Abstract
Background:
A naturally occurring loss-of-function mutation in the gene for C-C chemokine receptor type 5 (CCR5-Δ32) has recently been reported as a protective factor in post-stroke motor and cognitive recovery. We sought to examine whether this mutation also prevented the development of depressive symptoms up to 2 years after a stroke.
Methods:
Participants were survivors of a first-ever mild to moderate ischemic stroke or transient ischemic attack from the TABASCO prospective study who underwent a 3 T MRI at baseline and were examined by a multiprofessional team 6, 12 and 24 months after the event, including an evaluation of depressive symptoms using the Geriatric Depression Scale.
Results:
CCR5-Δ32 status and a baseline depression evaluation were available for 435 patients. Compared with noncarriers, CCR5-Δ32 carriers (16.1%) had fewer depressive symptoms at admission (p = 0.035) and at 6 months (p < 0.001), 12 months (p < 0.001) and 24 months (p = 0.006) after the index event. This association remained significant at 6 and 12 months after adjustment for age, sex, education, antidepressant use, ethnicity and the presence of cortical infarcts. These findings were more robust in women. Compared to baseline, depressive symptoms in CCR5-Δ32 noncarriers tended to remain stable or grow worse over time, but in CCR5-Δ32 carriers, symptoms tended to improve.
Limitations: A limitation of this study was the exclusion of patients who had a severe stroke or who had pre-stroke depression.
Conclusion:
Carriers of the CCR5-Δ32 allele had a lower tendency to develop depressive symptoms post-stroke, and this phenomenon was more prominent in women. These findings could have clinical implications; they suggest a mechanism-based treatment target for post-stroke depression. Drugs mimicking this loss-of-function mutation exist and could serve as a novel antidepressant therapy.
Introduction
Depression is a distressing and frequent neuropsychiatric complication of brain ischemia that affects 18% to 61% of patients after stroke.1–3 Post-stroke depression is associated with increased mortality,4 higher disability, lower quality of life5 and a greater risk of cognitive decline.6 Although it is of great clinical relevance, the relationship between stroke and depression remains relatively unexplained, and specific mechanism-based treatment targets are lacking.
The traditional monoamine hypothesis of depression, which focuses on the dysfunction of serotonin and noradrenaline (norepinephrine), fails to address the complexity of the pathogenesis of depression.7 Indeed, depression can be perceived as a psychoneuroimmunological disorder in which inflammatory mechanisms play a crucial role.8,9 Other theories focus on synaptic plasticity as an important means of recovery from depression through converging disrupted molecular and cellular mechanisms that cause dysfunction of the circuitry essential for mood regulation.10
We recently reported that C-C chemokine receptor type 5 (CCR5), an established factor involved in immune processes and neuromodulation,11 is a promising molecular target for post-stroke recovery. CCR5 knockdown in rodents promoted motor recovery after stroke and increased axonal sprouting.12 In humans, a naturally occurring mutation that leads to partial loss of function of CCR5 (CCR5-Δ32, rs333) improved stroke recovery based on distinct measures of cognitive and motor function.12
We sought to examine whether stroke survivors who were carriers of the CCR5-Δ32 mutation would also develop fewer depressive symptoms than noncarriers up to 2 years post-stroke.
Methods
Study population
Participants were consecutive eligible patients from the Tel Aviv Brain Acute Stroke Cohort (TABASCO) study,13 an observational cohort study aimed at identifying predictors for post-stroke cognitive decline and other neuropsychiatric sequelae using systematic neurocognitive evaluations, neuroimaging and genetic data. Patients included were men and women older than 50 years, admitted within 72 hours after a first-ever acute ischemic stroke or transient ischemic attack (TIA), with a total score on the National Institutes of Health Stroke Scale (NIHSS) of less than 17.14 Exclusion criteria were as follows: hemorrhagic stroke, cognitive impairment before the stroke (determined by an Informant Questionnaire on Cognitive Decline in the Elderly post-score of 3.3 or higher),15 substance abuse other than nicotine, and severe aphasia or physical disability that made the possibility of follow-up unlikely.
Additional exclusion criteria for the current substudy were a diagnosis of depression and/or an anxiety disorder before the stroke, or treatment with selective serotonin reuptake inhibitors or serotonin–norepinephrine reuptake inhibitors upon admission.
We collected information on demographics, medical history, physical activity, work, economic and marital status, and functional scores at baseline and follow-up, as previously reported.13
Protocol approvals, registrations and patient consent
This study was registered at ClinicalTrials.gov (NCT01926691). All participants signed informed consent forms, approved by the local ethics committee.
Determination of CCR5 genotype
Genomic DNA was extracted from white blood cells taken from citrated blood and then amplified by polymerase chain reaction (PCR) as previously described,12,16 using 5′-CCTGGCTGTCGTCCATGCTG-3′ and 5′-CTGATCTAGAGCCATGTGCACAACTCT-3′ as forward and reverse primers, respectively. These primers amplify a 735 bp fragment. Following PCR amplification of genomic DNA, the amplified products were digested using EcoRI (New England Biolabs), and the digested products were detected following electrophoresis on a 4% MetaPhor agarose gel stained with ethidium bromide. After restriction, the 735 bp PCR product was cleaved into a common band of 332 bp for both alleles, a 403 bp product for the wild-type allele and a 371 bp product for the mutant Δ32 allele. Heterozygous individuals had both wild-type and mutant alleles and demonstrated 3 bands (403, 371 and 332 bp).
Determination of the length of polymorphism repeats in the promoter region of the serotonin transporter gene
We explored the association between CCR5-Δ32 genotype and another candidate gene for the prevention of post-stroke depression — the gene for the serotonin-transporter-linked polymorphic region (5-HTTLPR), which is located in the promoter region of the serotonin transporter gene on chromosome 17 and consists of short (S) and long (L) alleles resulting from a 43 bp insertion or deletion. The 5-HTTLPR LL genotype has been negatively associated with post-stroke depression.17 We used primers flanking 5-HTTLPR to generate a 484/528 bp fragment and then determined genotype as previously described.18
Using PCR, we also analyzed APOE genotypes from blood sampled during the patient’s hospitalization.19
Depressive symptoms
Depressive symptoms were assessed within 72 hours of admission and again at 6, 12 and 24 months later, using the 15-item Geriatric Depression Scale (GDS),20 in which a score of 6 or higher may indicate a depressive state.20 The GDS has been shown to have acceptable internal consistency and reliability (Cronbach α ≥ 0.70) in older adults from a range of populations.21 All raters involved in conducting cognitive and depression assessments were blinded to genetic and clinical data.
MRI protocol
We acquired MRI images within 7 days of stroke onset on a 3 T GE scanner (GE Signa EXCITE) using an 8-channel head coil and according to a previously described protocol.13,22 All axial slices were prescribed on the same orientation, covering the whole brain, aligned along the fourth ventricle–orbitofrontal orientation. The MRI analyses have been previously reported.22
Statistical analyses
To track differences in depression trajectories over time, we used a repeated-measures approach based on a generalized linear model for the comparison of GDS scores at the 4 observation points between carriers and noncarriers. We analyzed statistical differences between the longitudinal GDS curves of the different genotypes using 2-way analysis of variance with Bonferroni correction. We used a multiple linear regression model to evaluate the relationship between CCR5-Δ32 alleles and GDS at admission and 6, 12 and 24 months post-stroke, adjusted for age, sex, education, administration of selective serotonin reuptake inhibitors or serotonin–norepinephrine reuptake inhibitors, ethnicity and the presence of cortical infarcts. We used multivariate analysis of variance to test for the main effects of CCR5 and 5-HTTLPR alleles, as well as for an interaction effect of CCR5 × 5-HTTLPR on GDS scores.
Further details of our statistical methods are provided in Appendix 1, available at jpn.ca/200197-a1.
Results
We initially evaluated 575 consecutive eligible cognitively intact patients at baseline who were admitted to the Department of Emergency Medicine at Tel Aviv Sourasky Medical Center from Apr. 1, 2008, to Dec. 1, 2014, within 72 hours of the onset of symptoms of a TIA or mild to moderate ischemic stroke. Of those, we determined CCR5 genotype in 564 participants; 482 completed GDS questionnaires at admission, and 47 were excluded based on the exclusion criteria. This left a total of 435 patients for analysis. Patients included in the study were slightly younger than those who were excluded (67 ± 9.7 yr v. 69.2 ± 10.6 yr; p = 0.031), but we observed no other differences in baseline characteristics between patients who were included and those who were excluded.
Participant characteristics
A summary of the baseline characteristics of study participants is presented in Table 1. Participants had a mean (± standard deviation) age of 67 ± 9.8 years and 62.1% were male. Of the total sample, 365 participants (83.9%) were homozygotes for the CCR5 wild-type allele (noncarriers); 66 (15.2%) were heterozygotes for the CCR5-Δ32 variant allele; and 4 (0.9%) were homozygotes for the variant allele. Allele frequencies were in Hardy–Weinberg equilibrium. Because only a small number of participants were CCR5-Δ32 homozygotes, we pooled all CCR5-Δ32 variant allele carriers into a single group (n = 70) and compared this group with the noncarriers (n = 365). Among patients who had a stroke (n = 302; 69.4% of the total sample), stroke etiologies (based on Trial of Org 10172 in Acute Stroke Treatment [TOAST] criteria) were as follows: 185 lacunar strokes (61.3% of stroke patients), 35 cardioembolic strokes (11.5%), 25 large-artery atherosclerotic strokes (8.3%), 57 strokes of other or undetermined etiology (18.9%). The rest of the participants had TIAs (n = 133; 30.6% of the total sample). We observed no differences in CCR5-Δ32 distribution across stroke subtypes or between stroke and TIA patients.
Table 1.
Baseline and follow-up characteristics of post-stroke survivors (n = 435)*
| Characteristic | CCR5-Δ32 noncarriers (n = 365) | CCR5-Δ32 carriers (n = 70)† | p value |
|---|---|---|---|
| Age, yr | 66.7 ± 9.7 | 68.9 ± 9.9 | 0.08 |
| Male | 232 (63.6) | 38 (54.3) | 0.030 |
| Education, yr | 12.7 ± 3.8 | 14.7 ± 3.8 | < 0.001 |
| Body mass index, kg/m2 | 27.1 ± 4.7 | 27.4 ± 3.1 | 0.49 |
| Ethnicity, Ashkenazi | 189 (51.8) | 60 (85.7) | < 0.001 |
| Current smoker | 85 (23.3) | 17 (24.3) | 0.69 |
| Diabetes mellitus | 108 (29.6) | 22 (31.4) | 0.76 |
| Dyslipidemia | 201 (55.1) | 38 (54.3) | 0.90 |
| Hypertension | 224 (61.4) | 41 (58.6) | 0.66 |
| Ischemic heart disease | 64 (17.5) | 15 (21.4) | 0.44 |
| Framingham Risk Score for stroke | 11.3 ± 5.5 | 12.4 ± 5.8 | 0.22 |
| APOE ɛ4 allele | 59 (16.2) | 11 (15.7) | 0.95 |
| NIHSS at hospital admission, median (interquartile range) | 2.00 (0.00–4.00) | 2.00 (0.00–3.00) | 0.005 |
| C-reactive protein at hospital admission, mg/L | 8.0 ± 16.0 | 4.4 ± 6.0 | 0.003 |
| Transient ischemic attack | 109 (29.9) | 24 (34.3) | 0.46 |
| Geriatric Depression Scale score, median (interquartile range) | |||
| At hospital admission | 2.00 (1.00–4.00) | 2.00 (0.00–3.00) | 0.035 |
| 6 months post-stroke | 2.00 (0.00–4.00) | 1.00 (0.00–3.00) | < 0.001 |
| 12 months post-stroke | 2.00 (0.00–4.00) | 1.00 (0.00–2.00) | < 0.001 |
| 24 months post-stroke | 2.00 (0.00–4.00) | 1.00 (0.00–2.25) | 0.006 |
| Brain MRI parameters | |||
| No MRI performed | 86 (23.6) | 14 (20) | 0.52 |
| Infarct type‡ | |||
| No infarct | 83 (29.7) | 19 (33.9) | 0.69 |
| Cortical | 61 (21.9) | 22 (39.3) | 0.005 |
| Subcortical | 101 (36.2) | 11 (19.6) | 0.07 |
| Subtentorial | 34 (12.2) | 4 (7.1) | 0.13 |
| Ischemic lesion volume, mm3 | 7459.1 ± 1202.8 | 7372.4 ± 1170.6 | 0.65 |
| Intracranial volume, mm3 | 1 458 197.3 ± 168 608 | 1 422 139.9 ± 195 470.8 | 0.19 |
| Total white matter volume, mm3 | 444 438 ± 64 184.7 | 442 681.9 ± 69 646.4 | 0.86 |
| Total grey matter volume, mm3 | 578 935.1 ± 60 663 | 564 562.1 ± 66 959.7 | 0.14 |
| Microbleed, lobar | 34 (9.3) | 10 (14.3) | 0.21 |
| Microbleed, deep | 15 (4.1) | 2 (2.9) | 0.62 |
| Lacunes | 112 (30.7) | 21 (30.0) | 0.91 |
| Enlarged perivascular spaces | 258 (70.7) | 54 (77.1) | 0.27 |
NIHSS = National Institutes of Health Stroke Scale.
Values are mean ± standard deviation or n (%), unless otherwise indicated.
66 heterozygote, 4 homozygote.
Percentages based on only patients who had an MRI (n = 279 for CCR5-Δ32 noncarriers; n = 56 for CCR5-Δ32 carriers).
CCR5-Δ32 carriers had more education but did not differ from noncarriers in terms of cardiovascular risk factors or frequency of the APOE ɛ4 allele. Of the CCR5-Δ32 carriers, 85.7% were Ashkenazi in origin, compared to 51.8% of noncarriers (the CCR5-Δ32 mutation has been described as more prevalent among Ashkenazi Jews23). CCR5-Δ32 carriers had lower C-reactive protein at admission. Among the patients who were excluded from the study because of antidepressant therapy at hospital admission or a diagnosis of depression and/or an anxiety disorder before their stroke or TIA (n = 47), the prevalence of CCR5-Δ32 was 14.9%, compared to 16.1% of patients included in the study. This difference was not statistically significant.
CCR5-Δ32 status, neurological findings at admission and MRI results
The neurological (NIHSS) scores of CCR5-Δ32 carriers were slightly but significantly lower at hospital admission compared to noncarriers (2.00 [0.00–3.00] v. 2.00 [0.00–4.00], respectively; p = 0.005; Table 1). CCR5-Δ32 carriers also had a higher frequency of lesions in cortical territories (39.3% of lesions in carriers v. 21.9% of lesions in noncarriers; p = 0.005). Both patient groups had low NIHSS scores, because only survivors of mild to moderate stroke were included in the study, but among patients with relatively more neurologic deficits (NIHSS ≥ 5) only a small number were CCR5-Δ32 carriers (5% carriers v. 95% noncarriers; p = 0.015). We observed no relationship between CCR5-Δ32 status and lesion side, infarct volume, stroke etiology or any other MRI finding, including measures of small vessel disease.
CCR5-Δ32 and depressive scores
All included participants had a completed GDS assessment during hospitalization; 322 had a completed GDS at 6 months, 293 at 12 months, and 268 at 24 months.
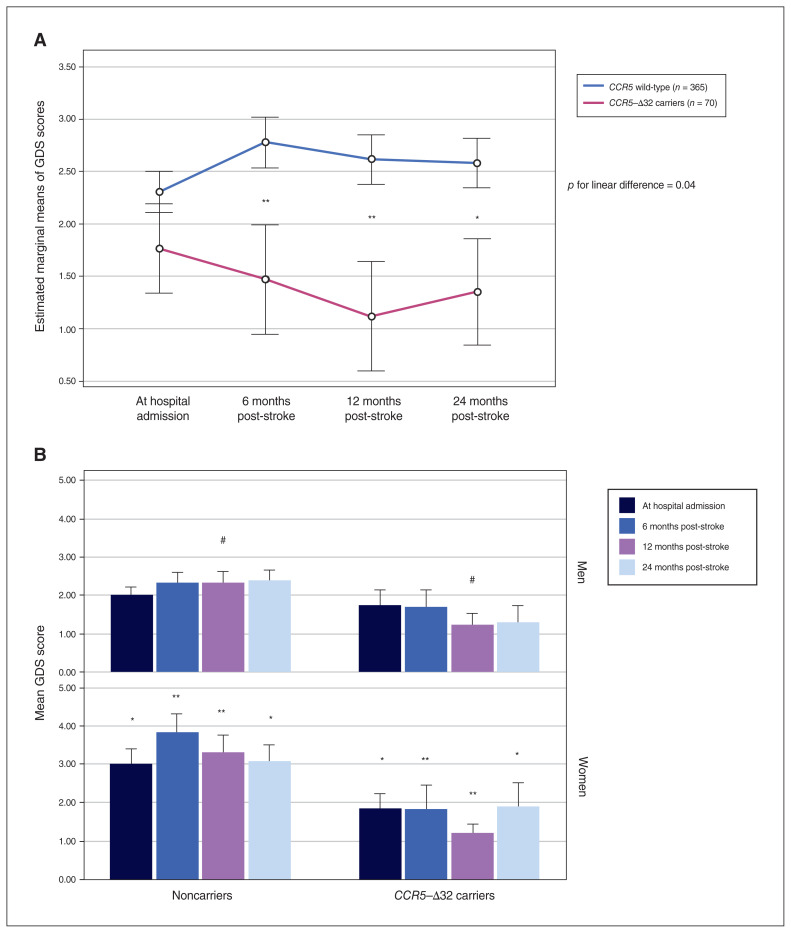
Compared to noncarriers, CCR5-Δ32 carriers had fewer depressive symptoms (lower GDS scores) at admission and at 6, 12 and 24 months after the index event (p = 0.035, p < 0.001, p < 0.001 and p = 0.006, respectively; Table 1). After adjustment for age, sex, education, administration of antidepressants during follow-up, ethnicity and the presence of cortical infarcts, the association between CCR5-Δ32 status and GDS score remained significant only at 6 and 12 months post-stroke (Table 2 and Figure 1A), although the trend was similar at the other time points. In terms of depressive symptoms, noncarriers tended to remain stable or develop worse symptoms over time, and CCR5-Δ32 carriers tended to improve (Figure 1A).
Table 2.
CCR5-Δ32 allele in relation to depressive scores after stroke or transient ischemic attack
| Model 1, unadjusted | Model 2, adjusted* | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Geriatric Depression Scale score | β | SE | p value | β | SE | p value |
| At admission | −0.081 | 0.357 | 0.09 | — | — | — |
| 6 months post-stroke | −0.149 | 0.490 | 0.011 | −0.111 | 0.475 | 0.044 |
| 12 months post-stroke | −0.171 | 0.506 | 0.003 | −0.117 | 0.495 | 0.040 |
| 24 months post-stroke | −0.130 | 0.556 | 0.033 | −0.070 | 0.543 | 0.24 |
SE = standard error.
Adjusted for age, sex, education, antidepressant use, ethnicity and the presence of cortical infarcts.
Figure 1.
(A) Generalized linear model analysis of repeated-measures of longitudinal scores on the Geriatric Depression Scale (GDS) throughout follow-up, comparing CCR5-Δ32 carriers to noncarriers (CCR5 wild-type). (B) Geriatric Depression Scale scores at hospital admission and at 6, 12 and 24 months post-stroke, comparing CCR5-Δ32 carriers to noncarriers by sex. #p = 0.058, *p < 0.05, **p < 0.001, for differences between CCR5-Δ32 carriers and noncarriers at each time point.
We observed a GDS score of 6 or higher in 64 participants (14.7%) immediately after the stroke/TIA, in 57 participants (17.7%) at 6 months, in 47 participants (16%) at 12 months, and in 41 participants (15.3%) at 24 months.
Among those with a higher GDS score (≥ 6) at baseline, 59 patients (92.2%) were noncarriers, compared to 365 patients (83.9%) in the whole cohort (χ2 = 3.81, p = 0.05). We made similar observations at 6 and 12 months: among those with a higher GDS at 6 months, 53 patients (93%) were noncarriers and only 4 (7%) were CCR5-Δ32 carriers (χ2 = 5.43, p = 0.02); among those with a higher GDS at 12 months, 46 patients (97.9%) were noncarriers and 1 (2.1%) was a CCR5-Δ32 carrier (χ2 = 9.36, p = 0.002). We also found this trend at 24 months (among those with a higher GDS, 37 patients [90.2%] were noncarriers, and only 4 [9.8%] were CCR5-Δ32 carriers) but the results at this time point were nonsignificant (χ2 = 1.28, p = 0.26).
On average, the noncarriers had higher GDS scores than CCR5-Δ32 carriers at all time points, and post hoc tests revealed that their scores differed significantly (p = 0.04 for the linear difference between curves; Figure 1A).
Participants who were CCR5-Δ32 homozygotes (n = 4) had lower GDS scores at hospital admission than CCR5-Δ32 heterozygotes and noncarriers (0.50 [0.00–2.50] v. 2.00 [0.75–3.25] and 2.00 [1.00–4.00], respectively), as well as at 6 months (0.00 [0.00–0.75] v. 1.00 [0.00–3.00] and 2.00 [0.00–4.00]), 12 months (0.00 [0.00–0.75] v. 1.00 [0.00–2.00] and 2.00 [0.00–4.00]), and 24 months (0.00 [0.00–0.75] v. 1.00 [0.00–3.00] and 2.00 [0.00–4.00]) after the index event. Therefore, we found a trend toward dose response for the number of CCR5-Δ32 alleles, but the differences were nonsignificant, probably because of the small sample.
Because our cohort included both stroke and TIA patients, we performed a subanalysis in patients with evidence of acute diffusion-weighted imaging lesions in neuroimaging (evidence of acute stroke), and the results were similar (Table 3).
Table 3.
Baseline and follow-up depression scores for post-stroke survivors with confirmed acute ischemic lesions in diffusion-weighted imaging (n = 233)*
| Characteristic | CCR5-Δ32 noncarriers (n = 195) | CCR5-Δ32 carriers (n = 38)† | p value |
|---|---|---|---|
| Age, yr | 66.8 ± 9.4 | 69.9 ± 10.4 | 0.07 |
| Education, yr | 12.2 ± 3.8 | 14.2 ± 3.1 | 0.003 |
| Geriatric Depression Scale score | |||
| At hospital admission | 2.00 (1.00–4.00) | 2.00 (0.00–3.00) | 0.017 |
| 6 months post-stroke | 2.00 (0.75–5.00) | 1.00 (0.00–3.00) | 0.015 |
| 12 months post-stroke | 2.00 (0.00–5.00) | 1.00 (0.00–2.00) | 0.009 |
| 24 months post-stroke | 1.00 (0.00–4.00) | 1.00 (0.00–3.00) | 0.06 |
Values are mean ± standard deviation or median (interquartile range).
35 heterozygote, 3 homozygote.
Sex differences in the CCR5-Δ32 association with depressive scores
In general, women had more depressive symptoms (higher GDS scores) than men at hospital admission and 6 months post-stroke/TIA, with similar trends at 12 and 24 months (2.00 [1.00–5.00] v. 2.00 [0.00–3.00], p = 0.01; 2.00 [1.00–5.00] v. 1.00 [0–4.00], p = 0.011; 2.00 [1.00–5.00] v. 1 [0.00–3.00], p = 0.13; and 2.00 [0.00–4.00] v. 1.00 [0.00–4.00], p = 0.42, respectively). Subanalysis by sex revealed that female CCR5-Δ32 carriers had lower GDS scores than noncarriers at hospital admission and at 6, 12 and 24 months post-stroke (p = 0.015, p < 0.001, p < 0.001 and p = 0.02, respectively). Male CCR5-Δ32 carriers showed the same trend as women at all time points, but this result came close to significance only at 12 months post-stroke (p = 0.058; Figure 1B).
CCR5-Δ32 and 5-HTTLPR polymorphisms and post-stroke depressive symptoms
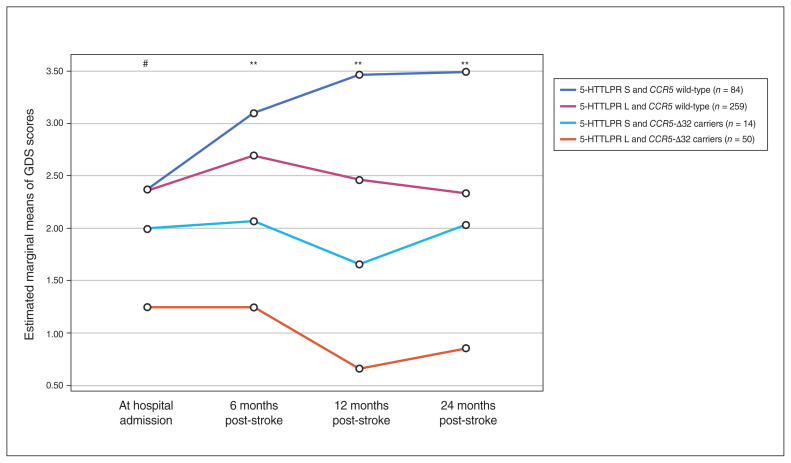
Univariate analyses demonstrated that participants with 1 or 2 copies of the 5-HTTLPR long variant (5-HTTLPR-L carriers) had fewer depressive symptoms (lower GDS scores) at only 12 months after the index event compared to noncarriers (p = 0.008). Figure 2 shows GDS scores in a generalized linear model analysis for the 4 allelic groups we evaluated (available for 407 participants). Carriers of both 5-HTTLPR-L and CCR5-Δ32 had the lowest level of depressive symptoms at all time points, followed by 5-HTTLPR-S and CCR5-Δ32 carriers, then by 5-HTTLPR-L and CCR5-Δ32 noncarriers, and then by 5-HTTLPR-S and CCR5-Δ32 noncarriers, who had the highest depressive symptoms at all time points (p = 0.052, p = 0.002, p = 0.001 and p = 0.009 for GDS scores at hospital admission and at 6, 12 and 24 months post-stroke, respectively). When we submitted these GDS scores to a multivariate analysis of variance with 2 between-subjects factors (5-HTTLPR [long, short] and CCR5 [wild-type, CCR5-Δ32]), we found no significant 5-HTTLPR × CCR5 interaction (p = 0.24).
Figure 2.
Generalized linear model analysis of repeated-measures of longitudinal scores on the Geriatric Depression Scale (GDS) throughout follow-up, comparing the 4 allelic groups for the 5-HTTLPR gene and CCR5. #p = 0.052, **p < 0.001, for differences between 5-HTTLPR S and CCR5 wild-type and 5-HTTLPR L and CCR5-Δ32.
We observed no differences between APOE ɛ4 carriers and noncarriers in terms of depressive scores.
Discussion
In this study, we report for the first time that carriers of a naturally occurring loss-of-function mutation of CCR5-Δ32 are relatively protected from developing depressive symptoms post-stroke. These findings extend those of our recent report, in which the same mutation was found to recovery of distinct measures of cognitive and motor function after stroke.12 Our results showed that the antidepressive effect of the mutation was more pronounced in women than in men.
To date, the exact etiology of post-stroke depression remains undetermined,6 and mechanism-targeted treatment options are lacking.24 Theories about the pathogenesis of post-stroke depression have expanded from focusing on alterations in ascending monoamine systems and abnormalities in the hypothalamic–pituitary–adrenal (HPA) axis to include alterations in neuroplasticity and glutamate neurotransmission, as well as an excess of proinflammatory cytokines.2,25
CCR5 blockade tends to suppress T-lymphocyte chemotactic activity toward chemokines and modifies the production of proinflammatory cytokines in vitro.26 In the primate brain, CCR5 blockade has been shown to attenuate activation of glial cells, maintain the integrity of the endothelial mono-layer, reduce the infiltration of T cells and attenuate neuroinflammation.27 CCR5 blockade may prevent the loss of synaptic connections after stroke or promote the formation of new connections through 2 intracellular signalling cascades: CREB and dual leucine zipper kinase signalling. Both targets mediate injury signals, dendritic spine morphogenesis and axonal regeneration in other systems.28 In a previous report, we showed that CCR5 knockdown in rodents stabilized dendritic spines after experimental stroke during the period of maximal spine loss in tissue adjacent to the infarct.12 CCR5 blockade induced a remarkable degree of axonal sprouting in the bihemispheric or callosal connections of premotor cortex after experimental stroke.29
Thus, we suggest 2 proposed mechanisms of action for impaired CCR5 activity in recovery from brain injury: reducing inflammatory reactions that appear after the ischemic insult, and improving synaptic plasticity through neuromodulation. In our population, CCR5-Δ32 carriers had lower C-reactive protein levels at admission, reflecting a decreased inflammatory profile.
Blockade of CCR5 with antagonists is increasingly being adopted to counteract inflammatory diseases and infections where this receptor plays a relevant role. In 2007, maraviroc, a negative allosteric modulator of the CCR5 receptor and therefore a competitive CCR5 inhibitor, was approved for clinical use as an HIV-1 entry inhibitor.30 Maraviroc modulates several parallel signalling cascades implicated in learning, memory and depression, including the suppression of adenyl cyclase31,32 and the activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and p44/42 mitogen-activated protein kinase (MAPK) signalling.33 We have recently shown in an experimental model of stroke and traumatic brain injury in rodents that administration of maraviroc improves motor and cognitive recovery after stroke and traumatic brain injury.12 In contrast, Sorce and colleagues34 reported in 2010 that CCR5 deficiency aggravates brain damage after cerebral stroke in mice and that CCR5 expression is neuroprotective. We cannot fully explain the differences between these 2 reports. A partial explanation may be attributed to the different animal model used in each study. Sorce’s team used a stroke model based on occlusion of the middle cerebral artery, and our model was based on focal cortical strokes through photothrombosis, resembling focal or smaller occlusions that probably better parallel the ischemic insults seen in our cohort of human patients.12
We also observed sex differences in the association of CCR5-Δ32 with depressive scores: female CCR5-Δ32 carriers showed greater protection against depressive symptoms. These findings cannot be attributed merely to differences in inflammatory profiles, because C-reactive protein levels at admission did not differ significantly between men and women (aside from a single measurement at 12 months). Interestingly, sex differences have also been reported in previous studies involving the role of CCR5 in different illnesses. Gade-Andavolu and colleagues35 found that CCR5-Δ32 carriers with multiple sclerosis had twice the mortality rate of patients with the noncarrier genotype, and this effect was more significant in females. Similarly, Mojtahedi and colleagues36 reported that the CCR5-Δ32 allele may be a genetic risk factor for Behçet disease in Iranian women but not in men. In type 1 diabetes, men with functional polymorphisms in CCR5 were at increased risk for diabetic nephropathy, but not women.37 In patients with HIV type 1, CCR5 expression was reduced in the lymph nodes of women compared to men.38 A possible explanation for this phenomenon suggests a role for estrogenic hormones in the pathogenic milieu of these diseases. Indeed, Mo and colleagues39 reported that estrogen is implicated in the regulation of CCR5 expression and T cell chemokine response in mice. They suggested that their results helped explain the increased susceptibility to and severity of autoimmune diseases in women. Notably, depressive disorders are also twice as common in women compared to men.40
We found a higher frequency of lesions in cortical territories among CCR5-Δ32 carriers versus noncarriers. We found no difference between the groups in terms of small-vessel disease burden, which may imply that the underlying mechanism is not only anti-inflammatory.
To eliminate speculation that among CCR5-Δ32 carriers some cerebrovascular events were actually “TIA mimics,” we performed a subanalysis in patients with evidence of acute diffusion-weighted imaging lesions in neuroimaging (evidence of acute stroke) and found results similar to the full cohort.
Our results showed that the CCR5-Δ32 allele had a risk-reducing effect for post-stroke depressive symptoms when accompanied by either the short or the long variant of the 5-HTTLPR gene, without evidence for an interaction effect. Thus, another striking and novel finding of the current study pointed to a protective role of CCR5-Δ32, mitigating if not reversing the risk effects of the 5-HTTLPR-S, which have been well documented in single-gene studies of post-stroke depression.17 Another variant, the APOE ɛ4 allele, has been associated with depression in an elderly Swedish population,41 but we found no relationship to post-stroke depressive symptoms in our population.
Finally, we found that genotype distributions for the CCR5-Δ32 polymorphism were in accordance with Hardy–Weinberg equilibrium, indicating that wild-type CCR5 has survival value. As mentioned above, CCR5 expression rather than deficiency has been reported as a beneficial factor in multiple sclerosis, Behçet disease and diabetic nephropathy.36,37 Glass and colleagues42 showed that CCR5 was essential for survival against West Nile virus infection in mice and humans and was associated with a less severe form of meningo-encephalitis in tick-borne encephalitis virus infections.43 Most likely, CCR5 facilitates the clearance of these infections by promoting leukocyte trafficking to the central nervous system, proof of its beneficial effects for human health. Still, CCR5-Δ32 homozygous individuals are seemingly healthy and have no apparent abnormalities.44 These individuals have acquired the mutation through a line of ancestry dating back thousands of years; it may be that this has allowed them to evolve and possibly adapt to the lack of CCR5 usage by shifting immune and other functions to alternative receptors or structures.
Strengths of our study included the systematic prospective follow-up and comprehensive data on participants’ clinical status and macrostructural MRI measures. The combination of these factors made this study unique. Other strengths included the availability of genetic and inflammatory data and the advantage of a cohort that consisted of a high percentage of Ashkenazi Jews, more of whom carry the CCR5-Δ32 mutation than other populations.
Limitations
Limitations of our study included the exclusion of participants with baseline depression. This group may involve etiologies other than vascular depression and should be analyzed separately from the post-stroke depression group. Nevertheless, this group had a slightly lower prevalence of the CCR5-Δ32 allele (14.9%) than the included patients (16.1%), supporting our hypothesis.
Another limitation was the exclusion of patients who had severe stroke, and who may experience higher levels of depression and inflammatory processes. Our findings cannot be generalized to this group of patients, and further research among them is warranted. However, the significant findings in our cohort with mild to moderate stroke support our hypothesis, as well as our finding that among patients with greater neurologic deficits (higher NIHSS) there were few CCR5-Δ32 carriers.
Our study did not aim to define post-stroke depression in this cohort — only to note the extent of depressive symptoms based on a depression scale. Reaching significant findings in this cohort was probably a greater challenge than working with patients who had a more severe clinical presentation of depression.
The fact that 57% of our population was Ashkenazi in origin may limit the results of this study to this demographic. However, the association of CCR5-Δ32 status with GDS scores remained significant after adjustment for ethnicity. In addition, previous studies have demonstrated that ligands of the CCR5 receptor, such as the macrophage inflammatory protein 1α (MIP-1α), mediated the effects of the depressive symptom burden (by the GDS) on dementia severity in a cohort that was not limited to Ashkenazi Jews.45 Thus, it is prudent to suggest that the role of the CCR5 receptor as a mediator of the effects of depression post-stroke will not be limited to our specific demographic.
Although loss to follow-up was relatively modest, there was likely some selection bias. We used multiple imputations to address this problem and provide an estimate of probable GDS scores in patients lost to follow-up. Because we used a large number of predictor variables, and because we observed no differences in baseline characteristics between participants who attended at follow-up and those who were missing or lost to follow-up, it seemed reasonable to assume that data were missing at random. Under the assumption of data missing at random, we may have obtained valid inferences by applying multiple imputation techniques.
Finally, because our findings are novel and contradict some of the previous data, we call for further exploration of the role of CCR5 in post-stroke depression and point to a need for replication of our findings in other cohorts.
Conclusion
Carriers of the CCR5-Δ32 allele may be relatively protected from the development of depressive symptoms post-stroke, and this phenomenon is more prominent in women.
These findings may have significant clinical implications, because they highlight a promising mechanism-based treatment target for post-stroke depression and possibly other depression types. Medications that mimic the action of the CCR5-Δ32 mutation are available.
Acknowledgements
This study is supported by grants RAG11482 from the American Federation for Aging Research, 2011344 from the U.S.–Israel Bi-national Science Foundation, AARG-16-442861 from the Alzheimer’s Association, PTCG-20-706182 from Part the Cloud-Gates Partnership Grant Program and the Alzheimer’s Association, and a grant from Rekanati Foundation.
Footnotes
Competing interests: None declared.
Contributors: O. Tene, N. Bornstein, S. Shenhar-Tsarfaty and E. Ben Assayag designed the study. O. Tene, H. Hallevi, J. Molad, S. Usher, N. Bornstein and E. Ben Assayag acquired the data, which O. Tene, J. Molad, E. Seyman and E. Ben Assayag analyzed. O. Tene and E. Bassayag wrote the article, which H. Hallevi, J. Molad, S. Usher, E. Seyman, N. Bornstein and S. Shenhar-Tsarfaty reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Clinical trial registration: http://clinicaltrials.gov/show/NCT01926691 Identifier: NCT01926691
References
- 1.Broomfield NM, Quinn TJ, Abdul-Rahim AH, et al. Depression and anxiety symptoms post-stroke/TIA: prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurol 2014;14:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry 2016;173:221–31. [DOI] [PubMed] [Google Scholar]
- 3.Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2014;9:1017–25. [DOI] [PubMed] [Google Scholar]
- 4.Morris PL, Robinson RG, Andrzejewski P, et al. Association of depression with 10-year poststroke mortality. Am J Psychiatry 1993;150:124–9. [DOI] [PubMed] [Google Scholar]
- 5.Ayerbe L, Ayis S, Wolfe CD, et al. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry 2013;202:14–21. [DOI] [PubMed] [Google Scholar]
- 6.Tene O, Shenhar-Tsarfaty S, Korczyn AD, et al. Depressive symptoms following stroke and transient ischemic attack: is it time for a more intensive treatment approach? Results from the TABASCO cohort study. J Clin Psychiatry 2016;77:673–80. [DOI] [PubMed] [Google Scholar]
- 7.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008;358:55–68. [DOI] [PubMed] [Google Scholar]
- 8.Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry 2011;24:519–25. [DOI] [PubMed] [Google Scholar]
- 9.Oglodek EA, Szota A, Just MJ, et al. Comparison of chemokines (CCL-5 and SDF-1), chemokine receptors (CCR-5 and CXCR-4) and IL-6 levels in patients with different severities of depression. Pharmacol Rep 2014;66:920–6. [DOI] [PubMed] [Google Scholar]
- 10.Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016;22:238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou M, Greenhill S, Huang S, et al. CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory. eLife 2016; 5:e20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joy MT, Ben Assayag E, Shabashov-Stone D, et al. CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell 2019;176:1143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben Assayag E, Korczyn AD, Giladi N, et al. Predictors for poststroke outcomes: the Tel Aviv Brain Acute Stroke Cohort (TABASCO) study protocol. Int J Stroke 2012;7:341–7. [DOI] [PubMed] [Google Scholar]
- 14.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. [DOI] [PubMed] [Google Scholar]
- 15.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994;24:145–53. [DOI] [PubMed] [Google Scholar]
- 16.Lucotte G. Frequencies of the CC chemokine receptor 5 delta 32 allele in various populations of defined racial background. Biomed Pharmacother 1997;51:469–73. [DOI] [PubMed] [Google Scholar]
- 17.Mak KK, Kong WY, Mak A, et al. Polymorphisms of the serotonin transporter gene and post-stroke depression: a meta-analysis. J Neurol Neurosurg Psychiatry 2013;84:322–8. [DOI] [PubMed] [Google Scholar]
- 18.Frodl T, Koutsouleris N, Bottlender R, et al. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol Psychiatry 2008;13:1093–101. [DOI] [PubMed] [Google Scholar]
- 19.Wenham PR, Newton CR, Price WH. Analysis of apolipoprotein E genotypes by the Amplification Refractory Mutation System. Clin Chem 1991;37:241–4. [PubMed] [Google Scholar]
- 20.Sheikh JI, Yesavage JA, Brooks JO, III, et al. Proposed factor structure of the Geriatric Depression Scale. Int Psychogeriatr 1991; 3:23–8. [DOI] [PubMed] [Google Scholar]
- 21.Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15-item Geriatric Depression Scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. J Am Geriatr Soc 2005;53:1570–6. [DOI] [PubMed] [Google Scholar]
- 22.Tene O, Hallevi H, Korczyn AD, et al. The price of stress: high bedtime salivary cortisol levels are associated with brain atrophy and cognitive decline in stroke survivors. results from the TABASCO prospective cohort study. J Alzheimers Dis 2018; 65: 1365–75. [DOI] [PubMed] [Google Scholar]
- 23.Maayan S, Zhang L, Shinar E, et al. Evidence for recent selection of the CCR5-delta 32 deletion from differences in its frequency between Ashkenazi and Sephardi Jews. Genes Immun 2000;1:358–61. [DOI] [PubMed] [Google Scholar]
- 24.Everson SA, Roberts RE, Goldberg DE, et al. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med 1998;158:1133–8. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Ling S, Yang Y, et al. Systematic hypothesis for poststroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuroendocrinol Lett 2014; 35:104–9. [PubMed] [Google Scholar]
- 26.Yuan J, Ren HY, Shi YJ, et al. In vitro immunological effects of blocking CCR5 on T cells. Inflammation 2015;38:902–10. [DOI] [PubMed] [Google Scholar]
- 27.Mondal S, Rangasamy SB, Roy A, et al. Low-dose maraviroc, an antiretroviral drug, attenuates the infiltration of T cells into the central nervous system and protects the nigrostriatum in hemiparkinsonian monkeys. J Immunol 2019;202:3412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostany R, Chowdhury TG, Johnston DG, et al. Local hemodynamics dictate long-term dendritic plasticity in peri-infarct cortex. J Neurosci 2010;30:14116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N, Daie K, Svoboda K, et al. Robust neuronal dynamics in premotor cortex during motor planning. Nature 2016;532:459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fatkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med 2008; 359:1442–55. [DOI] [PubMed] [Google Scholar]
- 31.Rasenick MM. Depression and adenylyl cyclase: sorting out the signals. Biol Psychiatry 2016;80:812–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price T, Brust TF. Adenylyl cyclase 7 and neuropsychiatric disorders: a new target for depression? Pharmacol Res 2019;143:106–12. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Wang L, Hu K, et al. Mechanisms and therapeutic targets of depression after intracerebral hemorrhage. Front Psychiatry 2018; 9:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorce S, Bonnefont J, Julien S, et al. Increased brain damage after ischaemic stroke in mice lacking the chemokine receptor CCR5. Br J Pharmacol 2010;160:311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gade-Andavolu R, Comings DE, MacMurray J, et al. Association of CCR5 delta32 deletion with early death in multiple sclerosis. Genet Med 2004;6:126–31. [DOI] [PubMed] [Google Scholar]
- 36.Mojtahedi Z, Ahmadi SB, Razmkhah M, et al. Association of chemokine receptor 5 (CCR5) delta32 mutation with Behcet’s disease is dependent on gender in Iranian patients. Clin Exp Rheumatol 2006; 24(Suppl 42):S91–4. [PubMed] [Google Scholar]
- 37.Mlynarski WM, Placha GP, Wolkow PP, et al. Risk of diabetic nephropathy in type 1 diabetes is associated with functional polymorphisms in RANTES receptor gene (CCR5): a sex-specific effect. Diabetes 2005;54:3331–5. [DOI] [PubMed] [Google Scholar]
- 38.Meditz AL, Folkvord JM, Lyle NH, et al. CCR5 expression is reduced in lymph nodes of HIV type 1-infected women, compared with men, but does not mediate sex-based differences in viral loads. J Infect Dis 2014;209:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mo R, Chen J, Grolleau-Julius A, et al. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol 2005; 174:6023–9. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013. [Google Scholar]
- 41.Skoog I, Waern M, Duberstein P, et al. A 9-year prospective population-based study on the association between the APOE*E4 allele and late-life depression in Sweden. Biol Psychiatry 2015; 78:730–6. [DOI] [PubMed] [Google Scholar]
- 42.Glass WG, Lim JK, Cholera R, et al. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med 2005;202:1087–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kindberg E, Mickiene A, Ax C, et al. A deletion in the chemokine receptor 5 (CCR5) gene is associated with tickborne encephalitis. J Infect Dis 2008;197:266–9. [DOI] [PubMed] [Google Scholar]
- 44.Barmania F, Pepper MS. C-C chemokine receptor type five (CCR5): an emerging target for the control of HIV infection. Appl Transl Genom 2013;2:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Royall DR, Al-Rubaye S, Bishnoi R, et al. Serum proteins mediate depression’s association with dementia. PLoS One 2017;12:e0175790. [DOI] [PMC free article] [PubMed] [Google Scholar]