Abstract
Autophagy is a lysosomal degradation pathway and the main clearance route of many toxic protein aggregates. The molecular pathology of Alzheimer's disease (AD) manifests in the form of protein aggregates—extracellular amyloid-β depositions and intracellular tau neurofibrillary tangles. Perturbations at different steps of the autophagy pathway observed in cellular and animal models of AD might contribute to amyloid-β and tau accumulation. Increased levels of autophagosomes detected in patients' brains suggest an alteration of autophagy in human disease. Autophagy is also involved in the fine-tuning of inflammation, which increases in the early stages of AD and possibly drives its pathogenesis. Mounting evidence of a causal link between impaired autophagy and AD pathology uncovers an exciting opportunity for the development of autophagy-based therapeutics.
Abbreviations
- 5-HT2AR
5-Hydroxytryptamine receptor 2A
- AKT (PKB)
Protein kinase B
- AMPK
AMP-activated protein kinase
- ANP32A
Acidic leucine-rich nuclear phosphoprotein 32 family member A
- APP
Amyloid precursor protein
- ATG
Autophagy-related gene
- ATP
Adenosine triphosphate
- BECN1
Beclin 1
- CBP
CREB-binding protein
- cIAP1/2
Cellular inhibitor of apoptosis protein 1/2
- CREB
cAMP response element-binding protein
- Erβ
Oestrogen receptor beta
- GSK3β
Glycogen synthase kinase 3 beta
- H3R
Histamine 3 receptor
- HDAC6
Histone deacetylase 6
- IFN-γ
Interferon gamma
- IL
Interleukin
- INHAT
Inhibitor of acetyltransferases
- IST1
Increased sodium tolerance protein 1
- LAMP1
Lysosomal-associated membrane protein 1
- LC3
Microtubule-associated proteins 1A/1B light chain 3B
- mGluR5
Metabotropic glutamate receptor 5
- mTOR
Mammalian target of rapamycin
- mTORC1
Mammalian target of rapamycin complex 1
- NBRF2
Neighbour of BRCA1 LncRNA 2
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- OPTN
Optineurin
- p300
Histone acetyltransferase p300
- p62/SQSTM1
Sequestosome-1
- PI3P
Phosphatidylinositol 3-phosphate
- PPARα
Peroxisome proliferator-activated receptor alpha
- PrPc
Cellular prion protein
- PSEN1/2
Presenilin 1/2
- RAB7
RAS-related protein Rab-7a
- RAPTOR
Regulatory associated protein of mTOR
- RIPK1
Receptor-interacting serine/threonine-protein kinase 1
- RPS6KB1
Ribosomal protein S6 kinase beta-1
- SIRT1
Sirtuin-1
- SNAPIN
SNARE-associated protein
- SNARE
SNAP receptor
- SSH1
Slingshot homologue 1
- TFEB
Transcription factor EB
- TNF
Tumour necrosis factor
- TRADD
TNF receptor type-1 associated death domain
- TRAF2
TNF Receptor-associated factor 2
- ULK1/2
Unc-51 like autophagy activating kinase
- VDAC1
Voltage-dependent anion-selective channel 1
- VPS34
Vacuolar protein sorting 34
- WIPI2
WD repeat domain phosphoinositide-interacting 2
- ZBTB16
Zinc finger and BTB domain-containing protein 16
Autophagy
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved pathway where portions of cytoplasm are sequestered in double-membraned autophagosomes and delivered to lysosomes for degradation. Autophagy allows recycling of nutrients to provide energy during starvation, but in complex multicellular organisms, it is also involved in other aspects of cellular stress management. Indeed, by degrading aggregate-prone proteins and damaged organelles, autophagy acts as aquality control machinery maintaining cellular homeostasis [1]. Furthermore, autophagy negatively regulates several key components of the immune response, playing a crucial role in counteracting inflammation [2]. Autophagy is particularly important in safeguarding the health of brain cells, and its impairment results in the neuronal accumulation of toxic proteins and excessive neuroinflammation—hallmarks of many neurodegenerative diseases [3]. Although disrupted autophagy may not be the primary cause of neurodegeneration, a growing body of evidence indicates that defects at different stages of this pathway may contribute to disease progression and pathology, suggesting that boosting autophagy might be a valuable therapeutic strategy.
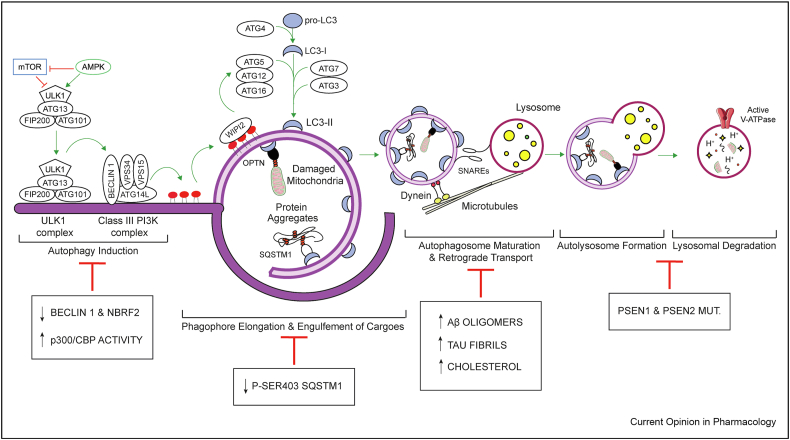
Autophagy is a multistep process that starts with the formation of a cup-shaped, double-membraned structure called a phagophore. On activation by Unc-51 like autophagy activating kinase (ULK1/2), the vacuolar protein sorting 34 (VPS34)/Beclin1 complex translocates on the nascent phagophore to produce phosphatidylinositol 3-phosphate, which enables the sequential recruitment of WD repeat domain phosphoinositide-interacting 2 and the autophagy-related gene (ATG)12–ATG5–ATG16L1 complex. The latter is an E3-like ligase protein complex required for the conjugation of Atg8/microtubule-associated proteins 1A/1B light chain 3B (LC3) on the developing autophagosome. In addition to enabling expansion and closure of the phagophore, LC3 facilitates the engulfment of cargo in autophagic vesicles by directly interacting with autophagy receptors, such as sequestosome-1 (p62/SQSTM1). After closure, autophagosomes travel towards lysosomes clustered at the microtubule organising centre via dynein motors, where SNAP receptors (SNAREs) mediate autophagosome–lysosome fusion, enabling degradation of the autophagic cargo (Figure 1). Autophagy is triggered by various stressor-signalling pathways. The most common are low energy production–mediated AMP-activated protein kinase (AMPK) activation and nutrient starvation–mediated mammalian target of rapamycin complex 1 (mTORC1) inhibition. Transcription factor EB (TFEB), a transcription factor controlled by mTORC1, coordinates both autophagosome biogenesis and lysosomal proteolysis, acting as a central regulator of the autophagy flux [4].
Figure 1.
Autophagy in the pathogenesis of AD. mTOR inhibition and AMPK activation are the most common signalling pathways inducing autophagy. Both events lead to the activation of the ULK1 complex (ULK1, ATG13, FIP200, ATG101), which, in turn, translocates to the pre-autophagosomal membrane where it starts the nucleation of the phagophore with the recruitment of the class III PI3K complex (BECLIN 1, VPS34, VPS15, ATG14L) and the production of PI3P. The latter interacts with WIPI2, which subsequently recruits the ATG5–ATG12–ATG16 complex (E3-like ligase). The coordinated action of this complex along with ATG7 (E1-like ligase) and ATG3 (E2-like ligase) allows the conjugation of LC3, previously cleaved by ATG4, to phosphatidylethanolamine (PE) lipids on the developing autophagosome. During the expansion, cytoplasmic materials (protein aggregates and damaged mitochondria) are engulfed in the phagophore through the interaction with specific adaptors (e.g. SQSTM1 and OPTN) directly binding to LC3-II. After closure, the newly formed autophagosome travels along microtubules, via dynein motor protein, to reach the lysosome. Autophagosome–lysosome fusion is aided by SNAREs and leads to the formation of the autolysosome. Proper acidification of lysosomes, mediated by v-ATPase, is necessary to activate the lysosomal hydrolases and stimulate proteolysis of the cytoplasmic materials. The white boxes describe the molecular events, which hinder the progression of the autophagy flux during the pathogenesis of AD.
In this review, we will consider how proteins linked to Alzheimer's disease (AD) affect autophagy and how autophagy disruption impacts the accumulation of the pathological amyloid-β (Aβ) and tau aggregates. Finally, we will discuss the most recent developments in autophagy-related therapeutics.
Alzheimer's disease
AD is a debilitating neurodegenerative condition clinically characterised by progressive dementia and cognitive impairment. Central pathologic events in AD are as follows: (i) the abnormal cleavage of amyloid precursor protein (APP), resulting in the accumulation of Aβ plaques in the extracellular space, and (ii) the hyperphosphorylation of microtubule-associated protein tau, leading to the formation of intracellular neurofibrillary tangles (NFTs). Converging evidence indicates that the deposition of Aβ is a likely initial pathological event in AD and is responsible for the spread of tau pathology. Clinically, the amount of tau NFTs correlates with the cognitive dysfunction of AD to a greater extent than Aβ plaque load does. Chronic neuroinflammation—recently identified as a third core feature of AD—has emerged as a potential bridge between these two events [5]. Although most patients present with ‘sporadic’ late onset disease (~80 years), a small number of cases (<1%) have inherited mutations in genes that affect processing of Aβ (APP, PSEN1 and PSEN2) and develop the disease much earlier (~45 years) [6]. The discovery of monogenic forms of AD has led to the development of faithful cellular and animal models of these Mendelian diseases, allowing a better investigation of the biological mechanisms underlying this condition, including autophagy.
Autophagy and Alzheimer's disease
Perturbations at different stages of autophagy might compromise the homeostasis of toxic aggregate-prone proteins (Aβ and tau) and exacerbate neuroinflammation, thus contributing to AD pathogenesis. Supporting a relationship between autophagy and AD, genetic variants relevant to the autophagy-lysosomal pathway have been identified as disease risk factors for late-onset AD [6], and conversely, pathogenic aggregate-prone toxic proteins can also affect autophagy (Figure 1). The accumulation of immature autophagic vacuoles observed in dystrophic neurites of brains of patients with AD is compatible with autophagy impairment [7]. Further studies in vivo and in vitro demonstrated that both autophagy induction and autophagosome clearance may be altered in AD.
Autophagy and Aβ
The amyloidogenic form of Aβ is generated from APP via sequential proteolytic cleavages performed by β- and γ-secretases. In AD, the production of Aβ exceeds its clearance, leading to Aβ accumulation and the formation of neurotoxic aggregates [6]. The latter might be caused, at least partially, by a progressive impairment of autophagy. Defects in autophagosome formation have been reported in brains of patients with AD and might be explained by reduced levels of BECLIN 1 and NBRF2, key regulators of autophagosome biogenesis. Genetic depletion of these factors in AD mouse models suppresses autophagy-mediated degradation of Aβ aggregates and accelerates cognitive impairment [8,9]. Accumulation of autophagosomes within patient dystrophic neurites suggests defective axonal retrograde transport. Studies in relevant mouse models demonstrate that interactions of Aβ oligomers with dynein motor protein hinder dynein recruitment to SNAPIN-loaded autophagosomes, thus impairing their trafficking towards lysosomes and reducing degradation of autophagy cargo [10]. Accordingly, dynein or SNAPIN overexpression in AD mice improved axonal transport, autophagosome maturation and Aβ clearance [10,11].
AD-related mutations in PSEN1 and PSEN2 not only cause pathogenic Aβ cleavage but also impact autophagy flux. PSEN1 maintains lysosomal acidification by targeting the v-ATPase V0a1 subunit to lysosomes, whereas PSEN2 controls RAB7 recruitment to autophagosomes by modulating ER Ca2+ homeostasis. Consequently, mutations in PSEN1 and PSEN2 impair lysosomal proteolysis and hinder autophagosome–lysosome fusion [12,13]. Environmental and genetic disease modifiers also contribute to AD by interfering with the autophagy–lysosomal pathway. For instance, elevated intracellular cholesterol levels in neurons exert a dual effect on autophagy and mitophagy. On the one hand, this stimulates auto/mitophagosome formation by exacerbating Aβ-induced mitochondrial oxidative stress, but, on the other hand, it alters the distribution of SNAREs and prompts the cytosolic aggregation of the mitophagy adaptor optineurin (OPTN), ultimately preventing cargo recognition and vesicle fusion necessary for the completion of auto/mitophagy [14,15]. Finally, components of the autophagic machinery display a protective role in AD via non-canonical pathways that use some components of the autophagy machinery. Murine studies show that LC3-associated phagocytosis and endocytosis are required for the recycling of Aβ receptors by microglia and defects in these processes contribute to Aβ accumulation, resulting in pervasive neurodegeneration, consistent with the human disease [16,17].
Autophagy and tau
Tau is involved in microtubule assembly and stability, and it is modulated by several post-translational modifications. Among these, phosphorylation plays a central role in the formation of NFTs because hyperphosphorylation decreases tau association with microtubules, leading to protein misfolding and oligomerisation. Tau is also secreted, and its aggregates can spread from cell to cell to seed new aggregates in healthy cells [18]. Tau hyperacetylation by histone acetyltransferase p300 (p300)/CREB-binding protein (CBP) promotes its accumulation and causes cognitive deficits in transgenic mice [19]. p300 also mediates the acetylation of regulatory associated protein of mTOR, a component of mTORC1, which inhibits autophagy [20]. Hyperactivity of p300/CBP inhibits autophagy in tauopathy brains and promotes tau secretion in neurons. Enhancing autophagic flux prevents p300-mediated tau secretion, and inhibition of p300/CBP can reduce the spread of aggregated tau [21].
Pathological transformations of tau may also affect its interaction with the autophagic machinery. While normal and soluble forms of tau are targeted by the cargo receptor OPTN, the degradation of insoluble species via selective autophagy appears to be assisted by p62/SQSTM1. Interestingly, overexpression of p62/SQSTM1 reduces pathological tau and spreading in tauopathy mouse models [22]. Furthermore, slingshot homologue 1–mediated dephosphorylation of p62/SQSTM1 phospho-Ser403—a post-translational modification that enhances its activity and is decreased in AD—impairs its affinity for ubiquitinated substrates and tau clearance. Knockdown of slingshot homologue 1 decreases phospho-tau in PS19 mice brains [23].
Pathogenic tau may also amplify its toxic effects by inhibiting autophagy. Tau accumulation upregulates acidic leucine-rich nuclear phosphoprotein 32 family member A, a component of the histone acetyl transferase INHAT complex that masks histone acetylation. This inhibits the expression of increased sodium tolerance protein 1, which facilitates the assembly of the ESCRT-III complex required for autophagosome maturation [24]. Moreover, WT tau seeds are more efficiently targeted by autophagy than the P301L mutant counterparts in neurons. This may, in part, result from the degree of inhibition of histone deacetylase 6, involved in autophagosome–lysosome fusion, by the different seeds [25].
Autophagy and inflammation
Microglia, the resident immune cells of the central nervous system, play a major role in regulating brain inflammation and have recently gained attention as key regulators of neurodegenerative processes. Coincidentally, autophagy is recognised as an essential modulator of inflammation. An example of the autophagic regulation of inflammation is the degradation of the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome, an intracellular complex responsible for the maturation of inflammatory cytokines interleukin-1β and interleukin-18 [26]. Beclin 1 insufficiency leads to an upregulation of NLRP3 in microglia, accompanied by elevated inflammatory cytokine expression [26]. Conversely, overexpression of TFEB promotes lysosomal function and decreases inflammatory protein expression in microglia from murine AD models [27]. These data suggest that the NLRP3 inflammasome is autophagy regulated and that autophagy deficiency directly causes inflammation. The autophagic machinery crosses paths with another key modulator of inflammation. Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) regulates tumour necrosis factor (TNF)–induced cell death, balancing between apoptosis and necroptosis, that is between non-inflammatory and highly inflammatory cell death [28]. ULK1 phosphorylates RIPK1, thereby reducing TNF-induced cell death. Accordingly, ULK1 knockdown enhances TNF-induced necroptosis, providing further evidence of the anti-inflammatory role of autophagy [29]. Further cellular studies suggest that RIPK1 is a negative regulator of mTORC1, as its ablation enhances mTORC1 activity and lysosomal dysfunction [30]. In contrast, inhibition of RIPK1 in an AD mouse model reduced amyloid burden, the levels of inflammatory cytokines, and promoted microglial degradation of Aβ in vitro [31].
Finally, inflammatory cytokines can also impact autophagy. Interferon gamma administration promotes microglial clearance of Aβ in a mouse model of AD. Interferon gamma upregulates ATG5 and ATG7 expression, while simultaneously downregulating mTOR activity, indicating that the Aβ clearance might be autophagy-dependent [32].
Autophagy-based therapeutic strategies for AD
Mounting evidence of a causal link between impaired autophagy and defective clearance of Aβ and tau has uncovered an exciting opportunity to develop autophagy-targeted therapy for the treatment of AD. As genetic manipulation inducing autophagy has been reviewed elsewhere [33], here, we will focus on the pharmacological approaches reported in recent years (Table 1). Small molecule enhancers of autophagy can be classified as mTOR-dependent or mTOR-independent. Classical allosteric mTOR inhibitors, including rapamycin and rapalogs, efficiently induce neuronal autophagic clearance of tau and Aβ and improve the cognitive function of several AD mouse models [33]. Similarly, treatment with ATP-competitive mTOR kinase inhibitors (OSI-027, AZD2014 and AZD8055) has been shown to persistently reduce tau levels in induced pluripotent stem cell-derived neurons from other tauopathies [34]. The development of compounds targeting upstream regulators of mTOR is another approach that can be used. Alborixin (an ionophore antibiotic) and nitazoxanide (an anti-parasitic drug) downregulate mTOR signalling via regulation of the PTEN/PI3K/AKT pathway, in the absence of toxicity. This results in increased autophagy-mediated clearance of Aβ in both neuronal and glial cells and improved learning and memory impairments of APP/PS1 transgenic mice [35,36]. Likewise, a tauopathy-homing nanoassembly containing PEGylated ceria nanoparticles modulates the mTOR–TFEB axis through AKT signalling. Treatment with this nanomaterial elicits autophagy-dependent tau proteolysis and ameliorates cognitive dysfunction in an AD rat model [37]. Finally, inhibition of p300-mediated acetylation by SMDC37892 also leads to a beneficial effect on tau turnover in human iPSC-derived excitatory neurons potentially due to mTORC1 inhibition caused by decreased raptor acetylation [20,21]. Although mTOR inhibition has shown promising effects, it may be important to consider the possibility raised by some studies suggesting that this strategy (and autophagy stimulation, in general) may only be beneficial if initiated early in the disease course [38].
Table 1.
Molecules tested for amelioration of AD pathology through modulation of autophagy.
| Molecule(s) | Target(s) | Mechanism of action | Effect in AD | Reference |
|---|---|---|---|---|
| OSI-027, AZD2014 and AZD8055 | mTOR | ATP-competitive mTOR kinase inhibitors | Decrease in tau levels in iPSC-derived neurons from tauopathies | [34] |
| Alborixin and nitazoxanide | mTOR | Downregulation of mTOR signalling | Increased clearance of Aβ in neuronal and glial cells; improved learning and memory impairments of APP/PS1 transgenic mice | [35,36] |
| SMDC37892 | mTOR | Modulation of p300-mediated acetylation | Tau turnover in human iPSC-derived excitatory neurons | [20,21] |
| Tauopathy homing nanoassembly | AKT/mTOR | mTOR–TFEB axis | Tau proteolysis; improvement of cognitive dysfunction in an AD rat model | [37] |
| Melatonin, metformin and crocetin | AMPK | Activation of AMPK | Aβ and tau clearance; improvement of cognitive decline in several tauopathies and APP/PS1 mice | [41, 42, 43] |
| Felodipine | AMPK | L-type Ca2+ channel antagonist | Reduces insoluble tau; ameliorates the morphologic abnormalities in zebrafish models of tauopathies | [45] |
| Sert | AMPK/mTOR | VDAC1-mediated mitochondrial transport of ATP | Tau degradation | [44] |
| Berberine | BECLIN 1/VPS34; CATHEPSIN D | Increases levels of BECLIN 1/VPS34; promotes maturation of lysosomal proteases cathepsin D | Tau clearance in 3 × Tg mice | [39] |
| ICCB-19 and Apt-1 | BECLIN 1/VPS34 complex | Disrupt interaction of TRADD with TRAF2, cIAP1 or cIAP2, to increase K63-linked ubiquitination of Beclin1 | Restores tau proteostasis in the PS19 mouse model | [40] |
| Curcumin analogue C1 | TFEB | Activates TFEB nuclear translocation | Reduces APP, Aβ and tau aggregates in AD mouse models | [46,47] |
| Wy14643 and gemfibrozil | PPARα | Activation of PPARα | Decreases Aβ deposition and attenuates the cognitive deficits in APP/PS1ΔE9 mice | [48] |
| Thioperamide | H3R | CREB-mediated upregulation of TFEB/ATG7/LAMP1 | Reduces Aβ levels in APP/PS1 mouse model | [49] |
| Desloratadine | 5-HT2AR | SIRT1 | Reduces Aβ levels in the APP/PS1 mouse model | [50] |
| CTEP | mGluR5 | Modulation of the GSK3β–ZBTB16 autophagy pathway | Rescues Aβ pathology in male APPswe/PS1ΔE9 mice | [51] |
See text for details.
The second category of compounds comprises mTOR-independent autophagy inducers. Berberine, an isoquinoline alkaloid, stimulates autophagy-mediated tau clearance in 3 × Tg AD mice by enhancing the level of BECLIN 1/VPS34 and promoting the maturation of lysosomal proteases CATHEPSIN D [39]. In addition, the small molecules ICCB-19 and Apt-1 enhance the autophagic turnover of tau by activating the BECLIN 1/VPS34 complex. Binding of these drugs to the adaptor protein TRADD displaces its interaction with TRAF2, cIAP1 or cIAP2, thus releasing these factors and allowing K63-linked ubiquitination of BECLIN 1. Treatment with Apt-1 also restored tau proteostasis in the PS19 mouse model [40].
Melatonin, metformin and crocetin increase both neuronal and microglial autophagy via activation of AMPK. Treatment with these compounds promotes Aβ and tau clearance and ameliorates cognitive decline in several tauopathies and APP/PS1 transgenic mouse models [41, 42, 43]. Sert, a serotonin selective reuptake inhibitor, modulates AMPK-mTOR-RPS6KB1 signalling by altering the VDAC1-mediated mitochondrial transport of ATP [44]. Similarly, the L-type Ca2+ channel antagonist felodipine diminished the neuronal levels of insoluble tau and ameliorates the morphologic abnormalities in zebrafish models of tauopathies [45].
TFEB is phosphorylated and inhibited by mTORC1; hence, most of the TFEB activators are mTOR inhibitors. Interestingly, the curcumin analogue C1 directly binds to and activates TFEB nuclear translocation without altering its phosphorylated state and acts independently of mTOR activity. This efficiently promotes autophagy flux and reduced APP, Aβ and tau aggregates in pre-pathological and symptomatic AD mouse models [46,47]. Similarly, drugs activating peroxisome proliferator-activated receptor alpha (Wy14643 and gemfibrozil), another transcription factor involved in the regulation of autophagy, decrease Aβ deposition and attenuate the cognitive deficits in APP/PS1ΔE9 mice [48]. Because of their wide expression in the brain and their role in modulating autophagy by sensing extracellular metabolites, G-protein–coupled receptors emerge as druggable targets in neurodegeneration. Chemical inhibition of H3R (thioperamide) and 5-HT2AR (desloratadine) promotes autophagy by CREB-mediated upregulation of TFEB/ATG7/LAMP1 (in neurons) and SIRT1 (in microglia), respectively, and significantly reduces Aβ levels in the APP/PS1 mouse model [49,50]. Interestingly, allosteric inhibition of mGluR5 by CTEP restarted autophagy and rescued Aβ pathology in male APPswe/PS1ΔE9 mice, but not in their female counterparts. This was due to the fact that the aberrant association of mGluR5 with Aβ and PrPC, which leads to the inactivation of the GSK3β–ZBTB16 autophagy pathway, occurs only in the male mouse brain [51]. In the context of gender-specific pathogenic events, the identification of the oestrogen receptor beta role in promoting autophagy flux by direct interaction with ATG7 might explain why low oestrogen levels during menopause correlate with an acceleration of AD onset [52]. Such insights will drive the future development of sex-tailored therapeutics.
Concluding remarks
Pharmacological induction of autophagy reduces the levels of toxic aggregate-prone proteins and improves the clinical signs of the disease in multiple AD animal models. However, most of the models used so far are based on the genetic forms of AD; hence, they do not phenocopy the complex pathophysiology of most patients affected by the ‘sporadic’ disease. A future challenge will be to develop models incorporating non-genetic factors and the common comorbidities associated with AD and exploring the role of autophagy in this multifactorial context [53]. Upstream signalling regulators (mTOR, TFEB, AMPK) impacting autophagy have been exploited as druggable targets for improving the clearance of pathogenic aggregate-prone proteins. However, these factors act as central signalling hubs for many other vital functions in the cells; therefore, chronic pharmacological alteration of their activities may result in undesirable side effects. Considering that autophagy-based therapies are more efficient when administered in early stage of the disease and for long periods, the identification of more specific modulators with a better safety profile may be desirable [38]. Finally, recent publications shed light on the canonical and non-canonical functions of autophagy proteins in modulating the activity of microglia, which play a prominent role in the prodromal phase of the disease [54]. Enhancing autophagy in microglia counteracts inflammation and increases their phagocytic activity, which, in turn, prolongs the survival of neurons and delays disease progression. The introduction of measures targeting microglial autophagy may also hold promise for the future management of the disease.
Conflict of interest statement
D.C.R. is a consultant for Aladdin Healthcare Technologies SE, Drishti Discoveries, PAQ Therapeutics and Nido Biosciences. None of the other authors have any potential competing interests.
Financial support and sponsorship
This work was supported by UK Dementia Research Institute (funded by the MRC, Alzheimer's Research UK and the Alzheimer's Society), Roger de Spoelberch Foundation, Alzheimer's Research UK, The Tau Consortium, Cambridge Centre for Parkinson-Plus, National Institute for Health Research Cambridge Biomedical Research Centre, Merck Sharp & Dohme (D.C.R.) and European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860035 (M.R.).
This review comes from a themed issue on Neuroscience (Dementia)
Edited by Damiana Scuteri, Pierluigi Nicotera and Giacinto Bagetta
References
- 1.Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B., Mizushima N., Virgin H.W. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubinsztein D.C., Mariño G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 5.Masters C.L., Bateman R., Blennow K., Rowe C.C., Sperling R.A., Cummings J.L. Alzheimer's disease. Nat Rev Dis Primer. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 6.Scheltens P., De Strooper B., Kivipelto M., Holstege H., Chételat G., Teunissen C.E., Cummings J., van der Flier W.M. Alzheimer's disease. Lancet Lond Engl. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nixon R.A., Wegiel J., Kumar A., Yu W.H., Peterhoff C., Cataldo A., Cuervo A.M. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 8.Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P.A., Small S., Spencer B., Rockenstein E., Levine B. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachance V., Wang Q., Sweet E., Choi I., Cai C.-Z., Zhuang X.-X., Zhang Y., Jiang J.L., Blitzer R.D., Bozdagi-Gunal O. Autophagy protein NRBF2 has reduced expression in Alzheimer's brains and modulates memory and amyloid-beta homeostasis in mice. Mol Neurodegener. 2019;14:43. doi: 10.1186/s13024-019-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tammineni P., Ye X., Feng T., Aikal D., Cai Q. Impaired retrograde transport of axonal autophagosomes contributes to autophagic stress in Alzheimer's disease neurons. eLife. 2017:6. doi: 10.7554/eLife.21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Xiong X., Li S., Liang J., Zhang X., Tian M., Li X., Gao M., Tang L., Li Y. Enhanced autophagic retrograde axonal transport by dynein intermediate chain upregulation improves Aβ clearance and cognitive function in APP/PS1 double transgenic mice. Aging. 2020;12:12142–12159. doi: 10.18632/aging.103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.-H., Yu W.H., Kumar A., Lee S., Mohan P.S., Peterhoff C.M., Wolfe D.M., Martinez-Vicente M., Massey A.C., Sovak G. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedeli C., Filadi R., Rossi A., Mammucari C., Pizzo P. PSEN2 (presenilin 2) mutants linked to familial Alzheimer disease impair autophagy by altering Ca2+ homeostasis. Autophagy. 2019;15:2044–2062. doi: 10.1080/15548627.2019.1596489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roca-Agujetas V., Barbero-Camps E., de Dios C., Podlesniy P., Abadin X., Morales A., Marí M., Trullàs R., Colell A. Cholesterol alters mitophagy by impairing optineurin recruitment and lysosomal clearance in Alzheimer's disease. Mol Neurodegener. 2021;16:15. doi: 10.1186/s13024-021-00435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbero-Camps E., Roca-Agujetas V., Bartolessis I., de Dios C., Fernández-Checa J.C., Marí M., Morales A., Hartmann T., Colell A. Cholesterol impairs autophagy-mediated clearance of amyloid beta while promoting its secretion. Autophagy. 2018;14:1129–1154. doi: 10.1080/15548627.2018.1438807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann B.L., Teubner B.J.W., Tummers B., Boada-Romero E., Harris L., Yang M., Guy C.S., Zakharenko S.S., Green D.R. LC3-Associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer's disease. Cell. 2019;178:536–551. doi: 10.1016/j.cell.2019.05.056. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]; Non-canonical function of autophagy proteins in microglia endocytosis can be exploited to improve Aβ clearance and ameliorate neurodegeneration in AD.
- 17.Heckmann B.L., Teubner B.J.W., Boada-Romero E., Tummers B., Guy C., Fitzgerald P., Mayer U., Carding S., Zakharenko S.S., Wileman T. Noncanonical function of an autophagy protein prevents spontaneous Alzheimer's disease. Sci Adv. 2020;6:abb9036. doi: 10.1126/sciadv.abb9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunello C.A., Merezhko M., Uronen R.-L., Huttunen H.J. Mechanisms of secretion and spreading of pathological tau protein. Cell Mol Life Sci CMLS. 2020;77:1721–1744. doi: 10.1007/s00018-019-03349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min S.-W., Chen X., Tracy T.E., Li Y., Zhou Y., Wang C., Shirakawa K., Minami S.S., Defensor E., Mok S.A. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med. 2015;21:1154–1162. doi: 10.1038/nm.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son S.M., Park S.J., Stamatakou E., Vicinanza M., Menzies F.M., Rubinsztein D.C. Leucine regulates autophagy via acetylation of the mTORC1 component raptor. Nat Commun. 2020;11:3148. doi: 10.1038/s41467-020-16886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X., Li Y., Wang C., Tang Y., Mok S.-A., Tsai R.M., Rojas J.C., Karydas A., Miller B.L., Boxer A.L. Promoting tau secretion and propagation by hyperactive p300/CBP via autophagy-lysosomal pathway in tauopathy. Mol Neurodegener. 2020;15:2. doi: 10.1186/s13024-019-0354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhang S., Zheng H. The cargo receptor SQSTM1 ameliorates neurofibrillary tangle pathology and spreading through selective targeting of pathological MAPT (microtubule associated protein tau) Autophagy. 2019;15:583–598. doi: 10.1080/15548627.2018.1532258. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that SQSTM1 is involved in targeted clearance of pathological tau, and increased expression of this cargo receptor ameliorates tau pathology.
- 23.Fang C., Woo J.-A.A., Liu T., Zhao X., Cazzaro S., Yan Y., Matlack J., Kee T., LePochat P., Kang D.E. SSH1 impedes SQSTM1/p62 flux and MAPT/Tau clearance independent of CFL (cofilin) activation. Autophagy. 2020 doi: 10.1080/15548627.2020.1816663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Q., Luo Y., Zhang X.-N., Yang X.-F., Hong X.-Y., Sun D.-S., Li X.-C., Hu Y., Li X.-G., Zhang J.-F. MAPT/Tau accumulation represses autophagy flux by disrupting IST1-regulated ESCRT-III complex formation: a vicious cycle in Alzheimer neurodegeneration. Autophagy. 2020;16:641–658. doi: 10.1080/15548627.2019.1633862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng J.-H., Ajit A., Tabassum Z., Patel N., Tian X., Chen Y., Prevatte A.W., Ling K., Rigo F., Meeker R.B. Tau seeds are subject to aberrant modifications resulting in distinct signatures. Cell Rep. 2021;35:109037. doi: 10.1016/j.celrep.2021.109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtman J., Freitag K., Gimber N., Schmoranzer J., Heppner F.L., Jendrach M. Beclin1-driven autophagy modulates the inflammatory response of microglia via NLRP 3. EMBO J. 2019:38. doi: 10.15252/embj.201899430. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study suggesting that NLRP3 is degraded through autophagy in primary murine microglia.
- Zhou W., Xiao D., Zhao Y., Tan B., Long Z., Yu L., He G. Enhanced autolysosomal function ameliorates the inflammatory response mediated by the NLRP3 inflammasome in Alzheimer's disease. Front Aging Neurosci. 2021;13:629891. doi: 10.3389/fnagi.2021.629891. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows elevated NLRP3 and microglial activation in two different AD mouse models, likely resulting from impaired autolysosomal function.
- 28.Delanghe T., Dondelinger Y., Bertrand M.J.M. RIPK1 kinase-dependent death: a symphony of phosphorylation events. Trends Cell Biol. 2020;30:189–200. doi: 10.1016/j.tcb.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Wu W., Wang X., Berleth N., Deitersen J., Wallot-Hieke N., Böhler P., Schlütermann D., Stuhldreier F., Cox J., Schmitz K. The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep. 2020;31:107547. doi: 10.1016/j.celrep.2020.107547. [DOI] [PubMed] [Google Scholar]
- 30.Najafov A., Luu H.S., Mookhtiar A.K., Mifflin L., Xia H.-G., Amin P.P., Ordureau A., Wang H., Yuan J. RIPK1 promotes energy sensing by the mTORC1 pathway. Mol Cell. 2021;81:370–385. doi: 10.1016/j.molcel.2020.11.008. e7. [DOI] [PubMed] [Google Scholar]
- 31.Ofengeim D., Mazzitelli S., Ito Y., DeWitt J.P., Mifflin L., Zou C., Das S., Adiconis X., Chen H., Zhu H. RIPK1 mediates a disease-associated microglial response in Alzheimer's disease. Proc Natl Acad Sci U S A. 2017;114:E8788–E8797. doi: 10.1073/pnas.1714175114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Yang Y., Xing Z., Zuo Z., Wang R., Gu H., Qi F., Yao Z. Intraperitoneal injection of IFN-γ restores microglial autophagy, promotes amyloid-β clearance and improves cognition in APP/PS1 mice. Cell Death Dis. 2020;11:440. doi: 10.1038/s41419-020-2644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors of this paper show that intraperitoneal administration of IFN-γ significantly decreases the Aβ load in APP/PS1 mice, which might depend on the activation of microglial autophagy.
- 33.Djajadikerta A., Keshri S., Pavel M., Prestil R., Ryan L., Rubinsztein D.C. Autophagy induction as a therapeutic strategy for neurodegenerative diseases. J Mol Biol. 2020;432:2799–2821. doi: 10.1016/j.jmb.2019.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Silva M.C., Nandi G.A., Tentarelli S., Gurrell I.K., Jamier T., Lucente D., Dickerson B.C., Brown D.G., Brandon N.J., Haggarty S.J. Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons. Nat Commun. 2020;11:3258. doi: 10.1038/s41467-020-16984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wani A., Gupta M., Ahmad M., Shah A.M., Ahsan A.U., Qazi P.H., Malik F., Singh G., Sharma P.R., Kaddoumi A. Alborixin clears amyloid-β by inducing autophagy through PTEN-mediated inhibition of the AKT pathway. Autophagy. 2019;15:1810–1828. doi: 10.1080/15548627.2019.1596476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan L., Qiu X.-X., Zhu Z.-Y., Lv J.-L., Lu J., Mao F., Zhu J., Wang J.-Y., Guan X.-W., Chen J. Nitazoxanide, an anti-parasitic drug, efficiently ameliorates learning and memory impairments in AD model mice. Acta Pharmacol Sin. 2019;40:1279–1291. doi: 10.1038/s41401-019-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun H., Zhong Y., Zhu X., Liao H., Lee J., Chen Y., Ma L., Ren J., Zhao M., Tu M. A tauopathy-homing and autophagy-activating nanoassembly for specific clearance of pathogenic tau in Alzheimer's disease. ACS Nano. 2021;15:5263–5275. doi: 10.1021/acsnano.0c10690. [DOI] [PubMed] [Google Scholar]
- 38.Majumder S., Richardson A., Strong R., Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PloS One. 2011;6:25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y., Chen Y., Liang Y., Chen H., Ji X., Huang M. Berberine mitigates cognitive decline in an Alzheimer's Disease Mouse Model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed Pharmacother. 2020;121:109670. doi: 10.1016/j.biopha.2019.109670. [DOI] [PubMed] [Google Scholar]
- Xu D., Zhao H., Jin M., Zhu H., Shan B., Geng J., Dziedzic S.A., Amin P., Mifflin L., Naito M.G. Modulating TRADD to restore cellular homeostasis and inhibit apoptosis. Nature. 2020;587:133–138. doi: 10.1038/s41586-020-2757-z. [DOI] [PubMed] [Google Scholar]; This study shows that small molecules release TRAF2 or cIAP1/2 from TRADD, which increases K63-linked ubiquitination of Beclin 1, promotes autophagy, and induces the degradation of mutant tau.
- 41.Luengo E., Buendia I., Fernández-Mendívil C., Trigo-Alonso P., Negredo P., Michalska P., Hernández-García B., Sánchez-Ramos C., Bernal J.A., Ikezu T. Pharmacological doses of melatonin impede cognitive decline in tau-related Alzheimer models, once tauopathy is initiated, by restoring the autophagic flux. J Pineal Res. 2019;67:12578. doi: 10.1111/jpi.12578. [DOI] [PubMed] [Google Scholar]
- 42.Wani A., Al Rihani S.B., Sharma A., Weadick B., Govindarajan R., Khan S.U., Sharma P.R., Dogra A., Nandi U., Reddy C.N. Crocetin promotes clearance of amyloid-β by inducing autophagy via the STK11/LKB1-mediated AMPK pathway. Autophagy. 2021 doi: 10.1080/15548627.2021.1872187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y., Zhao S., Fan Z., Li Z., Zhu Y., Shen T., Li K., Yan Y., Tian J., Liu Z. Metformin attenuates plaque-associated tau pathology and reduces amyloid-β burden in APP/PS1 mice. Alzheimer's Res Ther. 2021;13:40. doi: 10.1186/s13195-020-00761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang H.-Y., Shim J.S., Kim D., Kwon H.J. Antidepressant drug sertraline modulates AMPK-MTOR signaling-mediated autophagy via targeting mitochondrial VDAC1 protein. Autophagy. 2020 doi: 10.1080/15548627.2020.1841953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi F.H., Menzies F.M., Lopez A., Stamatakou E., Karabiyik C., Ureshino R., Ricketts T., Jimenez-Sanchez M., Esteban M.A., Lai L. Felodipine induces autophagy in mouse brains with pharmacokinetics amenable to repurposing. Nat Commun. 2019;10:1817. doi: 10.1038/s41467-019-09494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this paper, we shows that felodipine induces autophagy-dependent clearance of aggregate-prone proteins causing neurodegenerative diseases at plasma concentrations similar to those seen in human patients.
- 46.Song J.-X., Malampati S., Zeng Y., Durairajan S.S.K., Yang C.-B., Tong B.C.-K., Iyaswamy A., Shang W.-B., Sreenivasmurthy S.G., Zhu Z. A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and Tau pathology in Alzheimer's disease models. Aging Cell. 2020;19:e13069. doi: 10.1111/acel.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song J.-X., Sun Y.-R., Peluso I., Zeng Y., Yu X., Lu J.-H., Xu Z., Wang M.-Z., Liu L.-F., Huang Y.-Y. A novel curcumin analog binds to and activates TFEB in vitro and in vivo independent of MTOR inhibition. Autophagy. 2016;12:1372–1389. doi: 10.1080/15548627.2016.1179404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo R., Su L.-Y., Li G., Yang J., Liu Q., Yang L.-X., Zhang D.-F., Zhou H., Xu M., Fan Y. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy. 2020;16:52–69. doi: 10.1080/15548627.2019.1596488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Liu B., Xu Y., Yang M., Wang C., Song M., Liu J., Wang W., You J., Sun F. Activation of CREB-mediated autophagy by thioperamide ameliorates β-amyloid pathology and cognition in Alzheimer's disease. Aging Cell. 2021;20:13333. doi: 10.1111/acel.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu J., Zhang C., Lv J., Zhu X., Jiang X., Lu W., Lu Y., Tang Z., Wang J., Shen X. Antiallergic drug desloratadine as a selective antagonist of 5HT2A receptor ameliorates pathology of Alzheimer's disease model mice by improving microglial dysfunction. Aging Cell. 2021;20:13286. doi: 10.1111/acel.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Elrahman K.S., Albaker A., de Souza J.M., Ribeiro F.M., Schlossmacher M.G., Tiberi M., Hamilton A., Ferguson S.S.G. Aβ oligomers induce pathophysiological mGluR5 signaling in Alzheimer's disease model mice in a sex-selective manner. Sci Signal. 2020:13. doi: 10.1126/scisignal.abd2494. [DOI] [PubMed] [Google Scholar]; The authors show that autophagy modulation in AD can occur via different signalling in males and females, therefore this study provides important insights for the development of sex-tailored therapies.
- 52.Wei Y., Zhou J., Wu J., Huang J. ERβ promotes Aβ degradation via the modulation of autophagy. Cell Death Dis. 2019;10:565. doi: 10.1038/s41419-019-1786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dawson T.M., Golde T.E., Lagier-Tourenne C. Animal models of neurodegenerative diseases. Nat Neurosci. 2018;21:1370–1379. doi: 10.1038/s41593-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plaza-Zabala A., Sierra-Torre V., Sierra A., Autophagy, Microglia Novel partners in neurodegeneration and aging. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18030598. [DOI] [PMC free article] [PubMed] [Google Scholar]