Abstract
The Blomfild’s Beauty butterfly Smyrna blomfildia (Fabricius 1781) (Lepidoptera: Nymphalidae: Nymphalini) is a sexually dimorphic species found in Mexico, Central, and South America. Males are territorial and are more vibrantly colored than females. Genome skimming by Illumina sequencing allowed the assembly of a complete circular mitochondrial genome (mitogenome) of 15,149 bp from S. blomfildia consisting of 83.9% AT nucleotides, 13 protein-coding genes, 22 tRNAs, two rRNAs, and a control region in the typical butterfly gene order. The S. blomfilda COX1 gene features an atypical start codon (CGA) while ATP6, COX1, COX2, CYTB, ND1, ND3, ND4, and ND5 display partial stop codons completed by the addition of 3’ A residues to the mRNA. Bayesian phylogenetic reconstruction places Smyrna as a member of the tribe Nymphalini and sister to a clade containing genera Araschnia, Vanessa, Polygonia, and Aglais, which differs from its classic taxonomic placement in tribe Coeini.
Keywords: Illumina sequencing, mitogenomics, Lepidoptera, Nymphalidae, Nymphalini
The Blomfild’s Beauty butterfly, Smyrna blomfildia (Fabricius 1781) (Lepidoptera: Nymphalidae: Coeini), is found in Mexico, Central, and South America (Machado and Freitas 2001; Pfeiler et al. 2020). Adults display sexual dimorphism with territorial males being more vibrantly colored than females (Muyshondt and Muyshondt 1978). The color patterns forming within each wing sector (wing regions bounded by wing veins, also called wing ‘cells’ by entomologists) of S. blomfildia are homologous to those found in neighboring sectors, which develop independently and generally are weakly correlated with one another (Nijhout 1985). The few correlated wing sectors observed by Nijhout (1985) in S. blomfildia anticipated patterns of correlation later observed on the wings of other butterfly species (Monteiro et al. 2003, 2007; Kodandaramaiah 2009) that led to important discoveries regarding the developmental architecture underlying all insect wings (Abbasi and Marcus 2017; Banerjee and Monteiro 2020; McKenna et al. 2020).
The S. blomfildia caterpillars feed on nettle plant leaves of the genera Urera, Urticastrum, and other members of the family Urticaceae (Schaus 1884; Muyshondt and Muyshondt 1978; Dutra et al. 2006). Females lay pale green and white banded eggs that hatch five days after being laid (Muyshondt and Muyshondt 1978; Dutra et al. 2006). Females choose to lay eggs on nettle plants lacking fruit to reduce ant attraction, thereby reducing S. blomfildia larval mortality from ant attacks (Machado and Freitas 2001). Originally described as a member of the genus Papilio (Fabricius 1781), S. blomfildia has been referred to by other specific epithets including S. bella and S. pluto, now considered junior synonyms (Muyshondt and Muyshondt 1978). Here we report the complete mitochondrial genome (mitogenome) sequence of S. blomfildia from specimen Sb2017.1, collected in Tingo Maria, Peru (GPS 9.29616S, 75.99831 W) in October 2017 that has been pinned, spread, and deposited in the Wallis Roughley Museum of Entomology, University of Manitoba (http://www.wallisroughley.ca/, Jason Gibbs, Jason.Gibbs@umanitoba.ca) voucher WRME0507738.
DNA was prepared from a specimen leg using a DNeasy Blood and Tissue kit (Qiagen, Düsseldorf, Germany) with slight modifications to the standard protocol as described in McCullagh and Marcus (2015). DNA was sheared by sonication and a fragment library was prepared using the NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, Massachusetts) as previously described (Peters and Marcus 2017), before sequencing by Illumina NovaSeq6000 (San Diego, California) (Marcus 2018). The mitogenome of S. blomfildia (Genbank MZ151338) was assembled and annotated using Geneious Prime 2021.1.1 from an SRA library of 18,400,288 paired 150 bp reads (Genbank SRA PRJNA729786) using a Baeotus beotus reference mitogenome (Lepidoptera: Nymphalidae, MW566598) (Lalonde 2021). The S. blomfildia nuclear rRNA repeat (Genbank MZ198233) was also assembled and annotated using a B. beotus (MW571038) reference sequence. The rRNA repeat sequence is increasingly recognized as being very useful for phylogenetic comparisons based on nuclear markers (Dodsworth 2015; Coissac et al. 2016; Marcus 2018; Krehenwinkel et al. 2019), so we have chosen to release it here.
The S. blomfildia circular 15,149 bp mitogenome assembly was composed of 24,120 paired reads with nucleotide composition: 34.5% A, 10.6% C, 5.5% G, and 49.4% T. The gene composition and order in S. blomfildia is typical of the arrangement found in most butterfly mitogenomes (Park et al. 2016). The S. blomfildia protein coding gene start codons include: ATG (ATP6, COX2, COX3, CYTB, ND1, ND4, ND4L), ATT (ATP8, ND2, ND5, ND6), ATC, (ND3), and CGA, an atypical COX1 start codon that is also found in the COX1 gene of many other insects (Liao et al. 2010). The mitogenome contains four protein-coding genes (COX1, COX2, ND4 ND5) with single-nucleotide (T) stop codons, and four protein-coding gene (ATP6, CYTB, ND1 ND3) with two-nucleotide (TA) stop codons completed by post-transcriptional addition of 3′ A residues. All structures of the tRNAs were verified using ARWEN v.1.2 (Laslett and Canback 2008) and have typical cloverleaf secondary structures with the exception for trnS (AGN) where the dihydrouridine arm is replaced by a loop, whereas the control region and mitochondrial rRNAs are typical for Lepidoptera (McCullagh and Marcus 2015).
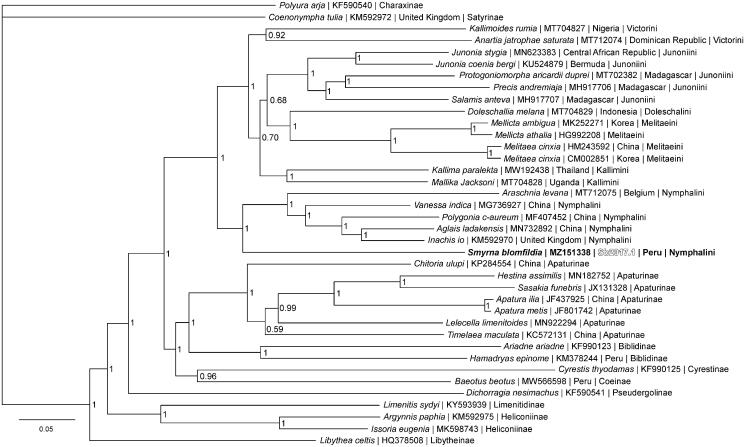
Phylogenetic reconstruction (Figure 1) was completed using the complete mitogenome of S. blomfildia and 37 other mitogenomes from the family Nymphalidae. Sequences were aligned in CLUSTALX 2.1 (Thompson et al. 1997; Larkin et al. 2007) and analyzed using Bayesian Inference with the GTR + I + G model (model selected using jModeltest 2.1.1 (Darriba et al. 2012)) in Mr. Bayes version 3.2.7 (Ronquist and Huelsenbeck 2003; Ronquist et al. 2012). Phylogenetic analysis places Smyrna as a member of the tribe Nymphalini and sister to a clade containing genera Araschnia, Vanessa, Polygonia, and Aglais, confirming the findings of some previous molecular phylogenetic analyses (Wahlberg et al. 2005; Wahlberg and Wheat 2008). Placing Smyrna in tribe Nymphalini is also supported by larval morphological characters (Muyshondt and Muyshondt 1978). This differs from the classical taxonomic placement of Smyrna with Baeotus beotus in the tribe Coeini based on adult morphology (Muyshondt and Muyshondt 1978) and supported by a different molecular phylogenetic analysis (Wahlberg et al. 2009). Based on our results, we agree with prior researchers who have reclassified Smyrna in tribe Nymphalini.
Figure 1.
The Bayesian phylogeny (GTR + I + G model, average Potential Scale Reduction Factor (PSRF) = 1, average deviation of split frequencies = 0.001523) of the Smyrna blomfildia mitogenome, 37 additional mitogenomes from within family Nymphalidae, including outgroup species Polyura arja (Charaxinae) and Coenonympha tullia (Satyrinae) (Alexiuk et al. 2020; Hamilton et al. 2020; Lalonde and Marcus 2020; Payment et al. 2020; Lalonde 2021), produced by 10 million MCMC generations in MrBayes, with sampling every 100 generations, and after discarding the first 250,000 generations as burn-in. At each node, the Bayesian posterior probability values determined by MrBayes are given.
Acknowledgements
Thanks to Genome Quebec for assistance with library preparation and sequencing.
Funding Statement
This work received support from NSERC under [Grant RGPIN-2016-06012] and from the University of Manitoba under the University Research Grants Program.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov] (https://www.ncbi.nlm.nih.gov/) under the accession nos. MZ151338 and MZ198233. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA729786, SRX10874928, and SAMN19163223 respectively.
References
- Abbasi R, Marcus JM.. 2017. A new A-P compartment boundary and organizer in holometabolous insect wings. Sci Rep. 7(1):16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexiuk MR, Marcus JM, Lalonde MML.. 2020. The complete mitochondrial genome of the Jackson’s Leaf butterfly Mallika jacksoni (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 5:3316–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee TD, Monteiro A.. 2020. Molecular mechanism underlying venation patterning in butterflies. bioRxiv. 2020.01.02.892760. [Google Scholar]
- Coissac E, Hollingsworth PM, Lavergne S, Taberlet P.. 2016. From barcodes to genomes: extending the concept of DNA barcoding. Mol Ecol. 25(7):1423–1428. [DOI] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D.. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodsworth S. 2015. Genome skimming for next-generation biodiversity analysis. Trends Plant Sci. 20(9):525–527. [DOI] [PubMed] [Google Scholar]
- Dutra HP, Freitas AVL, Oliveira PS.. 2006. Dual ant attraction in the Neotropical shrub Urera baccifera (Urticaceae): the role of ant visitation to pearl bodies and fruits in herbivore deterrence and leaf longevity. Funct Ecology. 20(2):252–260. [Google Scholar]
- Fabricius JC. 1781. Species Insectorum Exhibentes Eorum Differentia Specifica, Synonyma Auctorum, Loca Natalia, Metamorphosin Adiectis, Observationibus, Descriptionibus. Spec Ins. 2:1–494. [Google Scholar]
- Hamilton RV, Marcus JM, Lalonde MML.. 2020. The complete mitochondrial genome of the black dead leaf butterfly Doleschallia melana (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 5: 3306–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodandaramaiah U. 2009. Eyespot evolution: phylogenetic insights from Junonia and related butterfly genera (Nymphalidae: Junoniini). Evol Dev. 11(5):489–497. [DOI] [PubMed] [Google Scholar]
- Krehenwinkel H, Pomerantz A, Henderson JB, Kennedy SR, Lim JY, Swamy V, Shoobridge JD, Graham N, Patel NH, Gillespie RG, et al. 2019. Nanopore sequencing of long ribosomal DNA amplicons enables portable and simple biodiversity assessments with high phylogenetic resolution across broad taxonomic scale. GigaScience. 8(5):giz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde MML. 2021. Phylogenetic analysis of the complete mitochondrial genome of the graphic beauty butterfly Baeotus beotus (Doubleday 1849) (Lepidoptera: Nymphalidae: Nymphalinae: Coeini). Mitochondrial DNA B Resour. 6(4):1516–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde MML, Marcus JM.. 2020. The complete mitochondrial genome of the Malagasy clouded mother-of-pearl butterfly Protogoniomorpha ancardii duprei (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 5:3261–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics. 23(21):2947–2948. [DOI] [PubMed] [Google Scholar]
- Laslett D, Canback B.. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24(2):172–175. [DOI] [PubMed] [Google Scholar]
- Liao F, Wang L, Wu S, Li Y-P, Zhao L, Huang G-M, Niu C-J, Liu Y-Q, Li M-G.. 2010. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int J Biol Sci. 6(2):172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado G, Freitas AVL.. 2001. Larval defence against ant predation in the butterfly Smyrna blomfildia. Ecol Entomol. 26(4):436–439. [Google Scholar]
- Marcus JM. 2018. Our love-hate relationship with DNA barcodes, the Y2K problem, and the search for next generation barcodes. AIMS Genet. 5(1):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh BS, Marcus JM.. 2015. The complete mitochondrional genome of Lemon Pansy, Junonia lemonias (Lepidoptera: Nymphalidae: Nymphalinae). J Asia-Pacific Ent. 18(4):749–755. [Google Scholar]
- McKenna KZ, Kudla AM, Nijhout HF.. 2020. Anterior–posterior patterning in lepidopteran wings. Front Ecol Evol. 8:146. [Google Scholar]
- Monteiro A, Chen B, Scott LC, Vedder L, Prijs HJ, Belicha-Villanueva A, Brakefield PM.. 2007. The combined effect of two mutations that alter serially homologous color pattern elements on the fore and hindwings of a butterfly. BMC Genet. 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro A, Prijs J, Hakkaart T, Bax M, Brakefield PM.. 2003. Mutants highlight the modular control of butterfly eyespot patterns. Evol Dev. 5(2):180–187. [DOI] [PubMed] [Google Scholar]
- Muyshondt A, Jr., Muyshondt A.. 1978. Notes on the life cycle and natural historyof butterflies of El Salvador. IIC. Smyrna blomfildia and S. karwinskii (Nymphalidae: Coloburini). J Lepid Soc. 32:160–174. [Google Scholar]
- Nijhout HF. 1985. Independent development of homologous pattern elements in the wing patterns of butterflies. Dev Biol. 108(1):146–151. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim MJ, Jeong SY, Kim SS, Kim I.. 2016. Complete mitochondrial genomes of two gelechioids, Mesophleps albilinella and Dichomeris ustalella (Lepidoptera: Gelechiidae), with a description of gene rearrangement in Lepidoptera. Curr Genet. 62(4):809–826. [DOI] [PubMed] [Google Scholar]
- Payment JE, Marcus JM, Lalonde MML.. 2020. The complete mitochondrial genome of the African leaf butterfly Kallimoides rumia (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 5:3415–3417.33458190 [Google Scholar]
- Peters MJ, Marcus JM.. 2017. Taxonomy as a hypothesis: testing the status of the Bermuda buckeye butterfly Junonia coenia bergi (Lepidoptera: Nymphalidae). Syst Entomol. 42(1):288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiler E, Nazario-Yepiz NO, Markow TA.. 2020. Additional species records and nomenclature updates of butterflies from a threatened coastal habitat in Southern Sonora, Mexico. J Lepid Soc. 74(3):197–200. [Google Scholar]
- Ronquist F, Huelsenbeck JP.. 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19(12):1572–1574. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP.. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaus W. 1884. Early stages of Mexican Lepidoptera. Papilio. 4:100–103. [Google Scholar]
- Thompson DJ, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG.. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25(24):4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg N, Brower AVZ, Nylin S.. 2005. Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae). Biol J Linn Soc. 86(2):227–251. [Google Scholar]
- Wahlberg N, Leneveu J, Kodandaramaiah U, Peña C, Nylin S, Freitas AVL, Brower AVZ.. 2009. Nymphalid butterflies diversify following near demise at the Cretaceous/Tertiary boundary. Proc Biol Sci. 276(1677):4295–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg N, Wheat C.. 2008. Genomic outposts serve the phylogenomic pioneers: designing novel nuclear markers for genomic DNA extractions of lepidoptera. Syst Biol. 57(2):231–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov] (https://www.ncbi.nlm.nih.gov/) under the accession nos. MZ151338 and MZ198233. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA729786, SRX10874928, and SAMN19163223 respectively.