAI.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winston JA, Thanissery R, Montgomery SA, Theriot CM. 2016. Cefoperazone-treated mouse model of clinically-relevant Clostridium difficile strain R20291. J Vis Exp 118:e54850. 10.3791/54850. [DOI] [PMC free article] [PubMed] [Google Scholar]