Abstract
Background/Purpose
To compare the rate of retroprosthetic membrane (RPM) formation in Boston Keratoprosthesis (BKPro) with polymethyl methacrylate (PMMA) versus titanium backplates.
Design
Retrospective comparative chart review.
Methods
Multicenter study population: a total of 78 eyes with keratoprosthesis implants with either PMMA or titanium backplates were included in the study. To be included in the study, all subjects had to have completed a minimum of 6-month follow-up period. Incidence of RPM development at 6-month postoperative period was noted across the study population. PMMA and titanium backplates were then compared by their rate of association with subsequent RPM.
Results
Twenty-three out of 55 eyes (41.8%) with PMMA backplates and three out of 23 eyes (13.0%) with titanium backplates had developed an RPM at 6 months after implantation. The titanium backplates were associated with significantly less RPM formation than PMMA backplates (p=0.014, Chi-square test).
Conclusions
Titanium seems to be associated with less RPM formation than PMMA when used as a material for the BKPro back plate.
Keywords: Boston Keratoprosthesis, Keratoprosthesis, KPro, BKPro, Retroprosthetic membrane, RPM, PMMA, Titanium
Introduction
The Boston Keratoprosthesis (BKPro) offers a viable solution for potential corneal transplant candidates who are at high risk for graft failures such as those with a prior history of graft failure or severe neurotrophic diseases. The device is shaped like a collar button, modified from previous designs [1]. Until recently, the BKPro has been manufactured exclusively of medical-grade polymethyl methacrylate (PMMA). This material was introduced to the budding field of keratoprosthesis some 60 years ago and results have been satisfactory without overt toxicity [2].
In the constant search for improvements, it can be questioned whether materials other than PMMA might be superior. The stem of the device must obviously still be made from a transparent material and likewise the front plate, to allow for inspection of the central carrier cornea. The back plate, on the other hand, which presents a large surface area facing the anterior chamber, does not need to be transparent. Here, among several possibilities, titanium comes to mind as an alternative, considering its widespread successful use in joint replacements, tooth transplants, pacemakers, etc. Medical-grade titanium alloys have a significantly higher strength-to-weight ratio than stainless steel. The selection of titanium as a biomaterial for most biological implants is determined by a combination of several factors including its high resistance to corrosion, bio-inertness, ductility, lightness (4.5 g/cm3) and strength [3, 4]. The mechanical and chemical properties of titanium alloys combine to provide implants that are highly damage-tolerant [3, 4]. Applying this concept further to the human eye, where the natural anatomy limits the shape and allowable volume of implants, titanium might be a suitable implant material as it has proven high tissue tolerance and it can be easily machined to thin, flexible plates still with extraordinary strength.
Titanium is not new to the keratoprosthesis field. Russian surgeons have used a titanium plate or prongs for instrastromal keratoprosthesis positioning in patients [5]. Also, in a more recent rabbit study from Finland, instrastromal haptics of the same material was employed [6]. A recent in vitro study compared the tolerance of corneal epithelial cells to PMMA versus titanium in tissue culture. There was significantly increased cell proliferation and decreased cell death when the corneal cells were kept in contact with titanium as compared to PMMA [7].
In this retrospective study, we compare tissue reaction to PMMA backplates with titanium backplates in vivo in patients. A reliable measure of the degree of postoperative inflammation is the formation of retroprosthesis membrane (RPM) [1]. They can become dense and vision-impairing, often requiring opening with a YAG laser or by surgery. Here, PMMA and titanium back plates have been compared by their rate of triggering an RPM. These data have been presented at various scientific meetings [8].
Materials and methods
Two types of PMMA backplates were used. An earlier version of the BKPro had a PMMA back plate that was screwed on to the stem that had screw threads [1]. It was demonstrated that the torsional forces associated with the screw placement caused damage to the posterior graft. It has been hypothesized that this damage could be associated with an increased inflammatory response and the formation of an RPM [9]. Therefore, a modification was introduced where the screw threads were eliminated and the back plate was simply pressed down over the stem and a titanium locking ring was snapped into a groove behind the back plate in order to lock it into place [9]. This snap-on design is currently used for all of the backplates.
Titanium backplates are manufactured using medical-grade titanium. They are depicted in Fig. 1. The diameter of the central opening is 3.35 mm while the overall diameter is 8.5 mm; both of these dimensions are similar to PMMA backplates. Like the PMMA back plate, it has 16 holes, each hole being 1.8 mm in diameter. The titanium backplates can be machined very thin, the thickness at the edge being only 0.25 mm. The PMMA backplates, on the other hand, have an edge thickness of 0.9 mm. The titanium backplates have the same snap-on configuration as the later version of the PMMA backplates. Standard method for preparation of the donor graft-BKPro assembly was used in all cases and has been well described [10, 11].
Fig. 1.
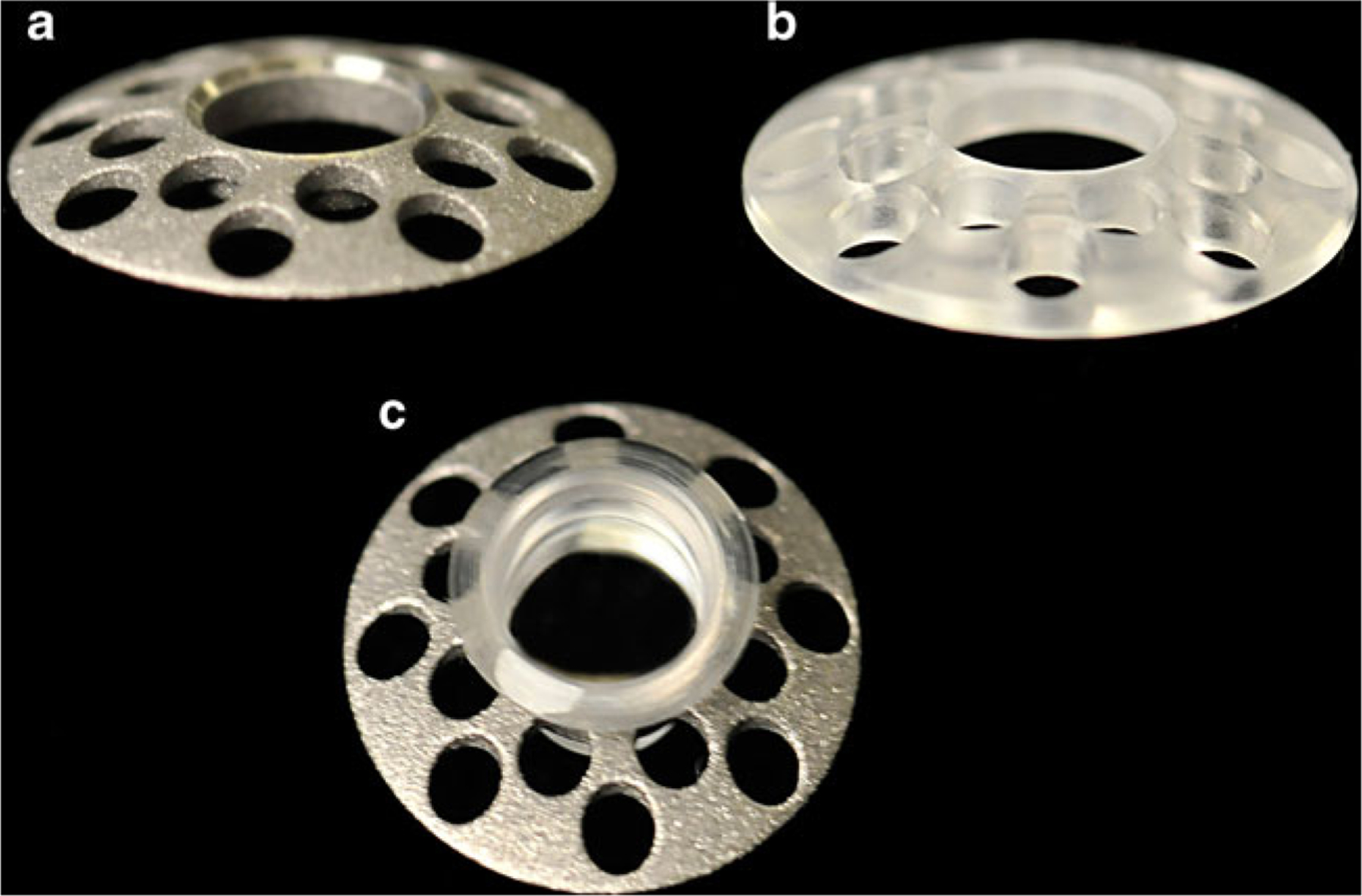
a Titanium back plate. b PMMA back plate. c Assembled Boston Keratoprosthesis with titanium back plate (corneal graft not included)
To be included in the study, patients needed to have completed a minimum of 6 months of follow-up. We have observed that most cases of RPM develop within the first 6 months postoperatively. A recent study by Chew et al. [12] also noted that mean time for RPM formation was 104 days (SD 77 days). Moreover, because of the limited number of eyes in the titanium group with follow-up beyond 6 months, we selected 6 months as an end-point and compared the rate of RPM formation between the groups over this time period.
The underlying pathology, for which the corneal transplant was indicated, was categorized as belonging to one of the three disease categories: autoimmune (ocular cicatricial pemphigoid, Stevens-Johnson, etc), chemical burns (acid and alkaline burns) and others (trauma, bullous keratopathy, infections such as herpes simplex, zoster, post-bacterial ulcers, aniridia, graft failures, etc.). The incidence of RPM formation at 6 months was noted in each of the different subgroups (Table 1). A Chi-square test was performed to detect any difference between the groups with respect to the rate of triggering an RPM.
Table 1.
Retroprosthetic membrane (RPM) formation with PMMA or titanium back plate at 6-month follow-up
| Disease category | Total cases | Total number of RPM |
|---|---|---|
| Group 1: Threaded PMMA | ||
| - Autoimmune | 6 | 2 (33.3%) |
| - Chemical burns | 8 | 5 (62.5%) |
| - Others | 25 | 11 (44.0%) |
| Overall: | 39 | 18 (46.1%) |
| Group 2: Threadless PMMA | ||
| - Autoimmune | 3 | 2 (66.6%) |
| - Chemical burns | 2 | 1 (50.0%) |
| - Others | 11 | 2 (18.1%) |
| Overall: | 16 | 5 (31.2%) |
| Group 3: Threadless titanium | ||
| - Autoimmune | 3 | 1 (33.3%) |
| - Chemical burns | 1 | 1 (100%) |
| - Others | 19 | 1 (5.2%) |
| Overall: | 23 | 3 (13.0%) |
A total number of 78 eyes of 73 patients (42 male, 31 female) were enrolled in the study (Table 1). This represented consecutive cases of three surgeons (CHD, KAC, and MWB) meeting the inclusion criteria. All surgeries were performed between 2001 and 2009. The average age of the subjects was 58.39 years (range 17–94 years). As all the patients had completed a minimum of 6-month follow-up, the rate of RPM formation at the 6-month period was evaluated. The study population was divided into three groups. Group 1 (n=39) consisted of the older threaded PMMA back plates. Most of these surgeries were performed between 2001 and 2003. Group 2 (n=16) consisted of the newer threadless PMMA back plates. All surgeries in this group were performed between 2003 and 2009. Group 3 (n=23) consisted of threadless titanium back plates. All the surgeries with titanium back plates were performed between 2005 and 2009, with a larger proportion of cases being done towards the end of this time period. Each of these groups was further divided into the three disease categories mentioned above.
Results
Table 1 summarizes the rate of RPM formation in each of the three back plate groups and disease categories. The PMMA material (groups 1 and 2 combined) was associated with significantly more RPM than titanium (group 3) (p=0.014, Chi-square test). Among the PMMA groups, although group 1 was associated with more RPM than group 2, this difference did not achieve statistical significance (p=0.309, Chi-square test).
Of the three cases of RPM in the titanium group, one occurred in an autoimmune patient and one in a chemical burn. Both of these conditions are known to have increased propensity for developing post-operative inflammation. In this group, only one in 19 eyes in the non-autoimmune and non-chemical burn category developed an RPM.
Discussion
The BKPro with titanium back plate (Fig. 1) was introduced by CHD at the Massachusetts Eye and Ear Infirmary, Boston, in 2005. Since then, a total of 145 such devices have been implanted. The overall clinical impression has been favorable: it has been the unanimous opinion of all collaborators that titanium seems to cause less postoperative reaction than PMMA (Fig. 2). Only three devices had to be replaced, all of which were implanted in autoimmune patients.
Fig. 2.
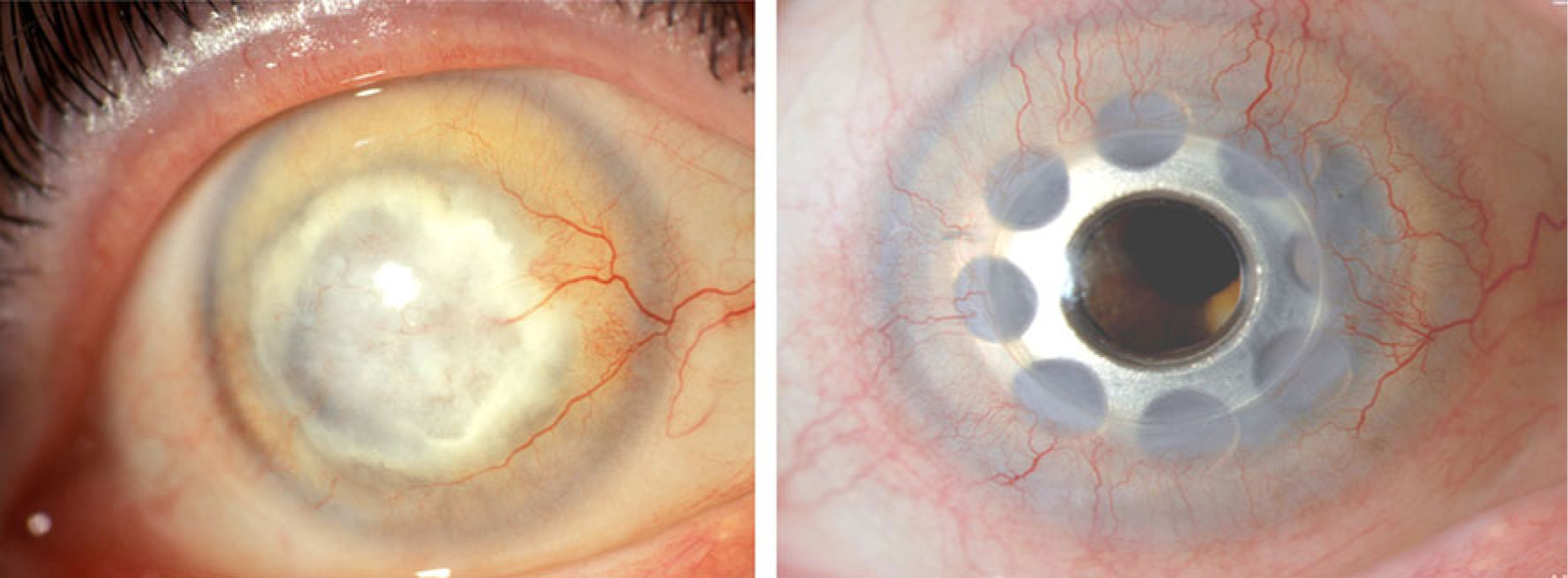
Pre- and post-operative (3 years) appearance after Boston Keratoprosthesis with a titanium back plate
One of the potential disadvantages with the titanium back plate is its cosmetic appearance. In our experience, this has not been a major concern to patients. Sandblasting, to a large extent, minimizes the metallic sheen and darkens the appearance of the metal. For those patients unhappy with the appearance of their eye after surgery, a colored or painted contact lens results in an excellent cosmetic appearance (Fig. 3).
Fig. 3.
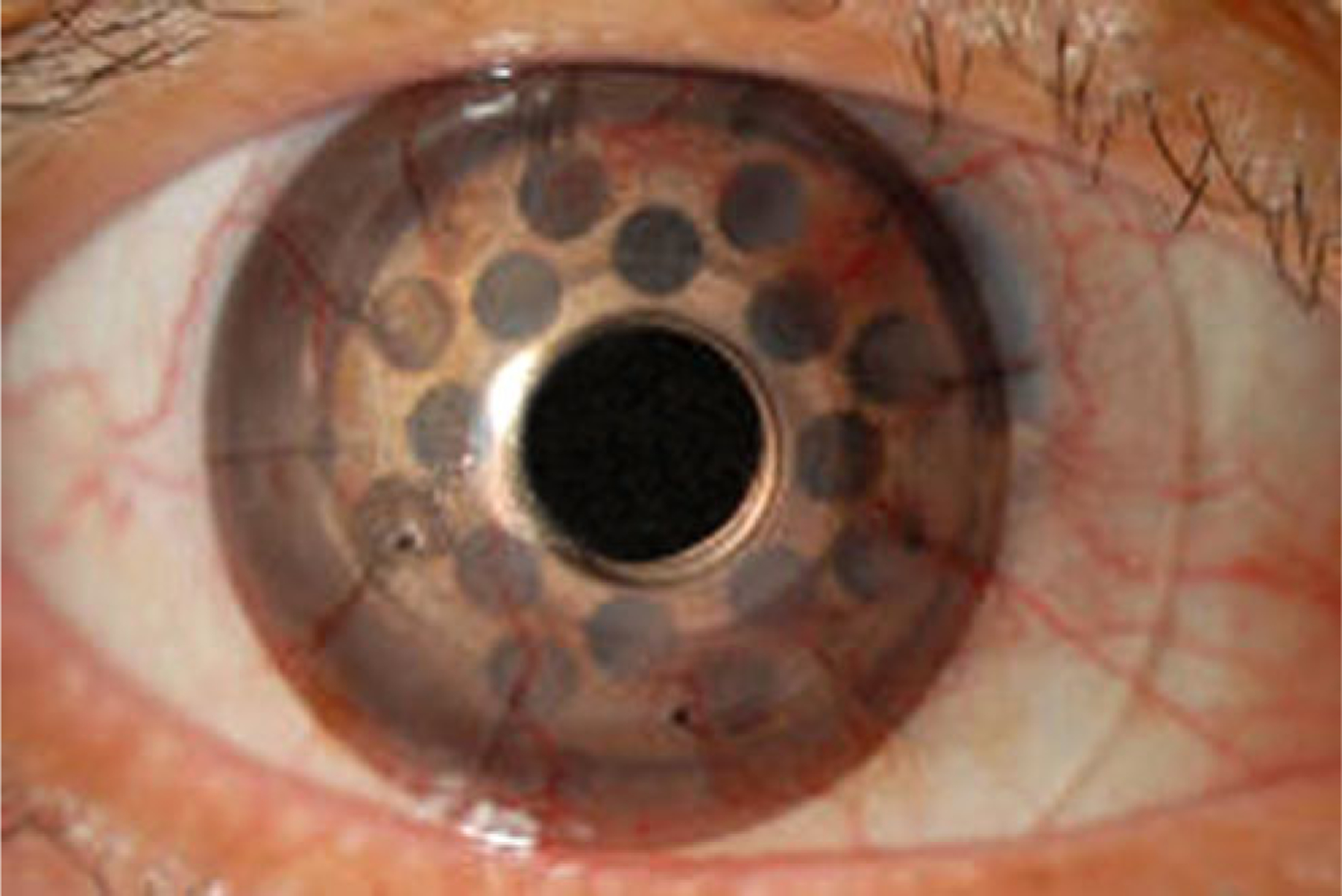
Appearance of an eye with the Boston Keratoprosthesis after fitting with a tinted contact lens. The tinted lens can improve cosmesis and reduce glare
The question has arisen whether patients with titanium back plates could be subjected to standard magnetic resonance imaging of the head. However, titanium is non-magnetic and several of our patients have had MRI without incident. Additionally, patients who have had this implant have not reported having any problems with inducing false security alarms from metal detectors at airports or other such locations.
The only other disadvantage of the titanium back plate is its relatively higher production cost. While little can be done to reduce the cost of the metal itself, the manufacturing cost can be substantially reduced by creating the backplates in bulk through a fully automated computerized lathe rather than thorough the process of individual manual cutting and polishing.
Given the overall favorable clinical impression and encouraging clinical and laboratory results, titanium appears to be a promising material for the keratoprosthesis backplate. The titanium back plate, however, has not yet (November 2010) been approved by the FDA for marketing.
Acknowledgement
Study was supported by the Keratoprosthesis Fund, Massachusetts Eye and Ear Infirmary.
Footnotes
Conflicts of interest All authors are full time employees of their listed institutes and otherwise have no financial interest in any companies or products mentioned in the article. Study approved by Institutional Review Board Committees of the individual institutions. Performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Presented in part at the European Association for Vision and Eye Research Conference 2009, Slovenia.
Electronic supplementary material The online version of this article (doi:10.1007/s00417-011-1684-y) contains supplementary material, which is available to authorized users.
Contributor Information
Michael W. Belin, Department of Ophthalmology & Vision Science, University of Arizona, 4232 West Summer Ranch Place, Marana, AZ 85658, USA
James V. Aquavella, Eye Institute, University of Rochester, 210 Crittenden Blvd, Rochester, NY 14642, USA
References
- 1.Dohlman CH, Harissi-Dagher M, Khan BF, Sippel KC, Aquavella JV, Graney JM (2006) Introduction to the use of the Boston Keratoprosthesis. Expert Rev Ophthalmol 1:41–48 [Google Scholar]
- 2.Stone W Jr, Herbert E (1953) Experimental study of plastic material as replacement for the cornea. Am J Ophthalmol 36:168–173 [DOI] [PubMed] [Google Scholar]
- 3.Wong JY, Bronzino JD (2007) Biomaterials. CRC Press, Boca Raton [Google Scholar]
- 4.Niinomi M (2008) Metallic biomaterials. J Artif Organs 11:105–110 [DOI] [PubMed] [Google Scholar]
- 5.Moroz ZI (1987) Artificial cornea. In: Fyodorov SN (ed) Microsurgery of the eye: main aspect. Mir, Moscow, p 27 [Google Scholar]
- 6.Linnola RJ, Happonen AP, Andersson OH, Vedel E, Yli-Urpa AU, Krause U, Laatikainen L (1996) Titanium and bioactive glass-ceramic coated titanium as materials for keratoprosthesis. Exp Eye Res 63:471–478 [DOI] [PubMed] [Google Scholar]
- 7.Ament JD, Spurr-Michaud S, Dohlman CH, Gipson IK (2009) The Boston Keratoprosthesis: comparing corneal cell compatibility with titanium and PMMA back plates. Cornea 28:808–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohlman CH, Todani A, Ament JD, Chodosh J, Ciolino JB, Colby KA, Pineda R, Belin MW, Aquavella JV, Graney J (2009) Titanium vs PMMA back plates for Boston keratoprosthesis: Incidence of retroprosthetic membrane. IOVS; ARVO poster #1505 [Google Scholar]
- 9.Dohlman CH, Harissi-Dagher M, Graney JM (2007) The Boston Keratoprosthesis: a new threadless design. D J Ophthalmol 13:3 [Google Scholar]
- 10.Todani A, Gupta P, Colby K (2009) Type I Boston Keratoprosthesis with cataract extraction and intraocular lens placement for visual rehabilitation of herpes zoster ophthalmicus: the “KPro Triple”. Br J Ophthalmol 93:119. [DOI] [PubMed] [Google Scholar]
- 11.Dohlman CH, Nouri M (2005) Keratoprosthesis surgery. In: Foster CS, Azar D, Dohlman CH (eds) Smolin and Thoft’s the cornea, 4th edn. Lippincott Williams and Wilkins, Philadelphia, pp 1085–1095 [Google Scholar]
- 12.Chew HF, Ayres BD, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS, Jin YP, Cohen EJ (2009) Boston Keratoprosthesis outcomes and complications. Cornea 28:989–996 [DOI] [PubMed] [Google Scholar]


