ABSTRACT
The immunomes of Ehrlichia chaffeensis and Ehrlichia canis have recently been revised to include immunodominant hypothetical proteins with conformational antibody epitopes. In this study, we examined 216 E. chaffeensis and 190 E. canis highly antigenic proteins according to ANTIGENpro and also performed a genome-wide hypothetical protein analysis (E. chaffeensis n = 104; E. canis n = 124) for immunoreactivity. Using cell-free protein expression and immunoanalysis, 118 E. chaffeensis and 39 E. canis proteins reacted with sera from naturally E. chaffeensis-infected patients or E. canis-infected dogs. Moreover, 22 E. chaffeensis and 18 E. canis proteins consistently and strongly reacted with a panel of patient or canine sera. A subset of E. chaffeensis (n = 18) and E. canis (n = 9) proteins were identified as immunodominant. Consistent with our previous study, most proteins were classified as hypothetical, and the antibody epitopes exhibited complete or partial conformation dependence. The majority (28/40, 70%) of E. chaffeensis and E. canis proteins contained transmembrane domains, and 19 (48%) were predicted to be secreted effectors. The antigenic repertoires of E. chaffeensis and E. canis were mostly diverse and suggest that the immunomes of these closely related ehrlichiae are dominated by species-specific conformational antibody epitopes. This study reveals a significant group of previously undefined E. chaffeensis and E. canis antigens and reaffirms the importance of conformation-dependent epitopes as targets of anti-Ehrlichia immune responses. These findings substantially expand our understanding of host-Ehrlichia immune responses, advance efforts to define the molecular features of protective proteins, and improve prospects for effective vaccines for the ehrlichioses.
KEYWORDS: Ehrlichia, immunoreactive, conformation-dependent, antibody, epitope
INTRODUCTION
Ehrlichia chaffeensis and Ehrlichia canis are tick-transmitted obligately intracellular bacteria that cause human monocytotropic ehrlichiosis (HME) and canine monocytic ehrlichiosis (CME), respectively (1, 2). HME is an emerging zoonosis in the United States, but it has also been reported in Europe, Asia, Africa, and South America. CME is a globally distributed disease in dogs. Clinical manifestations of HME and CME in humans and dogs range from subclinical to potentially life-threatening (1, 3). Currently, only limited therapeutic options are available, and no vaccine has been developed for HME or CME.
An important obstacle to vaccine development for HME and CME has been the very small repertoire of defined E. chaffeensis and E. canis immunoreactive and protective proteins (4), limited to several tandem repeat proteins (TRPs) (5–9), ankyrin protein (Ank200) (10, 11), and the major outer membrane proteins (OMPs) (12, 13). These major immunoreactive protein orthologs of E. chaffeensis and E. canis contain linear antibody epitopes that have been molecularly defined. During infection, these proteins are expressed by Ehrlichia and stimulate strong antibody responses in humans and dogs (14–17). However, these major immunoreactive proteins were identified using immunoscreening approaches that detect linear antibody epitopes and Ehrlichia propagation that relies on mammalian host cells which may bias pathogen protein discovery toward proteins expressed specifically in the mammalian host (18–20). Thus, the high potential exists for identification of many undiscovered antigenic proteins using new genomic and proteomic approaches.
Recently, we implemented a high-throughput antigen discovery strategy that combines genomics, bioinformatics, cell-free protein expression, and immunoscreening approaches to rapidly identify new antigens from E. chaffeensis and E. canis, including those that have conformation-dependent antibody epitopes (21). In our recent study, we focused on a group of ∼100 open reading frames (ORFs) classified as hypothetical and identified a new group of major immunoreactive E. chaffeensis and E. canis proteins with conformational epitopes. This major advance in understanding the ehrlichial immunome revealed the importance of conformational epitopes that have been largely unexplored and unstudied. Moreover, there was little knowledge regarding the existence of conformation-dependent antigens or their roles in Ehrlichia immunity. This new finding also suggested that there is a large group of immunoreactive proteins that remain undefined in Ehrlichia. For instance, a large number of new antigens have been identified from other pathogens by proteomic screening instead of conventional approaches (22–24). Additionally, 185 immunodominant proteins, including 75 hypothetical proteins, were identified from intracellular bacterium Chlamydia trachomatis by proteome microarray (25). More studies have surprisingly revealed that 7 to 20% of the proteins of other pathogens are immunoreactive (22–27).
The bioinformatic tool ANTIGENpro is a sequence-based predictor of protein antigenicity and has been successfully applied to many pathogens, including Salmonella, Leishmania, and Corynebacterium spp. (28, 29). In vitro transcription and translation (IVTT) is a simple, rapid, and efficient cell-free system which can produce soluble and native recombinant proteins for functional studies (30, 31). In this study, we used these approaches to screen the remaining hypothetical proteins in the genomes and annotated proteins identified by ANTIGENpro with high antigenicity scores from E. chaffeensis and E. canis ORFeomes. This study further extends the defined repertoire of immunoreactive proteins, most of which are hypothetical proteins and contain conformation-dependent epitopes not detectable by conventional immunoblotting approaches, and provides relatively complete antigenic repertoires of E. chaffeensis and E. canis, which will facilitate vaccine development for ehrlichioses.
RESULTS
Expression and immunoscreening of E. chaffeensis and E. canis proteins.
Previously, we predicted the antigenicity of 1105 E. chaffeensis (Arkansas strain) and 925 E. canis (Jake strain) ORFs (excluding RNA genes and pseudogenes) by ANTIGENpro and obtained respective antigenicity scores (between 0 and 1). The enrichment efficiency of ANTIGENpro was validated by known protective antigens of E. chaffeensis and E. canis (i.e., TRPs and OMPs). We also investigated the immunoreactivity of ∼100 hypothetical proteins distributed in the top 250 in each respective ORFeome (21). In order to further extend the knowledge regarding immunoreactive protein repertoires of E. chaffeensis and E. canis, we investigated proteins in the top 350 (including both hypothetical and annotated proteins but excluding known antigens and ribosomal proteins) and remaining hypothetical proteins present in E. chaffeensis and E. canis ORFeomes (Fig. S1 in the supplemental material). The antigenicity score threshold (∼0.6) leading to the identification of the top 350 E. chaffeensis and E. canis proteins provided a balance between high sensitivity and specificity in predicting protein antigenicity (29). In addition, our previous empirical data and other studies suggest that hypothetical proteins are frequent targets of the host immune response; hence, we included remaining E. chaffeensis and E. canis hypothetical proteins in the ORFeome regardless of ANTIGENpro rank. Due to different pipelines used for genomic annotation, there were some differences in gene assignments in the E. chaffeensis and E. canis ORFeomes between Integrated Microbial Genomes (IMG) and GenBank. We also included several hypothetical proteins (including proteins with domain of unknown function [DUF]) predicted by GenBank, but not by IMG. A few very small E. chaffeensis proteins (<42 amino acids [aa]) predicted by IMG were removed by the GenBank pipeline and were not included in this study. Therefore, the total number of proteins in E. chaffeensis ORFeome was adjusted to 882.
Whole-genome annotations for E. chaffeensis and E. canis currently assign 176 (20%) and 230 (25%) ORFs as hypothetical or DUF containing, respectively. Within the ANTIGENpro top 350, we have previously investigated 93 E. chaffeensis and 98 E. canis hypothetical proteins (21). Therefore, in this study, we further examined the immunoreactivity of 320 E. chaffeensis and 314 E. canis proteins in total, including all remaining hypothetical proteins (n = 104 and 124, respectively) regardless of ANTIGENpro ranking, which were not examined in our previous investigation. A cell-free IVTT system was used to express the ORFs, and protein expression was confirmed by dot blot of selected proteins (30 from E. chaffeensis and 25 from E. canis) (Fig. 1A). Although the protein expression levels varied, the identification of immunoreactive proteins was not influenced by expression levels due to the saturation of enzyme-linked immunosorbent assay (ELISA) plate wells by IVTT-expressed proteins as confirmed in our previous investigation (21).
FIG 1.
Expression of E. chaffeensis and E. canis proteins by IVTT and immunoreactivity screening by ELISA. (A) Detection of IVTT expression of selected proteins of E. chaffeensis and E. canis by dot immunoblot with anti-His tag antibody. CTL, negative control (IVTT reaction without plasmid template). (B and C) Pooled sera of HME patients or CME dogs were used to screen E. chaffeensis (B) and E. canis (C) proteins, respectively. ELISA OD650 values represent the mean optical density reading from three wells (± standard deviations) after subtracting negative-control (IVTT reaction with the empty vector template) reading. A sample OD650 of ≥0.2 was considered positive and ≥0.5 a strong positive.
The 320 IVTT-expressed E. chaffeensis proteins were screened for immunoreactivity by antigen capture ELISA using pooled convalescent HME sera (indirect fluorescent antibody assay [IFA] titer, 1,600), and a total of 118 (37%) E. chaffeensis proteins reacted with pooled HME sera (mean optical density at 650 nm [OD650] ≥ 0.2 with background subtracted). All E. chaffeensis proteins ranked based on the immunoreactivity (from high to low) are listed in Table S1, and Fig. 1B identifies 40 E. chaffeensis proteins that exhibited the highest immunoreactivity (mean OD650 > 0.8) with pooled sera. The 314 IVTT-expressed E. canis proteins were similarly screened for immunoreactivity by ELISA with pooled CME sera (IFA titer, 1,600), and 39 (12%) proteins were immunoreactive (mean OD650 ≥ 0.2; Fig. 1C). All E. canis proteins ranked according to the immunoreactivity (from high to low) are listed in Table S2. These immunoreactive E. chaffeensis and E. canis proteins were investigated further to determine consistency and overall immunoreactivity among a panel of HME or CME sera, respectively.
Identification of major immunoreactive E. chaffeensis and E. canis proteins.
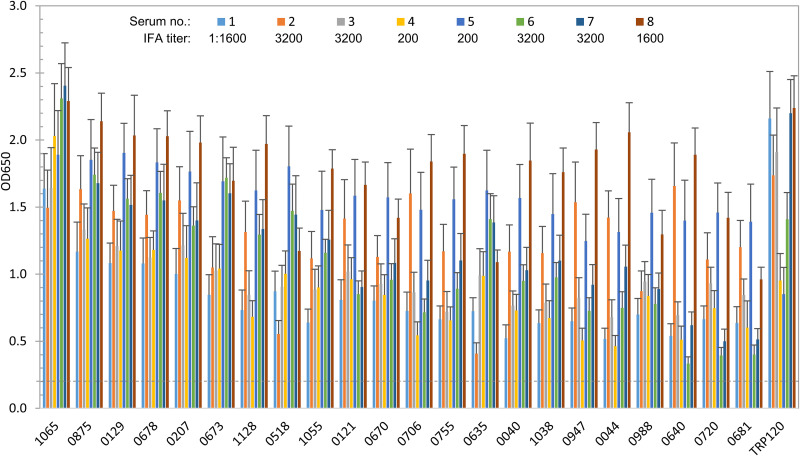
To define and compare protein immunoreactivity, we used a panel of eight HME and 10 CME sera to further investigate the E. chaffeensis and E. canis proteins by ELISA. All the patient and canine sera recognized E. chaffeensis or E. canis by IFA, and the antibody titers ranged from 200 to 3,200 (Fig. 2 and 3). Previously, we have demonstrated that E. chaffeensis TRP120 and E. canis TRP19 can provide sensitive and specific diagnosis of HME and CME infection comparable to the “gold-standard” IFA (10, 32). Moreover, TRP120 and TRP19 are well-defined immunodominant proteins with molecularly defined linear antibody epitopes that have been shown to react strongly and consistently with antibodies from infected patient and canine sera (21, 33, 34). Therefore, major immunoreactive proteins E. chaffeensis TRP120 and E. canis TRP19 were used as positive controls. Of the 118 E. chaffeensis immunoreactive proteins identified, we selected 40 proteins with the strongest reactions (OD650 > 0.8) for further reactivity characterization using multiple HME sera. Eighteen (45%) of these immunoreactive proteins were ranked in the top 350 by ANTIGENpro, and 25 proteins (63%) were hypothetical, consistent with our previous finding that more hypothetical proteins were immunoreactive than annotated proteins (21). All these proteins were recognized by eight HME sera. The top 18 proteins that reacted strongly with all or most HME sera (similar to gold-standard TRP120) were considered immunodominant based on mean ELISA OD650 values from multiple sera (≥1.0). An additional 22 proteins reacted strongly with some HME sera and consistently with all sera, but at lower levels (mean OD650, 0.5 to 1.0). Thus, these immunoreactive proteins were classified as subdominant. None of the HME sera reacted with the IVTT-negative-control protein (OD650 < 0.1) expressed from the empty plasmid used to clone ehrlichial proteins, and none of these proteins reacted with a normal human serum control (OD650 < 0.1). The top 22 immunoreactive proteins ranked by mean ELISA OD650 values (>0.8) are shown in Fig. 2 and Table 1.
FIG 2.
Immunoreactivity of 22 E. chaffeensis proteins and TRP120 comparison by ELISA. IVTT-expressed proteins were probed with a panel of convalescent-phase sera from eight HME patients. A normal human serum control did not recognize any of these proteins (OD650 < 0.1). ELISA OD650 values represent the mean optical density reading from three wells (± standard deviations) after subtracting negative-control (IVTT reaction with empty plasmid template) reading. A sample OD650 of ≥0.2 was considered positive and ≥0.5 a strong positive. The top 18 proteins with mean OD650 of ≥1.0 from multiple sera were considered immunodominant and proteins with mean OD650 of 0.5 to 1.0 subdominant.
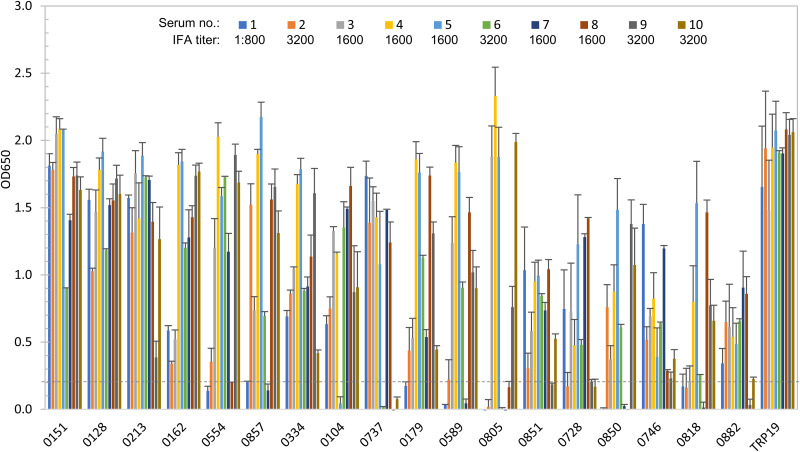
FIG 3.
Immunoreactivity of 18 E. canis proteins and TRP19 comparison by ELISA. IVTT-expressed proteins were probed with convalescent-phase sera from 10 CME dogs. A normal canine serum control did not recognize any of these proteins (OD650 < 0.1). OD650 values represent the mean optical density reading from three wells (± standard deviations) after subtracting negative-control (IVTT reaction with empty plasmid template) reading. A sample OD650 of ≥0.2 was considered positive and ≥0.5 a strong positive. The top nine proteins with mean OD650 of ≥1.0 from multiple sera were considered immunodominant and proteins with mean OD650 of 0.5 to 1.0 subdominant.
TABLE 1.
Immunoreactivity and ANTIGENpro analysis of E. chaffeensis proteins
| Protein (Ech_ tag no.) | Mean ELISA OD650a | ANTIGENpro rank | Antigenicity score | E. canis ortholog | E. canis ortholog ANTIGENpro rank |
E. canis ortholog imunoreactivityb |
|---|---|---|---|---|---|---|
| 1065 | 1.96 | 242 | 0.70 | 0857 | 194 | ++ |
| 0875 | 1.60 | 1,039 | 0.06 | 0223 | 803 | NA |
| 0129 | 1.50 | 849 | 0.16 | 0080 | 706 | − |
| 0678 | 1.48 | 424 | 0.54 | 0369 | 450 | − |
| 0207 | 1.42 | 1,013 | 0.06 | 0796 | 919 | − |
| 0673 | 1.33 | 876 | 0.14 | 0372 | 832 | + |
| 1128 | 1.23 | 524 | 0.44 | *c | * | * |
| 0518 | 1.15 | 1,040 | 0.05 | 0513 | 877 | − |
| 1055 | 1.15 | 181 | 0.76 | 0848 | 100 | − |
| 0121 | 1.15 | 722 | 0.26 | 0071, 0072 | 23, 53 | +, − |
| 0670 | 1.09 | 900 | 0.12 | 0373 | 835 | − |
| 0706 | 1.09 | 840 | 0.16 | 0349 | 890 | − |
| 0755 | 1.08 | 316 | 0.63 | 0319 | 411 | NA |
| 0635 | 1.08 | 429 | 0.53 | 0404 | 431 | − |
| 0040 | 1.07 | 106 | 0.83 | 0018 | 130 | NA |
| 1038 | 1.07 | 333 | 0.61 | 0838 | 18 | − |
| 0947 | 1.04 | 875 | 0.14 | 0172 | 861 | − |
| 0044 | 1.03 | 122 | 0.81 | 0022 | 115 | NA |
| 0988 | 0.97 | 528 | 0.44 | 0141 | 421 | − |
| 0640 | 0.96 | 467 | 0.50 | 0399 | 414 | − |
| 0720 | 0.90 | 559 | 0.42 | 0344 | 333 | − |
| 0681 | 0.82 | 771 | 0.22 | 0366 | 881 | − |
Mean OD650 from 8 HME patient sera.
++, immunodominant; +, immunoreactive in immunoscreening; −, not immunoreactive in immunoscreening; NA, not available.
*, E. canis ortholog not identified.
Of the 39 E. canis immunoreactive proteins identified (OD650 > 0.2), 28 (72%) proteins were ranked in the top 350 by ANTIGENpro, and 12 proteins (31%) were hypothetical. The reactivity of these proteins was further characterized by ELISA with multiple CME sera, and all these proteins were recognized by most CME sera. The top nine E. canis proteins reacted strongly with most canine sera at a level comparable to TRP19 (mean OD650 > 1.0) and thus were considered immunodominant. Another 30 E. canis proteins reacted with mean ELISA OD650 values of 0.5 to 1.0 and were classified as subdominant. None of the CME sera reacted with the IVTT-expressed negative-control protein (OD650 < 0.1), and none of these proteins reacted with a normal canine serum control (OD650 < 0.1). The top 18 proteins ranked by mean ELISA OD650 values (>0.5) are shown in Fig. 3 and Table 2. Some new immunoreactive proteins of E. chaffeensis and E. canis were outside the ANTIGENpro top 350 ranking and had low predicted antigenicity score and rank, demonstrating that many hypothetical proteins are potentially immunoreactive despite low antigenicity scores predicted by ANTIGENpro (Tables 1 and 2). This might be partially due to the databases and training model that ANTIGENpro uses for machine learning, which may have a bias for proteins with known function.
TABLE 2.
Immunoreactivity and ANTIGENpro analysis of E. canis proteins
| Protein (Ecaj_ tag no.) | Mean ELISA OD650a | ANTIGENpro rank | Antigenicity score | E. chaffeensis ortholog | E. chaffeensis ortholog ANTIGENpro rank |
E. chaffeensis ortholog imunoreactivityb |
|---|---|---|---|---|---|---|
| 0151 | 1.72 | 96 | 0.85 | 0976 | 161 | + |
| 0128 | 1.53 | 50 | 0.90 | 0189 | 51 | + |
| 0213 | 1.44 | 348 | 0.61 | *c | * | * |
| 0162 | 1.25 | 362 | 0.60 | 0960 | 551 | NA |
| 0554 | 1.20 | 103 | 0.84 | 0471 | 91 | − |
| 0857 | 1.19 | 194 | 0.75 | 1065 | 242 | ++ |
| 0334 | 1.10 | 76 | 0.87 | 0731 | 77 | + |
| 0104 | 1.02 | 577 | 0.39 | 0159 | 175 | + |
| 0737 | 1.00 | 397 | 0.56 | * | * | * |
| 0179 | 0.99 | 181 | 0.77 | 0939 | 150 | − |
| 0589 | 0.94 | 156 | 0.80 | 0432 | 258 | − |
| 0805 | 0.89 | 193 | 0.75 | 0997 | 301 | − |
| 0851 | 0.72 | 767 | 0.20 | * | * | * |
| 0728 | 0.69 | 466 | 0.50 | * | * | * |
| 0850 | 0.66 | 244 | 0.71 | 1058 | 287 | + |
| 0746 | 0.65 | 473 | 0.49 | * | * | * |
| 0818 | 0.60 | 213 | 0.73 | * | * | * |
| 0882 | 0.53 | 781 | 0.19 | 1104 | 483 | + |
Mean OD650 from 10 CME sera.
++, immunodominant; +, immunoreactive in immunoscreening; −, not immunoreactive in immunoscreening; NA, not available.
*, E. chaffeensis ortholog not identified.
Antibody epitopes of E. chaffeensis and E. canis immunoreactive proteins.
Immunoreactive proteins of Ehrlichia previously identified by immunoblot contain linear epitopes and are limited in number (4). Recently, we discovered new immunoreactive Ehrlichia proteins that are not detectable by conventional immunoblotting approaches, revealing the dominance of conformation-dependent antibody epitopes in these proteins (21). To further identify new immunoreactive E. chaffeensis and E. canis proteins in this study, we compared the immunoreactivity by ELISA of native proteins (IVTT products) with that of denatured proteins (IVTT products treated by urea) using the same panel of HME or CME sera.
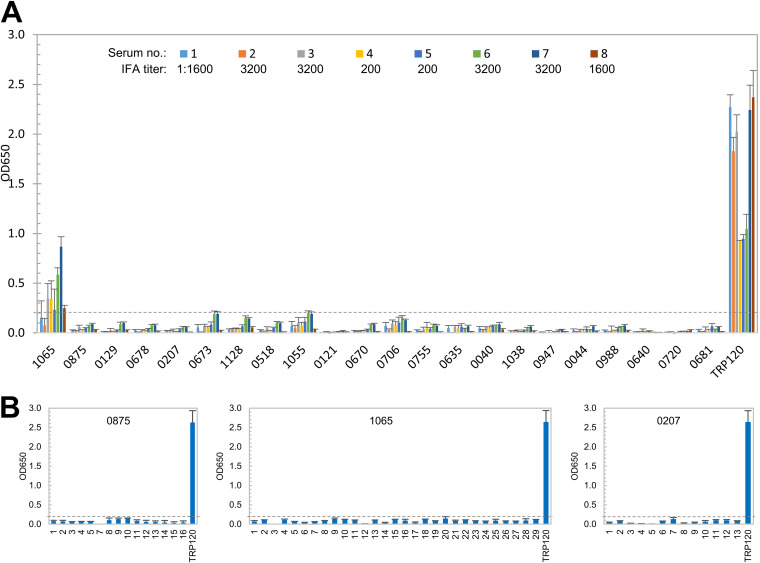
After denaturation, only one E. chaffeensis immunoreactive protein (Ech_1065) among the top 22 still reacted weakly (mean OD650, 0.36) with 5 HME sera compared to its native IVTT protein (mean OD650, 1.96). All other denatured E. chaffeensis proteins did not react with any HME serum, while the major immunoreactive protein control, TRP120, was not affected by denaturation since it contains a major linear epitope (Fig. 4A) (4). These results indicate that the new E. chaffeensis immunoreactive proteins are defined by conformation-dependent antibody epitopes.
FIG 4.
Conformation-dependent immunoreactivity of 22 E. chaffeensis proteins by ELISA. (A) Immunoreactivity of the denatured IVTT-expressed E. chaffeensis proteins compared with TRP120 by ELISA using a panel of eight HME sera. (B) Immunoreactivity of overlapping synthetic peptides spanning three E. chaffeensis immunoreactive proteins, as determined by ELISA with pooled HME sera. Positive control, a TRP120 epitope peptide. OD650 values represent the mean optical density reading from three wells (± standard deviations) after background subtraction. A sample OD650 of ≥0.2 was considered positive and ≥0.5 a strong positive after subtracting negative-control (IVTT reaction with empty plasmid template in panel A; negative peptide in panel B) reading.
To determine if the E. chaffeensis immunoreactive proteins contained linear epitopes, we used synthetic peptides to map linear epitopes in these proteins (5, 6, 9). Overlapping polypeptides (19 to 20 amino acids; 6-amino-acid overlap) were synthesized to cover the sequence of three selected E. chaffeensis immunoreactive proteins (Ech_0207, Ech_0875, and Ech_1065), except that peptide 6 of Ech_0875 was not synthesized successfully due to its strong hydrophobicity. The pooled HME sera used in our initial screening were used to test all peptides by ELISA (Fig. 4B). None of these peptides reacted with HME sera, demonstrating that these E. chaffeensis immunoreactive proteins do not contain major linear epitopes, consistent with ELISA using denatured and native IVTT products (Fig. 2 and Fig. 4A).
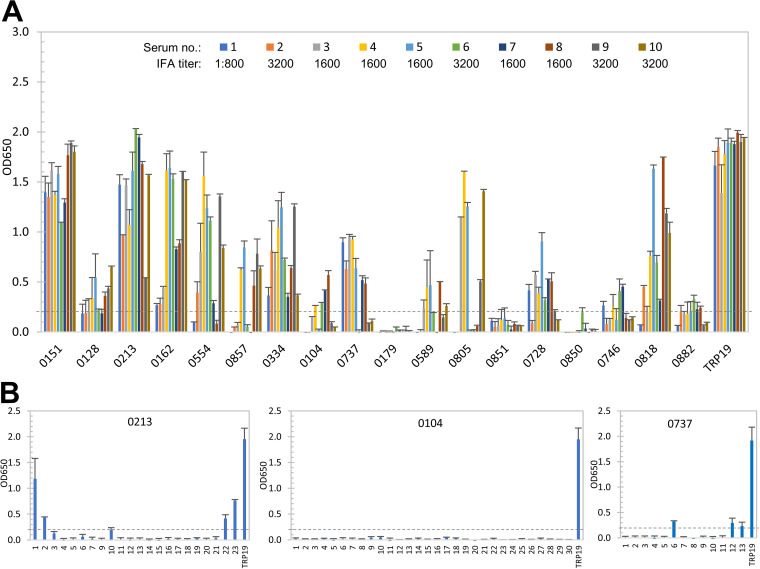
Among the top 18 E. canis immunoreactive proteins, the immunoreactivity of five proteins (Ecaj_0151, Ecaj_0213, Ecaj_0162, Ecaj_0818, and Ecaj_0563) was not reduced substantially after denaturation, similar to well-defined E. canis major immunoreactive protein TRP19 that has defined linear antibody epitope; however, three proteins, including Ecaj_0179, Ecaj_0851, and Ecaj_0850, did not react with any canine serum. An additional 10 E. canis proteins reacted with canine sera at a substantially lower level than native IVTT proteins (Fig. 5A). Thus, our results indicate that 13 of 18 E. canis immunoreactive proteins have conformation-dependent antibody epitopes.
FIG 5.
Conformation-dependent immunoreactivity of 18 E. canis proteins by ELISA. (A) Immunoreactivity of the denatured IVTT-expressed E. canis proteins compared with TRP19 by ELISA using convalescent-phase sera from 10 CME dogs. (B) Immunoreactivity of overlapping synthetic peptides spanning three E. canis immunoreactive proteins, as determined by ELISA with pooled CME sera. Positive control, a TRP19 epitope peptide. ELISA OD650 values represent the mean optical density reading from three wells (± standard deviations) after background subtraction. A sample OD650 of ≥0.2 was considered positive and ≥0.5 a strong positive after subtracting negative-control (IVTT reaction with empty plasmid template in panel A; negative peptide in panel B) reading.
We also selected three E. canis proteins (Ecaj_0213, Ecaj_0104, and Ecaj_0737) to investigate conformational dependence using overlapping synthetic peptides. These three proteins were predicted to contain different types of antibody epitope according to our ELISA results. By ELISA, peptide 1 of Ecaj_0213 reacted strongly with pooled CME sera, and four peptides of Ecaj_0213 (peptides 2, 10, 22, and 23) reacted with pooled CME sera, suggesting the presence of a major linear epitope and a few minor linear epitopes in this protein. Other peptides of Ecaj_0213 did not react with canine sera except that peptide 8 was not synthesized successfully due to its strong hydrophobicity. Three peptides of Ecaj_0737 (peptides 6, 12, and 13) reacted with pooled CME sera, but at a substantially lower level than the whole protein, suggesting the presence of a few minor linear epitopes. None of the Ecaj_0104 peptides reacted with CME sera, suggesting the absence of linear epitopes. These results support the conclusion that some E. canis immunoreactive proteins contain major conformational epitopes, while some others contain linear epitopes or both (Fig. 3 and Fig. 5A).
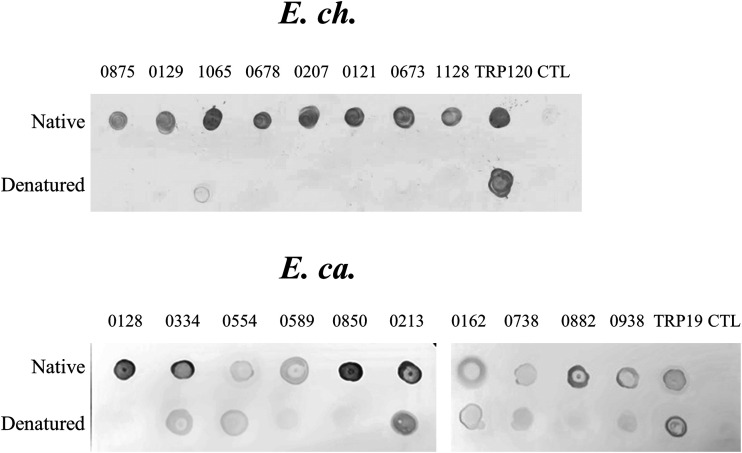
The conformational dependence of epitopes in the Ehrlichia immunoreactive proteins was also examined by dot immunoblot (Fig. 6). Top E. chaffeensis and E. canis immunoreactive proteins were selected, and the immunoreactivity of denatured proteins was compared with that of native proteins using an HME or a CME serum. After denaturation, none of the E. chaffeensis immunoreactive proteins reacted with the HME serum except that Ech_1065 reacted weakly. Consistent with our ELISA data in Fig. 4A, these results support the conclusion that these E. chaffeensis immunoreactive proteins are defined by conformation-dependent antibody epitopes. After denaturation, one E. canis protein (Ecaj_0213) reacted strongly with the CME serum, but four proteins (Ecaj_0128, Ecaj_0589, Ecaj_0850, and Ecaj_0882) did not react. Denatured Ecaj_0334 reacted with the CME serum at a substantially lower level than native proteins, whereas the other four proteins reacted at a level similar to the native proteins. These results are consistent with our ELISA data in Fig. 5A, supporting our conclusion that the E. canis immunoreactive proteins contain major conformational epitopes, linear epitopes, or both.
FIG 6.
Conformation-dependent immunoreactivity of E. chaffeensis and E. canis proteins by dot immunoblot. Immunoreactivity of the native and denatured proteins and TRPs was detected by dot immunoblot with serum from an HME patient or a CME dog. All proteins were IVTT expressed and purified. CTL, negative control (IVTT protein with empty plasmid template).
Bioinformatic analysis of E. chaffeensis and E. canis immunoreactive proteins.
We performed a comprehensive bioinformatic analysis of the top 22 E. chaffeensis and 18 E. canis immunoreactive proteins using multiple online prediction tools (Tables 3 and 4). Notably, 18 (82%) of E. chaffeensis and 10 (56%) of E. canis proteins were predicted to contain one to six transmembrane helixes by TMHMM 2.0 (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0). However, using SignalP 5.0 (https://services.healthtech.dtu.dk/service.php?SignalP-5.0) and SecretomeP 2.0 (http://www.cbs.dtu.dk/services/SecretomeP), only three E. chaffeensis (Ech_0678, Ech_0044, and Ech_1038) and one E. canis protein (Ecaj_0818) were predicted to be secreted by a standard secretory signal peptide or a nonclassical and leaderless mechanism. Ehrlichia has type I and type IV secretion systems (T1SS and T4SS); therefore, these proteins were analyzed as possible T1SS and T4SS substrates. No consensus sequence of type IV secretory motif R-X(7)-R-X-R-X-R (35) was identified in any of the proteins. Only three E. chaffeensis (Ech_0129, Ech_0040, and Ech_1038) and two E. canis proteins (Ecaj_0334 and Ecaj_0728) were predicted to be type IV substrates by the S4TE 2.0 tool (36). In contrast, a putative type I secretion signal (LDAVTSIF-enriched and KHPMWC-poor) (37, 38) was identified in the last 50 C-terminal residues of these proteins, suggesting that the majority of these proteins are type I secreted substrates. Ech_0875 and Ecaj_0104 proteins showed the greatest difference between the residue occurrences of LDAVTSIF (72% and 70%) and KHPMWC (8% and 6%) in the last 50 C-terminal amino acids, whereas the predicted type IV substrate Ech_0129 showed the least difference (44% versus 34%). Moreover, the PREFFECTOR server (http://draco.cs.wpi.edu/preffector) identified 11 (50%) E. chaffeensis and 8 (44%) E. canis proteins as effectors (39). This analysis supports the conclusion that many of these proteins are type I secreted effectors, although additional experimental validations are required (Tables 3 and 4).
TABLE 3.
Predicted features of E. chaffeensis immunoreactive proteins
| Protein (Ech_ tag no.) | No. of amino acids (mass [kDa]) | Transmembrane domainc,e | Secretion | T4Sd | Effectorf | Annotation |
|---|---|---|---|---|---|---|
| 1065 | 404 (44) | − | − | − | − | 2-Oxoglutarate dehydrogenase, E2 component |
| 0875 | 226 (25) | + | − | − | − | Phosphatidylglycerophosphatase A |
| 0129 | 416 (46) | + | − | + | − | HemY domain protein |
| 0678 | 230 (25) | + | +a | − | + | Hypothetical protein |
| 0207 | 176 (19) | + | − | − | + | Hypothetical protein |
| 0673 | 256 (28) | + | − | − | + | Hypothetical protein |
| 1128 | 196 (22) | + | − | − | + | Hypothetical protein |
| 0518 | 291 (32) | + | − | − | − | Hypothetical protein |
| 1055 | 175 (19) | + | − | − | − | Cytochrome C oxidase assembly protein |
| 0121 | 368 (40) | − | − | − | − | Hypothetical protein |
| 0670 | 266 (29) | + | − | − | − | Hypothetical protein |
| 0706 | 108 (12) | − | − | − | + | Hypothetical protein |
| 0755 | 828 (91) | + | − | − | − | Sensor histidine kinase/response regulator |
| 0635 | 357 (39) | + | − | − | + | Hypothetical protein |
| 0040 | 714 (79) | + | − | + | + | Type IV secretion system component VirD4 |
| 1038 | 1963 (216) | + | +a | + | + | EtpE |
| 0947 | 138 (15) | + | − | − | − | Hypothetical protein |
| 0044 | 237 (26) | + | +b | − | + | Type IV secretion system protein VirB8 |
| 0988 | 208 (23) | + | − | − | + | Hypothetical protein |
| 0640 | 295 (32) | − | − | − | + | Hypothetical protein |
| 0720 | 759 (80) | + | − | − | − | Hypothetical protein |
| 0681 | 221 (24) | + | − | − | − | Hypothetical protein |
Signal peptide predicted by SignalP.
Secretion predicted by SecretomeP.
Predicted by TMHMM.
Predicted by S4TE.
+, positive; −, negative.
Predicted by PREFFECTOR.
TABLE 4.
Predicted features of E. canis immunoreactive proteins
| Protein (Ecaj_ tag no.) | No. of amino acids (mass [kDa]) | Transmembrane domainb,d | Secretion | T4Sc | Effectore | Annotation |
|---|---|---|---|---|---|---|
| 0151 | 205 (23) | + | − | − | + | Electron transport protein SCO1/SenC |
| 0128 | 347 (39) | + | − | − | + | Extracellular solute-binding protein, family 1 |
| 0213 | 328 (36) | + | − | − | − | Hypothetical protein |
| 0162 | 395 (43) | − | − | − | − | Translation elongation factor 1A (EF-1A/EF-Tu) |
| 0554 | 634 (69) | − | − | − | − | Heat shock protein Hsp70 |
| 0857 | 400 (44) | − | − | − | − | 2-Oxoglutarate dehydrogenase E2 component |
| 0334 | 630 (72) | + | − | + | + | PpiC-type peptidyl-prolyl cis-trans isomerase |
| 0104 | 425 (48) | + | − | − | + | Hypothetical protein |
| 0737 | 182 (20) | + | − | − | − | Hypothetical protein |
| 0179 | 194 (21) | − | − | − | + | Formylmethionine deformylase |
| 0589 | 372 (41) | − | − | − | + | DNA-directed RNA polymerase subunit alpha |
| 0805 | 492 (54) | − | − | − | − | ATP-dependent protease ATP-binding subunit |
| 0851 | 221 (24) | + | − | − | − | Hypothetical protein |
| 0728 | 228 (26 | + | − | + | − | Hypothetical protein |
| 0850 | 451 (51) | − | − | − | + | Insulinase-like peptidase M16, C terminus |
| 0746 | 243 (27) | + | − | − | − | Hypothetical protein |
| 0818 | 211 (24) | − | +a | − | + | LexA family transcriptional regulator |
| 0882 | 527 (59) | + | − | − | − | Hypothetical protein |
Secretion predicted by SecretomeP.
Predicted by TMHMM.
Predicted by S4TE.
+, positive; −, negative.
Predicted by PREFFECTOR.
Among the top 22 E. chaffeensis and 18 E. canis immunoreactive proteins, the majority (21/40; 53%) were hypothetical, consistent with our previous findings. In addition, eight E. chaffeensis and 11 E. canis proteins were annotated with putative function, and, interestingly, most were enzymes involved in important biological processes, such as phosphatase, dehydrogenase, oxidase, kinase, isomerase, polymerase, deformylase, and peptidase. Notably, two type IV secretion system components (VirD4 and VirB8) and an entry-triggering protein of E. chaffeensis (EtpE) were also identified as immunoreactive.
DISCUSSION
Traditional protein approaches such as Western immunoblot have been used to identify a small subset of major immunoreactive ehrlichial proteins (TRPs, Anks, and OMPs) from E. chaffeensis- or E. canis-infected mammalian cells, which contain linear antibody epitopes (2). These proteins have been the primary focus of Ehrlichia immunomolecular studies for decades. In this investigation, we used bioinformatic antigen prediction, cell-free IVTT expression, and immunoanalysis to identify many previously undiscovered immunodominant and subdominant ehrlichial proteins, most of which were hypothetical and characterized by conformation-dependent immunoreactivity and consistent with our previous study (21). These proteins vastly expand the defined immunoreactive proteins of E. chaffeensis and E. canis and have the potential to accelerate vaccine and diagnostic development for ehrlichioses.
Both E. chaffeensis and E. canis ORFeomes contain many genes that encode proteins with unknown function. Some well-known immunoreactive proteins of E. chaffeensis and E. canis, including TRPs, were formally hypothetical but, during the last decade, have been demonstrated to be secreted effectors that have various distinct functions during infection (40–43). Recently, many hypothetical proteins of E. chaffeensis and E. canis have been identified to be immunodominant or subdominant (21). A large number of hypothetical proteins in other intracellular pathogens have also been found to be immunoreactive, indicating that many antigenic proteins still have unknown function (25, 44). Our strategy of protein screening prioritization and results of antigen identification in this study support this conclusion.
We also identified Ehrlichia major immunoreactive proteins that were annotated with putative function, including eight E. chaffeensis and 11 E. canis proteins (Tables 3 and 4). Most of these proteins were predicted to be enzymes involved in multiple important biological processes, such as signal transduction, energy metabolism, protein synthesis and metabolism, glycometabolism, and lipid metabolism, suggesting that immunoreactive Ehrlichia antigens are not always associated with outer membrane and may also play important roles in cellular reprogramming strategy to promote survival. Not surprisingly, two type IV secretion system proteins (VirD4 and VirB8) and an entry-triggering protein of E. chaffeensis (EtpE) were also identified as immunoreactive. Anaplasma marginale type IV secretion system proteins have been found to be immunogenic components of a protective bacterial membrane vaccine in cattle, including VirB2, VirB7, VirB11, and VirD4 (45). E. chaffeensis EtpE has also shown partial protection in dogs (46). However, the E. canis ortholog of EtpE was not identified to be immunoreactive, suggesting that the antibody epitope in EtpE may not be conserved in E. canis (Table 1).
Prior to the application of IVTT in Ehrlichia antigen identification, almost all E. chaffeensis and E. canis immunoreactive proteins were defined by major linear epitopes due to limitations in traditional experimental approaches previously used and difficulties in studying protein conformation (4). Current application of IVTT allowed us to directly express soluble proteins in native conformation, particularly those difficult to express in cell-based systems. Not surprisingly, conformation-dependent epitopes previously undiscovered predominated in this study, consistent with our recent report, suggesting that the Ehrlichia immunomes have a predominance of epitopes with conformation dependence. Some conformational immunoreactive proteins have been experimentally identified in other human pathogens, such as bacteria Campylobacter jejuni, Yersinia pseudotuberculosis, dengue virus, and hepatitis E virus (44, 47–49). Moreover, it has been estimated that the vast majority of B-cell epitopes are conformational (50). The proteins identified in the current and previous studies elicit strong host antibody responses and thus may play an important role in infection. This study further highlights the gap in knowledge and the need to define the antigenic repertoire and antibody epitopes involved for effective vaccine development.
The upregulated gene expression of a large number of E. chaffeensis proteins has been reported in tick cells compared to human cells. We also reported that gene expression of some Ehrlichia proteins is detectable exclusively in tick cells, including Ech_0635, Ech_0988, and Ech_1038 found in this study (18), which may be some of the first antigens to elicit human immune responses when Ehrlichia from infected ticks (tick phenotype) is transmitted to humans. In addition, it has also been demonstrated that differential expression of known ehrlichial antigens, such as TRPs and OMPs, occurs in tick and mammalian hosts (18–20). Divergent protein immunoreactivity in Ehrlichia cultivated in mammalian and tick cells illuminates the differences in Ehrlichia proteomes expressed in the different host cell environments (19). Thus, many of our Ehrlichia antigens may have not been identified previously because they are predominately expressed in tick cells. In a previous study, immunoreactive membrane proteins, including four hypothetical proteins, were identified from E. chaffeensis-infected mammalian and/or tick cells by SDS-PAGE and mass spectrometry; however, we found only one of them, Ech_0040 (VirD4) to be immunoreactive in this study (19). These differences could be due to different animal hosts or inoculation for infection (needle versus tick) to generate the sera used to screen. We expect that these Ehrlichia antigens may be useful in immunodiagnostics for early detection of antibodies against tick-specific ehrlichial proteins or for developing transmission-blocking vaccines.
Some previously identified immunoreactive proteins of Ehrlichia consist of ortholog pairs, such as TRPs, Anks, and OMPs, all of which contain major linear epitopes, suggesting that E. chaffeensis and E. canis have similar orthologous immunomes (51). However, in this study, although we have identified E. canis and E. chaffeensis orthologs for most of the E. chaffeensis and E. canis immunoreactive proteins, only one ortholog pair (Ech_1065 and Ecaj_0857), annotated as 2-oxoglutarate dehydrogenase E2 component, was found to react strongly and consistently with HME or CME sera (Tables 1 and 2). We also found that some other E. canis and E. chaffeensis orthologs were both immunoreactive, but these pairs did not react similarly with HME or CME sera. Moreover, some E. chaffeensis/E. canis antigens do not have corresponding orthologs, and more antigens were found from E. chaffeensis than E. canis. These findings suggest that the antibody epitopes in the majority of immunoreactive proteins are not conserved among corresponding ortholog pairs from E. chaffeensis and E. canis. Most of the E. canis immunoreactive proteins exhibited conformational epitopes; however, major epitopes of all new E. chaffeensis immunoreactive proteins appeared to conformation dependent. These observations also highlight the fundamental difference from previously defined major linear epitopes of Ehrlichia (4). These results further demonstrated that E. chaffeensis and E. canis proteins have a divergence in antibody recognition and different conformational immunomes, a principle that is potentially valuable in development of effective vaccines.
In this study, the majority of the immunoreactive proteins of E. chaffeensis and E. canis are predicted to be membrane proteins that contain at least one transmembrane domain. This interesting feature has not been previously reported, and the significance of transmembrane domains in immunoreactive proteins is still unclear, although it further highlights the importance of ehrlichial proteins with transmembrane domains as targets of the host immune response. In addition, only three E. chaffeensis and two E. canis proteins were predicted to be T4SS substrates by S4TE. However, the majority of immunoreactive proteins were predicted to be type I secreted effectors, consistent with our recent report (21). We have previously demonstrated that some ehrlichial major immunoreactive proteins, including TRPs and Ank200, are T1SS substrates, indicating that type I secretion system plays an important role in not only Ehrlichia pathobiology but also immunity (38). We also found that TRPs are effectors interacting with many host proteins associated with important cellular processes (51, 52). Therefore, Ehrlichia T1SS effector proteins may be predominantly targeted by the host immune response and elicit protective antibody responses against infection. Similarly, these immunoreactive proteins may be involved in many different interactions with the host cell during infection and thus be targets that can be neutralized by antibody. Further studies are needed to verify the conclusions regarding these immunoreactive proteins and to determine their roles in ehrlichial pathobiology and immunity.
In this and recent studies, we have analyzed about half of E. chaffeensis and E. canis ORFeomes, including all proteins in the top 350 according to ANTIGENpro and all hypothetical proteins present in ORFeomes, and discovered numerous immunoreactive proteins, including 24 E. chaffeensis and 16 E. canis immunodominant proteins. We expect that the significant expansion of known Ehrlichia immunoreactive proteins represents relatively complete antigenic repertoires of E. chaffeensis and E. canis and also highlights the likely importance of conformation-dependent antibody epitopes in immunity. These immunoreactive proteins will provide many potential candidates that rival TRPs for developing both sensitive diagnostics and effective subunit vaccines for HME and CME.
MATERIALS AND METHODS
Gene synthesis.
E. chaffeensis (Arkansas strain) and E. canis (Jake strain) gene sequences are available in the Integrated Microbial Genomes (IMG) (https://img.jgi.doe.gov/) (53) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/). E. chaffeensis and E. canis genes were codon optimized and chemically synthesized by GenScript (Piscataway, NJ).
HME and CME antisera.
HME patient sera were kind gifts from the Centers for Disease Control and Prevention (Atlanta, GA), Vanderbilt University (Nashville, TN), Washington University, and St. Louis Children’s Hospital (St. Louis, MO). CME sera were obtained from naturally infected dogs from the United States and Colombia. In order to avoid the possibility of polyreactive antibodies (IgM), which have been previously described (54), only convalescent-phase sera and anti-IgG secondary antibodies were used to examine the immunoreactivity.
IVTT.
In vitro expression of ehrlichial proteins was performed using the S30 T7 high-yield protein expression system (Promega, Madison, WI) according to the instructions from the manufacturer. The ehrlichial ORFs were cloned into pET-14b vector containing a 6×His tag sequence, and crude plasmids were extracted by GenScript. Each plasmid was transformed into Stellar competent cells (TaKaRa, Mountain View, CA) and purified from culture of a single colony using QIAprep spin miniprep kit (Qiagen, Germantown, MD). The purified plasmid was mixed with the Escherichia coli extract and a reaction premix and incubated at 37°C for 3 h.
Dot immunoblot.
The expression of ehrlichial proteins by IVTT was confirmed by dot immunoblot with horseradish peroxidase (HRP)-labeled His tag mouse antibody (1:500; GenScript) as described previously (21). The immunoreactivity of native and denatured ehrlichial proteins was also examined by dot immunoblot with HME or CME serum, for which IVTT-expressed proteins were purified by MagneHis protein purification system (Promega) according to the instructions from the manufacturer. TMB 1-component substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was used for all dot immunoblots.
ELISA immunoscreening.
The immunoreactivity of Ehrlichia IVTT-expressed proteins with a 6×His tag was determined by a His-tagged antigen-capture ELISA as described previously (21). HME or CME sera were diluted 1:200. Alkaline phosphatase-labeled rabbit anti-human IgG(H+L) secondary antibody (1:5,000; Abcam, Cambridge, MA) and BluePhos substrate (Kirkegaard & Perry) were used, and optical density was measured at 650 nm (OD650). Dilution buffer containing 4 M urea was used to denature IVTT-expressed proteins, and the diluted protein was incubated for 10 min at 99°C. ELISA OD650 values represent the mean OD650 reading from three wells (± standard deviation) after background subtraction. Since negative controls (an IVTT negative protein control and a normal human or canine serum control) generally had ELISA OD650 values of <0.1, a sample ELISA OD650 value of ≥0.2 was considered positive and ≥0.5 a strong positive after subtracting the negative-control ELISA OD650 reading (background). The top proteins with mean OD650s of ≥1.0 from multiple sera were considered immunodominant, and proteins with mean OD650 of 0.5 to 1.0 subdominant.
Peptide ELISA.
To identify linear antibody epitopes in Ehrlichia immunoreactive proteins, ELISAs were performed using overlapping peptides (19 to 20 amino acids; 6 amino acids overlapped) commercially synthesized by GenScript (5). All peptides were supplied as a lyophilized powder and resuspended in molecular biology-grade water (1 mg/ml).
Indirect fluorescent antibody assay.
The antibody titers of sera from HME patients and CME dogs were determined by IFA as previously described (21). Antigen slides were prepared from E. chaffeensis (Arkansas)-infected THP-1 cells or E. canis (Jake)-infected DH82 cells. A fluorescein isothiocyanate (FITC)-labeled rabbit anti-human or anti-dog IgG(H+L) secondary antibody (Kirkegaard & Perry Laboratories) was used. Slides were examined with a BX61 epifluorescence microscope (Olympus, Japan).
ACKNOWLEDGMENTS
This work was supported by the Clayton Foundation for Research.
T.L. designed and performed the experiments, analyzed the data, and wrote and revised the original draft of the manuscript. J.G.P. and X.Z. designed and performed the experiments and contributed to data analysis. D.H.W. contributed to conception of the project and manuscript revisions. J.W.M. conceived the project, provided funding, supervised the project, and assisted with data analysis and interpretation, writing, reviewing, and editing of the manuscript.
We declare the existence of a financial/nonfinancial competing interest, including a pending patent application.
Footnotes
Supplemental material is available online only.
Contributor Information
Jere W. McBride, Email: jemcbrid@utmb.edu.
Guy H. Palmer, Washington State University
REFERENCES
- 1.Paddock CD, Childs JE. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev 16:37–64. 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBride JW, Walker DH. 2011. Molecular and cellular pathobiology of Ehrlichia infection: targets for new therapeutics and immunomodulation strategies. Expert Rev Mol Med 13:e3. 10.1017/S1462399410001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrus S, Waner T. 2011. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet J 187:292–296. 10.1016/j.tvjl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 4.McBride JW, Walker DH. 2010. Progress and obstacles in vaccine development for the ehrlichioses. Expert Rev Vaccines 9:1071–1082. 10.1586/erv.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo T, Zhang X, McBride JW. 2009. Major species-specific antibody epitopes of the Ehrlichia chaffeensis p120 and E. canis p140 orthologs in surface-exposed tandem repeat regions. Clin Vaccine Immunol 16:982–990. 10.1128/CVI.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo T, Zhang X, Wakeel A, Popov VL, McBride JW. 2008. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect Immun 76:1572–1580. 10.1128/IAI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle CK, Nethery KA, Popov VL, McBride JW. 2006. Differentially expressed and secreted major immunoreactive protein orthologs of Ehrlichia canis and E. chaffeensis elicit early antibody responses to epitopes on glycosylated tandem repeats. Infect Immun 74:711–720. 10.1128/IAI.74.1.711-720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBride JW, Doyle CK, Zhang X, Cardenas AM, Popov VL, Nethery KA, Woods ME. 2007. Identification of a glycosylated Ehrlichia canis 19-kilodalton major immunoreactive protein with a species-specific serine-rich glycopeptide epitope. Infect Immun 75:74–82. 10.1128/IAI.01494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBride JW, Zhang X, Wakeel A, Kuriakose JA. 2011. Tyrosine-phosphorylated Ehrlichia chaffeensis and Ehrlichia canis tandem repeat orthologs contain a major continuous cross-reactive antibody epitope in lysine-rich repeats. Infect Immun 79:3178–3187. 10.1128/IAI.01347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo T, Zhang X, Nicholson WL, Zhu B, McBride JW. 2010. Molecular characterization of antibody epitopes of Ehrlichia chaffeensis ankyrin protein 200 and tandem repeat protein 47 and evaluation of synthetic immunodeterminants for serodiagnosis of human monocytotropic ehrlichiosis. Clin Vaccine Immunol 17:87–97. 10.1128/CVI.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nethery KA, Doyle CK, Zhang X, McBride JW. 2007. Ehrlichia canis gp200 contains dominant species-specific antibody epitopes in terminal acidic domains. Infect Immun 75:4900–4908. 10.1128/IAI.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi N, Unver A, Zhi N, Rikihisa Y. 1998. Cloning and characterization of multigenes encoding the immunodominant 30-kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol 36:2671–2680. 10.1128/JCM.36.9.2671-2680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun 66:132–139. 10.1128/IAI.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SM, Cullman LC, Walker DH. 1997. Western immunoblotting analysis of the antibody responses of patients with human monocytotropic ehrlichiosis to different strains of Ehrlichia chaffeensis and Ehrlichia canis. Clin Diagn Lab Immunol 4:731–735. 10.1128/cdli.4.6.731-735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JS, Yager E, Reilly M, Freeman C, Reddy GR, Reilly AA, Chu FK, Winslow GM. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J Immunol 166:1855–1862. 10.4049/jimmunol.166.3.1855. [DOI] [PubMed] [Google Scholar]
- 16.Kuriakose JA, Zhang X, Luo T, McBride JW. 2012. Molecular basis of antibody mediated immunity against Ehrlichia chaffeensis involves species-specific linear epitopes in tandem repeat proteins. Microbes Infect 14:1054–1063. 10.1016/j.micinf.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride JW, Corstvet RE, Gaunt SD, Boudreaux C, Guedry T, Walker DH. 2003. Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect Immun 71:2516–2524. 10.1128/IAI.71.5.2516-2524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuriakose JA, Miyashiro S, Luo T, Zhu B, McBride JW. 2011. Ehrlichia chaffeensis transcriptome in mammalian and arthropod hosts reveals differential gene expression and post transcriptional regulation. PLoS One 6:e24136. 10.1371/journal.pone.0024136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo GM, Cheng C, Tomich J, Ganta RR. 2008. Total, membrane, and immunogenic proteomes of macrophage- and tick cell-derived Ehrlichia chaffeensis evaluated by liquid chromatography-tandem mass spectrometry and MALDI-TOF methods. Infect Immun 76:4823–4832. 10.1128/IAI.00484-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singu V, Peddireddi L, Sirigireddy KR, Cheng C, Munderloh U, Ganta RR. 2006. Unique macrophage and tick cell-specific protein expression from the p28/p30-outer membrane protein multigene locus in Ehrlichia chaffeensis and Ehrlichia canis. Cell Microbiol 8:1475–1487. 10.1111/j.1462-5822.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 21.Luo T, Patel JG, Zhang X, Walker DH, McBride JW. 2020. Ehrlichia chaffeensis and E. canis hypothetical protein immunoanalysis reveals small secreted immunodominant proteins and conformation-dependent antibody epitopes. NPJ Vaccines 5:85. 10.1038/s41541-020-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felgner PL, Kayala MA, Vigil A, Burk C, Nakajima-Sasaki R, Pablo J, Molina DM, Hirst S, Chew JS, Wang D, Tan G, Duffield M, Yang R, Neel J, Chantratita N, Bancroft G, Lertmemongkolchai G, Davies DH, Baldi P, Peacock S, Titball RW. 2009. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. Proc Natl Acad Sci USA 106:13499–13504. 10.1073/pnas.0812080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigil A, Chen C, Jain A, Nakajima-Sasaki R, Jasinskas A, Pablo J, Hendrix LR, Samuel JE, Felgner PL. 2011. Profiling the humoral immune response of acute and chronic Q fever by protein microarray. Mol Cell Proteomics 10:M110.006304. 10.1074/mcp.M110.006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vigil A, Davies DH, Felgner PL. 2010. Defining the humoral immune response to infectious agents using high-density protein microarrays. Future Microbiol 5:241–251. 10.2217/fmb.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Fisher MI, Cheng C, Sun G, Pal S, Teng A, Molina DM, Kayala MA, Vigil A, Baldi P, Felgner PL, Liang X, de la Maza LM. 2011. Identification of immunodominant antigens by probing a whole Chlamydia trachomatis open reading frame proteome microarray using sera from immunized mice. Infect Immun 79:246–257. 10.1128/IAI.00626-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, Baldi P, Felgner PL. 2008. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun 76:3374–3389. 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vigil A, Ortega R, Jain A, Nakajima-Sasaki R, Tan X, Chomel BB, Kasten RW, Koehler JE, Felgner PL. 2010. Identification of the feline humoral immune response to Bartonella henselae infection by protein microarray. PLoS One 5:e11447. 10.1371/journal.pone.0011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin CF, Teh BA, Anthony AA, Aziah I, Ismail A, Ong EB, Lim TS. 2014. Overexpression, purification and validation of antigenic Salmonella enterica serovar Typhi proteins identified from LC-MS/MS. Appl Biochem Biotechnol 174:1897–1906. 10.1007/s12010-014-1173-y. [DOI] [PubMed] [Google Scholar]
- 29.Magnan CN, Zeller M, Kayala MA, Vigil A, Randall A, Felgner PL, Baldi P. 2010. High-throughput prediction of protein antigenicity using protein microarray data. Bioinformatics 26:2936–2943. 10.1093/bioinformatics/btq551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson ED, Gan R, Hodgman CE, Jewett MC. 2012. Cell-free protein synthesis: applications come of age. Biotechnol Adv 30:1185–1194. 10.1016/j.biotechadv.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu Y, Kuruma Y, Ying BW, Umekage S, Ueda T. 2006. Cell-free translation systems for protein engineering. FEBS J 273:4133–4140. 10.1111/j.1742-4658.2006.05431.x. [DOI] [PubMed] [Google Scholar]
- 32.Cardenas AM, Doyle CK, Zhang X, Nethery K, Corstvet RE, Walker DH, McBride JW. 2007. Enzyme-linked immunosorbent assay with conserved immunoreactive glycoproteins gp36 and gp19 has enhanced sensitivity and provides species-specific immunodiagnosis of Ehrlichia canis infection. Clin Vaccine Immunol 14:123–128. 10.1128/CVI.00361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taques I, Koiyama MFG, Campos ANS, Costa JS, Hongyu K, Aguiar DM. 2020. Canonical correlative analyses among an enzyme-linked immunosorbent assay using synthetic peptides, an indirect fluorescent antibody test, and hematologic measurements in dogs infected with Ehrlichia canis. Vet Clin Pathol 49:574–582. 10.1111/vcp.12908. [DOI] [PubMed] [Google Scholar]
- 34.Pritt B, Dumler JS. 2019. Ehrlichia, Anaplasma, and related intracellular bacteria, p 1173. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12 ed. ASM Press, Washington, DC. [Google Scholar]
- 35.Vergunst AC, van Lier MC, den Dulk-Ras A, Stuve TA, Ouwehand A, Hooykaas PJ. 2005. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc Natl Acad Sci USA 102:832–837. 10.1073/pnas.0406241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noroy C, Lefrancois T, Meyer DF. 2019. Searching algorithm for type IV effector proteins (S4TE) 2.0: improved tools for type IV effector prediction, analysis and comparison in proteobacteria. PLoS Comput Biol 15:e1006847. 10.1371/journal.pcbi.1006847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delepelaire P. 2004. Type I secretion in gram-negative bacteria. Biochim Biophys Acta 1694:149–161. 10.1016/j.bbamcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Wakeel A, den Dulk-Ras A, Hooykaas PJ, McBride JW. 2011. Ehrlichia chaffeensis tandem repeat proteins and Ank200 are type 1 secretion system substrates related to the repeats-in-toxin exoprotein family. Front Cell Infect Microbiol 1:22. 10.3389/fcimb.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhroso A, Eidson S, Korkin D. 2018. Genome-wide prediction of bacterial effector candidates across six secretion system types using a feature-based statistical framework. Sci Rep 8:17209. 10.1038/s41598-018-33874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo T, Kuriakose JA, Zhu B, Wakeel A, McBride JW. 2011. Ehrlichia chaffeensis TRP120 interacts with a diverse array of eukaryotic proteins involved in transcription, signaling, and cytoskeleton organization. Infect Immun 79:4382–4391. 10.1128/IAI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo T, McBride JW. 2012. Ehrlichia chaffeensis TRP32 interacts with host cell targets that influence intracellular survival. Infect Immun 80:2297–2306. 10.1128/IAI.00154-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo T, Mitra S, McBride JW. 2018. Ehrlichia chaffeensis TRP75 Interacts with host cell targets involved in homeostasis, cytoskeleton organization, and apoptosis regulation to promote infection. mSphere 3:e00147-18. 10.1128/mSphere.00147-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakeel A, Kuriakose JA, McBride JW. 2009. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect Immun 77:1734–1745. 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Parrish JR, Hines J, Mansfield L, Finley RL. Jr., 2019. A proteome-wide screen of Campylobacter jejuni using protein microarrays identifies novel and conformational antigens. PLoS One 14:e0210351. 10.1371/journal.pone.0210351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutten EL, Norimine J, Beare PA, Heinzen RA, Lopez JE, Morse K, Brayton KA, Gillespie JJ, Brown WC. 2010. Anaplasma marginale type IV secretion system proteins VirB2, VirB7, VirB11, and VirD4 are immunogenic components of a protective bacterial membrane vaccine. Infect Immun 78:1314–1325. 10.1128/IAI.01207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budachetri K, Teymournejad O, Lin M, Yan Q, Mestres-Villanueva M, Brock GN, Rikihisa Y. 2020. An entry-triggering protein of Ehrlichia is a new vaccine candidate against tick-borne human monocytic ehrlichiosis. mBio 11:e00895-20. 10.1128/mBio.00895-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Portnyagina O, Zelepuga E, Khomenko V, Solov'eva E, Solov'eva T, Novikova O. 2018. In silico and in vitro analysis of cross-reactivity between Yersinia pseudotuberculosis OmpF porin and thyroid-stimulating hormone receptor. Int J Biol Macromol 107:2484–2491. 10.1016/j.ijbiomac.2017.10.133. [DOI] [PubMed] [Google Scholar]
- 48.Andrade DV, Warnes C, Young E, Katzelnick LC, Balmaseda A, de Silva AM, Baric RS, Harris E. 2019. Tracking the polyclonal neutralizing antibody response to a dengue virus serotype 1 type-specific epitope across two populations in Asia and the Americas. Sci Rep 9:16258. 10.1038/s41598-019-52511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He B, Zhang Z, Zhang X, Tang Z, Liu C, Zheng Z, Li S, Zhang J, Xia N, Zhao Q. 2020. Functional epitopes on hepatitis E virions and recombinant capsids are highly conformation-dependent. Hum Vaccin Immunother 16:1554–1564. 10.1080/21645515.2019.1703454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Regenmortel MHV. 1996. Mapping epitope structure and activity: from one-dimensional prediction to four-dimensional description of antigenic specificity. Methods 9:465–472. 10.1006/meth.1996.0054. [DOI] [PubMed] [Google Scholar]
- 51.Lina TT, Farris T, Luo T, Mitra S, Zhu B, McBride JW. 2016. Hacker within! Ehrlichia chaffeensis effector driven phagocyte reprogramming strategy. Front Cell Infect Microbiol 6:58. 10.3389/fcimb.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunphy PS, Luo T, McBride JW. 2013. Ehrlichia moonlighting effectors and interkingdom interactions with the mononuclear phagocyte. Microbes Infect 15:1005–1016. 10.1016/j.micinf.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen IA, Chu K, Palaniappan K, Pillay M, Ratner A, Huang J, Huntemann M, Varghese N, White JR, Seshadri R, Smirnova T, Kirton E, Jungbluth SP, Woyke T, Eloe-Fadrosh EA, Ivanova NN, Kyrpides NC. 2019. IMG/M v.5.0: an integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res 47:D666–D677. 10.1093/nar/gky901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones DD, DeIulio GA, Winslow GM. 2012. Antigen-driven induction of polyreactive IgM during intracellular bacterial infection. J Immunol 189:1440–1447. 10.4049/jimmunol.1200878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00224-21-s0001.pdf, PDF file, 0.4 MB (388.9KB, pdf)