Abstract
Background
An unexpected high prevalence of enterococcal bloodstream infection (BSI) has been observed in critically ill patients with COVID-19 in the intensive care unit (ICU).
Materials and methods
The primary objective was to describe the characteristics of ICU-acquired enterococcal BSI in critically ill patients with COVID-19. A secondary objective was to exploratorily assess the predictors of 30-day mortality in critically ill COVID-19 patients with ICU-acquired enterococcal BSI.
Results
During the study period, 223 patients with COVID-19 were admitted to COVID-19-dedicated ICUs in our centre. Overall, 51 episodes of enterococcal BSI, occurring in 43 patients, were registered. 29 (56.9%) and 22 (43.1%) BSI were caused by Enterococcus faecalis and Enterococcus faecium, respectively. The cumulative incidence of ICU-acquired enterococcal BSI was of 229 episodes per 1000 ICU admissions (95% mid-p confidence interval [CI] 172–298). Most patients received an empirical therapy with at least one agent showing in vitro activity against the blood isolate (38/43, 88%). The crude 30-day mortality was 42% (18/43) and 57% (4/7) in the entire series and in patients with vancomycin-resistant E. faecium BSI, respectively. The sequential organ failure assessment (SOFA) score showed an independent association with increased mortality (odds ratio 1.32 per one-point increase, with 95% confidence interval 1.04–1.66, p = .021).
Conclusions
The cumulative incidence of enterococcal BSI is high in critically ill patients with COVID-19. Our results suggest a crucial role of the severity of the acute clinical conditions, to which both the underlying viral pneumonia and the enterococcal BSI may contribute, in majorly influencing the outcome.
KEY MESSAGES
The cumulative incidence of enterococcal BSI is high in critically ill patients with COVID-19.
The crude 30-day mortality of enterococcal BSI in critically ill patients with COVID-19 may be higher than 40%.
There could be a crucial role of the severity of the acute clinical conditions, to which both the underlying viral pneumonia and the enterococcal BSI may contribute, in majorly influencing the outcome.
Keywords: Enterococcus, VRE, BSI, COVID-19, SARS-CoV-2
Background
Critically ill patients with acute hypoxemic respiratory failure due to coronavirus disease 2019 (COVID-19) requiring mechanical ventilation in intensive care units (ICU) have been reported to possibly be at increased risk of developing bloodstream infection (BSI) compared with other non-COVID-19 critically ill patient populations [1–3]. Although the exact causal pathways of this increased risk are still not completely clear, different non-mutually exclusive mechanisms have been proposed: (i) the use of immunomodulatory agents; (ii) the impairment of antigen presentation and the presence of acquired immunosuppression due to SARS-CoV-2; (iii) the impairment of microcirculation due to the endothelial dysfunction and coagulopathy occurring during COVID-19 [1,4,5].
Regarding the aetiology of BSI in critically ill patients with COVID-19, an unexpectedly high prevalence of enterococcal BSI has been previously observed [3,6,7]. Against this background and considering the frequent use of antimicrobials in critically ill patients with COVID-19 [8,9], the risk of selecting difficult-to-treat resistant strains such as vancomycin-resistant enterococci (VRE) may be non-negligible [10], in turn possibly impacting patients’ outcomes.
The present, descriptive cases series was aimed to better depict the characteristics of ICU-acquired enterococcal BSI in critically ill patients with COVID-19, especially in the terms of cumulative incidence, causative microorganisms, and outcome.
Methods
This retrospective, single-centre study was conducted in two ICU wards (up to a maximum of 39 beds for COVID-19 patients, with their number being dynamically reduced/increased according to the local COVID-19 epidemiology) at San Martino Policlinico Hospital, a 1200-bed teaching hospital in Genoa, Italy. From 1 January 2020 to 31 December 2020, all patients with COVID-19 and ICU-acquired enterococcal BSI were included in the study.
The primary objective was to describe the characteristics of ICU-acquired enterococcal BSI in critically ill patients with COVID-19, in terms of cumulative incidence, causative microorganisms, clinical characteristics, antimicrobial treatment, and 30-day mortality. A secondary objective was to exploratorily assess the predictors of 30-day mortality in critically ill COVID-19 patients with ICU-acquired enterococcal BSI.
The collection of anonymized data for the present study was approved by the local Ethics Committee (Liguria Region Ethics Committee, registry number 163/2020) and specific informed consent was waived due to the retrospective nature of the study.
Definitions
COVID-19 was defined as at least one real-time polymerase chain reaction assay positive for SARS-CoV-2 on a respiratory specimen. ICU-acquired enterococcal BSI was defined as at least one blood culture drawn at >48 h after ICU admission positive for enterococci [11].
Data collection
The following data were collected from the laboratory database and the patients’ medical records as they were at the time of the first enterococcal BSI episode: age in years; gender; Charlson score [12]; diabetes mellitus; chronic obstructive pulmonary disease; chronic kidney disease [13]; previous myocardial infarction; presence of solid neoplasm; presence of hematological malignancy; solid organ transplant; haematopoietic stem cell transplantation; admission from a long-term care facility; previous hospitalization (within 6 months); previous vancomycin-resistant enterococci (VRE) isolation (within 6 months); acute respiratory distress syndrome (ARDS) at hospital admission (at least mild according to Berlin criteria [14]); need for invasive mechanical ventilation (before the development of enterococcal BSI); previous therapy with glycopeptides (within 6 months); anti-inflammatory treatment for COVID-19 (steroids and/or tocilizumab); ICU stay in days before the development of the first enterococcal BSI episode; recent treatment with cephalosporins (during hospital stay before the development of BSI), presence of neutropenia (defined as an absolute neutrophil count <500 cell/mm3); presence of a central venous catheter (CVC); Pitt bacteraemia score [15]; sequential organ failure assessment (SOFA) score [16]; presence of septic shock [17]; causative agent of the enterococcal BSI; presence of a polymicrobial BSI (and type of concomitant aetiological agent other than Enterococcus spp., with at least two consecutive cultures positive for the same pathogen being necessary for defining BSI due to coagulase-negative staphylococci or other common skin contaminants); DENOVA score [18]; presence of a CVC-related BSI (CRBSI) [19]; concomitant endocarditis (the presence of IE was defined according to the modified Duke’s criteria [20]); adequate source control, that is, unnecessary, removal of infected devices, or drainage of infected fluid collections; administration of an empirical therapy; administration of an appropriate empirical therapy (defined as administration of at least one agent with in vitro activity against the given blood isolate).
Microbiology
The Vitek MS MALDI-TOF mass spectrometry (bioMérieux, Craponne, France) was routinely used for identifying Enterococcus spp. as causative microorganisms of ICU-acquired BSI, whereas the Vitek 2 automated system (bioMérieux, Craponne, France) was routinely used for susceptibility testing. The results of the susceptibility tests were interpreted according to the criteria of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (breakpoint tables for interpretation of minimum inhibitory concentrations [MIC] and zone diameters, version 10.0, 2020; http://www.eucast.org). For daptomycin, isolates were considered susceptible in the case of MIC ≤2 mg/L [21].
Statistical analysis
No sample size calculations a priori were performed for this descriptive, exploratory study. For the primary descriptive analysis, the cumulative incidence of ICU- acquired enterococcal BSI was calculated as the number of events per 1000 ICU admissions of COVID-19 patients, with exact mid-p 95% confidence interval (CI) [22]. In the case of multiple episodes of enterococcal BSI from the same species occurring in the same patient, a novel event was considered as independent from the previous one if developed at least 30 days after the last positive culture related to the previous episode. With regard to the demographic and clinical characteristics of single patients, categorical variables were summarized with numbers and percentages, and continuous variables with medians and interquartile ranges. The 95% CI was calculated for all estimates [23,24].
For the secondary exploratory analysis of predictors of 30-day mortality, the first ICU-acquired enterococcal BSI per patient was considered. The possible association between clinical variables and 30-day mortality was first tested in univariable logistic regression models. Then, variables potentially associated with the outcome in univariable comparisons (p < .20) were included in an initial logistic regression multivariable model, and then further selected for the final multivariable model through a stepwise backward procedure based on the Akaike information criterion. The survival of patients with ICU-acquired BSI was also summarized graphically through the Kaplan–Meier method, with the time of origin set at the day when the first positive culture of the first enterococcal BSI episode was drawn. Statistical analyses were performed using the R Statistical Software (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
During the study period, 223 patients with COVID-19 were admitted to the participating ICUs. Overall, 51 episodes of enterococcal BSI, occurring in 43 patients, were registered. 29 (56.9%) and 22 (43.1%) BSI were caused by Enterococcus faecalis and Enterococcus faecium, respectively. The cumulative incidence of ICU-acquired enterococcal BSI was of 229 episodes per 1000 ICU admissions (95% mid-p confidence interval [CI] 172–298). The cumulative incidence of E. faecalis and E. faecium BSI was 130 (95% mid-p CI 89–184) and 99 (95% mid-p CI 63–147) episodes per 1000 ICU admissions, respectively.
The demographic and clinical characteristics of the study population are shown in Table 1. As shown in the table, the median age of patients with ICU-acquired Enterococcus spp. BSI was of 63 years (interquartile range [IQR] 58–69) and 77% were males (33/43). The first episode of ICU-enterococcal BSI mainly occurred late during ICU stay (median 18 days from ICU admission, interquartile range 12–32). Overall, a moderate burden of baseline comorbidities was observed, with a median Charlson score of 3 (IQR 2–5). Most patients received an empirical therapy with at least one agent showing in vitro activity against the blood isolate (38/43, 88%).
Table 1.
Demographic and clinical characteristics of COVID-19 patients with ICU-acquired Enterococcus spp. BSI.
| Variable | No. of patientsa | % | 95% CI |
|---|---|---|---|
| Demographic variables | |||
| Age in years, median (IQR) | 63 (58–69) | 59–66 | |
| Male gender | 33/43 | 77 | 62–88 |
| Medical history | |||
| Charlson score, median (IQR) | 3 (2–5) | 2–4 | |
| Diabetes mellitus | 9/43 | 21 | 11–36 |
| Chronic obstructive pulmonary disease | 3/43 | 7 | 2–18 |
| Chronic kidney disease | 8/43 | 19 | 9–33 |
| Previous myocardial infarction | 4/43 | 9 | 3–22 |
| Presence of solid neoplasm | 1/43 | 2 | 0–12 |
| Presence of hematological malignancy | 1/43 | 2 | 0–12 |
| Solid organ transplant | 0/43 | 0 | 0–7 |
| Haematopoietic stem cell transplantation | 0/43 | 0 | 0–7 |
| Admission from LTCF | 4/43 | 9 | 3–22 |
| Previous hospitalisation (within 6 months) | 7/43 | 16 | 7–30 |
| Previous VRE isolation (within 6 months) | 5/43 | 12 | 5–25 |
| Previous therapy with glycopeptides (within 6 months) | 5/43 | 12 | 5–25 |
| COVID-19 pneumonia | |||
| ARDS at hospital admissionb | 40/43 | 93 | 82–98 |
| Need for invasive mechanical ventilationc | 43/43 | 100 | 93–100 |
| Anti-inflammatory treatment for COVID-19 | |||
| Treatment with steroidsd | 34/43 | 79 | 64–89 |
| Treatment with tocilizumabe | 12/43 | 28 | 16–43 |
| Variables at BSI onsetf | |||
| Duration of ICU stay before BSI onset in days, median (IQR) | 18 (12–32) | 13–23 | |
| Recent treatment with cephalosporinsg | 34/43 | 79 | 64–89 |
| Neutropenia (ANC < 500 cell/mm3) | 0/43 | 0 | 0–7 |
| Presence of CVC | 42/43 | 98 | 88–100 |
| Pitt bacteraemia score | 6 (4–8) | 6–8 | |
| SOFA score | 8 (6–11) | 7–10 | |
| Septic shock | 21/43 | 49 | 33–64 |
| Infection variablesh | |||
| Aetiological agent | |||
| Enterococcus faecalis | 24/43 | 56 | 41–70 |
| Enterococcus faecium | 19/43 | 44 | 30–59 |
| Ampicillin-resistant E. faecalis | 0/24 | 0 | 0–13 |
| Vancomycin-resistant E. faecium | 7/19 | 37 | 17–61 |
| Polymicrobial BSIi | 19/43 | 44 | 30–59 |
| DENOVA score | 1 (0–2) | 1–1 | |
| CRBSI | 31/43 | 72 | 57–84 |
| Endocarditis | 0/43 | 0 | 0–7 |
| Source control | |||
| Performed/unnecessaryj | 40/43 | 93 | 82–98 |
| Not performed | 3/43 | 7 | 2–18 |
| Empirical therapyk | 41/43 | 95 | 84–99 |
| In vitro active empirical therapyl | 38/43 | 88 | 75–95 |
ANC, absolute neutrophil count; ARDS, acute respiratory distress syndrome; BSI, bloodstream infection; COVID-19, coronavirus disease 2019; CRBSI, catheter-related bloodstream infection; CI, confidence intervals; CVC, central venous catheter; DENOVA, long Duration of symptoms/Embolization/Number of positive cultures/Origin of infection unknown/Valve disease/Auscultation of murmur; ICU, intensive care unit; IQR, interquartile range; LTCF, log-term care facility; SOFA, sequential organ failure assessment; VRE, vancomycin-resistant enterococci.
aResults are presented as No. of patients/Total of patients unless otherwise indicated.
bAt least mild according to Berlin criteria [14].
cBefore the development of enterococcal BSI.
dMethylprednisolone 1 mg/kg/die (30/34, 88%), dexamethasone 8 mg/die (4/34, 12%).
eTocilizumab was administered at the dosage of 8 mg/kg (single intravenous infusion or repeated once).
fThe day when the first positive blood culture for Enterococcus spp. was drawn.
gDuring hospital stay before the development of BSI.
hRelated to the first episode of ICU-acquired enterococcal BSI in each patient.
iCoagulase-negative Staphylococcus spp. (n = 9), Bacillus cereus (n = 1), Candida auris (n = 1), Pseudomonas aeruginosa (n = 1), Morganella morganii (n = 1), Viridans Group Streptococcus spp. (n = 1), Candida albicans plus Candida auris (n = 1), Candida albicans plus Staphylococcus aureus (n = 1), coagulase-negative Staphylococcus spp. plus Bacteroides fragilis (n = 1), coagulase-negative Staphylococcus spp. plus Enterobacter aerogenes (n = 1), Enterobacter aerogenes plus Pseudomonas aeruginosa (n = 1).
jCVC removal (n = 28, performed in all cases within 96 h after collection of the first positive culture), not necessary (n = 12).
kVancomycin (n = 17), daptomycin (n = 10), linezolid (n = 3), tigecycline (n = 1), daptomycin plus linezolid (n = 6), daptomycin plus ampicillin (n = 1), daptomycin plus gentamicin (n = 1), vancomycin plus linezolid (n = 1), vancomycin plus amikacin plus linezolid (n = 1).
lWith at least one agent showing in vitro activity against the blood isolate: vancomycin (n = 14), daptomycin (n = 10), linezolid (n = 3), tigecycline (n = 1), daptomycin plus linezolid (n = 6), daptomycin plus ampicillin (n = 1), daptomycin plus gentamicin (n = 1), vancomycin plus linezolid (n = 1), vancomycin plus amikacin plus linezolid (n = 1).
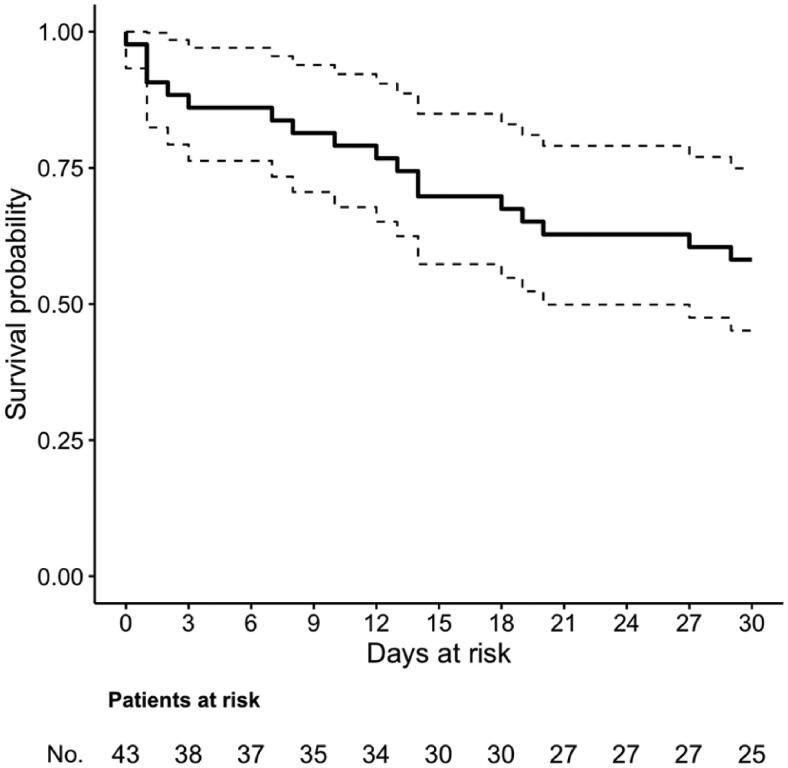
The crude 30-day mortality in our series was 42% (18/43), as also shown in Figure 1. According to the causative agent of the first enterococcal BSI episode, 30-day mortality was 42% (10/24), 42% (8/19), and 57% (4/7) in patients with E. faecalis BSI, E. faecium BSI, and vancomycin-resistant E. faecium BSI, respectively. The results of the univariable and multivariable analyses of predictors of 30-day mortality are shown in Table 2. In univariable analyses, previous hospitalization and SOFA score showed an association with increased mortality. In the final multivariable model, only the SOFA score retained an independent association with increased mortality (odds ratio 1.32 per one-point increase, with 95% confidence interval 1.04–1.66, p = .021).
Figure 1.
Survival in critically ill COVID-19 patients with ICU-acquired Enterococcus spp. BSI. The time of origin was set at the day when the first blood culture positive for Enterococcus spp. was drawn.
Table 2.
Univariable and multivariable analysis 30-day mortality predictors in COVID-19 patients with ICU-acquired Enterococcus spp. BSI.
| Variable | Non-survivorsa 18 (42) | Survivorsa 25 (58) | Univariable analysis |
Multivariable analysis** |
||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Age in years, median (IQR) | 65 (60–70) | 61 (54–67) | 1.06 (0.98–1.14) | .150 | 1.07 (0.98–1.17) | .128 |
| Male gender | 14 (78) | 19 (76) | 1.11 (0.26–4.67) | .892 | ||
| Charlson score, median (IQR) | 3 (2–5) | 3 (1–4) | 1.23 (0.87–1.73) | .246 | ||
| Diabetes mellitus | 4 (22) | 5 (20) | 1.14 (0.26–5.03) | .860 | ||
| Chronic obstructive pulmonary disease | 2 (11) | 1 (4) | 3.00 (0.25–35.91) | .386 | ||
| Chronic kidney disease | 3 (17) | 5 (20) | 0.80 (0.16–3.88) | .782 | ||
| Previous myocardial infarction | 1 (6) | 3 (12) | 0.43 (0.04–4.52) | .483 | ||
| Presence of solid neoplasm | 1 (6) | 0 (0) | Model not converging | .419* | ||
| Presence of hematological malignancy | 1 (6) | 0 (0) | Model not converging | .419* | ||
| Solid organ transplant | 0 (0) | 0 (0) | – | 1.000* | ||
| Haematopoietic stem cell transplantation | 0 (0) | 0 (0) | – | 1.000* | ||
| Admission from LTCF | 3 (17) | 1 (4) | 4.80 (0.46–50.50) | .191 | ||
| Previous hospitalisation (within 6 months) | 6 (33) | 1 (4) | 12.00 (1.29–111.32) | .029 | 6.52 (0.62–68.44) | .118 |
| Previous VRE isolation (within 6 months) | 3 (17) | 2 (8) | 2.30 (0.34–15.44) | .391 | ||
| Previous therapy with glycopeptides (within 6 months) | 1 (6) | 4 (16) | 0.31 (0.03–3.03) | .313 | ||
| ARDS at hospital admission | 16 (89) | 24 (96) | 0.33 (0.03–3.99) | .386 | ||
| Need for invasive mechanical ventilation | 18 (100) | 25 (100) | – | 1.000* | ||
| Treatment with steroids | 15 (83) | 19 (76) | 1.58 (0.34–7.38) | .562 | ||
| Treatment with tocilizumab | 5 (28) | 8 (32) | 0.82 (0.22–3.09) | .766 | ||
| Duration of ICU stay before BSI onset in days, median (IQR) | 17 (12–32) | 21 (11–32) | 1.01 (0.98–1.05) | .476 | ||
| Recent treatment with cephalosporins | 15 (83) | 19 (76) | 1.58 (0.34–7.38) | .562 | ||
| Neutropenia (ANC < 500 cell/mm3) | 0 (0) | 0 (0) | – | 1.000* | ||
| Presence of CVC | 17 (94) | 25 (100) | Model not converging | .419* | ||
| Pitt bacteraemia score, median (IQR) | 8 (7–8) | 6 (4–8) | 1.24 (0.97–1.58) | .086 | ||
| SOFA score, median (IQR) | 11 (8–13) | 7 (6–10) | 1.31 (1.07–1.60) | .010 | 1.32 (1.04–1.66) | .021 |
| Septic shock | 10 (56) | 11 (44) | 1.59 (0.47–5.39) | .456 | ||
| VRE as aetiological agent | 4 (22) | 3 (12) | 2.10 (0.41–10.80) | .377 | ||
| Polymicrobial BSI | 7 (39) | 12 (48) | 0.69 (0.20–2.36) | .553 | ||
| DENOVA score, median (IQR) | 0 (1–2) | 1 (1–2) | 0.92 (0.45–1.86) | .808 | ||
| CRBSI | 12 (67) | 19 (76) | 0.63 (0.16–2.42) | .502 | ||
| Endocarditis | 0 (0) | 0 (0) | – | 1.000* | ||
| Source control performed/unnecessary | 15 (83) | 25 (100) | Model not converging | .066* | ||
| Empirical therapy | 17 (94) | 24 (96) | 0.71 (0.04–12.13) | .812 | ||
| In vitro active empirical therapy | 16 (89) | 22 (88) | 1.09 (0.16–7.31) | .929 | ||
ANC, absolute neutrophil count; ARDS, acute respiratory distress syndrome; BSI, bloodstream infection; COVID-19, coronavirus disease 2019; CRBSI, catheter-related bloodstream infection; CI, confidence intervals; CVC, central venous catheter; DENOVA, long Duration of symptoms/Embolization/Number of positive cultures/Origin of infection unknown/Valve disease/Auscultation of murmur; ICU, intensive care unit; IQR, interquartile range; LTCF, log-term care facility; SOFA, sequential organ failure assessment; VRE, vancomycin-resistant enterococci.
aResults are presented as No. of patients/total of patients unless otherwise indicated.
*In the case of zero events in both groups and non-converging univariable logistic regression models, p values are from Fisher exact test. Nonconvergence was also observed when including the variable source control performed/unnecessary (p < .20 in univariable analysis) in the multivariable logistic regression model, that was eventually built without this variable. An additional, penalised, multivariable logistic regression model with Firth’s correction, which included source control performed/unnecessary plus all the variables included in the final standard multivariable model, confirmed the results observed in the standard model (age: OR 1.07, 95% CI 0.99–1.18, p = .112; previous hospitalisation: OR 2.82, 95% CI 0.40–31.13, p = .302; SOFA score: OR 1.26, 95% CI 1.03–1.61, p = .025) and no independent association with treatment failure was observed for source control performed/unnecessary (OR 0.23, 95% CI 0.00–3.27, p = .296). The additional analysis with Firth’s correction was performed using the logistf package for R Statistical Software version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
**Only results for variables retained in the final multivariable model are presented. The discriminatory performance and the calibration of the model were evaluated using the C-statistic (area under the curve [AUC] 0.835, with 95% CI from 0.702 to 0.967) and the Hosmer–Lemeshow’s test (p = .149), respectively.
Discussion
During 2020, we registered 51 episodes of ICU-acquired enterococcal BSI in 43 different COVID-19 patients, with a high cumulative incidence of 229 episodes per 1000 ICU admissions. A high crude 30-day mortality of 42% was observed, despite the lack of a heavy burden of baseline comorbidities and a high rate of appropriate empirical therapy.
The high cumulative incidence of enterococcal BSI we observed is in line with the results of Bonazzetti and colleagues, who previously registered 55 episodes of enterococcal BSI among 96 critically patients with COVID-19, equal to a cumulative incidence of 573 episodes per 1000 ICU admissions, which was even greater than the high cumulative incidence we observed in our centre [6]. In addition, Enterococcus spp., together with coagulase-negative staphylococci, was the most frequently responsible for BSI among critically ill COVID-19 patients in four different previous series [1,3,6,7]. Some possible explanations for an increased predisposition of critically ill COVID-19 patients to develop enterococcal BSI have been proposed. For example, the use of ceftriaxone or ceftaroline (which is inactive against E. faecium, although some in vitro activity against E. faecalis has been reported) as empirical agents in the suspicion of community-acquired bacterial pneumonia superimposed to the viral disease, since this could have exerted a selective pressure increasing the risk of enterococcal BSI [3]. However, large use of cephalosporins may have not been the rule in all series reporting a high cumulative incidence of enterococcal BSI in critically ill COVID-19 patients [1,6]. An alternative, non-mutually exclusive possible explanation is an increased risk of cross-transmission of Enterococcus spp. due to possibly relaxed infection-control measures (i.e. during the peaks of the COVID-19 pandemic the protection of healthcare personnel from the virus was prioritized with respect to the allocation of personal protective equipment, with a possible consequent risk of cross-transmission of bacteria across patients [25–27]), although this explanation is not fully in line with the fact that a similar increase in the transmission of several other bacteria (e.g. Enterobacterales, staphylococci) was apparently not observed [3,6]. Finally, an intriguing hypothesis is that of an increased bacterial translocation from the gut due to the presence/worsening of intestinal wall damage/inflammation related to the viral infection and/or the host response to the virus [1], which nonetheless deserves further investigation in the light of, again, the apparent absence of a parallel increase in the risk of BSI due to Enterobacterales and the lack of a clear elucidation of the underlying causal pathway and mechanisms.
Despite the above-discussed uncertainty in deciphering the true reasons for the high cumulative incidence of enterococcal BSI we and others observed in critically ill COVID-19 patients, the high number of such infections remains, and with the present series, we tried to better depict the characteristics and outcomes of enterococcal BSI in this peculiar population. Notably, despite the high median age of patients in our cohort (63 years), we ultimately registered only a moderate burden of baseline comorbidities (frequency <10% for most of the registered comorbidities, as shown in Table 1), likely reflecting the fact that severe respiratory insufficiency by SARS-CoV-2 may present not only in patients with already existing severe conditions but also in old patients with none or few comorbidities [28]. Another interesting aspect worth discussing is that mortality was high despite most patients (88%) received an in vitro active empirical therapy since the onset of BSI, suggesting a possible role of the severity of the underlying viral disease in unfavourably influencing the outcome. Of note, two patients did not receive prompt empirical therapy, possibly due to initially unclear or unrecognized infections in presence of mitigated clinical signs and inflammatory markers at the onset of BSI after previous treatment with anti-inflammatory and immunomodulatory agents [29,30].
The prognostic impact of the patients’ critical conditions is in line with the independent association we observed between higher SOFA score and 30-day mortality in the multivariable logistic regression model, although it should be recognized that this secondary analysis was burdened by the small sample size and the consequent low power for detecting other possible clinically relevant associations. For example, the potential unfavourable impact of vancomycin resistance in E. faecium, since, in crude numbers, 30-day mortality was higher in the small subgroup of patients with VRE BSI (57% vs. 42% in the entire series), possibly connected to the fact that 3 of the 5 in vitro inactive empirical therapies in the entire series were administered to patients with VRE BSI. Finally, it should be noted that we detected a high number of CRBSI (72%). However, we are unsure whether the CVC was the primary site of infection since we cannot exclude that colonization and infection of the device may have occurred after an initial translocation into the bloodstream from another site (e.g. the gut). Another factor showing a non-statistically significant direction of effect towards increased mortality and deserving further investigation in larger cohorts is the Pitt bacteraemia score (reflecting the severity of BSI presentation), whereas in our cohort there was no apparent association between baseline comorbidities (summarized by means of the Charlson score) and an unfavorable prognosis. The absence of endocarditis diagnoses and the low values of the DENOVA score (reflecting the risk of endocarditis) did not allow us to explore their possible prognostic effect in our cohort.
Besides the low power of the secondary, exploratory analysis of predictors of 30-day mortality (discussed above), the present study has some other important limitations. First, this was a case series of COVID-19 patients with enterococcal BSI, thus no comparison was made with critically ill COVID-19 patients with no BSI or with BSI caused by other microorganisms. However, such a possible comparison was not the aim of our study, which was instead conceived to depict the characteristics and outcome of ICU-acquired enterococcal BSI, once recognized their high cumulative incidence in the target population. Second, owing to the observational nature of the study, which implies the existence of unmeasured confounding, and the small sample size, which precludes more sophisticated adjustment for measurable confounding, the present study was not structured to assess the impact of antibacterial therapy (and the type of employed drug/s) on mortality in a completely reliable way. Other not included data that we were eventually unable to reliably collect retrospectively from all patients were: (i) presence and type of recent chemotherapy; (ii) the time elapsed from the last CVC substitution and development of enterococcal BSI. In addition, we cannot exclude the incompleteness of the information on previous glycopeptide use and previous VRE isolation (e.g. in case of the previous hospitalization in other centers). Finally, the single-centre nature of the study may reduce the generalizability of our findings.
In conclusion, our results are complementary to those of other series reporting a high cumulative incidence of enterococcal BSI in critically ill patients with COVID-19. Our hypothesis-generating findings suggest a crucial role of the severity of the acute clinical conditions, to which both the underlying viral pneumonia and the enterococcal BSI may contribute, in majorly influencing the outcome.
Acknowledgments
We would like to thank the GECOVID study group.
Authors’ contributions
Conceptualization, D.R.G., M.B.; methodology, D.R.G.; formal analysis, D.R.G., L.L., C.Ru., F.Br. and F.Ba.; data curation, S.T., C.Ro., L.B., S.D., C.D., L.M., F.C., E.W., D.B., A.M., N.P., and I.B.; writing – original draft preparation, D.R.G.; writing – review and editing, D.R.G., L.L., C.Ru., F.Br., F.Ba., S.T., C.Ro., L.B., S.D., C.D., L.M., A.M., F.C., E.W., D.B., N.P., I.B., P.P. M.B.; supervision, D.R.G, A.M., P.P., M.B. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
DRG is a section editor of Annals of Medicine. He had no role in the editorial process and decisions regarding the present article. Outside the submitted work, DRG reports honoraria from Stepstone Pharma GmbH and unconditional grants from MSD Italia, Correvio Italia and Pfizer Inc. Outside the submitted work, MB has received funding for scientific advisory boards, travel, and speaker honoraria from Angelini, Astellas, AstraZeneca, Basilea, Bayer, BioMérieux, Cidara, Correvio, Cubist, Menarini, Molteni, MSD, Nabriva, Paratek, Pfizer, Roche, Shionogi, Tetraphase, Thermo Fisher, and The Medicine Company. The other authors have no conflicts of interest to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Buetti N, Ruckly S, de Montmollin E, et al. . COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.d’Humieres C, Patrier J, Lortat-Jacob B, et al. . Two original observations concerning bacterial infections in COVID-19 patients hospitalized in intensive care units during the first wave of the epidemic in France. PLoS One. 2021;16(4):e0250728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacobbe DR, Battaglini D, Ball L, et al. . Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobesh PP, Trujillo TC.. Coagulopathy, venous thromboembolism, and anticoagulation in patients with COVID-19. Pharmacotherapy. 2020;40(11):1130–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. . Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonazzetti C, Morena V, Giacomelli A, et al. . Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an italian ICU: an observational study. Crit Care Med. 2021;49(1):e31–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Scaravilli V, Mangioni D, et al. . Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160(2):454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lansbury L, Lim B, Baskaran V, et al. . Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawson TM, Moore LSP, Zhu N, et al. . Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kampmeier S, Tonnies H, Correa-Martinez CL, et al. . A nosocomial cluster of vancomycin resistant enterococci among COVID-19 patients in an intensive care unit. Antimicrob Resist Infect Control. 2020;9(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laupland KB, Kirkpatrick AW, Church DL, et al. . Intensive-care-unit-acquired bloodstream infections in a regional critically ill population. J Hosp Infect. 2004;58(2):137–145. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Eckardt K-U, Tsukamoto Y, et al. . Definition and classification of chronic kidney disease: a position statement from kidney disease: Improving global outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100. [DOI] [PubMed] [Google Scholar]
- 14.Force ADT, Ranieri VM, Rubenfeld GD, et al. . Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 15.Rhee JY, Kwon KT, Ki HK, et al. . Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the pitt bacteremia score and the acute physiology and chronic health evaluation II scoring systems. Shock. 2009;31(2):146–150. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, et al. . The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berge A, Krantz A, Ostlund H, et al. . The DENOVA score efficiently identifies patients with monomicrobial Enterococcus faecalis bacteremia where echocardiography is not necessary. Infection. 2019;47(1):45–50. [DOI] [PubMed] [Google Scholar]
- 19.Mermel LA, Allon M, Bouza E, et al. . Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of america. Clin Infect Dis. 2009;49(1):1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JS, Sexton DJ, Mick N, et al. . Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. [DOI] [PubMed] [Google Scholar]
- 21.Satlin MJ, Nicolau DP, Humphries RM, et al. . Development of daptomycin susceptibility breakpoints for Enterococcus faecium and revision of the breakpoints for other enterococcal species by the clinical and laboratory standards institute. Clin Infect Dis. 2020;70(6):1240–1246. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ, Boice JD, Jr.. Epidemiologic analysis with a programmable calculator. Bethesda (MD): NIH Publication; 1979. [Google Scholar]
- 23.Pereira CB, Polpo A. MedOr: Order of Medians Based on Confidence Statements. arXiv.org 2012. http://arxiv.org/abs/1212.5405.
- 24.Blaker H. Confidence curves and improved exact confidence intervals for discrete distributions. Can J Statistics. 2000;28(4):783–798. [Google Scholar]
- 25.Bassetti M, Giacobbe DR.. A look at the clinical, economic, and societal impact of antimicrobial resistance in 2020. Expert Opin Pharmacother. 2020;21(17):2067–2071. [DOI] [PubMed] [Google Scholar]
- 26.Rawson TM, Ming D, Ahmad R, et al. . Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol. 2020;18(8):409–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund B, Agvald-Ohman C, Hultberg A, et al. . Frequent transmission of enterococcal strains between mechanically ventilated patients treated at an intensive care unit. J Clin Microbiol. 2002;40(6):2084–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grasselli G, Greco M, Zanella A, et al. . Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kooistra EJ, van Berkel M, van Kempen NF, et al. . Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. Crit Care. 2021;25(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taramasso L, Magnasco L, Portunato F, et al. . Clinical presentation of secondary infectious complications in COVID-19 patients in intensive care unit treated with tocilizumab or standard of care. Eur J Int Med. 2021. doi: 10.1016/j.ejim.2021.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.