ABSTRACT
Neutrophils are required for host resistance against Streptococcus pneumoniae, but their function declines with age. We previously found that CD73, an enzyme required for antimicrobial activity, is downregulated in neutrophils (also known as polymorphonuclear leukocytes [PMNs]) from aged mice. This study explored transcriptional changes in neutrophils induced by S. pneumoniae to identify pathways controlled by CD73 and dysregulated with age. Pure bone marrow-derived neutrophils isolated from wild-type (WT) young and old and CD73 knockout (CD73KO) young mice were mock challenged or infected with S. pneumoniae ex vivo. RNA sequencing (RNA-Seq) was performed to identify differentially expressed genes (DEGs). We found that infection triggered distinct global transcriptional changes across hosts that were strongest in CD73KO neutrophils. Surprisingly, there were more downregulated than upregulated genes in all groups upon infection. Downregulated DEGs indicated a dampening of immune responses in old and CD73KO hosts. Further analysis revealed that CD73KO neutrophils expressed higher numbers of long noncoding RNAs (lncRNAs) than those in WT controls. Predicted network analysis indicated that CD73KO-specific lncRNAs control several signaling pathways. We found that genes in the c-Jun N-terminal kinase (JNK)-mitogen-activated protein kinase (MAPK) pathway were upregulated upon infection in CD73KO mice and in WT old mice, but not in WT young mice. This corresponded to functional differences, as phosphorylation of the downstream AP-1 transcription factor component c-Jun was significantly higher in neutrophils from infected CD73KO mice and old mice. Importantly, inhibition of JNK/AP-1 rescued the ability of these neutrophils to kill S. pneumoniae. Together, our findings revealed that the ability of neutrophils to modify their gene expression to better adapt to bacterial infection is in part regulated by CD73 and declines with age.
KEYWORDS: RNA-Seq, PMNs, pneumonia, extracellular adenosine, JNK/AP-1, MAPK, aging, pneumococcus
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is an opportunistic pathogen that normally resides in the human nasopharynx but has the capacity to cause life-threatening infections that result in more than a million deaths annually (1). Pneumococcal infections are particularly a problem for elderly individuals. Despite the availability of vaccines and antibiotic therapies, S. pneumoniae remain a leading cause of community-acquired bacterial pneumonia in individuals older than 65 years of age (2). According to a recent Active Bacterial Core surveillance report, individuals of ≥50 years of age accounted for 71% of S. pneumoniae cases and 82% of associated deaths (3). Novel interventions are thus required to prevent a significant loss of life in the elderly and to combat the health and economic burdens posed by this infection (4).
Neutrophils (also known as polymorphonuclear leukocytes [PMNs]) play a central role in the clearance of S. pneumoniae infections. We and others have found previously that PMNs are required for host resistance against pneumococcal infections (5–7), as depletion of PMNs prior to pneumococcal pulmonary challenge results in significantly higher bacteria burden in the lungs and increases lethality (7). It is well known that PMN antibacterial function declines with age (8, 9). We previously found that this could be recapitulated in mouse models, where we observed a significant decrease in opsonophagocytic killing of S. pneumoniae by PMNs isolated from old mice compared to that by those from young controls (10). Strikingly, adoptive transfer of PMNs from young mice reversed the susceptibility of aged mice to pneumococcal pneumonia (10). This emphasizes the importance of PMNs in immunity and highlights their potential as targets for interventions that boost the resistance of elderly hosts against infection. However, the host pathways that drive the age-associated decline in PMN function remain to be fully elucidated.
The extracellular adenosine (EAD) pathway plays an important role in host resistance to pneumococcal infection (7). Upon infection, ATP released by damaged or injured cells is converted into EAD by the sequential action of two extracellular enzymes, CD39, which converts ATP to AMP, and CD73, which then dephosphorylates AMP to EAD (11). We previously found that genetic ablation or pharmacological inhibition of CD73 in mice results in higher pulmonary pneumococcal loads and systemic spread of infection (7). CD73 is required for the ability of PMNs to kill S. pneumoniae, as PMNs isolated from young CD73 knockout (CD73KO) mice fail to kill pneumococci ex vivo (7, 10, 12). Importantly, age-driven changes in the EAD pathway impair the antibacterial function of PMNs. PMNs from old mice express significantly less CD73 than PMNs from young controls, and supplementation with EAD reverses the age-driven decline in the ability of PMNs to kill S. pneumoniae (10).
The aim of this study was to investigate how aging impairs the antimicrobial activity of PMNs and what aspect of this is regulated by CD73. Although PMNs have prepackaged antimicrobial compounds that can readily kill bacteria, studies have demonstrated that PMNs also undergo significant changes in their transcriptome in response to inflammation and bacterial infection (13, 14). Therefore, we examined global transcriptional changes in PMNs in response to S. pneumoniae infection ex vivo and how these responses are altered with aging and the absence of CD73. We found that infection with S. pneumoniae significantly altered the transcriptional profiles of PMNs from all host groups and that, importantly, active transcription was required for the ability of PMNs to kill bacteria. Surprisingly, we found that many more genes were downregulated than upregulated in response to infection. Downregulated genes indicated a dampening of proinflammatory immune responses in PMNs from CD73KO and wild-type (WT) old mice, but not in young hosts. Interestingly, larger numbers of long noncoding RNAs (lncRNAs) were found to be differentially expressed upon pneumococcal challenge in PMNs from CD73KO mice compared to PMNs from WT mice. Predicted network analysis of these lncRNAs indicated that various immune signaling pathways are potentially regulated downstream of the EAD pathway. We also found an increased expression of mitogen-activated protein kinase (MAPK) signaling pathway genes in PMNs from old and CD73KO mice but not in those from young hosts. We confirmed that the activation of c-Jun N-terminal kinase/activator protein-1 (JNK/AP-1), one of the MAPK signaling pathways, was significantly upregulated in response to S. pneumoniae infection in PMNs from CD73KO and old mice compared to young controls. Importantly, pharmacological inhibition of JNK/AP-1 reversed the defect in pneumococcal killing by PMNs from old and CD73KO mice, indicating that this pathway can potentially be targeted to reverse the age-related dysregulation of PMN responses.
RESULTS
Active transcription and translation are important for the ability of PMNs to kill S. pneumoniae.
We previously reported that PMNs from old mice fail to efficiently kill S. pneumoniae, in part due to a decline in the surface expression of CD73 and extracellular adenosine production (10). In this study, we wanted to explore whether CD73 and age-driven changes in the transcriptome impair PMN antimicrobial function. As PMNs are known to have antimicrobial products presynthesized and packaged during maturation for rapid immune response (15), we investigated the importance of active transcription and translation in the ability of PMNs to kill S. pneumoniae. To do this, we used a well-established ex vivo opsonophagocytic killing assay (7, 16) in which we isolated PMNs (Ly6G+) from the bone marrow of young C57BL/6 wild-type (WT) mice and treated them prior to infection with S. pneumoniae with either actinomycin D (a transcription inhibitor [17]) or cycloheximide (a translation inhibitor [18]) at concentrations that do not impair cellular viability (17, 19). We found that treating PMNs with actinomycin D caused a significant 2-fold decrease in bacterial killing compared to vehicle control (VC), while treatment with cycloheximide completely abrogated the ability of PMNs to kill bacteria and instead enabled bacterial growth in the presence of PMNs (Fig. 1A). These findings suggest that active transcription of new mRNAs and formation of new proteins is crucial for optimal antipneumococcal responses.
FIG 1.
Active transcription and translation are required for the ability of polymorphonuclear leukocytes (PMNs) to kill Streptococcus pneumoniae. (A) PMNs isolated from the bone marrow of C57BL/6 young wild-type (WT) mice were treated with 5 μg/ml of actinomycin D (Act.D), 10 μg/ml of cycloheximide (CHX), or phosphate-buffered saline (PBS; vehicle control [VC]) for 30 min at 37°C. Treated neutrophils were then infected with S. pneumoniae TIGR4 cells preopsonized with homologous serum for 45 min at 37°C. Reactions were plated on blood agar plates, and the percentage of bacteria killed compared to a no-PMN control under the same conditions was calculated. Positive percent killing indicates bacterial death, while negative percent indicates bacterial growth. (B and C) PMNs isolated from the bone marrow of C57BL/6 young WT, old WT, and CD73 knockout (CD73KO) mice were infected with 103 (B) or 4 × 105 (C) S. pneumoniae TIGR4 CFU preopsonized with homologous serum for 45 min at 37°C. MOI, multiplicity of infection. Reaction mixtures were plated on blood agar plates, and the percentage of bacteria killed compared to a no-PMN control under the same condition was calculated for each strain. Data shown are pooled from (A and B) six separate experiments (n = 6 biological replicates) or from (C) three separate experiments (n = 3 biological replicates) where each condition was tested in triplicate (n = 3 technical replicates) per experiment. Asterisks indicate significant differences between the indicated groups as calculated by Student’s t test.
Profiling of mRNA expression.
We wanted to test whether there are age-related changes in mRNA expression that render PMNs ineffective in their antimicrobial function. In addition, we were interested in investigating whether any of the age-driven changes were shared by PMNs that lack CD73. We first reconfirmed that aging and lack of CD73 significantly blunts the ability of PMNs to kill S. pneumoniae ex vivo using a multiplicity of infection (MOI) of 0.01, typically used in standard published opsonophagocytic (OPH) killing assays (Fig. 1B) (7, 10, 12, 20). For transcriptional analysis, we wanted to use a higher MOI to enhance bacterial interaction with PMNs, and therefore we tested bacterial killing by PMNs from the different hosts at an MOI of 4. We found that PMNs from old and CD73KO mice were significantly impaired in their ability to kill S. pneumoniae compared to those from young controls (Fig. 1C), confirming that aging and lack of CD73 significantly blunt PMN antibacterial function regardless of the bacterial MOI. Next, RNA sequencing (RNA-Seq) was used to compare the transcriptional profiles of PMNs from young WT, old WT, and young CD73KO mice at baseline and upon infection. For RNA isolation, we obtained a pure PMN population (approximately 99% purity; see Fig. S1 in the supplemental material) from the bone marrow of mice using negative selection (see Materials and Methods). Three mice were used per strain. Efficient killing of pneumococci by PMNs from young controls requires opsonization (21). Therefore, to more closely mimic in vivo conditions and the opsonophagocytic killing assay (Fig. 1C), PMNs isolated from each mouse were either challenged with the S. pneumoniae TIGR4 strain (at an MOI of 4) opsonized with homologous mouse serum from the same mouse for 40 min or mock challenged with serum containing buffer. We focused on the 40-min time point, as this is a standard time period used in ex vivo killing assays (7, 10, 12) and it allows us to examine differences in antimicrobial function (Fig. 1B and C) while maintaining PMN viability (≤20% PMN necrosis [PI+]; see Fig. S4B in the supplemental material). Detailed methods on pure PMN isolation and subsequent RNA sequencing workflow are given in Materials and Methods and are summarized in Fig. 2A. Differentially expressed genes (DEGs) were analyzed using DESeq2, and significant differential expression of a gene was defined as expression with a fold change value of ≥2.0 and a false-discovery rate (FDR) of <0.05.
FIG 2.
RNA sequencing experimental approach. (A) Schematic diagram of PMN isolation, sample preparation, and RNA sequencing analysis. (B and C) Principal-component analysis (PCA) plot showing variance in mRNA expression (post data normalization) in uninfected or S. pneumoniae-challenged samples, presented as separate plots for each mouse strain (B) or showing all infected samples on the same plot (C).
Mock-infected PMNs from young, old, and CD73KO mice show limited differences in mRNA profiles.
To determine if there is an intrinsic age-driven change in expression of genes that shape antimicrobial responses, we compared the mRNA expression profiles of mock-challenged PMNs from old WT mice to those of young WT controls. Keeping the expression of PMNs from young WT mice as a baseline, we found a total of 23 DEGs to be upregulated in PMNs from old mice (Table 1). Surprisingly, 15 of these DEGs corresponded to the category of either immunoglobulin heavy chain variable regions or the immunoglobulin kappa chain variable region (Table 1). mRNA levels of certain variable region genes have been previously shown to differ in PMNs, although these cells do not express immunoglobulins (22). PMNs from old WT mice also showed upregulation of a few other genes, which included Calca (calcium regulation and cAMP activity), Mt2 (metal ion regulation), Ces1d (lipase activity), Col5a1 (type V collagen), and C130026l21Rik (unannotated lncRNA) (Table 1). Interestingly, none of the genes known for their role in PMN antimicrobial function showed an age-driven differential expression under baseline conditions.
TABLE 1.
Differentially expressed genes in mock-challenged PMNs from old WT mice compared to those from young WT micea
| Old_WT_SpNeg_vs_Young_WT_SpNeg (as base) | Log2FC | P value | Padj (FDR)b |
|---|---|---|---|
| Ighv2-9 | 5.324005446 | 4.76E−05 | 0.044462976 |
| Igkv4-91 | 5.232587656 | 5.49E−05 | 0.049161656 |
| Igkv4-79 | 5.05316031 | 4.04E−05 | 0.042456147 |
| Igkv8-19 | 4.536847197 | 3.60E−09 | 1.11E−05 |
| Ighv14-3 | 4.456074702 | 4.35E−05 | 0.042456147 |
| Igkv12-89 | 3.968347624 | 2.66E−08 | 6.36E−05 |
| Igkv14-126 | 3.631253191 | 7.12E−13 | 3.83E−09 |
| Ighv11-2 | 3.557189056 | 2.33E−26 | 5.02E−22 |
| Calca | 3.212945532 | 4.51E−14 | 3.23E−10 |
| Igkv6-15 | 3.135262255 | 2.49E−07 | 0.000485531 |
| Igkv8-27 | 3.022666053 | 4.31E−05 | 0.042456147 |
| Ighv5-6 | 2.975616563 | 8.41E−06 | 0.012907276 |
| Gata3 | 2.949808135 | 7.68E−10 | 2.75E−06 |
| Ighv1-53 | 2.750705075 | 4.67E−10 | 2.01E−06 |
| Igha | 2.691611019 | 6.17E−15 | 6.63E−11 |
| Igkv6-32 | 2.666982201 | 2.17E−05 | 0.027412136 |
| Ighv5-17 | 2.381609479 | 1.09E−07 | 0.000233962 |
| Mt2 | 2.296737618 | 2.84E−06 | 0.005077141 |
| Ly6a | 2.155448841 | 1.31E−05 | 0.01870331 |
| C130026I21Rik | 1.641618531 | 1.54E−05 | 0.020715214 |
| Ces1d | 1.384138134 | 3.24E−06 | 0.005347714 |
| Rn18s-rs5 | 1.077561976 | 2.60E−05 | 0.031055339 |
| Col5a1 | 1.060211245 | 2.48E−08 | 6.36E−05 |
List of differentially expressed (DE) genes (log2 fold change [log2FC] ≥ 1.0 or log2FC ≤ −1.0; false-discovery rate [FDR] < 0.05) in mock-challenged PMNs from old wild-type (WT) mice compared to mock-infected PMNs from young WT mice.
Padj, adjusted P value.
To determine if there is an intrinsic CD73-driven change in the expression of genes that shape antimicrobial responses, we then compared the mRNA expression profiles of mock-stimulated PMNs from young CD73KO mice to those of young WT mice. As shown in Table 2, we noted that only 8 genes were differentially expressed in resting PMNs, with equal numbers of upregulated and downregulated DEGs. Upregulated DEGs included Gm11868 (an lncRNA with predicted histone demethylase activity in Drosophila), Gm13456 (a pseudogene related to somatic muscle development activity in Drosophila), Gm6548 (an unannotated pseudogene), and Ighv9-4 (corresponding to the category of immunoglobulin heavy chain variable regions). As expected, the downregulated DEGs included NT5E (which encodes CD73). Other downregulated DEGs included Fam63b (ubiquitin carboxyl-terminal hydrolase activity), Aqp9 (transmembrane transporter activity), and Cyb5r4 (NADPH-cytochrome reductase activity). As observed in old mice, none of the known antimicrobial genes showed a differential expression in mock-challenged CD73KO PMNs. In summary, we found limited differences in mRNA expression in mock-challenged PMNs from WT and CD73KO mice, as well as limited differences across host age.
TABLE 2.
Differentially expressed genes in mock-challenged PMNs from CD73KO mice compared to those from young WT micea
| CD73KO_SpNeg_vs_Young_WT_SpNeg (as base) | Log2FC | P value | Padj (FDR) |
|---|---|---|---|
| Gm11868 | 5.382471685 | 4.98E−30 | 3.69E−26 |
| Ighv9-4 | 5.305558233 | 1.53E−06 | 2.84E−03 |
| Gm13456 | 2.127675061 | 1.06E−32 | 1.57E−28 |
| Gm6548 | 1.275332385 | 2.14E−07 | 4.52E−04 |
| Fam63b | −1.142265331 | 5.00E−21 | 2.47E−17 |
| Aqp9 | −1.14542389 | 8.13E−06 | 1.20E−02 |
| Cyb5r4 | −1.393240659 | 9.15E−17 | 2.71E−13 |
| Nt5e | −2.880728481 | 2.53E−18 | 9.39E−15 |
List of differentially expressed (DE) genes (log2FC ≥ 1.0 or log2FC ≤ −1.0; FDR < 0.05) in mock-challenged CD73KO PMNs compared to mock-infected WT PMNs from young mice.
S. pneumoniae induces global changes in transcriptome profiles.
We next wanted to determine whether S. pneumoniae infection induced any transcriptional changes in PMNs. To do this, the global transcriptome profiles of infected and mock-challenged PMNs were characterized for each mouse group. Principal-component analysis (PCA) was done prior to and after pneumococcal infection to investigate changes in patterns of mRNA expression between the different groups. We found that infection with S. pneumoniae resulted in major transcriptome changes in all three PMN types (Fig. 2B). Analysis of PMNs from each mouse group clearly showed distinct patterns of mRNA expression between the mock-infected and infected samples, with a combined total variance of 49% (PC1 and PC2), suggesting a distinct response of PMNs to S. pneumoniae. In PMNs from all three mouse groups, the mock-challenged samples formed a cluster separate from the corresponding infected samples (Fig. 2B). When we compared infected PMNs across the different mouse groups, we found that while CD73KO PMNs showed variation, PMNs from young WT mice clustered distinctly from those of the corresponding old mice (Fig. 2C). In summary, infection with S. pneumoniae triggers global changes in PMN transcriptome profiles that differs across host age.
Genes and functional categories upregulated in response to S. pneumoniae.
We then explored the genes whose expression was upregulated upon PMN infection and how this varied among the different host groups. By selecting DEGs with at least a 2-fold change in expression compared to mock-infected controls for each mouse group, we surprisingly found only a small number of genes (10 per group) that were upregulated in PMNs from WT mice in response to pneumococcal challenge, regardless of age (Fig. 3A and Fig. 4A). In contrast, CD73KO PMNs showed the strongest transcription response to bacterial infection, with 36 upregulated genes (Fig. 5A). Some of the upregulated DEGs were common in PMNs from all three mouse groups (Fig. 6A). The six overlapping DEGs (Osm, Fos, Jun, Zfp36, Egr1, and Atf3) belonged to the categories of growth regulators, transcription factors, and transcription and translation regulators. When we compared DEGs that were commonly upregulated in PMNs from old WT and CD73KO mice, but not in those from young WT mice, we found only two DEGs (Fig. 6A), Slfn1, which has a known role in cell proliferation and immune response, and Nr4a1, a transcription factor. When we examined the DEGs that were upregulated in response to infection that were specific to CD73KO PMNs, we found increased expression of Btg2 (regulation of the cell cycle), Zcchc4 (nucleic acid binding and methyltransferase activity), Dusp1 (phosphatase activity), Klhl42 (ubiquitin-protein transferase activity), Snai1 and Hlx (sequence-specific DNA binding activity), F3 (phospholipid binding and cytokine receptor activity), Hspa1a and Hspa1b (ubiquitin protein ligase binding and protein folding chaperone), Tacstd2 (calcium signaling), and Rhob (GDP and GTP binding activity). A number of upregulated DEGs from CD73KO PMNs belonged to the category of lncRNAs that have not been functionally annotated, and thus their roles in cellular function are currently unknown.
FIG 3.
Analysis of differentially expressed genes (DEGs) in response to S. pneumoniae infection in PMNs from young WT mice. (A) Volcano plot representing differential gene expression (false-discovery rate [FDR], <0.05) in response to ex vivo challenge with S. pneumoniae TIGR4 compared to a mock-challenged control in PMNs isolated from the bone marrow of young WT mice. Genes marked in green represent significantly downregulated DEGs (log2 fold change [log2FC] ≤ −1.0; FDR < 0.05), and genes marked in red represent significantly upregulated DEGs (log2FC ≥ 1.0; FDR < 0.05). (B and C) Gene ontology (GO) enrichment analysis using DAVID, indicating the top 10 significant (P ≤ 0.05) biological process (BP), molecular function (MF), cellular component (CC), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway terms for significantly downregulated DEGs (B) and significantly upregulated DEGs (C).
FIG 4.
Analysis of differentially expressed genes in response to S. pneumoniae infection in PMNs from old WT mice. (A) Volcano plot representing differential gene expression (FDR, <0.05) in response to ex vivo infection with S. pneumoniae TIGR4 compared to a mock-infected control in PMNs isolated from the bone marrow of old WT mice. Genes marked in green represent significantly downregulated DEGs (log2FC ≤ −1.0; FDR < 0.05), and genes marked in red represent significantly upregulated DEGs (log2FC ≥ 1.0; FDR < 0.05). (B and C) Gene ontology (GO) enrichment analysis using DAVID, indicating the top 10 significant (P ≤ 0.05) biological process (BP), molecular function (MF), cellular component (CC), and KEGG pathway terms for significantly downregulated DEGs (B) and significantly upregulated DEGs (C).
FIG 5.
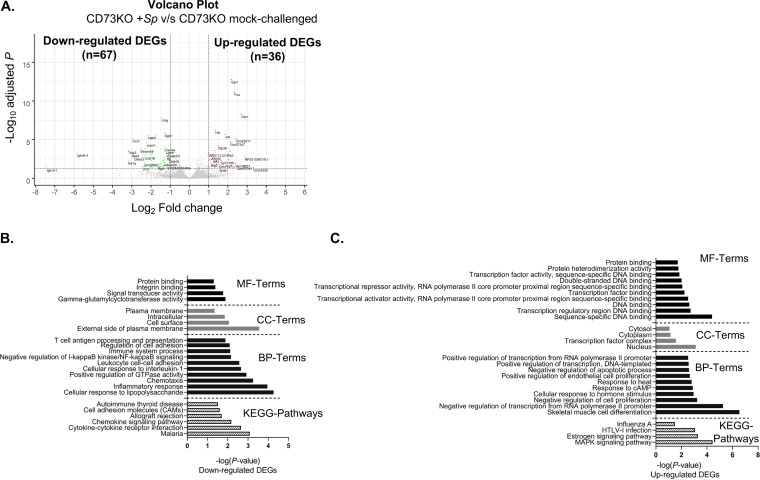
Analysis of differentially expressed genes in response to S. pneumoniae infection in PMNs from young CD73KO mice. (A) Volcano plot representing differential gene expression (FDR, <0.05) in response to ex vivo challenge with S. pneumoniae TIGR4 compared to a mock-infected control in PMNs isolated from the bone marrow of CD73KO mice. Genes marked in green represent significantly downregulated DEGs (log2FC ≤ −1.0; FDR < 0.05), and genes marked in red represent significantly upregulated DEGs (log2FC ≥ 1.0; FDR < 0.05). (B and C) Gene ontology (GO) enrichment analysis using DAVID, indicating the top 10 significant (P ≤ 0.05) biological process (BP), molecular function (MF), cellular component (CC), and KEGG pathway terms for significantly downregulated DEGs (B) and significantly upregulated DEGs (C).
FIG 6.
Venn diagrams showing distribution of significantly upregulated or downregulated genes across host groups in response to S. pneumoniae infection. Distribution of significantly upregulated (log2FC ≥ 1.0; FDR < 0.05) (A) and significantly downregulated (log2FC ≤ −1.0; FDR < 0.05) (B) DEGs in PMNs from young WT versus old WT versus CD73KO mice upon pneumococcal challenge.
We next grouped upregulated genes into different functional categories (see Tables S2 to S4 in the supplemental material). Overall, there was a significant overlap in the annotated processes between PMNs from the three mouse groups with DEGs falling mainly into the categories of DNA binding, transcription regulation, and transcription factor activity (Fig. 3C and Fig. 4C and Fig. 5C), as many of these DEGs are known to regulate gene expression as coactivators, regulators, or transcription factors (Fos, Jun, Egr1, Atf3, Sertad3, Nr4a1, F3, and Hlx). These data indicate that upon challenge with S. pneumoniae, PMNs may have undergone transcriptional reprogramming, indicated by upregulation of genes involved in transcription activation or transcription regulation.
Genes and functional categories downregulated in response to S. pneumoniae.
Genes whose expression was downregulated upon PMN infection were examined, including their variation among the different host groups. Interestingly, we found more genes (2- to 3-fold more) that were downregulated than upregulated in PMNs in response to infection in all mouse groups (Fig. 3A and Fig. 4A and Fig. 5A). A total of 56 genes were downregulated in response to pneumococcal challenge in PMNs from young mice, while only 35 genes were downregulated in PMNs from old mice (Fig. 3A and Fig. 4A). As observed with the upregulated DEGs, CD73KO PMNs showed the strongest transcriptional response following S. pneumoniae challenge, with 67 downregulated DEGs (Fig. 5A). Overall, there was considerable overlap observed between PMNs from the three mouse groups (Fig. 6B). The 24 overlapping DEGs belonged to the categories of immune and inflammatory response (Tnip3, Icam1, Sgk1, Prdm1, Cxcl16, and Prdm1), MAPK signaling (Dusp4), cell surface signaling (Adora2b, Treml4, P2ry10, and Itga5), transcription regulation (Jmy, Rora, and Nab2), microtubule organization (Kif1a), protein regulation (Trim13), cell cycle and cell-cell adhesion (Avp1 and Serpinb8), actin cytoskeleton (Phldb1), podocyte function (Schip1), apoptosis (Ggct), metallopeptidase (Astl), embryonic development function and tumorigenesis (Olfml3), and Notch signaling (Chac1). Comparison of DEGs that were commonly downregulated in PMNs from old WT and CD73KO mice showed 6 overlapping downregulated DEGs that were not differentially expressed in PMNs from young WT mice (Fig. 6B). These included Tubb6 (microtubule organization), Rgl1 (guanine nucleotide exchange factor), Rrad (GTPase activity), Cd40 (immune and inflammatory response), Tnip1 (inflammatory response), and Emp1 (cell-cell interaction and cell proliferation). These findings point toward an overall age-related decrease in immune and inflammatory response, characteristics of which are also shared by CD73KO PMNs.
To further understand how CD73 regulates the transcriptional profile during infection, we examined the distribution of DEGs that were downregulated in CD73KO but not in WT PMNs (Fig. 6B). These included the migration-related genes Cxcr5 (C-X-C-chemokine receptor activity), Cccl2 (CCR2 chemokine receptor binding), and Icam4 (integrin binding); the G protein-coupled receptor-related genes S1pr1 (G protein-coupled receptor binding) and Gpr84 (G protein-coupled peptide receptor activity); the GTP-related genes Gbp5 (GTP hydrolysis), Rnd1 (GTPase activity), and Tbc1d4 (GTPase activator activity); the kinase-related genes Sdc4 (protein kinase C binding), Itk (tyrosine kinase activity), Nuak1, and Pim2 (serine/threonine protein kinase activity); and genes involved in other processes, namely Bcl2a1a (apoptotic process), Gpatch3 (nucleic acid binding), Clec2d (transmembrane signaling receptor activity), Lgmn (endopeptidase activity), Lfng (acetylglucosaminyl transferase activity), and F10 (calcium and phospholipid binding). These data suggest potential dysregulation in PMN migration in response to S. pneumoniae in the absence of CD73, which is consistent with our previous findings (7).
To elucidate the PMN responses dampened upon pneumococcal challenge in susceptible versus resistant hosts, we compared the distribution of DEGs that were downregulated in PMNs from young WT mice but not in PMNs from old WT or CD73KO mice. These included Dusp8 (tyrosine/serine/threonine phosphatase activity), Ctla4 (negative regulator of T-cell responses), Bhlhe40 (transcriptional repressor activity), Tnfrsf8 (transmembrane signaling receptor activity), Cish (1-phosphatidylinositol-3-kinase regulator activity), Rgs1 (GTPase activator activity), and Jag2 (calcium ion binding and growth factor activity). These data suggest that select genes that inhibit immune responses are downregulated in young WT hosts to better respond to S. pneumoniae challenge.
We further categorized downregulated genes into different functional categories (Tables S2 to S4). As expected, many genes were shared by more than one functional category. Overall, the functional categories which were commonly downregulated in all three PMN types included cellular response to lipopolysaccharide, inflammatory response, gamma-glutamylcyclotransferase activity, and components of cell surface and external side of the plasma membrane (Fig. 3B and Fig. 4B and Fig. 5B). Interestingly, PMNs from both old WT and CD73KO mice, but not young WT mice, showed downregulation of NF-κB signaling regulation upon S. pneumoniae challenge. In summary, the majority of downregulated DEGs across all three PMN types belonged to the categories of transcription regulators and immune regulators.
S. pneumoniae induces changes in lncRNA expression in the absence of CD73.
Further analysis identified a total of 22 lncRNAs that were either significantly up- or downregulated in CD73KO PMNs upon pneumococcal challenge (Fig. 7). We observed a smaller number of lncRNAs (n = 5) in WT PMNs from young mice (Fig. 8), while PMNs from WT old mice had none. We made several searches in all available gene ontology (GO) and annotation databases and found to the best of our knowledge that these lncRNAs have not been previously functionally annotated. We therefore performed prediction and network analysis (see Materials and Methods) of CD73KO-specific lncRNAs and found a total of 105 potential target interactions, including 3 genes (Il10, Icam1, and Rora) identified in our RNA sequencing analysis (Fig. 7; see also Table S5 in the supplemental material). Since lncRNAs could directly bind to the target mRNA through complementary base pairing and thus determine the regulation of gene expression, we therefore inferred the biological functions of our lncRNAs based on their direct interaction with the gene targets, which in turn perturb the biological process in the disease pathway. For example, Gm37747 can bind to several gene targets, including Cers6-205, Atp8a1-207, Spc25, Lpr2, Il10, Icam1, Atf3 and Ldb2-204, that perturb signal transduction, regulation of cell adhesion, and cellular response to tumor necrosis factor. This would signify that Gm37747 is an important lncRNA in these pathways. Importantly, we identified 5 pathways (longevity regulation pathway, MAPK signaling pathway, apoptosis signaling pathway, nuclear receptor transcription pathway, and metabolic pathway) that were regulated by these lncRNAs (Fig. 7). Among the predicted biological processes (Fig. 7) were several signaling pathways, including signal transduction by protein phosphorylation cascade, positive regulation of MAPK, interferon signaling, cytokine signaling in the immune system, cellular response to tumor necrosis factor, positive regulation of protein kinase C signaling, and negative regulation of protein kinase B signaling. For young WT PMNs, lncRNA network analysis predicted different target genes (Table S5), but only one biological process was found and it was connected to Reep3 (olfactory signaling pathway and cellular component organization or biogenesis) in the network (Fig. 8). These findings suggest that during S. pneumoniae infection, expression of lncRNAs in PMNs is controlled by CD73.
FIG 7.
CD73KO PMN-specific lncRNA-target network and biological process. In the network, red diamonds represent lncRNAs in CD73KO PMNs that are significantly upregulated in response to S. pneumoniae infection, while green diamonds represent the downregulated lncRNAs. Blue ovals indicate predicted gene targets for the lncRNAs, while the green ovals are the genes identified in actual RNA sequencing analysis. The big blue rectangles represent the predicted significantly impacted biological processes and pathways.
FIG 8.
Young WT PMN-specific lncRNA-target network and biological process. In the network, green diamonds represent the lncRNAs in young WT PMNs that are downregulated in response to S. pneumoniae infection. Blue ovals are predicted gene targets for the lncRNAs. The big rectangle represents the predicted significantly impacted biological pathway.
RT-PCR validation.
To validate our RNA sequencing data, we tested the expression of a subset of differentially expressed genes through real-time PCR (RT-PCR). The selection of genes tested was based on role in PMN function (Il10 and Adora2b) or role in MAPK pathways (Fos, Jun, Hspa1a, and Atf3) or was selected randomly (Rrad and Rgl1). The same samples on which RNA sequencing was performed were converted to cDNA for RT-PCR validation. Data were analyzed by the comparative threshold cycle (2−ΔΔCT) method, normalizing the threshold cycle (CT) values obtained for target gene expression to those for GAPDH of the same sample. The average of fold change values of target mRNA expression in infected samples was calculated relative to uninfected controls and then converted to a log2 scale, as described for the RNA sequencing data. Multiple targets were tested in the CD73KO RNA samples, as this group showed the strongest transcriptional response to S. pneumoniae challenge. Overall, the average log2 fold change values obtained during RT-PCR and RNA sequencing were consistent for the tested target genes (Fig. 9C and D; see also Fig. S2A in the supplemental material) with a Pearson correlation coefficient of 0.8632 and a P value of <0.01 (Fig. S2B).
FIG 9.
Validation of MAPK signaling pathway differentially expressed genes by real-time PCR. Lists of KEGG pathways and the corresponding genes retrieved from DAVID software using significantly upregulated DEGs (log2FC ≥ 1.0; FDR < 0.05), in response to infection with S. pneumoniae TIGR4 compared to a mock-infected control, in PMNs isolated from the bone marrow of old WT (A) or CD73KO (B) mice are shown. Expression of select upregulated DEGs corresponding to the MAPK signaling pathway identified during RNA sequencing (white bars) was validated by real-time PCR (RT-PCR) (black bars) for old WT (C) and CD73KO PMNs (D). The data shown are the log2 value of the average of fold change values of target mRNA expression in infected samples relative to that in mock-infected controls. The relative fold change in target mRNA expression was calculated using three separate biological samples. Data were analyzed using the comparative threshold cycle (2−ΔΔCT) method, normalizing the threshold cycle (CT) values obtained for target gene expression to those for GAPDH of the same sample.
The MAPK signaling pathway is differentially upregulated in PMNs from CD73KO and old mice in response to S. pneumoniae infection.
DEGs significantly downregulated or upregulated in PMNs from young WT, old WT, and CD73KO mice upon pneumococcal challenge were analyzed separately to identify pathways responsive to S. pneumoniae challenge. As the number of significant DEGs (FDR value, <0.05) identified was low, we first used a liberal approach to perform functional category analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID), in which all functional categories and pathways with a P value of <0.05 were considered significant. We found that for PMNs from young WT mice, autoimmune thyroid disease and cytokine-cytokine receptor interaction pathway terms were downregulated (Fig. 3B). No downregulated Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway was observed in PMNs from old WT mice. In contrast, downregulated pathways were identified in CD73KO PMNs (Table 3) and included malaria, cytokine-cytokine receptor interaction, the chemokine-signaling pathway, allograft rejection, cell adhesion molecules, and autoimmune thyroid disease (Fig. 5B). When we compared pathways that were upregulated, we did not find any in PMNs from young WT mice. In contrast, in PMNs from old WT mice, the upregulated pathway terms included HTLV-1 infection, the MAPK signaling pathway, and leishmaniasis and colorectal cancer (Fig. 4C), while the upregulated pathways in CD73KO PMNs included the MAPK signaling pathway, the estrogen signaling pathway, HTLV-1 infection, and influenza A (Fig. 5C).
TABLE 3.
Downregulated KEGG pathways in PMNs isolated from young WT or CD73KO mice in response to S. pneumoniae infectiona
| KEGG pathway term | Downregulation in: |
|||||
|---|---|---|---|---|---|---|
| Young WT PMNs upon S. pneumoniae challenge |
CD73KO PMNs upon S. pneumoniae challenge |
|||||
| No. of genes | Genes | P value | No. of genes | Genes | P value | |
| Auto-immune thyroid disorder | 3 | CTLA4, GZMB, IL10 | 1.70E−02 | 3 | GZMB, CD40, IL10 | 3.1E−02 |
| Cytokine-cytokine receptor interaction | 4 | CCR7, CXCL16, TNFRSF8, IL10 | 3.0E−02 | 6 | CCR7, CCL2, CXCR5, CXCL16, CD40, IL10 | 2.20E−03 |
| Malaria | 4 | ICAM1, CCL2, CD40, IL10 | 8.20E−04 | |||
| Chemokine signaling pathway | 5 | ITK, CCR7, CCL2, CXCR5, CXCL16 | 6.7E−03 | |||
| Allograft rejection | 3 | GZMB, CD40, IL10 | 2.0E−02 | |||
| Cell adhesion molecules (CAMs) | 4 | PVR, ICAM1, CD40, SDC4 | 2.5E−02 | |||
Significantly (P ≤ 0.05) downregulated Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways with genes involved and P values based upon DAVID analysis of significantly downregulated differentially expressed genes (DEGs) (log2FC ≥ 1.0; FDR < 0.05), compared to mock-challenged controls, in PMNs isolated from young WT or CD73KO mice in response to S. pneumoniae TIGR4 infection.
Importantly, PMNs from old WT and CD73KO mice shared two common upregulated pathways, which included the MAPK signaling pathway (Fig. 9A and B). This pathway was also significantly upregulated in CD73KO PMNs upon infection when the analysis was performed with an FDR value of <0.05 as a criterion. KEGG analysis indicated that S. pneumoniae induced upregulation of JNK as one of the common MAPK pathways in PMNs from old WT and CD73KO mice (see Fig. S3 in the supplemental material). We observed upregulation of Fos and Jun, the components of the activator protein-1 (AP-1) transcription complex, which is regulated downstream of JNK signaling (23). Differential expression of select genes (Fos, Jun, and Hspa1a) in this pathway upon infection of PMNs from WT old and CD73KO mice was further confirmed using RT-PCR (Fig. 9C and D). To determine whether changes at the gene expression level translated to functional differences in JNK pathway signaling, we quantified the proportion of c-Jun that undergoes phosphorylation in response to pneumococcal challenge. When phosphorylated, c-Jun forms part of the AP-1 transcription factor complex that is activated downstream of JNK signaling (23). We found increased phosphorylation of c-Jun in response to S. pneumoniae infection (Fig. 10) and, importantly, the proportion of c-Jun that was phosphorylated was significantly higher in infected PMNs from old WT and CD73KO mice than that in young controls (Fig. 10B). These findings demonstrate age- and CD73-driven changes in MAPK signaling in PMNs in response to pneumococcal infection.
FIG 10.
Phosphorylated c-Jun pools are higher in PMNs from old and CD73KO mice following S. pneumoniae infection. PMNs isolated from the bone marrow of the indicated strains of mice were incubated for 30 min at 37°C with S. pneumoniae TIGR4 preopsonized with matching serum at an MOI of 4 or were mock treated (uninfected) with 3% matching mouse serum only. Flow cytometry was used to determine the effect of bacterial infection on phospho-c-Jun (Ser73) levels. (A) Gating strategy followed during analysis of flow cytometry in young WT mice. We gated on PMNs (Ly6G+ cells) and measured the expression (mean fluorescent intensity [MFI]) of phospho-c-Jun (Ser73) and total c-Jun. (B) PMNs from young WT mice were either mock challenged, treated with anisomycin (JNK/AP-1 pathway activator), or infected with S. pneumoniae in the absence or presence of JNK-IN-8 (JNK/AP-1 pathway inhibitor). The ratio of phosphorylated c-Jun to the total cellular levels of c-Jun is presented. (C) PMNs from young WT, old WT, and CD73KO mice were infected with S. pneumoniae, and the ratio of phosphorylated c-Jun with respect to the total cellular levels of c-Jun was compared. Representative data (B and C) from one of five separate experiments in which each condition was tested in triplicate (n = 3 technical replicates) per experiment are shown. Asterisks indicate significant differences as calculated by Student’s t test.
Blocking JNK/AP-1 signaling pathway boosts bacterial killing in PMNs from old and CD73KO mice.
We then wanted to explore whether the age- and CD73-driven changes in the JNK MAPK pathway had an effect on PMN function. The JNK/AP-1 signaling pathway is well known for its role in stress-induced apoptotic cell death (24–26). Therefore, we tested whether there were differences in apoptosis between the mouse groups. Using annexin V/propidium iodide (PI) staining and flow cytometry, we found that the percentage of apoptotic PMNs increased following infection (see Fig. S4A to C in the supplemental material); however, there were no differences among the mouse groups. This was further confirmed using a lactate dehydrogenase (LDH) release assay (Fig. S4D).
To determine whether JNK/AP-1 signaling played a role in PMN antibacterial function, we treated PMNs from young WT mice with the JNK stimulator anisomycin and measured their ability to kill bacteria using our opsonophagocytic killing assay. The ability of anisomycin to activate the JNK pathway was confirmed by measuring the extent of c-Jun phosphorylation by flow cytometry (Fig. 10B). Interestingly, we found a significant 2-fold reduction in the ability of PMNs from young mice to kill S. pneumoniae upon treatment with anisomycin (Fig. 11A). Anisomycin had no additive effect on the impaired PMN antibacterial function observed in CD73KO and old mice (Fig. 11B and C). As activation of JNK signaling blunted PMN antimicrobial function in young hosts, we then asked whether the function of PMNs from old WT and CD73KO mice can be rescued by inhibiting this pathway. To do this, PMNs from old WT or CD73KO mice were treated with JNK-IN-8 or SR11302 prior to infection. JNK-IN-8 is a selective and high-affinity inhibitor that irreversibly blocks the catalytic domain of JNK (27), while SR11302 is a selective inhibitor of the AP-1 complex (28). The ability of JNK-IN-8 to inhibit the phosphorylation of c-Jun was also confirmed by flow cytometry (Fig. 10B). We found that, strikingly, treatment of PMNs with SR11302 or JNK-IN-8 significantly enhanced their ability to kill S. pneumoniae by 5- and 10-fold, respectively, in both old and CD73KO mice (Fig. 11 B and C). None of the JNK pathway inhibitors or activators had any significant direct effect on bacterial viability (see Fig. S5 in the supplemental material). These data indicate that blocking the JNK/AP-1 pathway reverses the defect in pneumococcal killing by PMNs from old WT and CD73KO mice. To test whether this improved killing was specific to PMNs that displayed enhanced activation of the JNK/AP-1 pathway, we then measured the effects of SR11302 and JNK-IN-8 on PMN function in young mice. We found that inhibition of JNK/AP-1 resulted in a 1.8-fold reduction in the ability of PMNs from young mice to kill S. pneumoniae (Fig. 11A). Taken together, these data suggest that balanced activation of the JNK/AP-1 pathway is required for bacterial killing by PMNs and that any aberrant changes in this pathway (over- or underactivation) impairs PMN function.
FIG 11.
Blocking JNK/AP-1 pathway boosts the antimicrobial function of PMNs isolated from CD73KO and old WT mice. PMNs isolated from the bone marrow of young WT (A), CD73KO (B), and old WT (C) mice were treated with the indicated JNK stimulator anisomycin (20 μM), JNK inhibitor JNK-IN-8 (20 μM), AP-1 inhibitor SR11302 (20 μM), or enriched Hanks’ balanced salt solution (HBSS+) (vehicle control [VC]) for 30 min at 37°C. Treated PMNs were then challenged with the S. pneumoniae TIGR4 strain preopsonized with homologous serum for 45 min at 37°C. Reaction mixtures were plated on blood agar plates, and the percentage of bacteria killed compared to a no-PMN control under the same conditions was calculated. The fold change in bacterial killing with respect to the controls was then calculated by dividing the value of the treatment group by that of the vehicle control for each strain. Data shown are pooled from three separate experiments (n = 3 biological replicates) in which each condition was tested in triplicate (n = 3 technical replicates) per experiment. Asterisks indicate significantly different from 1 by one-sample t test.
DISCUSSION
PMN antimicrobial function declines with aging and is in part driven by changes in extracellular adenosine production and signaling (10). The aim of this study was to examine transcriptional changes in PMNs in response to S. pneumoniae infection across different hosts to better understand how aging impairs PMN function and what aspect of this was controlled by the extracellular adenosine-producing enzyme CD73. We found very limited differences in mRNA expression in mock-stimulated PMNs across the different hosts, indicating that either the intrinsic age-related defect in PMN function occurs at the protein level, that it is the transcriptional response following external stimulation which drives the difference in PMN responses, or both. In fact, S. pneumoniae infection triggered global transcriptional changes that were distinct across the different hosts.
A surprising finding was that there were 2- to 3-fold more downregulated than upregulated genes in response to infection across all host groups. Sixty percent of the upregulated genes in WT mice were the same regardless of host age, while the majority of the downregulated DEGs were shared across the three different hosts, suggesting an overall blunting of transcriptional activity and expression of only select transcripts in activated PMNs. This is reminiscent of stress responses observed in yeast cells in which only genes required for resistance against a particular stressor are expressed while the rest are shut off, possibly to conserve energy (29–32). Overall, the number of DEGs in response to infection was not high (ranging from 45 to 103 genes across the different hosts), which is consistent with the smaller amount of mRNA and lower overall transcriptional activity observed in PMNs compared to those in other immune cells (33, 34). However, even these relatively moderate changes were key for efficient antimicrobial function, as inhibition of transcription significantly impaired the ability of PMNs to kill pneumococci. It is possible that larger changes in gene expression would be observed with time, as indicated by upregulation of genes involved in transcription activation or transcription regulation across all hosts. Here, we limited our study to observing changes within 40 min of infection due to concerns about the effects of bacterial infection on PMN viability in culture (35). In summary, this study shows that PMNs undergo transcriptional reprogramming, which is required for their ability to efficiently kill bacteria.
CD73KO neutrophils displayed the strongest transcriptional response to S. pneumoniae, with 40% more differentially expressed genes during infection compared to those in WT age-matched controls. This correlated with significant changes in expression of more than 20 lncRNAs in response to infection, 77% of which were upregulated. In contrast, PMNs from young WT controls displayed only 5 differentially expressed lncRNAs, all of which were downregulated, while PMNs from old mice had none. These findings suggest that during S. pneumoniae infection, lncRNA expression in PMNs is negatively controlled by CD73 or extracellular adenosine production. Extracellular adenosine was previously shown to activate expression of MEG3, an lncRNA in a liver cancer cell line (36). This study, to our knowledge, is the first to report a link between the EAD pathway and lncRNA expression in PMNs in response to infection. Furthermore, our data suggest that in the absence of CD73, changes in lncRNA expression dysregulate several biological processes in the cell, including those important for PMN antimicrobial activity. Recent studies have highlighted the role of lncRNAs in transcriptional regulation of inflammatory responses of several immune cells (37), including macrophages (38, 39) and human PMNs (40, 41). Interestingly, polymorphisms in lncRNAs expressed in neutrophils were associated with pneumococcal bacteremia in children in Kenya (42).
Of particular interest in our study were genes that were up- and downregulated only in PMNs from WT old and CD73KO mice but not in the young controls. Among genes that were downregulated were Rrad and CD40, that have a role in oxidative responses. Binding of CD40 to its ligand activates downstream PI3K/NF-κB, leading to a PMN oxidative burst (43), and a defect in CD40 signaling is associated with blunted respiratory burst and antimicrobial activity in human PMNs (43). We previously found that CD73KO PMNs were defective in reactive oxygen species (ROS) production upon pneumococcal challenge (12). While PMNs from old mice do not show a defect in ROS production (10), aging is often associated with a buildup of reactive oxygen species, which, if not controlled, can lead to cellular damage (44). The Ras-related associated with diabetes (Rrad) protein is a GTP binding and calmodulin binding protein involved in reducing oxidative stress and preventing cellular senescence (45). Thus, reduction in Rrad expression could indicate an age-related decline in the ability of PMNs to counteract the oxidative stress induced following S. pneumoniae challenge. Among the genes upregulated only upon infection in PMNs from old WT and CD73KO mice were Slfn1 and Nr4a1. Slfn-1 is known for its role as inducer of cell cycle arrest in immune cells (46). Nr4a1, on the other hand, belongs to a family of nuclear receptor proteins that are rapidly induced under stress conditions and play an important role in DNA repair. Members of this family show aberrant expression in inflamed tissues and have emerged as key regulators of various diseases affecting the aging population (47). Interestingly, DNA damage and cell cycle arrest are characteristic features of cellular senescence (44). Overall, shared changes in gene expression in PMNs from old WT and CD73KO mice in response to infection suggest an overall decline in the ability of these cells to aptly adapt to infection-mediated stress, which is in part regulated by CD73.
KEGG pathway analysis showed that S. pneumoniae upregulated MAPK pathways in PMNs from both CD73KO and old mice but not in PMNs from young hosts. The MAPK pathways include JNK, p38, and ERK1/2, all of which regulate various cellular processes in response to external stimuli (48). Importantly, certain aspects of PMN function are attributed to different MAPK pathways. These include p38 MAPK- and ERK-mediated chemotaxis and respiratory burst (49, 50), MEK/ERK-mediated oxidative burst and phagocytosis (51), and p38 MAPK-mediated degranulation (52). Here, we observed upregulation of Fos and Jun, the components of the activator protein-1 (AP-1) transcription complex, which is regulated downstream of JNK signaling (23). We found that upon infection, c-Jun is phosphorylated in all mouse groups; however, the proportion of c-Jun undergoing phosphorylation was significantly higher in PMNs from old WT and CD73KO mice in comparison to that in young controls, indicating an increase in MAPK activation in these PMNs. Interestingly, host aging has been reported to be associated with an increase in basal levels of activation of other mitogen-activated protein kinase (MAPK) pathways, including ERK1/2 and p38MAPK in human PMNs (53–56). Importantly, these changes impair the ability of PMNs to respond to acute stimuli and impair their function (53, 57). For example, elevated activation of ERK1/2 impairs the ability of inflammatory signals to delay apoptosis in PMNs from elderly donors (54, 58). In fact, pharmacologically targeting these pathways has been shown to improve PMN function in elderly hosts. In sterile injury of the skin, oral administration of a p38 MAPK inhibitor resulted in enhanced PMN clearance in elderly human volunteers (59). Similarly, we found here that stimulation of JNK/AP-1 blunted PMN antipneumococcal responses in young hosts, while inhibition of this pathway rescued the ability of PMNs from old and CD73KO mice to kill S. pneumoniae, indicating that overactivation of JNK/AP-1 impairs PMN antimicrobial function. Overall, our findings indicate that balanced activation of the JNK/AP-1 pathway is required for optimal bacterial killing by PMNs, which fits with reports showing that inhibition of the JNK pathway decreased ROS production and release of neutrophil extracellular traps (NETs) by PMNs from young hosts in response to the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa (60).
In conclusion, this study demonstrated that the ability of PMNs to modify their gene expression to better adapt to bacterial infection is in part regulated by CD73 and found that this capacity declines with age. Importantly, we identified JNK/AP-1 signaling as a potential target for therapeutic intervention that can boost resistance of vulnerable hosts against S. pneumoniae infection.
MATERIALS AND METHODS
Mice.
Wild-type (WT) young (4 months) and old (22 to 24 months) C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and the National Institute on Aging colonies. CD73 knockout (CD73KO) mice on a C57BL/6 background (11) were purchased from Jackson Laboratories and bred at a specific pathogen-free facility at the University at Buffalo. Young (4 months) CD73KO mice were used. Due to the limited availability of aged animals, male mice were used in all experiments. This work was performed in accordance with the recommendations from the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University at Buffalo.
Bacteria.
S. pneumoniae TIGR4 AC316 strain (serotype 4) (61) was a kind gift from Andrew Camilli. Bacteria were grown at 37°C in 5% CO2 in Todd-Hewitt broth supplemented with 0.5% yeast extract and Oxyrase until cultures reached the mid-exponential phase. Aliquots were frozen at −80°C in growth medium with 20% (vol/vol) glycerol. Aliquots were thawed on ice, washed, and diluted in phosphate-buffered saline (PBS) prior to use. Bacterial CFU were enumerated by serial dilution and dribble plating on tryptic soy agar (TSA) plates supplemented with 5% sheep blood (Northeast Laboratory Services).
PMN isolation.
Femurs and tibias of uninfected mice were flushed with RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 2 mM EDTA, and bone marrow cells were resuspended in PBS as described previously (12). PMNs were obtained through density gradient centrifugation using Histopaque 1119 and Histopaque 1077 as previously described (62). This method yields PMNs with 85% to 90% purity (12). To obtain pure PMNs for RNA sequencing, the negative-selection EasySep mouse neutrophil enrichment kit (catalog no. 19762; Stemcell Technologies) was used following the manufacturer’s protocol. PMN purity was determined through flow cytometry, and >98% of cells were Ly6G positive (Ly6G+) (see Fig. S1 in the supplemental material).
PMN infection and total RNA extraction.
Pure PMNs were isolated from young WT, old WT, and young CD73KO mice. From each mouse, 106 pure PMNs were either infected with S. pneumoniae TIGR4 strain (MOI of 4) opsonized with 3% homologous mouse serum or were mock treated with 3% serum in buffer alone for 40 min at 37°C. Three mice per strain were used to obtain three distinct biological replicates of infected and mock-treated PMNs for a total of 18 samples. Following bacterial challenge, RNA was extracted from PMNs using the RNeasy minikit (Qiagen) per the manufacturer’s protocol. A Turbo DNA-free kit (Invitrogen) was used to digest DNA from the samples. RNA concentrations and 260/280 ratios were determined using a NanoDrop 1000 instrument (Thermo Fisher Scientific).
Illumina library preparation and RNA sequencing.
An Agilent 2100 Bioanalyzer was used to determine the integrity, purity, and concentration of RNA samples. An RNA integrity (RIN) score of 6.5 or above was considered acceptable for further analysis. Quality checking revealed improper fragmentation of one sample (one mock-infected CD73KO sample), which was omitted from further analysis. Total RNA was enriched for mRNA using poly(A) selection (Illumina). A stranded RNA library prep kit (NEB) and an Ultra II RNA library prep kit (NEB) were used to prepare cDNA libraries for the remaining 17 samples, according to the manufacturer’s protocol. RNA sequencing was carried out at the Genomics and Bioinformatics core facility at the University at Buffalo on a HiSeq 2500 instrument (Illumina) with a mid-output 75-cycle paired-end run with 10 to 20 million reads per sample. Details of the RNA samples, along with RNA concentration and RIN scores, are provided in Table S1 in the supplemental material.
Differential gene expression analysis.
Per-cycle base call (BCL) files generated by the Illumina HiSeq 2500 instrument were converted to per-read FASTQ files using bcl2fastq version 2.20.0.422 with default settings. FastQC version 0.11.5 was used to review the sequencing quality, while FastQ Screen version 0.11.1 was used to determine any potential contamination. FastQC and FastQ Screen quality reports were summarized using MultiQC version 1.5 (63). Genomic alignments were performed using HISAT2 version 2.1.0 with default parameters (64). To differentiate between bacterial and mammalian RNA, the resulting reads were aligned to NCBI genome assembly GRCh38 as the reference genome. Sequence alignments were compressed and sorted into binary alignment map (BAM) files using SAMtools version 1.3. Counting of mapped reads for genomic features was performed using Subread FeatureCounts version 1.6.2 (65) (parameters: -s2-g gene_id -t exon -Q 60), and the annotation file specified with “-a” was the NCBI GRCh38 reference sequence provided by Illumina iGenomes. MultiQC software was used to summarize alignment as well as feature assignment statistics (63). Differentially expressed genes were detected using the Bioconductor package DESeq2 version 1.20.0 (66). Genes with one count or less were filtered out, and alpha was set to 0.05. Log2 fold changes were calculated using DESeq2 using a negative binomial generalized linear model, dispersion estimates, and logarithmic fold changes integrated with the Benjamini-Hochberg procedure to control the false-discovery rate (FDR). A list of differentially expressed genes (DEGs) was generated using DESeq2. We defined significant up- or downregulation of gene expression as an absolute fold change of ≥2 with an FDR value of <0.05. The PCA plots were generated using the ggplot2 package, and the volcano plots were made using the Bioconductor package EnhancedVolcano.
Gene ontology enrichment analysis.
Functional enrichment analysis of significantly up- or downregulated DEGs was performed on the Database for Annotation, Visualization and Integrated Discovery (DAVID) (67) using the default settings. For each comparison, gene functions were categorized into biological process, molecular function, and cellular components. These gene functions were analyzed separately for up- or downregulated DEGs. DAVID was used to further perform pathway analysis and to retrieve pathway maps based on the identified DEGs. All functional categories and pathways with P values of <0.05 were considered significant. The complete data are available in the supplemental material and on the NCBI website under GEO series accession number GSE150811.
lncRNA network analysis.
In order to elucidate the possible function and biological process of long noncoding RNAs (lncRNAs) identified in this screen, we performed computational prediction of the potential lncRNA-target interaction. lncRNAs bind to complementary sequence of neighboring or target genes to repress expression. Thus, if an lncRNA is upregulated, it is predicted to downregulate the expression of the target gene and vice versa. We performed computational prediction of lncRNA-target interactions using LncTar software for prediction of lncRNA-RNA interactions through free energy minimization. Using the normalized binding free energy (ndG), we selected a value of −0.02 as the cutoff for the analysis, as previously described (68). In order to confirm the reliability of our prediction analysis, we further used LncRRIsearch, an online server for prediction of lncRNA-target interactions, to validate the result from the previous analysis. Briefly, we searched the genomic locations of all our lncRNAs from the mouse genome (GRCm38.p6), and nucleotide sequences of the lncRNAs and their neighboring genes were retrieved for prediction of potential lncRNA-RNA interactions. In order to gain understanding of the possible biological process and physiological pathways, we catalogued all potential target genes and performed functional enrichment analysis to identify significantly affected pathways using a combination of gene ontology (GO) terms, PANTHER, and KOBAS (http://kobas.cbi.pku.edu.cn/kobas3), as previously described (69, 70). Networks were then generated, indicating the likelihood of the focus lncRNAs, gene targets, and biological processes in the network being found together by chance and including concomitant lncRNAs coregulating one or more targets (71). The networks, pathways, and biological functional classification were generated using Cytoscape version 3.7.2.
Real-time PCR.
RT-PCR was used to validate the expression of some of the differentially expressed genes identified in RNA sequencing. For this purpose, the same RNA samples previously used to prepare Illumina libraries were used. Following treatment with indicated inhibitors and challenge with S. pneumoniae, RNA was extracted from 1 × 106 PMNs/condition, and DNA was digested as described above. For all RT-PCRs, 500 ng of each sample was converted into cDNA using the SuperScript Vilo cDNA synthesis kit (Life Technologies) according to the manufacturer’s protocol. RT-PCR was performed using the CFX96 Touch real-time PCR detection system from Bio-Rad. Threshold cycle (CT) values were determined using the following TaqMan probes from Life Technologies (Thermo Fisher Scientific): GAPDH (Mm99999915_m1), interleukin 10 (IL-10; Mm01288386_m1), c-FOS (Mm00487425_m1), Cybr4 (Mm01144487_m1), Hsp72 (Mm01159846_s1), Rgl1 (Mm00444088_m1), ADOR2B (Mm00839292_m1), Rrad (Mm00451053_m1), Tnip1 (Mm01288484_m1), DUSP1 (Mm00457274_g1), c-JUN (Mm00495062_s1), Nr4a1 (Mm01300401_m1), Sifn1 (Mm00624380_m1), Tubb6 (Mm00660543_m1), and Atf3 (Mm00476032_m1). All samples were run in duplicates. Data were analyzed by the comparative threshold cycle (2−ΔΔCT) method, normalizing the CT values obtained for target gene expression to those for GAPDH of the same sample. For comparison of expression levels upon infection, relative quality (RQ) of transcripts was calculated by the ΔΔCT method by using the formula RQ = 2−ΔΔCT (72). ΔΔCT values were obtained by using the formula ΔΔCT = ΔCT(infected) − ΔCT(uninfected).
Opsonophagocytic killing assay.
The ability of PMNs to kill S. pneumoniae ex vivo was measured using a well-established opsonophagocytic (OPH) killing assay as previously described (7, 10, 12, 20). Briefly, 1 × 105 PMNs were incubated with 1 × 103 bacteria (unless indicated otherwise) grown to the mid-log phase and preopsonized with 3% mouse serum in 100-μl reaction mixtures of Hanks’ balanced salt solution (HBSS) containing 0.1% gelatin. Reaction mixtures were then rotated at 37°C for 45 min. Where indicated, PMNs were incubated with actinomycin D (transcription inhibitor), cycloheximide (translation inhibitor), anisomycin (JNK stimulator), SR11302 (AP-1 inhibitor), JNK-IN-8 (JNK inhibitor), or HBSS (vehicle control) for 30 min prior to infection. Anisomycin and SR11302 were purchased from Tocris Biosciences, and actinomycin D, cycloheximide, and JNK-IN-8 were purchased from Sigma. Percent killing was determined by dribble plating on blood agar plates and was calculated in comparison to the no-PMN control under the same conditions (with or without treatments).
Phosphorylated c-Jun measurement.
The ability of S. pneumoniae to induce phosphorylation of c-Jun was measured by flow cytometry. Briefly, 5 × 105 PMNs were challenged with preopsonized S. pneumoniae TIGR4 at an MOI of 4 in 100-μl reaction mixtures of HBSS containing 0.1% gelatin. Reaction mixtures were then rotated at 37°C for the indicated time periods. Where indicated, PMNs were incubated with anisomycin (JNK stimulator), SR11302 (AP-1 inhibitor), JNK-IN-8 (JNK inhibitor), or HBSS (vehicle control) for 30 min prior to infection. Following incubation, cells were fixed with Cytofix (BD Bioscience) and permeabilized with ice-cold methanol. Cells were then stained for fluorophore-tagged antibodies against Ly6G (catalog no. 5605991; BD Biosciences), phospho-c-Jun (Ser73, catalog no. 12714S; Cell Signaling) (73), and total c-Jun (catalog no. 15683S; Cell Signaling) at 1:50 dilutions per the manufacturers’ protocols. Fluorescence intensities were measured on a BD LSRFortessa instrument, and at least 10,000 events were analyzed using FlowJo.
Measurement of apoptosis.
PMNs (2 × 105) were infected with preopsonized S. pneumoniae TIGR4 (MOI of 4) at 37°C for 5 and 40 min or were mock treated with HBSS buffer containing 3% mouse serum. Flow cytometry was used to determine the percentages of live, apoptotic, or necrotic cells using the fluorescein isothiocyanate (FITC) annexin V apoptosis detection kit with propidium iodide (PI) (BioLegend) following the manufacturer’s protocol.
Lactate dehydrogenase cell cytotoxicity assay.
The cytotoxic effect of S. pneumoniae infection on PMNs was evaluated by measuring the levels of the released cytoplasmic enzyme lactate dehydrogenase (LDH). Briefly, 2 × 105 PMNs were infected the with preopsonized S. pneumoniae TIGR4 strain (MOI of 4) at 37°C for 5 and 40 min or were mock treated with HBSS buffer containing 3% mouse serum. The reaction mixtures were briefly centrifuged at 1,000 relative centrifugal force (rcf) for 5 min, and supernatants were collected to measure the release of LDH using the CytoTox 96 assay kit (Promega, Madison, WI).
Flow cytometry.
Anti-Ly6G (IA8; BioLegend) antibodies were used to determine the purity of isolated PMNs. Staining was performed in the presence of Fc block (BD Biosciences). Fluorescence intensities were measured on a BD LSRFortessa instrument, and at least 2,000 events were analyzed using FlowJo.
Statistics.
OPH and flow cytometry data were analyzed using Prism 8 (GraphPad Software, Inc.). Bar graphs represent the mean values of technical replicates for representative data or the mean values of biological replicates (where conditions were tested in triplicates per experiment) for pooled data plus or minus standard deviation (SD), as indicated in the figure legends. A one-sample t test or Student’s t test, as indicated, was used to determine significant differences. Correlation of mRNA expression by RNA-Seq and quantitative PCR (qPCR) was assessed by Pearson correlation analysis. All P values of <0.05 were considered significant (indicated by asterisks).
Data availability.
The data presented and discussed in this study, along with all of the RNA sequencing files and raw data files, are available on the NCBI website under Gene Expression Omnibus (GEO) series accession number GSE150811.
ACKNOWLEDGMENTS
RNA sequencing was performed at the Genomics and Bioinformatics Core facility at the University at Buffalo. We thank Donald Yergeau for helpful discussions. We also thank Sujith A. Valiyaparambil for technical and logistical assistance.
This work was supported by National Institutes of Health grants R00AG051784 and R56 AG068568-01A1 and by University at Buffalo Clinical and Translational Science Institute pilot award CTSA1153519 to E.N.B.G. This work was also supported by American Heart Association grant number 827322 to M.B. O.B.M. was supported by an American Association of Immunologists Careers in Immunology fellowship and by a College of Health Sciences and Technology bridge grant.
We declare that no conflicts of interest exist.
Footnotes
Supplemental material is available online only.
Contributor Information
Elsa N. Bou Ghanem, Email: elsaboug@buffalo.edu.
Nancy E. Freitag, University of Illinois at Chicago
REFERENCES
- 1.Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z, Adetifa IMO, Adhikari TB, Akibu M, Al Lami FH, Al-Eyadhy A, Alvis-Guzman N, Amare AT, Amoako YA, Antonio CAT, Aremu O, Asfaw ET, Asgedom SW, Atey TM, Attia EF, Avokpaho EFGA, Ayele HT, Ayuk TB, Balakrishnan K, Barac A, Bassat Q, Behzadifar M, Behzadifar M, Bhaumik S, Bhutta ZA, Bijani A, Brauer M, Brown A, Camargos PAM, Castañeda-Orjuela CA, Colombara D, Conti S, Dadi AF, Dandona L, Dandona R, Do HP, Dubljanin E, Edessa D, Elkout H, Endries AY, Fijabi DO, GBD 2016 Lower Respiratory Infections Collaborators, et al. 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18:1191–1210. 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wunderink RG, Waterer G. 2017. Advances in the causes and management of community acquired pneumonia in adults. BMJ 358:j2471. 10.1136/bmj.j2471. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control. 2018. Active Bacterial Core surveillance report, Emerging Infections Program Network, Streptococcus pneumoniae, 2018. Centers for Disease Control, Atlanta, GA. [Google Scholar]
- 4.Boe DM, Boule LA, Kovacs EJ. 2017. Innate immune responses in the ageing lung. Clin Exp Immunol 187:16–25. 10.1111/cei.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garvy BA, Harmsen AG. 1996. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 20:499–512. 10.1007/BF01487042. [DOI] [PubMed] [Google Scholar]
- 6.Hahn I, Klaus A, Janze AK, Steinwede K, Ding N, Bohling J, Brumshagen C, Serrano H, Gauthier F, Paton JC, Welte T, Maus UA. 2011. Cathepsin G and neutrophil elastase play critical and nonredundant roles in lung-protective immunity against Streptococcus pneumoniae in mice. Infect Immun 79:4893–4901. 10.1128/IAI.05593-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bou Ghanem EN, Clark S, Roggensack SE, McIver SR, Alcaide P, Haydon PG, Leong JM. 2015. Extracellular adenosine protects against Streptococcus pneumoniae lung infection by regulating pulmonary neutrophil recruitment. PLoS Pathog 11:e1005126. 10.1371/journal.ppat.1005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons SR, Bhalla M, Herring SE, Tchalla EYI, Bou Ghanem EN. 2021. Older but not wiser: the age-driven changes in neutrophil responses during pulmonary infections. Infect Immun 89:e00653-20. 10.1128/IAI.00653-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simell B, Vuorela A, Ekstrom N, Palmu A, Reunanen A, Meri S, Kayhty H, Vakevainen M. 2011. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 29:1929–1934. 10.1016/j.vaccine.2010.12.121. [DOI] [PubMed] [Google Scholar]
- 10.Bhalla M, Simmons SR, Abamonte A, Herring SE, Roggensack SE, Bou Ghanem EN. 2020. Extracellular adenosine signaling reverses the age-driven decline in the ability of neutrophils to kill Streptococcus pneumoniae. Aging Cell 19:e13218. 10.1111/acel.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. 2004. Crucial role for ecto-5′-Nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med 200:1395–1405. 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siwapornchai N, Lee JN, Tchalla EYI, Bhalla M, Yeoh JH, Roggensack SE, Leong JM, Bou Ghanem EN. 2020. Extracellular adenosine enhances the ability of PMNs to kill Streptococcus pneumoniae by inhibiting IL-10 production. J Leukoc Biol 108:867–882. 10.1002/JLB.4MA0120-115RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subrahmanyam YVBK, Yamaga S, Prashar Y, Lee HH, Hoe NP, Kluger Y, Gerstein M, Goguen JD, Newburger PE, Weissman SM. 2001. RNA expression patterns change dramatically in human neutrophils exposed to bacteria. Blood 97:2457–2468. 10.1182/blood.v97.8.2457. [DOI] [PubMed] [Google Scholar]
- 14.Gomez JC, Dang H, Kanke M, Hagan RS, Mock JR, Kelada SNP, Sethupathy P, Doerschuk CM. 2017. Predicted effects of observed changes in the mRNA and microRNA transcriptome of lung neutrophils during S. pneumoniae pneumonia in mice. Sci Rep 7:11258. 10.1038/s41598-017-11638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. 2014. Granule protein processing and regulated secretion in neutrophils. Front Immunol 5:448. 10.3389/fimmu.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lysenko ES, Clarke TB, Shchepetov M, Ratner AJ, Roper DI, Dowson CG, Weiser JN. 2007. Nod1 signaling overcomes resistance of S. pneumoniae to opsonophagocytic killing. PLoS Pathog 3:e118. 10.1371/journal.ppat.0030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang FY, Shaio MF. 1990. In vitro effect of actinomycin D on human neutrophil function. Microbiol Immunol 34:311–321. 10.1111/j.1348-0421.1990.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 18.Hsu HY, Nicholson AC, Hajjar DP. 1996. Inhibition of macrophage scavenger receptor activity by tumor necrosis factor-alpha is transcriptionally and post-transcriptionally regulated. J Biol Chem 271:7767–7773. 10.1074/jbc.271.13.7767. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh V, Kim M-J, Gelissen IC, Brown AJ, Sandoval C, Hallab JC, Kockx M, Traini M, Jessup W, Kritharides L. 2014. Cellular cholesterol regulates ubiquitination and degradation of the cholesterol export proteins ABCA1 and ABCG1. J Biol Chem 289:7524–7536. 10.1074/jbc.M113.515890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Standish AJ, Weiser JN. 2009. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol 183:2602–2609. 10.4049/jimmunol.0900688. [DOI] [PubMed] [Google Scholar]
- 21.Dalia AB, Standish AJ, Weiser JN. 2010. Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils. Infect Immun 78:2108–2116. 10.1128/IAI.01125-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez JC, Dang H, Martin JR, Doerschuk CM. 2016. Nrf2 modulates host defense during Streptococcus pneumoniae pneumonia in mice. The J Immunology 197:2864–2879. 10.4049/jimmunol.1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeke A, Misheva M, Reményi A, Bogoyevitch MA. 2016. JNK signaling: regulation and functions based on complex protein-protein partnerships. Microbiol Mol Biol Rev 80:793–835. 10.1128/MMBR.00043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt B, Abou-Eladab EF, Tiedge M, Walzel H. 2010. Role of the JNK/c-Jun/AP-1 signaling pathway in galectin-1-induced T-cell death. Cell Death Dis 1:e23. 10.1038/cddis.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolomeichuk SN, Terrano DT, Lyle CS, Sabapathy K, Chambers TC. 2008. Distinct signaling pathways of microtubule inhibitors—vinblastine and Taxol induce JNK-dependent cell death but through AP-1-dependent and AP-1-independent mechanisms, respectively. FEBS J 275:1889–1899. 10.1111/j.1742-4658.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- 26.Dhanasekaran DN, Reddy EP. 2008. JNK signaling in apoptosis. Oncogene 27:6245–6251. 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T, Xie T, Marto JA, Kim N, Sim T, Laughlin JD, Park H, LoGrasso PV, Patricelli M, Nomanbhoy TK, Sorger PK, Alessi DR, Gray NS. 2012. Discovery of potent and selective covalent inhibitors of JNK. Chem Biol 19:140–154. 10.1016/j.chembiol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanjul A, Dawson MI, Hobbs PD, Jong L, Cameron JF, Harlev E, Graupner G, Lu XP, Pfahl M. 1994. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature 372:107–111. 10.1038/372107a0. [DOI] [PubMed] [Google Scholar]
- 29.Leipheimer J, Bloom ALM, Campomizzi CS, Salei Y, Panepinto JC. 2019. Translational regulation promotes oxidative stress resistance in the human fungal pathogen Cryptococcus neoformans. mBio 10:e02143-19. 10.1128/mBio.02143-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom ALM, Jin RM, Leipheimer J, Bard JE, Yergeau D, Wohlfert EA, Panepinto JC. 2019. Thermotolerance in the pathogen Cryptococcus neoformans is linked to antigen masking via mRNA decay-dependent reprogramming. Nat Commun 10:4950. 10.1038/s41467-019-12907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López-Maury L, Marguerat S, Bähler J. 2008. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet 9:583–593. 10.1038/nrg2398. [DOI] [PubMed] [Google Scholar]
- 32.Murray JI, Whitfield ML, Trinklein ND, Myers RM, Brown PO, Botstein D. 2004. Diverse and specific gene expression responses to stresses in cultured human cells. Mol Biol Cell 15:2361–2374. 10.1091/mbc.e03-11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monaco G, Lee B, Xu W, Mustafah S, Hwang YY, Carré C, Burdin N, Visan L, Ceccarelli M, Poidinger M, Zippelius A, Pedro de Magalhães J, Larbi A. 2019. RNA-Seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep 26:1627–1640.e7. 10.1016/j.celrep.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vafadarnejad E, Rizzo G, Krampert L, Arampatzi P, Arias-Loza A-P, Nazzal Y, Rizakou A, Knochenhauer T, Bandi SR, Nugroho VA, Schulz DJJ, Roesch M, Alayrac P, Vilar J, Silvestre J-S, Zernecke A, Saliba A-E, Cochain C. 2020. Dynamics of cardiac neutrophil diversity in murine myocardial infarction. Circ Res 127:e232–e249. 10.1161/CIRCRESAHA.120.317200. [DOI] [PubMed] [Google Scholar]
- 35.Zysk G, Bejo L, Schneider-Wald BK, Nau R, Heinz H. 2000. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin Exp Immunol 122:61–66. 10.1046/j.1365-2249.2000.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L-X, Deng W, Zhou X-T, Chen R-P, Xiang M-Q, Guo Y-T, Pu Z-J, Li R, Wang G-F, Wu L-F. 2016. The mechanism of adenosine-mediated activation of lncRNA MEG3 and its antitumor effects in human hepatoma cells. Int J Oncol 48:421–429. 10.3892/ijo.2015.3248. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Ao L, Yang J. 2019. Long non-coding RNAs in diseases related to inflammation and immunity. Ann Transl Med 7:494–494. 10.21037/atm.2019.08.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB, O'Neill LAJ, Moore MJ, Caffrey DR, Fitzgerald KA. 2013. A long noncoding RNA mediates both activation and repression of immune response genes. Science 341:789–792. 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atianand MK, Hu W, Satpathy AT, Shen Y, Ricci EP, Alvarez-Dominguez JR, Bhatta A, Schattgen SA, McGowan JD, Blin J, Braun JE, Gandhi P, Moore MJ, Chang HY, Lodish HF, Caffrey DR, Fitzgerald KA. 2016. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell 165:1672–1685. 10.1016/j.cell.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang N, Zhang X, He Y, Luo B, He C, Liang Y, Zeng J, Li W, Xian Y, Zheng X. 2019. Identification of key protein-coding genes and lncRNAs in spontaneous neutrophil apoptosis. Sci Rep 9:15106. 10.1038/s41598-019-51597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang K, Sun X, Chen Y, Shen Y, Jarvis JN. 2015. RNA sequencing from human neutrophils reveals distinct transcriptional differences associated with chronic inflammatory states. BMC Med Genomics 8:55. 10.1186/s12920-015-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rautanen A, Pirinen M, Mills TC, Rockett KA, Strange A, Ndungu AW, Naranbhai V, Gilchrist JJ, Bellenguez C, Freeman C, Band G, Bumpstead SJ, Edkins S, Giannoulatou E, Gray E, Dronov S, Hunt SE, Langford C, Pearson RD, Su Z, Vukcevic D, Macharia AW, Uyoga S, Ndila C, Mturi N, Njuguna P, Mohammed S, Berkley JA, Mwangi I, Mwarumba S, Kitsao BS, Lowe BS, Morpeth SC, Khandwalla I, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Duncanson A, Jankowski J, Markus HS, Mathew CG, Palmer CNA, Plomin R, Sawcer SJ, Trembath RC, Viswanathan AC, Wood NW, Deloukas P, Kilifi Bacteraemia Surveillance Group, et al. 2016. Polymorphism in a lincRNA associates with a doubled risk of pneumococcal bacteremia in Kenyan children. Am J Hum Genet 98:1092–1100. 10.1016/j.ajhg.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin R, Yu S, Song Z, Zhu X, Wang C, Yan J, Wu F, Nanda A, Granger DN, Li G. 2013. Soluble CD40 ligand stimulates CD40-dependent activation of the β2 integrin Mac-1 and protein kinase C zeda (PKCζ) in neutrophils: implications for neutrophil-platelet interactions and neutrophil oxidative burst. PLoS One 8:e64631. 10.1371/journal.pone.0064631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153:1194–1217. 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Z, Guo H, Qin J, Lu S, Liu Q, Zhang X, Zou Y, Gong Y, Shao C. 2019. Pan-senescence transcriptome analysis identified RRAD as a marker and negative regulator of cellular senescence. Free Radic Biol Med 130:267–277. 10.1016/j.freeradbiomed.2018.10.457. [DOI] [PubMed] [Google Scholar]
- 46.Jang S, Ryu SM, Lee J, Lee H, Hong SH, Ha KS, Park WS, Han ET, Yang SR. 2019. Bleomycin inhibits proliferation via Schlafen-mediated cell cycle arrest in mouse alveolar epithelial cells. Tuberc Respir Dis (Seoul) 82:133–142. 10.4046/trd.2017.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeanneteau F, Barrère C, Vos M, De Vries CJM, Rouillard C, Levesque D, Dromard Y, Moisan MP, Duric V, Franklin TC, Duman RS, Lewis DA, Ginsberg SD, Arango-Lievano M. 2018. The stress-induced transcription factor NR4A1 adjusts mitochondrial function and synapse number in prefrontal cortex. J Neurosci 38:1335–1350. 10.1523/JNEUROSCI.2793-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Kim SC, Yu T, Yi Y-S, Rhee MH, Sung G-H, Yoo BC, Cho JY. 2014. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm 2014:352371–352371. 10.1155/2014/352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benna JE, Han J, Park J-W, Schmid E, Ulevitch RJ, Babior BM. 1996. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phoxby p38 and ERK but not by JNK. Arch Biochem Biophys 334:395–400. 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki K, Hino M, Kutsuna H, Hato F, Sakamoto C, Takahashi T, Tatsumi N, Kitagawa S. 2001. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1β. J Immunol 167:5940–5947. 10.4049/jimmunol.167.10.5940. [DOI] [PubMed] [Google Scholar]
- 51.Downey GP, Butler JR, Tapper H, Fialkow L, Saltiel AR, Rubin BB, Grinstein S. 1998. Importance of MEK in neutrophil microbicidal responsiveness. J Immunol 160:434–443. [PubMed] [Google Scholar]
- 52.Mócsai A, Jakus Z, Vántus T, Berton G, Lowell CA, Ligeti E. 2000. Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. J Immunol 164:4321–4331. 10.4049/jimmunol.164.8.4321. [DOI] [PubMed] [Google Scholar]
- 53.Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O, Khalil A, Dupuis G. 2004. Signal transduction and functional changes in neutrophils with aging. Aging Cell 3:217–226. 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 54.Fortin CF, Larbi A, Dupuis G, Lesur O, Fulop T, Jr.. 2007. GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology 8:173–187. 10.1007/s10522-006-9067-1. [DOI] [PubMed] [Google Scholar]
- 55.Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T, Jr.. 2006. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol 79:1061–1072. 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- 56.Larbi A, Douziech N, Fortin C, Linteau A, Dupuis G, Fulop T, Jr.. 2005. The role of the MAPK pathway alterations in GM-CSF modulated human neutrophil apoptosis with aging. Immun Ageing 2:6. 10.1186/1742-4933-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM. 2014. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 123:239–248. 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tortorella C, Simone O, Piazzolla G, Stella I, Cappiello V, Antonaci S. 2006. Role of phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways in granulocyte macrophage-colony-stimulating factor failure to delay fas-induced neutrophil apoptosis in elderly humans. J Gerontol A Biol Sci Med Sci 61:1111–1118. 10.1093/gerona/61.11.1111. [DOI] [PubMed] [Google Scholar]
- 59.De Maeyer RPH, van de Merwe RC, Louie R, Bracken OV, Devine OP, Goldstein DR, Uddin M, Akbar AN, Gilroy DW. 2020. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol 21:615–625. 10.1038/s41590-020-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan MA, Farahvash A, Douda DN, Licht J-C, Grasemann H, Sweezey N, Palaniyar N. 2017. JNK activation turns on LPS- and Gram-negative bacteria-induced NADPH oxidase-dependent suicidal NETosis. Sci Rep 7:3409. 10.1038/s41598-017-03257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greene NG, Narciso AR, Filipe SR, Camilli A. 2015. Peptidoglycan branched stem peptides contribute to Streptococcus pneumoniae virulence by inhibiting pneumolysin release. PLoS Pathog 11:e1004996. 10.1371/journal.ppat.1004996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swamydas M, Lionakis MS. 2013. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp (77):e50586. 10.3791/50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ewels P, Magnusson M, Lundin S, Käller M. 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32:3047–3048. 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360. 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 66.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang DW, Sherman BT, Lempicki RA. 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Ma W, Zeng P, Wang J, Geng B, Yang J, Cui Q. 2015. LncTar: a tool for predicting the RNA targets of long noncoding RNAs. Brief Bioinform 16:806–812. 10.1093/bib/bbu048. [DOI] [PubMed] [Google Scholar]
- 69.Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P, Floros E, Dalamagas T, Hatzigeorgiou AG. 2016. DIANA-LncBase v2: indexing microRNA targets on non-coding transcripts. Nucleic Acids Res 44:D231–D238. 10.1093/nar/gkv1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senn V, Bassler D, Choudhury R, Scholkmann F, Righini-Grunder F, Vuille-Dit-Bille RN, Restin T. 2021. Computational network analysis identifies evolutionarily conserved miRNA gene interactions potentially regulating immune response in bovine trypanosomosis. Front Cell Infect Microbiol 11:715671. 10.3389/fcimb.2021.715671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buza T, Arick M, Wang H, Peterson DG. 2014. Computational prediction of disease microRNAs in domestic animals. BMC Res Notes 7:403. 10.1186/1756-0500-7-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 73.Suman S, Rachakonda G, Mandape SN, Sakhare SS, Villalta F, Pratap S, Lima MF, Nde PN. 2018. Phospho-proteomic analysis of primary human colon epithelial cells during the early Trypanosoma cruzi infection phase. PLoS Negl Trop Dis 12:e0006792. 10.1371/journal.pntd.0006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00258-21-s0001.pdf, PDF file, 2.4 MB (2.4MB, pdf)
Data Availability Statement
The data presented and discussed in this study, along with all of the RNA sequencing files and raw data files, are available on the NCBI website under Gene Expression Omnibus (GEO) series accession number GSE150811.