Abstract
1. Purpose:
To assess the ability of latanoprost-eluting contact lenses to lower the intraocular pressure (IOP) of glaucomatous eyes of cynomolgus monkeys. We also compared the IOP reduction to that achieved with daily 0.005% latanoprost ophthalmic solution.
2. Design:
Preclinical efficacy study of three treatment arms in a crossover design.
3. Subjects:
Female cynomolgus monkeys with glaucoma-induced in one eye by repeated argon laser trabeculoplasty.
4. Methods:
Low dose (CLLO) and high dose (CLHI) latanoprost eluting contact lenses were produced by encapsulating a thin latanoprost-polymer film within the periphery of a methafilcon hydrogel, which was lathed into a contact lens. In this crossover study, we assessed the IOP lowering effect of CLLO, CLHI, or daily latanoprost ophthalmic solution in a single eye in the same four female monkeys. Each monkey consecutively received 1 week of continuous wear CLLO, 3 weeks without treatment, 5 days of latanoprost drops, 3 weeks without treatment, and 1 week of continuous wear CLHI. On two consecutive days prior to initiation of each study arm, the IOP of anesthetized monkeys was measured using a calibrated pneumatonometer every hour over 7 consecutive hours from 9:30 AM to 3:30 PM to establish the baseline IOP at the predetermined time points and to account for any diurnal variations that may have occurred over the time period studied. Two-tailed Student t-tests and repeated measures ANOVA were used for statistical analysis with two-tailed values of p < 0.05 considered statistically significant.
5.
Main Outcome Measure: Intraocular pressure.
6. Results:
Latanoprost ophthalmic solution resulted in IOP reduction of 5.4 ± 1.0 mmHg on days 3 and peak IOP reduction of 6.6 ± 1.3 mmHg on day 5. CLLO reduced IOP by 6.3 ± 1.0, 6.7 ± 0.3, and 6.7 ± 0.3 mmHg on days 3, 5, and 8 respectively. CLHI lowered IOP by 10.5 ± 1.4, 11.1 ± 4.0, and 10.0 ± 2.5 mmHg on days 3, 5, and 8 respectively. For the CLLO and CLHI treatment groups, the IOP was statistically significantly reduced compared to the untreated baseline at most time points measured. CLHI demonstrated significantly greater IOP reduction than latanoprost ophthalmic solution on Day 3 (p = 0.001) and Day 5 (p = 0.015), and at several time points on Day 8 (p < 0.05). There was no statistically significant difference in IOPs when comparing CLHI and CLLO.
7. Conclusions:
Sustained delivery of latanoprost by contact lenses is at least as effective as delivery with daily latanoprost ophthalmic solution. More research is needed to determine the optimal continuous release dose that would be well tolerated and maximally effective. Contact lens drug delivery may become an option for the treatment of glaucoma and a platform for ocular drug delivery.
Sustained delivery of latanoprost by contact lenses reduced intraocular pressure as effectively as daily latanoprost drops in the glaucomatous eyes of cynomolgus monkeys.
Introduction:
The first line of treatment for glaucoma in the United States is typically topical ophthalmic medications (eye drops) intended to reduce intraocular pressure (IOP); these ophthalmic solutions are associated with burning, stinging and difficulty with self-administration.(1–4) Unfortunately, adherence with glaucoma eye drops is poor and patients with lower adherence rates have worse outcomes.(5,6) Therefore, improving patient adherence with glaucoma medications has been described as a top public health priority.(7,8) Providing an effective method of sustained drug delivery has been identified as a major unmet need for the treatment of glaucoma.(7)
Since their introduction over 50 years ago, contact lenses have been proposed as a method of ocular drug delivery. Historically, providing controlled and sustained drug release from a contact lens has proven problematic.(9,10) We have developed a drug-eluting contact lens that demonstrated the ability to provide sustained release of latanoprost, a prostaglandin analog glaucoma medication, for a month in rabbits.(11) As measured by aqueous humor drug concentrations, the latanoprost-eluting contact lenses delivered about the same amount of medication over the course of a day as a daily drop of 0.005% latanoprost solution. However, that study could not demonstrate whether the contact lens could effectively lower IOP since latanoprost does not have that effect in rabbits.(12,13) It was particularly important to provide direct proof of the efficacy of the drug-eluting lens since its drug release kinetics differed from that of drops and the effect of drug release kinetics on efficacy is not clear. Latanoprost ophthalmic solution demonstrated a large initial bolus followed by rapidly decreasing drug levels over 12 hours.(11) In contrast, the latanoprost-eluting contact lenses demonstrate an initial burst on day 1 followed by a sustained daily rate of delivery over the course of a month.(11) Here we examined the efficacy of sustained latanoprost delivery from drug-eluting contact lenses in glaucomatous cynomolgus monkeys.
Methods:
Materials.
High molecular weight (119 kDa) 50:50 PLGA (poly(lactic-co-glycolic) acid; 50 glycolide: 50 L-Lactide) was obtained from DURECT Corporation (Birmingham, AL). Irgacure 2959 was purchased from Ciba Specialty Chemicals Corporation (Tarrytown, NY). For incorporation into the contact lenses, latanoprost was obtained in methyl acetate (10 mg/mL, Cayman Chemical, Ann Arbor, MI). Commercially available latanoprost aqueous solution (50 μg/mL) with benzalkonium (BAK) preservatives was obtained from Sandoz Inc. (Princeton, NJ). Unpolymerized methafilcon was purchased in liquid form from Kontur Kontact Lens Company (Hercules, CA). Ethyl acetate and all the other reagents were purchased from Sigma Aldrich (St. Louis, MO). Phosphate buffered saline (PBS, pH 7.4) was obtained from Invitrogen (Carlsbad, CA). Biopsy punches (2mm and 3 mm) were obtained from Sklar Instruments (West Chester, PA).
Animals.
The study protocol was approved by the Institutional Animal Care and Utilization Committee of The Icahn School of Medicine at Mount Sinai (New York, NY). All animals were treated according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research (ARVO Handbook, 1993). Four adult female cynomolgus monkeys, each weighing 3–5 kg, were included in this study. In one eye elevated IOP and glaucoma had been previously induced by photocoagulation of the midtrabecular meshwork with either an argon laser (65–120 spots; power 1.1–1.5 W; size 50 μm; duration 0.5 sec) or diode laser (50–120 spots; power 1.2 W; size 75 μm; duration 0.5 sec).(9,11,14) The contralateral eye remained untreated. Only the glaucomatous eye of each animal was studied.
Contact Lens Retention Study.
To determine the shape of the contact lens that would be able to be worn on the glaucomatous eyes of monkeys, contact lenses were fabricated with a combination of back radius of curvatures (base curves) and diameters (Table 1) based on prior reports of the ocular dimensions of cynomolgus monkeys.(15,16) Contact lenses were lathed from cylinders of methafilicon, a co-polymer of poly(hydroxyethylmethacrylate) and methacrylic acid, which is one of the hydrogels that is approved for use as a contact lens in the United States and internationally; this hydrogel contains 55% water when hydrated. The trial set of contact lenses was composed of only methafilcon and lacked a drug-polymer film.
Table 1.
Contact lens trial set parameters.
| Base Curve | Diameter |
|---|---|
| 6.3 | 11.8 |
| 12.5 | |
| 13.0 | |
| 6.5 | 11.8 |
| 12.5 | |
| 13.0 | |
| 6.8 | 11.8 |
| 12.5 | |
| 13.0 |
A contact lens trial set was manufactured with 3 different base curves, each with 3 different diameters. The lens underlined in bold was retained for 5 days and was therefore used in studies of drug effect.
Prior to the initiation of the study to determine the best fit of lenses in the monkeys, the baseline IOP of their right, glaucomatous eye was measured at 9:30 AM on two consecutive days under anesthesia (ketamine hydrochloride 2–5 mg/kg of body weight administered intramuscularly). They were examined by slit lamp biomicroscopy and the corneal diameter was measured with vernier calipers. A contact lens was placed on the right glaucomatous eye and observed for proper fit while the animals were anesthetized. Once the monkeys recovered from anesthesia, they were observed for signs of discomfort, such as blepharospasm or eye rubbing. On day 3, the animals were anesthetized and the eyes underwent slit lamp examination without removal of the contact lenses. On day 5, the contact lenses that remained in place were removed and the eyes were examined with fluorescein to evaluate the corneal surface.
Fabrication of Latanoprost-eluting Contact Lenses.
Pharmaceutical-grade latanoprost was supplied within a methyl acetate solution (10 mg/ mL, Cayman Chemical). 400 μL of the latanoprost solution and good manufacturing practice (GMP)-grade PLGA (60 mg, 50:50; DURECT corporation) were added to 600 μL of ethyl acetate. 30 μL of the combined solution was then pipetted onto a concavity that had been lathed into a cylinder of dry polymerized methafilcon (Kontur Kontact Lens Company). 40 μL of the drug-polymer solution was used to create a film with greater drug loading. After rotation on a spin coater (Model SC100B, Best Tools, LLC, St Louis, MO,) for 6 minutes, the ethyl acetate evaporated and only a drug-polymer film remained. A central aperture was cut from the film using a 3 mm biopsy punch for films created from the 30 μL drug-polymer solution (low dose contact lenses; CLLO) and a 2 mm biopsy punch for films created from 40 μL of solution (high dose contact lenses; CLHI; Table 2). Following desiccation for 1 day and lyophilization for 1 day, the drug-polymer film was encapsulated in methafilcon by ultraviolet (UV) photopolymerization (400 W metal halide bulb, Loctite Corporation, Rocky Hill, CT) to recreate a hydrogel cylinder. The methafilcon block was then lathed into a contact lens that consisted of the drug-PLGA film fully encapsulated in methafilcon. To remove any surface excipients, the contact lenses were placed in 5 mL sterile PBS solution and rotated at approximately 64 RPM at 37° Celsius for two hours. The lenses were removed from PBS, dried at room temperature, and stored in air-tight glass vials sealed with a sealed plastic screw top. Finally, the glass containers holding the lenses were placed in a temperature-controlled container and terminally sterilized by irradiation in a Gamma Cell 220E Cobalt 60 Irradiation Unit (Atomic Energy of Canada LTD, Ottawa, Canada) with a total dose administration of 25 kGy.
Table 2.
Characteristics of the drug-polymer film within latanoprost-eluting contact lenses designed for glaucomatous monkeys.
| Lens Type | Estimated Drug Content (μg) | Film Inner Diameter (Aperture; mm) | Film Outer Diameter (mm) | Film Surface Area (mm2) |
|---|---|---|---|---|
| Low Dose CL | 97 | 4.2 | 9.5 ± 0.3 | 57.4 ± 4.1 |
| High Dose CL | 149 | 2.8 | 10.2 ± 0.1 | 76.7 ± 2.2 |
Drug-polymer film dimensions are reported as means ± one standard deviation. Both sets of contact lenses had a 12.5 mm outer diameter and a 6.5 mm base curve.
Coherence Topography of Contact Lenses.
The morphologies of the contact lenses containing the drug-polymer films were imaged using an anterior segment optical coherence tomographer. (OCT; RTVue, Optovue, Fremont, CA). Dry contact lenses were positioned with the convex side of the lens facing the OCT camera. Raster scanning imaging was used in four segments for each contact lens to obtain cross sectional images of the hydrogel and drug-polymer film. Film thickness was quantified using the RTVue software linear measurement tool as per the operator’s instructions.
Efficacy Evaluation of Latanoprost Ophthalmic Solution and Latanoprost-eluting Contact Lenses in Glaucomatous Monkeys.
Four female cynomolgus monkeys were included in this study. The animals previously had glaucoma-induced in one eye by repeated argon laser trabeculoplasty. Each animal consecutively received the following treatment arms: a CLLO, commercially available latanoprost ophthalmic solution, and a CLHI in a cross-over design with a 3-week interval between treatment modalities (washout period). On two consecutive days prior to initiation of each study arm, the IOP of anesthetized monkeys was measured using a calibrated pneumatonometer (Mentor, Model 30 Classic, Norwell, MA) every hour over 7 consecutive hours from 9:30 AM to 3:30 PM to establish the baseline IOP at the predetermined time points and to account for any diurnal variations that may have occurred over the time period studied.
The CLLO study arm.
Contact lenses were initially hydrated in 5 mL of sterile saline. On Day 1 the lenses were inserted onto the right, glaucomatous eye of anesthetized monkeys and worn continuously through Day 8. IOP was measured on Days 3, 5, and 8. The time of day that IOP was measured alternated between mornings and afternoons in order to capture diurnal variations. Therefore, IOP was measured at 10:30 AM on day 3, 2:30 PM on day 5, and then hourly from 9:30 AM to 3:30 PM on day 8 following removal of the contact lens at 9:00 AM (Table 4). IOP was also measured once daily on days 9 and 11, i.e. 1 day and 3 days respectively after the lenses were removed. A slit-lamp examination was performed to assess ocular status, tolerance and contact lens fit at baseline and on the same days as IOP measurements. Throughout the study the lenses were only temporarily removed twice for IOP measurement, which occurred on Day 3 and Day 5. While temporarily removed, the lenses were stored in 5 mL of sterile saline for approximately 2 minutes. On study day 8 the lenses were permanently removed.
Table 4.
Change in intraocular pressure.
| Change in IOP (mm Hg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 9:30 AM | 10:30 AM | 11:30 AM | 12:30 PM | 1:30 PM | 2:30 PM | 3:30 PM | ||
| Latanoprost Solution | ||||||||
| Treatment | Day 3 | −5.4 ± 1.0 | ||||||
| Day 5 | −3.4 ± 0.3 | −5.3 ± 0.5 | −6.6 ± 1.3 | −5.4 ± 1.0* | −5.1 ± 1.4 | −4.0 ± 0.6 | −2.9 ± 1.0 | |
| Post-treatment | Day 3 | −0.6 ± 1.4 | ||||||
| CLLO Treatment | Day 3 | −6.3 ± 1.0* | ||||||
| Day 5 | −6.7 ± 0.3 § (n=3) | |||||||
| Day 8 | −7.8 ± 3.8* | −7.4 ± 3.4* | −7.8 ± 3.0* | −6.9 ± 1.3* | −6.6 ± 2.1 | −5.3 ± 1.7 | −4.0 ± 1.1 | |
| Post-Treatment | Day 1 | −3.0 ± 3.4 | ||||||
| Post-Treatment | Day 3 | −1.8 ± 3.9 | ||||||
| CLHI Treatment | Day 3 | −10.5 ± 1.4**§ | ||||||
| Day 5 | −11.1 ± 4.0*§ | |||||||
| Day 8 | −10.0 ± 2.5* § (n = 3) | −9.3 ± 4.2* (n=3) | −8.8 ± 3.5** (n=3) | −9.2 ± 3.2* (n=3) | −10.2 ± 2.4*§ (n=3) | −8.2 ± 2.3 *§ (n=3) | −6.0 ± 4.4 (n=3) | |
| Post-Treatment | Day 1 | −4.0 ± 3.1 (n=3) | ||||||
| Post-Treatment | Day 3 | −3.7 ± 4.0 (n=3) | ||||||
| Post-T reatment | Day 8 | −1.0 ± 1.3 (n=3) | ||||||
Data are mean changes in Intraocular pressure (IOP) ± standard deviation from baseline
p<0.05
p<0.005 in comparison to baseline (2-tailed Student-test);
p<0.05 in comparison to latanoprost drop treatment at the same time, using Student t-test. Changes in IOP for the CLHI on Day 8 were compared to the last day of treatment (Day 5) with latanoprost drops. CLLO = Low-dose contact lens. CLHI = High-dose CL; n = 4 unless otherwise noted.
The latanoprost ophthalmic solution study arm.
Treatment began 3 weeks after the CLLO arm ended. Diurnal IOP was measured on two consecutive days prior to initiation of the drops to establish the baseline and to confirm that the IOP returned to the same levels as measured prior to initiation of the CLLO study. 50μl (25 μl × 2) of BAK-preserved 0.005% latanoprost ophthalmic solution (Sandoz Inc. Princeton, NJ) was topically applied once daily to the glaucomatous eye only at 9:30 am for 5 consecutive days.(14,17) As with the CLLO, IOP of anesthetized monkeys was measured on Days 3, 5, and 8. The IOP was measured at 10:30 am on treatment day 3 and hourly for 6 hours on day 5. 3 days after the receiving the last drop of latanoprost (day 8) the IOP was measured again at 10:30 am.
The CLHI study arm.
This arm was initiated at least 3 weeks after the discontinuation of treatment with latanoprost ophthalmic solution. After the baseline IOP was measured on two consecutive days, hydrated CLHI were inserted onto the glaucomatous eye of 4 monkeys in the same manner as CLLO. As with CLLO and latanoprost ophthalmic solution, the IOP was also measured once daily on Day 3, Day 5, and Day 8 at the same time of day. After the contact lenses were removed, IOP was measured on Day 9 and 11, and on day 16 (8 days after the respective lenses were removed) since the mean IOP did not return to baseline on day 11.
Statistical Analysis.
Two-tailed Student t-tests and repeated measures ANOVA were used for statistical analysis with two-tailed values of p < 0.05 considered statistically significant.(18) Nonparametric testing was also used for comparing median changes in IOP and the results were similar. Power analysis indicated that for paired comparisons between latanoprost drops versus low-dose CL and high-dose CL, a sample size of 4 monkeys provided 80% power for detecting large differences in IOP reduction (ie, effect sizes of 1.5 or greater, which translate into 3–4 mmHg in IOP, assuming a variance of 4 mmHg) based on a Student t-tests at each time point (version 7.0, nQuery Advisor, Statistical Solutions, Cork, Ireland). Data analysis was performed using IBM Corporation SPSS Statistics version 23.0 (IBM, Armonk, NY). No adjustments were applied to the p-value criterion for multiple comparisons based on the rationale of not wanting to be overly conservative and missing important treatment effects due to reduced statistical power.(19)
Results.
Lens retention study.
In order to establish the dimensions for drug-eluting contact lenses that would be suitable for continuous wear on the glaucomatous eyes of monkeys, animals were examined under anesthesia. Using vernier calipers, the average vertical and horizontal corneal diameters of four glaucomatous eyes were 11.5 ± 0.5 mm and 10.5 ± 0.5 mm, respectively. These measures were consistent with reported dimensions.(15,16) Concurrently, the animals were examined by slit lamp biomicroscopy to assess their baseline ocular health and the IOP was measured with a calibrated pneumatonometer.
The measures obtained were used to determine the parameters for a contact lens trial set that contained 3 different base curves (back radius of the lens curvature), each with 3 lens diameters (9 combinations; Table 1). The selection of these parameters was based on the reported dimensions of the corneas from the healthy eyes of cynomolgus monkeys.(11,15) The lenses were composed of methafilcon, a high water content copolymer of pHEMA and methacrylic acid, and did not contain a drug-polymer film. They were evaluated in a 5-day retention study.
Lenses were inserted onto the corneas of anesthetized monkeys. Regardless of the diameter, lenses with a base curve of 6.3 were found to have excessive vaulting of the lens over the cornea (too steep) and base curves of 6.8 were found to be too flat, with the edge of the lenses lifting off the eye. Three lenses with 6.5 mm base curves and diameters of 11.8 mm (n = 1), 12.5 mm (n = 1), or 13.0 mm (n = 1) maintained a proper orientation on the eye; all of these lenses remained in place for the first 3 days of the 5-day retention study. On Day 3 of the retention study, all three eyes were found to be normal by slit lamp examination. On Day 5 only the lens with a 12.5 mm diameter was retained. Slit lamp examination with fluorescein revealed only trace vortex-pattern punctate staining of the cornea that was consistent with contact lens wear. The IOP of the same eye was unchanged from the baseline IOP (36 mm Hg) that had been measured at the same time of day (9:30 am) prior to the initiation of the contact lens retention study. Contact lenses that contained a drug-polymer film were manufactured with base curves of 6.5 mm and a diameter of 12.5 mm for subsequent studies.
Latanoprost eluting contact lenses.
CLLO and CLHI were produced as described in the Methods section. Both sets of lenses contained drug-polymer films that were encapsulated in methafilcon and lathed into contact lenses. The films within the CLHI had a greater outer diameter and smaller central aperture than those in CLLO (Table 2). CLLO and CLHI were loaded with approximately 97 μg and 149 μg of latanoprost, respectively. By ocular coherence tomography (OCT) the cross-section of the drug-polymer film could be seen within the cross-section of the hydrogel (Figure 1).
Figure 1.
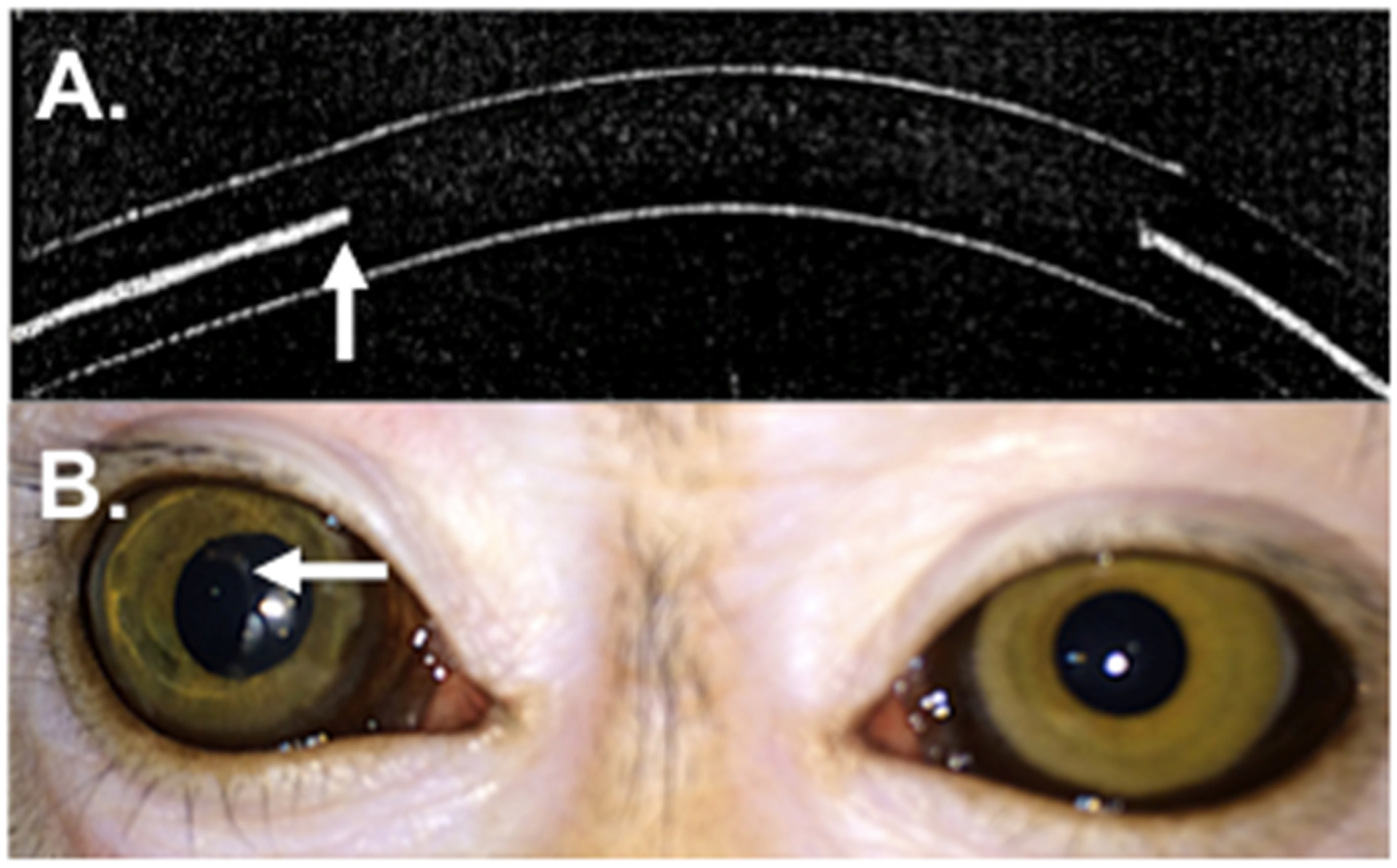
The latanoprost-eluting contact lens. (A) Representative ocular coherence tomography image of the cross-section of the contact lens. The arrow points to the inner edge of the drug-polymer film within the hydrogel. (B) Photograph of latanoprost-eluting contact lens on the right monkey eye. The clear central aperture is surrounded by a translucent ring of drug-polymer film. The arrow points to the inner margin of the drug polymer film.
Non-human primate efficacy study.
We compared the IOP lowering effect of CLLO, CLHI, and daily latanoprost (0.005%) drops in a single eye in the same four female glaucomatous cynomolgus monkeys. Each monkey underwent the following regimen in sequence:
- CLLO worn continuously for 7 days
-
1aNo treatment for three weeks
-
1a
- Latanoprost one drop daily for 5 days
-
2aNo treatment for three weeks
-
2a
CLHI worn continuously for 7 days
Baseline Circadian IOP Measurements.
Primates, such as monkeys and humans, have circadian IOP variations.(17,20) In order to determine the baseline IOP for any given time of day, we measured the IOP of untreated eyes hourly over the course of 7 hours (from 9:30 AM to 3:30 PM) on 2 consecutive days prior to the initiation of each treatment. The average IOP for each animal at each time point served as the baseline IOP to which measurements obtained during subsequent treatment were compared for that animal (Table 3). No differences were found between the baseline measurements.
Table 3.
Baseline intraocular pressure.
| Baseline IOP (mm Hg) | |||||||
|---|---|---|---|---|---|---|---|
| Treatment Arm | 9:30 AM | 10:30 AM | 11:30 AM | 12:30 PM | 1:30 PM | 2:30 PM | 3:30 PM |
| CL LO | 31.1 ± 3.1 | 30.4 ± 3.4 | 30.8 ± 3.9 | 30.6 ± 3.0 | 31.4 ± 3.7 | 30.0 ± 4.3 | 28.3 ± 3.7 |
| Latanoprost Solution | 31.1 ± 3.6 | 30.5 ± 3.4 | 30.9 ± 4.5 | 30.4 ± 3.6 | 31.1 ± 3.8 | 30.5 ± 4.2 | 28.9 ± 4.2 |
| CL HI | 31.3 ± 4.2 | 31.0 ± 4.6 | 31.1 ± 3.5 | 31.1 ± 4.5 | 31.1 ± 4.3 | 30.6 ± 4.3 | 30.0 ± 5.1 |
Intraocular pressure (IOP) was measured hourly from 9:30 AM to 3:30 PM on 2 consecutive days prior to the initiation of each treatment. The average IOP for each animal at each time point served as the baseline IOP for that animal in calculating changes in IOP due to treatments. Data are the means ± standard deviations of the measurements at each time point in 4 animals. CLLO = Low-dose contact lens. CLHI = High-dose CL.
Latanoprost ophthalmic solution.
Commercial 0.005% latanoprost solution was administered daily for 5 days. When IOP was first measured at 10:30 AM (1 hour after drop application) on Day 3 (Table 4), there was a reduction of 5.4 ± 1.0 mmHg.. On day 5 IOP was measured immediately prior to drop administration and then hourly for a total of 7 measurements. Compared to the baseline (Table 3), the IOP was reduced by 3.4 ± 0.3 mmHg at the initial morning time point (9:30 am). The peak IOP reduction of 6.6 ± 1.3 mmHg occurred at 11:30 AM. The IOP was reduced by 2.9 ± 1.0 mmHg at 3:30 pm. The peak and trough IOP reduction from latanoprost ophthalmic solution in this study was the same as previously reported in this glaucomatous monkey colony.(12,13,17) IOP was measured again on Day 8, three days after the last drop was given. There was no IOP reduction (+0.0 ± 0.6 mmHg).
Latanoprost-eluting Contact Lenses.
The IOP lowering effect of CLLO and CLHI were studied separately (see Methods for details of treatment schedules). In both contact lens groups, IOP was statistically significantly reduced compared to baseline (Table 3) at most time points tested on Days 3, 5 and 8 (p<0.05 by Student t-test at those time points, see Table 4). For example, the IOP was reduced by 6.3 ± 1.0 mmHg for CLLO and 10.5 ± 1.4 mmHg for CLHI on day 3 at 9:30 am (Figure 2). On day 5 at 2:30 pm, IOP was reduced by 6.7 ± 0.3 mmHg for CLLO and by 11.1 ± 4.0 mmHg for CLHI. The contact lenses were removed on day 8 at 9:30 am and at that time the IOP was reduced by 7.8 ± 3.8 mmHg CLLO and by 10.0 ± 2.5 mmHg for CLHI. Even after the contact lenses were removed on day 8, sustained lowering of IOP was observed for both groups (p<0.05 by Student t-test compared to the baseline at most time points).
Figure 2.
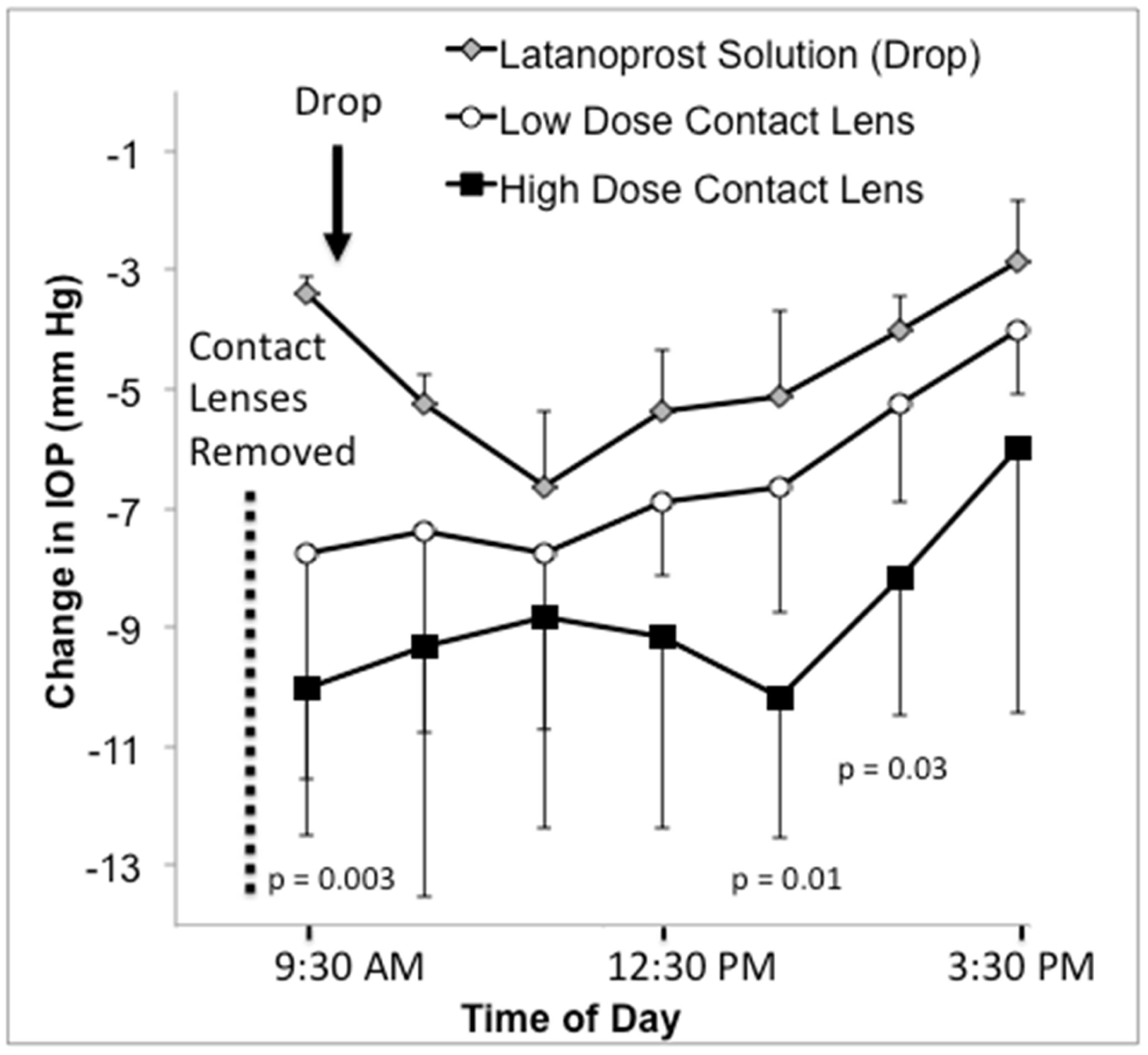
IOP change from baseline over 7 hours on the last day of treatment in glaucomatous monkey eyes. Latanoprost drop data represent the change in IOP before and following the 5th consecutive morning dose of 0.005% latanoprost solution. The contact lens data represents the change in IOP after removing the lenses following 7 days of continuous wear. P-values were calculated using Student t-tests comparing high dose contact lenses and latanoprost drops at the same time point. Data are means ± standard deviation. n = 4 for the latanoprost drop and the low dose contact lens and n = 3 for the high dose contact lenses. IOP = intraocular pressure.
CLHI demonstrated statistically significantly greater IOP reduction than latanoprost ophthalmic solution on Day 3 (p = 0.001, Student t-test) and Day 5 (p = 0.015). On the last day of treatment (Day 5 for drops and Day 8 for CLHI) IOPs were lower after treatment with CLHI than with latanoprost ophthalmic solution at 9:30 am (p = 0.003), 1:30 pm (p = 0.01), and 2:30 pm (p = 0.02, Table 4). There was no difference in IOPs when comparing CLLO and drops (p>0.05) or CLHI and CLLO (p >0.05). One and 3 days after the contact lenses were removed, the IOP returned to baseline (p>0.05, Student t-test).
Continuous-wear soft contact lenses in monkeys are associated with a notoriously high rate of corneal infiltrates and infections.(11,15,16) The animals were monitored daily for signs of ocular discomfort and the treatment eyes were examined by slit lamp biomicroscopy with and without fluorescein. In the CLLO group, punctate staining appeared in the central cornea in one monkey on day 8. Some conjunctival mucus was noted in 2 of the 4 monkey eyes on days 3 and was not observed on day 5 in any of the animals. On day 8, two of the 4 monkeys were found to have mild mucus along the inferior conjunctiva. In the CLHI group, mild conjunctival mucus and hyperemia of the lid margin appeared in one monkey on day 2 and in two monkeys on days 3 and day 5. On day 5, two monkeys also developed mild hyperemia and one of these monkeys developed central punctate staining of the cornea on day 5. A corneal infiltrate and an epithelial defect were found in the same animal on day 8. Staphylococcus capitis, a species that is commonly present on human skin and rarely isolated from the skin of monkeys or other animals,(11,21) was identified from a culture of the infiltrate, which resolved with topical ofloxacin therapy. The IOP measurements from day 8 and thereafter were not included in the study for that animal.
Discussion.
We have developed a latanoprost-eluting contact lens and previously demonstrated sustained release of the drug for one month in rabbits, at levels thought to be therapeutic.(9,11,14) The efficacy of the lenses could not be assessed since latanoprost does not effectively reduce IOP in rabbits.(12,15,16) The purpose of this study was to test the efficacy of latanoprost-eluting contact lenses in glaucomatous eyes of monkeys, which, like humans, demonstrate IOP reduction when administered latanoprost eye drops.(14,17) We created two different latanoprost-eluting contact lens formulations that were made specifically for monkey eyes. Both contact lens formulations contained a latanoprost-polymer film completely encapsulated within a contact lens hydrogel. Keeping the drug loading thickness of the films the same, differences in drug loading were accomplished by increasing the film surface area of the CLHI compared to the CLLO.
CLLO and CLHI both produced sustained IOP reductions in glaucomatous monkey eyes, as did latanoprost ophthalmic solution at some time points. This study was only powered to detect large differences in IOP reduction between treatment groups. Nevertheless, CLHI produced a statistically significantly greater IOP reduction than latanoprost solution at some time points (Table 4). The IOP reduction from CLLO was similar to 0.005% latanoprost ophthalmic solution that was administered daily. More differences in IOP between groups might be observed in a study with larger sample sizes.
It was unexpected to find that the CLHI resulted in greater IOP reduction than latanoprost ophthalmic solution at multiple time points (Table 4). For instance, on Day 3 the CLHI resulted in an IOP reduction of 34% compared to the 10% IOP reduction observed with latanoprost ophthalmic solution. On the last treatment day, the maximum IOP reduction observed with the CLHI was 32% and 21% with latanoprost ophthalmic solution. These findings were surprising because latanoprost solution given at a concentration of 0.005% (50 μg/mL) and administered once daily is reported to be the optimal dosing regimen.(12) More frequent administration of 0.005% latanoprost solution does not produce additional IOP reduction (22) and latanoprost has been shown in multiple studies to be less effective when administered twice a day instead of once a day.(20,23,24) A clinical dose-response study was performed using 35, 60 or 115 μg/mL latanoprost ophthalmic solution in ocular hypertensive humans and found no clear effect of increasing dose at the concentrations tested.(25)
Reduction of IOP throughout the day is the desired therapeutic effect of drug treatment. The latanoprost-eluting contact lenses demonstrated relatively constant IOP lowering throughout the study period. For example, CLHI achieved IOP reductions of −10.5 ± 1.4 mm Hg on Day 3, −11.1 ± 4.0 mm Hg on Day 5, and −10.0 ± 2.5 mm Hg on Day 8 when measured immediately after the contact lenses were removed at 10:30 AM, 2:30 PM, and 9:30 AM, respectively. In contrast, latanoprost ophthalmic solution resulted in more variation in IOP reduction during diurnal IOP measurements. On the 5th treatment day with latanoprost solution, IOP reduction prior to drop installation was −3.0 ± 0.4 mm Hg at 9:30 AM, peaked two hours after drop installation at −6.5 ± 0.6 mm Hg at 11:30 am, and then decreased to 3.5 ± 0.3 mm Hg by 3:30 PM. The same peak and trough IOP profile has been observed in other studies of 0.005% latanoprost ophthalmic solution in glaucomatous monkeys. (14,17)
This efficacy study was performed in glaucomatous cynomolgus monkeys because the animals can wear contact lenses and have a similar physiological response to latanoprost as do humans.(12) However, studying drug-eluting contact lenses in monkeys presented some challenges. Compared to human eyes, the eyes of cynomoglus monkeys have a more shallow superior and inferior fornix and a cornea that has a smaller diameter, is more prominent, and steeper than that of humans. As a result of the smaller ocular surface, the monkey eye holds less of an eye drop than that of humans. Therefore, instead of a single 50 μl drop that would be administered to a human eye from a commercially available bottle of latanoprost, two 25 μl drops of 0.005% latanoprost ophthalmic solution were pipetted into the monkeys’ eyes, 5 minutes apart, as has been reported.(14,17) For contact lenses, the smaller ocular surface prevented us from using contact lenses with the same lens parameters as those designed for human use. Instead, we were required to manufacture contact lenses with a smaller diameter and a steeper base curve. As a result, the film within the CLHI and CLLO had a smaller visual aperture that respectively measured 2.0 mm and 3.0 mm. If drug-eluting contact lenses were designed for human eyes with a larger corneal diameter, the drug-polymer films would have a visual aperture of 6 mm or 8 mm, while achieving the same drug-polymer loading as CLHI and CLLO, respectively. From a drug delivery perspective, the larger diameter of human contact lenses would also provide the capacity to load a larger drug-polymer film.
Monkeys are prone to contact lens related infections at a far higher rate than in humans.(16,26) A study of pHEMA based soft contact lenses in cynomologus monkeys found that 9 of the 12 animals developed complications that required discontinuation of wear and 8 of the 12 animals developed corneal infiltrates.(16) In another investigation in rhesus monkeys with contact lenses, all 4 of the animals studied developed cornea problems.(26) Given the propensity for contact lens related corneal infections in monkeys, future studies may consider the prophylactic use of antibiotics in monkeys wearing contact lenses.
The primary focus of this study was on the efficacy of the treatment arms and while the eyes were examined by fluorescein-assisted slit lamp biomicroscopy, one of the limitations to this study was that additional methods of ocular safety (e.g. endothelial cell count) were not assessed. From a systemic safety standpoint, the overall heath, activity, and weight of the animals were monitored, but the serum drug levels were not measured in the treatment groups and is another limitation of this study.
We have found that sustained delivery of latanoprost by contact lenses is at least as effective as daily latanoprost eye drops, and possibly more so. Research is needed to determine the optimal continuous release dose that would be well tolerated and maximally effective. Contact lens drug delivery may become an option for the treatment of glaucoma and a platform for ocular drug delivery.
Acknowledgments
This research was funded by NEI 1K08EY019686-01 (JBC), Massachusetts Lions Eye Research Fund (JBC), New England Cornea Transplant Fund (JBC), Eleanor and Miles Shore Foundation (JBC), Technology Development Grant from Boston Children’s Hospital (JBC, DSK), and by a Career Development Award from Research to Prevent Blindness, Inc., NY, NY (JBC). This study was supported in part by an unrestricted grant from Research to Prevent Blindness Inc., New York, NY (JBS).
Disclosures: JBC and DSK are inventors on a patent for the drug-eluting contact lens evaluated in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The data were presented in part at the Glaucoma 360: 5th Annual New Horizons Forum (2016), San Francisco, CA and at the ARVO Annual Meeting (2016), Seattle, WA.
References
- 1.Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. British Journal of Ophthalmology. 1990. August;74(8):477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghate D, Edelhauser H. Barriers to glaucoma drug delivery. J Glaucoma. 2008;17(2):147. [DOI] [PubMed] [Google Scholar]
- 3.Hennessy AL, Katz J, Covert D, Kelly CA, Suan EP, Speicher MA, et al. A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am J Ophthalmol. 2011. December;152(6):982–8. [DOI] [PubMed] [Google Scholar]
- 4.Stone JL, Robin AL, Novack GD, Covert DW, Cagle GD. An objective evaluation of eyedrop instillation in patients with glaucoma. Archives of Ophthalmology. 2009. June;127(6):732–6. [DOI] [PubMed] [Google Scholar]
- 5.Sleath B, Blalock S, Covert D, Stone JL, Skinner AC, Muir K, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011. December;118(12):2398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse AR. Improving Medication Adherence to Reduce Vision Loss in Patients with Glaucoma: Low Hanging Fruit? Ophthalmology. 2015. July;122(7):1280–2. [DOI] [PubMed] [Google Scholar]
- 7.Quigley HA. Glaucoma. Lancet. 2011. April 16;377(9774):1367–77. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Survey of Ophthalmology. 2008. November;53 Suppl1:S57–68. [DOI] [PubMed] [Google Scholar]
- 9.Ciolino JB, Dohlman CH, Kohane DS. Contact lenses for drug delivery. Semin Ophthalmol. 2009. May;24(3):156–60. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho IM, Marques CS, Oliveira RS, Coelho PB, Costa PC, Ferreira DC. Sustained drug release by contact lenses for glaucoma treatment-a review. J Control Release. 2015. March 28;202:76–82. [DOI] [PubMed] [Google Scholar]
- 11.Ciolino JB, Stefanescu CF, Ross AE, Salvador-Culla B, Cortez P, Ford EM, et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials. 2014. January;35(1):432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stjernschantz JW. From PGF(2alpha)-isopropyl ester to latanoprost: a review of the development of xalatan: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2001. May;42(6):1134–45. [PubMed] [Google Scholar]
- 13.Paschalis EI, Cade F, Melki S, Pasquale LR, Dohlman CH, Ciolino JB. Reliable intraocular pressure measurement using automated radio-wave telemetry. Clin Ophthalmol. 2014;8:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serle JB, Podos SM, Kitazawa Y, Wang RF. A comparative study of latanoprost (Xalatan) and isopropyl unoprostone (Rescula) in normal and glaucomatous monkey eyes. Japanese Journal of Ophthalmology. 1998. March;42(2):95–100. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman PL, Calkins BT, Erickson KA. Ocular biometry of the cynomolgus monkey. Curr Eye Res. 1981;1(5):307–9. [DOI] [PubMed] [Google Scholar]
- 16.Carney LG, Kiely PM, Brennan NA, Crewther SG, Nathan J. Extended contact lens wear in the developing cynomolgus monkey eye. Recovery from adverse responses. Cornea. 1989. September;8(3):182–7. [PubMed] [Google Scholar]
- 17.Daull P, Buggage R, Lambert G, Faure M-O, Serle J, Wang R-F, et al. A comparative study of a preservative-free latanoprost cationic emulsion (Catioprost) and a BAK-preserved latanoprost solution in animal models. J Ocul Pharmacol Ther. 2012. October;28(5):515–23. [DOI] [PubMed] [Google Scholar]
- 18.Muller KE, Barton CN. Approximate power for repeated-measures ANOVA lacking sphericity. Journal of the American Statistical …. 1989. [Google Scholar]
- 19.Althouse AD. Adjust for Multiple Comparisons? It’s Not That Simple. Ann Thorac Surg. 2016. May;101(5):1644–5. [DOI] [PubMed] [Google Scholar]
- 20.Diestelhorst M, Krieglstein GK, Lusky M, Nagasubramanian S. Clinical dose-regimen studies with latanoprost, a new ocular hypotensive PGF2 alpha analogue. Survey of Ophthalmology. 1997. February;41 Suppl 2:S77–81. [DOI] [PubMed] [Google Scholar]
- 21.Kloos WE, Zimmerman RJ, Smith RF. Preliminary studies on the characterization and distribution of Staphylococcus and Micrococcus species on animal skin. Appl Environ Microbiol. 1976. January;31(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linden C, Alm A. The effect on intraocular pressure of latanoprost once or four times daily. British Journal of Ophthalmology. 2001. October;85(10):1163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden C, Alm A. Effects on intraocular pressure and aqueous flow of various dose regimens of latanoprost in human eyes. Acta Ophthalmologica Scandinavica. 1997. August;75(4):412–5. [DOI] [PubMed] [Google Scholar]
- 24.Nagasubramanian S, Sheth GP, Hitchings RA, Stjernschantz J. Intraocular pressure-reducing effect of PhXA41 in ocular hypertension. Comparison of dose regimens. OPHTHA. 1993. September 1;100(9):1305–11. [DOI] [PubMed] [Google Scholar]
- 25.Alm A, Villumsen J, Törnquist P, Mandahl A, Airaksinen J, Tuulonen A, et al. Intraocular pressure-reducing effect of PhXA41 in patients with increased eye pressure: a one-month study. Discussion. Ophthalmology. 1993;100(9):1312–7. [DOI] [PubMed] [Google Scholar]
- 26.Gammon JA, Boothe RG, Chandler CV, Tigges M, Wilson JR. Extended-wear soft contact lenses for vision studies in monkeys. Invest Ophthalmol Vis Sci. 1985. November;26(11):1636–9. [PubMed] [Google Scholar]


