Abstract
Isonitrile group has been identified in many natural products. Due to the broad reactivity of N≡C triple bond, these natural products have valuable pharmaceutical potentials. This review summarizes the current biosynthetic pathways and the corresponding enzymes that are responsible for isonitrile-containing natural product generation. Based on the strategies utilized, two fundamentally distinctive approaches are discussed. In addition, recent progress in elucidating isonitrile group formation mechanisms is also presented.
Keywords: Biosynthesis, Enzymes, Natural products, Reaction mechanisms, Biotransformations
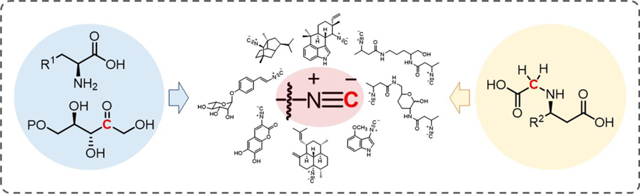
Graphical Abstract
1. Introduction
Since the discovery of penicillin,[1] small molecules produced by natural sources have attracted (bio)chemists’ attention due to their broad pharmaceutical potentials and structural complexity. In addition to classical approaches that include isolation of the compounds from the producing sources and chemical synthesis, molecular understanding of natural product biosynthetic pathways and the associated enzyme mechanism provides an alternative and attractive approach in preparing targeted molecules in a time-effective and cost-efficient manner. During the natural product assembly, in addition to those transformations that are involved in building the skeleton of the molecules, the structural complexity is expanded through the introduction of functional groups. In this review, we summarize the current understanding of natural products that are decorated with the isonitrile group, also known as isocyanide group, a novel functional group that contains a nitrogen-carbon triple bond (−N≡C), with a focus on the biosynthesis and the corresponding enzymatic mechanisms involved.
From a chemistry point of view, isonitrile group has the zwitterionic character and therefore can serve as a nucleophile, an electrophile as well as a metal ligand.[2] Isonitrile is a useful synthon and has been widely used in organic synthesis and organometallic chemistry. For example, isonitrile group participates in the Ugi reaction and the Passerini reaction to build heterocycles.[2–5] Moreover, isonitrile group containing molecule, such as kalihinol F, has been applied to alleviate the copper toxicity in Wilson’s disease.[6] From a biology point of view, many isonitrile-containing natural products have been demonstrated to preserve attractive biological properties including antibacterial activity and anti-malarial activity.[7–8]
Due to the unique chemical structure and broad biological activities of isonitrile-containing natural products, their biosynthetic pathways and the corresponding mechanism(s) have attracted chemical biologists’ and medicinal chemists’ attention. Before the discovery of axisonitrile-1 from marine sponge Axinella cannabina in 1973,[9] xanthocillin isolated from Penicillium notatum was the only known isonitrile-containing natural product[10] (Figure 1). To date, isonitrile-containing compounds have been isolated from terrestrial as well as marine sources that include fungi,[11] bacteria,[12] nudibranch molluscs,[13] and marine sponges.[7,14] For some of those obtained from terrestrial sources, the isonitrile group is biosynthesized through modification of the amino group of aromatic amino acids, e.g., tyrosine and tryptophan.[8,15–16] Alternatively, a different strategy involving decarboxylation of glycine has also been utilized to install the isonitrile functionality.[17–18] Since the majority of the marine isonitrile-containing compounds contain a terpenoid carbon scaffold, it has been postulated that the isonitrile group, along with other N-atom containing functional groups such as isocyanate, isothiocyanate, and formamide, can be installed through the decoration of the terpenoid skeleton.[7,14] In the meantime, the corresponding biosynthesis remains to be elucidated. In addition to terrestrial and marine sources, isocyalexin A was isolated from UV-irradiated rutabaga roots of Brassica napus L. ssp. rapifera.[19–20] To date, isocyalexin A, a unique cruciferous phytoalexin that protects plants from pathogen attack and UV radiation, is the only isonitrile-containing compound isolated from the plant.
Figure 1.
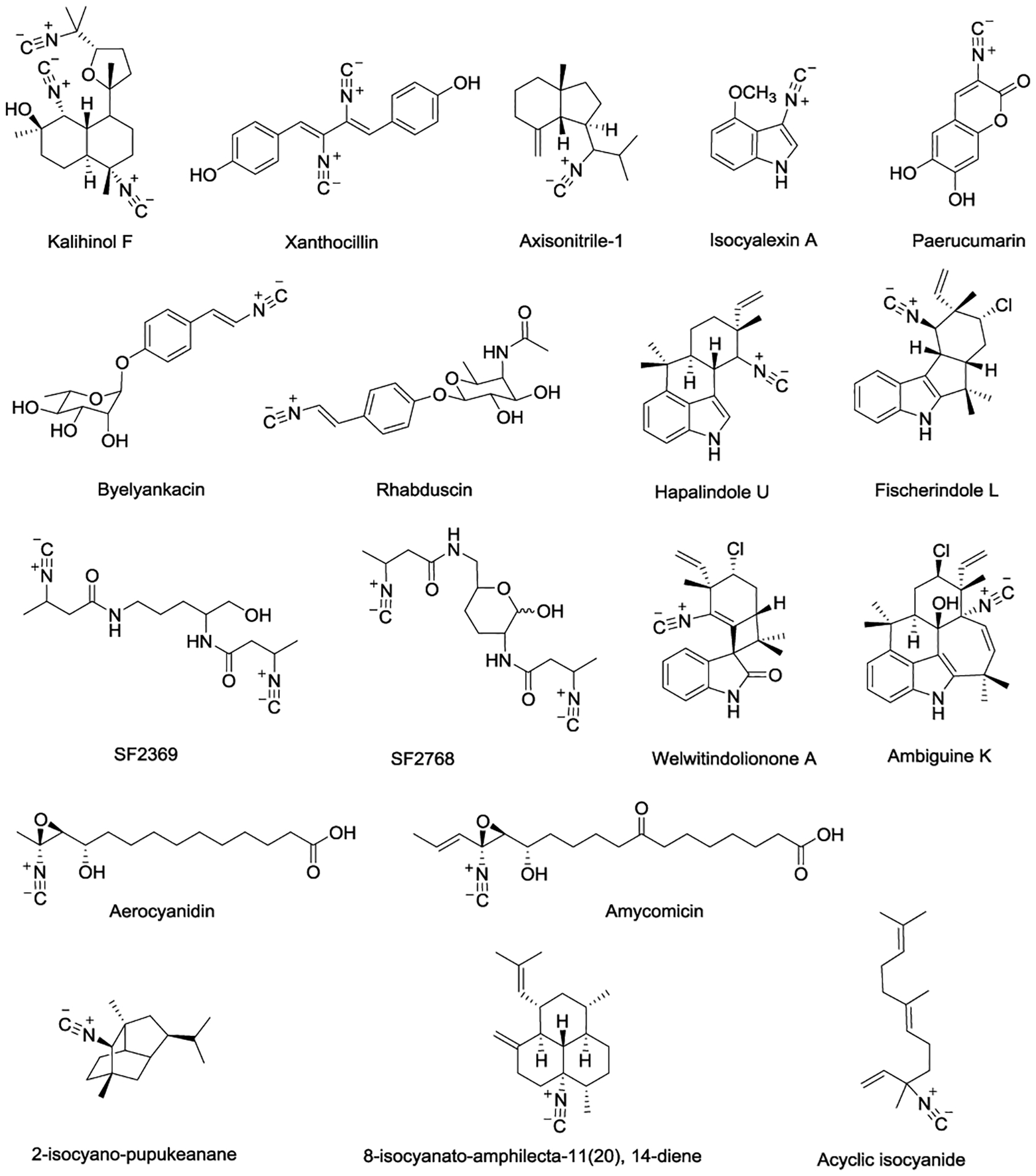
Examples of isonitrile-containing natural products.[19,21–30]
Based on different strategies utilized, two types of enzymes are known to introduce the isonitrile group in nature. The first type of enzymes catalyzes a condensation reaction between the primary amino group from the l-tryptophan/tyrosine and the carbonyl group of ribulose-5-phosphate. Several enzymes that include IsnA[8,12,15–16], PvcA[31–38], AmbI1/2[39], WelI1[40–41], Dit1,[42–44] and XanB[45] are involved, or have been proposed, to catalyze this type of isonitrile group installation. The second type of enzymes is iron and 2-oxoglutarate (Fe/2OG) dependent enzyme including ScoE,[17,46–47] and SfaA.[18,48] During the second type of isonitrile group installation, the N-alkyl glycine is converted to the corresponding isonitrile and the reaction involves a four-electron oxidation process (Figure 2).
Figure 2.
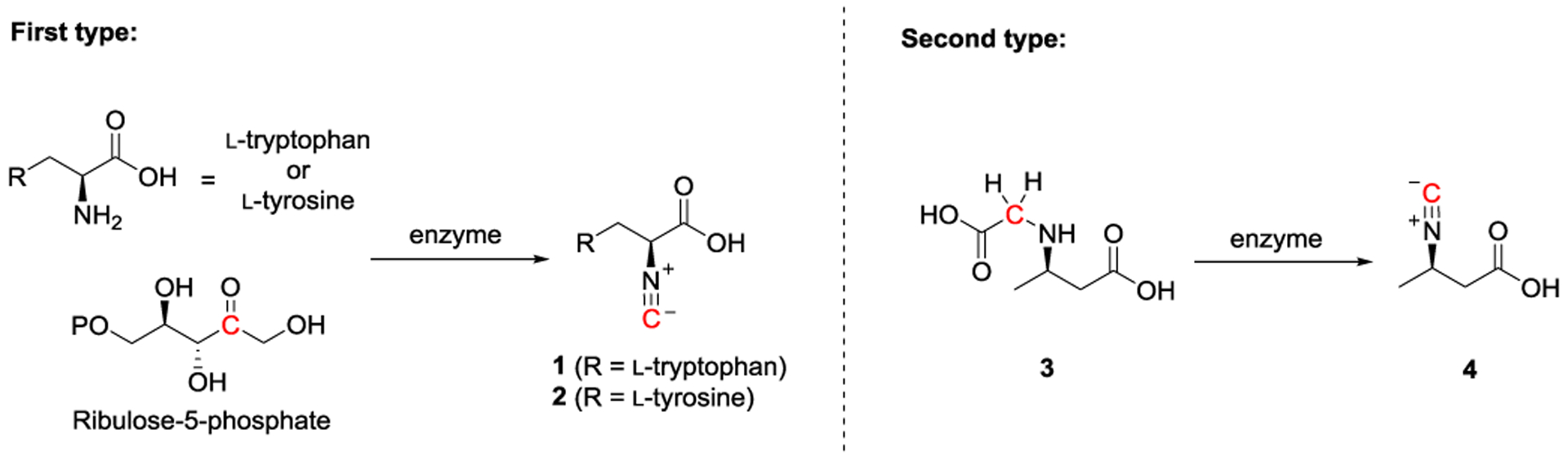
Two different strategies are utilized to install the isonitrile group. The first type involves a condensation reaction between l-tryptophan/tyrosine and ribulose-5-phosphate, while the second type undergoes a four-electron oxidation process by converting the glycine moiety into the corresponding isonitrile group.
2. Isonitrile Formation through Condensation Reaction
The first isonitrile-forming enzyme, IsnA, was characterized by Brady and Clardy in 2005.[15] Through an approach by screening DNA from a variety of environmental samples (eDNA), two genes, isnA (isonitrile synthase) and isnB (2-oxoglutarate dependent oxygenase), were identified and shown to be responsible for the production of (E)-3-(2-isocyanovinyl)-1H-indole ((E)-5) (Figure 3A). In the biosynthesis of 5, IsnA is proposed to catalyze isonitrile group formation to produce 1. Subsequently, 1 undergoes IsnB-catalyzed oxidative decarboxylation to form (E)-5, which has the olefin group in a trans-orientation. To identify the origin of the isonitrile carbon source, inverse-labeling feeding was used to eliminate the need to prepare possible isotope-labeled precursors.[16] Specifically, various possible 12C-labeled precursors were added to a 13C-enriched glucose environment during the feeding studies. Utilization of E. coli strains that carry specific modification in the sugar biosynthetic pathway, the isonitrile carbon was identified to originate from the carbonyl carbon of the ribulose-5-phosphate (Figure 3B). Briefly, when 12C-tryptophan, 12C-3-phos- phate-glycerol, 13C-glucose were fed with isnA/B encoded fba mutant, no 12C-labeled (E)-5 was observed. In contrast, a mixture of 13C-labeled and unlabeled 5 was detected when either glmS or manA mutant was employed, suggesting the isonitrile carbon was derived from the carbon source that can flux into the pentose phosphate pathway. After investigating possible sugars involved in the pentose phosphate pathway, the C2 carbon, the carbonyl carbon, of ribulose-5-phosphate was identified as the carbon source for the isonitrile group. Additionally, ribose-5-phosphate and arabinose-5-phosphate, the tautomerically equivalent sugars to ribulose-5-phosphate, can also serve as a substrate.
Figure 3.
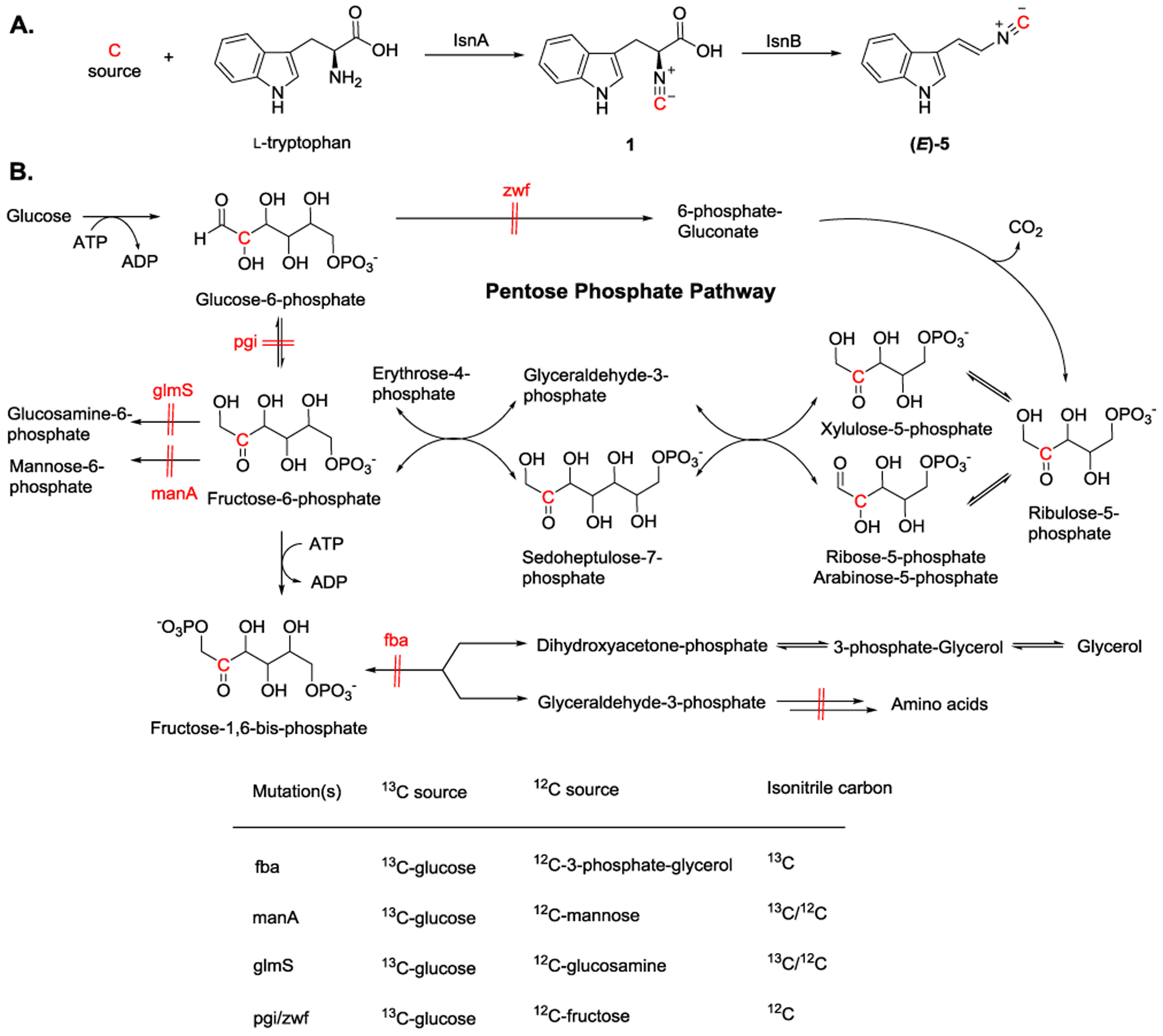
A) Proposed biosynthetic pathways for (E)-5 formation through IsnA and IsnB catalyzed reactions. B) Using inverse-labeling feeding approach, the isonitrile carbon is originated from the carbonyl carbon (C2) of ribulose-5-phosphate.
Applying the eDNA approach, in 2007, Brady et al. identified several new isnA/isnB-related genes and obtained a few isonitrile-containing molecules (7–10) that have not been isolated previously.[12,15] These compounds share a common structural feature where an isonitrile group or its derivative, such as N-formyl group, is connected to an aromatic ring (Figure 4A). Although the detailed biosynthetic mechanism remains to be carefully elucidated, due to the structural similarity of these molecules, analogous biosynthetic pathways are likely to be utilized to install the isonitrile group. IsnA/IsnB homologs were found to be involved in (E)-4-(2-isocyanovinyl) phenol (6) production, the common precursor of rhabduscin and byelyankacin.[8] Instead of l-tryptophan used for (E)-5 production, l-tyrosine is utilized as the substrate to construct 6 (Figure 4B). Rhabduscin has been shown to inhibit insect phenoloxidase activity, an important immunity protein that is involved in cellular and humoral defense. Thus, rhabduscin may serve as a potential insecticide candidate.[49–50] The other structurally similar compound, byelyankacin, isolated from Enterobacter sp. exhibits strong melanogenesis-inhibitory activity through inhibition of tyrosinase.[51]
Figure 4.
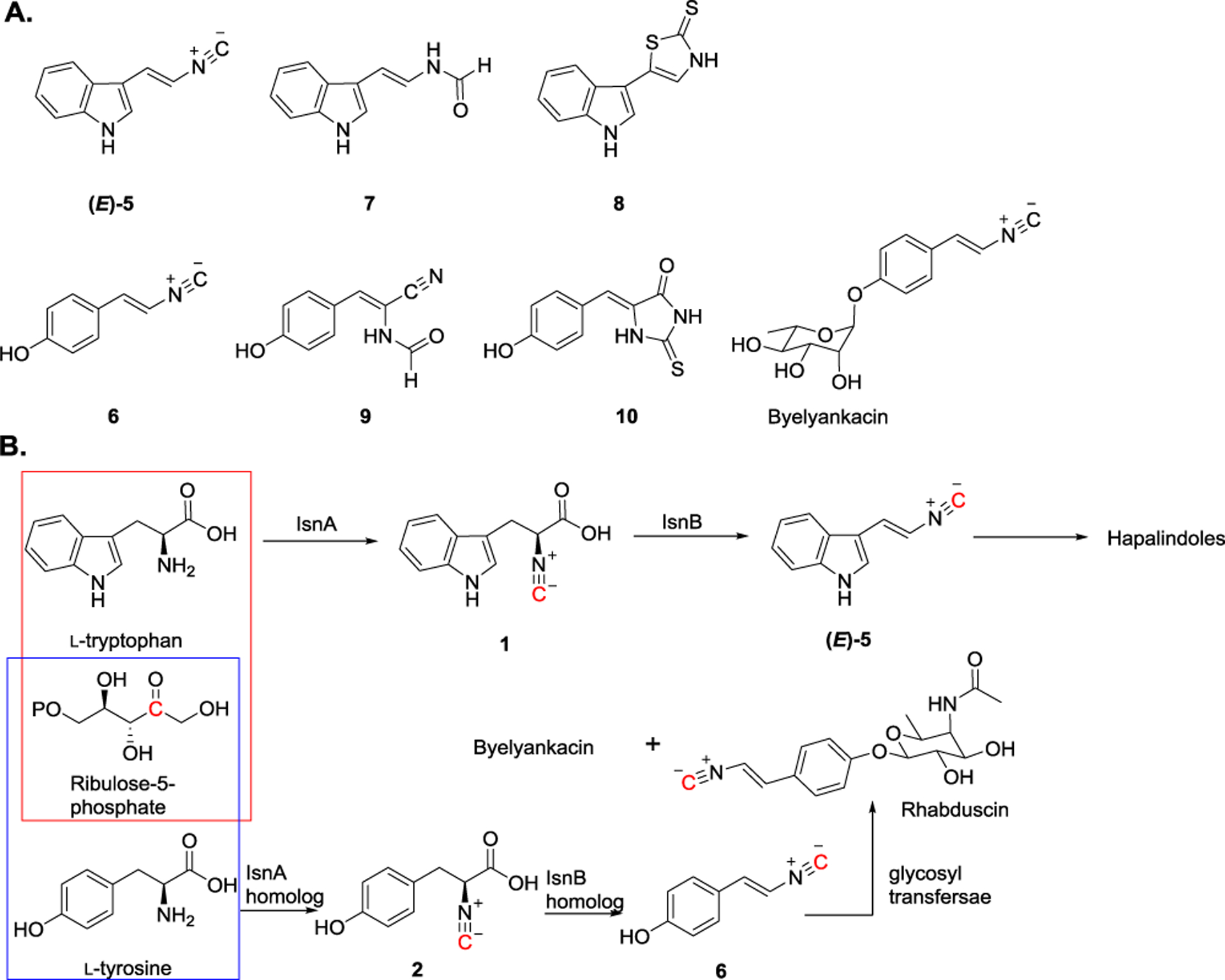
A) Through the eDNA approach, novel isonitrile-containing natural products were isolated. B) Proposed biosynthetic pathways for (E)-5 and 6 formation: IsnA and its homolog use ribulose-5-phosphate and l-tryptophan/l-tyrosine to install an isonitrile group. Subsequently, IsnB and its homolog catalyzes decarboxylation-assisted olefination using 1 and 2 as substrates to produce (E)-5 and 6, respectively.
Stintzi et al. discovered the potential pyoverdine chromophore biosynthetic gene cluster, pvcABCD (Figure 5A).[33,52] Using l-tyrosine as the substrate, pyoverdine chromophore has been proposed to be biosynthesized involving possible intermediates 11 and 12. Pyoverdine represents a group of green-fluorescent molecules, comprising a cyclic peptide moiety and a chromophore (Figure 5B). In addition to serving as an iron transporter,[31–32] pyoverdine also plays a crucial role in virulence and infection of Pseudomonas aeruginosa, which causes severe acute and chronic infections in human. Based on the regulation studies, pvcABCD genes expression is negatively regulated by iron concentration and is positively regulated by the regulatory factors PvdS and PtxR.[33] However, the function of the genes involved in pyoverdine chromophore formation has not been verified. On the other hand, Clarke-Pearson and Brady demonstrated that pvcABCD is responsible for producing paerucumarin, an isonitrile functionalized coumarin (Figure 5A).[35] Sequence analysis reveals that PvcA and PvcB are homologs of IsnA and IsnB. Thus, PvcA’s function has been proposed to introduce the isonitrile group. It is then followed by PvcB-catalyzed desaturation to form 13. Finally, PvcC and PvcD catalyzed reactions produce paerucumarin via intermediate 14. In addition, the crystal structure of PvcA was reported with a resolution of 2.1 Å, where PvcA adopts a modified Rossmann fold with a smaller C-terminal domain.[36] However, since the activity of isonitrile synthase was suggested through the coupled reaction with oxidase reaction, the product of PvcA has only been inferred but not yet been directly observed.
Figure 5.
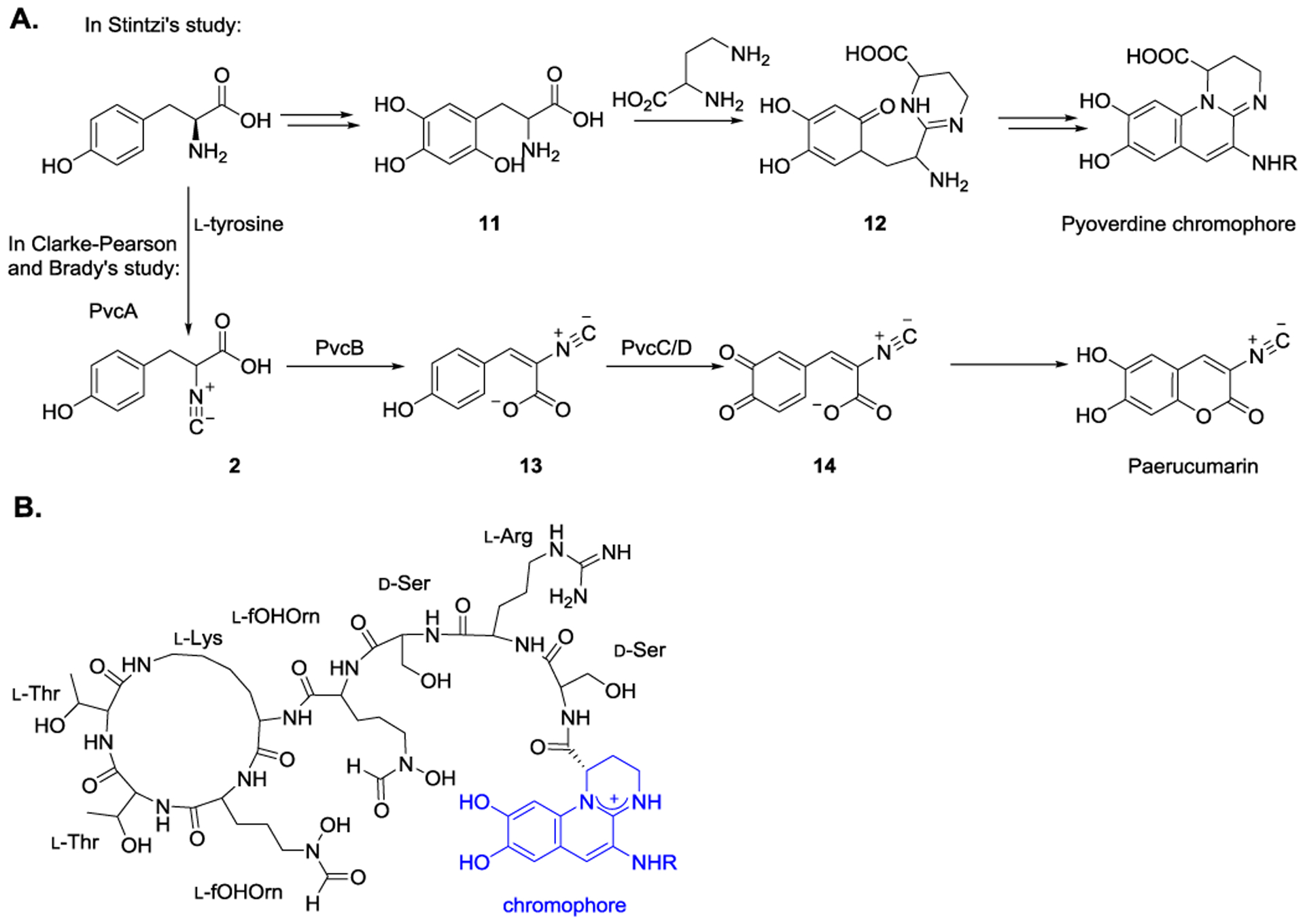
A) Proposed biosynthetic pathways for the formation of pyoverdine chromophore and paerucumarin. B) Representative structure of Pyoverdine. Pyoverdines is a group of green-fluorescent molecules composed of three parts: (i) a conserved fluorescent dihydroxyquinoline chromophore; (ii) an acyl side chain bound to the amino group of pyoverdine chromophore (R is either a glutamic acid, a α-ketoglutaric acid, a succinic acid or a malic acid); (iii) a variable peptide chain bound to the carboxyl group of pyoverdine chromophore by an amide group. The “fOHOrn” represses N5-formyl-N5-hydroxyornithine.
The role of pvc operon (pvcABCD) is associated with biofilm formation in Pseudomonas aeruginosa.[34,37] The mutation studies indicate the pvc operon affects biofilm formation through regulating chaperon/usher pathway.[37] Paerucumarin, the proposed pvcABCD product, enhances the expression of the chaperon/usher pathway (cup) proteins. Adding paerucumarin exogenously in the pvcA mutant significantly increased the expression level of cup proteins. Besides, it has been proposed that the expression of the iron-regulated genes was enhanced by the pvc operon through the iron-chelation by paerucumarin.[53] Recently, the modulation of swarming and quorum sensing of 2 and paerucumarin were reported.[54] Exogenously added 2 enhances the production of rhamnolipid, an extracellular surfactant which helps bacteria to swarm on semi-solid surfaces in P. aeruginosa, while adding paerucumarin does not affect rhamnolipid production. Since the enhanced swarming motility reduces biofilm production, this result implies the potential relationship between the isonitrile containing molecule and biofilm formation.
More recently, ambiguine (amb) biosynthetic gene cluster identified from Fischerella ambigua UTEX1903 provides the foundation for the biosynthetic studies of hapalindole-type natural products.[39] This type of natural product was first isolated from Stigonemataceae in 1984.[55] The family of hapalindole-type molecules constitutes a class of more than 70 homologs that include hapalindoles,[55–56] fischerindoles,[57] welwitindolinones[58] and ambiguines[59] (Figure 6A). These natural products share a common scaffold constructed using an indole with a monoterpene unit, and most of them are decorated with the isonitrile group or its derivative such as isothiocyanate group. Natural products belonging to hapalindoles are featured with their broad range of biological activities including insecticidal activity, antibacterial activity, and antimycotic activity.[60–63] Due to their structural diversities/complexities and attractive biological properties, numerous total synthetic strategies have been reported to chemically synthesize hapalindoles.[64–68] On the other hand, the corresponding biosynthesis remains unknown since the associated gene cluster had not been identified until 2013. AmbI1 and AmbI2, originated from Fischerella ambigua UTEX1903, are homologs to IsnA, while AmbI3 is a homolog to IsnB, thus suggesting that a similar strategy is used to install isonitrile group in ambiguines and hapalindoles (Figure 6B).[39] Later on, feeding experiments demonstrated that incubating l-tryptophan and ribulose-5-phosphate with AmbI1-3 resulted in the formation of the cis-vinyl isonitrile product (Z)-5.[39] (Z)-5 is proposed to be involved in amb-catalyzed biosynthesis of hapalindoles and ambiguines.[39]
Figure 6.
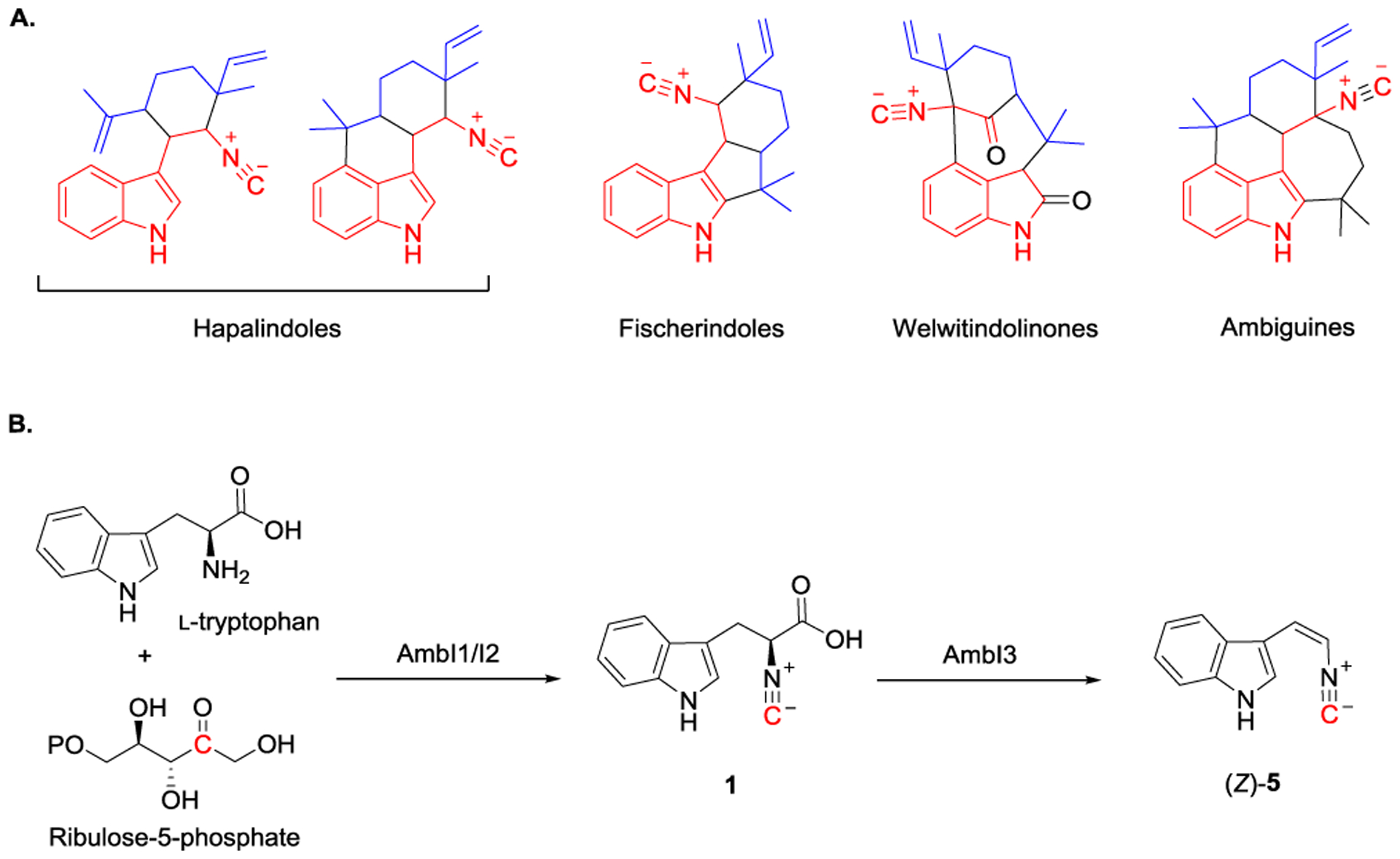
A) The family of hapalindole-type natural products include hapalindoles, fischerindoles, welwitindolinones and ambiguines. B) Proposed biosynthetic pathways of (Z)-5 formation catalyzed by AmbI1-3..
In addition to ambiguine, welwitindolinone biosynthetic gene cluster was identified from Hapalosiphon welwitschii UTEX B1830 in 2014.[40] Welwitindolinone was first isolated from the blue-green algae Hapalosiphon welwitschii.[58] Most of welwitindolinones possess a 3,4-disubstituted oxindole with bicyclo[4.3.1]-decane ring system (Figure 6A). Genetic analysis revealed that the wel gene cluster shows significant similarity with amb gene cluster, indicating a similar biosynthetic strategy is used in hapalindole-type natural product biosynthetic pathways. The feeding studies reveal that (Z)-isomer of 5 was also produced when AmbI1-3 homologs originated from UTEX B1830 (WelI1-3) were tested. However, Micallef et al. reported that WelI1-3 from Westiella intricata (UH strain HT-29-1) produce both (Z) and (E)-5.[41]
In addition to bacteria, an analogous approach in introducing isonitrile group is also utilized by yeasts and fungi. In Saccharomyces cerevisiae, Dit1 and Dit2 are proposed to be responsible for the production of dityrosine (15), a major component of yeast spore wall that protects spores during extreme environmental conditions.[42–43] Dit1, a homolog of IsnA, is proposed to catalyze isonitrile group installation. Dit2, a cytochrome P450 enzyme, is predicted to be involved in dimerization reaction of 2 to produce 16 (Figure 7A). Additionally, compound 17 and its potential pre-cursor 18 are also isolated in the sporulating cells. Taken together, the possible biosynthetic pathway leading to dityrosine (15) production was proposed.[44] Dit1 converts tyrosine to 2. The reaction is then followed by Dit2-catalyzed dimerization to form 16. Intermediate 16 could be further converted to 17, which leads to dityrosine (15) production. Alternatively, Dit2 could also use 18 as the substrate to produce 17.
Figure 7.
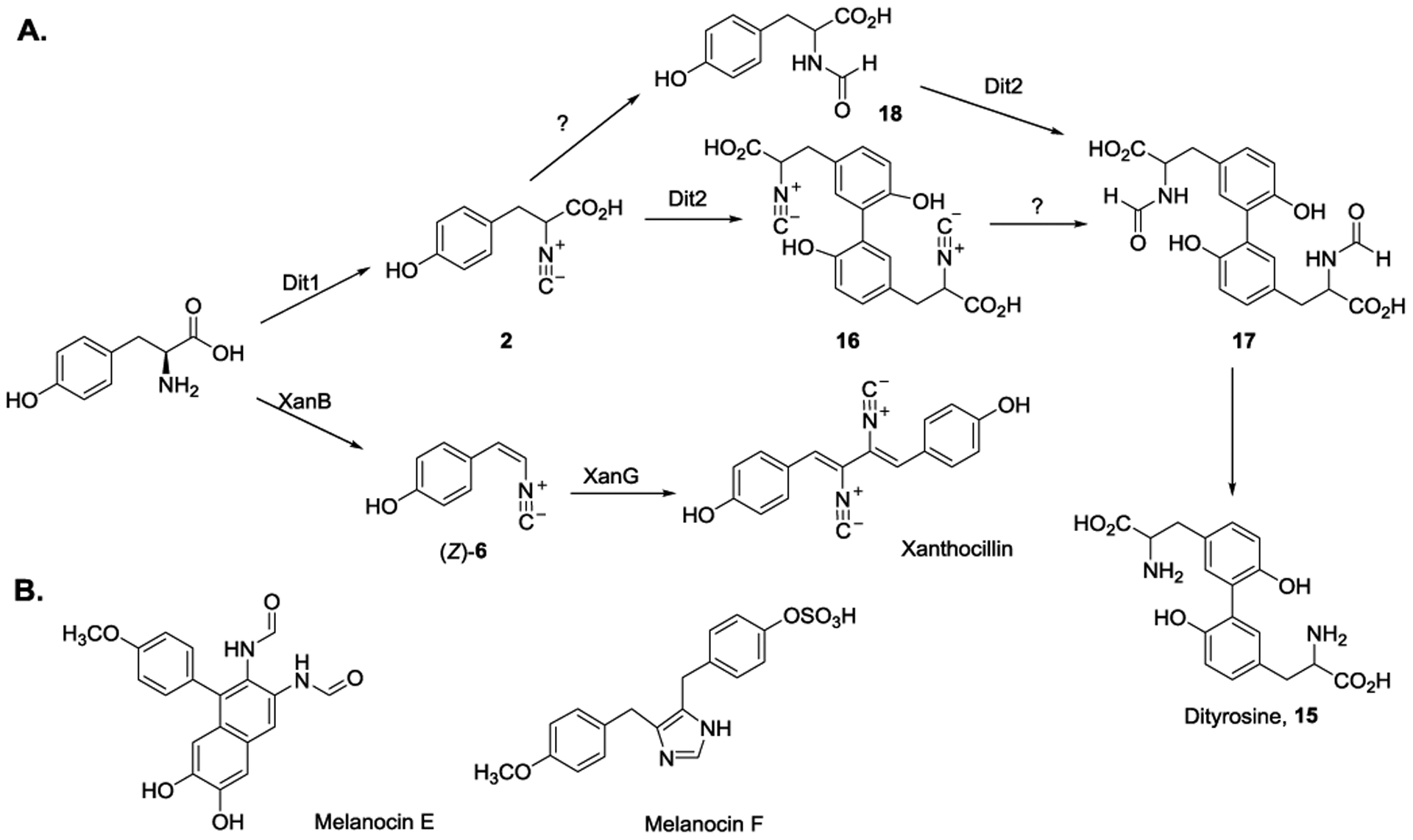
A) Proposed biosynthetic pathways of Dit1/Dit2- and XanB/XanG-catalyzed reactions that are involved in dityrosine (15) and xanthocillin formation. B) Structures of melanocin E and melanocin F isolated from Aspergillus fumigatus.
Recently, the biosynthesis of xanthocillin in human pathogen Aspergillus fumigatus was identified.[45] During copper starvation conditions, several xanthocillin-related compounds, including two novel molecules, melanocin E and melanocin F were isolated (Figure 7B). Under copper-depleted condition, the production of these compounds increases. These observations suggest possible copper-responsive metabolism and imply the possible biosynthetic pathway of these molecules. In the xanthocillin biosynthetic pathway, XanB and XanG are the homologs of Dit1 and Dit2. Thus, a similar pathway in producing dityrosine may be utilized in xanthocillin biosynthesis. Interestingly, XanB has both isonitrile-forming enzyme and Fe/2OG enzyme domains and is likely to catalyze vinyl isonitrile (Z)-6 formation (Figure 7A). To date, this is the only example where both isonitrile and olefin formation can be performed in a single enzyme. The reaction is then followed by XanG catalyzed dimerization to form xanthocillin.
Even though quite a few isonitrile-forming enzymes have been identified or suggested through bioinformatic analysis, isonitrile formation and detailed mechanism of this novel reaction remain to be elucidated. The first in vitro enzymatic production of (S)-3-(1H-indol-3-yl)-2-isocyanopropanoic acid (1), the proposed IsnA and AmbI1/AmbI2 reaction product, was achieved by Chang, Liu et al. in 2017.[69] To confirm the function of AmbI1 and AmbI2, deuterium-labeled tryptophan (d5-l-tryptophan) and ribulose-5-phosphate were employed as the substrates. The AmbI1/AmbI2 catalyzed reaction was monitored by liquid chromatography-mass spectrometry. A peak with a mass shift of +5 and having the same retention time as of standard 1 was observed. When AmbI3, the downstream Fe/2OG enzyme was introduced to the reaction, depletion of d5-1 along with the formation of (Z)-d5-5 were detected (Figure 8A). Based on these experimental observations, the activity of isonitrile-forming enzyme was verified and a potential mechanism accounting for the isonitrile group formation was proposed (Figure 8B). Starting with a condensation reaction between the amino group of l-tryptophan and the carbonyl group of ribulose-5-phosphate, an imine intermediate (19) is generated. After removal of the phosphate group, the carbon-carbon bond of β-keto imine (20) is cleaved via retro-aldol reaction pathway. The resulting isonitrile-containing intermediate (21) could then be fragmented into 1 with the release of formaldehyde as the co-product. Through the stepwise reconstitution of AmbI1/I2/I3 reactivities, these observations provide experimental supports of vinyl isonitrile biosynthetic pathways and pave the way for future mechanistic studies.
Figure 8.
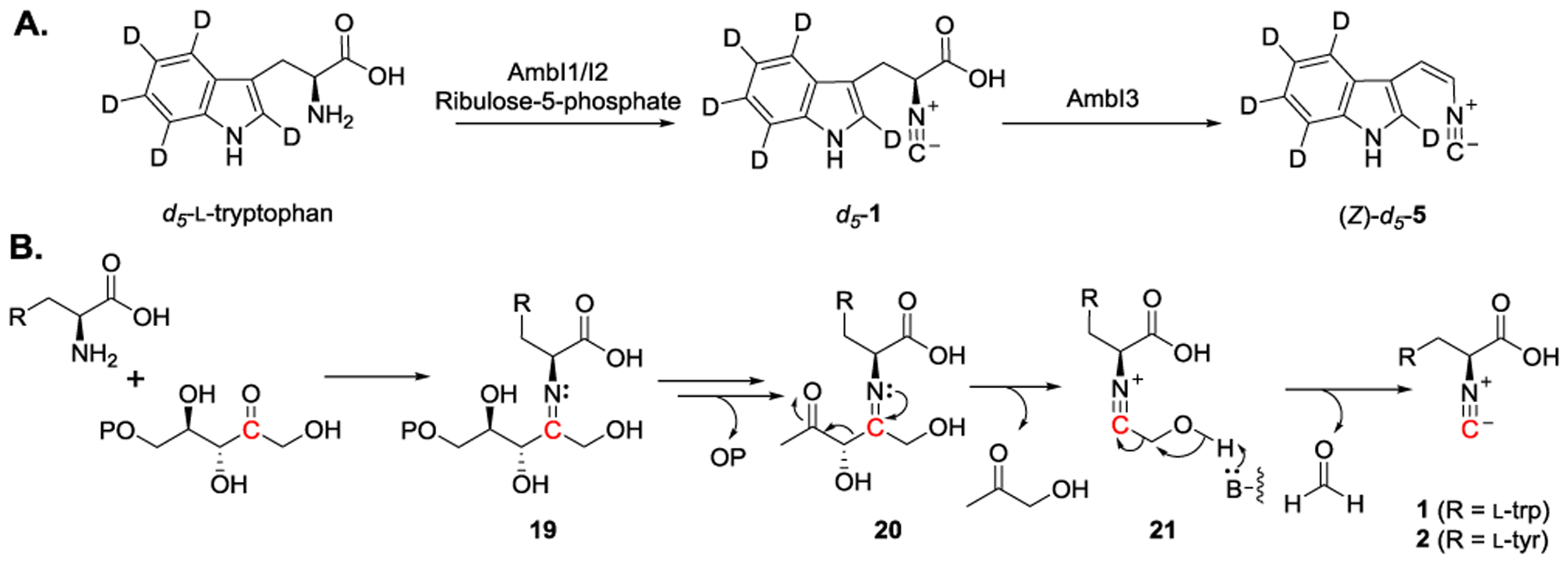
A) Detection of the elusive intermediate (d5-1.) from in vitro enzymatic reaction using AmbI1/I2. AmbI3 then catalyzes decarboxylation-assisted olefination to generate (Z)-d5-5. as the final product. B) Proposed mechanism accounting for the formation of isonitrile.
3. Isonitrile Formation through Decarboxylation-Assisted Desaturation
Recently, two structurally similar isonitrile-containing lipopeptides (22 and 23) were isolated via ScoA-E catalyzed biotransformation (Figure 9A).[17,46] Compounds 22 and 23 share the structural similarity to the other two isonitrile-containing antibiotics, SF2369 and SF2768, which were isolated from Actinomycetes and Streptomyces, respectively.[25,70] The corresponding biosynthetic pathway for 22 production is illustrated in Figure 9B. Briefly, ScoC, an acyl-acyl carrier protein ligase, starts the assembly line by introducing crotonic acid onto ScoB, an acyl carrier protein. The carboxylic acid moiety of crotonic acid is activated through adenylation using ATP. It is then followed by a substitution reaction to form a thioester where the crotonic acid moiety is loaded onto ScoB. Subsequently, ScoD is proposed to catalyze the addition of glycine at the β-position of ScoB-tethered crotonic acid. ScoE then catalyzes isonitrile formation by converting the glycine moiety to the corresponding isonitrile group. It is followed by ScoA, a nonribosomal peptide synthetase (NRPS), catalyzed condensation between the isonitrile-containing intermediate and l-lysine. The resulting isonitrile-containing peptide is then reductively released via the reductase domain to afford product 22.
Figure 9.
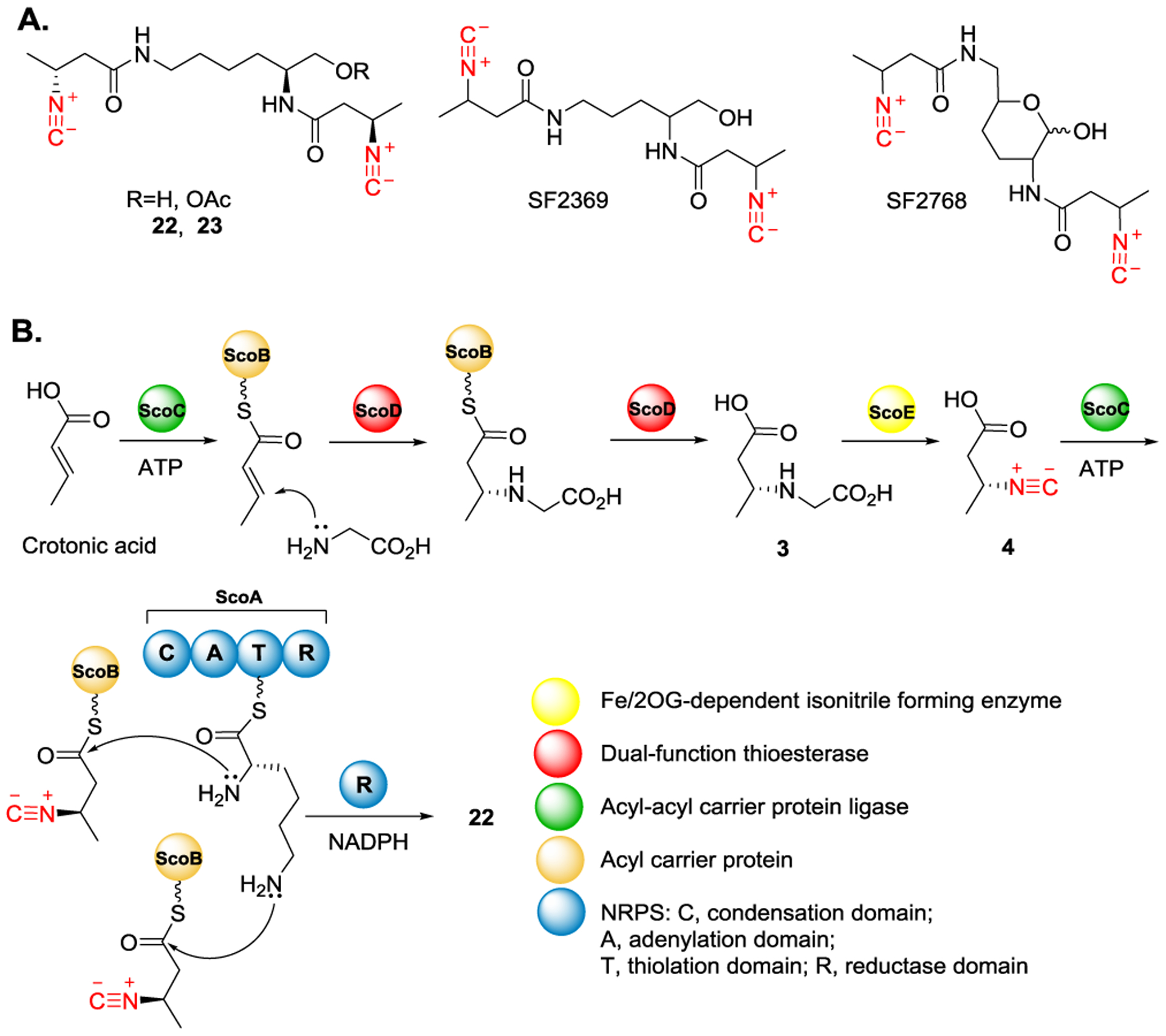
A) Structures of 22 and 23 that were isolated from ScoA-E-catalyzed reactions. 22 and 23 are structurally similar with SF2369 and SF2768. B) Proposed functions of ScoA-E in the biosynthesis of 22.
In comparison with ScoA-E originated from Streptomyces coeruleorubidus NRRL18370, similar biosynthetic clusters in Mycobacterium marinum strain M,[46] M. tuberculosis H37Rv[71–72], S. avermitilis MA-4680,[73–74] and S. thioluteus DSM 40027[48] were also identified (Figure 10A). Thus, similar biosynthetic machineries and possible correlation of these enzymatic reactions are used to produce other isonitrile-containing natural products. Within the multi-drug resistant tuberculosis strain, M. tuberculosis H37Rv, the genes (rv0096-0101) homologous to scoA-E, are associated with the survival of M. tuberculosis in the mouse macrophages through mutation studies.[71–72,75–77] Even though the plausible function of Rv0097, a ScoE homolog, has not been confirmed, in vivo studies suggest that the function of Rv0096/Rv0097 is associated with M. tuberculosis drug resistance in the lungs and spleens.[78] The crystal structure of Rv0098, a homolog to ScoD, in the presence of dodecanoyl-CoA reveals that Rv0098 is a long-chain fatty acyl-CoA thioesterase.[79–80] Moreover, SAV606, a homolog to Rv0098, identified from S. avermitilis MA-4680, has a dual function.[81] In addition to triggering the hydrolysis of the corresponding thioester to form N-carboxymethyl-3-aminononanoate (3), it also catalyzes the addition of glycine onto 24 (Figure 10B). Rv0099, also known as FadD10, a ScoC homolog, likely serves a function of as fatty acyl-AMP ligase. It was originally designated as a fatty acyl-CoA ligase.[82] However, Rv0099 transfers dodecanoyl moiety onto Rv0100, an acyl carrier protein, suggesting the function of Rv0099 as acyl-acyl carrier protein ligase.[83–84] Rv0101, a ScoA homolog, is a NRPS containing seven catalytic domains and may serve the similar function as ScoA.[83]
Figure 10.
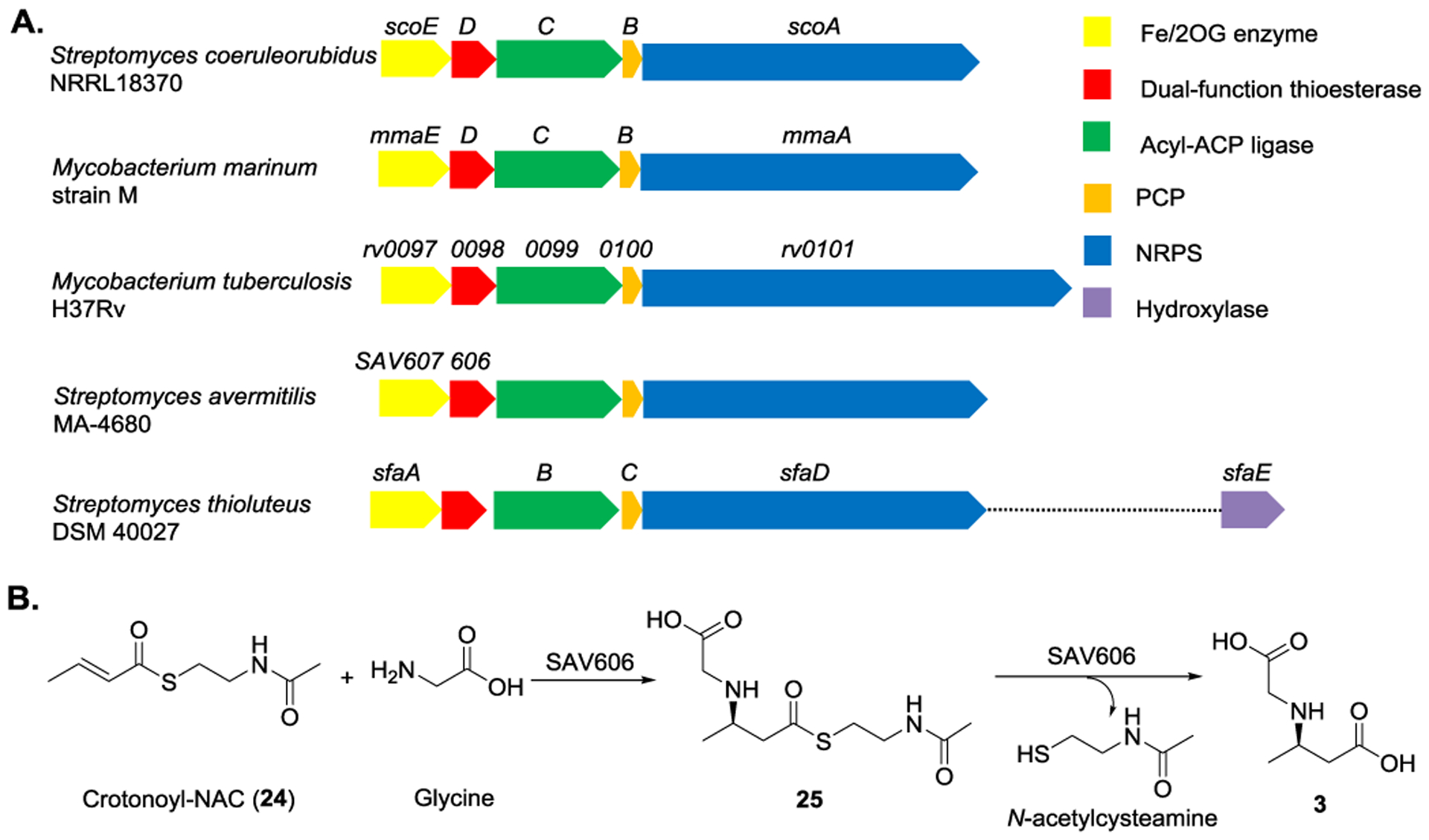
A) Schematic representation of the conserved biosynthetic gene clusters that are proposed to be involved in the biosynthesis of isonitrile-containing peptides. B) SAV606 is a dual function enzyme that can catalyze addition of glycine onto 24 followed by thioester hydrolysis of 25 to form 3.
Another gene cluster (SfaA-E) responsible for the biosynthesis of SF2768 was recently identified in S. thioluteus DSM 40027.[48] Following the analogous biosynthetic pathway used in biosynthesizing 22 and 23 by ScoA-E, SF2768 has been isolated and characterized as the product of SfaA-E reaction (Figure 11). Specifically, a putative dioxygenase, SfaA, was proposed to be involved in 4 formation. In addition, SfaE, a predicted asparaginyl beta-hydroxylase (as labeled with purple color in Figures 10A and 11), was proposed to catalyze δ-hydroxylation of the lysine residue of 26 to form 27. It is then followed by spontaneous hemiacetal formation to afford SF2768. SF2768 shows a preferential binding affinity toward copper than other metal ions.[48] Moreover, the intracellular cupric ion concentration in S. thioluteus was increased when incubating with copper-SF2768 complex. Therefore, SF2768 has been assigned as a chalkophore in S. thioluteus and its function may involve extracellular copper transportation into the cell.[48,85–86]
Figure 11.
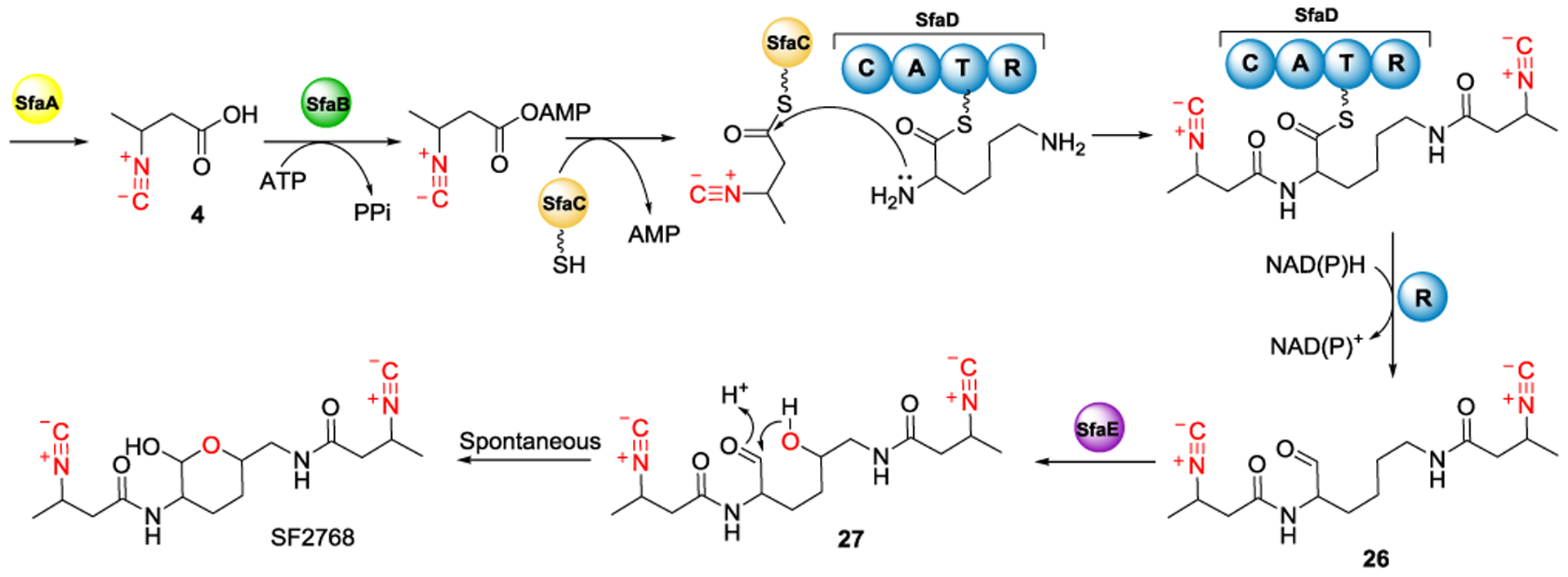
Proposed biosynthetic pathway accounting for the formation of SF2768 and its derivatives. Please refer to Figure 9 for the abbreviations.
4. Investigation of Fe/2OG Enzyme Catalyzed Isonitrile Formation Mechanism
The first mechanistic investigation of Fe/2OG enzyme-catalyzed isonitrile formation was reported in 2019. As revealed by the X-ray crystal structure of the zinc-bound ScoE, a possible reaction mechanism accounting for isonitrile group installation was proposed.[17] In the proposed mechanism, the reaction proceeds through an imine intermediate (28) formation. Following hydroxylation (28 → 29), decarboxylation and dehydration afford 4 (Figure 12A). Due to the absence of the substrate (3) and 2OG in the active site, the mechanistic details were not provided in the report. In 2020, based on the previously reported ScoE structure, Li et al. constructed the enzyme-substrate complex model.[47] Using molecular dynamic simulation and quantum mechanical/molecular mechanical calculation, they proposed that subsequent to C—H bond activation of 28, a reaction pathway involving hydroxylation (28 → 30 → 29) and then decarboxylation (29 → 4) is less likely due to a high energy barrier (Figure 12B, pathway I). Instead, an alternative pathway where the iron center acts as an electron relay mediator to lower the energy barrier for decarboxylation step (30 → 4) has been suggested (Figure 12B, pathway II).
Figure 12.
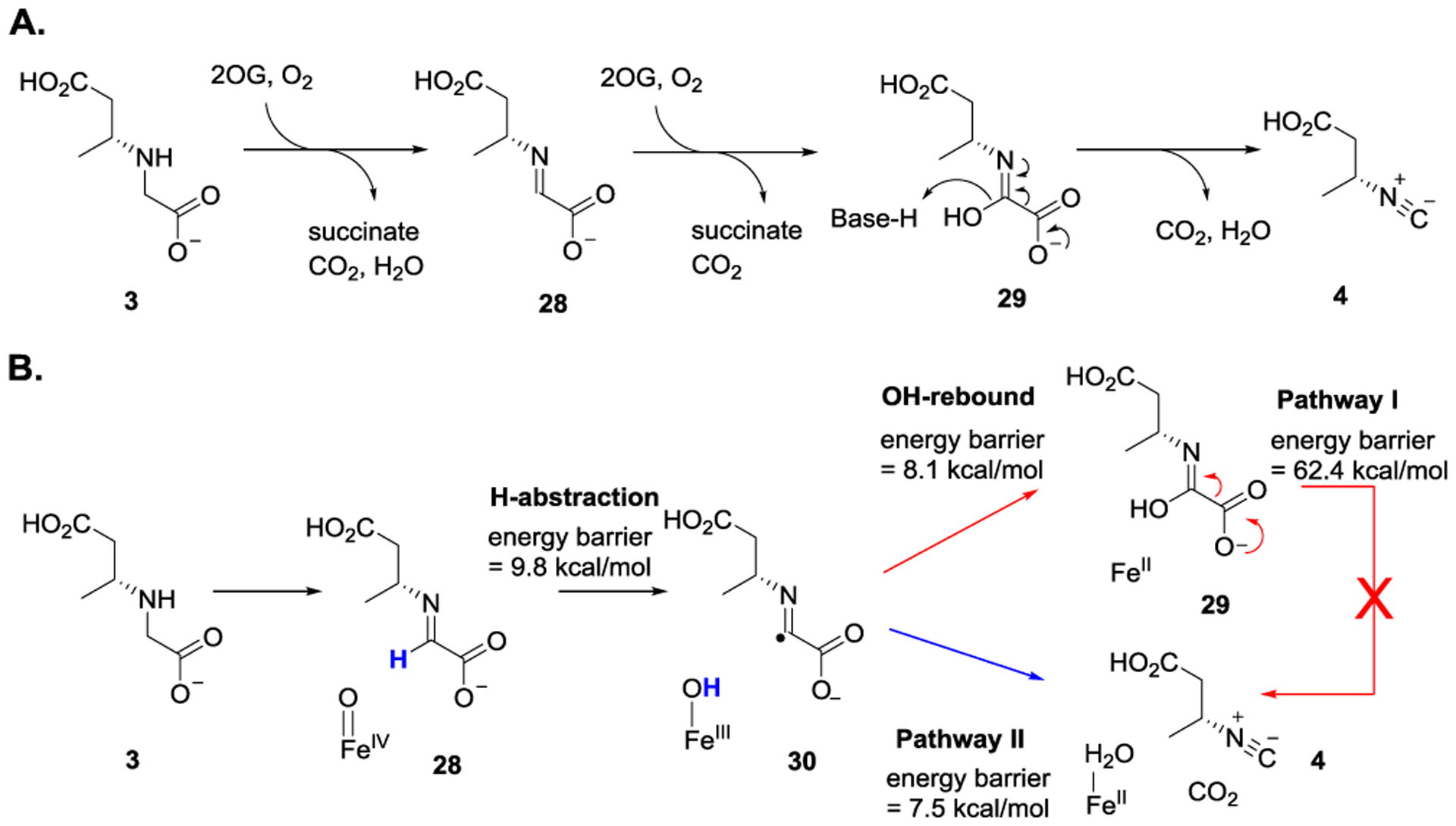
A) Possible reaction mechanism accounting for ScoE-catalyzed isonitrile formation. B) Based on molecular dynamic simulation and quantum mechanical/molecular mechanical calculation, the alternative decarboxylation pathway was proposed.
In 2020, in studying ScoE, SAV607, and SfaA, the three Fe/2OG enzymes that catalyze isonitrile formation, the requirement of two equivalents 2OG to complete an isonitrile group installation was established. This observation indicates that two sequential reactions are likely to be involved in isonitrile group installation (Figure 13A).[18] The substrate (3) and putative intermediate (31) bound ScoE structures solved at a resolution of 2.18 and 2.17 Å provide the structural information to hint the plausible reaction mechanism of this novel transformation (Figure 13B). In the substrate-bound structure, one of C5 hydrogen of 3 points toward the iron, suggesting C5 is the initial C—H activation site. This mechanistic hypothesis was confirmed using freeze-quench coupled Mössbauer analysis with the detection of the reactive intermediate, an Fe(IV)-oxo species. Observation of greater accumulation of Fe(IV)-oxo intermediate when deuterated isotopologue (5-2H2-3) was used indicated the possible C—H activation site. It is likely due to the H/2H kinetic isotope effect with the replacement of the targeted C5-H bond with C5-2H bond. Also, the structure of 31 and its plausible function were verified using the 13C-NMR. During the enzymatic reaction, by controlling the molar ratio of 5-13C-3 to 2OG, the newly formed peak with a chemical shift of ~91 ppm was detected by 13C NMR. Under the C—H coupling mode, this peak shows as a doublet indicating a single C—H bond connection. These observations are consistent with X-ray structural results. When the ratio of 5-13C-3 to 2OG was changed from 1 to 1.5 to 1 to 2.5, a decrease of 31 and the formation of a product (4) peak at δ ~150 ppm were observed in 13C NMR. Thus, compound 31 likely serves as the on-pathway intermediate during the isonitrile group installation.
Figure 13.
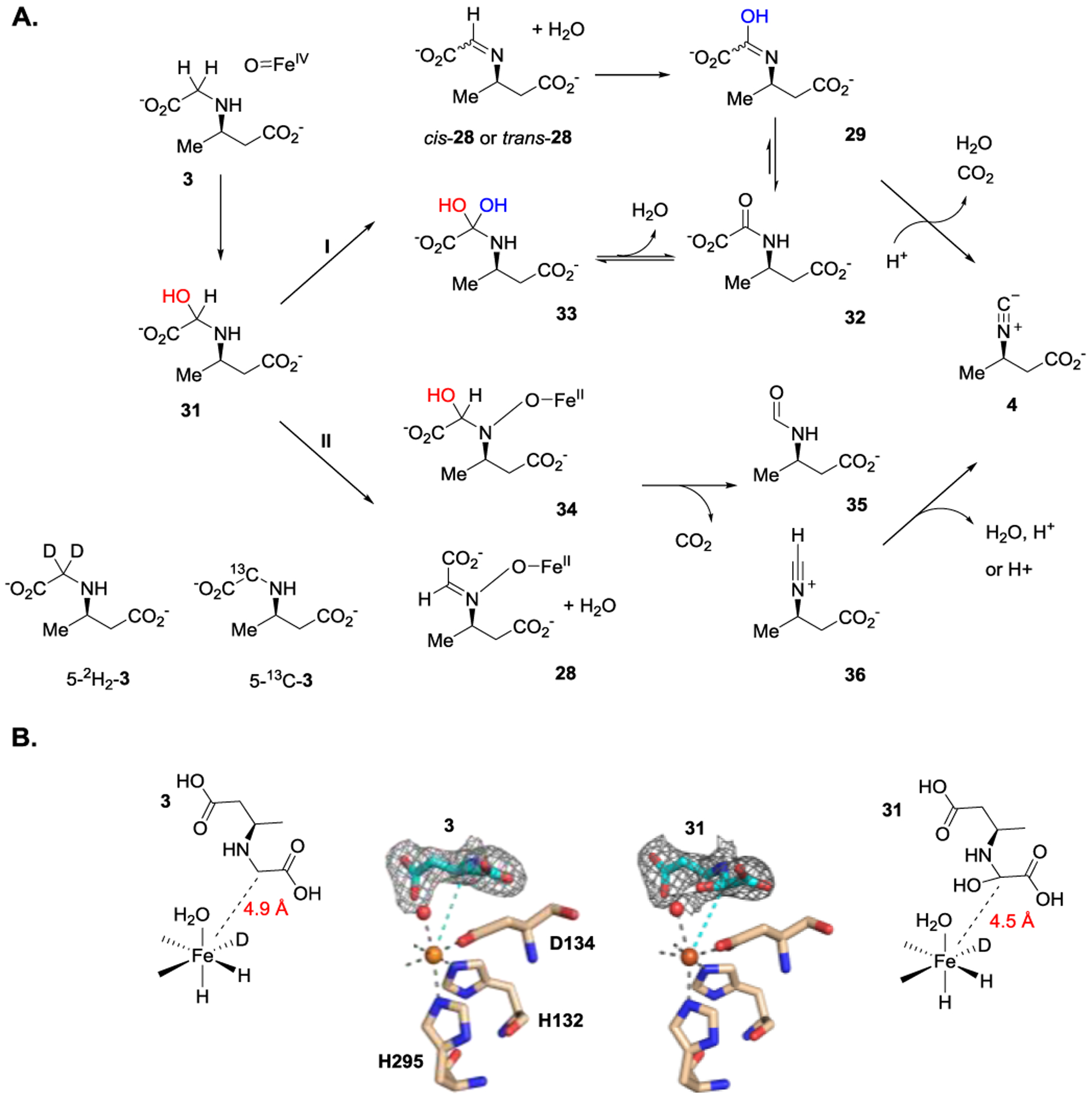
A) Proposed biosynthetic pathway accounting for isonitrile formation. B) The crystal structures of the substrate (3) and a putative intermediate (31) bound ScoE.
Taken together, the possible biosynthetic pathway is proposed in Figure 13A. The formation of 31 follows the classical hydroxylation reaction mechanism catalyzed by Fe/2OG enzymes. Subsequently, the reaction can proceed through dehydration to form 28. It is then followed by hydroxylation to form 29, which likely rapidly tautomerizes to its carbonyl from (32). The reaction then undergoes dehydration and decarboxylation to complete isonitrile group formation. Alternatively, the reaction can proceed through hydroxylation and then dehydration to produce 32 (31 → 33 → 32). To test these mechanistic hypotheses, cis-28 and 32 were prepared and tested. No obvious production of isonitrile 4 can be detected when cis-28 or 32 was used as an enzyme substrate, which indicates that a pathway involving cis-28 or 32 as the intermediate product is less likely (Figure 13A, pathway I). Different from a reaction pathway involving second C5 hydroxylation, the presumptive Fe(IV)-oxo species can trigger oxidative decarboxylation by activating the nitrogen atom. Nitrogen atom activation has been reported in iron-containing enzyme catalyzed reactions.[87–88] In this alternative pathway, the reaction proceeds through decarboxylation and dehydration to form isonitrile product (34 → 35 → 4). In addition, 31 can be dehydrated first. It is then followed by similar decarboxylation to generate isonitrile product (28 → 36 → 4). Although the detailed mechanism remains to be carefully evaluated, these results shed light on plausible mechanisms in constructing the isonitrile group found in isonitrile-containing peptides.
5. Conclusions
Natural products decorated with isonitrile group(s) have a wide variety of biological activities and potential pharmaceutical applications. Herein, we summarize the reported enzymes and the biosynthetic machineries that are responsible for isonitrile formation. To date, two fundamentally different approaches are used to install the isonitrile group. The first type involves a condensation reaction between the amino group of l-tryptophan/tyrosine and the carbonyl group of ribulose-5-phosphate, while the second type involves a decarboxylation-assisted desaturation. Although significant progress has been made in the biosynthetic and mechanistic studies of various systems, there is still considerable room for the field to grow to understand (bio)chemical logics toward isonitrile biosynthesis.
Acknowledgement
This work is supported by the Harry C. Kelly Memorial Fund for U.S.-Japan Scientific Cooperation, through the Office of Global Engagement and the NC Japan Center, at NC State University, National Institute of Health (GM127588 to W.-c. C., and Y.G.) and MOST (2019YFA09005000 and 2018YFA0901900 to J.Z).
Biography

Tzu-Yu Chen (top left) received her B.S. in Chemistry under the guidance of Professor Ken-Tsung Wong in 2018. She then joined the Ph.D program at North Carolina State University under the guidance of Prof. Wei-Chen Chang. Her research focuses on understanding the chemical logics of enzyme-catalyzed transformations.
Jinfeng Chen (top middle) was born in 1994 in Sichuan, China. In 2016, she received her B.S. in the Department of Chemistry from Sichuan Normal University. She is currently working towards completing of her Ph.D. under the guidance of Professor Jiahai Zhou at Shanghai Institute of Organic Chemistry, Chinese academy of Sciences. Her current researches focus on metalloenzyme crystallography.
Yijie Tang (top right) is a Ph.D. candidate under the supervision of Professor Yisong Guo at Carnegie Mellon University. He obtained the B.S degree at University of Science and Technology of China in 2014. His research concentrates on the mechanistic investigation of iron-containing metalloenzymes via transient kinetics techniques, bioinorganic spectroscopies, and molecular modeling.
Jiahai Zhou (bottom left) obtained his Ph.D. under the supervision of Professor Hai-Bao Chen at SIOC, CAS, in 2000. He did postdoctoral research with Professor Zhaohui Xu at University of Michigan‐Ann Arbor. In 2006, he started his independent academic career at SIOC. In 2020, he moved to Shenzhen Institutes of Advanced Technology. His current research interests focus on the structural enzymology on biosynthesis and biocatalysis, and AI assisted enzyme engineering on synthetic biology.
Yisong Guo (bottom middle) obtained his Ph.D. under Professor Stephen P. Cramer at UC, Davis, in 2009. During post-doctoral research, Dr. Guo first worked with Professor Eckard Münck and then with Professors Carsten Krebs and J. Martin Bollinger. In 2014, Dr. Guo started his independent career at Carnegie Mellon University. He is now an Associate Professor. Dr. Guo’s research interests lie at the intersection of bioinorganic/biophysical chemistry, and mechanistic enzymology.
Wei-chen Chang (bottom right) obtained his BS under Professor Tien-Yau Luh. He received his Ph.D. under Professor Hung-wen Liu at UT Austin in 2011. During 2011—2015, he worked with Professors J. Martin Bollinger and Carsten Krebs as postdoc fellow. In 2016, Dr. Chang started his independent career at North Carolina State University. His research focuses on mechanistic investigation of novel biological transformations found in natural product biosynthetic pathways.
References
- [1].American Chemical Society International Historic Chemical Landmarks. Discovery and Development of Penicillin. 1999. http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/flemingpenicillin.html (accessed October 12, 2020).
- [2].Giustiniano M; Basso A; Mercalli V; Massarotti A; Novellino E; Tron GC; Zhu J To each his own: isonitriles for all flavors. Functionalized isocyanides as valuable tools in organic synthesis. Chem. Soc. Rev 2017, 46, 1295–1357. [DOI] [PubMed] [Google Scholar]
- [3].Dömling A; Ugi I Multicomponent Reactions with Isocyanides. An- gew. Chem. Int. Ed 2000, 39, 3168–3210. [DOI] [PubMed] [Google Scholar]
- [4].Wang Q; Wang DX; Wang MX; Zhu J Still Unconquered: Enantioselective Passerini and Ugi Multicomponent Reactions. Acc. Chem. Res 2018, 51, 1290–1300. [DOI] [PubMed] [Google Scholar]
- [5].Zhang J; Yu P; Li SY; Sun H; Xiang SH; Wang JJ; Houk KN; Tan B Asymmetric phosphoric acid-catalyzed four-component Ugi reaction. Science 2018, 361, eaas8707. [DOI] [PubMed] [Google Scholar]
- [6].Sandoval IT; Manos EJ; Van Wagoner RM; Delacruz RG; Edes K; Winge DR; Ireland CM; Jones DA Juxtaposition of chemical and mutation-induced developmental defects in zebrafish reveal a copper-chelating activity for kalihinol F. Chem. Biol 2013, 20, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schnermann MJ; Shenvi RA Syntheses and biological studies of marine terpenoids derived from inorganic cyanide. Nat. Prod. Rep 2015, 32, 543–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Crawford JM; Portmann C; Zhang X; Roeffaers MB; Clardy J Small molecule perimeter defense in entomopathogenic bacteria. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 10821–10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cafieri F; Fattorusso E; Magno S; Santacroce C; Sica D Isolation and structure of axisonitrile-1 and axisothiocyanate-1 two unusual sesquiterpenoids from the marine spongeAxinella cannabina. Tetra- hedron 1973, 29, 4259–4262. [Google Scholar]
- [10].Rothe VW Das neue Antibiotikum Xanthocillin. Dtsch. Med. Wochenschr 1949, 79, 1080–1081. [DOI] [PubMed] [Google Scholar]
- [11].Gloer JB; Poch GK; Short DM; McCloskey DV Structure of Brassicicolin A: A Novel Isocyanide Antibiotic from the Phylloplane Fungus Alternaria brassicicola. J. Org. Chem 1988, 53, 3758–3761. [Google Scholar]
- [12].Brady SF; Bauer JD; Clarke-Pearson MF; Daniels R Natural Products from isnA-Containing Biosynthetic Gene Clusters Recovered from the Genomes of Cultured and Uncultured Bacteria. J. Am. Chem. Soc 2007, 129, 12102–12103. [DOI] [PubMed] [Google Scholar]
- [13].Manzo E; Ciavatta ML; Gavagnin M; Mollo E; Guo Y-W; Cimino G Isocyanide Terpene Metabolites of Phyllidiella pustulosa, a Nudibranch from the South China Sea. J. Nat. Prod 2004, 67, 1701–1704. [DOI] [PubMed] [Google Scholar]
- [14].Emsermann J; Kauhl U; Opatz T Marine Isonitriles and Their Related Compounds. Mar. Drugs 2016, 14, 1–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brady SF; Clardy J Cloning and heterologous expression of isocyanide biosynthetic genes from environmental DNA. Angew. Chem. Int. Ed 2005, 44, 7063–7065. [DOI] [PubMed] [Google Scholar]
- [16].Brady SF; Clardy J Systematic investigation of the Escherichia coli metabolome for the biosynthetic origin of an isocyanide carbon atom. Angew. Chem. Int. Ed 2005, 44, 7045–7048. [DOI] [PubMed] [Google Scholar]
- [17].Harris NC; Born DA; Cai W; Huang Y; Martin J; Khalaf R; Drennan CL; Zhang W Isonitrile Formation by a Non-Heme Iron(II)-Dependent Oxidase/Decarboxylase. Angew. Chem. Int. Ed 2018, 57, 9707–9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen TY; Chen J; Tang Y; Zhou J; Guo Y; Chang WC Pathway from N-Alkylglycine to Alkylisonitrile Catalyzed by Iron(II) and 2-Oxoglutarate-Dependent Oxygenases. Angew. Chem. Int. Ed 2020, 59, 7367–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pedras MS; Yaya EE The first isocyanide of plant origin expands functional group diversity in cruciferous phytoalexins: synthesis, structure and bioactivity of isocyalexin A. Org. Biomol. Chem 2012, 10, 3613–3616. [DOI] [PubMed] [Google Scholar]
- [20].Pedras MS; Yaya EE Dissecting metabolic puzzles through isotope feeding: a novel amino acid in the biosynthetic pathway of the cruciferous phytoalexins rapalexin A and isocyalexin A. Org. Biomol. Chem 2013, 11, 1149–1166. [DOI] [PubMed] [Google Scholar]
- [21].Moore RE; Cheuk C; Yang X-QG; Patterson GML Hapalindoles, Antibacterial and Antimycotic Alkaloids from the Cyanophyte Hapalosiphon fontinalis. J. Org. Chem 1987, 52, 1036–1043. [Google Scholar]
- [22].Park A; Moore RE; Patterson GML Fischerindole L, a New Isonitrile from the Terrestrial Blue-Green Alga Fischerella muscicola. Tetrahedron Lett 1992, 33, 3257–3260. [Google Scholar]
- [23].Stratmann K; Moore RE; Bonjouklian R; Deeter JB; Patterson GML; Shaffer S; Smith CD; Smitka TA Welwitindolinones, Unusual Alkaloids from the Blue-Green Algae Hapalosiphon welwitschii and Westiella intricata. Relationship to Fischerindoles and Hapalindoles. J. Am. Chem. Soc 1994, 116, 9935–9942. [Google Scholar]
- [24].Mo S; Krunic A; Chlipala G; Orjala J Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod 2009, 72, 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tabata Y; Hatsu M; Amano S; Shimizu A; Imai S SF2768, a new isonitrile antibiotic obtained from Streptomyces. Sci. Rep. Meiji Seika Kaisha 1995, 34, 1–9. [Google Scholar]
- [26].Parker WL; Rathnum ML; Johnson JH; Wells JS; Principe PA; Sykes RB Aerocyanidin, a new antibiotic produced by Chromo-bacterium violaceum. J. Antibiot 1988, 41, 454–460. [DOI] [PubMed] [Google Scholar]
- [27].Pishchany G; Mevers E; Ndousse-Fetter S; Horvath DJ Jr.; Paludo CR; Silva-Junior EA; Koren S; Skaar EP; Clardy J; Kolter R Amycomicin is a potent and specific antibiotic discovered with a targeted interaction screen. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 10124–10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hagadone MR; Burreson BJ; Scheuer PJ; Finer JS; Clardy J Defense Allomones of the Nudibranch Phyllidia varicosa Lamarck 1801. Helv. Chim. Acta 1979, 62, 2484–2494. [Google Scholar]
- [29].Mitome H; Shirato N; Miyaoka H; Yamada Y; Soest R. W. M.v. Terpene Isocyanides, Isocyanates, and Isothiocyanates from the Okinawan Marine Sponge Stylissa sp. J. Nat. Prod 2005, 67, 833–837. [DOI] [PubMed] [Google Scholar]
- [30].Burreson BJ; Scheuer PJ Isolation of a Diterpenoid Isonitrile from a Marine Sponge. J. Chem. Soc., Chem. Commun 1974, 1035–1036. [Google Scholar]
- [31].Meyer JM; Abdallah MA The Fluorescent Pigment of Pseudomonas fluorescens: Biosynthesis, Purification and Physicochemical Properties. J. Gen. Microbiol 1978, 107, 319–328. [Google Scholar]
- [32].Meyer JM; Hornspreger JM Role of Pyoverdinepf, the Iron-binding Fluorescent Pigment of Pseudomonas Jluorescens, in Iron Transport. J. Gen. Microbiol 1978, 107, 329–331. [Google Scholar]
- [33].Stintzi A; Johnson Z; Stonehouse M; Ochsner U; Meyer J-M; Vasil ML; Poole K The pvc Gene Cluster of Pseudomonas aeruginosa: Role in Synthesis of the Pyoverdine Chromophore and Regulation by PtxR and PvdS. J. Bacteriol 1999, 181, 4118–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Visca P; Imperi F; Lamont IL Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol 2007, 15, 22–30. [DOI] [PubMed] [Google Scholar]
- [35].Clarke-Pearson MF; Brady SF Paerucumarin, a new metabolite produced by the pvc gene cluster from Pseudomonas aeruginosa. J. Bacteriol 2008, 190, 6927–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Drake EJ; Gulick AM Three-dimensional structures of Pseudomonas aeruginosa PvcA and PvcB, two proteins involved in the synthesis of 2-isocyano-6,7-dihydroxycoumarin. J. Mol. Biol 2008, 384, 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Qaisar U; Luo L; Haley CL; Brady SF; Carty NL; Colmer-Hamood JA; Hamood AN The pvc Operon Regulates the Expression of the Pseudomonas aeruginosa Fimbrial Chaperone/Usher Pathway (Cup) Genes. Plos One 2013, 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhu J; Lippa GM; Gulick AM; Tipton PA Examining Reaction Specificity in PvcB, a Source of Diversity in Isonitrile-Containing Natural Products. Biochemistry 2015, 54, 2659–2669. [DOI] [PubMed] [Google Scholar]
- [39].Hillwig ML; Zhu Q; Liu X Biosynthesis of Ambiguine Indole Alkaloids in Cyanobacterium Fischerella ambigua. ACS Chem. Biol 2013, 9, 372–377. [DOI] [PubMed] [Google Scholar]
- [40].Hillwig ML; Fuhrman HA; Ittiamornkul K; Sevco TJ; Kwak DH; Liu X Identification and characterization of a welwitindolinone alkaloid biosynthetic gene cluster in the stigonematalean Cyanobacterium Hapalosiphon welwitschii. ChemBioChem 2014, 15, 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Micallef ML; Sharma D; Bunn BM; Gerwick L; Viswanathan R; Moffitt MC Comparative analysis of hapalindole, ambiguine and welwitindolinone gene clusters and reconstitution of indole-isonitrile biosynthesis from cyanobacteria. BMC Microbiol 2014, 14, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Briza P; Breitenbach M; Ellinger A; Segall J Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Gen. Dev 1990, 4, 1775–1789. [DOI] [PubMed] [Google Scholar]
- [43].Briza P; Eckerstorfer M; Breitenbach M The sporulation-specific enzymes encoded by the DITI and DIT2 genes catalyze a two-step reaction leading to a soluble LL-dityrosine-containing precursor of the yeast spore wall. Proc. Natl. Acad. Sci. U. S. A 1994, 91, 4524–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Briza P; Kalchhauser H; Pittenauer E; Allmaier G; Breitenbach MN,N’-Bisformyl dityrosine is an in vivo precursor of the yeast ascospore wall. Eur. J. Biochem 1996, 239, 124–131. [DOI] [PubMed] [Google Scholar]
- [45].Lim FY; Won TH; Raffa N; Baccile JA; Wisecaver J; Rokas A; Schroeder FC; Keller NP Fungal Isocyanide Synthases and Xanthocillin Biosynthesis in Aspergillus fumigatus. mBio 2018, 9, e00785–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Harris NC; Sato M; Herman NA; Twigg F; Cai W; Liu J; Zhu X; Downey J; Khalaf R; Martin J; Koshino H; Zhang W Biosynthesis of isonitrile lipopeptides by conserved nonribosomal peptide synthetase gene clusters in Actinobacteria. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 7025–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li H; Liu Y Mechanistic Investigation of Isonitrile Formation Catalyzed by the Non-Heme Iron/α-KG Dependent Decarboxylase (ScoE). ACS Catal 2020, 10, 2942–2957. [Google Scholar]
- [48].Wang L; Zhu M; Zhang Q; Zhang X; Yang P; Liu Z; Deng Y; Zhu Y; Huang X; Han L; Li S; He J Diisonitrile Natural Product SF2768 Functions As a Chalkophore That Mediates Copper Acquisition in Streptomyces thioluteus. ACS Chem. Biol 2017, 12, 3067–3075. [DOI] [PubMed] [Google Scholar]
- [49].González-Santoyo I; Córdoba-Aguilar A Phenoloxidase: a key component of the insect immune system. Entomol. Exp. Appl 2012, 142, 1–16. [Google Scholar]
- [50].Lu A; Zhang Q; Zhang J; Yang B; Wu K; Xie W; Luan YX; Ling E Insect prophenoloxidase: the view beyond immunity. Front. Physiol 2014, 5, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Takahashi S; Iwai H; Kosaka K; Miyazaki T; Osanai Y; Arao N; Tanaka K; Nagai K; Nakagawa A Byelyankacin: A Novel Melanogenesis Inhibitor Produced by Enterobactersp. B20. J. Antibiot 2007, 60, 717–720. [DOI] [PubMed] [Google Scholar]
- [52].Stintzi A; Cornelis P; Hohnadel D; Meyer J-M; Dean C; Poole K; Kourambas S; Krishnapillai V Novel pyoverdine biosynthesis gene(s) of Pseudomonas aeruginosa PA0. Microbiology 1996, 142, 1181–1190. [DOI] [PubMed] [Google Scholar]
- [53].Qaisar U; Kruczek CJ; Azeem M; Javaid N; Colmer-Hamood JA; Hamood AN The Pseudomonas aeruginosa extracellular secondary metabolite, Paerucumarin, chelates iron and is not localized to extracellular membrane vesicles. J. Microbiol 2016, 54, 573–581. [DOI] [PubMed] [Google Scholar]
- [54].Asif A; Iftikhar A; Hamood A; Colmer-Hamood JA; Qaisar U Isonitrile-functionalized tyrosine modulates swarming motility and quorum sensing in Pseudomonas aeruginosa. Microb. Pathog 2019, 127, 288–295. [DOI] [PubMed] [Google Scholar]
- [55].Moore RE; Cheuk C; Patterson GML Hapalindoles: New Alkaloids from the Blue-Green Alga Hapalosiphon fontinalis. J. Am. Chem. Soc 1984, 106, 6456–6457. [Google Scholar]
- [56].Moore RE; Cheuk C; Yang X-QG; Patterson GML; Bonjouklian R; Smitka TA; Mynderse JS; Foster RS; Jones ND; Swartzendruber JK; Deeter JB Hapalindoles, Antibacterial and Antimycotic Alkaloids from the Cyanophyte Hapalosiphon fontinalis. J. Org. Chem 1987, 52, 1036–1043. [Google Scholar]
- [57].Park A; Moore RE; Patterson GML Fischerindole-L, a New Isonitrile from the Terrestrial Blue-Green-Alga Fischerella-Muscicola. Tetrahedron Lett 1992, 33, 3257–3260. [Google Scholar]
- [58].Stratmann K; Moore RE; Bonjouklian R; Deeter JB; Patterson GML; Shaffer S; Smith CD; Smitka TA Welwitindolinones, Unusual Alkaloids from the Blue-Green Algae Hapalosip hon welwitschii and West iella intrica ta. Relationship to Fischerindoles and Hapalindoles. J. Am. Chem. Soc 1994, 116, 9935–9942. [Google Scholar]
- [59].Smitka TA; Bonjouklian F; Doolin L; Jones ND; Deeter JB; Yoshida WY; Prinsep MR; Moore RE; Patterson GML Ambiguine Isonitriles, Fungicidal Hapalindole-Type Alkaloids from Three Genera of Blue-Green Algae Belonging to the Stigonemataceae. J. Org. Chem 1992, 57, 857–861. [Google Scholar]
- [60].Mo SY; Krunic A; Chlipala G; Orjala J Antimicrobial Ambiguine Isonitriles from the Cyanobacterium Fischerella ambigua. J. Nat. Prod 2009, 72, 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Becher PG; Keller S; Jung G; Sussmuth RD; Juttner F Insecticidal activity of 12-epi-hapalindole J isonitrile. Phytochemistry 2007, 68, 2493–2497. [DOI] [PubMed] [Google Scholar]
- [62].Raveh A; Carmeli S Antimicrobial Ambiguines from the Cyanobacterium Fischerella sp. Collected in Israel. J. Nat. Prod 2007, 70, 196–201. [DOI] [PubMed] [Google Scholar]
- [63].Mo S; Krunic A; Santarsiero BD; Franzblau SG; Orjala J Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry 2010, 71, 2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Richter JM; Ishihara Y; Masuda T; Whitefield BW; Llamas T; Pohjakallio A; Baran PS Enantiospecific Total Synthesis of the Hapalindoles, Fischerindoles, and Welwitindolinones via a Redox Economic Approach. J. Am. Chem. Soc 2008, 130, 17938–17954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bhat V; Allan KM; Rawal VH Total synthesis of N-methylwelwitindolinone D isonitrile. J. Am. Chem. Soc 2011, 133, 5798–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Allan KM; Kobayashi K; Rawal VH A unified route to the welwitindolinone alkaloids: total syntheses of (−)-N-methylwelwitindolinone C isothiocyanate, (−)-N-methylwelwitindolinone C isonitrile, and (−)-3-hydroxy-N-methylwelwitindolinone C isothiocyanate. J. Am. Chem. Soc 2012, 134, 1392–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Styduhar ED; Huters AD; Weires NA; Garg NK Enantiospecific total synthesis of N-methylwelwitindolinone D isonitrile. Angew. Chem. Int. Ed 2013, 52, 12422–12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bhat V; Dave A; MacKay JA; Rawal VH The Chemistry of Hapalindoles, Fischerindoles, Ambiguines, and Welwitindolinones. Alkaloids Chem. Biol 2014, 73, 65–160. [DOI] [PubMed] [Google Scholar]
- [69].Chang W-C; Sanyal D; Huang JL; Ittiamornkul K; Zhu Q; Liu X In Vitro Stepwise Reconstitution of Amino Acid Derived Vinyl Isocyanide Biosynthesis: Detection of an Elusive Intermediate. Org. Lett 2017, 19, 1208–1211. [DOI] [PubMed] [Google Scholar]
- [70].Amano SI; Sakurai T; Endo K; Takano H; Beppu T; Furihata K; Sakuda S; Ueda K A cryptic antibiotic triggered by monensin. J. An- tibiot. (Tokyo) 2011, 64, 703. [DOI] [PubMed] [Google Scholar]
- [71].Cole ST; Brosch R; Parkhill J; Garnier T; Churcher C; Harris D; Gordon SV; Eiglmeier K; Gas S; III CEB; Tekaia F; Badcock K; Basham D; Brown D; Chillingworth T; Connor R; Davies R; Devlin K; Feltwell T; Gentles S; Hamlin N; Holroyd S; Hornsby T; Jagels K; Krogh A; McLean J; Moule S; Murphy L; Oliver K; Osborne J; Quail MA; Rajandream M-A; Rogers J; Rutter S; Seeger K; Skelton J; Squares R; Squares S; Sulston JE; Taylor K; Whitehead S; Barrell BG Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [DOI] [PubMed] [Google Scholar]
- [72].Camus J-C; Pryor MJ; Medigue C; Cole ST Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 2002, 148, 2967–2973. [DOI] [PubMed] [Google Scholar]
- [73].Omura S; Ishikawa HIJ; Hanamoto A; Takahashi C; Shinose M; Takahashi Y; Horikawa H; Nakazawa H; Osonoe T; Kikuchi H; Shiba T; Sakaki Y; Hattori M Genome sequence of an industrial microorganism Streptomyces avermitilis: Deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. U. S. A 2001, 98, 12215–12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ikeda H; Ishikawa J; Hanamoto A; Shinose M; Kikuchi H; Shiba T; Sakaki Y; Hattori M; Omura S Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol 2003, 21, 526–531. [DOI] [PubMed] [Google Scholar]
- [75].Sassetti CM; Rubin EJ Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. U. S. A 2003, 100, 12989–12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sassetti CM; Boyd DH; Rubin EJ Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol 2003, 48, 77–84. [DOI] [PubMed] [Google Scholar]
- [77].Rengarajan J; Bloom BR; Rubin EJ Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A 2005, 102, 8327–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dhar N; McKinney JD Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 12275–12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wang F; Langley R; Gulten G; Wang L; Sacchettini JC Identification of a type III thioesterase reveals the function of an operon crucial for Mtb virulence. Chem. Biol 2007, 14, 543–551. [DOI] [PubMed] [Google Scholar]
- [80].Maity K; Bajaj P; Surolia N; Surolia A; Suguna K Insights into the substrate specificity of a thioesterase Rv0098 of mycobacterium tuberculosis through X-ray crystallographic and molecular dynamics studies. J. Biomol. Struct. Dyn 2012, 29, 973–983. [DOI] [PubMed] [Google Scholar]
- [81].Chisuga T; Miyanaga A; Kudo F; Eguchi T Structural analysis of the dual-function thioesterase SAV606 unravels the mechanism of Michael addition of glycine to an alpha,beta-unsaturated thioester. J. Biol. Chem 2017, 292, 10926–10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sourjik V; Berg HC Functional interactions between receptors in bacterial chemotaxis. Nature 2004, 428, 437–441. [DOI] [PubMed] [Google Scholar]
- [83].Chhabra A; Haque AS; Pal RK; Goyal A; Rai R; Joshi S; Panjikar S; Pasha S; Sankaranarayanan R; Gokhale RS Nonprocessive [2 + 2]e-off-loading reductase domains from mycobacterial nonribosomal peptide synthetases. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 5681–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Liu Z; Ioerger TR; Wang F; Sacchettini JC Structures of Mycobacterium tuberculosis FadD10 protein reveal a new type of adenylate-forming enzyme. J. Biol. Chem 2013, 288, 18473–18483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhu M; Wang L; Zhang Q; Ali M; Zhu S; Yu P; Gu X; Zhang H; Zhu Y; He J Tandem Hydration of Diisonitriles Triggered by Isonitrile Hydratase in Streptomyces thioluteus. Org. Lett 2018, 20, 3562–3565. [DOI] [PubMed] [Google Scholar]
- [86].Zhu M; Wang L; He J Chemical Diversification Based on Substrate Promiscuity of a Standalone Adenylation Domain in a Reconstituted NRPS System. ACS Chem. Biol 2019, 14, 256–265. [DOI] [PubMed] [Google Scholar]
- [87].He H-Y; Henderson AC; Du Y-L; Ryan KS Two-Enzyme Pathway Links l-Arginine to Nitric Oxide in N-Nitroso Biosynthesis. J. Am. Chem. Soc 2019, 141, 4026–4033. [DOI] [PubMed] [Google Scholar]
- [88].Ng TL; Rohac R; Mitchell AJ; Boal AK; Balskus EP An N-nitrosating metalloenzyme constructs the pharmacophore of streptozotocin. Nature 2019, 566, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


