Abstract
Adoptive cell transfer of ex vivo-expanded regulatory T cells (Treg) has shown immense potential in animal models of auto- and allo-immunity. However, the effective translation of such Treg therapies to the clinic has been slow. As Treg homeostasis is known to require continuous T-cell receptor (TCR) ligation and exogenous interleukin (IL-)2, some investigators have explored the use of low-dose IL-2 injections to increase endogenous Treg responses. Systemic IL-2 immunotherapy, however, can also lead to the activation of cytotoxic T lymphocytes and natural killer (NK) cells, causing adverse therapeutic outcomes. Here, we describe a drug-delivery platform which can be engineered to autostimulate Tregs with IL-2 in response to TCR-dependent activation and thus activate these cells in sites of antigen encounter. To this end, protein nanogels (NGs) were synthesized with cleavable bis-N-hydroxy succinimide crosslinkers and IL-2/Fc fusion (IL-2) proteins to form particles that release IL-2 under reducing conditions, as found at the surface of T cells receiving stimulation through the T-cell receptor. Tregs surface-conjugated with IL-2 NGs were found to have preferential, allograft-protective effects relative to unmodified Tregs or Tregs stimulated with systemic IL-2. We demonstrate that murine and human NG-modified Tregs carrying an IL-2 cargo perform better than conventional Tregs in suppressing alloimmunity in murine and humanized mouse allotransplantation models. In all, the technology presented in this study has the potential to improve Treg transfer therapy by enabling the regulated spatiotemporal provision of IL-2 to antigen-primed Tregs.
One Sentence Summary:
Redox-sensitive cytokine cargos backpacked on regulatory T cells promote local immune regulation in areas of rejection.
Introduction
Regulatory T cells (Tregs) are a subset of helper CD4+ T cells that co-express the high-affinity interleukin (IL)-2 receptor alpha, CD25, and the transcription factor Forkhead box p3 (Foxp3) (1–3). Notably, as modulators of innate and adaptive immune responses to both foreign and self-antigens, Treg therapies are considered to have numerous clinical applications (4, 5). Despite the slow translation of early pre-clinical findings, however, recent years have been marked by encouraging efforts to translate Treg immunotherapies to the clinic for preventing acute and chronic graft rejection, graft-versus-host disease (GvHD), and autoimmune diseases such as type 1 diabetes (6–8). Specifically, in the context of alloimmunity, adoptive Treg therapies tip the immunological scales in favor of immunoregulation by promoting intragraft Treg dominance and attenuating the pro-inflammatory processes that precipitate graft loss (9–11).
For therapeutic Treg strategies, peripheral Tregs are isolated from the blood of patients, expanded ex vivo, and adoptively transferred back (12–14). Critical for the success of these therapies is the ex vivo expansion of Tregs, as induction of immune tolerance by Tregs relies both on the qualitative and quantitative inhibition of pro-inflammatory cells (15). Attaining relevant therapeutic outcomes in a lymphoreplete individual can thus require as many as 50×109 polyclonal Tregs (16); although, antigen-specific Tregs are known to be more effective in suppressing alloimmunity at lower cell numbers (17, 18). Apart from quantitative impediments, clinical Treg strategies are additionally hampered by qualitative constraints. Particularly, the unstable expression of the master Treg regulator, Foxp3, predisposes Tregs to convert into pro-inflammatory counterparts upon in vivo injection (19–22). To drive Treg expansion, maintain constant Foxp3 expression, and to sustain immunosuppressive qualities, Tregs require continuous T-cell receptor (TCR) ligation (23, 24) and a favorable environment of exogenously produced factors such as IL-2 (25–27).
The dependence of Tregs on IL-2 and its high-affinity receptor CD25 underpinned the discovery of regulatory T cells by Sakaguchi et al. (1). Notably, in contrast to conventional CD4+ T cells, Tregs are dependent on external sources of IL-2 since they cannot endogenously synthesize it (28, 29). Research on the dependence of peripheral Tregs on IL-2 recently culminated in a clinically relevant alternative to adoptive Treg transfer. In various auto- and allo-immune disease models, systemic, low-dose IL-2 proved capable of preferentially stimulating Tregs over effector T cells, due to the selective enrichment of CD25 on Tregs (30–32). Nevertheless, a crucial caveat to this approach lies in supplying IL-2 to the Tregs at the right site and time, since the systemically administered immunomodulator can spark the simultaneous activation of other pro-inflammatory cells that share surface expression of CD25 (33). A recent clinical study on patients with chronic GvHD that were treated with low-dose IL-2, for example, showed synchronous activation of both Tregs and natural killer (NK) cells circulating in the peripheral blood of these patients (34).
Here, we demonstrate a drug-delivery platform that combines the virtues of adoptive Treg transfer and exogenous IL-2 supplementation by spatiotemporally linking IL-2 release to tissue-specific TCR triggering of adoptively-transferred Tregs. We recently demonstrated a strategy (35) to create TCR-signaling-responsive “backpacks” that sensitively released IL-15 to tumor-specific CD8α+ T cells by exploiting the increased cell surface reducing potential of antigenically-primed, activated T cells (36, 37). Importantly, once T cells—including Tregs—are mitogenically stimulated, cytoplasmic and cell surface reducing agents are upregulated to counter the oxidative stresses secondary to cellular proliferation (35, 37–39). Presently, we demonstrate the engineering of immunoregulatory CD4+Foxp3+ T cells with redox-sensitive IL-2 nanoparticles that provide cytokine-mediated survival stimuli to adoptively-transferred Tregs undergoing alloantigen-TCR-specific activation in antigen-rich areas; such as the allograft and allograft-draining lymph nodes (Fig. 1A). We hypothesized that these membrane-conjugated backpacks would improve the therapeutic outcomes of Treg therapy without the need for genetic engineering, and, thus, keep costs low and safety margins high, while having realistic translational applicability. In our experiments, we found that in addition to the platform foregoing the use of state-of-the-art equipment or techniques and requiring only a simple 45-minute incubation step to be conjugated onto Tregs, the effects of engineered Tregs markedly outlasted those of unmodified Tregs.
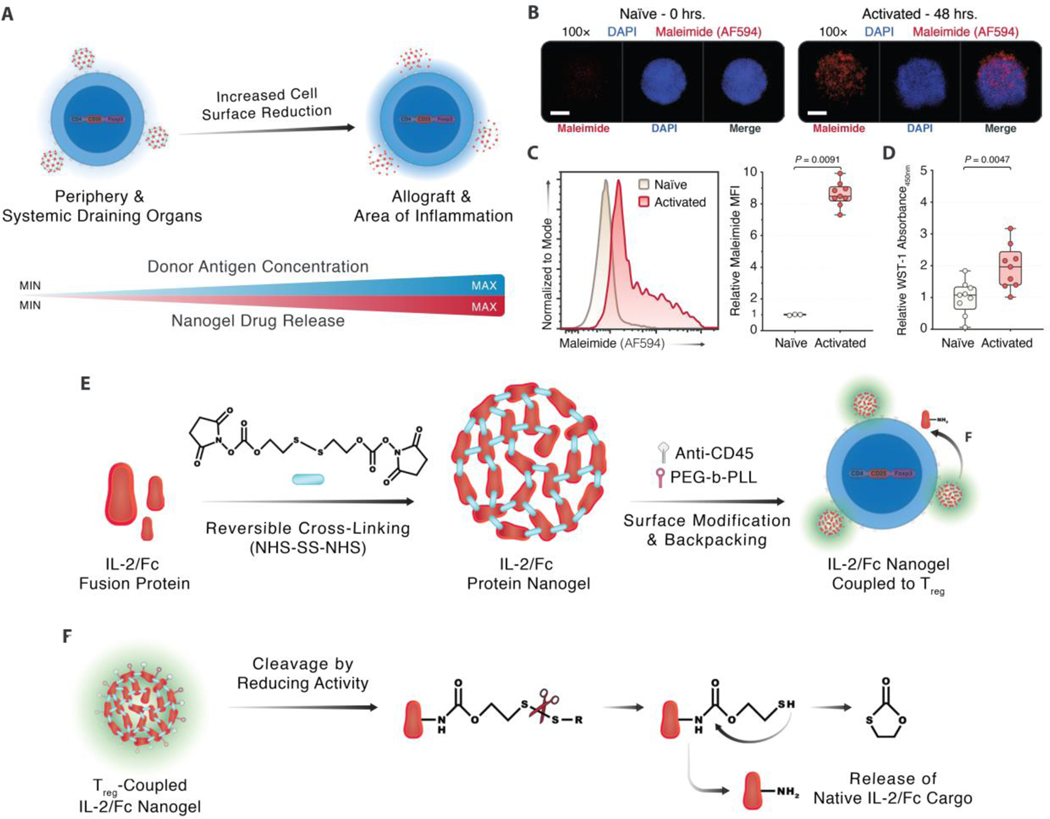
Fig. 1. Designing a TCR-signaling-responsive cytokine-delivery platform for Tregs.
(A) Circulating T cells increase their reducing activity in proportion to the donor antigen concentration, which is highest in areas of inflammation. Nanoparticle backpacks that release their payload in response to a heightened redox potential provide controlled spatiotemporal release of adjuvants. (B) Confocal microscopy of free surface thiol staining on naïve Tregs versus Tregs primed with 4 μg/mL each of soluble anti-CD3ε and anti-CD28 for 48 hours to determine if Tregs upregulate cell surface redox agents (5 μm scale bar). (C) Flow cytometry staining of free surface thiol expression on activated Tregs compared with resting, non-activated Tregs among viable CD4+ events (n = 3 technical replicates/condition, 3 experiments). (D) Colorimetric WST-1 assay on naïve and activated Tregs measuring cell surface reductive activity (n = 3 technical replicates/condition, 3 experiments). (E) Synthesis of reduction-sensitive nanoparticles through reversible crosslinking of IL-2/Fc fusion protein, a survival cytokine for Tregs, with bis-N-hydroxy succinimide crosslinkers, and further surface modification with monoclonal anti-CD45 and PEG-PLL for prolonged surface retention. (F) Cleavage of the disulfide bond that maintains the integrity of the backpack, through reducing agents originating from activated Tregs, making the native cargo protein, IL-2/Fc, available to the host Treg. Fold changes were normalized against the CT condition. Throughout, data represent boxplots with median, interquartile range, minimum, maximum, and all individual data points of the denoted experimental groups. P values were calculated with independent samples two-tailed Student t-tests, and non-parametric Kolmogorov-Smirnov tests were performed when the assumption of homoscedasticity could not be met.
MFI, mean fluorescence intensity; NHS-SS-NHS, bis-N-hydroxy succinimide.
Results
Functionalizing Tregs with a TCR-Signaling-Responsive Drug-Delivery Platform
While we previously proved the efficacy of a redox-sensitive, cytokine-backpacking platform in potentiating effector T cells to curb tumor engraftment (35), it was unknown whether Tregs could be similarly functionalized to suppress alloimmunity. First, we examined if Tregs increase their free surface thiol expression and reducing activity upon TCR ligation. We found that CD4+CD25+ Tregs magnetically sorted from C57BL/6 murine splenocytes and stimulated for 48 hours with monoclonal antibodies (mAbs) against CD3ε and CD28 increased the expression of unbound surface thiols by confocal microscopy (Fig. 1B). In parallel, using flow cytometry, we observed an increase in the free surface thiol expression on activated Tregs versus naïve Tregs (Fig. 1C, fig. S1A), similar to the increase on CD4+ and CD8α+ T cells upon CD3/CD28-mediated stimulation (fig. S1B–D). Finally, we confirmed the increased reducing activity at the surface of activated Tregs using colorimetric WST-1 assays, which measure the conversion of the stable WST-1 salt into the fluorescent formazan. This conversion is driven by reducing agents that are secreted from metabolically active cells, thus gauging the activation state of cells through their redox potential. In these WST-1 assays, we delineated a ~2-fold increase in the redox potential of activated Tregs compared with naïve Tregs (Fig. 1D). Building on these findings, we used disulfide-containing bis-N-hydroxysuccinimide crosslinkers, which are redox-sensitive, to weave together cytokine or protein cargo as adducted prodrugs in the form of nanoscale gels (nanogels, NGs). Conjugating these redox-sensitive NGs onto Tregs would then allow for the release of the native cargo upon cleavage of the self-immolative linkers (40, 41). To understand if redox-responsive nanogels made from IL-2 could autostimulate and stabilize Tregs, we synthesized IL-2/Fc NGs. The rationale behind using IL-2/Fc over wildtype IL-2 was its improved stability and half-life compared to the latter, allowing for longer-lasting in vitro and in vivo effects. Additionally, we included a single amino acid mutation (D265A) in the Fc sequence to prevent the deposition of complement and the formation of humoral immunity against the Fc portion (42). We focused our efforts on IL-2 in particular as its roles in promoting Treg homeostasis and stability are well-established (25–27). Additionally, to promote the stable anchoring of the NGs onto the Treg surface and to prevent the premature internalization of the NGs, we further surface modified the NGs with monoclonal anti-CD45 and PEG-PLL (Fig. 1E). We previously showed that binding NGs to the surface of T cells through CD45 provides a non-internalizing surface anchor expressed exclusively on leukocytes; and, CD45 ligation in itself does not affect T cell proliferation (35, 43, 44). The cleavage of the integral disulfide bond in the nanogel crosslinkers is ultimately driven by the increased reducing activity at the surface of activated Tregs, thus releasing the native IL-2/Fc cargo for autocrine capture by cell-surface CD25 and subsequent CD25-mediated survival signaling (Fig. 1F).
Surface Engineering of Tregs with IL-2/Fc Nanogel Does Not Alter Treg Phenotype
Next, we examined whether IL-2/Fc NGs could be conjugated to Tregs and if this conjugation would affect the Treg phenotype. Using flow cytometry (fig. S1A), we found that incubating magnetically-isolated Tregs with fluorophore-labeled, anti-CD45-bearing IL-2/Fc NGs at 4°C for 45 minutes did not reduce the co-expression of CD25 and Foxp3 among CD4+ T cells compared with control Tregs (Fig. 2A). Among the Tregs that were incubated with IL-2/Fc NGs, however, >90% acquired FITC fluorescence, indicating successful membrane conjugation of the NGs (Fig. 2B–D, Movie S1–2). In in vitro release assays, we then found that the release of IL-2/Fc from NGs was driven by glutathione (GSH; a reducing agent), with ~90% of the crosslinked IL-2/Fc being released within 6 days in the presence of GSH (fig. S2A). We additionally studied the duration of IL-2/Fc NG retention on the surface of NG-coupled Tregs stimulated in vitro for 3 and 7 days with anti-CD3ε and anti-CD28 to appraise the degree of NG dilution on the Treg surface with mitosis. Using flow cytometry, we found that ~30% and ~20% of Tregs cultured in the presence of anti-CD3ε/CD28 for 3 and 7 days respectively retained surface expression of IL-2/Fc NGs (fig. S2B). Although expectedly, the majority of the NG-engineered Tregs lost surface expression of the NGs after activation, the cells that maintained NG expression retained phenotypic markers related to Treg functionality, namely CD25 and CTLA-4. In contrast, Tregs that lost NG surface expression downregulated the same markers (fig. S2C–D).
Fig. 2. Surface engineering of Tregs with IL-2/Fc nanogel does not alter Treg phenotype.
(A–B) Co-expression of CD25 and Foxp3 among CD4+ T cells (A) and the expression of FITC-labelled NGs on these CD4+CD25+Foxp3+ cells (B) comparing control, unmodified Tregs (CT) and IL-2/Fc nanogel-conjugated Tregs (NG; n = 5 experiments). (C–D) Confocal microscopy images of CT (C) and NG (D) Tregs (100 μm scale bar). (E) Flow cytometric analysis of CT Tregs, CT Tregs cultured with soluble IL-2/Fc (CT+IL-2/Fc), and NG Tregs stimulated in vitro with 1 μg/mL plate-bound anti-CD28 and titrated concentrations (0–330 ng/mL) of plate-bound anti-CD3ε for 3 days, assaying CD25+Foxp3+ percentages among CD4+ T cells. (F) Box plots of CD25+Foxp3+ percentages among CD4+ events, CD4+CD25hiFoxp3+ percentages among all live events, and counts of total live CD4+CD25hiFoxp3+ events (n = 3 technical replicates/condition, 2 experiments). (G–H) Flow cytometric analysis of CT, CT+IL-2/Fc, and NG Tregs cultured for 7 days (G) and assayed across the same metrics as described in (E–F; H) (n = 3 technical replicates/condition, 2 experiments). (I–J) Dye-dilution suppression assays with suppressor Tregs and CellTrace Violet-labeled effector splenocytes in the presence of 4 μg/mL each of soluble anti-CD3ε and anti-CD28, assessing the CD4+ (I) and CD8α+ (J) compartments respectively (n= 3 technical replicates/condition, 3 experiments). All fold changes were normalized against the CT condition. Throughout, data represent boxplots with median, interquartile range, minimum, maximum, and all individual data points of the denoted experimental groups. P values were calculated with independent samples two-tailed Student t-tests, and non-parametric Kolmogorov-Smirnov tests were performed when the assumption of homoscedasticity could not be met. For experimental groups with matched data points across multiple time points or concentrations, mixed-effects model analyses with the Geisser-Greenhouse correction were performed, followed by Holm-Šídák multiple comparison tests.
CT, control; NG, nanogel.
To delineate the effects of IL-2/Fc NGs on Treg survival, we studied Treg survival among unmodified, untouched Tregs (CT), Tregs receiving soluble IL-2/Fc stimulation (CT+IL-2/Fc), and NG-conjugated Tregs (NG) in in vitro stimulation assays. In these experiments, all Treg conditions were cultured for 3 and 7 days with a constant concentration of anti-CD28 and titrated concentrations of anti-CD3ε to appraise the relation between TCR-signaling strength and NG-dependent survival outcomes. We postulated that the redox-sensitive IL-2/Fc NGs would release IL-2/Fc under reducing conditions at the Treg surface, which would be contingent on the CD3-dose-dependent activation of Tregs, and, thus, selectively promote pro-survival effects at higher CD3ε concentrations. To characterize the survival outcomes, we compared the maintenance of the CD4+CD25+Foxp3+ phenotype, the absolute counts of Tregs, and the mean expression of surface-expressed co-inhibitory and stimulatory glycoproteins tied to Treg function (45–49). At day 3 post-stimulation and across all concentrations of anti-CD3ε, Tregs cultured with free IL-2/Fc and IL-2/Fc NGs maintained a higher expression of CD25 and Foxp3 compared to the untouched CT Tregs, without the off-target proliferation of non-CD4+ T cells (Fig. 2E, fig. S3A–C). Additionally, the percentages and absolute counts of CD4+CD25hiFoxp3+ Tregs were higher for the CT+IL-2/Fc and NG conditions compared to the CT condition (Fig. 2F). Only at the highest concentration of anti-CD3ε, however, there were >25 and >50-fold CD25hiFoxp3+ Tregs in the CT+IL-2/Fc and NG conditions respectively compared to the CT condition. At day 7 post-stimulation, there were similar CD3-dependent increases in the absolute counts of CD25hiFoxp3+ Tregs in the CT+IL-2/Fc and NG conditions, but, again, without discernible CD3-dependent effects among the CT Tregs (Fig. 2G–H, fig. S3D–E). Additionally, at the highest concentration of anti-CD3ε, there were >10 and >11-fold CD25hiFoxp3+ Tregs in the CT+IL-2/Fc and NG conditions respectively compared to the CT condition, with fewer phenotype-positive Tregs at lower anti-CD3ε concentrations for the CT+IL-2/Fc and NG Tregs compared with the highest anti-CD3ε concentration.
Next, we assessed if IL-2/Fc from the NG Tregs could stimulate neighboring effector T cells. At sites of immune activation, predominantly in the draining lymph nodes and allografts, Tregs are often mingled with conventional CD8 T cells that are also IL-2-responsive (50). In fact, patients who are given Treg-tailored IL-2 therapy specifically receive low-dose injections to prevent the off-target activation of pro-inflammatory CD8 T cells, which can undermine desired immunosuppression end goals (31, 50). Therefore, we appraised the possible IL-2-responsiveness of CD8α+ T cells to IL-2/Fc NGs by co-culturing CT, CT+IL-2/Fc, and NG Tregs with MACS-sorted CD8α+ T cells in a ratio of 1:5 Tregs-to-CD8 T cells and stimulated the cells with anti-CD3ε/CD28 for 3 and 24 hours. A physiological ratio of Tregs-to-CD8 T cells was chosen to obviate the natural suppression of the CD8α+ T cells by the Tregs and thus maintain a balance between the suppressive effects of the Tregs, Treg homeostasis, and CD8 homeostasis. IL-2-mediated signaling in Tregs and CD8α+ T cells was assessed through flow cytometric analysis (fig. S3F) of phosphorylated signal transducer and activator of transcription 5 (STAT5), which is the key transcription factor actuated by IL-2 receptor signaling (51). Within 24 hours, in the NG co-culture samples, despite the presence of IL-2/Fc backpacks on the Tregs, we observed no discernible STAT5 phosphorylation among the CD8α+ T cells, contrasted by the majority of the Tregs being pSTAT5+ (fig. S3G–H). Compared with the NG co-culture samples, however, a subset of the CT+IL-2/Fc CD8α+ T cells showed a distinct increase in the phosphorylation of STAT5 at the 24-hour time point. The latter suggests that IL-2/Fc released from backpacked NG Tregs is consumed by the high-affinity IL-2 receptor, CD25, before it can spill over to neighboring CD8α+ T cells, while IL-2/Fc access, and subsequent IL-2-mediated signaling, is more equivalent for the Tregs and the CD8α+ T cells in the CT+IL-2/Fc co-culture samples.
Additionally, for the in vitro cultured Tregs we mapped the Treg phenotype by studying the mean expressions of PD-1, CTLA-4, and LAG-3, which are co-inhibitory molecules known to be essential for Treg suppressive function (45–48), and CD69, which is a general marker of lymphocytic activation including Tregs (49). At day 3 post-stimulation, we observed no CD3-dose-dependent effects on the expression of the co-inhibitory or activation-induced markers on CT Tregs (fig. S3I–M). In the same timeframe, however, we found that NG Tregs expressed higher concentrations of these markers compared to CT and CT+IL-2/Fc Tregs, while CT+IL-2/Fc Tregs also showed higher expression of these markers compared to CT Tregs. At day 7 post-stimulation, the results were similar in showing higher, CD3-dose-dependent expression of the co-inhibitory and activation-induced markers on NG and CT+IL-2/Fc Tregs compared to the CT Tregs (fig. S3N–R).
Finally, to assess the functionality of NG-coupled Tregs and to rule out a decrease in their suppressive function, we employed an in vitro dye-dilution assay. We isolated murine splenocytes as target cells and stained them with a trace dye to track the number of divisions of these splenocytes in culture. We then co-cultured these target cells for 3 days with either unmodified, untouched CT Tregs or NG Tregs (both freshly isolated) in the presence of anti-CD3ε/CD28 mAbs, culturing a fixed number of target splenocytes with increasing numbers of effector Tregs. Upon assessing the target CD4+ and CD8α+ T cells compartments of the cultured splenocytes, we observed a similar dose-dependent suppression of the splenic T cells by both CT and NG Tregs (R>0.900, P<0.001) (Fig. 2I–J, fig. S4).
Nanogel-Engineered Tregs Suppress In Vivo Alloimmunity Better than Conventional Tregs
After exploring the in vitro effects of IL-2/Fc NGs on Treg homeostasis, we evaluated the in vivo suppressive function of NG Tregs in a murine allotransplantation model. Opting for a stringent, fully MHC-mismatched skin transplant model, we grafted BALB/c skin allografts onto the dorsal trunks of immunodeficient Rag1−/− mice on a C57BL/6 (B6) background. Three days later, we intravenously injected an equal number of immunogenic B6 CD8α+ T cells that drive the rejection process and immunoregulatory B6 CD4+CD25+ Tregs, either modified with IL-2/Fc NGs (NG) or unmodified control Tregs (CT), which mitigate the alloimmune cascades (Fig. 3A). In light of recent studies that established the validity of systemic IL-2 therapy (30–32), we also administered soluble IL-2/Fc with CT Tregs in a separate condition (CT+IL-2/Fc). The IL-2/Fc concentration injected per mouse in this condition was dosed equivalent to the IL-2/Fc released from the transferred NG Tregs per mouse (more details provided in the Materials and Methods). Seven days after the adoptive T cell transfer, we analyzed the non-draining lymph nodes and spleens (distal to the site of alloimmunity) and the draining lymph nodes and skin allografts (proximal to the site of alloimmunity) (Fig. 3B), based on our hypothesis that NG Tregs would proliferate better than conventional Tregs within the alloimmune microenvironment.
Fig. 3. Nanogel-engineered Tregs suppress in vivo alloimmunity better than conventional Tregs.
Rag1−/− mice on a C57BL/6 background were transplanted with fully MHC-mismatched BALB/c allografts 3 days before the adoptive transfer of 5.0×105 C57BL/6 CD8α+ cells and 5.0×105 C57BL/6 Tregs, with or without IL-2/Fc NG. Additionally, a group of control mice was treated with both CD8α+ cells, Tregs, and systemic IL-2/Fc. On day 7, the mice were euthanized, and various tissues were analyzed by histology, immunofluorescence, and flow cytometry (n = 7 biological replicates/condition, 2 experiments). (A–B) Experimental mouse model (A) and timeline (B). (C) H&E and CD3ε immunofluorescence staining of the experimental conditions as well as naïve, untreated Rag1−/− skin (4× H&E, 1.00 mm scale bar; 20× H&E, 150 μm scale bar; 20× immunofluorescence, 100 μm scale bar). (D) Macroscopic images of each murine replicate’s allograft for all three treatment conditions at the experimental endpoint with red box borders indicating rejection (10.0 mm scale bar), complemented with the survival curves and percentages at day 7. (E–F) Flow cytometric analyses of CD25+Foxp3+ Tregs among viable CD4+ events (E) and CD8α+ T cells among viable events (F) across the four distal-to-proximal tissue compartments for CT-, CT+IL-2/Fc-, and NG-treated mice. (G) Fold changes of the Treg-to-CD8α ratios in the distal-to-proximal draining sites. (H) Fold changes of the skin allograft surface areas. (I) Fold changes of the [3H]-Thymidine incorporation in mixed lymphocyte reactions of host Rag1−/− splenocytes with irradiated donor BALB/c splenocytes. All fold changes were normalized against the CT condition. Throughout, data represent boxplots with median, interquartile range, minimum, maximum, and all individual data points of the denoted experimental groups. P values were calculated with one-way analyses of variance followed by Holm-Šídák multiple comparison tests.
CT, control; LNs, lymph nodes; NG, nanogel.
Histological analysis of the allografts of the CT and CT+IL-2/Fc-treated conditions 7 days after the adoptive Treg transfer showed signs of epidermal thickening and perivascular lymphocytic infiltrates, compared to naïve, untransplanted BALB/c skin. T cell infiltration in these conditions was then gauged by immunofluorescence staining for CD3ε, the T cell co-receptor expressed on both CD4+ and CD8α+ T cells, showing greater CD3ε+ infiltrates in the CT and CT+IL-2/Fc conditions (Fig. 3C). Additionally, in these groups, there was a disruption of the adnexal structures and the native skin architecture, which are in line with high grades of rejection. The skin grafts of NG-treated mice, on the contrary, showed rare lymphocytic infiltrates, had mild signs of inflammation in the overlying epidermis, and had intact adnexal structures (for example, the hair follicles). Macroscopic examination of the skin showed attenuated signs of allograft rejection in the NG-treated mice compared to CT and CT+IL-2/Fc-treated mice by day 7 post adoptive transfer, with ~70% of the NG grafts looking healthy compared to ~40% of the CT and ~30% of the CT+IL-2/Fc grafts (Fig. 3D).
Using flow cytometry (fig. S5), we then assayed the percentages of CD4+CD25+Foxp3+ Tregs and CD8α+ T cells within the distally and proximally draining tissue sites, as well as the ratio of Tregs-to-CD8α T cells in each draining site, normalizing the fold changes of the Treg-to-CD8 ratios against the CT condition. We observed that systemic IL-2/Fc increased Treg percentages and decreased CD8α+ percentages in the spleen (Fig. 3E–F, fig. S6A–B), which resulted in increased Treg-to-CD8α ratios in the spleen (Fig. 3G). The systemic IL-2/Fc, however, provided limited advantages over lone CT Treg transfer in the allograft-draining lymph nodes and the allograft in terms of the Treg-to-CD8α ratio. NG treatment, in contrast, precipitated no increase of the Treg-to-CD8α ratios in the distal lymph nodes and spleens. The NG treatment did, however, show an increase in Treg-to-CD8α ratios in the graft-draining lymph nodes and allografts, which constitute the proximal sites of alloimmunity. Several other observations supported the histological and phenotypic findings that NG-treated mice had healthier allografts, including the increased allograft sizes among NG-treated mice (Fig. 3H). Moreover, splenocytes isolated from the different groups (CT, CT+IL-2/Fc, and NG) were stimulated ex vivo for 48 hours with irradiated naïve donor BALB/c splenocytes to test for alloimmune memory responses. After 48 hours, the co-cultured splenocytes were pulsed for another 12–16 hours in the presence of [Methyl-3H]-Thymidine to measure alloantigen-specific cell proliferation. We observed that, compared with the CT and CT+IL-2/Fc-treated conditions, there was a reduction (>2-fold) of alloimmune memory proliferation among the host splenocytes of NG-treated mice (Fig. 3I).
Finally, clinical studies using systemic IL-2 injections as a tolerogenic Treg-centric therapy have shown that systemic IL-2 treatment not only stimulates Tregs but can also induce off-target expansion of NK cells (33, 34), among other pro-inflammatory cells. To ensure that our IL-2/Fc NGs delivered IL-2/Fc specifically to Tregs, we additionally assessed the effects of the IL-2/Fc NGs on the endogenously present NK compartment in our transplanted Rag1−/− mice (fig. S6C). Despite no discernible increases in the percentages of NK cells across all distal-to-proximal tissue sites, we observed lower absolute counts of NK cells in the draining lymph nodes of the NG Treg-treated mice compared with the CT and CT+IL-2/Fc Treg-treated mice, suggesting the selective release of IL-2/Fc to the Tregs in the NG Treg-treated mice compared to the other conditions (fig. S6D–E).
Nanogel-Engineered Tregs Prolong Murine Allograft Survival Better than Conventional Tregs
Next, we compared the therapeutic effects of conventional Tregs, Tregs supplemented with systemic IL-2/Fc, and NG-engineered Tregs by transplanting new groups of Rag1−/− mice using the same mismatched BALB/c to B6 allotransplant model as described earlier (Fig. 4A–B). While the mean survival time of the allotransplants for both CT and CT+IL-2/Fc-treated conditions was 6 days post adoptive transfer, the allograft survival of mice treated with 10 μg IL-2/Fc NG/106 Tregs was lengthened to 10 days mean survival time post adoptive transfer (Fig. 4C). By increasing the surface-conjugated dose of IL-2/Fc NG to 20 μg/106 Tregs, we observed a further prolongation of the allograft survival to 18 days post adoptive transfer, effectively increasing allograft survival 3-fold compared with the CT-treated mice (Fig. 4C).
Fig. 4. Nanogel-engineered Tregs prolong murine allograft survival better than conventional Tregs and differentially promote antigen-specific Tregs.
Allograft survival was studied using the same Rag1−/− to BALB/c model as described in Figure 3, adoptively transferring 5.0×105 C57BL/6 CD8α+ cells and 5.0×105 C57BL/6 Tregs on day 3 post-transplant, without IL-2/Fc NG, with systemic IL-2/Fc, or with IL-2/Fc NG (n = 6 mice/group). (A–B) Experimental mouse survival model (A) and timeline (B). (C) Survival curves of each of the treatment groups. In vivo T cell responses were tracked by transplanting BALB/c skin grafts onto Rag1−/− mice and adoptively transferring 5.0×105 C57BL/6 CD8α+ cells transduced with Cypridina luciferase (CLuc) and 5.0×105 C57BL/6 Tregs transduced with Gaussia luciferase (GLuc), either unmodified or decorated with IL-2/Fc NGs, on day 3 post-transplant (n = 3 biological replicates/condition, 1 representative experiment). (D) Experimental bioluminescent T cell tracking model and timeline. (E) In vivo imaging of CT and NG Treg GLuc signals following intravenous coelenterazine administration. (F) In vivo imaging of CD8α+ T cell CLuc signals following intravenous Cypridina luciferin administration. (G) Ratios of Treg-GLuc signal in the skin grafts compared with the whole animals, and fold changes of Treg-to-CD8α (GLuc-to-CLuc) signal in the skin grafts compared with the whole animals. Finally, antigen-specific alloimmunity was assessed with an OVA-OT-1-OT-2 model. Rag1−/− mice were transplanted with OVA allografts 3 days before the adoptive transfer of 5.0×105 OT-1 CD8α+ T cells and 2.5×105 OT-1 CD4+CD25+ Tregs and 2.5×105 OT-2 CD4+CD25+ Tregs, left untouched or coupled IL-2/Fc NGs. On day 7, the mice were euthanized, and various tissues were analyzed by flow cytometry (n = 7 biological replicates/condition, 2 experiments). (H–I) Antigen-specific mouse model (H) and timeline (I). (J) Fold changes of the ratio of OT-2 Tregs to OT-1 CD8α in the distal-to-proximal draining sites. (K) Fold changes of the [3H]-Thymidine incorporation in mixed lymphocyte reactions of host Rag1−/− splenocytes with irradiated donor OVA splenocytes. All fold changes were normalized against the CT condition. Throughout, data represent boxplots with median, interquartile range, minimum, maximum, and all individual data points of the denoted experimental groups. P values were calculated with log-rank Mantel-Cox tests, or with independent samples two-tailed Student t-tests, and non-parametric Kolmogorov-Smirnov tests were performed when the assumption of homoscedasticity could not be met.
CT, control; CLuc, Cypridina luciferase; GLuc, Gaussia luciferase; LNs, lymph nodes; MLR, mixed lymphocyte reactions; NG, nanogel.
These improved mechanistic and survival results for NG-engineered Tregs highlight the functional differences between unmodified CT and NG Tregs. However, these results did not allow us to visualize the Tregs and CD8 T cells in vivo to understand the local impact of IL-2/Fc NGs on Treg homeostasis and the suppression of alloimmunity. To answer this question, we developed a dual imaging T cell tracking assay to follow the Treg and CD8 T cell populations in vivo. Using retroviral vectors, we transduced two separate bioluminescent luciferase reporters encoding for Gaussia luciferase (GLuc) and Cypridina luciferase (CLuc; fig. S7A) into pre-activated Tregs and CD8α+ T cells respectively (fig. S7B–C). As each luciferase can only catalyze the conversion of its own substrate, we could monitor one luciferase without affecting the other luciferase in the same biological sample (fig. S7D–G). Following the T cell transductions, we transferred 5.0×105 CD8α-CLuc cells and Treg-GLuc cells each (with or without IL-2/Fc NGs) into Rag1−/− mice that were transplanted with BALB/c skin 3 days prior (Fig. 4D). Seven days after the adoptive T cell transfer, we gauged the in vivo bioluminescent signal intensities for GLuc and CLuc. We observed that NG-engineered Tregs expressing GLuc co-localized more readily within the skin grafts of the transplanted mice compared to the GLuc-transduced CT Tregs, which remained predominantly within the spleens (Fig. 4E). Additionally, we observed that the allograft infiltration of CD8α+ T cells, as measured by the CLuc signal, was higher for CT-treated mice compared with NG-treated mice (Fig. 4F). Furthermore, the ratio of Treg-GLuc signals arising from the skin grafts to the Treg-GLuc signals from the whole animals was higher for the NG-treated mice compared to the CT-treated mice, and, similarly, the ratio of the Treg-GLuc signal to CD8α-CLuc signal was higher for NG-treated mice compared to CT-treated mice (Fig. 4G).
Finally, we investigated whether the NG platform was promoting activated, antigen-specific Tregs in sites of alloimmunity or that, instead, the IL-2 release from the NGs was antigen agnostic and milieu dependent. For this question, we compared the effects of CT Tregs and NG Tregs in an OVA-OT-1-OT-2 skin allotransplantation model. We transplanted skin grafts with ubiquitous ovalbumin (OVA) expression onto the dorsal trunks of immunodeficient Rag1−/− mice 3 days before the adoptive transfer of a 2:1:1 ratio of OT-1 CD8α+ T cells, OT-1 CD4+CD25+ Tregs, and OT-2 CD4+CD25+ Tregs (with or without IL-2/Fc NG; Fig. 4H). The model allowed us to study the role of the NG platform in the homeostasis of non-specific OT-1 Tregs compared to antigen-specific OT-2 Tregs. The CD4+ T cells from OT-2 mice can undergo OVA323–339-specific clonal expansion through MHC-II-dependent presentation of OVA323–339 peptides, while the CD4+ T cells from OT-1 mice cannot. Thus, due to the non-specificity of OT-1 CD4+CD25+ Tregs for the relevant MHC-II-presented OVA peptides, these Tregs can only become activated due to environmental stimuli. Then, to be able to distinguish the OT-1 cells from the OT-2 cells by flow cytometry, we used a fluorescent tetrameric OT-1 antibody that was specific for the SIINFEKL peptide. Since the Rag1−/− hosts lack endogenous T and B cells, the T cells negative for OT-1 were considered to be the remaining adoptively-transferred CD4+ T cells, which were the Tregs from the OT-2 mice (more details provided in the Materials and Methods). Seven days after the adoptive T cell transfer, we euthanized the mice and analyzed various distal-to-proximal tissues as described before (Fig. 4I).
Using flow cytometry (fig. S8), we determined the percentages of CD4+Foxp3+OT-2+ Tregs and OT-1+CD8α+ T cells within the distally and proximally draining tissue sites, as well as the ratio of OT-2 Tregs to OT-1 CD8α T cells in each tissue site. Comparing the ratios of OT-2 Tregs to OT-1 CD8α T cells across all four compartments, we observed the highest increase in the ratio of OT-2 Tregs to OT-1 CD8α of as much as 150-fold and on average 35-fold in the skin allografts of the NG-treated mice compared with the CT-treated mice (Fig. 4J). To understand which Treg responses were dominant in each draining site, that is, non-specific OT-1 Tregs versus antigen-specific OT-2 Tregs, we assessed the percentage of OT-1+ versus OT-2+ CD4+Foxp3+ Tregs. We found that the dominant Treg type for both CT and NG-treated mice in all draining sites was the antigen-specific OT-2 Treg. The percentage of OT-2 Tregs out of the total Tregs (OT-1 + OT-2) in the draining lymph nodes and skin allografts, however, was higher for NG-treated mice compared to CT-treated mice, suggesting that the IL-2/Fc NGs promoted the homeostasis of antigen-specific Tregs more efficiently in these proximal draining sites compared to untreated antigen-specific CT Tregs (fig. S9A). Furthermore, NG-treated mice had lower counts of antigen-specific OT-1 CD8α+ T cells across all draining sites (fig. S9B). Additionally, splenocytes isolated from the two treatment groups (CT and NG) were stimulated ex vivo for 48 hours with irradiated naïve donor OVA splenocytes to test for alloimmune memory responses. This assay showed attenuated memory responses (>2-fold) in the NG-treated mice versus the CT-treated mice (Fig. 4K).
IL-2/Fc NGs Conjugated to Human Tregs Improve Their Function and Homeostasis In Vitro and In Vivo and Promote Intragraft Tregs
Ultimately, we tested the ability of IL-2/Fc NGs to promote human Treg homeostasis in vitro and in vivo. For the in vitro experiments, magnetically sorted human CD4+CD25hiCD127lo Tregs were either left untouched (CT) or coupled with anti-human-CD45 IL-2/Fc NGs (NG). The Tregs were then stimulated with mAbs against CD3ε/CD28 and analyzed by flow cytometry (fig. S10A) 3 and 7 days after stimulation. Compared with the human CT Tregs, the NG Tregs had >2-fold and >5-fold absolute counts of CD4+CD25+CD127loFoxp3+ T cells within 3 (fig. S10B) and 7 days (fig. S10C) respectively. Building on these in vitro findings, we opted for a pre-clinical humanized mouse model in which we grafted discarded healthy donor skin from patients undergoing cosmetic surgery onto the dorsal trunks of NOD-scid IL-2 receptor-γnull (NSG) mice. These mice have a severe grade of immunodeficiency, which allows for the engraftment of mature human immune cells. Once engrafted, the human cells can then mount alloimmunity against the transplanted human skin grafts. Seven days after the skin transplants, we intravenously injected Treg-depleted PBMCs and Tregs from the same donor, with or without IL-2/Fc NG conjugation, or we systemically supplemented the control Tregs with soluble IL-2/Fc (Fig. 5A). On day 21 following the adoptive T cell transfer, we analyzed the spleens and skin grafts of the NSG mice (Fig. 5B, fig. S11), being restricted to these draining sites due to the atrophy of secondary lymphoid tissues apart from the spleen in NSG mice.
Fig. 5. Human Tregs coupled with IL-2/Fc nanogels have improved function and homeostasis in vivo and promote intragraft Tregs in a humanized mouse model of skin transplantation.
NSG mice were transplanted with skin grafts from healthy donors 7 days before the adoptive transfer of 5.0×106 CD25+CD127lo-depleted PBMCs and 1.0×106 Tregs, with or without IL-2/Fc NG, or with systemic IL-2/Fc. On day 21, the mice were euthanized, and various tissues were analyzed by histology, immunofluorescence, and flow cytometry (n = 8–10 biological replicates/condition, 2 experiments). (A–B) Experimental humanized mouse model (A) and timeline (B). (C) H&E and CD3ε immunofluorescence staining of the experimental conditions as well as naïve, healthy human skin (10× H&E, 400 μm scale bar; 40× H&E, 100 μm scale bar; 40× immunofluorescence, 40.0 μm scale bar). (D) Macroscopic images of each human skin allograft for all three treatment conditions at the experimental endpoint with red box borders indicating rejection (10.0 mm scale bar), in addition to the survival curves and percentages on day 21. (E–F) Flow cytometric analyses of Foxp3+ Tregs among CD4+ lymphocytes (E) and CD8α+ T cells among all lymphocytes (F) in the spleens and skin grafts of the CT-, CT+IL-2/Fc-, and NG-treated NSG mice. (G–I) Box plots of Foxp3+ percentages among CD4+ events (G), CD8α+ T cells among all events (H), and fold changes of the Treg-to-CD8α ratios in the distal and proximal draining sites (I). All fold changes were normalized against the CT condition. Throughout, data represent boxplots with median, interquartile range, minimum, maximum, and all individual data points of the denoted experimental groups. P values were calculated with one-way analyses of variance followed by Holm-Šídák multiple comparison tests.
CT, control; NG, nanogel; NSG, NOD-scid IL-2 receptor-γnull; PBMC, peripheral blood mononuclear cell.
By histology, the allografts of the CT and CT+IL-2/Fc-treated conditions compared with healthy, untreated, untransplanted “naïve” human skin showed signs of epidermal-dermal interface changes, the abolishment of the rete ridges, adnexal disruption, spongiosis, dense lymphocytic infiltrates, and overall epidermal necrosis—all markers of severe rejection (Fig. 5C). The infiltration of T cells in these conditions was assessed by CD3ε+ immunofluorescence staining, validating the presence of CD3ε+ T cells in both CT and CT+IL-2/Fc-treated mice (Fig. 5C). However, despite early signs of flattening of the rete ridges and mild spongiosis among the allografts of the NG-treated mice, there was no noticeable involvement of the overlying epidermis, no or rare lymphocytic infiltrates, limited adnexal involvement, and a well-demarcated reticular dermis, indicating healthier skin than the CT-treated conditions with only mild grades of alloimmune-associated damage. Additionally, using confocal microscopy, we observed Foxp3+ cells in the allografts of NG-treated mice at the interface of the dermis and the graft bed (fig. S12A). Of note, there were also Foxp3+ cells in the allografts of the CT+IL-2/Fc-treated mice; however, the incidence of allograft rejection was not attenuated in these mice, with 25% of the CT+IL-2/Fc grafts looking healthy versus 40% of the CT grafts and 80% of the NG grafts (Fig. 5D). Moreover, in bulk RNA extracted from the intact human skin allografts of CT and NG Treg-treated mice, we screened for the mRNA of several secreted and surface-bound proteins in the graft microenvironment, finding increased mRNA concentrations of IL-1α, IL-7, TGF-β, and Foxp3, as well as decreased E-selectin concentrations in the NG grafts compared with the CT grafts (fig. S12B).
By flow cytometry (fig. S11), we then analyzed the percentages of human CD4+Foxp3+ Tregs and CD8α+ T cells within the distal spleens and proximal skin allografts, as well as the ratio of Treg-to-CD8α T cells in both draining sites, in which the Treg-to-CD8α ratios were normalized against the CT-treated group to report the fold change differences. Similar to the in vivo murine Treg results, we found that the systemic IL-2/Fc injection increased human Treg percentages in the spleens of the NSG mice (Fig. 5E) and decreased CD8α+ percentages (Fig. 5F). This increase in Tregs and decrease in CD8α+ T cells improved the Treg-to-CD8α ratios for the CT+IL-2/Fc mice in the spleens (Fig. 5G–I). Preferential on-target, allograft-specific effects, as judged by increased Treg-to-CD8α ratios in the allografts of the transplanted mice, however, were only observed in the NG-treated condition (Fig. 5E–I). Finally, complementing these histological and phenotypic findings was the observation that NG-treated mice had larger allografts at the time of the study endpoint (fig. S12C).
Discussion
In recent years, cell-based Treg therapy has moved to the frontiers of treatment strategies for auto- and allo-immune-mediated diseases (6). In contrast to drug-based approaches, adoptive Treg transfer has the potential to induce permanent antigen-specific regulation of the immune system, which is a clinically unmet need in autoimmunity and transplantation. Nonetheless, the clinical success of this regulatory immunotherapy has been hampered by the poor in vivo homeostasis of the adoptively-transferred Tregs, as Tregs rapidly disappear from the circulation upon adoptive transfer (52). Alternative immunotherapeutic strategies include the boosting of native Tregs within the immune system through systemic IL-2 injections, although this approach is accompanied by its own limitations. Excess concentrations of circulating IL-2 cause indirect endothelial cell damage through cytokines and vasoactive mediators originating from the non-specific activation of NK cells, and can also cause direct vascular damage secondary to the binding of IL-2 to CD25+ endothelial cells (50). For over 20 years, this sequence of events has been termed vascular leak syndrome (VLS), and it remains one of the most serious side-effects of IL-2 immunotherapy, known to precipitate organ failure due to the extravasation of fluids and proteins with subsequent interstitial edema (53). Here, we described an immunotherapeutic platform that can improve the potency of adoptive Treg transfer as well as the on-target effects of systemic IL-2 therapy, by forming a combination therapy in which IL-2 release is linked explicitly to TCR-ligation of adoptively-transferred, antigen-specific Tregs in the allografts and graft-draining lymph nodes.
The goal of this technology is to improve the therapeutic outcomes of Treg therapy without the need for genetic engineering, where cell surface-conjugated nanogels (fig. S13A) provide a simple platform to enhance Treg therapy. The critical factor in realizing this technology is the fact that Tregs, similar to other T lymphocytes, increase their cell surface reducing activity upon TCR-ligation (fig. S13B–C). The latter enables the exploitation of the naturally augmented redox potential on the surface of Tregs interacting with cognate antigens in the alloimmune environment and, thus, to spatiotemporally control IL-2 supplementation in a redox-sensitive, TCR-dependent fashion (fig. S13D–E). Another essential characteristic of the NG platform is the short time required to modify the Tregs destined for adoptive transfer: A 45-minute incubation was sufficient to conjugate >90% of Tregs with IL-2 NGs, with in vivo improvements in cell function and homeostasis and as much as 3 times increased survival outcomes in a murine skin allotransplantation model. The intuitive redox-sensitive backbone of the nanogels provides a simple solution to a complex problem of enhancing Treg functionality in proximal sites of inflammation, enabling on-target effects despite systemic injections.
In our in vivo murine experiments, we observed clear spatiotemporal benefits provided to the Tregs conjugated with IL-2 NGs, as there was an increase in the activation of NG Tregs in those tissues with the highest concentrations of alloantigen. Additionally, across all in vivo experiments, an inverse relationship between the number of Tregs and CD8α T cells was only observed in the allografts and graft-draining lymph nodes of NG-treated mice. Furthermore, comparing the Treg-to-CD8α ratios in the skin grafts of the transplant recipients to the Treg-to-CD8α ratios in the spleens, we only found a >10-fold increase in the skin-to-spleen ratio in the mice treated with IL-2/Fc NG-engineered Tregs. This preferential on-target effect is intriguing, as mice treated with systemic IL-2/Fc had similarly increased numbers of Tregs but lower ratios of Foxp3+ Tregs to CD8α T cells. Isolated increases of Treg percentages in the CD4+ compartment, importantly, are not a reliable marker of downstream therapeutic effects. Clinically, increases of Foxp3 expression in the grafts and urine of kidney transplant recipients are, in fact, used as biomarkers of allograft rejection (54, 55). Through the restricted release of survival cytokines to alloantigen-specific Tregs, the NG platform proved capable of improving the ratio of Foxp3+ Tregs to CD8α T cells—a more clinically relevant metric of therapeutic outcome (56).
From a translational perspective, we found that the murine results could be reproduced in human in vitro and humanized mouse in vivo settings. Besides confirming the preferential on-target effects of the IL-2/Fc NG-conjugated Tregs, there was an upregulation of mRNA signatures in line with immune tolerance in NG-treated skin allografts. We observed increased TGF-β, known to inhibit antigen-presenting cell (APC) activity and confer “infectious tolerance” to neighboring cells (57, 58), and Foxp3, a hallmark of the regulatory T cell population (1–3). Additionally, we found a decrease in the E-selectin expression, which facilitates the binding of immune cells to endothelial cells at sites of inflammation (59) and is positively correlated with the severity of allograft rejection (60). Finally, we observed an increase of IL-7 mRNA, which is a compelling finding in the context of recent studies that established IL-7 signaling as a key factor in maintaining the local T cell homeostasis of intragraft Tregs in mice (61, 62). Although these findings remain to be validated in humans, it is becoming increasingly evident that the enhancement of adoptive T cell therapies requires a myriad of cytokine signals at different stages and in distinct locations. Since IL-7 is known for its role in stabilizing T cells of the memory phenotype, heightened IL-7 signaling could improve the maintenance of Tregs that have committed to peripheral tissues. Importantly, the IL-2/Fc NGs were a testbed drug cargo to establish whether NGs could quickly and stably be tethered onto the Treg surface without a loss of the host cell’s phenotype or functionality.
Regarding the limitations, a fundamental constraint of the NG platform is perhaps its inherent self-limiting nature, as every division of the conjugated host cell dilutes the total surface-bound NGs (fig. S14A). Although we observed that IL-2/Fc NG-engineered Tregs ameliorated allograft acceptance outcomes in in vivo models compared with unmodified Tregs and Tregs supplemented with systemic IL-2/Fc, the effects of the IL-2/Fc NGs can last only as long as the supply of IL-2/Fc in the NG backpacks. Considering Treg viability is innately linked to the continuity of IL-2 signaling, one of our future efforts will be to optimize the release kinetics of the IL-2/Fc cargo from backpacked NGs. We postulate that altering the biochemical qualities of the crosslinkers that make up the IL-2/Fc NGs could further improve the homeostasis of NG Tregs and enable an equilibrium between the suppressive effects of the NG Tregs and their longevity. Alternatively, the self-limiting element of the NGs can be considered a natural safety mechanism in which the lifespan of the host cells and the number of cell divisions limit the effects of the membrane-tethered NGs. Additionally, we can envision the option of multiple therapeutic injections of NG-coupled Tregs to realize longer-lasting effects of IL-2/Fc NG-conjugated Tregs while balancing the inherent temporal limitations of the nanogels. Finally, future experiments are essential to expand on the preliminary results we presented regarding the potential paracrine effects facilitated by IL-2/Fc NG-backpacked Tregs. As the release of IL-2/Fc from the NG backpacks is mediated by reducing agents at the surface of the backpacked Tregs, robust activation of the IL-2/Fc NG-engineered Tregs could result in the off-target, paracrine IL-2-mediated stimulation of neighboring effector T cells by IL-2/Fc shedding from the nanogel backpacks (fig. S14B). On the other hand, activated effector T cells in the vicinity of naïve IL-2/Fc NG-engineered Tregs could non-specifically consume the backpacked IL-2/Fc through an increased redox potential at their cell surface (fig. S14C).
Beyond the current limitations of the IL-2/Fc NG platform that require to be addressed in future studies, the over-arching allure of this approach is its modularity and the potential to backpack alternative cytokines or biomolecules as adducted prodrugs for release in sites of antigen encounter (fig. S14D). For example, it can be envisioned that alternative cytokine-nanogel formulations such as IL-7 NGs can enhance the maintenance of tissue-specific Tregs or perhaps that formulations with IL-10 or TGF-β can promote the induction of paracrine infectious tolerance within the alloimmune microenvironment and, finally, that formulations with adjuvants or immunosuppressive drugs can allow backpacked Tregs to act as mules in delivering immunostimulatory or -suppressive payloads in situ.
In all, the NG platform holds potential therapeutic merit in tackling diseases of an auto- or allo-immune nature, providing its effects in controlled spaces by linking innate TCR-signaling to the release of immunomodulatory proteins. Additionally, it is critical to note that most of the results in this work demonstrate the efficacy of the platform in improving the outcomes of polyclonal Treg transfer. Using an artificial antigen-specific allotransplant model in this study, we already observed the heightened effects of engineering IL-2/Fc NGs on antigen-specific Tregs. As such, we can foresee our platform being complementary to chimeric antigen-specific (CAR) Tregs (63, 64) to create a combination therapy that has the potential to induce tolerogenic responses and achieve even longer-lasting allograft acceptance (fig. S14E).
Materials and Methods
Study Design
This study was designed to investigate Tregs engineered with surface-conjugated, nanoscale IL-2 particles compared with unmodified Tregs as immunoregulatory adoptive cell therapy in the context of allotransplantation. To this end, nanogel IL-2/Fc fusion nanogel (NG) particles were synthesized with reduction-sensitive bis-N-hydroxy succinimide (NHS) crosslinkers that degrade upon TCR-ligation-dependent T-cell activation. Then, the ability to couple NG particles on Tregs was verified, and unaltered functional characteristics of coupled Tregs were confirmed with in vitro survival and suppression assays. NG Treg functionality in vivo was tested in Rag1−/− mice transplanted with fully MHC-mismatched skin and humanized NSG mice transplanted with human skin. Specifically, we focused on the efficacy of NG-coupled Tregs to suppress local pro-inflammatory T cells in sites of antigen encounter, including the allograft, without activating peripheral Tregs. To assess the survival benefits to the allograft, we performed survival studies with an allogeneic skin transplant model. Animals were assigned to treatment groups randomly and age-matched between conditions where necessary. The investigators were not blinded, sample sizes were not predetermined by power analysis in light of prior experience, and no animals were excluded because of illness.
Mice
C57BL/6 (#000664), BALB/c (#000651), B6.129S7-Rag1tm1Mom (Rag1−/−, #002216), C57BL/6-Tg(CAG-OVA)916Jen (OVA, #005145), C57BL/6-Tg(TcraTcrb)1100Mjb (OT-1, #003831), B6.Cg-Tg(TcraTcrb)425Cbn (OT-2, #004194), and NOD.Cg-Prkdcscid Il2rgtm1Wjl/Sz (NSG, #005557) mice were purchased from The Jackson Laboratories. All murine strains in this study were maintained in specific-pathogen-free (SPF) conditions at the Brigham and Women’s Hospital animal facility in accordance with federal, state, and institutional guidelines. The study protocol was approved by the Brigham and Women’s Hospital Institutional Animal Care and Use Committee (IACUC). Mice were sex- and age-matched (8–12 weeks old) apart from several NSG mice that were up to 6 months of age (these mice were equally randomized into the treatment groups for the humanized experiments).
Human subjects and blood samples
Concentrated, leukapheresed blood from healthy individuals was obtained from the Brigham and Women’s Hospital Blood Bank for the in vitro human and in vivo humanized experiments. Peripheral blood mononuclear cells (PBMCs) were isolated from the leukapheresed concentrates within 4 hours by SepMate tubes (#85450, STEMCELL Technologies). Healthy skin was obtained from patients undergoing cosmetic surgery procedures as discarded tissues at the Brigham and Women’s Hospital Plastic Surgery Division. The study protocol was approved by an Institutional Review Board at the Brigham and Women’s Hospital and was performed in accordance with the principles of the Declaration of Helsinki.
Synthesis of nanogels
NHS-SS-NHS crosslinkers (details provided in the Supplementary Materials and Methods) were dissolved at 10 mg/mL in DMSO and added to an IL-2/Fc solution (10 mg/mL in 1× DPBS) at a molar equivalent of 15:1 crosslinker-to-IL-2/Fc. The cocktail was rotated at 25°C for 30 min, at which point 1× DPBS was added to bring the final protein concentration to 1 mg/mL. Subsequently, anti-CD45 in 1× DPBS was added to the diluted solution at a 1:10 molar equivalent of anti-CD45-to-IL-2/Fc, and the reaction mixture was rotated at 25°C for another 30 min. The resulting nanoscale gels (nanogels, NGs), were then washed thrice with 1.5 mL of 1× DPBS in a 100 kDa molecular weight cut-off Amicon ultracentrifugal filter (#UFC910024, Millipore). To enhance conjugation of anti-CD45/IL-2/Fc-NGs to T cells, before T cell coupling, polyethylene glycol-b-polylysine (PEG5k-PLL33k) in 1× DPBS was mixed with freshly prepared nanogels at a 12.5:100 molar equivalent of PEG-PLL-to-IL-2/Fc. The mixture was rotated at 25°C for 2–16 hours and used without further purification.
Coupling of nanogels
Engineering of the Treg surface with IL-2/Fc NGs was performed immediately before intravenous adoptive transfer. In short, freshly magnetically-sorted Tregs were divided into two groups, control (CT) and NG. NG Tregs were routinely coupled with 10–20 μg NG/106 Tregs in 200 μL of 1× HBSS (#14175095, Gibco). CT Tregs were similarly suspended in 200 μL 1× HBSS, however, in the absence of NGs. Both Treg groups were then incubated at 4°C on an intermediate speed plate shaker (setting 7/10) for 45 min. Consistently, nearly half of all CT and NG Tregs were lost during the coupling step. Following coupling, CT and NG Tregs were washed twice with 1× DPBS and used for downstream in vitro and in vivo applications.
Skin transplant models
To study the efficacy of the NG platform in controlling alloimmune responses, we used a fully MHC-mismatched murine skin transplant model. Full-thickness trunk skin grafts (1.0 cm × 1.5 cm) from BALB/c donors were harvested at the level of the areolar connective tissue, and, connective, adipose, and panniculus carnosus tissues were cleared using blunt-tipped forceps. The fur of each anesthetized recipient Rag1−/− mouse was shaven at the dorsal trunk, 1.0–by–1.5 cm of the recipient mouse’s skin was excised, and an equally sized skin graft was sutured onto the graft bed with PERMA-HAND 4–0 Silk Suture (#1677G, Ethicon). Skin transplants were secured with dry gauze and bandaged for 7–10 days. For survival studies, grafts were monitored daily for the presence of erythema, erosion, contraction, or necrosis of the allografts.
Additionally, antigen-specific alloimmune responses were studied with OVA donor grafts and Rag1−/− recipients, while human alloimmune responses were assessed with human skin grafts (obtained as described above) and NSG recipients, as described above.
Adoptive Treg transfer models
For the polyclonal Treg experiments, 5.0×105 CD8α+ cells and 5.0×105 CD4+CD25+ Tregs with or without IL-2/Fc NG from C57BL/6 mice were injected retro-orbitally in 90-μL total volumes of 1× DPBS at day 3 post-transplant. For methodological reasons, larger cell numbers could not be obtained. As a control condition, a group of CT Treg-treated mice received intravenous free IL-2/Fc at the time of the adoptive transfer, in the same total concentration as that would be released from the IL-2/Fc NG coupled to the NG Tregs. With an average IL-2/Fc release of 90% from the NG particles after 7 days and 90% of the NG Tregs being conjugated with IL-2/Fc NGs, together with 10 μg of NGs being conjugated with 106 Tregs, for every mouse injected with 5.0×105 NG Tregs, 5.0×105 CT Tregs were co-injected with 4.1 μg of free IL-2/Fc and referred to in the manuscript as CT+IL-2/Fc Tregs. This is was considered the equivalent dose of IL-2/Fc in the CT+IL-2/Fc and NG conditions.
Animals were euthanized at day 7 post adoptive transfer for mechanistic studies and left until allograft rejection in survival studies. For the antigen-specific Treg experiments, 5.0×105 CD8α+ cells and 2.5×105 CD4+CD25+ Tregs with or without IL-2/Fc NG from OT-1 mice, and 2.5×105 CD4+CD25+ Tregs with or without IL-2/Fc NG from OT-2 mice were injected retro-orbitally in 90-μL total volumes of 1× DPBS 3 days post-transplant. Animals were euthanized at day 7 post adoptive transfer for mechanistic studies.
For the humanized Treg experiments, 5.0×106 CD25+CD127lo-depleted PBMCs and 1.0×106 CD25+CD127lo Tregs from a healthy donor were injected retro-orbitally in 90-μL total volumes of 1× DPBS 7 days post-transplant. Animals were euthanized at day 21 post adoptive transfer for mechanistic studies.
OVA-OT-1-OT-2 model details
CD4+ T cells from OT-2 mice can undergo OVA323–339-specific clonal expansion through MHC-II-dependent presentation of OVA323–339 peptides by the allograft, while CD4+ T cells from OT-1 mice cannot. Thus, due to the non-specificity of OT-1 CD4+CD25+ Tregs for the relevant MHC-II-presented OVA peptides, OT-1 Tregs can only become activated due to environmental stimuli such as cytokines secreted in sites of antigen encounter. OT-2 CD4+CD25+ Tregs, on the contrary, will primarily be activated by the OVA323–339 peptides. Observing OT-2 Tregs in the proximal sites of alloimmunity, then, ties the activation and release of IL-2/Fc from the NGs to the presence of antigen, especially with the absence of OT-1 Tregs in any of the proximal sites of alloimmunity. The OT-1 CD8α+ T cells, meanwhile, are added to recognize the OVA257–264 peptides presented in association with class I MHC antigens to induce allograft rejection. For the discrimination between OT-1 and OT-2 T cells by flow cytometry using OT-1-specific tetrameric SIINFEKL antibodies, it is important to note that in the absence of prior ovalbumin sensitization in the OT-2 mice, a subset of polyclonal Tregs with low to negligible OT-2 receptors can be included in the OT-1 tetramer-negative pool of the adoptively transferred OT-2 Tregs. Thus, when OT-2 Tregs are referenced, the potential inclusion of polyclonal Tregs should be factored in, especially in the spleen and non-draining lymph nodes where the exposure to the OT-2-specific OVA323–339 peptide is sparser compared with the higher concentrations of OVA323–339 in the draining lymph nodes and skin allografts.
Statistics
Differences between two normally distributed groups were analyzed with independent samples two-tailed Student t-tests, and non-parametric Kolmogorov-Smirnov tests were performed when the assumption of homoscedasticity could not be met. Statistical analyses of multiple groups were performed with one-way analyses of variance followed by Holm-Šídák multiple comparison tests, or mixed-effects model analyses with the Geisser-Greenhouse correction followed by Holm-Šídák multiple comparison tests for experimental groups with matched data points across multiple time points or concentrations. Fold changes were normalized against the CT condition unless otherwise specified. To study the correlation between the suppressive profiles of CT and NG Tregs, CT and NG proliferation indices at the same suppressor-to-responder ratios were plotted against each other, and through linear regression, Pearson’s correlation coefficient (R) was calculated. For survival analyses, Kaplan-Meier graphs were analyzed with log-rank Mantel-Cox tests. P < 0.05 was considered significant for all analyses. Data analysis and graphing were performed with Prism 8.0 (GraphPad Software). Graphs show boxplots with median, interquartile range, minimum, maximum, and all individual data points of the denoted experimental groups.
Additional experimental details are described in the Supplementary Materials and Methods.
Supplementary Material
Fig. S1. Murine in vitro Treg and T cell phenotyping strategy.
Fig. S2. IL-2/Fc release from nanogels and nanogel surface retention on engineered Tregs.
Fig. S3. IL-2/Fc NGs promote the maintenance of Treg survival and phenotype in a CD3ε-dose-dependent fashion.
Fig. S4. Splenic T cell suppression correlation analysis.
Fig. S5. Murine in vivo skin transplant model phenotyping strategy.
Fig. S6. Nanogel-conjugated Tregs proliferate optimally in proximal sites of alloimmunity with decreased CD8α T cells and unaffected NK cells.
Fig. S7. Synchronously tracking Tregs and CD8 T cells with bioluminescent luciferase reporters.
Fig. S8. Antigen-specific mouse model of skin transplantation phenotyping strategy.
Fig. S9. Antigen-specific nanogeled T cells proliferate better in proximal sites of alloimmunity than non-antigen-specific nanogeled T cells.
Fig. S10. IL-2/Fc NGs conjugated to human Tregs improve their in vitro homeostasis compared to conventional Tregs.
Fig. S11. In vivo humanized mouse model of human skin transplantation phenotyping strategy.
Fig. S12. In vivo IL-2/Fc NG treatment of humanized skin transplanted mice favors graft size maintenance and intragraft Treg dominance over conventional Treg treatments.
Fig. S13. Nanogel backpacks are quickly and stably tethered on Tregs to facilitate the spatiotemporal release of adducted prodrugs at sites of antigen encounter.
Fig. S14. Limitations and future perspectives for IL-2/Fc nanogel-based immunotherapy.
Table S1. Full list of the confocal microscopy antibodies used in this study.
Table S2. Full list of the flow cytometry antibodies used in this study.
Table S3. Full list of the mRNA transcript targets and primers used in this study.
Table S4. Two-step quantitative real-time PCR protocol.
Data File S1. Original data.
Movie S1. 3D volume view of control Treg post in vitro coupling.
Movie S2. 3D volume view of nanogel-conjugated Treg post in vitro coupling.
Acknowledgments:
We thank our colleagues at the Schuster Family Transplantation Research Center at the Brigham and Women’s Hospital for their assistance and collaboration; we thank the anonymous reviewers for their constructive feedback; we thank the patients who made this work possible in trusting us with their donated skin and blood samples; we thank the Talal and Maha Shair Foundation for their generous support to science; we thank the Harvard Department of Immunology’s Flow Cytometry Facility for assistance with the flow cytometric analyses; and, we thank S. Tripathi, N. Murakami, F. Ordikhani, A. Akbarzadeh, F. Juillard, and B. Liu for their technical assistance in this study. J.Y. Choi was supported by the National Institutes of Health (T32 DK007527).
Funding: American Heart Association (AHA Award 13FTF17000018 to J.R.A.), American Diabetes Association (ADA Award 1-17-IBS-206 to J.R.A.), Qatar Foundation Grant (NPRP8-1744-3-357X to J.R.A.), and the National Institutes of Health (RO1 AI134842 to J.R.A.).
Footnotes
Competing Interests: D.J.I. and L.T. are inventors on U.S. Patent no. 2017/0080104 A1 (“Cell Surface Coupling of Nanoparticles”) related to the nanogel technology. D.J.I. is a co-founder of Torque Therapeutics, which has licensed patents related to the nanogel platform. J.R.A, S.K.E., and D.J.I. are inventors on U.S. Provisional Patent no. 62/887,805 (“Engineering Regulatory T Cells with TCR-Signaling-Responsive IL-2 Nanogels and Methods of Use”) related to the IL-2 nanogels and methods thereof for improving regulatory T cell therapy described in this manuscript. All other authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
References and Notes
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M, Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol 155, 1151–1164 (1995). [PubMed] [Google Scholar]
- 2.Hori S, Nomura T, Sakaguchi S, Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY, Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol 4, 330–336 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Wood KJ, Sakaguchi S, Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol 3, 199–210 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Yamaguchi T, Nomura T, Ono M, Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Wood KJ, Bushell A, Hester J, Regulatory immune cells in transplantation. Nat. Rev. Immunol 12, 417–430 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A, First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin. Immunol 133, 22–26 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, Owczuk R, Szadkowska A, Witkowski P, Mlynarski W, Jarosz-Chobot P, Bossowski A, Siebert J, Trzonkowski P, Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin. Immunol 153, 23–30 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Graca L, Cobbold SP, Waldmann H, Identification of regulatory T cells in tolerated allografts. J. Exp. Med 195, 1641–1646 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho-Gaspar M, Jones ND, Luo S, Martin L, Brook MO, Wood KJ, Location and time-dependent control of rejection by regulatory T cells culminates in a failure to generate memory T cells. J. Immunol 180, 6640–6648 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK, Response to self antigen imprints regulatory memory in tissues. Nature 480, 538–542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riley JL, June CH, Blazar BR, Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 30, 656–665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald-Hyman C, Turka LA, Blazar BR, Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci. Transl. Med 7, 280rv2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safinia N, Grageda N, Scottà C, Thirkell S, Fry LJ, Vaikunthanathan T, Lechler RI, Lombardi G, Cell Therapy in Organ Transplantation: Our Experience on the Clinical Translation of Regulatory T Cells. Front. Immunol 9, 354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Q, Lee K, Regulatory T-cell therapy for transplantation: how many cells do we need? Curr. Opin. Organ. Transplant 17, 349–354 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Tang Q, Bluestone JA, Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb Perspect. Med 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP, Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat. Med 14, 88–92 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allos H, Al Dulaijan BS, Choi J, Azzi J, Regulatory T Cells for More Targeted Immunosuppressive Therapies. Clin. Lab. Med 39, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S, Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl. Acad. Sci. U. S. A 106, 1903–1908 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA, Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol 10, 1000–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK, Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Josefowicz SZ, Lu LF, Rudensky AY, Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol 30, 531–564 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, Riess D, Hein MY, Buch T, Polic B, Schonle A, Zeiser R, Schmitt-Graff A, Kretschmer K, Klein L, Korn T, Sakaguchi S, Schmidt-Supprian M, Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity 41, 722–736 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Levine AG, Arvey A, Jin W, Rudensky AY, Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol 15, 1070–1078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setoguchi R, Hori S, Takahashi T, Sakaguchi S, Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med 201, 723–735 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J, Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311, 1924–1927 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA, IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol 178, 280–290 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Thornton AM, Shevach EM, CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med 188, 287–296 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY, Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VT, Alyea EP, Armand P, Blazar BR, Antin JH, Soiffer RJ, Ritz J, Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci. Transl. Med 5, 179ra43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klatzmann D, Abbas AK, The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol 15, 283–294 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Koreth J, Kim HT, Jones KT, Lange PB, Reynolds CG, Chammas MJ, Dusenbury K, Whangbo J, Nikiforow S, Alyea EP 3rd, Armand P, Cutler CS, Ho VT, Chen YB, Avigan D, Blazar BR, Antin JH, Ritz J, Soiffer RJ, Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood 128, 130–137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camirand G, Riella LV, Treg-Centric View of Immunosuppressive Drugs in Transplantation: A Balancing Act. Am. J. Transplant 17, 601–610 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Hirakawa M, Matos TR, Liu H, Koreth J, Kim HT, Paul NE, Murase K, Whangbo J, Alho AC, Nikiforow S, Cutler C, Ho VT, Armand P, Alyea EP, Antin JH, Blazar BR, Lacerda JF, Soiffer RJ, Ritz J, Low-dose IL-2 selectively activates subsets of CD4(+) Tregs and NK cells. JCI Insight 1, e89278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang L, Zheng Y, Melo MB, Mabardi L, Castano AP, Xie YQ, Li N, Kudchodkar SB, Wong HC, Jeng EK, Maus MV, Irvine DJ, Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol 36, 707–716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noelle RJ, Lawrence DA, Modulation of T-cell function. II. Chemical basis for the involvement of cell surface thiol-reactive sites in control of T-cell proliferation. Cell. Immunol 60, 453–469 (1981). [DOI] [PubMed] [Google Scholar]
- 37.Lawrence DA, Song R, Weber P, Surface thiols of human lymphocytes and their changes after in vitro and in vivo activation. J. Leukoc. Biol 60, 611–618 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Mougiakakos D, Johansson CC, Kiessling R, Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood 113, 3542–3545 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Mougiakakos D, Johansson CC, Jitschin R, Böttcher M, Kiessling R, Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood 117, 857–861 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Wang J, Luft JC, Tian S, Owens G Jr, Pandya AA, Berglund P, Pohlhaus P, Maynor BW, Smith J, Hubby B, Napier ME, DeSimone JM, Rendering protein-based particles transiently insoluble for therapeutic applications. J. Am. Chem. Soc 134, 8774–8777 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riber CF, Smith AA, Zelikin AN, Self-Immolative Linkers Literally Bridge Disulfide Chemistry and the Realm of Thiol-Free Drugs. Adv. Healthc. Mater 4, 1887–1890 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Baudino L, Shinohara Y, Nimmerjahn F, Furukawa J, Nakata M, Martinez-Soria E, Petry F, Ravetch JV, Nishimura S, Izui S, Crucial role of aspartic acid at position 265 in the CH2 domain for murine IgG2a and IgG2b Fc-associated effector functions. J. Immunol 181, 6664–6669 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Fabre JW, Williams AF, Quantitative serological analysis of a rabbit anti-rat lymphocyte serum and preliminary biochemical characterisation of the major antigen recognised. Transplantation 23, 349–359 (1977). [DOI] [PubMed] [Google Scholar]
- 44.Thomas ML, Barclay AN, Gagnon J, Williams AF, Evidence from cDNA clones that the rat leukocyte-common antigen (T200) spans the lipid bilayer and contains a cytoplasmic domain of 80,000 Mr. Cell 41, 83–93 (1985). [DOI] [PubMed] [Google Scholar]
- 45.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH, PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med 206, 3015–3029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asano T, Meguri Y, Yoshioka T, Kishi Y, Iwamoto M, Nakamura M, Sando Y, Yagita H, Koreth J, Kim HT, Alyea EP, Armand P, Cutler CS, Ho VT, Antin JH, Soiffer RJ, Maeda Y, Tanimoto M, Ritz J, Matsuoka KI, PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood 129, 2186–2197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P, CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol 3, 1097–1101 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S, CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Cibrián D, Sánchez-Madrid F, CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol 47, 946–953 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arenas-Ramirez N, Woytschak J, Boyman O, Interleukin-2: Biology, Design and Application. Trends Immunol. 36, 763–777 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Fujii H, Nakagawa Y, Schindler U, Kawahara A, Mori H, Gouilleux F, Groner B, Ihle JN, Minami Y, Miyazaki T, Activation of Stat5 by interleukin 2 requires a carboxyl-terminal region of the interleukin 2 receptor beta chain but is not essential for the proliferative signal transmission. Proc. Natl. Acad. Sci. U. S. A 92, 5482–5486 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q, Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med 7, 315ra189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baluna R, Vitetta ES, Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology 37, 117–132 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Mansour H, Homs S, Desvaux D, Badoual C, Dahan K, Matignon M, Audard V, Lang P, Grimbert P, Intragraft levels of Foxp3 mRNA predict progression in renal transplants with borderline change. J. Am. Soc. Nephrol 19, 2277–2281 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, Hartono C, Li B, Sharma VK, Seshan SV, Kapur S, Hancock WW, Schwartz JE, Suthanthiran M, Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N. Engl. J. Med 353, 2342–2351 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, Goode EL, Knutson KL, The Ratios of CD8(+) T Cells to CD4(+)CD25(+) FOXP3(+) and FOXP3(−) T Cells Correlate with Poor Clinical Outcome in Human Serous Ovarian Cancer. PLoS One 8, e80063. doi: 10.1371/journal.pone.0080063 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kingsley CI, Karim M, Bushell AR, Wood KJ, CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol 168, 1080–1086 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM, CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J. Exp. Med 205, 1975–1981 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A, P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature 385, 81–83 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Hautz T, Zelger B, Grahammer J, Krapf C, Amberger A, Brandacher G, Landin L, Muller H, Schon MP, Cavadas P, Lee AW, Pratschke J, Margreiter R, Schneeberger S, Molecular markers and targeted therapy of skin rejection in composite tissue allotransplantation. Am. J. Transplant 10, 1200–1209 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Carrette F, Surh CD, IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin. Immunol 24, 209–217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, Rosenblum MD, Cutting Edge: memory regulatory T cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol 190, 4483–4487 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MA, Hannen RF, Cooper D, Marelli-Berg FM, Watt FM, Lechler RI, Maher J, Smyth LA, Lombardi G, Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am. J. Transplant 17, 931–943 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Noyan F, Zimmermann K, Hardtke-Wolenski M, Knoefel A, Schulde E, Geffers R, Hust M, Huehn J, Galla M, Morgan M, Jokuszies A, Manns MP, Jaeckel E, Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am. J. Transplant 17, 917–930 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ, In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Control. Release 172, 426–435 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Chaumont F, Dallongeville S, Chenouard N, Herve N, Pop S, Provoost T, Meas-Yedid V, Pankajakshan P, Lecomte T, Le Montagner Y, Lagache T, Dufour A, Olivo-Marin JC, Icy: an open bioimage informatics platform for extended reproducible research. Nat. Methods 9, 690–696 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Metsalu T, Vilo J, ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 43, W566–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Murine in vitro Treg and T cell phenotyping strategy.
Fig. S2. IL-2/Fc release from nanogels and nanogel surface retention on engineered Tregs.
Fig. S3. IL-2/Fc NGs promote the maintenance of Treg survival and phenotype in a CD3ε-dose-dependent fashion.
Fig. S4. Splenic T cell suppression correlation analysis.
Fig. S5. Murine in vivo skin transplant model phenotyping strategy.
Fig. S6. Nanogel-conjugated Tregs proliferate optimally in proximal sites of alloimmunity with decreased CD8α T cells and unaffected NK cells.
Fig. S7. Synchronously tracking Tregs and CD8 T cells with bioluminescent luciferase reporters.
Fig. S8. Antigen-specific mouse model of skin transplantation phenotyping strategy.
Fig. S9. Antigen-specific nanogeled T cells proliferate better in proximal sites of alloimmunity than non-antigen-specific nanogeled T cells.
Fig. S10. IL-2/Fc NGs conjugated to human Tregs improve their in vitro homeostasis compared to conventional Tregs.
Fig. S11. In vivo humanized mouse model of human skin transplantation phenotyping strategy.
Fig. S12. In vivo IL-2/Fc NG treatment of humanized skin transplanted mice favors graft size maintenance and intragraft Treg dominance over conventional Treg treatments.
Fig. S13. Nanogel backpacks are quickly and stably tethered on Tregs to facilitate the spatiotemporal release of adducted prodrugs at sites of antigen encounter.
Fig. S14. Limitations and future perspectives for IL-2/Fc nanogel-based immunotherapy.
Table S1. Full list of the confocal microscopy antibodies used in this study.
Table S2. Full list of the flow cytometry antibodies used in this study.
Table S3. Full list of the mRNA transcript targets and primers used in this study.
Table S4. Two-step quantitative real-time PCR protocol.
Data File S1. Original data.
Movie S1. 3D volume view of control Treg post in vitro coupling.
Movie S2. 3D volume view of nanogel-conjugated Treg post in vitro coupling.