ABSTRACT
During chronic infection with Helicobacter pylori, Schlafen 4-expressing myeloid-derived suppressor cells (SLFN4+ MDSCs) create a microenvironment favoring intestinal metaplasia and neoplastic transformation. SLFN4 can be induced by alpha interferon (IFN-α), which is mainly secreted from plasmacytoid dendritic cells (pDCs). This study tested the hypothesis that Helicobacter pylori infection promotes SLFN4+ MDSC differentiation by inducing pDCs to secrete IFN-α. C57BL/6 mice were gavaged with H. pylori, and infection lasted 2, 4, or 6 months. Mouse pDCs were isolated from bone marrow of wild-type C57BL/6J mice. The results showed that H. pylori infection increased the number of SLFN4+ MDSCs by inducing IFN-α expression in mice. Further mechanistic experiments unraveled that IFN-α induced SLFN4 transcription by binding to the Slfn4 promoter. Furthermore, H. pylori infection stimulated pDCs to secrete IFN-α by activating the TLR9-MyD88-IRF7 pathway. Collectively, Helicobacter pylori infection promotes SLFN4+ MDSC differentiation by inducing secretion of IFN-α from pDCs.
KEYWORDS: IFN-α, Helicobacter pylori, SLFN4+ MDSC, plasmacytoid dendritic cells
INTRODUCTION
Gastric cancer is one of the most common malignant tumors in the world (1). Chronic Helicobacter pylori infection is the main risk factor of gastric cancer. From chronic superficial gastritis to chronic atrophic gastritis caused by chronic H. pylori infection, once intestinal metaplasia and dysplasia occur, these precancerous lesions may eventually develop into gastric cancer (2). Therefore, it is of great clinical significance to block the occurrence and development of gastric precancerous lesions. However, the exact molecular mechanism of how chronic gastritis progresses to intestinal epithelial dysplasia after H. pylori infection remains unclear.
H. pylori infection induces chronic superficial gastritis, which eventually leads to chronic atrophic gastritis, precancerous lesions (e.g., intestinal metaplasia and dysplasia), and then gastric cancer in some infected patients (2–4). The myeloid cell differentiation factor Schlafen 4 (SLFN4) marks a subset of CD11b+ Gr-1+ myeloid-derived suppressor cells (MDSCs) in the stomach during H. pylori-induced spasmolytic polypeptide-expressing metaplasia (SPEM), which is an early committed step appearing before gastric cancer (5). During chronic infection with H. pylori, SLFN4+ MDSCs induce the expression or secretion of some cytokines, such as inducible nitric oxide synthase (iNOS), Arg-1, and miR-130b. Furthermore, SLFN4+ MDSCs can suppress T cell function and create a microenvironment favoring intestinal metaplasia and neoplastic transformation (5, 6). Therefore, understanding the molecular mechanism of SLFN4+ MDSC differentiation is important to prevent intestinal metaplasia.
SLFN4 belongs to the Schlafens (SLFNs) family, a family of proteins strongly induced by type 1 interferons, i.e., IFN-α, which has been implicated in lymphoid and myeloid cell differentiation (7). Plasmacytoid dendritic cells (pDCs) are the major IFN-α-producing subset of hematopoietic cells and play an important role in connecting innate and adaptive immunity (8). Toll-like receptor 9 (TLR9) is an important pattern recognition receptor in pDCs (9). Through the activation of TLR9, pDCs secrete cytokines such as IFN-α, interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) and activate natural killer cells, B cells, and T cells, thus exerting a stronger immune response (10). Then, whether H. pylori infection regulates SLFN4+ MDSC differentiation by inducing secretion of IFN-α from pDCs is not known.
Thus, in the present study, we tested the hypothesis that H. pylori infection promotes SLFN4+ MDSC differentiation by inducing secretion of IFN-α from pDCs. Furthermore, we explored the underlying mechanisms.
RESULTS
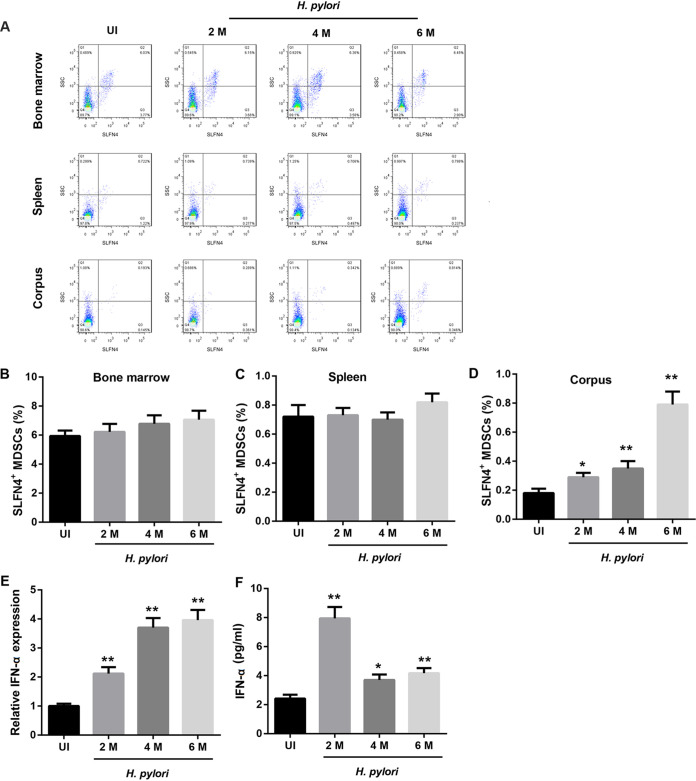
Infection with H. pylori increased SLFN4+ MDSC percentage and IFN-α expression in mice.
Infection with H. pylori had no obvious effect on the percentage of SLFN4+ MDSCs in mouse bone marrow and spleen (Fig. 1A to C). However, compared to that in the uninfected mice, the percentage of SLFN4+ MDSCs in the gastric corpus from infected mice was significantly increased, which was dependent on the duration of H. pylori infection (Fig. 1A and D). Furthermore, infection with H. pylori notably upregulated the mRNA level of IFN-α in the mouse gastric corpus, and this regulation was dependent on the duration of H. pylori infection (Fig. 1E). Moreover, compared to that in uninfected mice, the serum level of IFN-α was significantly increased in infected mice, especially those with H. pylori infection of >2 months (Fig. 1F).
FIG 1.
Infection with H. pylori increased SLFN4+ cell percentage and IFN-α expression in mice. Representative by flow cytometry plots (A) showing the percentage of SLFN4+ MDSC cells in bone marrow (B), spleen (C), and corpus (D) samples. IFN-α mRNA levels in the gastric corpus determined by qRT-PCR analysis (E) and IFN-α levels in mouse sera determined by ELISA (F) from uninfected (UI) mice and mice infected with H. pylori for 2, 4, or 6 months. *, P < 0.05, **, P < 0.01 versus UI. N = 5 mice/per group.
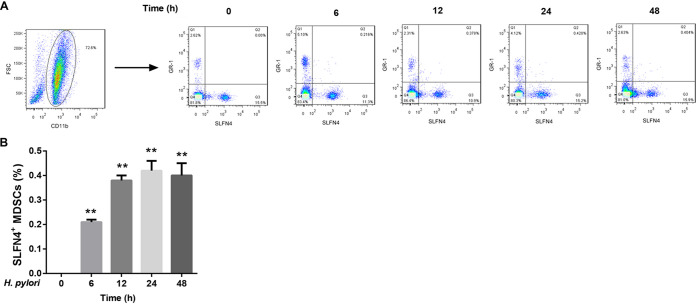
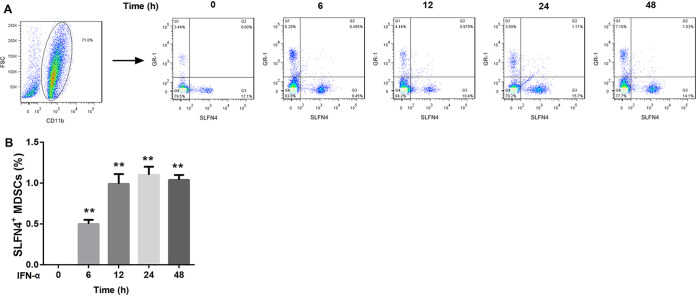
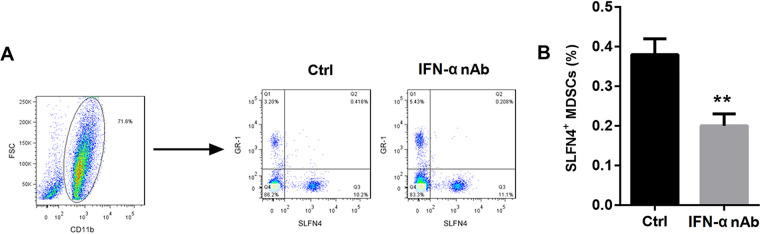
H. pylori infection increased the number of SLFN4+ MDSCs by inducing IFN-α expression.
To further clarify the role of IFN-α in regulating the number of SLFN4+ MDSCs in the presence of H. pylori infection, we isolated bone marrow cells from wild-type mice and infected these cells with H. pylori or treated them with IFN-α for different durations. CD11b+ Gr-1+ cells were now known as MDSCs. After infection with H. pylori, the percentage of SLFN4+ MDSCs among bone marrow cells presented a gradual upward trend with the infection time and peaked at 24 h, followed by a decline at 48 h (Fig. 2). Similarly, IFN-α exposure remarkably increased the percentage of SLFN4+ MDSCs among bone marrow cells (Fig. 3). Importantly, pretreatment with IFN-α-neutralizing antibody attenuated the H. pylori infection-mediated upregulation of SLFN4+ MDSCs (Fig. 4). These results indicated that H. pylori infection increased the number of SLFN4+ MDSCs by inducing IFN-α expression.
FIG 2.
Effect of H. pylori infection on the percentage of SLFN4+ MDSCs. Representative flow cytometry plots (A) and the percentages of SLFN4+ MDSCs determined by flow cytometry (B) in wild-type mouse bone marrow cells infected with 100 CFU of H. pylori for different durations (0, 6, 12, 24, and 48 h). **, P < 0.01 versus 0 h. The experiments were repeated independently three times.
FIG 3.
Effect of IFN-α exposure on the percentage of SLFN4+ MDSCs. Representative flow cytometry plots (A) and the percentages of SLFN4+ MDSCs determined by flow cytometry (B) in wild-type mouse bone marrow cells exposed to 800 U/ml of IFN-α for different durations (0, 6, 12, 24, and 48 h). **, P < 0.01 versus 0 h. The experiments were repeated independently three times.
FIG 4.
Effect of IFN-α neutralization on the percentage of SLFN4+ MDSCs. Wild-type mouse bone marrow cells were pretreated with IFN-α-neutralizing antibody (nAb) for 24 h and then infected with 100 CFU of H. pylori for 12 h. Cells only infected with H. pylori and treated with PBS served as controls. Then, the percentage of SLFN4+ MDSCs (CD11b+ GR-1+) was determined by flow cytometry (A), shown in a representative histogram (B). **, P < 0.01 versus control (Ctrl). The experiments were repeated independently three times.
IFN-α induced Slfn4 transcription by binding to ISREs in the Slfn4 promoter.
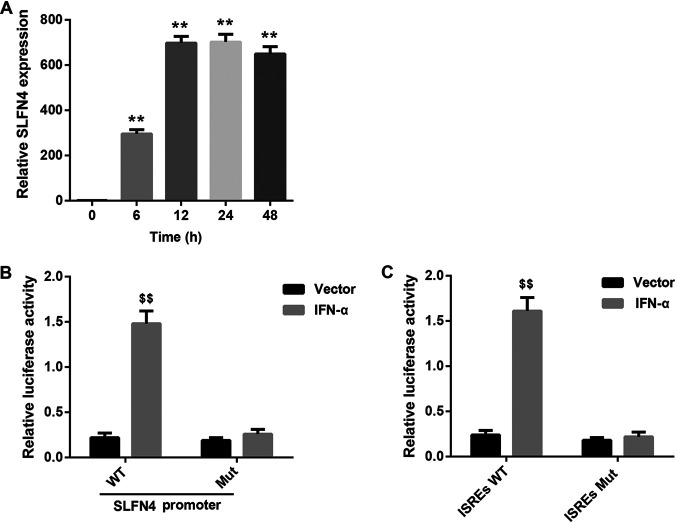
After IFN-α exposure (Fig. 5A) in bone marrow cells, the mRNA level of SLFN4 increased gradually, peaked at 24 h, and finally slightly decreased at 48 h (Fig. 5A). Then, we elucidated whether IFN-α induces the transcription and expression of SLFN4 by binding to interferon-sensitive response elements (ISREs) in the promoter region of the Slfn4 gene. A luciferase activity assay showed that IFN-α overexpression remarkably increased the luciferase activity of the SLFN4 wild-type (WT) promoter-reporter construct, whereas it had no obvious effect on that of the SLFN4 mutant (Mut) promoter-reporter construct (Fig. 5B). Moreover, IFN-α overexpression did not have a significant effect on luciferase activity after mutation of ISREs in the promoter region of the Slfn4 gene (Fig. 5C). These findings suggested that IFN-α induced Slfn4 transcription by binding to ISREs in the Slfn4 promoter. This may be the mechanism by which IFN-α increased the number of SLFN4+ MDSCs.
FIG 5.
IFN-α induced SLFN4 transcription by binding to ISREs in the Slfn4 promoter. (A) Relative SLFN4 mRNA level determined by qRT-PCR analysis in wild-type mouse bone marrow cells exposed to 800 U/ml of IFN-α for different durations (0, 6, 12, 24, and 48 h). **, P < 0.01 versus 0 h. N = 3. (B) Luciferase activity in HEK293 cells cotransfected with the IFN-α expression vector/empty vector and wild-type (WT)/mutant (Mut) Slfn4 promoter-luciferase constructs. (C) Luciferase activity in HEK293 cells cotransfected with the IFN-α expression vector/empty vector and luciferase constructs containing wild-type (WT)/mutant (Mut) ISREs in the promoter region of the Slfn4 gene. $$, P < 0.01 versus vector. The experiments were repeated independently three times.
H. pylori infection stimulated pDCs to secrete IFN-α.
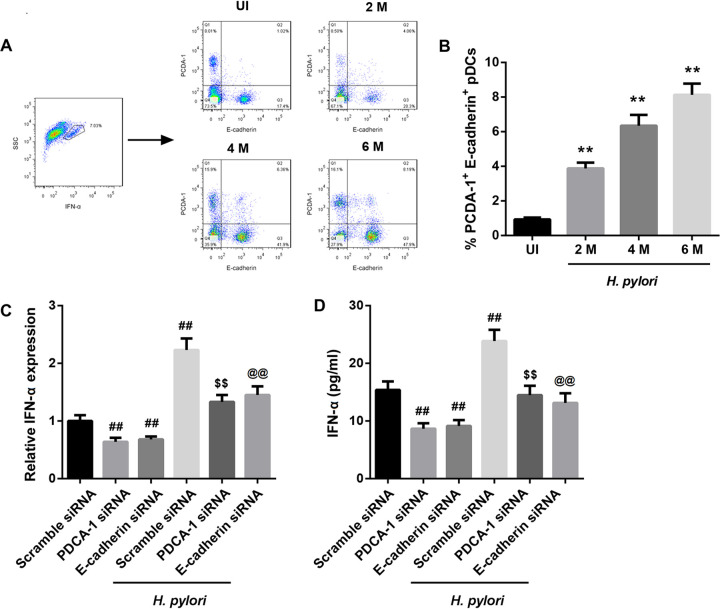
Next, we determined whether pDCs are a possible source of IFN-α. We previously reported that IFN-α was mainly secreted from pDCs that highly express plasmacytoid dendritic cell antigen-1 (PDCA-1) and E-cadherin (5). Therefore, we detected the number of PDCA-1+ E-cadherin+ pDCs in mice infected with H. pylori. Compared to that in the uninfected mice, the percentage of PDCA-1+ E-cadherin+ pDCs in the gastric corpus from infected mice was significantly increased, which was dependent on the duration of H. pylori infection (Fig. 6A and B). Furthermore, in mouse pDCs, the upregulation of IFN-α expression by H. pylori infection was counteracted by silencing of either PDCA-1 or E-cadherin (Fig. 6C and D). These results indicated that H. pylori infection upregulated IFN-α expression by increasing the number of PDCA-1+ E-cadherin+ pDCs.
FIG 6.
H. pylori infection stimulated pDCs to secrete IFN-α. Representative flow cytometry plots (A) showing the percentages of PDCA-1+ E-cadherin+ pDCs in the gastric corpus samples (B) from uninfected (UI) mice and mice infected with H. pylori for 2, 4, or 6 months. **, P < 0.01 versus UI. N = 5 per group. IFN-α mRNA levels determined by qRT-PCR analysis (C) and IFN-α levels determined by ELISA (D) in mouse pDCs transfected with PDCA-1 siRNA, E-cadherin siRNA, or scramble siRNA in the absence or presence of H. pylori. ##, P < 0.01 versus scramble siRNA; $$, P < 0.01 versus PDCA-1 siRNA; @@, P < 0.01 versus E-cadherin siRNA. The experiments were repeated independently three times.
H. pylori stimulated pDCs to secret IFN-α by activating the TLR9-MyD88-IRF7 pathway.
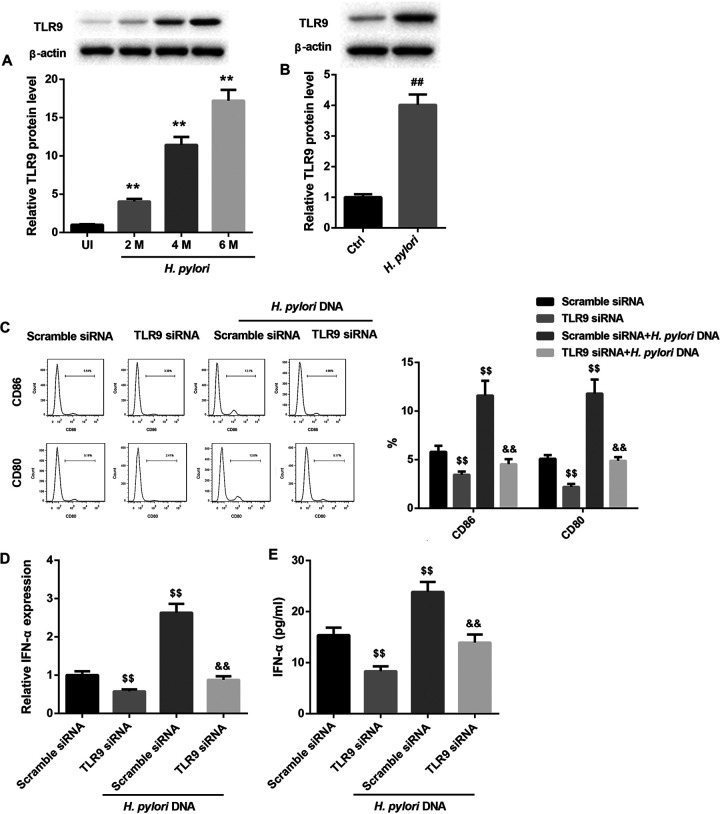
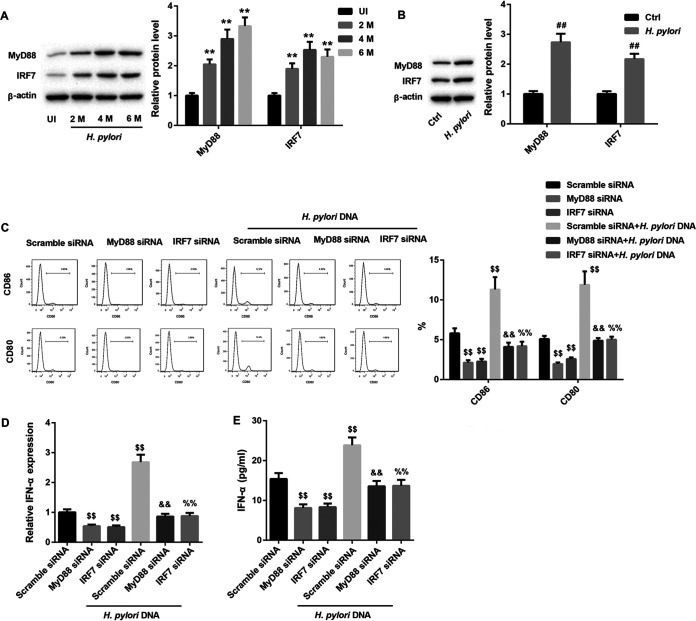
Finally, we determined the molecular mechanisms by which H. pylori infection stimulated pDCs to secret IFN-α. Evidence indicates that H. pylori DNA can be recognized by TLR9 that is highly expressed on pDCs (11, 12). TLR9 can induce IFN-α expression via the MyD88-interferon regulatory factor 7 (IRF7) signaling pathway (9). Thus, we investigated whether H. pylori infection stimulates pDCs to secret IFN-α by activating the TLR9-MyD88-IRF7 pathway. The protein levels of TLR9, MyD88, and IRF7 in the gastric corpus samples from infected mice were significantly higher than those in the uninfected mice (Fig. 7A and 8A). Furthermore, in mouse pDCs, the protein levels of TLR9, MyD88, and IRF7 were also increased after infection with H. pylori (Fig. 7B and 8B). Activated pDCs highly express CD80 and CD86. The results from the flow cytometry assays showed that with or without H. pylori DNA infection, TLR9 silencing significantly decreased the percentages of cells expressing CD80 and CD86, which suggested that TLR9 silencing suppressed the activity of pDCs (Fig. 7C). TLR9 silencing also notably downregulated IFN-α expression in mouse pDCs (Fig. 7D and E). Importantly, the H. pylori DNA infection-induced activation of pDCs and increased secretion of IFN-α were abolished by TLR9 silencing (Fig. 7C to E). Moreover, silencing of MyD88 and IRF7 alone produced effects similar to those from silencing of TLR9 (Fig. 8C to E). Together, these results manifested that H. pylori stimulated pDCs to secret IFN-α by activating the TLR9-MyD88-IRF7 pathway.
FIG 7.
TLR9 silencing abrogated the H. pylori DNA infection-induced activation of pDCs and increased secretion of IFN-α. (A) Western blot analysis of TLR9 protein levels in the gastric corpus samples from uninfected (UI) mice and mice infected with H. pylori for 2, 4, or 6 months. **, P < 0.01 versus UI. N = 5 per group. (B) Western blot analysis of TLR9 protein levels in pDCs from mice infected with H. pylori. The percentages of CD80 and CD86 on the surfaces of pDCs determined by flow cytometry (C), IFN-α mRNA levels determined by qRT-PCR analysis (D), and IFN-α levels determined by ELISA (E) in mouse pDCs transfected with TLR9 siRNA or scramble siRNA in the absence or presence of H. pylori DNA. ##, P < 0.01 versus Ctrl; $$, P < 0.01 versus scramble siRNA; &&, P < 0.01 versus scramble siRNA plus H. pylori DNA. The experiments were repeated independently three times.
FIG 8.
Silencing of MyD88 and IRF7 abrogated the H. pylori DNA infection-induced activation of pDCs and increased secretion of IFN-α. (A) Western blot analysis of MyD88 and IRF7 protein levels in the gastric corpus samples from uninfected (UI) mice and mice infected with H. pylori for 2, 4, or 6 months. **, P < 0.01 versus UI. N = 5 per group. (B) Western blot analysis of MyD88 and IRF7 protein levels in pDCs from mice infected with H. pylori. The percentages of CD80 and CD86 on the surfaces of pDCs determined by flow cytometry (C), IFN-α mRNA levels determined by qRT-PCR analysis (D), and IFN-α levels determined by ELISA (E) in mouse pDCs transfected with MyD88 siRNA, IRF7 siRNA, or scramble siRNA in the absence or presence of H. pylori DNA. ##, P < 0.01 versus Ctrl; $$, P < 0.01 versus scramble siRNA; &&, P < 0.01 versus MyD88 siRNA; %%, P < 0.01 versus IRF7 siRNA. The experiments were repeated independently three times.
DISCUSSION
An in-depth study of the molecular mechanism of the progression of chronic H. pylori infection to gastric precancerous lesions is of great significance for the prevention and treatment of gastric cancer (13). Early studies by Merchant et al. demonstrated that chronic infection of H. pylori affects gastric mucosal surface homeostasis through the Shh/Gli1 (Sonic hedgehog/Glioma-associated protein 1) signaling pathway, leading to the occurrence of intestinal metaplasia (14, 15). Further investigation revealed that CD11b+ Gr1+ MDSCs exert immunosuppressive function by expressing a myeloid cell differentiation factor, SLFN4 (5). SLFN4+ MDSCs can create a microenvironment favoring intestinal metaplasia and neoplastic transformation during chronic infection with H. pylori (5, 6). In this study, we found that the percentage of SLFN4+ MDSCs in the gastric corpus samples from H. pylori infected mice was significantly increased, which was consistent with the previous report. In addition, we also proved that H. pylori infection increased the number of SLFN4+ MDSCs by inducing IFN-α expression.
The SLFN4 promoter consists of a Gli binding site at −3323 (16) and several STAT/ISRE elements at −239, near the start site of transcription (17). Our previous study proved that Slfn4 promoter activity required Gli1; however, elevated Shh levels did not appear sufficient to boost SLFN4 expression in the corpus, whereas peak induction required IFN-α (6). Evidence indicates that IFN-α induces gene expression by binding to ISREs in the promoter region of the gene. Bioinformatics analysis showed that ISRE sites existed in most human SLFN genes (18). Therefore, in this study, we tested the hypothesis that IFN-α might induce SLFN4 transcription and expression by binding to ISREs in the promoter region of the Slfn4 gene. The results from luciferase activity assays confirmed that IFN-α directly bound to ISREs in the Slfn4 promoter. This may be the mechanism by which IFN-α promoted the differentiation of SLFN4+ MDSCs.
Our previous study reported that IFN-α was mainly secreted from pDCs that highly express PDCA-1 and E-cadherin (5). Here, the results of flow cytometry showed that the percentage of PDCA-1+ E-cadherin+ pDCs in the gastric corpus samples from infected mice was significantly increased. Using PDCA-1 or E-cadherin knockout technology, we demonstrated that H. pylori infection upregulated IFN-α expression by increasing the number of PDCA-1+ E-cadherin+ pDCs. In addition, evidence indicates that H. pylori DNA can be recognized by TLR9 that is highly expressed on pDCs (11, 12). pDCs preferentially express TLR9, which is an intracellular endosomal receptor that allows pDCs to respond to single-stranded DNA viruses and H. pylori by triggering signal transduction through the adaptor protein MyD88. TLR9 can induce pDCs to secret IFN-α expression via the MyD88-IRF7 signaling pathway (9, 10). In the present study, we performed rescue experiments by silencing TLR9, MyD88, and IRF7 to investigate the involvement of the TLR9-MyD88-IRF7 pathway in the H. pylori-induced activation of pDCs and secretion of IFN-α. Our results revealed that H. pylori infection induced activation of pDCs and secretion of IFN-α by activating the TLR9-MyD88-IRF7 pathway.
In conclusion, this study demonstrates that H. pylori infection induces pDCs to secrete IFN-α by activating the TLR9-MyD88-IRF7 pathway, while IFN-α promotes SLFN4 transcription and expression by binding to the Slfn4 promoter and thereby promotes SLFN4+ MDSC differentiation. These findings provide new insights into the mechanisms underlying the SLFN4+ MDSC differentiation during chronic infection with H. pylori and shed new light on the prevention of gastric metaplasia that could progress to gastric cancer.
MATERIALS AND METHODS
H. pylori infection in mice.
C57BL/6 mice (4 months old) purchased from the Department of Experimental Animals of Central South University were maintained in a room with controlled temperature (23 ± 2°C), at 45 to 55% relative humidity, with a 12-h day/night cycle, and had free access to food and water. Animals were gavaged 3 times over 5 days with H. pylori (CS1 strain), 108 CFU per 100 μl. Control uninfected mice were gavaged with 100 μl saline alone. Infection lasted 2, 4, or 6 months (5). This study was approved by the Ethical Committee of Xiangya Hospital, Central South University.
The corpus tissues were minced for total RNA and protein extraction as well as for tissue dissociation in EDTA for flow cytometry. Mouse bone marrow cells were isolated from the femurs of mice and processed by red blood cell lysis. Cells were collected by centrifugation at 400 × g and resuspended in 200 μl phosphate-buffered saline (PBS) containing 5% bovine serum albumin. Spleens were removed from mice and dissociated into a single-cell suspension as previously described (5).
Mouse pDC culture and transfection.
For generation of mouse pDCs, bone marrow was isolated from wild-type C57BL/6J mice. pDCs were sorted from bone marrow cultures using a pDC isolation kit II (Miltenyi Biotec, USA) according to the manufacturer’s instructions.
Mouse pDCs were transfected with PDCA-1 small interfering RNA (siRNA), E-cadherin siRNA, TLR9 siRNA, or scramble siRNA (GenePharma, Shanghai, China) (Table 1) using Lipofectamine 3000 (Invitrogen, USA) in the absence or presence of H. pylori.
TABLE 1.
siRNA sequences
| siRNA | Sequence |
|---|---|
| PDCA-1 siRNA | GCCCTCTTTCTATCACTATCT |
| E-cadherin siRNA | GCTTCAGTTCCGAGGTCTACA |
| TLR9 siRNA | GCTGAACTTGAGCTATAATGG |
| MyD88 siRNA | GGAAAGCAGTGTCCCACAAAC |
| IRF7 siRNA | GCTGTTTGGAGACTGGCTATT |
Flow cytometry.
According to our previously reported method (5), SLFN4+ cells were sorted from H. pylori-infected mouse stomach, bone marrow, or spleen. For detection of the percentage of SLFN4+ MDSCs among bone marrow cells, bone marrow cells were isolated from control mice and then infected with 100 CFU of H. pylori or exposed to 800 U/ml of IFN-α (R&D, number 12125-1) for different durations (0, 6, 12, 24, and 48 h). In another experiment, bone marrow cells from control mice were pretreated with IFN-α-neutralizing antibody (R&D, number 22100-1) for 24 h and then infected with 100 CFU of H. pylori for 12 h. After that, bone marrow cells were incubated with anti-CD11b-fluorescein isothiocyanate (FITC) antibody (BioLegend, USA), and then gated CD11b+ cells were analyzed for SLFN4-tdTomato fluorescence and the GR-1+ surface marker using anti-Gr-1-allophycocyanin (APC) antibody (BioLegend, USA) as previously described (5).
For detection of the percentages of PDCA-1+ E-cadherin+ pDCs, corpus cells were stained with anti-IFN-α-FITC antibody (Interferon Source), and then gated IFN-α+ cells were incubated with anti-PDCA-1-Alexa Fluor 647 (eBioscience, USA) and anti-E-cadherin-AF647 (BioLegend, USA) antibodies as previously described (5).
For detection of the expression of CD80 and CD86 on the surfaces of pDCs, pDCs were isolated from control mice and then transfected with TLR9 siRNA, MyD88 siRNA, IRF7 siRNA, or scramble siRNA in the absence or presence of H. pylori DNA. After that, pDCs were suspended in 100 μl PBS and then incubated with 3 μl anti-CD80 (BioLegend) and 3 μl anti-CD86 (BioLegend) antibodies at room temperature in the dark for 30 min. Cells were analyzed by flow cytometry (FACSCalibur; Becton, Dickinson, USA).
Quantitative real-time PCR analysis.
Total RNA was extracted from gastric corpus, bone marrow cells, and pDCs using TRIzol reagent (Invitrogen, USA). Subsequently, RNA was then reverse transcribed into cDNAs using the iScript cDNA synthesis kit (Bio-Rad, USA). Then, the mRNA levels of IFN-α and SLFN4 were detected by quantitative real-time PCR (qRT-PCR) using the Platinum Taq DNA polymerase (Invitrogen, USA). β-Actin levels served as the internal control of mRNA expression. The relative expression was calculated using the comparative threshold cycle (2−ΔΔCT) method. The primers were as follows: IFN-α forward, 5′-CGCATCAAAGGACTCATCTGCTG-3′; IFN-α reverse, 5′-CTGCTGCATCAGACAACCTTGC-3′; SLFN4 forward, 5′-GCCCTCTGTTCAAGTCAAGTGTCC-3′; SLFN4 reverse, 5′-CCCAGATGAAATCCTTTCCACGA-3′; β-actin forward, 5′-GGCTGTATTCCCCTCCATCG-3′; β-actin reverse, 5′-CCAGTTGGTAACAATGCCATGT-3′.
Enzyme-linked immunosorbent assay.
The level of IFN-α in mouse sera or cell supernatant was measured with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Abcam, USA) according to the manufacturer’s instructions. The optical densities were measured at 405 nm using a microplate reader (μQuanti). The IFN-α level was determined using a standard curve established with the appropriate recombinant IFN-α (expressed in picograms per milliliter).
Luciferase reporter assay.
Evidence indicates that IFN-α induces gene expression by binding to interferon-stimulated response elements (ISREs) in the promoter region of the gene (18). A luciferase reporter assay was performed to determine the interaction between IFN-α and ISREs in the promoter region of Slfn4. The promoter region of Slfn4 was synthesized and obtained from GenePharma (Shanghai, China). The fragment was individually cloned into the pGL3-Basic vector (catalog number E1751; Promega) to generate the wild-type or mutant type of reporter vectors containing the Slfn4 promoter. Briefly, HEK293 cells were cotransfected with IFN-α expression vector/empty vector and wild-type (WT)/mutant (Mut) Slfn4 promoter-luciferase plasmid using Superfect transfection reagent (Qiagen, Germany). In another experiment, HEK293 cells were cotransfected with the IFN-α expression vector/empty vector and luciferase plasmid containing WT/Mut ISREs in the promoter region of the Slfn4 gene (in which there was a mutation or wild-type sequence at the binding site of ISREs). At 24 h posttransfection, luciferase activity was measured using a dual-luciferase reporter assay system (Promega, USA) according to the manufacturer’s instructions and normalized using Renilla luciferase activity.
Western blotting.
Proteins were extracted from mouse corpus tissues or cultured pDCs by using radioimmunoprecipitation assay lysis buffer (Beyotime, Haimen, China). Proteins were then subjected to Western blotting as previously described (5). The primary antibodies were as follows: anti-TLR9 (1:1,000; Abcam, USA), anti-MyD88 (1:1,000; Abcam, USA), anti-IRF7 (1:1,000; Santa Cruz Biotechnology, USA), and β-actin (1:1,000; Abcam, USA) antibodies. β-Actin was used as the loading control. Following incubation with horseradish peroxidase-conjugated secondary antibodies, the immunoactivities were visualized with an enhanced chemiluminescence (ECL) kit (Thermo Scientific, USA) and semiquantified using ImageJ software.
Statistical analysis.
Statistical analyses were performed using SPSS 25.0 (SPSS, Inc., USA). The differences among groups were analyzed using the Student’s t test and one-way analysis of variance (ANOVA). P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
This study was supported by grants from National Natural Science Foundation of China (no. 81974064).
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Qian Li, Email: prliqian68@163.com.
Igor E. Brodsky, University of Pennsylvania
REFERENCES
- 1.Jonaitis P, Kupcinskas L, Kupcinskas J. 2021. Molecular alterations in gastric intestinal metaplasia. Int J Mol Sci 22:5758. 10.3390/ijms22115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu JY, Lee YC, Graham DY. 2019. The eradication of Helicobacter pylori to prevent gastric cancer: a critical appraisal. Expert Rev Gastroenterol Hepatol 13:17–24. 10.1080/17474124.2019.1542299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa P. 2013. Gastric cancer: overview. Gastroenterol Clin North Am 42:211–217. 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu KS, Wong IO, Leung WK. 2016. Helicobacter pylori associated gastric intestinal metaplasia: treatment and surveillance. World J Gastroenterol 22:1311–1320. 10.3748/wjg.v22.i3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding L, Hayes MM, Photenhauer A, Eaton KA, Li Q, Ocadiz-Ruiz R, Merchant JL. 2016. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest 126:2867–2880. 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding L, Li Q, Chakrabarti J, Munoz A, Faure-Kumar E, Ocadiz-Ruiz R, Razumilava N, Zhang G, Hayes M, Sontz R, Mendoza Z, Mahurkar S, Greenson J, Perez-Perez G, Hanh N, Zavros Y, Samuelson L, Iliopoulos D, Merchant J. 2020. MiR130b from Schlafen 4 MDSCs stimulates epithelial proliferation and correlates with preneoplastic changes prior to gastric cancer. Gut 69:1750–1761. 10.1136/gutjnl-2019-318817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geserick P, Kaiser F, Klemm U, Kaufmann SH, Zerrahn J. 2004. Modulation of T cell development and activation by novel members of the Schlafen (slfn) gene family harbouring an RNA helicase-like motif. Int Immunol 16:1535–1548. 10.1093/intimm/dxh155. [DOI] [PubMed] [Google Scholar]
- 8.Saitoh T, Satoh T, Yamamoto N, Uematsu S, Takeuchi O, Kawai T, Akira S. 2011. Antiviral protein Viperin promotes Toll-like receptor 7- and Toll-like receptor 9-mediated type I interferon production in plasmacytoid dendritic cells. Immunity 34:352–363. 10.1016/j.immuni.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Asselin-Paturel C, Trinchieri G. 2005. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med 202:461–465. 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puig M, Tosh KW, Schramm LM, Grajkowska LT, Kirschman KD, Tami C, Beren J, Rabin RL, Verthelyi D. 2012. TLR9 and TLR7 agonists mediate distinct type I IFN responses in humans and nonhuman primates in vitro and in vivo. J Leukoc Biol 91:147–158. 10.1189/jlb.0711371. [DOI] [PubMed] [Google Scholar]
- 11.Varga MG, Peek RM. 2017. DNA transfer and Toll-like receptor modulation by Helicobacter pylori. Curr Top Microbiol Immunol 400:169–193. 10.1007/978-3-319-50520-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauppila JH, Karttunen TJ, Saarnio J, Nyberg P, Salo T, Graves DE, Lehenkari PP, Selander KS. 2013. Short DNA sequences and bacterial DNA induce esophageal, gastric, and colorectal cancer cell invasion. APMIS 121:511–522. 10.1111/apm.12016. [DOI] [PubMed] [Google Scholar]
- 13.Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM. 2014. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol 20:5461–5473. 10.3748/wjg.v20.i18.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant JL. 2012. Hedgehog signalling in gut development, physiology and cancer. J Physiol 590:421–432. 10.1113/jphysiol.2011.220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merchant JL, Ding L. 2017. Hedgehog signaling links chronic inflammation to gastric cancer precursor lesions. Cell Mol Gastroenterol Hepatol 3:201–210. 10.1016/j.jcmgh.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Zaatari M, Kao J, Tessier A, Bai L, Hayes M, Fontaine C, Eaton K, Merchant J. 2013. Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets. PLoS One 8:e58935. 10.1371/journal.pone.0058935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Zuylen W, Garceau V, Idris A, Schroder K, Irvine K, Lattin J, Ovchinnikov D, Perkins A, Cook A, Hamilton J, Hertzog P, Stacey K, Kellie S, Hume D, Sweet M. 2011. Macrophage activation and differentiation signals regulate Schlafen-4 gene expression: evidence for Schlafen-4 as a modulator of myelopoiesis. PLoS One 6:e15723. 10.1371/journal.pone.0015723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puck A, Aigner R, Modak M, Cejka P, Blaas D, Stöckl J. 2015. Expression and regulation of Schlafen (SLFN) family members in primary human monocytes, monocyte-derived dendritic cells and T cells. Results Immunol 5:23–32. 10.1016/j.rinim.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00407-21-s0001.pdf, PDF file, 0.2 MB (206.8KB, pdf)