ABSTRACT
Preerythrocytic vaccines prevent malaria by targeting parasites in the clinically silent sporozoite and liver stages and preventing progression to the virulent blood stages. The leading preerythrocytic vaccine, RTS,S/AS01E (Mosquirix), entered implementation programs in 2019 and targets the major sporozoite surface antigen, circumsporozoite protein (CSP). However, in phase III clinical trials, RTS,S conferred partial protection with limited durability, indicating a need to improve CSP-based vaccination. Previously, we identified highly expressed liver-stage proteins that could potentially be used in combination with CSP; they are referred to as preerythrocytic vaccine antigens (PEVAs). Here, we developed heterologous prime-boost CSP vaccination models to confer partial sterilizing immunity against Plasmodium yoelii (protein prime-adenovirus 5 [Ad5] boost) and Plasmodium berghei (DNA prime-Ad5 boost) in mice. When combined as individual antigens with P. yoelii CSP (PyCSP), three of eight P. yoelii PEVAs significantly enhanced sterile protection against sporozoite challenge, compared to PyCSP alone. Similar results were obtained when three P. berghei PEVAs and P. berghei CSP were combined in a single vaccine regimen. In general, PyCSP antibody responses were similar after CSP alone versus CSP plus PEVA vaccinations. Both P. yoelii and P. berghei CSP plus PEVA combination vaccines induced robust CD8+ T cell responses, including signature gamma interferon (IFN-γ) increases. In the P. berghei model system, IFN-γ responses were significantly higher in hepatic versus splenic CD8+ T cells. The addition of novel antigens may enhance the degree and duration of sterile protective immunity conferred by a human vaccine such as RTS,S.
KEYWORDS: circumsporozoite protein, malaria, preerythrocytic, sterile protection, vaccine
INTRODUCTION
The burden of malaria remains intolerable, causing over 400,000 deaths and 200 million clinical cases each year, primarily among children in Africa (1). Antimalarial drugs and insecticide-treated nets have effectively reduced malaria cases over the past few decades, but progress has stalled in recent years and been reversed in some countries with the heaviest burden of malaria. Vaccination remains a key strategy to bolster control efforts toward reduction and elimination of this scourge.
Vaccines that confer long-lasting sterile immunity would prevent morbidity and death and could eliminate malaria in mass vaccination campaigns. Such vaccines target sporozoite (SPZ)-stage parasites inoculated by mosquitoes and/or clinically silent liver-stage (LS) parasites to avert the pathogenic and transmissible blood-stage forms. The most advanced malaria vaccine, called RTS,S or Mosquirix, targets the Plasmodium falciparum SPZ major surface protein (circumsporozoite protein [CSP]) and confers partial protection from clinical malaria but not sterilizing immunity in young children (2, 3). While RTS,S entered WHO-requested implementation programs in 2019, efforts continue to improve on the partial protective efficacy conferred by RTS,S.
CSP-based vaccines can be enhanced by combination with other preerythrocytic vaccine antigens (PEVAs). We previously demonstrated in preclinical models that vaccine efficacy to reduce LS parasite burden (LPB) is enhanced when CSP is combined with PEVAs identified as preferentially transcribed by LS parasites in microarray studies (4). Whereas RTS,S-induced protection is thought to be mediated primarily by antibodies, protection by novel PEVAs delivered as DNA vaccines is mediated by CD8-dependent and independent mechanisms in rodents (4, 5).
Here, using new mouse immunization-challenge models of partial sterilizing immunity independently developed for Plasmodium yoelii and for Plasmodium berghei, we evaluated eight of the PEVA candidates that had greatest activity in our prior study (4) for their ability to enhance sterilizing CSP protection. Eight P. yoelii PEVA (PyPEVA) candidates (administered via heterologous protein prime-adenovirus 5 [Ad5] boost) and three P. berghei PEVA (PbPEVA) candidates (administered via heterologous DNA prime-Ad5 boost) were tested in combination with CSP to determine whether such combination regimens increased the frequency of sterile protection, compared to CSP alone. According to our results, regardless of immunization approach, combinations with PEVA candidates in both P. yoelii and P. berghei models enhanced sterile protection induced with CSP.
RESULTS
Establishment of a “partial sterile protection” model in mice.
To improve on the protection conferred by DNA vaccinations, measured as reduction in LPB in our previous study (4), we evaluated different CSP immunization regimens in outbred mice for evidence of sterile protection (i.e., no evidence of patent parasitemia) (see Table S3 in the supplemental material). Although priming with either protein or DNA followed by recombinant Ad5 boost conveyed sterile protection against P. yoelii challenge in a variable number of mice, the protein prime with recombinant Ad5 boost regimen showed the highest level of protection in CD1 or CB6F1 mice. We adapted this regimen to induce partial sterile immunity (i.e., sterile immunity in a fraction of animals) in BALB/c mice, which were immunized subcutaneously twice with 10 μg of green fluorescent protein (GFP) and 10 μg of P. yoelii CSP (PyCSP), formulated with complete Freund’s adjuvant (CFA)/incomplete Freund’s adjuvant (IFA), 21 days apart. On day 42, mice were immunized intramuscularly (IM) with 1 × 1010 virus particles (VP) Ad5 empty vector (EV) and 1 × 1010 VP Ad5 PyCSP (OD260 Inc.), and 50% of the animals were sterilely protected from P. yoelii SPZ challenge (see Tables S4 and S5). This model of partial sterile immunity offered the possibility to observe enhancement of sterile immunity by combination of PyCSP with other PEVAs versus PyCSP alone.
Expression, purification, and immunization of PEVAs.
Based on our earlier work (4), eight PyPEVA candidates (Table 1) were short-listed to analyze their ability to confer sterile protection against infectious SPZ challenge in an animal model. Figure 1A shows a Coomassie-stained gel of all of the purified proteins used in this study, along with purified insect cell enhanced GFP (eGFP). Depending on the amino acid composition of the proteins, there were considerable differences in the observed and calculated molecular weights (Table 1) (6, 7). Liver-specific protein 1 (LISP1) (100 kDa), PY04162 (conserved Plasmodium protein of unknown function) (80 kDa), PY03000 (AP-4 complex subunit β) (120 kDa), and middle domain of eukaryotic initiation factor 4G (MIF4G) (180 kDa) migrated higher than their calculated molecular weights, likely due to the presence of relatively larger proportions of negatively charged amino acids. TATA-binding protein (TBP) (50 kDa), PY01495 (conserved Plasmodium protein of unknown function) (90 kDa), serine hydroxymethyltransferase (SHMT) (70 kDa), and SPZ and LS asparagine-rich protein (SLARP) (115 kDa) are highly hydrophilic and thus bind less SDS, migrating higher than their calculated molecular weights (Table 1). PY04162 showed an additional band at 120 kDa that was not seen in the Western blot with His-tagged antibody, indicating that it might be pulled down with PY04162 as a complex or by nonspecific binding. MIF4G and SLARP showed the presence of some lower-molecular-weight bands, which could be degradation products. The identities of all of the vaccine antigens were confirmed by mass spectrometric analysis (see Data Set S2). To examine the ability of PEVA candidates to enhance sterile protection when coadministered with PyCSP, BALB/c mice received primary immunizations with recombinant proteins in combination with PyCSP, followed by booster immunization with recombinant Ad5 expressing the same antigens as used for primary immunizations (Fig. 1B).
TABLE 1.
Preerythrocytic vaccine candidates selected for sterile protection analysis
| P. yoelii orthologue (PlasmoDB identification no.)a | Protein name | Protein function | P. yoelii amino acid positions in vaccine | Predicted size (kDa) | Type of recombinant protein |
|---|---|---|---|---|---|
| PY03000 (Pf3D7_0730200) | AP-4 complex subunit β, putative | 6–891 | 104 | Insect cell expression | |
| PY00162/Pb103100 (Pf3D7_1411500) | MIF4G | Conserved Plasmodium protein, unknown function (MIF4G domain) | 3–980 | 117 | Insect cell expression for P. yoelii; in vivo expression by GG-delivered plasmid for P. berghei |
| PY04499 (Pf3D7_1418100) | LISP1 | LS egress | 1172–1732 | 66 | Insect cell expression |
| PY04162 (Pf3D7_1207400) | Conserved Plasmodium protein, unknown function | 1–387 | 46 | Insect cell expression | |
| PY01495 (Pf3D7_1241500) | Conserved Plasmodium protein, unknown function | 1–467 | 55 | E. coli expression | |
| PY03269/Pb090210 (PF3D7_1147000) | SLARP | LS development and transmission regulator | 1356–1989 | 74 | E. coli expression for P. yoelii; in vivo expression by GG-delivered plasmid for P. berghei |
| PY00669/Pb131980 (PF3D7_1456100) | SHMT | Serine hydroxymethyltransferase, putative | 1–484 | 57 | Insect cell expression for P. yoelii; in vivo expression by GG-delivered plasmid for P. berghei |
| PY00712 (Pf3D7_0506200) | TBP | Transcription initiation factor | 1–277 | 33 | E. coli expression |
FIG 1.
(A) Purification of PEVAs. Coomassie-stained gel showing purified proteins expressed in either insect cells or E. coli with a His tag at the C terminus and purified using Ni-NTA resin. (B) Protein prime-Ad5 boost PyPEVA/PyCSP combination study design. Mice were immunized as described in Materials and Methods. On day 56, spleens were harvested posteuthanasia from 5 mice/group. The remaining 14 mice/group were challenged with 300 P. yoelii SPZ on day 63. Mice were monitored for parasitemia via blood smears for 14 days postchallenge. (C) Protection conferred by PEVAs combined with CSP. Following challenge with P. yoelii SPZ, mice were monitored by blood smears from day 4 to day 7 and on day 14 for the presence of parasites. Mice that stayed parasite free were considered protected. *, P < 0.004; ***, P < 0.0001. P values were based on the time to parasitemia, and P values of <0.004 were considered significant after Bonferroni correction for multiple comparisons. (D) Protection after rechallenge of protected animals. Protected animals (challenged animals that did not develop blood-stage parasitemia) were rechallenged, 16 weeks after the last vaccine dose, with 300 P. yoelii 17XNL SPZ to assess the duration of protection. Mice were monitored by blood smears from day 4 to day 7 and on day 14 postrechallenge for the presence of parasites. *, P < 0.006. P values were based on the time to parasitemia, and P values of <0.006 were considered significant after Bonferroni correction for multiple comparisons.
Increased sterile protection by combination vaccine with CSP and PEVAs.
Three weeks after the final immunizations, mice were challenged with 300 P. yoelii 17XNL SPZ and were monitored for parasitemia via blood smears from day 4 to day 7 and on day 14 postchallenge. Mice that remained parasite-free during this period were considered protected. In combined data from two independent animal studies (total of 28 mice per group), the comparator PyCSP-GFP group showed approximately 20% protection (Fig. 1C). All of the PyCSP-PyPEVA combinations other than PyCSP-PY04162 yielded a greater proportion of protected mice than in the PyCSP-GFP group. All antigens yielded significant protection (measured as prolonged time to infection), compared to naive controls (GFP alone) (Fig. 1C). As a post hoc analysis, we compared the top three performing antigens (LISP1, SHMT, and TBP) to GFP plus CSP, and each conferred significantly greater protection (P < 0.004 for all antigens). Upon rechallenge of protected animals 16 weeks after the last vaccine dose, LISP1, SHMT, TBP, and PY01495 conferred significant protection, compared to naive controls, while GFP plus CSP did not (Fig. 1D). The data indicate that PyCSP-PyPEVA heterologous prime-boost immunizations can confer sterilizing immunity that is durable and superior to that achieved with PyCSP alone.
To generalize the observation that PyPEVA could enhance protection induced by PyCSP, we also evaluated P. berghei orthologues of PyPEVA as vaccine candidates in inbred B6 mice, in parallel with the ongoing P. yoelii studies. B6 mice are highly susceptible to P. berghei infection, and sterile protection is difficult to achieve in this model; therefore, we initially measured protection as a reduction of LPB. B6 mice were immunized at 3-week intervals twice with DNA (5 μg) by gene gun (GG) and boosted with 4 × 1010 VP of Ad5 vector by IM injection (Fig. 2A). We immunized different groups of mice with P. berghei CSP (PbCSP) alone, a combination of three PbPEVAs (P. berghei SHMT [PbSHMT], P. berghei SLARP [PbSLARP], and P. berghei MIF4G [PbMIF4G]) (3Ag), and PbCSP combined with 3Ag (Fig. 2B). Control groups received DNA EV and Ad5 EV according to the same immunization regimen. LPB was determined by measuring P. berghei 18S rRNA by quantitative PCR (qPCR) at 40 h after the SPZ challenge. LPB was significantly lower in all three study groups (PbCSP, 3Ag, and PbCSP plus 3Ag), compared to control groups (naive and EV) (Fig. 2B). The 3Ag showed greater protection than PbCSP alone and, when combined with PbCSP, significantly (P < 0.01) reduced LPB, in comparison to all other groups.
FIG 2.
(A) DNA prime-Ad5 boost PbPEVA/PbCSP combination study design. (B) P. berghei LS antigens combined with CSP reduce parasite burden in B6 mice. B6 mice (5 mice per group) were immunized at 3-week intervals twice with DNA by GG (5 μg) and boosted with 4 × 1010 VP of Ad5 vector by IM injection. Control groups received DNA EV and Ad5 EV according to the same immunization regimen. Fourteen days after the last boost, mice were challenged with 10,000 P. berghei SPZ by IV injection. Animals were euthanized and livers were harvested 40 h after the challenge for LPB determination by real-time qPCR. Data represent the ratio of expression of P. berghei 18SrRNA to that of mouse β-actin for individual mice. **, P < 0.01. (C) Partial sterile protection in CD1 mice immunized with a combination of three PbPEVAs and CSP. CD1 outbred mice (14 animals per group) were immunized as for panel B. Fourteen days after the last boost, mice were challenged with 300 P. berghei SPZ by IV injection. Mice were monitored for parasitemia via blood smears for 14 days postchallenge. Mice that stayed parasite free were considered protected. Data represent percentages of surviving mice.
We also evaluated the ability of 3Ag with PbCSP to induce partial sterile protection in outbred mice. CD1 mice were immunized at 3-week intervals twice with DNA (5 μg) by GG and later boosted with 4 × 1010 VP of Ad5 vector by IM injection. Two weeks after the last immunization, CD1 mice were challenged with 300 P. berghei SPZ by intravenous (IV) injection, and mice were monitored for parasitemia via blood smears. Mice that received PbCSP alone or 3Ag achieved 50% protection, compared to 7% and 14% protection in the naive group and the group immunized with EV, respectively (Fig. 2C). The combination of 3Ag with PbCSP increased protection (73%), compared to the group immunized with PbCSP alone. Thus, both P. berghei and P. yoelii models show that the addition of PEVAs can enhance the level of sterile protection achieved by immunization with CSP alone.
Immunogenicity.
In two independent animal studies with the eight PyPEVAs, sera were collected 2 weeks after the final immunization. Antibody responses to PyCSP measured by enzyme-linked immunosorbent assay (ELISA) were similar across all groups that received different PyCSP-PyPEVA combinations (Fig. 3A). In contrast, antibody responses to different PyPEVAs varied; most PyPEVAs ranged from moderate (∼2 optical density at wavelength 405 nm [OD405] units) to high (∼4 OD405 units) antibody responses, while MIF4G antibody levels were low (Fig. 3B).
FIG 3.
Antibody responses to PyPEVAs combined with PyCSP measured against CSP antigen (A) or individual PyPEVAs (B). Box and whisker plots show antibodies to CSP antigens or PEVAs measured by direct ELISA using sera from vaccinated mice collected 14 days after the last vaccine dose.
CD8+ T cell activation by PyPEVAs.
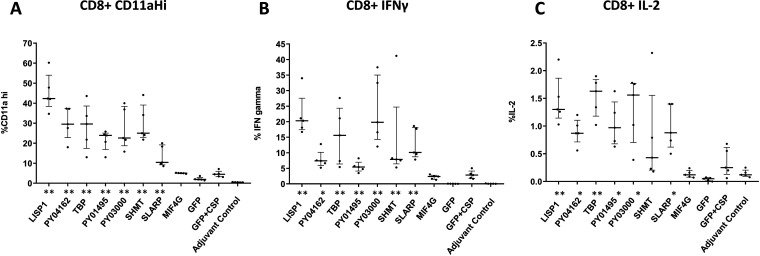
The involvement of CD8+ T cells as effectors against preerythrocytic-stage malaria has been widely documented (8, 9). We previously demonstrated that reduction of LPB as a consequence of PyPEVA DNA immunization is in part mediated by CD8+ T cells, but this varies between antigens (4), Here, we examined CD8+ T cell activation by PyPEVAs, as assessed by flow cytometry, using splenocytes harvested from mice 14 days after the final immunization (see gating strategy in Fig. S1). CD8+ CD11ahi, CD8+ gamma interferon (IFN-γ)+, and CD8+ interleukin 2 (IL-2)+ responses were recalled with peptides corresponding to each immunizing antigen (Fig. 4). CD8+ responses against each antigen were analyzed for significant differences from GFP plus CSP. All antigens tested elicited significantly higher CD8+ T cell responses than GFP plus CSP, except for MIF4G plus CSP (all CD8+ responses) and SHMT plus CSP (CD8+ IL-2 response). Levels of CD8+ T cell activation and cytokine production correlated significantly with antibody responses when their mean responses were compared with the individual PEVAs by Pearson test (see Fig. S2).
FIG 4.
CD8+ T cell recall responses to PEVAs after immunization with PyPEVA/PyCSP combinations. Fourteen days after the final immunization, spleens were harvested posteuthanasia from 5 mice per group. Single-cell suspensions of splenocytes were used for in vitro stimulation with pools of antigen-specific peptides. Samples from PyPEVA- plus PyCSP-immunized animals were stimulated with peptides from the corresponding PyPEVA and PyCSP together; splenocytes from P. yoelii GFP- plus PyCSP-treated control animals and adjuvant-only-treated animals were stimulated with peptides only from CSP. To determine levels of CD8+ T cell activation, cells were stained for markers, including CD3ε, CD4, CD8α, CD11a, IL-2, and IFN-γ. Levels of expression of various T cell activation markers against each PyPEVA are shown as medians with interquartile ranges and were compared between each PyPEVA plus CSP group and the GFP plus CSP group using a nonparametric Mann-Whitney test. *, P < 0.05; **, P < 0.01.
We previously showed that CD8+ T cell depletion completely abrogated protection induced by P. yoelii SHMT (PySHMT) that reduced LPB (4). Consistent with this, we observed here that CD8+ T cell depletion ablated protection in a rechallenge of animals that were protected by CSP plus SHMT immunization, 16 weeks after the last vaccine dose; 2 of 2 CD8+ T cell-depleted animals developed parasitemia on day 4, versus 0 of 2 animals that were sham depleted (see Fig. S3). Small sample sizes precluded statistical testing.
IFN-γ responses in spleens and livers of mice immunized with P. berghei LS antigens.
According to the majority of published studies, IFN-γ plays a significant role in eliminating LS malaria parasites (10). We tested the induction of IFN-γ responses in spleens and livers of B6 mice immunized with PbSHMT plus PbSLARP plus PbMIF4G (3Ag) as a combined vaccine and with a combination of PbCSP and 3Ag. We stimulated splenocytes and intrahepatic mononuclear cells (IHMC) in vitro with pools of H-2Kb-restricted peptides derived from 3Ag, and we measured IFN-γ production by enzyme-linked immunosorbent spot assay (ELISPOT) and intracellular cytokine staining (ICS). Both immunizations recalled specific IFN-γ+ CD8+ T cell responses among spleens and IHMC; however, the magnitudes of responses were significantly higher in the livers (Fig. 5A and B) than in the spleens (Fig. 5C and D). Neither spleen cells nor IHMC showed IFN-γ responses recalled with the PbPEVA peptides in EV-immunized mice.
FIG 5.
Higher IFN-γ responses in IHMC than in spleens of mice immunized with three PbPEVAs or three PbPEVAs plus CSP. B6 mice (5 mice per group) were immunized using the same immunization schedule as for Fig. 2A. Fourteen days after the last boost immunization, IHMC and spleen cells were analyzed for IFN-γ production with ELISPOT and ICS assays (see Materials and Methods). Data represent IFN-γ responses in livers (A and B) and spleens (C and D) in ELISPOT (A and C) and ICS (B and D) assays.
DISCUSSION
The most advanced malaria vaccine, called RTS,S or Mosquirix, targets the P. falciparum SPZ major surface protein CSP and entered implementation programs in 2019. RTS,S confers partial protection from clinical malaria but not sterile immunity in young children (2, 3); therefore, improvements are needed for CSP-based vaccination. Including other PEVAs (for example, those expressed during LS development) may provide a viable route to improvement, because those antigens would induce broader specificities of the responding B cells and T cells. We previously identified novel PEVAs that improve on the activity of CSP alone to reduce LPB (4), and here we sought to evaluate whether these PEVAs, in combination with CSP, improve on the rates of sterilizing immunity achieved with CSP alone.
In our previous report (4), we identified candidate vaccine antigens based on exclusive or preferential expression during LS parasite development, using microarray analysis of P. falciparum parasites during in vitro development in HC-04 cells (an immortalized cell line derived from human liver tissue that displays normal hepatocyte morphology and gene expression [11]). In murine DNA immunization studies, we demonstrated that orthologues for many of these genes conferred protection against P. yoelii in BALB/c mice and/or P. berghei in C57BL/6 mice, measured by qPCR as a reduction in LPB ∼40 h after SPZ challenge. Two antigens, LISP1 and SLARP, when combined with CSP provided significantly greater protection than CSP alone in both models. The results of the current study, using new immunization modalities, confirm these previous findings and demonstrate for the first time that antigen combinations can enhance the level of sterilizing immunity conferred by CSP-based vaccines.
In our current study, we measured both the PyCSP and PyPEVA antibody responses in mice after the Ad5 boost. Since antibody responses are important for a CSP-based vaccine, it is critical that PEVA proteins do not interfere with the CSP response and broaden specificity to target other antigens besides CSP. Our data demonstrate that, in mice coimmunized with PyCSP and PyPEVA, the CSP antibody response is high and remains unperturbed when combined with any PyPEVA tested. Anti-PyPEVA responses were induced by most antigens with varying titers. Three candidates, LISP1, TBP, and PY03000, induced the strongest antibody responses, with OD values higher than those observed with PyCSP; however, among these, only LISP1 and TBP enhanced protection when combined with CSP. PySHMT induced high levels of sterile protection but only moderate antibody titers. In contrast, in the PbPEVA B6 mouse model, we observed strong antibody responses in sera from mice immunized with PbSHMT, as determined by immunofluorescence assay (see Fig. S4 in the supplemental material). Sera from mice immunized with PbCSP also showed strong reactivity by immunofluorescence assay, but the pattern (or reaction) was distinct from that observed when combined with PbSHMT (see Fig. S4A and B). Although our previous work and our current work suggest that the mechanism of protection induced by both PySHMT and PbSHMT is conferred by CD8+ T cells, it is quite possible that antibody response plays an auxiliary role in protection.
PEVA combinations substantially increased total CD8+ T cell responses.
CD8+ T cells are generally considered the primary effectors against preerythrocytic-stage malaria (9). We previously demonstrated that delivery of PEVAs as DNA vaccines induced reduction in LPB that was in part mediated by CD8+ T cells (4). Apart from determining antibody responses induced by PyPEVAs as proteins in combination with Ad5-vectored P. yoelii antigens, we also determined the levels of antigen-induced CD8+ T cells by measuring the expression of CD11a; as shown in Fig. 4, the levels of CD8+ T cells expressing CD11ahi were quite variable, from 45% for LISP1 to 5% for MIF4G. Although we did not determine the expression of CD11a on CD8+ T cells in the P. berghei model, immunizations with combined PbPEVAs also activated CD8+ T cells responses. Therefore, it appears that, despite disparate immunization regimens, sterile protection and antibody and CD8+ T cell responses in the P. berghei DNA-Ad5 vaccine model were consistent with PEVA combination studies with P. yoelii protein-Ad5 vaccines.
Multiple mechanisms have been proposed for the effector function of CD8+ T cells in mediating protection against preerythrocytic-stage malaria infection. One of the primary mechanisms is the production of cytokines, and IFN-γ (10, 12) is considered one of the major CD8+ T cell-produced cytokines that mediates protection against LS parasites. It has been established that hepatocytes respond to the secretion of IFN-γ by producing nitric oxide (13), which decreases in mice with depleted CD8+ T cells and also in mice treated with neutralizing antibodies against IFN-γ (12). Furthermore, IFN-γ increases the efficiency of the innate immune response through the increase of the expression of Toll-like receptors on the surface of dendritic cells and macrophages, making them hyperresponsive to Plasmodium parasite antigens (14). According to the published observations, we have noted strong IFN-γ responses in both the P. yoelii and P. berghei models, even though we used different regimens of immunization. Our observations thus support the role of IFN-γ+ CD8+ T cells as having a key role in the protective response against preerythrocytic-stage malaria. Because we observed significantly higher levels of PbPEVA IFN-γ+ CD8+ T cells in livers than in spleens, this supports the notion that the targets of PEVA peptide-specific CD8+ T cells are most likely infected hepatocytes, which express parasite LS-derived antigenic peptides in association with MHC class I molecules (pMHCI) on their surface (15).
In this work, we identified three P. yoelii candidates that increase sterilizing immunity conferred by CSP vaccines in mice, i.e., LISP1, SHMT, and TBP. Because in the P. berghei system we immunized mice with a combination of three PbPEVAs plus PbCSP, it is not possible to assign a hierarchical order of protection inducers to any single antigen. However, based on our observations from a limited number of experiments with a single DNA PbPEVA plus PbCSP (see Table S6), we consider PbSHMT to be a significant contributor to protection. Another point that is clear from this study is that, while PbCSP administered three times as a DNA vaccine failed to induce sterile protection, it induced nearly 50% sterile protection when given as DNA with Ad5-vectored PbCSP (Fig. 2). The same conclusion regarding improved protective outcomes with the addition of Ad5-vectored antigens is based on more extensive results (see Table S3) from experiments in which immunization with 3× DNA CSP failed to induce protection in CD1 and F1 (progeny of BALB/c × C57BL/6 breeding pairs) mice, but addition of Ad5-vectored CSP enhanced protection. It has been amply demonstrated that booster immunization with a viral vector induces a strong cell‐mediated immune response (16). Among viral vectors, Ad5 expressing Plasmodium antigens such as thrombospondin-related adhesion protein (TRAP) has been successfully used to induce elevated levels of protection (17). Ad5 has the unusual property of chemotropism targeting the liver (18), and this single feature makes Ad5 an excellent platform for improved protective efficacy.
LISP-1 is specifically involved in parasite egress from hepatocytes and is expressed late during parasite development, localizing to the parasitophorous vacuole membrane (PVM). LISP1-deficient LS parasites do not rupture the PVM and remain trapped inside hepatocytes (19). LISP-1 is the first Plasmodium protein shown by gene targeting to be involved in lysis of the PVM (19). We previously demonstrated that immunization with LISP1 in combination with CSP significantly reduced LPB, compared with CSP alone, in both P. berghei and P. yoelii rodent challenge models (4).
SHMT is a pyridoxal-5-phosphate-dependent enzyme that catalyzes the conversion of l-serine and tetrahydrofolate to glycine and 5,10-methylenetetrahydrofolate (20). Expression of the Plasmodium SHMT gene is noticeably increased during late trophozoite to schizont stages, when high levels of folate and nucleotides are needed for cell multiplication processes, emphasizing the indispensable role of this enzyme (20, 21). We previously demonstrated that SHMT in combination with CSP conferred significantly greater protection (reduced LPB) than that seen with CSP alone, which may be mediated by the induction of CD8+ T cells required for protection (4).
TBP (PF0305w) is a transcriptional factor required by all three types of RNA polymerases in eukaryotic cells, and its highly conserved nature in Plasmodium makes it a viable candidate as a vaccine antigen (22). In our previous study, TBP was among the four antigens identified that improved on protection afforded by CSP alone; unlike the other three, however, TBP did not elicit IFN-γ responses from CD8+ T cells, and CD8+ T cell depletion did not cause any loss of protection in mice immunized with TBP (4).
Of note, P. falciparum orthologues of MIF4G and SLARP antigens were highlighted in our previous publication because each reduced LPB after SPZ challenge in two rodent models (P. yoelii in BALB/c mice and P. berghei in B6 mice). Although the combinations including SLARP and CSP antigens induced sterile protection in the P. berghei model, as single P. yoelii antigens in combination with CSP they failed to significantly increase sterile protection in P. yoelii studies. These observations offer a cautionary note regarding any recommendations for continued future development. Based on these studies, we propose that the CSP-based partial sterile protection model using a protein prime-Ad5 boost regimen could be used to define preerythrocytic vaccine improvements that completely block infection.
Other efforts to identify novel PEVA candidates have taken different approaches than ours. Some studies examined CD8+ T cell responses to immunogens expressed as viral vectors or plasmid DNA and/or their ability to independently confer protection against SPZ or blood-stage parasite challenge (23, 24). Ours is the first study to attempt to identify PEVAs that can enhance protection conferred by CSP vaccine in preclinical studies. In clinical studies, a CD8+ T cell epitope-based multiantigen vaccine, ME-TRAP, failed to enhance protection conferred by the RTS,S vaccine (25).
In summary, we recommend continued evaluation of LISP-1, SHMT, and TBP in human clinical trials to determine the safety and efficacy of these antigens in combination with CSP. These antigens may improve on the partial protection afforded by RTS,S, toward a more effective preerythrocytic vaccine that confers durable sterilizing immunity against malaria.
MATERIALS AND METHODS
Plasmid construction and expression of PyPEVA proteins.
All proteins were synthesized by GeneArt. For PY03000, MIF4G, LISP1, PY04162, and SHMT, synthetic gene sequences corresponding to the indicated amino acid regions (Table 1; also see Data Set S1 in the supplemental material) were cloned into insect cell expression vector pDEST8 (number 11804010; Thermo Fisher Scientific) using Gateway Cloning Technology (Thermo Fisher Scientific). All predicted N-linked glycosylation sites were removed by N-to-Q amino acid substitution, and a 6×His tag was included at the C terminus. Bacmid DNA was generated by transformation of the pDEST8-PyPEVA into the Escherichia coli strain DH10Bac (number 10361012; Themo Fisher Scientific). The high-molecular-weight Bacmid DNA was purified and transfected into Sf9 insect cells (number 10359016; Thermo Fisher Scientific) for baculovirus production. The baculovirus was quantified by the viral plaque assay (Thermo Fisher Scientific), amplified for one passage in Sf9 cells, and then used for protein production. High Five insect cells (number B85502; Thermo Fisher Scientific) were cultured to log-phase growth, and 200 ml of 1 × 106 cells/ml were infected with second-passage baculovirus. Infected cells were grown for 48 h at 27°C, and cells were pelleted by centrifugation and stored at −80°C until purification.
For E. coli expression of TBP and PY01495, the codon-optimized synthetic genes with a carboxy-terminal His tag (Gene Art) (Table 1; also see Data Set S1) were cloned at the NdeI and XhoI sites of pET-24a(+) (number 69749; Millipore), and proteins were expressed using T7 Express competent E. coli (number C2566I; New England Biolabs). To express SLARP in E. coli, a synthetic gene corresponding to Asn1355 to Gln1988 was cloned into the NdeI and XhoI sites of pET-24a(+) with a His tag at the C terminus.
Purification of PyPEVA proteins from E. coli and insect cells.
PySHMT, PY03000, and PyLISP1 proteins were purified from baculovirus-infected High Five insect cells (number B85502; Thermo Fisher Scientific). Two hundred milliliters of insect cell culture at 11 × 106 cells/ml was infected with recombinant baculovirus and harvested 48 h postinfection. Infected cells were then lysed by freeze-thaw cycles in the presence of 40 ml buffer containing 1% NP-40, 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole [pH 8.0], cOmplete mini EDTA-free tablet protease inhibitor cocktail (number 05892953001; Roche), and benzonase nuclease (number 70664; Millipore). The insoluble fraction in the lysate was pelleted at 12,000 rpm for 10 min. To further enrich the insoluble fraction, the pellet was washed three times by resuspension for 30 min in buffer containing 2% Triton X-100, 0.5 M NaCl, and 50 mM Tris [pH 7.5], with stirring. The enriched pellet fraction was solubilized overnight by stirring in the presence of 6 M guanidinium chloride, 100 mM NaH2PO4, 10 mM Tris, 10 mM imidazole, and 2-mercaptoethanol [pH 8.0]. The solubilized protein was then purified using Ni-nitrilotriacetic acid (NTA) resin (number 30210; Qiagen) under denaturing conditions. The purified protein was refolded by dilution and stirring in QuickFold protein refolding buffer 4 (number 0612-4; Athena Enzyme Systems) (50 mM morpholineethanesulfonic acid [MES] [pH 6.0], 240 mM NaCl, 10 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 0.5 M arginine, 0.5% Triton X-100, 1 mM reduced glutathione [GSH], 0.1 mM oxidized glutathione disulfide [GSSG]), and the refolded protein was concentrated 20-fold. To facilitate formulation of the protein with complete and incomplete Freund's adjuvant, the detergent in the sample was removed by consecutive passes through two 4-ml Pierce detergent removal spin columns (number 87779; Thermo Fisher Scientific) equilibrated with QuickFold protein refolding buffer 4 without Triton X-100, GSH, and GSSG.
PY04162 and PyMIF4G were soluble in the cytoplasm of High Five baculovirus-infected cells. Cells were lysed in 50 mM phosphate buffer, 300 mM NaCl, 10 mM imidazole [pH 7.4]. Cell debris was pelleted at 12,000 × g for 12 min. The clarified lysate was mixed with 1 ml of HisPur cobalt resin (number 89964; Thermo Fisher Scientific). The resin was washed twice with 50 ml of 50 mM phosphate buffer, 300 mM NaCl, 20 mM imidazole (pH 7.4), and eluted with 50 mM phosphate buffer, 300 mM NaCl, 200 mM imidazole (pH 7.4).
P. yoelii SLARP (PySLARP), P. yoelii TBP (PyTBP), and PY01495 were expressed as insoluble proteins in E. coli. Overnight cultures selected with 30 μg/ml kanamycin were diluted into LB supplemented with 5% glucose and 30 μg/ml kanamycin and were grown to an OD600 of 1. Protein synthesis was induced by addition of isopropyl-β-d-thiogalactoside (IPTG) (number I6758; Sigma-Aldrich). After 3 h of induction, cells were harvested by centrifugation at 10,000 × g for 30 min. The pellets were stored at −80°C until purification. The frozen pellets were thawed and resuspended overnight in 4 M guanidinium chloride to lyse the cells and to solubilize inclusion bodies. The resuspended fraction was centrifuged at 10,000 × g for 45 min to pellet cell debris, and the resolubilized inclusion bodies were loaded onto three 5-ml NTA columns (number 17524802; Cytiva). The NTA columns were washed, and then purified proteins were eluted and stored at −80°C until refolding. All buffers contained guanidinium chloride to keep the inclusion bodies denatured. Proteins were refolded by loading onto a 16/60 S300 size exclusion column (number 17116701; Cytiva) equilibrated with QuickFold protein refolding buffer 4 (0612-4; Athena Enzyme Systems). Pooled fractions were concentrated and loaded onto a 16/60 S75 size exclusion column equilibrated with 1× phosphate-buffered saline (PBS), 0.44 M sucrose, 0.55 M l-arginine [pH 7.4], for further purification and buffer exchange. Proteins were aliquoted and stored at −80°C.
PyCSP was produced as described in published methods (26). Briefly, amino acids 20 to 369 of the PyCSP (PYYM_0405600) with a C-terminal 6×His tag were expressed as a secreted protein in Saccharomyces cerevisiae and purified by NTA chromatography.
All purified PEVA proteins along with purified insect cell-expressed eGFP (1 μg of each protein) were run on a Coomassie-stained gel under reducing conditions (10% 2-mercaptoethanol in 4× gel loading dye) using an Invitrogen NuPage 4% to 12%, Bis-Tris 12-well mini-protein gel (Thermo Fisher Scientific). The identity of all PyPEVA proteins was confirmed by mass spectrometry (see Data Set S2).
Mass spectrometry and protein identification.
Identification of all gel-separated proteins was performed on reduced and alkylated, trypsin-digested samples prepared by standard mass spectrometry protocols. The supernatant and two washes (5% formic acid in 50% acetonitrile) of the gel digests were pooled and concentrated with a SpeedVac concentrator (Labconco, Kansas, MO) to dryness directly in 200 μl polypropylene autosampler vials (Sun Sri, Rockwood, TN). The recovered peptides were resuspended in 5 μl of solvent A (0.1% formic acid, 2% acetonitrile, and 97.9% water). Prior to mass spectrometry analysis, peptides were separated using AQ C18 reverse-phase medium packed in a pulled-tip, nano-chromatography column (Precision Capillary Columns, San Clemente, CA). The chromatography was performed using a Proxeon Easy-nLC II multidimensional liquid chromatograph and temperature-controlled Ion Max nanospray source in-line with an LTQ-Velos Orbitrap mass spectrometer (Thermo Fisher Scientific). Computer-controlled data-dependent automated switching to tandem mass spectrometry by Xcalibur v2.1 software was used for data acquisition and provided the peptide sequence information. Data processing and database searches were performed using the SEQUEST HT search module within Proteome Discoverer v1.4 (Thermo Fisher Scientific) against a targeted database containing the proteins of interest and common contaminant proteins (cRAP.fasta; The Global Proteome Machine). Oxidation (M) and deamidation (N/Q) were used as dynamic modifications and carbamidomethylation (C) was used as a fixed modification. Precursor and fragment mass tolerances were set to 10 ppm and 0.6 Da, respectively. Proteins were identified using a false discovery rate of 1%, calculated using a decoy database approach, and a minimum of 2 peptides per protein (see Data Set S2 for protein sequences and peptide coverage).
P. berghei adenoviral and plasmid DNA constructs.
Ad5 vectors with human cytomegalovirus (HCMV) promoters and pCI plasmid vectors expressing PbPEVA were purchased from GenScript (Piscataway, NJ, USA). Lipopolysaccharide (LPS) contamination in E. coli and plasmid DNA antigens was tested by GenScript and found to be well within the acceptable range for immunization.
Ethics statement.
Animal studies were performed under the guidelines of protocol LMIV 1E ASP approved by the Institutional Animal Care and Use Committee (IACUC) of the National Institute of Allergy and Infectious Diseases, NIH. The studies that were performed at the Walter Reed Army Institute of Research (WRAIR) animal facility were reviewed and approved by the WRAIR/Naval Medical Research Center (NMRC) IACUC. Research was conducted in an AAALAC International-accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals (27).
SPZ challenges.
P. yoelii 17XNL and P. berghei ANKA (28) were maintained by cyclical transmission in mice and Anopheles stephensi mosquitoes. Twenty-five to 50 P. yoelii 17XNL- or P. berghei ANKA-infected A. stephensi mosquitoes (14 to 21 days postinfection for P. yoelii and 18 to 30 days postinfection for P. berghei) were dissected using a 29-gauge insulin syringe and forceps. The dissected glands were placed in a LoBind 1.5-ml Eppendorf tube containing 0.1 ml of dissection medium (medium E-199 without l-glutamine [Quality Biological, Inc., Gaithersburg, MD] containing 0.2% bovine serum albumin [BSA]). The dissected glands were triturated 20 times using a 1-ml syringe with a 26-gauge needle to release the SPZ, and then 0.9 ml of dissecting medium was added to the released SPZ to achieve a 1.0-ml volume. The SPZ were counted using a disposable hemocytometer, and the SPZ yield per milliliter was calculated. A challenge dose of 300 SPZ (for P. yoelii in BALB/c mice and P. berghei in CD1 mice) or 10,000 SPZ (for P. berghei in C57BL/6 mice) in a 0.2-ml volume per animal was administered IV via the tail vein to immunized and naive mice.
Mouse immunizations and challenges.
Female BALB/c mice (6 to 7 weeks of age) and CD1 female mice (6 to 8 weeks of age) were purchased from Charles River Laboratories (Wilmington, MA, USA). Female C57BL/6J (B6) mice (6 to 8 weeks of age) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained under pathogen-free conditions in animal facilities and were fed autoclaved food ad libitum.
For protection studies, 19 BALB/c mice/group were immunized subcutaneously twice with 10 μg of PyPEVA protein and 10 μg of PyCSP, formulated with CFA/IFA, 21 days apart. On day 42, mice were immunized IM with 1 × 1010 VP Ad5 PyPEVA and 1 × 1010 VP Ad5 PyCSP (OD260 Inc.). The GFP plus CSP control group was immunized as above with the respective proteins and boosted with 1 × 1010 VP Ad5 EV and 1 × 1010 VP Ad5 PyCSP. The GFP-alone group received 20 μg GFP and then 2 × 1010 VP Ad5 EV for the protein prime and Ad5 boost immunizations, respectively. The adjuvant control group received only formulations of CFA/IFA or PBS for the protein and Ad5 immunizations. Three weeks after the last vaccine dose (challenge 1), 14 mice/group were challenged with 300 P. yoelii SPZ by IV injection. Mice were monitored for parasitemia via thin blood smears from day 4 to day 7 and on day 14 postchallenge. The slides were stained with 10% Giemsa stain and checked for the presence of blood-stage parasites. The protected animals (challenged animals that did not develop blood-stage parasitemia) were rechallenged 16 weeks after last vaccine dose with 300 P. yoelii 17XNL SPZ to assess the duration of protection (challenge 2).
For protection studies in the PbPEVA model, CD1 outbred mice (14 mice/group) and C57BL/6 mice (B6) (5 mice/group) were immunized at 3-week intervals twice with DNA (5 μg) by GG and boosted with 4 × 1010 VP of Ad5 vector by IM injection. Control groups received DNA EV and Ad5 EV according to the same immunization regimen. For sterile protection, CD1 mice were challenged with 300 P. berghei SPZ by IV injection. Mice were monitored for parasitemia via blood smears for 14 days postchallenge. For determination of LPB (4), B6 mice were challenged with 10,000 P. berghei SPZ by IV injection; livers were harvested at 40 h postchallenge for determination of P. berghei 18S rRNA. The EV-immunized control group was immunized as above with respective DNA EV and Ad5 EV and challenged with the same dose of P. berghei SPZ. Naive mice that received 10,000 P. berghei SPZ served as infectivity controls.
Antibody responses to PyPEVA and PyCSP by ELISA.
Antigen-specific antibody titers against PyPEVA or PyCSP were analyzed by ELISA using recombinant proteins as coating antigens. Ninety-six-well ELISA plates (Immunlon 4HBX, number 3855; Thermo Fisher Scientific) were coated with 100 μl/well of PyPEVA or PyCSP at 1 μg/ml overnight. Wells were washed and blocked with 5% milk for 2 h at room temperature. After washing, serum samples at different dilutions were added and incubated for 2 h at room temperature. Wells were washed, alkaline phosphatase-labeled goat anti-mouse IgG secondary antibody (KPL) was added, and plates were incubated for 2 h. After incubation and washing, 100 μl of phosphatase substrate solution (Sigma-Aldrich) was added to each well and plates were incubated for 20 min at room temperature. Absorbance readings at 405 nm were captured with SoftMax Pro v7.
Antibody responses to PbSHMT and PbCSP by immunofluorescence assay.
Hand-dissected P. berghei SPZ were resuspended at 500,000 SPZ/ml in PBS containing 1% BSA (number SH30073.03; HyClone), and 10 to 20 μl of SPZ suspension was added to each well of a 12-well immunofluorescence assay slide. After centrifugation, the medium was removed, and sera from B6 mice immunized with CSP plus EV, CSP plus SHMT, or SHMT plus EV as DNA prime-Ad5 boost regimens were added to each well at serial dilutions from 1:40 to 1:1,280. After 1 h of incubation at 37°C, the slides were washed three times and the secondary antibody, goat anti-mouse IgG-Alexa Fluor (number A11029; Invitrogen), at 1:100 dilution was added to each well for 30 min at 37°C. After several washes with PBS, the slides were mounted with a coverglass with 4 or 5 drops of VectaShield mounting medium (number H-2000; Vector Laboratories). The slides were stored at room temperature in the dark until they were imaged on a fluorescence microscope equipped with a digital camera (Olympus, Tokyo, Japan).
CD8+ T cell responses. (i) P. yoelii studies.
Fourteen days after the final PyPEVA immunizations, spleens were harvested from 5 BALB/c mice per group posteuthanasia. After the lysis of red blood cells, single-cell suspensions were used for in vitro stimulation with pools of peptides corresponding to the antigens used for immunization (see Table S1). Peptide pools were generated based on MHC Class 1 epitopes predicted by the artificial neural network (ANN) method (www.iedb.org).
After 1 h of peptide stimulation, brefeldin A (2 μg/ml) was added to cell cultures and incubation continued for another 16 h. Cells were then initially stained for surface markers, including CD3ε, CD4, CD8α, and CD11a. Subsequently, cells were fixed, permeabilized, and stained for intracellular markers, including IL-2 and IFN-γ. Flow cytometric acquisition of cells was performed using an LSRII system (BD Biosciences, San Jose, CA), and data were analyzed by FlowJo v10.0.07 software (Tree Star Inc., Ashland, OR).
(ii) P. berghei studies.
Fourteen days after the last PbPEVA administration, livers from B6 mice were perfused and IHMC were prepared as described previously (29). IHMC were resuspended in complete RPMI 1640 medium (number SH30027.01; HyClone) containing 10% heat-inactivated FBS (number SH30070.03IH25-40; HyClone), 1% penicillin-streptomycin, 1% GlutaMAX, 1% HEPES, 1% nonessential amino acids (Gibco), and 50 μM 2-mercaptoethanol (Sigma).
Spleens were harvested from B6 mice posteuthanasia at 14 days after the last boost immunization, and single-cell suspensions were prepared from red blood cell-lysed splenocytes. Cell pellets were resuspended in 5 ml of complete RPMI 1640 medium and diluted to a final concentration of 106 cells/100 μl.
IFN-γ ELISPOT.
BD ELISPOT plates were prepared using the mouse IFN-γ ELISPOT kit (number 551083; BD Biosciences) according to the manufacturer’s instructions. Briefly, ELISPOT plates were coated with anti-IFN-γ antibodies overnight at 4°C, subsequently washed with PBS, blocked using RPMI 1640 medium with 10% FBS for 2 h at room temperature, and washed prior to use. Isolated spleen cells and IHMC were resuspended in complete RPMI 1640 medium, transferred to ELISPOT plates at a concentration of 200 × 103 to 300 × 103 cells/well in 200 μl, and stimulated for 42 h at 37°C with either 1 μg/ml of P. berghei peptide pools corresponding to the immunizing PbPEVAs (see Table S2) or medium alone. Peptide pools were generated based on MHC class I epitopes predicted by the ANN method (www.iedb.org). Peptides were purchased from Peptide 2.0 Inc. (Chantilly, VA, USA). Results were quantified as the number of IFN-γ-specific spots per 106 cells after subtraction of results from medium control wells.
ICS assay.
For ICS, spleen cells and IHMC were harvested and cell cultures were stimulated for 14 h at 37°C with 1 μg/ml of peptide pools (as described above); GolgiPlug (number 555029; BD Biosciences) was added for the last 12 h of incubation. Cells were then stained for surface markers with the following anti-mouse antigen antibodies from BD Biosciences: phycoerythrin-conjugated anti-CD3e (clone 145-2C11), V500-conjugated anti-CD4 (clone GK1.5), and BUV397-conjugated anti-CD8α (clone 53-6.7). The Zombie NIR fixable viability kit (number 423106; BioLegend) was used to exclude dead cells. Cells were fixed and permeabilized with BD fixation/permeabilization buffer (number 554714; BD Biosciences), stained in permeabilization/wash buffer (number 554723; BD Biosciences) with allophycocyanin-conjugated anti-IFN-γ antibody (clone XMG1.2) according to the manufacturer’s instructions, washed with PBS, and fixed with 4% formaldehyde in PBS. The gating strategy is shown in Fig. S1 in the supplemental material. Flow cytometry was performed using an LSRII system (BD Biosciences), and data were analyzed with FlowJo v9.9.3 software (Tree Star Inc.).
CD8+ T cell depletion prior to third SPZ challenge.
To assess the involvement of CD8+ T cells in protection, four animals immunized with SHMT plus CSP and shown to be protected in two SPZ challenges were allocated to two groups for a third challenge 22 weeks after the last vaccine dose. One day before the third challenge, the test group received 100 μg of CD8β monoclonal antibody to deplete their CD8+ T cells; the control group received 100 μg of nonimmune purified hamster control IgG2κ (sham isotype) antibody. CD8+ T cell-depleted, CD8+ T cell sham-depleted, and naive mice were challenged with 300 SPZ IV 1 day after antibody depletions. The protective efficacy of the challenged animals was determined as described above.
Evaluation of vaccine efficacy by qPCR in the P. berghei model.
Total RNA was extracted from liver samples using TRIzol (number 15596026; Thermo Fisher Scientific) according to the manufacturer’s instructions. cDNA was synthesized using the high-capacity cDNA reverse transcription kit (number 4368814; Thermo Fisher Scientific). Reaction samples contained the following reagents in a 25-μl volume: 12.5 μl of SYBR green PCR master mix (Applied Biosystems), 0.1 μM either P. berghei 18S rRNA primers (5′-AAGCATTAAATAAAGCGAATACATCCTTAC-3′ and 5′-GGAGATTGGTTTTGACGTTTATGTG-3′) or mouse β-actin gene primers (5′-GGCTGTATTCCCCTCCAT-3′ and 5′-CCAGTTGGTAACAATGCAAT-3′), and 2 μl of a 1:10 dilution of the cDNA sample. The reaction was run on a 7500 Fast qPCR system (Applied Biosystems) using the following conditions: 15 min at 95°C and 40 cycles of 95°C for 20 s, 60°C for 30 s, and 72°C for 50 s. cDNA standards were prepared as 10-fold serial dilutions from 108 to 105 copies of purified PCR product for both 18S rRNA and β-actin; 18S rRNA was used as an internal control, and each reaction was set up in triplicate. Naive mouse liver served as a negative control. The parasite load was calculated as the ratio of 18S rRNA gene expression to host β-actin expression. Protection was defined as a statistically significant reduction in LPB in experimental mice, compared to mice immunized with EV, and was computed as described below.
Statistical analysis.
A Kaplan-Meier test with Bonferroni correction for multiple comparisons was used to assess differences in protective efficacy between naive controls and all PEVA combination candidates, as described previously (30). For experimental data presented here, the Shapiro-Wilk normality test was performed prior to statistical analysis, and no significant departures from normality were detected. Significant differences between data points were compared by ordinary one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test, two-way ANOVA followed by Sidak’s multiple-comparison test, or the Kruskal-Wallis test followed by Dunn’s multiple-comparison test. P values of <0.05 were considered significant. Statistical data analysis was performed using GraphPad Prism v6.07 for Windows (GraphPad Software, San Diego, CA, USA).
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health PATH Malaria Vaccine Initiative, the Military Infectious Diseases Research Program (MIDRP), and U.S. Army Materiel Command.
The material has been reviewed by WRAIR, and there is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
Footnotes
Supplemental material is available online only.
Contributor Information
Patrick E. Duffy, Email: patrick.duffy@nih.gov.
De'Broski R. Herbert, University of Pennsylvania
REFERENCES
- 1.World Health Organization. 2020. World malaria report 2020. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.RTS,S Clinical Trials Partnership. 2014. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med 11:e1001685. 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RTS,S Clinical Trials Partnership. 2015. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386:31–45. 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speake C, Pichugin A, Sahu T, Malkov V, Morrison R, Pei Y, Juompan L, Milman N, Zarling S, Anderson C, Wong-Madden S, Wendler J, Ishizuka A, MacMillen ZW, Garcia V, Kappe SHI, Krzych U, Duffy PE. 2016. Identification of novel pre-erythrocytic malaria antigen candidates for combination vaccines with circumsporozoite protein. PLoS One 11:e0159449. 10.1371/journal.pone.0159449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pichugin A, Zarling S, Perazzo L, Duffy PE, Ploegh HL, Krzych U. 2018. Identification of a novel CD8 T cell epitope derived from Plasmodium berghei protective liver-stage antigen. Front Immunol 9:91. 10.3389/fimmu.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirai A, Matsuyama A, Yashiroda Y, Hashimoto A, Kawamura Y, Arai R, Komatsu Y, Horinouchi S, Yoshida M. 2008. Global analysis of gel mobility of proteins and its use in target identification. J Biol Chem 283:10745–10752. 10.1074/jbc.M709211200. [DOI] [PubMed] [Google Scholar]
- 7.Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM. 2009. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci USA 106:1760–1765. 10.1073/pnas.0813167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krzych U, Zarling S, Pichugin AV. 2018. Pre-erythrocytic Immunity in Malaria, p 1–11. In Kremsner PG, Krishna S (ed), Encyclopedia of malaria. Springer, New York, NY. [Google Scholar]
- 9.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA 85:573–576. 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira A, Schofield L, Enea V, Schellekens H, van der Meide P, Collins WE, Nussenzweig RS, Nussenzweig V. 1986. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science 232:881–884. 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- 11.Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, Rasameesoraj M, Jenwithisuk R, Coleman RE, Udomsangpetch R, Cui L, Brewer TG. 2006. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg 74:708–715. 10.4269/ajtmh.2006.74.708. [DOI] [PubMed] [Google Scholar]
- 12.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330:664–666. 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 13.Seguin MC, Klotz FW, Schneider I, Weir JP, Goodbary M, Slayter M, Raney JJ, Aniagolu JU, Green SJ. 1994. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med 180:353–358. 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin BS, Parroche P, Ataíde MA, Lauw F, Ropert C, de Oliveira RB, Pereira D, Tada MS, Nogueira P, da Silva LHP, Bjorkbacka H, Golenbock DT, Gazzinelli RT. 2009. Malaria primes the innate immune response due to interferon-gamma induced enhancement of Toll-like receptor expression and function. Proc Natl Acad Sci USA 106:5789–5794. 10.1073/pnas.0809742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira-Ferreira J, Daniel-Ribeiro C. 2001. Protective CD8+ T cell responses against the pre-erythrocytic stages of malaria parasites: an overview. Mem Inst Oswaldo Cruz 96:221–227. 10.1590/s0074-02762001000200014. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues M, Li S, Murata K, Rodriguez D, Rodriguez JR, Bacik I, Bennink JR, Yewdell JW, Garcia-Sastre A, Nussenzweig RS. 1994. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes: comparison of their immunogenicity and capacity to induce protective immunity. J Immunol 153:4636–4648. [PubMed] [Google Scholar]
- 17.Hill AVS, Reyes-Sandoval A, O'Hara G, Ewer K, Lawrie A, Goodman A, Nicosia A, Folgori A, Colloca S, Cortese R, Gilbert SC, Draper SJ. 2010. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin 6:78–83. 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- 18.Arnberg N. 2009. Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol 19:165–178. 10.1002/rmv.612. [DOI] [PubMed] [Google Scholar]
- 19.Ishino T, Boisson B, Orito Y, Lacroix C, Bischoff E, Loussert C, Janse C, Menard R, Yuda M, Baldacci P. 2009. LISP1 is important for the egress of Plasmodium berghei parasites from liver cells. Cell Microbiol 11:1329–1339. 10.1111/j.1462-5822.2009.01333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sopitthummakhun K, Thongpanchang C, Vilaivan T, Yuthavong Y, Chaiyen P, Leartsakulpanich U. 2012. Plasmodium serine hydroxymethyltransferase as a potential anti-malarial target: inhibition studies using improved methods for enzyme production and assay. Malar J 11:194. 10.1186/1475-2875-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nirmalan N, Wang P, Sims PF, Hyde JE. 2002. Transcriptional analysis of genes encoding enzymes of the folate pathway in the human malaria parasite Plasmodium falciparum. Mol Microbiol 46:179–190. 10.1046/j.1365-2958.2002.03148.x. [DOI] [PubMed] [Google Scholar]
- 22.Ruvalcaba-Salazar OK, Romero-Ramirez H, Santos-Argumedo L, Vargas M, Hernandez-Rivas R. 2006. Preparation and characterization of a monoclonal antibody specific to Plasmodium falciparum TATA binding protein. Hybridoma (Larchmt) 25:367–371. 10.1089/hyb.2006.25.367. [DOI] [PubMed] [Google Scholar]
- 23.Limbach K, Aguiar J, Gowda K, Patterson N, Abot E, Sedegah M, Sacci J, Richie T. 2011. Identification of two new protective pre-erythrocytic malaria vaccine antigen candidates. Malar J 10:65. 10.1186/1475-2875-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doolan DL, Hoffman SL. 2001. DNA-based vaccines against malaria: status and promise of the Multi-Stage Malaria DNA Vaccine Operation. Int J Parasitol 31:753–762. 10.1016/s0020-7519(01)00184-9. [DOI] [PubMed] [Google Scholar]
- 25.Rampling T, Ewer KJ, Bowyer G, Edwards NJ, Wright D, et al. 2018. Safety and efficacy of novel malaria vaccine regimens of RTS,S/AS01B alone, or with concomitant ChAd63-MVA-vectored vaccines expressing ME-TRAP. NPJ Vaccines 3:49. 10.1038/s41541-018-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawla B, Mahajan B, Oakley M, Majam VF, Belmonte A, Sedegah M, Shimp RL, Jr, Kaslow DC, Kumar S. 2019. Antibody-dependent, gamma interferon-independent sterilizing immunity induced by a subunit malaria vaccine. Infect Immun 87:e00236‐19. 10.1128/IAI.00236-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Research Council, Washington, DC. [Google Scholar]
- 28.Lanar DE. 1990. Sequence of the circumsporozoite gene of Plasmodium berghei ANKA clone and NK65 strain. Mol Biochem Parasitol 39:151–153. 10.1016/0166-6851(90)90018-h. [DOI] [PubMed] [Google Scholar]
- 29.Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Williams J, Krzych U. 2003. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J Immunol 171:2024–2034. 10.4049/jimmunol.171.4.2024. [DOI] [PubMed] [Google Scholar]
- 30.Tripathi A, Pandey A. 2017. Post-hoc comparison in survival analysis: an easy approach. J Biosci Med 5:112–119. 10.4236/jbm.2017.53012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00165-21-s0001.pdf, PDF file, 0.8 MB (865.8KB, pdf)