Abstract
Patients with cancer have been identified in several studies to be at high risk of developing severe COVID-19; however, rates of SARS-CoV-2 IgG seroconversion and its association with cancer types and anti-cancer therapy remain obscure. We conducted a retrospective cohort study in patients with cancer that underwent SARS-CoV-2 IgG testing. Two hundred and sixty-one patients with a cancer diagnosis underwent SARS-CoV-2 IgG testing and demonstrated a high rate of seroconversion (92%). However, significantly lower seroconversion was observed in patients with hematologic malignancies (82%), patients that received anti-CD-20 antibody therapy (59%) and stem cell transplant (60%). Interestingly, all 17 patients that received immunotherapy, including 16 that received anti-PD-1/PD-L1 monoclonal antibodies, developed SARS-Cov-2 IgG antibodies (100% seroconversion). These data show differential rates of seroconversion in specific patient groups and bear importance for clinical monitoring and vaccination strategies that are being developed to mitigate the COVID-19 pandemic.
Keywords: SARS-CoV-2, seroconversion, cancer
Introduction
The coronavirus pandemic that started in December 2019 in Wuhan, China continues to send waves of COVID-19 disease throughout the world[1, 2]. Several observational studies have identified patients with cancer as being at higher risk of contracting the virus and higher rates of manifesting a severe form of COVID-19 disease [3–5]. We have previously reported a higher case fatality rate in patients with hematologic malignancies compared to solid malignancies in patients with cancer[6]. A pooled meta-analysis of 52 studies involving patients with cancer and COVID-19 reported a mortality rate of 25.6%[7]. While the mortality rates of patients with cancer are higher than the general population, it appears that about 70-80% of patients with cancer survive COVID-19 and therefore, it is important to understand the natural history of COVID-19 in this high-risk patient population. Of particular importance is the fact that this patient population often receives immunosuppressive cancer-directed therapy which may impact their ability to mount a humoral immune response to the virus. It is therefore prudent to study the rate of formation of such antibodies to SARS-CoV-2 in patients with cancer who survived the illness to properly inform and develop treatment, surveillance and monitoring strategies in this vulnerable patient population.
Results
Patient selection
We collected data for all patients with a cancer diagnosis cared for at the Montefiore Health System (MHS) starting March 1, 2020 (first observed COVID-19 infection at MHS) until September 15, 2020. Figure 1 represents cohort selection for this study. A total of 4302 patients were identified, of which 3562 were excluded as they did not have a SARS-CoV-2 RT-PCR test result in our system leaving 740 patients. Of the 740 patients 460 were excluded as 8 patient records were duplicates and 452 did not have a SARS-CoV-2 IgG test. After excluding the aforementioned patients, 280 patients were identified of which, 15 were excluded as they did not have a confirmed diagnosis of malignancy. Three more patients were excluded as they had a negative SARS-CoV-2 PCR and a negative SARS-CoV-2 IgG and one patient was excluded as negative SARS-CoV-2 IgG test preceded a positive SARS-CoV-2 PCR. Finally, 261 patients with a confirmed diagnosis of malignancy and at least one SARS-CoV-2 IgG test performed during their care at MHS were included for analysis.
Figure 1: Cohort description and patient inclusion criteria in the present study.
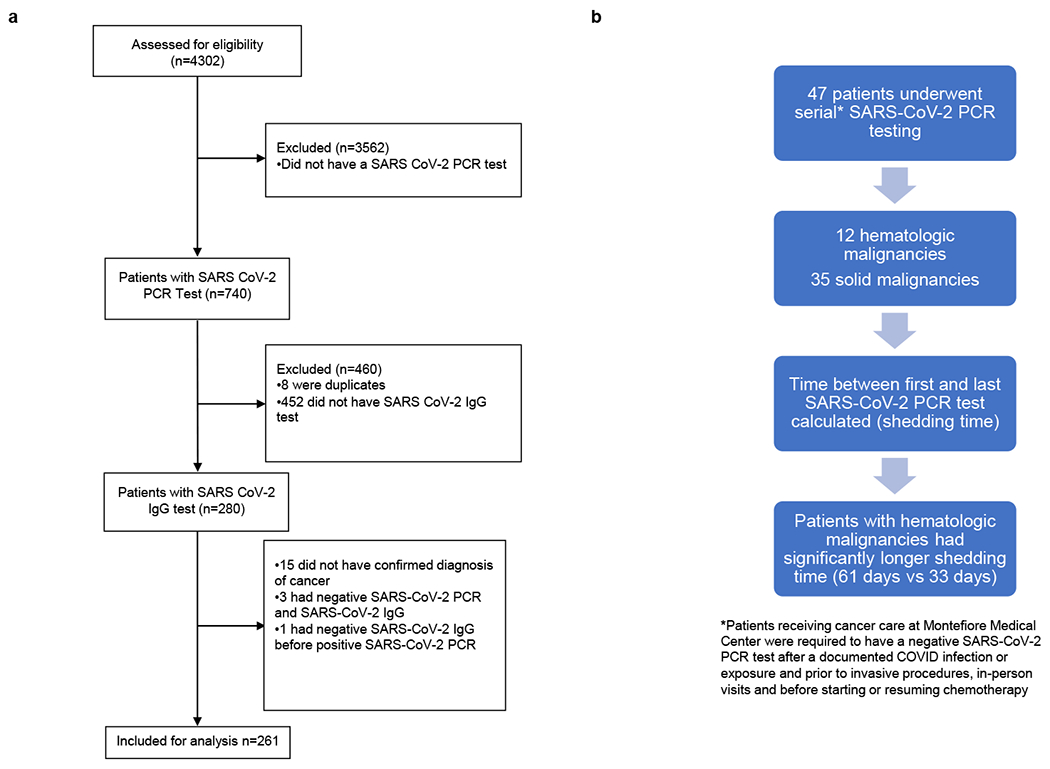
A) Consort Diagram representing patient selection into the final cohort, listing the selection criteria for inclusion into the present study. N=number of patients at each step.
B) Diagram representing patients undergoing serial SARS-CoV-2 PCR testing
Baseline Characteristics
A total of 261 patients with a confirmed diagnosis of malignancy were included in this study. The median age of the cohort was 64 years (range 20-90 years). Seventy-seven percent (201/261) had a diagnosis of solid malignancy and 23% (60/261) had hematologic malignancy. Fifty-one percent (134/261) of patients were female and 49% (127/261) were male. Forty-one percent (106/261) of patients were African-American, 37% (98/261) were Hispanic, 13% (33/261) were Caucasian, 3% (8/261) were Asian and 6% (16/261) belonged to other ethnicities.
As expected, we had a preponderance of patients with solid malignancies; 22% (58/261) had breast cancer, 22%(57/261) had genitourinary cancer, 17% (44/261) had gastrointestinal cancer, 9% (24/261) had thoracic and head and neck cancer, 4% (10/261) had gynecologic cancer, 2% (5/261) had central nervous system cancer, 1% (3/261) had skin/musculoskeletal cancer. Among patients with hematologic malignancies 10% (26/261) had lymphoid disorders, 8% (20/261) had plasma cell disorders, and 5% (14/261) had myeloid disorders.
We divided our cohort into patients who had active malignancy within 90 days of a SARS-CoV-2 test and those who did not. Of 261 patients, 68% (177/261) patients had active malignancy and 32% (84/261) did not. Of the patients with active malignancy, 135 had an initial diagnosis, 23 had progressive disease and 19 had relapsed disease. Of the inactive malignancy subgroup, 71 patients were in remission and 13 patients had malignancy that did not warrant therapy (e.g. monoclonal gammopathy of unknown significance). Patients were divided into three categories based on their comorbidities, 0-1, 2-3, and >3 comorbidities (co-morbidities curated by chart review). Cancer diagnosis itself was not included as a comorbidity. The distribution of patients in the comorbidity categories was 26% (68/261), 30% (78/261) and 44% (115/261), respectively.
In addition, we also calculated a modified Charlson Comorbidity Index (CCI) for all included patients. Given that malignant conditions comprise 4 out of 19 criteria for the CCI, these were excluded while calculating the CCI for our patients, an approach that has been used in a previous study focused on patients with cancer diagnoses [8]. We then divided the entire cohort into three categories: CCI 0-1, 2-3 and 4+. The distribution of patients by CCI categories is as follows, 0-1 26% (68/261), 2-3 38% (100/261) while 36% patients had a score of 4+ (93/261)
Overall, 92% patients (239/261) had a positive SARS-CoV-2 IgG test and 8% (22/261) patients had a negative SARS-CoV-2 IgG test. Fifty six percent (147/261) had symptomatic SARS-CoV-2 infection while 44% (114/261) patients had an asymptomatic infection. Symptomatic infection rate was 53% (106/201) among patients with solid malignancies and 68% (41/60) among those with hematologic malignancies. There was a significant association seen between patients with hematologic malignancy and symptomatic infection compared to the solid malignancies (p=0.04)
Twenty-three percent patients (61/261) had steroid use at baseline. Of these, 21 patients were on steroids daily and 40 patients received steroids occasionally. The indications and frequencies of steroid use are available in the supplement. The median time between SARS-CoV-2 PCR and SARS-CoV-2 IgG test was 40 days and mean was 46 days.
The baseline characteristics and frequencies of asymptomatic infection of the cohort are summarized in table 1 and supplement table 2 respectively.
Table 1:
Baseline characteristics
| Baseline Characteristics | ||
|---|---|---|
| Total number of patients | 261 | |
| Median Age (Range) | 64 years (20-90 years) | |
| Sex | n | % |
| Male | 127 | 49% |
| Female | 134 | 51% |
| Comorbidities | n | % |
| 0-1 | 68 | 26% |
| 2-3 | 78 | 30% |
| >3 | 115 | 44% |
| Charlson Comorbidity Index | n | % |
| 0-1 | 68 | 26% |
| 2-3 | 100 | 38% |
| >3 | 93 | 36% |
| Ethnicity | n | % |
| African-American | 106 | 41% |
| Hispanic | 98 | 37% |
| Caucasian | 33 | 13% |
| Asian | 8 | 3% |
| Other | 16 | 6% |
| Type of Cancer | n | % |
| Solid | 201 | 77% |
| Breast cancer | 58 | 22% |
| Gastrointestinal cancer | 44 | 17% |
| Thoracic & Head and Neck Cancers | 24 | 9% |
| Central Nervous System Cancers | 5 | 2% |
| Genitourinary cancer | 57 | 22% |
| Gynecologic cancer | 10 | 4% |
| Skin/Musculoskeletal cancer | 3 | 1% |
| Hematologic | 46 | 23% |
| Lymphoid malignancy | 26 | 10% |
| Plasma cell malignancy | 20 | 8% |
| Myeloid malignancy | 14 | 5% |
| SARS CoV-2 IgG | n | % |
| SARS CoV-2 IgG positive | 239 | 92% |
| SARS CoV-2 IgG negative | 22 | 8% |
| Active Cancer | n | % |
| Active | 177 | 68% |
| Inactive | 84 | 32% |
| Cancer Status | n | % |
| Initial diagnosis | 135 | 52% |
| Remission | 71 | 27% |
| Progressive disease | 23 | 9% |
| Relapse | 19 | 7% |
| Inactive | 13 | 5% |
| Active cancer by cancer group | n | % |
| Solid malignancy | 142 | 54% |
| Hematologic malignancy | 35 | 13% |
| Active cancer treatment within 90 days | n | % |
| Solid malignancy | 89 | 34% |
| Hematologic malignancy | 21 | 8% |
| Baseline Steroid use | n | % |
| 61 | 23% | |
Table 1 summarizes the baseline characteristics of the cohort. N=number of patients
Cancer Treatment History
We collected data for all cancer treatment that was received by each patient. We classified the treatments into the following categories, chemotherapy, immunotherapy, tyrosine-kinase inhibitors, anti-HER therapy, antibody-drug conjugate, anti-CD20 antibody, anti-CD38 antibody, proteasome inhibitors, immunomodulator, BTK inhibitor, IDH1 inhibitor, BCL2 inhibitor, mTOR inhibitors, PARP inhibitor, TGF-β inhibitor, AR-targeted therapy, bispecific T-cell engager therapy, anti-EGFR monoclonal antibody, anti-VEGF monoclonal antibody therapy and history of stem cell transplant and CAR-T and cellular therapy. If a patient received 2 agents falling in the same category, they were classified only once (for ex, if a patient received degarelix and leuprolide both, we classified them once in ‘endocrine therapy’). Combination and sequential treatment was classified in the appropriate category (for example, patient receiving rituximab-based chemoimmunotherapy would be classified as received anti-CD-20 antibody, chemotherapy and steroids). CAR-T and cellular therapy included two patients who received CAR-T cell therapy and one patient who received sipuleucel-T for prostate cancer. The most common treatment modality was cytotoxic chemotherapy in 46% (119/261) patients followed by endocrine therapy in 27% (71/261) patients. In the 90 days preceding a SARS-CoV-2 test 110 patients had received medical cancer treatment, including 89 patients with a solid malignancy and 21 patients with a hematologic malignancy. The frequencies of all treatments andtreatments within 90 days of COVID test have been summarized in Table 2. The median lines of therapy in the seropositive and seronegative cohorts is 1.
Table 2 :
Cancer Directed Therapy
| Cancer-directed therapy of the entire cohort | 261 | % |
| Chemotherapy | 119 | 46% |
| Immunotherapy | 17 | 7% |
| Anti-CD-20 antibody therapy | 17 | 7% |
| Anti-CD-38 antibody therapy | 2 | 1% |
| Immunomodulator | 6 | 2% |
| Proteasome inhibitor | 6 | 2% |
| Anti-VEGF antibody therapy | 5 | 2% |
| Anti-EGFR antibody therapy | 2 | 1% |
| Antibody-drug conjugate | 2 | 1% |
| Anti-HER antibody | 10 | 4% |
| Tyrosine-kinase inhibitor | 13 | 5% |
| Bispecific T-cell engager | 2 | 1% |
| Androgen receptor-targeted therapy | 11 | 4% |
| PARP-inhibitor | 1 | 0% |
| TGF-β therapy | 1 | 0% |
| BTK inhibitor | 3 | 1% |
| BCL2 inhibitor | 1 | 0% |
| IDH1 inhibitor | 1 | 0% |
| CDK 4/6 inhibitors | 3 | 1% |
| mTOR inhibitor | 1 | 0% |
| Endocrine therapy | 71 | 27% |
| Stem cell transplant | 10 | 4% |
| CAR-T/Cellular therapy | 3 | 1% |
| Cancer-directed therapy within 90 days of COVID test | n | % |
|---|---|---|
| Chemotherapy | 43 | 16% |
| Immunotherapy | 10 | 4% |
| Anti-CD-20 antibody therapy | 5 | 2% |
| Anti-CD-38 antibody therapy | 2 | 1% |
| Immunomodulator | 3 | 1% |
| Proteasome inhibitor | 1 | 0% |
| Anti-VEGF antibody therapy | 2 | 1% |
| Anti-EGFR antibody therapy | 2 | 1% |
| Antibody-drug conjugate | 0 | 0% |
| Anti-HER antibody | 1 | 0% |
| Tyrosine-kinase inhibitor | 6 | 2% |
| Bispecific T-cell engager | 1 | 0% |
| Androgen receptor-targeted therapy | 15 | 6% |
| PARP-inhibitor | 1 | 0% |
| TGF-β therapy | 0 | 0% |
| BTK inhibitor | 0 | 0% |
| BCL2 inhibitor | 0 | 0% |
| IDH1 inhibitor | 0 | 0% |
| CDK 4/6 inhibitors | 2 | 1% |
| mTOR inhibitor | 0 | 0% |
| Endocrine therapy | 40 | 15% |
| Stem cell transplant | 2 | 1% |
| CAR-T/Cellular therapy | 0 | 0% |
| Supportive care | 2 | 1% |
| Treated at outside institution (details unknown) | 2 | 1% |
Tables 2 summarizes frequencies of cancer-directed therapies in The overall cohort and within 90 days of COVID test. N=number of patients
Clinical course of patients with absent seroconversion
All 22 patients who had a negative SARS-CoV-2 IgG had a preceding SARS-CoV-2 PCR that was positive. Sixteen of 22 patients had symptomatic infections whereas 6 were asymptomatic. In the symptomatic subgroup, 11 patients were treated on general medical floor, 1 patient needed ICU level of care, 3 patients were quarantined at home and details of the treatment setting for 1 patient are unknown. In the asymptomatic group, 1 patient was on the general medical floor for different acute issue, one patient was transferred to ICU for close observation despite no symptoms, one patient was quarantined at home and details of 3 patients’ treatment settings are not available to us. Overall, in the seronegative cohort of patients, we observed high symptomatic infection rate, high rates of hospitalization with some needing ICU level of care.
Eleven of 22 patients had a hematologic malignancy and eleven had solid malignancy. In the seronegative group, 14 patients had chemotherapy, 7 had received anti-CD-20 antibody, 4 had received stem cell transplant, 3 had received a tyrosine kinase inhibitor, 2 patients each had received BiTE and CAR-T and one patient each had received immunomodulator, proteasome inhibitor, antibody-drug conjugate, PARP inhibitor and BTK inhibitor. These treatments are summarized in supplement table 3.
Association between seroconversion and cancer type
Given that patients with hematologic malignancies tend to be more immunosuppressed, and as several series have suggested, carry higher morbidity with COVID-19, we wanted to investigate differences in seroconversion in patients with hematologic versus solid malignancies. Among the 60 patients with hematologic malignancies, 49 (81.7%) manifested SARS-CoV-2 IgG positivity while 190 of the 201 (94.5%) patients with solid malignancy manifested SARS-CoV-2 IgG positivity (Fisher exact test OR 3.8, p value =0.005). Taken together, in our cohort, patients with hematologic malignancies had a higher frequency of manifesting symptomatic COVID-19 infection, and significantly lesser likelihood of seroconversion.
Association between seroconversion and cancer therapy
Furthermore, we aimed to investigate if seroconversion was associated with type of cancer therapy received by a patient. In our analysis, we observed a significant association between prior use of anti-CD20 antibody therapy and SARS-CoV-2 IgG. A total of seventeen patients had received anti-CD20 therapy, of which 7 patients had a negative SARS-CoV2 IgG. (Fisher exact test OR 0.09, p=0.00013). A similar finding was observed in the cohorts of patients who had a history of stem cell transplant. Ten patients had received a stem cell transplant in our cohort of which, 4 remained negative for SARS-CoV-2 IgG (Fisher exact test OR 0.1, p=0.0057). The above odds ratios refer to comparisons with the entire cohort of patients with cancer. The p-values are not adjusted for multiple hypothesis testing, however FDR correction is provided for variables with more than two levels of analysis in the supplement (tables 9, 11, 12). We also noted reduced seroconversion in patients who received CAR-T cell therapy (33%) and BiTE therapy (0%) however given small number of patients in these cohorts, statistical analysis was not performed.
In contrast, we observed very high seroconversion rates in patients who received immunotherapy or endocrine therapy. Seventeen patients received prior immunotherapy for their cancer and all seventeen of them manifested a positive SARS-CoV-2 antibody response. Despite this 100% seroconversion rate, the OR did not reach statistical significance (p=0.38), likely due to high baseline frequency of seroconversion for the entire patient population. Of 71 patients who received endocrine therapy for their cancer, 70 manifested a positive SARS-CoV-2 IgG (Fisher exact test OR 8.6=0.01 compared to those without endocrine therapy). These results have been summarized in Table 3.
Table 3:
Associations of therapies and cancer types with SARS-CoV-2 IgG positivity
| Results* | ||||||
|---|---|---|---|---|---|---|
| Type of Cancer | SARS CoV2 IgG positive | SARS CoV-2 IgG negative | Odds Ratio (univariate) | p value† (univariate) | Odds Ratio (multivariate) | p value (multivariate) |
| Hematologic malignancies | 49 | 11 | 3.8 | 0.005 | 4.004 | 0.0026 |
| Solid malignancies | 190 | 11 | ||||
| Type of Cancer-directed therapy | ||||||
| Anti-CD-20 antibody therapy | 10 | 7 | 0.09 | 0.00013 | ||
| Stem cell transplant | 6 | 4 | 0.1 | 0.0057 | ||
| Endocrine therapy | 70 | 1 | 8.6 | 0.01 | ||
| Immunotherapy | 17 | 0 | 0.38 | |||
Univariate analysis was carried out between all treatment categories and seroconversion, only the statistically significant results are shown here
Univariate statistical test used is 2-sided Fisher test and multivariate is 2-sided Wald test on logistic model
p-values are uncorrected for multiple hypothesis testing and FDR correction is provided for variables where more than two levels of analysis was performed in supplement tables 9,11 and 12
The above results indicate that patients with hematologic malignancies, anti-CD-20 antibody therapy, CAR-T cell therapy and stem cell transplant are associated with reduced seroconversion in patients with SARS-CoV-2. On the other hand, endocrine therapy has a strong association with positive antibody response in patients with SARS-CoV-2.
Active cancer and treatment within 90 days of SARS-CoV-2 IgG
We aimed to investigate for potential confounders in the differential seroconversion rates noted for solid and hematologic malignancies. We identified cancer status and cancer-directed therapy received within 90 days as potential key confounders and performed a multivariate logistic regression analysis. These variables were chosen as these would be biologically plausible to have an effect on seroconversion. Results indicate that the association between solid versus hematologic malignancy and SARS-CoV-2 remain significant after accounting for active cancer and active cancer-directed treatment in the preceding 90 days of the COVID test, OR 4.004, p=0.0026 (Table 3 and supplement table 10).
Association of steroid use and SARS-CoV-2 IgG test
We investigated baseline use of corticosteroids in our cohort. Twenty three percent (61/261) of the patients had steroid exposure prior to the SARS-CoV-2 test. Of these, 40 patients had occasional steroid use whereas 21 patients had daily steroid use. Ten additional patients received steroids for COVID-19 infection. In a univariate analysis, steroid use at baseline showed a notable trend with lack of seroconversion (p=0.06). Similarly, while the low numbers limit strong conclusions, steroid use for COVID-19 management also showed an association with absent seroconversion. The indications, frequencies and results of this analysis are summarized in the supplement.
Persistent SARS-CoV-2 PCR positivity
Eighteen percent (47/261) of patients underwent serial SARS-CoV-2 PCR testing as per institutional policies to document clearance of infection (Figure 2). Thirty-five patients had a solid malignancy and 12 had hematologic malignancy. The mean shedding time, calculated as the time between first and last positive SARS-CoV-2 PCR, was significantly higher in patients with hematologic than in patients with solid malignancies (61 days vs 33 days, p = 0.007, table 4). Seropositivity was noted in 31 solid malignancy and 9 hematologic malignancy. Remainder 4 solid malignancy and 3 hematologic malignancy patients remained seronegative (Table 4). This observation again stresses the importance of close follow-up and monitoring of patients with hematologic malignancies and may be impactful in designing quarantine strategies for these patients after clinical improvement from acute COVID-19 illness.
Table 4:
Shedding time and associations with malignancy type
| Type of malignancy | Hematologic malignancy | Solid malignancy | p value |
|---|---|---|---|
| SARS-CoV-2 PCR shedding time (mean) | 61 days | 33 days | 0.007 |
| Number of patients | 12 | 35 | |
| SARS-CoV-2 IgG test | Hematologic malignancy | Solid malignancy | |
| SARS-CoV-2 IgG positive | 9 | 31 | |
| SARS-CoV-2 IgG negative | 3 | 4 |
Statistical test used is Anova Chi-square test (sidedness is not applicable)
Data was collected using Microsoft Excel and analyzed using R 3.6.2 (See Methods)
Serial SARS-CoV-2 IgG testing
Fifty-six patients underwent serial SARS-CoV-2 IgG testing. Of these, 53 had an initial positive test and 3 patients had an initial negative test. We collected data for time between first and last test available in our system. In this cohort, 44 of 53 patients remained persistently positive whereas 9 patients turned seronegative. Eight of 9 had solid malignancy and one had hematologic malignancy. Seven of 9 patients received treatment for cancer in the 90 days preceding a COVID test. Of the 3 initial seronegative patients, one patient turned seropositive and 2 remained persistently seronegative. The median time between first and last test in this cohort was 49 days (supplement table 13)…
Outcomes
Twenty nine of 261 patients had died by the time of data cut-off date. Among them, 14 died due to progressive malignancy and 1 died of sequelae of COVID-19 infection. (Supplement table 14).
Discussion
COVID-19 disease caused by SARS-CoV-2 has now affected more than 68 million humans worldwide, including over 27 million in the US, and caused more than 400,000 deaths in the United States alone (Johns Hopkins Coronavirus Resource Center as of February 5, 2021). Older age and having multiple comorbid conditions have been identified as predictors of mortality in this disease[9]. Several observational cohorts have identified patients with cancer have a longer, protracted course with COVID-19 necessitating hospitalization and intensive care. Patients with hematologic malignancies have been reported in many series, including our own, to have higher mortality compared to solid malignancies [6, 10]. While it was hypothesized in many cohorts that a diagnosis of cancer predicts mortality, data on this particular aspect is still evolving as recent matched-studies report similar mortality in patients with cancer compared to age-matched controls without a diagnosis of cancer[11]. Nevertheless, concern about seroconversion in this patient population, which often receives immunosuppressive treatments, has been raised as mounting a humoral immunity is crucial in not only recovery from the infection, but to also establish and maintain herd immunity through effective vaccination strategies.
To our knowledge, this is the first large cancer cohort reporting seroconversion rates following SARS-CoV-2 infection. Ninety-two percent patients manifested a positive antibody response in our study focused on a large cohort of ethnically diverse patients who have survived a SARS-CoV-2 infection. With the same SARS-CoV-2 IgG assay, seroconversion rates in the general population have been reported as 90-100%[12–14]. Indeed, in an unselected cohort of 1008 patients with SARS-CoV-2 PCR positivity in our health system who had subsequent antibody testing, seroconversion rate was 91%, nearly identical with the overall seroconversion rate of our cohort of patients with cancer providing reassurance that most patients with cancer are able to mount an antibody response to SARS-CoV-2 similar to the general population (Suppl materials).
In an observational study from Spain involving 43 patients with cancer, seroconversion was noted in 83% patients and was absent in 17% (6) patients. Four of the 6 patients were on immunosuppressive therapy, of which 2 received rituximab and 2 received cisplatin-based therapy[15]. Studies comparing seroconversion in patients with cancer versus controls, report seroconversion rates ranging from 72.5% [16] (retrospective) to 87.9 % (prospective) [17]. A similar finding in an anti-CD20 antibody-treated patient was noted in a recently published case report [18].
In our study, we observed significant and clinically meaningful differences in seroconversion rates in patients who had received anti-CD20 antibody therapy, and stem cell transplants. The biologic basis of this can be explained by the fact that anti-CD-20 antibody therapy, such as rituximab, does deplete native B-cells not only in lymphoid tissue but also in the bone marrow[19]. A statistically significant association was also seen with CAR-T cell therapy (supplement) and BiTE therapy, however given the small sample size, further validation in prospective cohorts is needed. CAR-T cells directed toward CD-19 also deplete native B cells leading to hypogammaglobulinemia, often needing intravenous immunoglobulin replacement[20]. Patients who are recipients of stem cell transplantation are often subject to myeloablative doses of chemotherapy and total body irradiation which contributes to profound immunosuppression in these patients. Our study in conjunction with existing literature highlights that patients with hematologic malignancies who have received the aforementioned therapies, will need close follow-up and monitoring to document clearance of infection. Among these patients, seroconversion might not occur, possibly raising the concern of recurrent infections. As vaccines against SARS-CoV-2 are planned to be distributed on a large scale, monitoring SARS-CoV-2 IgG, immunoglobulin levels and lymphocyte subsets may be warranted in this patient population. Booster dosing may need to be studied in future trials and considered for this patient population should initial antibody responses be blunted.
Encouragingly, our study demonstrated high rates of seroconversion in patients with solid malignancies, in particular those who received immunotherapy and endocrine therapy for cancer treatment. Immunotherapy continuation has been specifically raised as a concern for patients with COVID-19 as immune-mediated pneumonitis is a significant side effect. Moreover, immunotherapy, specifically among patients with lung cancer, was associated with increased risk of ICU admission in one series of 275 patients[21]. On the contrary, two large cohorts, the UK Coronavirus Cancer Monitoring Project (UKCCMP) and the COVID-19 and Cancer Consortium (CCC 19) reported that mortality was not affected in patients with cancer and COVID-19 by type of anti-cancer therapy, including immunotherapy[4, 22]. It is also hypothesized that immune-checkpoint inhibitors may induce immunocompetence in patients infected with SARS-CoV-2 [23] based on prior data from human immunodeficiency virus and immunotherapy and ongoing trials with nivolumab in patients with sepsis[24, 25]. Our 100% seroconversion rate provides supportive evidence that immunotherapy is not deleterious and rather, may support the hypothesis of restoring immunocompetence in patients with COVID-19. In addition, we note strong trends towards inferior seropositivity rates among patients receiving steroid therapy both prior to SARS-CoV-2 infection as well as for the management of Covid-19.
It is worth highlighting that patients receiving endocrine therapy for their malignancy (mostly breast cancer and prostate cancer) typically tend to have limited or no other cancer therapy exposure and therefore, less immunosuppression. This may explain our observation of strong positive seroconversion in patients treated with endocrine therapy.
A subset of patients in our cohort underwent serial SARS-CoV-2 IgG testing at provider discretion and 9 of 53 patients who were initially seropositive turned seronegative over time. These observations clearly need further validation in a larger cohort. Importantly, these may have implications for guidelines and possible advocacy for continued vaccination of this vulnerable population.
Asymptomatic infection has been identified as a significant factor in the community spread of SARS-CoV-2, which in turn, continues to propagate the pandemic[26]. As discussed previously, patients with cancer are prone to more symptomatic and serious illness. However, in our cohort, we found a surprisingly high rate of asymptomatic infections, with a higher frequency noted among patients with solid tumors. This finding is logical as patients with hematologic malignancies are known to be prone to more serious illness and poorer outcomes. Many patients in our cohort tested positive as part of routine screening prior to procedures, or during admission for unrelated acute problems. In some cases, patients who had contact with family members who were symptomatic with COVID-19 remained asymptomatic themselves. In a recent small study, seroconversion was noted in patients with cancer only if they had a symptomatic infection [27] In our cohort 41% patients were defined as asymptomatic (114/261). Of these, 108 had positive SARS-CoV-2 IgG (supplement table 4) and 6 had negative SARS-CoV-2 IgG. This finding suggests that asymptomatic infection also does lead to seroconversion in the majority of cases and possibly contributes to expansion of the pandemic and herd immunity.
Another significant finding noted in our study is the tendency towards more persistent shedding of SARS-CoV-2 in patients with hematologic malignancies with a mean of 61 days despite clinical improvement in many cases. While we are unable to confirm if virus was live in each patient our findings appear concordant with a recent study that reported patients who had received stem cell transplant and CAR-T cell therapy, shed viable virus for up to 2 months from onset of symptoms[28].
Our study has a few limitations warranting discussion, including its retrospective design and a small cohort among patients who received specific therapies which predicted seroconversion, calling for further validation in larger cohorts focused on these unique associations. Another limitation of the study may be a slight overestimation of the asymptomatic infection rate given the manner asymptomatic infection needed to be defined in a retrospective design. Our cohort also represents standard of care practice wherein testing was done at provider discretion and not as part of a prospective, controlled study, however as PCR negativity was required for patients to be able to resume cancer management in our practices, testing was frequent in the majority of patients.
In summary, we present the largest known cohort of patients with malignancy who underwent SARS-CoV-2 IgG testing. Statistically significant absent seroconversion was observed in patients with hematologic malignancies, patients receiving anti-CD-20 antibody therapy, CAR-T cell therapy and stem cell transplant. These findings may be impactful not only for clinical monitoring and surveillance, but also in designing and tailoring vaccination for this high-risk patient population. These findings should be investigated in larger, prospective studies for further validation but should provide immediate guidance for clinicians and researchers.
METHODS
Study Objectives
The primary objectives were to study the rate of seroconversion for SARS-CoV-2 IgG for patients with cancer and its association with type of malignancy and type of anti-cancer therapy. Additionally, we also aimed to study patterns in the natural history of COVID-19 and patients with cancer. Specifically, we studied the rate of symptomatic and asymptomatic infection in patients with cancer and COVID-19 and its association with type of malignancy and treatment received.
Study Design
This was a real-world, observational, retrospective exploratory cohort study of the entire pool of patients with a cancer diagnosis managed at our institution with the pre-specified criteria of positivity of one COVID test without prior hypotheses testing/power analyses. We collected data on demographic variables (age, sex, cancer diagnosis), comorbidities (excluding cancer itself), SARS-CoV-2 IgG result, SARS-CoV-2 RT-PCR result, cancer treatment history, onset of symptoms of COVID-19, subsequent disease course, treatment setting, complications and outcomes. The data were extracted through a retrospective chart-level medical record review using Montefiore Medical Center’s EPIC electronic health record system. All patient information was de-identified. The study was approved by the Institutional Review Board of Albert Einstein College of Medicine/MHS. Informed consent was waived by Montefiore-Einstein Institutional Review Board as this was a retrospective chart review study. Institutional Review Board at Montefiore-Einstein provided ethics oversight. IRB # 2020-11814.
Definitions
Asymptomatic infection
Patients were classified as having an asymptomatic infection if a) there was clear documentation at the time of a positive SARS-CoV-2 test that patient had no symptoms b) if there was documentation at the time of a SARS-CoV-2 IgG test that patient had no symptoms or c) a test result of SARS-CoV-2 PCR or IgG was present in the patient’s chart and documentation was unable to confirm that patient had any symptoms (i.e. symptoms unknown- as these patients could not have had more than minimal symptoms they were clustered with the “asymptomatic cohort”).
Active cancer
We noted patient’s malignancy status within 90 days preceding a SARS-CoV-2 test. Patients were classified as having an active malignancy if it was their initial diagnosis, relapsed or progressive disease. Patients were classified as having an inactive malignancy if their cancer was in remission or if they carried a diagnosis that did not warrant therapy (example, monoclonal gammopathy of unknown significance).
COVID-19 test methods (assay)
SARS-CoV-2 RT-PCR
Real time RT-PCR for SARS-CoV-2 was performed on nasopharyngeal swabs collected in viral transport media using one of three testing platforms. These include the Hologic Panther Fusion, Abbott m2000 and Cepheid GenXpert SARS-COV-2 assays. All testing was performed in accordance with manufacturer or laboratory EUA instructions. Each assay is designed to amplify two separate regions within the SARS-CoV-2 viral genome and one amplification control in a single multiplex reaction. The target regions of amplification differ by platform with Hologic amplifying 2 separate regions of ORF1a, Abbott amplifying RdRp and N genes and Cepheid amplifying portions of the N and E genes.
SARS-CoV-2 IgG test
IgG testing was performed using the Abbott SARS-COV-2 IgG assay which has received emergency authorization form the FDA. The assay is a high throughput chemiluminescent microparticle immunoassay (CMIA) designed to detect IgG antibodies to the nucleocapsid of SARS-CoV-2. Recombinant SARS-CoV-2 antigen is incubated with a patient serum or plasma sample. The presence of patient IgG in a sample reacts with anti-human IgG acridinium-labeled conjugate to produce a chemiluminescent reaction measured as relative light units (RLU). The greater the IgG present the higher the RLU value. This relationship is reflected in the calculated signal-to-cutoff index (S/C) produced upon comparing patient RLU to the assay calibrator. Positive results for IgG antibodies are determined when the S/C is >=1.4
Statistics and Reproducibility
Associations between pairs of variables were assessed with standard statistical procedures. In the case of two-level categorical variables, a Fisher’s exact test was used. For a two-level categorical and one numerical variable, we used a two-sample t-test and results were then re-tested by Wilcoxon testing. For a multi-level categorical and one numerical variable, an ANOVA test was carried out and results re-tested by non-parametric Kruskal-Wallis Rank Sum test. Pairings between a two-level and a multi-level categorical variable were summarized in a table where each row tests the association of a single multi-level category to the remaining, split by the two-level categories. We also performed a multivariate logistic regression analysis to account for key confounding variables, such as active cancer treatment and active versus inactive malignancy. Multiple hypothesis adjustments were not made for all analyses however to account for multiple testing, a FDR correction is provided in the supplement where more than two level testing was performed. Statistical analyses were not performed on cohorts of less than 5 subjects given instability of results in such small groups. Data was collected using Microsoft Excel and all analyses were run in R software version 3.6.2.
Supplementary Material
Acknowledgements
We would like to acknowledge cancer center grant P30 CA013330 and NCORP grant 2UG1CA189859-06 in providing funding for this project. This work was supported partly by the Jane A. and Myles P. Dempsey fund and the Pelka family fund. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Competing interests statement
The authors declare no competing interests
Code Availability Statement: The utilized computer code has been deposited in github (https://github.com/kith-pradhan/CovidCancerReport). All analyses were done with built-in and freely available R packages. Further information on research design is available in the Nature Research Reporting Summary linked to this article
Data Availability Statement:
Primary data will be made available from the corresponding authors upon request to protect patient privacy. Data availability may be subject to consultation with and contingent of approval from the Montefiore-Einstein IRB
References
- 1.Liu J, et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care, 2020. 10(1): p. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.-j., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine, 2020. 382(18): p. 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Annals of Oncology, 2020. 31(7): p. 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer NM, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. The Lancet, 2020. 395(10241): p. 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai M, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discovery, 2020. 10(6): p. 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta V, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discovery, 2020. 10(7): p. 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saini KS, et al. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer, 2020. 139: p. 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jørgensen TL, et al. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. British Journal of Cancer, 2012. 106(7): p. 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du R-H, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. European Respiratory Journal, 2020. 55(5): p. 2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee LYW, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. The Lancet Oncology, 2020. 21(10): p. 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brar G, et al. COVID-19 Severity and Outcomes in Patients With Cancer: A Matched Cohort Study. J Clin Oncol, 2020. 38(33): p. 3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryan A, et al. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol, 2020. 58(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manalac J, et al. Evaluation of Abbott anti-SARS-CoV-2 CMIA IgG and Euroimmun ELISA IgG/IgA assays in a clinical lab. Clin Chim Acta, 2020. 510: p. 687–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew KL, et al. Clinical evaluation of serological IgG antibody response on the Abbott Architect for established SARS-CoV-2 infection. Clin Microbiol Infect, 2020. 26(9): p. 1256.e9–1256.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garde-Noguera J, et al. Impact of SARS-CoV-2 Infection on Patients with Cancer: Retrospective and Transversal Studies in Spanish Population. Cancers (Basel), 2020. 12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, et al. Low prevalence of IgG antibodies to SARS-CoV-2 in cancer patients with COVID-19. International Journal of Cancer, 2020. 147(11): p. 3267–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marra A, et al. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Annals of oncology : official journal of the European Society for Medical Oncology, 2020: p. S0923-7534(20)42965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda H, et al. Persistent COVID-19 Pneumonia and Failure to Develop Anti-SARS-CoV-2 Antibodies During Rituximab Maintenance Therapy for Follicular Lymphoma. Clin Lymphoma Myeloma Leuk, 2020. 20(11): p. 774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houot R, et al. Could anti-CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine? Eur J Cancer, 2020. 136: p. 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brudno JN and Kochenderfer JN, Toxicities of chimeric antigen receptor T cells: recognition and management. Blood, 2016. 127(26): p. 3321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robilotti EV, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med, 2020. 26(8): p. 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee LY, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet (London, England), 2020. 395(10241): p. 1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivarelli S, et al. Cancer Management during COVID-19 Pandemic: Is Immune Checkpoint Inhibitors-Based Immunotherapy Harmful or Beneficial? Cancers (Basel), 2020. 12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day CL, et al. PD-1 expression on HIV-specific Tcells is associated with T-cell exhaustion and disease progression. Nature, 2006. 443(7109): p. 350–4. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, et al. Immune checkpoint inhibition in sepsis: a Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med, 2019. 45(10): p. 1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolai LA, et al. Asymptomatic SARS Coronavirus 2 infection: Invisible yet invincible. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 2020. 100: p. 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuereder T, et al. SARS-CoV-2 seroprevalence in oncology healthcare professionals and patients with cancer at a tertiary care centre during the COVID-19 pandemic. ESMO Open, 2020. 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aydillo T, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. New England Journal of Medicine, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data will be made available from the corresponding authors upon request to protect patient privacy. Data availability may be subject to consultation with and contingent of approval from the Montefiore-Einstein IRB


