Abstract
Objectives:
To determine the incidence and major drivers of catastrophic costs among TB-affected households in Zimbabwe.
Methods:
We conducted a nationally representative health facility-based survey with random cluster sampling among consecutively enrolled drug susceptible (DS-TB) and drug resistant TB (DR-TB) patients. Costs incurred and income lost due to TB illness were captured using an interviewer administered standardised questionnaire. We used multivariable logistic regression to determine the risk factors for experiencing catastrophic costs.
Results:
A total of 841 patients were enrolled and were weighted to 900 during data analysis. There were 500 (56%) males and 46 (6%) DR-TB patients. Thirty-five (72%) DR-TB patients were HIV co-infected. Overall, 80% (95% CI:77-82) of TB patients and their households experienced catastrophic costs. The major cost drivers pre-TB diagnosis were direct medical costs. Nutritional supplements were the major cost driver post-TB diagnosis, with a median cost US$360 (IQR: 240-600). Post-TB median diagnosis costs were three-times higher among DR-TB (US$1,659 [653-2,787]) versus drug DS-TB affected households (US$537 [204-1,134]). Income loss was five-times higher among DR-TB versus DS-TB patients. In multivariable analysis, household wealth was the only covariate that remained significantly associated with catastrophic costs: the poorest households had sixteen times the odds of incurring catastrophic costs compared to wealthiest households (adjusted odds ratio [aOR:15.7 95% CI:7.5-33.1]).
Conclusion:
The majority of TB-affected households, especially those affected by DR-TB experienced catastrophic costs. Since the major cost drivers fall outside the healthcare system, multi-sectoral approaches to TB control and linking TB patients to social protection may reduce catastrophic costs.
Keywords: patient cost, tuberculosis, financial protection, Social protection, Zimbabwe, universal health coverage
Introduction
In 2019 approximately 10 million people developed tuberculosis (TB) globally and 1.6 million of them died, making TB one of the top ten killer diseases worldwide and the leading cause from a single infectious agent. (1) In the same year, Africa accounted for 25% of the global TB notifications. (1) The prevalence of human immune-deficiency virus (HIV) is high in countries of sub-Saharan Africa (SSA), and many of them have generalised HIV epidemics. Zimbabwe, a low-and middle income country (LMIC) in SSA is among the high TB, TB/HIV and multi-drug resistant TB (MDR-TB) burdened countries. In 2019, Zimbabwe had an estimated TB incidence of 199 per 100,000 population (95% CI: 147–258). The prevalence of MDR-TB/rifampicin resistant TB is around 3.9% among new and 14% among previously treated TB cases. (2,3)
Traditionally TB control has been the responsibility of the health care sector with a focus on TB diagnoses and curative treatment. Until recently, socioeconomic determinants of TB disease have received limited attention. Addressing these determinants has not been an integral part of TB control and would require a multi-sectoral response. The End TB Strategy has encouraged thinking beyond the biomedical model by including one milestone specifically relating to costs (no TB patients or households should experience catastrophic costs by 2020). (4) Total TB-related costs are defined as catastrophic if they exceed 20% of a household’s annual income.
Tuberculosis-related catastrophic costs are a public health challenge requiring urgent attention. (5) Such costs may plunge households into poverty and financial catastrophes. (6) For this reason, countries were encouraged to set baseline measurements of incident catastrophic costs by 2020. The measurements are based on three types of costs: direct medical, direct non-medical and indirect costs such as income loss. Direct medical costs include money spent on consultations, laboratory tests and hospitalisation. Direct non-medical costs are money spent on transport and food during health seeking. Income loss is money foregone by the patient or carers during illness.
Global efforts to ameliorate TB-related catastrophic costs lie in i) provision of free TB treatment ii) decentralisation of TB services to ensure equity of access and iii) advocacy for social protection and universal health coverage by the United Nations, national and international stakeholders. Despite interventions aimed at cushioning TB patients against direct medical costs, surveys in Africa and Asia have shown high incidence of TB-related catastrophic costs especially among i) drug resistant TB (DR-TB) patients, ii) poorest households, iii) cases where the patients were breadwinners and iv) those co-infected with HIV.(7–10)
In 2016, Zimbabwe embraced the End TB targets of eliminating TB-related catastrophic costs by 2020. The national TB control programme (NTP) has decentralised TB services, provided cash transfers to DR-TB patients and adopted active case finding to detect TB cases early. However, the country had no baseline measure of TB-related catastrophic costs or the major drivers of such costs. We therefore aimed to determine among TB-affected households in Zimbabwe, the incidence of catastrophic costs and its risk factors, and the major drivers of costs incurred because of accessing TB-related services.
Methods
Study design
A nationally representative, health facility-based survey with random cluster sampling among TB patients across Zimbabwe.
Setting
Zimbabwe is a southern African country with an estimated population of 14.9 million in 2020. (11) Classified as a low income country, it has suffered from an economic and humanitarian crisis for much of the last decade. Zimbabwe belongs to the 14 countries with a triple-burden of TB, TB/HIV and multi-drug resistant TB. (12)
Study population
Patients of all age groups who were on treatment for drug susceptible TB (DS-TB) or DR-TB for any type of TB (pulmonary, extra-pulmonary and/or disseminated), and who attended their scheduled appointments within the sampled health facilities from 23 July to 31 August 2018 were eligible for inclusion in the study. Patients were recruited consecutively when they attended their scheduled appointments. The study included patients who had been on treatment for at least two weeks in their current treatment phase (intensive or continuation phase). For patients who had been on treatment for <2 weeks at the time the facility was visited, interviews were rescheduled to a time when the patients had been on treatment for at least two weeks. Patients who had completed treatment were ineligible for inclusion in the study. This was to minimise recall bias and to ease logistics during recruitment.
Study procedures
Sample size calculation, sampling and patient enrolment
The sample was based on 26,677 TB patients notified in Zimbabwe in 2016. We assumed an absolute precision of 5% and a priori estimate of 50% for the incidence of households experiencing TB-related catastrophic costs. We used the standard formula for sample size calculation for a cluster sampled TB prevalence survey.(13) After factoring a design effect of 2.0, the sample size was 780 patients across 60 clusters, each contributing 13 patients. The sample size was adjusted to 900 assuming a non-response rate of 10%.
Cluster sampling was used to select health facilities (clusters). First, a list of clusters and their corresponding 2016 notifications was compiled. The number of TB notifications per cluster was used as a proxy for the size of clusters. Clusters that notified <10 patients were merged with adjacent clusters. Second, cumulative notifications were compiled and 60 clusters were selected by a probability proportional to size sampling method using a randomly defined starting point and sampling interval.
The study questionnaire was adapted from the WHO generic instrument and was created in CSPro® (Census Bureau, USA). Data collectors, one per facility, were trained by the NTP and partner organisations. They comprised TB focal nurses and Environmental Health Technicians. All clinical and economic data were collected for the respective phase only. In case of minors (<18 years), costs were obtained from their parents and legal guardians. Interviews were conducted after obtaining assent from minors and consent from their parents and/or legal guardians. While indirect costs like loss of income were not applicable for this group, costs for diagnosis, treatment and food were obtained from the guardians. Data on dissavings (use of savings and sale of assets) were also obtained from guardians. At the end of each day, data from tablets were synchronised electronically with a central server at central level. Zimbabwe National Statistics Agency staff monitored the server and promptly highlighted errors for clarification. Periodic data quality checks and support visits were conducted by the steering committee. A WhatsApp group for data collectors and steering committee members was created to aid in addressing operational challenges, mostly related to patient recruitment and syncing electronic records in real-time.
Data variables, source of data and data collection
Socio-demographic and clinical data (age, HIV status, type of TB patient) were extracted from treatment registers and patient treatment booklets prior to the interview. Data on hospital visits, costs incurred, household assets and coping strategies were collected by trained data collectors during face-to-face interviews with patients. All the interviews were conducted in separate rooms within health facilities to ensure confidentiality.
Data analysis
Anonymised data were exported to Stata version 15.0 (StataCorp, College Station, TX, USA) for cleaning and analysis. Categorical variables were summarised using frequencies (proportions), while continuous variables were summarised using medians and inter-quartile ranges (IQR) stratified by DR status. We summed up the direct medical costs (consultations fees, laboratory tests) and direct non-medical costs (transport, food). Costs that were in South African Rand were converted to USD using the prevailing conversion rate obtained from the Oanda currency converter (http://www.oanda.com). Productivity losses due to TB treatment were estimated using the output approach, where the difference in monthly income before and after TB diagnosis was extrapolated over the treatment period. Household income was based on self-reports. A sensitivity analysis of indirect costs estimation was done using valuation of the time lost by the patient in each phase of treatment. To estimate patient costs for the entire TB episode, including costs for all phases of treatment, we extrapolated costs based on data from patients in other phases of illness. We used the approach recommended by WHO, whereby we replaced missing cost data with median costs of the phase of illness among those in that phase with available data. Comparisons between categorical variables were done using the chi-square test. Multivariable logistic regression was used to determine risk factors for patients experiencing TB-related catastrophic costs after adjusting for sex, age, DR status; treatment phase, HIV status, breadwinner status, household income and location of health facility. The level of significance was set at P< 0.05.
Results
A total of 860 patients (96% of the target sample size) were reached and consented (Figure 1). Of these, 19 records were excluded from analysis for being on treatment for <14 days and not being able to reschedule an appointment (17) and for failure to complete the interview (two). Overall, the response rate was 841 (93%). The figure was 900 after factoring in non-response weights.
Figure 1:

Flow of patients who were enrolled in the Zimbabwe patient cost survey, 2018
Of the 900 patients, 851 (94%) had DS-TB (Table 1). The mean age was 36.9 years and 500 (56%) were men. A greater proportion of DR-TB than DS-TB patients were in the continuation phase (66% vs 56%) and were HIV-positive (72% vs 61%). Almost all patients were new, rather than retreatment. DR-TB patients were more likely to live in urban locations than DS-TB patients (69% vs 59%).
Table 1.
Socio-demographic and clinical profile of patients with DR-TB and DS-TB who were enrolled in the Zimbabwe patient cost survey, 2018
| Characteristic | DR-TB | DS-TB | Total | |||
|---|---|---|---|---|---|---|
|
|
||||||
| N | (%) | n | (%) | n | (%) | |
| 49 | (6) | 851 | (94) | 900‡ | ||
| Sex | ||||||
| Male | 28 | (56) | 472 | (56) | 500 | (56) |
| Female | 21 | (44) | 379 | (44) | 400 | (44) |
| Age group (years) | ||||||
| <15 | 0 | (0) | 52 | (6) | 52 | (6) |
| 15-24 | 4 | (8) | 99 | (12) | 103 | (12) |
| 25-34 | 21 | (42) | 209 | (24) | 230 | (26) |
| 35-44 | 15 | (31) | 277 | (33) | 292 | (33) |
| 45-54 | 7 | (14) | 124 | (15) | 131 | (15) |
| 55-64 | 0 | (0) | 53 | (6) | 53 | (6) |
| ≥65 | 2 | (5) | 37 | (4) | 39 | (4) |
| Mean (SD) | 36.4 | (11.9) | 36.9 | (14.8) | 36.9 | (14.7) |
| Treatment phase | ||||||
| Intensive | 17 | (34) | 376 | (44) | 392 | (44) |
| Continuation | 32 | (66) | 475 | (56) | 508 | (56) |
| HIV Status | ||||||
| Positive | 35 | (72) | 522 | (61) | 557 | (62) |
| Negative | 14 | (28) | 321 | (38) | 335 | (37) |
| Unknown | 0 | (0) | 8 | (1) | 8 | (1) |
| Type of TB patient | ||||||
| New | 45 | (91) | 796 | (94) | 841 | (94) |
| Retreatment | 4 | (9) | 55 | (6) | 59 | (6) |
| Facility location | ||||||
| Urban | 34 | (69) | 506 | (59) | 540 | (60) |
| Rural | 15 | (31) | 345 | (41) | 360 | (40) |
=weighted sample size;
DR-TB=drug resistant TB; DS-TB=drug susceptible TB; SD=Standard deviation; HIV = Human Immunodeficiency Virus
The proportion of households who experienced TB-related catastrophic costs was 80% (95% CI: 77-82) (Figure 2). The incidence of TB-related catastrophic costs was 90% among DR-TB patients and 79% among DS-TB patients, P=0.06 (Table 2). Overall, 95% of patients in the poorest income quintile experienced TB-related catastrophic costs. Income quintile was strongly associated with catastrophic costs in a dose-response relationship after adjusting for other variables. Compared to the wealthiest group, the poorest households had higher odds of incurring TB-related catastrophic costs, (adjusted odds ratio (aOR): 15.7 [7.5-33.1]). Sex, age, type of TB, treatment phase, treatment delay (≤ four weeks), HIV status, being a breadwinner and location of health facility were not associated with TB-related catastrophic costs in either univariable or multivariable analysis.
Figure 2:
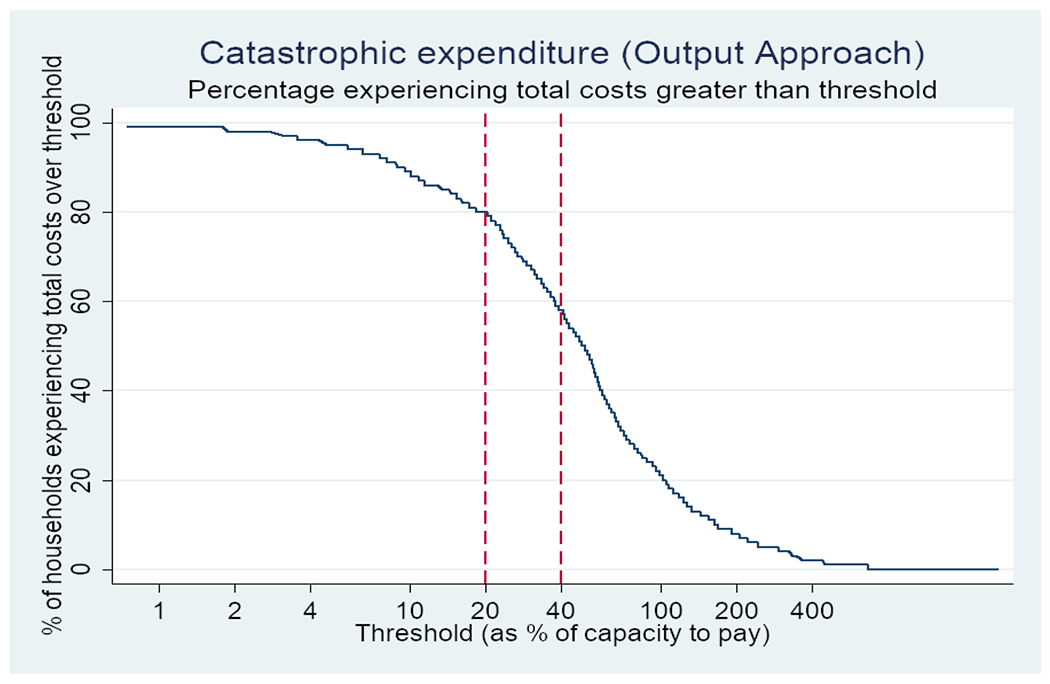
Proportion of tuberculosis-affected households who experienced TB-related catastrophic costs during the Zimbabwe tuberculosis patient cost survey 2018
Table 2:
Factors associated with catastrophic costs among tuberculosis-affected households enrolled in the Zimbabwe tuberculosis patient cost survey, 2018
| Characteristic | Total | Number (%) who incurred catastrophic costs | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|---|
|
| |||||
| 900 | 720 | (80) | |||
|
| |||||
| Sex | |||||
| Male | 500 | 392 | (78) | 0.8 (0.6 - 1.1) | 1.0 (0.7 - 1.5) |
| Female | 400 | 328 | (82) | Reference | Reference |
| Age group | |||||
| <15 | 52 | 47 | (90) | Reference | Reference |
| 15-24 | 103 | 79 | (77) | 0.4 (0.1 - 1.1) | 0.5 (0.2 - 1.4) |
| 25-34 | 230 | 171 | (74) | 0.3 (0.1 - 0.9) | 0.4 (0.1 - 1.2) |
| 35-44 | 292 | 240 | (82) | 0.5 (0.2 - 1.3) | 0.6 (0.2 - 1.8) |
| 45-54 | 131 | 102 | (78) | 0.4 (0.1 - 1.1) | 0.4 (0.1 - 1.2) |
| 55-64 | 53 | 45 | (85) | 0.6 (0.2 - 2.0) | 0.6 (0.2 - 2.0) |
| >65 | 39 | 37 | (95) | 2.0 (0.3 - 11.9) | 1.7 (0.3 - 10.7) |
| Type of TB | |||||
| DR-TB | 49 | 44 | (90) | 2.2 (0.7 - 6.7) | 0.3 (0.1 - 1.0) |
| DS-TB | 851 | 676 | (79) | Reference | Reference |
| Treatment phase | |||||
| Intensive | 392 | 313 | (80) | 1.0 (0.7 - 1.4) | 0.9 (0.6 - 1.3) |
| Continuation | 508 | 407 | (80) | Reference | Reference |
| Treatment delay (>4 weeks) | |||||
| Yes | 232 | 192 | (83) | 1.3 (0.8 - 1.9) | 1.3 (0.9 - 1.9) |
| No | 668 | 528 | (79) | Reference | Reference |
| HIV status | |||||
| Positive | 557 | 450 | (81) | 1.1 (0.8 - 1.7) | 1.4 (0.8 - 2.2) |
| Negative | 343 | 270 | (79) | Reference | Reference |
| Bread winner | |||||
| Yes | 457 | 364 | (80) | 1.1 (0.8 - 1.5) | 1.1 (0.7 - 1.9) |
| No | 443 | 356 | (80) | Reference | Reference |
| Income quintile | |||||
| Poorest | 191 | 181 | (95) | 14.5 (7.1 - 29.6) | 15.7 (7.5 - 33.1) |
| Less poor | 199 | 180 | (90) | 7.0 (3.9 - 12.4) | 7.2 (3.9 - 13.1) |
| Average | 159 | 125 | (79) | 2.8 (1.6 - 4.7) | 2.8 (1.6 - 4.8) |
| Less wealthy | 174 | 133 | (76) | 2.4 (1.5 - 3.9) | 2.5 (1.5 - 4.2) |
| Wealthiest | 177 | 101 | (57) | Reference | Reference |
| Location of health facility | |||||
| Rural | 360 | 295 | (82) | 1.2 (0.6 - 2.5) | 0.9 (0.4 - 1.8) |
| Urban | 540 | 425 | (79) | Reference | Reference |
DR-TB=drug resistant TB; DS-TB=drug susceptible TB; OR=Odds ratio; aOR=adjusted odds ratio; CI=confidence interval; HIV = Human Immunodeficiency Virus.
The major cost drivers in the pre-diagnosis phase were direct medical costs with a median of US$25 (IQR:6-58) followed by food US$18 (IQR:2.2-27) (Table 3). During the pre-diagnostic phase, DS-TB patients incurred higher direct costs than DR-TB patients (median US$54 vs. US$35). The median direct costs post-TB diagnosis were US$555 (IQR:220-600), and three times higher among DR-TB (US$1659 [IQR:653–2787]) vs DS-TB patients US$537 (IQR:204–1134).The major cost drivers post-TB diagnosis were nutritional supplements (US$360 [IQR:240-528]) vs (960 [IQR:640-1680]), for DR-TB vs DS-TB patients respectively], medical and travel costs. Median travel costs were five times higher among DR-TB compared to DS-TB patients.
Table 3:
Median costs (US$) incurred by TB patients enrolled in Zimbabwe patient cost survey (2018)
| Phase | Type of cost | Median | DR-TB (IQR) | Median | DS-TB (IQR) | Median | Total (IQR) |
|---|---|---|---|---|---|---|---|
| Pre-diagnosis | Medical | 13 | (4-39) | 25 | (6.5-60) | 25 | (6 -58) |
| Travel | 5 | (2-15) | 5 | (2-10.4) | 5 | (2- 10.7) | |
| Accommodation | 0 | (0-0) | 0 | (0- 0) | 0 | (0- 0) | |
| Food | 9 | (0-18) | 18 | (3.6- 36) | 18 | (2.2- 27) | |
| Nutritional supplements | 2 | (0-5) | 0 | (0- 2) | 0 | (0-2) | |
| Total median direct costs | 35 | (22-70) | 54 | (23-116) | 52 | (23-111) | |
|
| |||||||
| Post-diagnosis | Medical | 207 | (129 - 295) | 91 | (61.8- 134) | 91.2 | (63.4-151.2) |
| Travel | 152 | (11.3-552) | 32 | (3.1- 178) | 33.6 | (3.3- 193) | |
| Accommodation | 0 | (0-0) | 0 | (0- 0) | 0 | (0-0) | |
| Food | 100 | (0-480) | 25 | (0- 169.3) | 28.1 | (0-180) | |
| Nutritional supplements | 960 | (640-1,680) | 360 | (240-528) | 360 | (240-600) | |
| Total median direct costs | 1,659 | (653 -2,787) | 537 | (204-1,134) | 555 | (220- 1,197) | |
|
| |||||||
| Medical costs | 207 | (136-295) | 103 | (62-173) | 109 | (63- 194) | |
| Non-medical costs | 1545 | (461- 2,477) | 411 | (120-948) | 434.6 | (121-1,018) | |
| Indirect costs* | 1200 | (100-3,000) | 240 | (0-1,080) | 300 | (0-1,200) | |
| Total costs | 3,569.2 | (1,692-5,859) | 1185 | (523-2,222) | 1247 | (543-2,405) | |
=calculated based on the output approach.
Overall, the total median cost per TB episode was US$1,247 (IQR: 545-2405) (Table 3). The median total costs incurred by DR-TB patients were three times higher than those of DS-TB patients. Costs as a proportion of total costs were: non-medical costs (51%); indirect costs (36%) and medical costs (13%). During treatment, patients lost productive time with a median value of US$249.1 (IQR: 128.3-486.1). The losses were greater for DR-TB patients with a median of US$1,827.2 (IQR: 432.6-3,819.6) as compared to DS-TB patients US$238.3 (IQR: 113.3–441). Only 1.2% of households reported that they had received social protection.
Discussion
We found a high incidence of catastrophic costs among TB-affected households. The poorest households experienced the highest incidence of catastrophic costs. A higher proportion of DR-TB households incurred catastrophic costs compared to DS-TB household, albeit not reaching statistical significance. The major drivers of catastrophic costs were direct non-medical and indirect costs related to productivity loss. Indirect costs were five-times higher among DR-TB compared to DS-TB patients.
Previous surveys done in Africa and Asia have shown that TB patients incur huge catastrophic costs despite free TB treatment.(7,8,10,13) Our study provides additional evidence to this finding. However, catastrophic costs were not homogeneous among the different groups as the poorest households were disproportionally affected and risk of catastrophic costs increased as wealth quintile decreased. (6–8,10) This makes intuitive sense, and may be attributed to reduced resilience to external shocks such as TB. Unlike studies done elsewhere, we did not find a significant difference in catastrophic costs by DR status. We had low numbers of DR-TB patients, and our study may not have been sufficiently powered to detect the difference. Also, in our context even DS-TB patients experienced higher catastrophic costs than overall costs reported from other low-and middle income countries. (8, 9,14) High catastrophic costs may negatively impact on both access and adherence to TB treatment.
The major drivers of catastrophic costs lay outside the healthcare sector, a consistent finding with studies wherein non-medical costs were reported to account for up to 80% of catastrophic costs. (8,9,14) Our study highlights an urgent need to address socio-economic cost drivers such as income loss due to loss of productivity time, travel costs and nutritional supplements. These social determinants of TB have a major impact on TB health outcomes. Future studies should unravel both the type and source of nutritional supplements that are purchased by TB patients in Zimbabwe.
Patients on DR-TB treatment experienced far higher income losses than DS-TB patients in this study. The reasons could be three-fold: lengthy treatment requiring frequent visits to health facilities; loss of productivity time since TB affects mostly the economically productive age groups (25-44 years); lack of income replacement due to the informal nature of businesses in Zimbabwe and lack of social protection. Even in contexts where social protection is available, income loss poses the greatest financial risk to TB patients.(6) Fuady et al observed that TB patients in Indonesia needed money for transport and food and to protect themselves against income loss. (15) Social protection and income replacement are therefore key to protecting TB patients and their households against financial catastrophes. Our results have significant policy implications regarding social protection for TB patients in Zimbabwe. DR-TB and DS-TB patients experienced comparable catastrophic costs and may resort to harmful coping strategies like selling productive household assets or taking children out of school. This may affect household economics for years. In Zimbabwe, social protection (conditional cash transfers) is provided for DR-TB patients only. The NTP needs to provide and facilitate the uptake of social protection for TB patients regardless of drug resistance status.(16) Against the backdrop of a very low proportion of MDR-TB patients receiving social support, the NTP needs to evaluate the cash transfer programme for MDR-TB patients focussing on coverage, timeliness of disbursements and impact from the perspective of patients. The NTP has held a preliminary stakeholder consultation to identify priority actions to mitigate catastrophic costs. This was followed with a social protection mapping exercise which identified barriers to accessing social protection such as lack of knowledge about availability of services and cumbersome registration processes. (17)
This study is strengthened by the fact that we recruited patients consecutively to minimise selection bias. We minimised data entry errors through use of validated electronic questionnaires with check functions. However, there were limitations in that patients who sought TB care outside Zimbabwe were not represented in this survey. Moreover, we interviewed the patients once and had to estimate most of the costs. Recall bias could affect cost estimates for the pre-treatment period, leading to under-or overestimation of the costs. However, patients may not forget about the painful experiences they went through especially those related to selling productive assets. We minimised recall bias by interviewing persons in the intensive phase about diagnostic costs and the costs that were incurred prior to diagnosis. We could not capture both direct and indirect costs after treatment outcomes (including burial costs). These costs can extend well beyond the treatment period, even for people who are declared cured from TB.
Conclusion
TB patients and their households incur huge catastrophic costs in Zimbabwe despite free TB treatment. The major cost drivers could be ameliorated through social protection and universal health coverage. A multi-sectoral approach to TB control holds great promise to reducing catastrophic costs due to TB in Zimbabwe.
Acknowledgements
We would like to thank the participants who took part in this survey, the survey steering committee members and staff who collected the data.
Funding
This study was funded by the US Agency for International Development (USAID) Challenge TB through the World Health Organisation, Zimbabwe Country Office (Grant number AID-OAA-A-14-00029. Publication costs for this article were supported by the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, under award Number U01AI069924. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
List of abbreviations
- TB
Tuberculosis
- HIV
human immune-deficiency versus
- DR-TB
drug resistant TB
- DS-TB
drug susceptible TB
- MDR-TB
multi-drug resistant TB
- OOP
out of pocket
- IQR
inter-quartile range
- aOR
adjusted odds ratio
- WHO
World Health Organisation
- NTP
National Tuberculosis Control Programme
- SD
standard deviation
- US$
United States Dollar
Footnotes
Ethics approval and consent to participate
Written informed consent and/or assent was obtained from patients and their guardians prior the interviews. Ethics approval was obtained from the Medical Research Council of Zimbabwe (MRCZ/A/2290).
Consent for publication
Not applicable
Competing interests
The authors declare they have no competing interests
Availability of data and materials
The datasets are available from the corresponding author on reasonable request.
References
- 1.World Health Organisation. Global Tuberculosis Report 2020 [Internet]. Geneva, Switzerland; 2020. Available from: https://www.who.int/publications/i/item/9789240013131 [Google Scholar]
- 2.Timire C, Metcalfe JZ, Chirenda J, Scholten JN, Manyame-murwira B, Ngwenya M, et al. International Journal of Infectious Diseases Prevalence of drug-resistant tuberculosis in Zimbabwe: A health facility-based cross-sectional survey. Int J Infect Dis [Internet]. 2019;87:119–25. Available from: 10.1016/j.ijid.2019.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. Global Tuberculosis Report 2018 [Internet]. Geneva, Switzerland; 2018. Available from: WHO/CDS/TB/2018.20 [Google Scholar]
- 4.STOP TB Partnership. The paradigm shift 2016-2020: Global plan to End TB [Internet]. Geneva, Switzerland; 2015. Available from: http://www.stoptb.org/global/plan/plan2/ [Google Scholar]
- 5.Wingfield T, Tovar MA, Huff D, Boccia D, Montoya R, Ramos E, et al. The economic effects of supporting tuberculosis-affected households in Peru. Eur Radiol [Internet]. 2016;48:1396–410. Available from: 10.1183/13993003.00066-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J. 2014;43(6): 1763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuady A, Houweling TAJ, Mansyur M, Richardus JH. Catastrophic total costs in tuberculosis- affected households and their determinants since Indonesia ’ s implementation of universal health coverage. Infect Dis Poverty. 2018;7(3): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health Kenya. The First Kenya Tuberculosis Patient Cost Survey, 2017. Nairobi, Kenya; 2017. [Google Scholar]
- 9.Pedrazzoli D, Siroka A, Boccia D, Bonsu F, Nartey K, Houben R, et al. How affordable is TB care? Findings from a nationwide TB patient cost survey in Ghana. Trop Med Int Heal. 2018;23(8):870–8. [DOI] [PubMed] [Google Scholar]
- 10.Ukwaja KN, Alobu I, Abimbola S, Hopewell PC. Household catastrophic payments for tuberculosis care in Nigeria : incidence , determinants , and policy implications for universal health coverage. Infect Dis Poverty [Internet]. 2013;2(21):1–9. Available from: http://www.idpjournal.com/content/2/1/21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worldometer. Zimbabwe population (live) [Internet]. 2020. [cited 2020 Sep 21]. Available from: https://www.worldometers.info/world-population/zimbabwe-population/
- 12.World Health Organisation. Global Tuberculosis Report 2019 [Internet]. Geneva, Switzerland; 2019. Available from: WHO/CDS/TB/2019.15 [Google Scholar]
- 13.World Health Organization. Tuberculosis patient cost surveys: a handbook. Geneva, Switzerland; 2018. [Google Scholar]
- 14.Nhung NV, Hoa NB, Anh NT, Anh LTN, Siroka A, Lönnroth K, et al. Measuring catastrophic costs due to tuberculosis in Viet Nam. Int J Tuberc Lung Dis [Internet]. 2018;22(9):983–90. Available from: 10.5588/ijtld.17.0859. [DOI] [PubMed] [Google Scholar]
- 15.Fuady A, Houweling TAJ, Mansyur M, Burhan E, Richardus JH. Effect of financial support on reducing the incidence of catastrophic costs among tuberculosis-affected households in Indonesia : eight simulated scenarios. Infect Dis Poverty [Internet]. 2019;8(10):1–14. Available from: 10.1186/s40249-019-0519-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudgard WE, das Chagas NS, Gayoso R, Barreto ML, Boccia D, Smeeth L, et al. Uptake of governmental social protection and financial hardship during drug-,,resistant tuberculosis treatment in Rio de Janeiro, Brazil. Eur Respir J [Internet]. 2018;51(100274): 1–5. Available from: 10.1183/13993003.00274-2018 [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health and Child Care. Availability and coverage of social protection services for TB patients in Zimbabwe. Harare, Zimbabwe; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.


