Abstract
Glaucoma is the leading cause of irreversible blindness worldwide. The perspective of clinicians who treat the disease is important and may ultimately dictate the adoption of new treatment modalities, such as drug-eluting contact lenses. Recent advances have enabled contact lenses to serve as a sustained-release drug-delivery platform capable of treating glaucoma. This review covers the medical treatment of glaucoma, suboptimal adherence rates to treatment, and factors that may influence the clinical applicability of drug-eluting contact lenses. Ophthalmologists who treat glaucoma were surveyed to determine their perspective on treatment adherence, bandage contact lens use and the use of a drug-eluting contact lens to treat glaucoma. Given the challenge of treating glaucoma and the clinical need for improved drug delivery, drug-eluting contact lenses appear to be a promising treatment option.
Glaucoma is the leading cause of irreversible blindness worldwide, affecting over 60 million people [1]. Glaucoma is a group of progressive optic neuropathies characterized by slow progressive degeneration of retinal ganglion cells, resulting in a distinct appearance of the optic disc and a concomitant pattern of visual field loss. Although the pathogenesis of the disease is not fully understood, intraocular pressure (IOP) is still recognized as a major risk factor for glaucoma [2]. It is well established that lowering IOP slows the progression of glaucomatous optic neuropathy [3] and consistency in lowering the IOP has been reported to minimize the risk of progressive visual field loss [4].
Glaucoma therapy & adherence
Topical medications, or eye drops, are the mainstay of medical glaucoma therapy, which primarily aims to lower IOP. Unfortunately, eye drops are an inefficient delivery system and the eye absorbs only 1–7% of the medication in solution [5]. The remaining solution washes down the nasolacrimal duct, is absorbed by periocular tissues or spills onto the cheek. The excess solution contributes to side effects, such as symptomatic ocular surface toxicity, conjunctiva hyperemia, hyperpigmentation of the periocular skin and periocular fat atrophy [6–8].
Adherence with glaucoma eye drops is suboptimal. The Travatan Dosing Aid Study [9] reported that 44% of the participants took less than 75% of intended doses, despite the fact that they knew they were using a bottle equipped with an electronic monitoring device. Similarly, a study using pharmacy records demonstrated that 24% of patients admitted to omitting eye drops either occasionally or frequently and 51% were found to have had insufficient drops dispensed to comply with treatment as prescribed [10]. Overall, persistence rates have been found to be less than 50% after 1 year of starting treatment (Figure 1) [11].
Figure 1. Percentage of glaucoma patients remaining on therapy after treatment initiation.
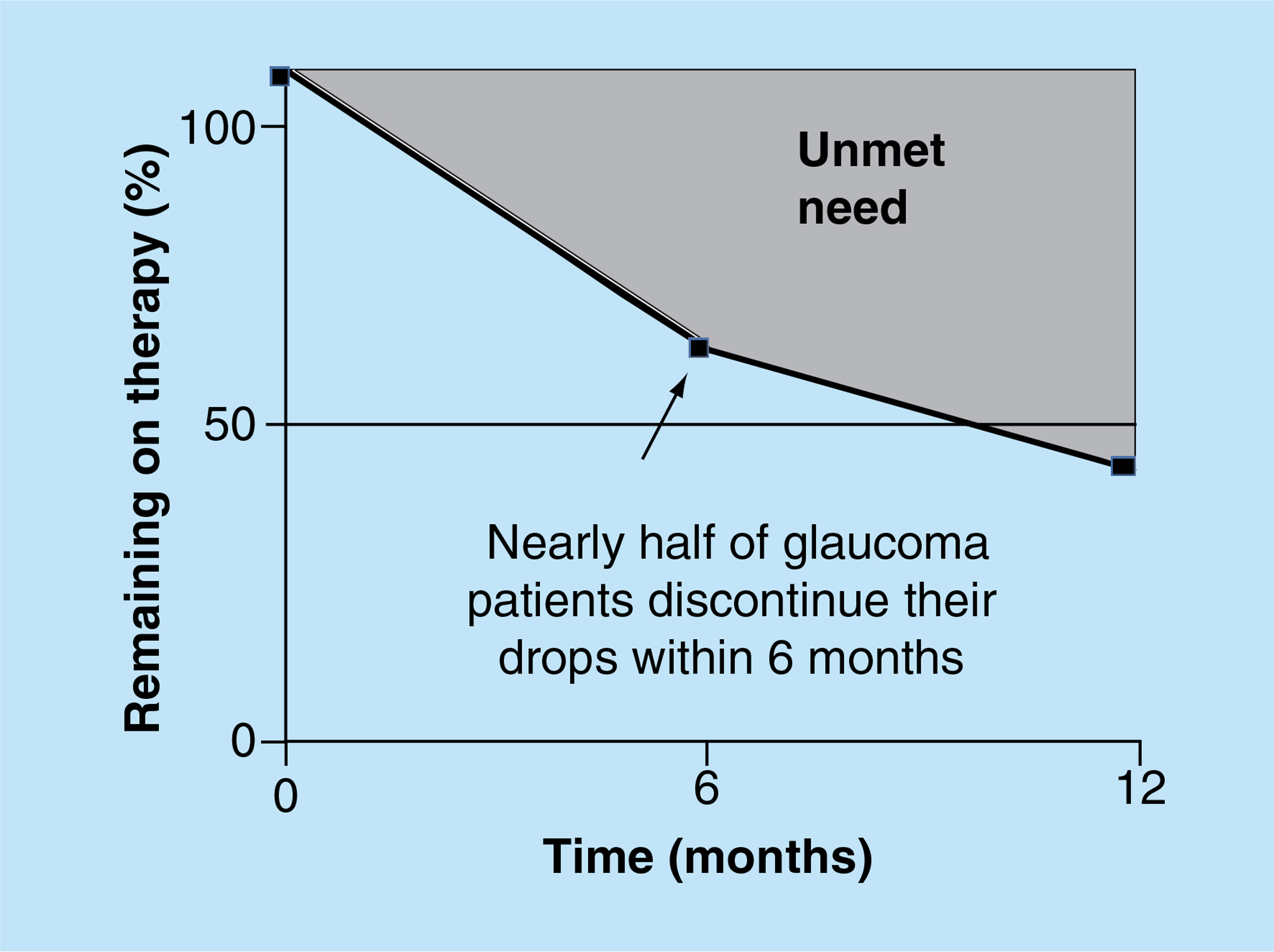
Data taken from [11].
There are many factors that likely affect the poor adherence. From the patients’ perspective, the disincentives associated with the use of glaucoma drops may outweigh the incentives for adherence. Eye drops frequently sting, burn and cause a transient blurring of vision upon application [12]. Dosing regimens can become complex, costly, and taxing when more than one medication is prescribed. Since glaucoma initially has a protracted asymptomatic phase when patients are unaware of their visual dysfunction [1], patients lack an organic form of positive feedback that motivates adherence [13,14].
Adherence with topical therapy is also dependent on proper drop administration. Glaucoma is typically associated with advanced age and patients often have low visual acuity and other physical limitations, such as poor manual dexterity due to rheumatoid disease [15]. Reduced skill in eye drop instillation may lead to underdosing or overdosing, which can lead to increased local side effects. Overdosing can also lead to a treatment gap since using more than one drop at a time can lead to a more rapid use of fluid in the bottle, which is typically dispensed monthly with limited volume. Only 21% of glaucoma patients properly instill their eye drops [16] and 29% miss their eye drops entirely [17]. When considering all these factors, it is not surprising that 38% of glaucoma patients have someone else administering their medication [18].
Unfortunately, physician estimation of patients’ adherence is an inaccurate reflection of true behavior [9], and nonadherence could be misinterpreted as nonefficacy of medication. This obstacle is exacerbated by ‘white-coat’ adherence, or patients who take their drops only during the week prior to eye exams [11]. Electronic monitoring devices show that patient adherence is significantly higher (p < 0.0001) the day prior to their eye exam [19]. In these instances, IOP may be temporarily controlled in the doctor’s office, but evidence of glaucoma progression may result in additional medications and/or surgical procedures. Therefore, a major unmet need in glaucoma treatment is a method of drug delivery that improves patient adherence and simplifies the treatment decision process for practitioners [1,20].
Sustained drug delivery for glaucoma treatment
The idea of sustained-release drug delivery for the treatment of glaucoma dates back over four decades [21], and studies have demonstrated the applicability of innovative sustained-release drug-delivery systems, such as fornix inserts, punctal plugs (tear duct plugs) and contact lenses.
Using a polymeric membrane system, Ocusert (Alza Corporation, CA, USA) was a device introduced in 1975 and was inserted in the inferior fornix – also known as the inferior ocular cul-de-sac – which is positioned between the lower lid and the eyeball. The Ocusert released a therapeutic amount of pilocarpine for 7 days, but was never widely adapted despite the fact that manual dosing may require application four times per day. Its limited use has been attributed to factors such as discomfort, awareness of device movement and lack of device retention. With the introduction of newer classes of glaucoma medications, the use of the device decreased considerably [22]. Analogous to the Ocusert, other fornix-based inserts have been used for glaucoma drug delivery. A fornix insert formed from chitosan, a natural biodegradable polymer, was loaded with bimatoprost and released the drug in a sustained manner for over a month. Over the same duration, it demonstrated the ability to lower IOP in glaucomatous eyes of rats [23]. Another fornix insert composed of a nondegradable polyhydroxyl ethyl methacrylate matrix incorporated with timolol-based nanoparticles demonstrated in vitro release for over a month [24].
Punctal plugs are conventionally used to block the drainage of tears for the treatment of aqueous deficient dry eye disease, but have also been used experimentally to deliver glaucoma drugs, such as latanoprost [25] and timolol [26]. Research on the efficacy of punctal plug delivery systems has advanced to human studies that were initiated as early as 2008. To date, results from these studies have not been reported within the peer-reviewed scientific literature.
Drug-eluting contact lenses
The concept of drug-eluting contact lenses dates back to the 1960s [27]; however, sustained and controlled delivery has historically been a significant challenge [28]. Drug-eluting contact lenses could improve patient adherence by providing a simplified monthly treatment regimen; reducing side effects related to drop overflow and preservatives; and by serving as a treatment reminder through the ability to correct refractive errors, which are present in the majority of glaucoma patients [29]. Sustained-release drug-eluting contact lenses may be especially attractive for the 38% of glaucoma patients who require assistance placing their eye drops [18]. Similar to drops, drug-eluting contact lenses could be inserted by family members or caregivers who are not health care providers; this differentiates drug-eluting contact lenses from other proposed sustained-release technologies, such as punctal plugs or intraocular implants. Moreover, advances in contact lens technology, such as the introduction of silicone hydrogels, has enabled 30-day continuous wear and this feature may be particularly important when designing a contact lens intended for prolonged and sustained drug release.
Commercial contact lenses can be used to absorb and release medications, but the release rate is rapid and little drug is eluted after the first several hours [27,30]. To extend the duration of drug release, novel contact lens designs have been developed. For example, molecularly-imprinted cavities, nanoparticles, microparticles, drug–polymer films, vitamin E barriers, liposomes, b-cyclodextrins and ionic polymers have been incorporated into contact lenses and have demonstrated varying drug release profiles within in vitro studies [28,30–33]. While there are relatively few in vivo studies showing sustained drug release from contact lenses [34–37], the studies that have been published explored the use of contact lenses for the delivery of glaucoma agents. In the eyes of rabbits, single latanoprost-eluting contact lenses maintained, for 1 month, intraocular drug concentrations that were comparable to those achieved by the daily application of topical latanoprost solution [34]. In the eyes of glaucomatous beagles, timolol has been released from contact lenses loaded with nanoparticles [35] or vitamin E [30,36,37].
Clearly, safety and efficacy will need to be demonstrated in human studies before drug-eluting contact lenses are available for patient use. However, the long-term success of drug-eluting contact lenses may ultimately be determined by practitioners and their adoption of the technology as treatment for ophthalmic diseases.
Practitioners’ thoughts on drug-eluting contact lenses to treat glaucoma
A study published in 2004 found that 93% of eye care practitioners would be interested in using a contact lens to deliver therapeutics if such a device was available [38]. While the study found that providers were enthusiastic about the concept of contact lens drug delivery, it did not explore the practitioners’ views on using contact lenses to treat specific ocular conditions, such as glaucoma. Another unanswered question was whether practitioners who treat glaucoma also use contact lenses, such as bandage contact lenses, which are typically used for therapeutic purposes and lack the ability for refractive correction. This is an important question since eye care providers may be more likely to adapt to a new drug-delivery technology if it involves using a device that is already part of their practice pattern. Since researchers have explored the possibility of using drug-eluting contact lenses to treat glaucoma [30], a survey was developed to answer these questions and others related to providers’ perspective on glaucoma adherence, bandage contact lens use, and the potential use of a drug-eluting contact lens to treat glaucoma.
A voluntary one-page survey (Table 1) was distributed to ophthalmologists who stated that they treat glaucoma, during glaucoma symposiums at the 2014 American Society of Cataract and Refractive Surgery (ASCRS) meeting. Questions concerned practitioners’ perspectives on glaucoma adherence, bandage contact lens use and the use of a drug-eluting contact lens to treat glaucoma. In total, 100 of the surveys were completed, the results were evaluated and a Chi-square test was carried out to compare the answers from glaucoma surgeons and nonsurgeons.
Table 1.
Survey responses from ophthalmologists that treat glaucoma.
| Yes (%) | No (%) | |
|---|---|---|
| Do you perform glaucoma filtration surgery? (n = 100) | 72 | 28 |
| Do you use bandage contact lenses? (n = 100) | 79 | 21 |
| If used, are bandage (scleral) contact lenses worn overnight? (n = 86) | 94.2 | 5.8 |
| If used, are bandage contact lenses well tolerated by your patients? (n = 83) | 95.2 | 4.8 |
| Do you think that glaucoma progression can be slowed or prevented by improving patient adherence? (n = 99) | 98 | 2 |
| When treating glaucoma patients, are you confronted with the challenge of differentiating the efficacy of topical therapy (eye drops) from lack of adherence? (n = 100) | 96 | 4 |
| If available, would you consider using a sustained-release drug-eluting contact lens to help differentiate the efficacy of topical medication (eye drop) from lack of adherence? (n = 99) | 94.9 | 5.1 |
| If available, would you consider using a sustained-release drug-eluting contact lens as a glaucoma treatment modality? (n = 99) | 88.9 | 11.1 |
| Do you think that patient adherence could be improved by prescribing a drug-eluting contact lens that has the ability to also correct refractive errors? (n = 99) | 87.9 | 12.1 |
Most of the respondents reported that they perform glaucoma filtration surgery, which suggests that they treat advanced forms of glaucoma that have failed medical therapy. Not surprisingly, the survey results indicate that the eye care providers believe glaucoma therapy can be improved through better patient adherence to the prescribed treatment regimen. When asked about the use of bandage contact lenses, the vast majority of practitioners responded that they use the devices in their practice and that the lenses are worn overnight. While most conventional contact lenses are worn instead of glasses to correct refractive errors, bandage contact lenses are typically used to promote cornea healing [39] or as an adjunctive therapy following glaucoma filtration surgery [40]. The results of the survey indicate that contact lenses are already part of the clinical practice of ophthalmologists who treat glaucoma and that the lenses are well tolerated by their patients.
If a sustained-release drug-eluting contact lens was available, the overwhelming majority of the surveyed ophthalmologists would consider using it to treat glaucoma or to differentiate the efficacy of a topical medication from lack of adherence (e.g., patients not taking glaucoma drops as prescribed). Because a drug-eluting contact lens could potentially delivery a glaucoma medication to the eye for a duration as long as 1 month, the survey asked practitioners to determine the wear times that they would consider using in their practice. The results indicate that the respondents would use, with nearly an equivalent preference, a drug-eluting contact lens continuously for 1 month, 2 weeks, 1 week or would recommend daily lens removal (Figure 2A). Nearly 80% of the respondents would use a drug-eluting contact lens continuously for 1 week or longer (Figure 2B). Interestingly, ophthalmologists who perform glaucoma filtration surgery were less likely to use a daily-wear lens than ophthalmologists who do not perform glaucoma filtration surgery (p<0.05, Chi-square test) (Figure 2B). A limitation of this study is that only ophthalmologists were surveyed. Future studies could potentially also survey optometrists who treat glaucoma, who may be more likely to use refractive contact lenses and less likely to use bandage contact lenses because of the patient populations that they typically assist.
Figure 2. (A) Ophthalmologist preference for the duration of release for a drug-eluting contact lens to treat glaucoma. (B) 80% of ophthalmologists would use a drug-eluting contact lens for a duration of 1 week or longer.
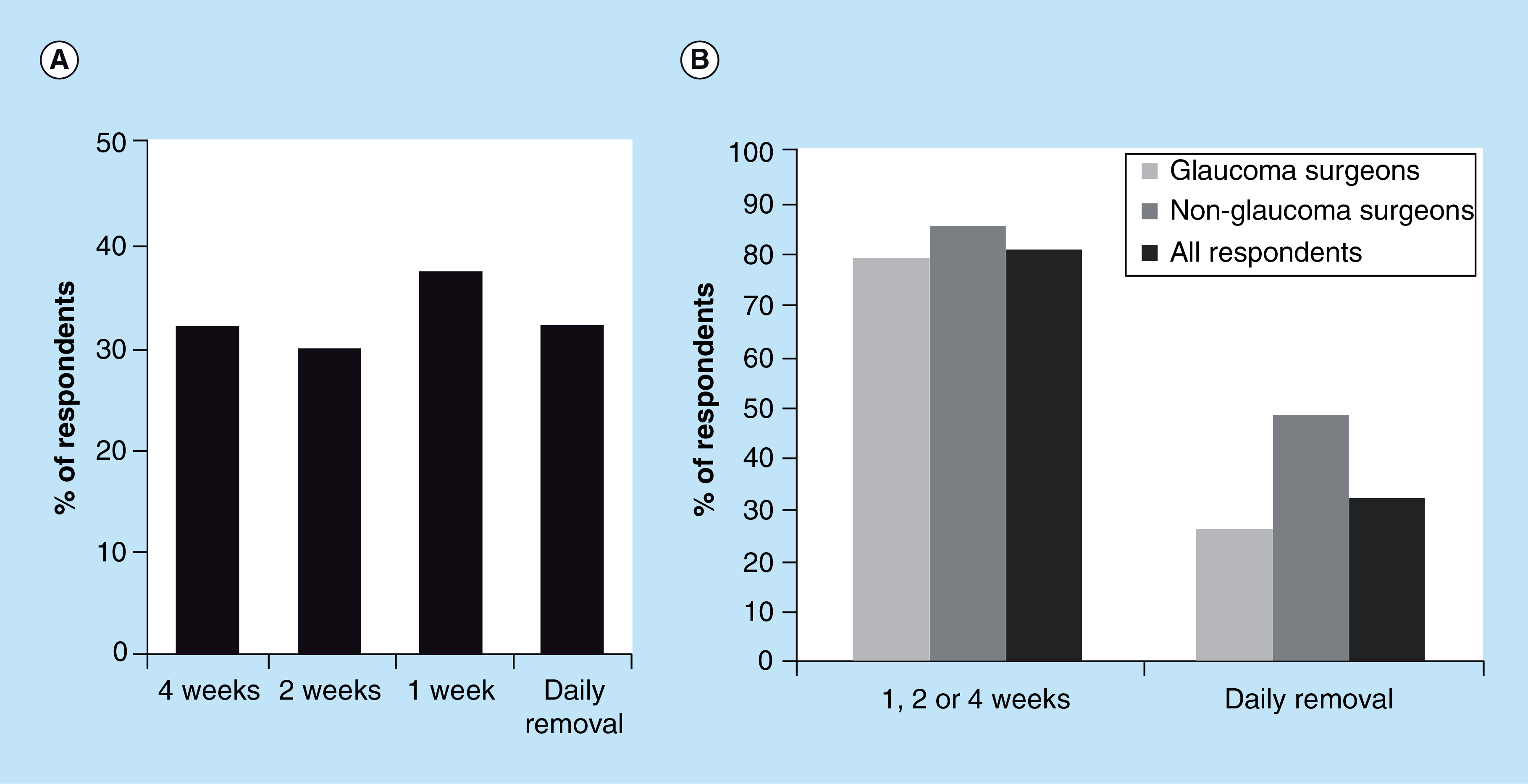
Ophthalmologists who do not perform glaucoma filtration surgery are more likely to use a drug-eluting contact lens that is removed daily (p < 0.05).
Expert opinion
A major unmet need for the treatment of glaucoma is a method of sustained drug delivery that would improve adherence with medical therapy. Drug-eluting contact lenses could achieve this goal by facilitating topical administration, by reducing dosing frequency from daily to as infrequent as monthly, and by minimizing the side effects associated with glaucoma drops. When surveyed, the vast majority of ophthalmologists who treat glaucoma use bandage contact lenses; they reported that the devices are well tolerated by their patients, and would consider using a drug-eluting contact lens for the treatment of glaucoma if such a device were available.
Future perspective
Currently, topical ophthalmic drug delivery is limited to eye drops and gels. In the future, there may be a wide range of drug delivery options that will personalize glaucoma therapy for each patient. A drug-eluting contact lens, containing a refractive correction, is one potential option that may encourage better patient compliance and reduce glaucomatous vision loss. Achieving regulatory approval is one of the most challenging obstacles for any new medical treatment. Over the next 5–10 years a drug-eluting contact lens could meet this challenge and become available for patient care. During the next decade it is also conceivable that drug-eluting contact lenses could potentially be used to deliver medications that are difficult to deliver by drops and may replace the need for some ocular injections. Drug delivery to the back of the eye is a question that needs future investigation.
Executive summary.
Glaucoma
Glaucoma is a group of progressive optic neuropathies characterized by slow progressive degeneration of retinal ganglion cells, resulting in a distinct appearance of the optic disc and a concomitant pattern of visual field loss.
Consistency in lowering the intraocular pressure has been reported to minimize the risk of progressive visual field loss.
Glaucoma therapy & adherence
Topical medications, or eye drops, are the mainstay of medical glaucoma therapy, which primarily aims to lower intraocular pressure.
Adherence with glaucoma eye drops is suboptimal.
A major unmet need in glaucoma treatment is a method of drug delivery that improves patient adherence and simplifies the treatment decision process for practitioners.
Sustained drug delivery for glaucoma treatment.
Recent studies have demonstrated the applicability of innovative sustained-release drug-delivery systems, such as fornix inserts, punctal plugs (tear duct plugs) and contact lenses.
Drug-eluting contact lenses
Drug-eluting contact lenses could improve patient adherence by providing a simplified monthly treatment regimen; reducing side effects related to drop overflow and preservatives; and by serving as a treatment reminder through the ability to correct refractive errors.
Recent in vivo studies have explored the use of contact lenses for the delivery of glaucoma agents.
Clearly, safety and efficacy will need to be demonstrated in human studies before drug-eluting contact lenses are available for patient use.
Practitioners’ thoughts on drug-eluting contact lenses to treat glaucoma.
Contact lenses are already part of the clinical practice of ophthalmologists who treat glaucoma and they are well tolerated by their patients.
The overwhelming majority of the surveyed ophthalmologists would consider using a sustained-release drug-eluting contact lens to treat glaucoma if it was available.
Expert opinion
Given the challenge of treating glaucoma and the clinical need for improved drug delivery, drug-eluting contact lenses appear to be a promising treatment option.
Future Perspective
Over the next 5–10 years a drug-eluting contact lens could become a medical reality and a therapeutic option to help prevent glaucomatous vision loss.
Financial & competing interests disclosure:
Both DS Kohane & JB Ciolino are listed as investors on a patent ifor a drug-eluting contact lens. Supported by NEI 1K08EY019686–01 (J.B.C.), Massachusetts Lions Eye. Research Fund (J.B.C.), New England Cornea Transplant Fund (J.B.C.), KPro Fund (J.B.C.), and by a Career Development Award from Research to Prevent Blindness, Inc. (J.B.C.). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
No writing assistance was utilized in the production of this manuscript.
References
- 1.Quigley HA. Glaucoma. Lancet 377(9774), 1367–1377 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 363(9422), 1711–1720 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Heijl A, Leske MC, Bengtsson B et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 120(10), 1268–1279 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Musch DC, Gillespie BW, Niziol LM et al. CIGTS Study Group. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 118(9), 1766–1773 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghate D, Edelhauser HF. Barriers to glaucoma drug delivery. J. Glaucoma 17(2), 147–156 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Digiuni M, Fogagnolo P, Rossetti L. A review of the use of latanoprost for glaucoma since its launch. Expert Opin Pharmacother. 13(5), 723–745 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Filippopoulos T, Paula JS, Torun N et al. Periorbital changes associated with topical bimatoprost. Ophthal. Plast. Reconstr. Surg. 24(4), 302–307 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Shah M, Lee G, Lefebvre DR et al. A cross-sectional survey of the association between bilateral topical prostaglandin analogue use and ocular adnexal features. PLoS ONE 8(5), e61638 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okeke CO, Quigley HA, Jampel HD et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology 116(2), 191–199 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Rotchford AP, Murphy KM. Compliance with timolol treatment in glaucoma. Eye (Lond.) 12(Pt 2), 234–236 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv. Ophthalmol. 53(Suppl. 1), S57–S68 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Friedman DS, Hahn SR, Gelb L et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology 115(8), 1320–1327 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Rosentreter A, Jablonski KS, Mellein AC et al. A new rebound tonometer for home monitoring of intraocular pressure. Graefes Arch. Clin. Exp. Ophthalmol. 249(11), 1713–1719 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Hsiao Y-C, Dzau JR, Flemmons MS et al. Home assessment of diurnal intraocular pressure in healthy children using the Icare rebound tonometer. J. AAPOS 16(1), 58–60 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology 116(Suppl.11), S30–S36 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Stone JL, Robin AL, Novack GD et al. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch. Ophthalmol. 127(6), 732–736 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Hennessy AL, Katz J, Covert D et al. A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am. J. Ophthalmol. 152(6), 982–988 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Winfield AJ, Jessiman D, Williams A et al. A study of the causes of non-compliance by patients prescribed eyedrops. Br. J. Ophthalmol. 74(8), 477–480 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kass MA, Meltzer DW, Gordon M. A miniature compliance monitor for eyedrop medication. Arch. Ophthalmol. 102(10), 1550–1554 (1984). [DOI] [PubMed] [Google Scholar]
- 20.Realini T A history of glaucoma pharmacology. Optom. Vis. Sci. 88(1), 36–38 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Macoul KL, Pavan-Langston D. Pilocarpine ocusert system for sustained control of ocular hypertension. Arch. Ophthalmol. 93(8), 587–590 (1975). [DOI] [PubMed] [Google Scholar]
- 22.Knight OJ, Lawrence SD. Sustained drug delivery in glaucoma. Curr. Opin Ophthalmol. 25(2), 112–117 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Franca JR, Foureaux G, Fuscaldi LL et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: in vitro and in vivo evaluation. PLoS ONE 9(4), e95461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung HJ, Chauhan A. Extended release of yimolol from nanoparticle-loaded fornix insert for glaucoma therapy. J. Ocul. Pharmacol. Ther. 29(2), 229–235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mati therapeutics inc. Safety and efficacy of punctum plug delivery system in subjects with open-angle glaucoma or ocular hypertension (CORE). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [2014 Jun 01]. NLM Identifier: NCT00650702http://clinicaltrials.gov/show/NCT00650702 [Google Scholar]
- 26.Mati therapeutics inc. Comparison of latanoprost PPDS with timolol maleate GFS in subjects with ocular hypertension or open-angle glaucoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000-[cited 2014 Jun 01]. Identifier: NCT02014142. http://clinicaltrials.gov/show/NCT02014142 NLM [Google Scholar]
- 27.González-Chomón C, Concheiro A, Alvarez-Lorenzo C. Soft contact lenses for controlled ocular delivery: 50 years in the making. Ther. Deliv. 4(9), 1141–1161 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Ciolino JB, Hoare TR, Iwata NG et al. A drug-eluting contact lens. Invest. Ophthalmol. Vis. Sci. 50(7), 3346–3352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GrØdum K, Heijl A, Bengtsson B. Refractive error and glaucoma. Acta Ophthalmol. Scand. 79(6), 560–566 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Bengani LC, Hsu K-H, Gause S et al. Contact lenses as a platform for ocular drug delivery. Expert Opin. Drug Deliv. 10(11), 1483–1496 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Bengani LC, Chauhan A. Extended delivery of an anionic drug by contact lens loaded with a cationic surfactant. Biomaterials 34(11), 2814–2821 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Gulsen D, Li C-C, Chauhan A. Dispersion of DMPC liposomes in contact lenses for ophthalmic drug delivery. Curr. Eye Res. 30(12), 1071–1080 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Karlgard CCS, Wong NS, Jones LW et al. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int. J. Pharm. 257(1–2), 141–151 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Ciolino JB, Stefanescu CF, Ross AE et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 35(1), 432–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung HJ, Abou-Jaoude M, Carbia BE et al. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 165(1), 82–89 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Peng C-C, Ben-Shlomo A, Mackay EO et al. Drug delivery by contact lens in spontaneously glaucomatous dogs. Curr. Eye Res. 37(3), 204–211 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Peng C-C, Burke MT, Carbia BE et al. Extended drug delivery by contact lenses for glaucoma therapy. J. Control. Release 162(1), 152–158 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Karlgard CCS, Jones LW, Moresoli C. Survey of bandage lens use in North America, October–December 2002. Eye Contact Lens 30(1), 25–30 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Kaufman HE, Gasset AR. Therapeutic soft bandage lenses. Int. Ophthalmol. Clin. 10(2), 379–385 (1970). [PubMed] [Google Scholar]
- 40.Grohe RM, Wyse TB. Fitting contact lenses in eyes with filtering blebs. J. Glaucoma 7(6), 439–445 (1998). [PubMed] [Google Scholar]


